Antibacterial, Antibiofilm, and Antiadhesive Properties of Different Quaternized Chitosan Derivatives
Abstract
1. Introduction
2. Results
2.1. Evaluation of MICs and MBCs Values of Quaternized Ch-Derivatives against P. aeruginosa and S. epidermidis
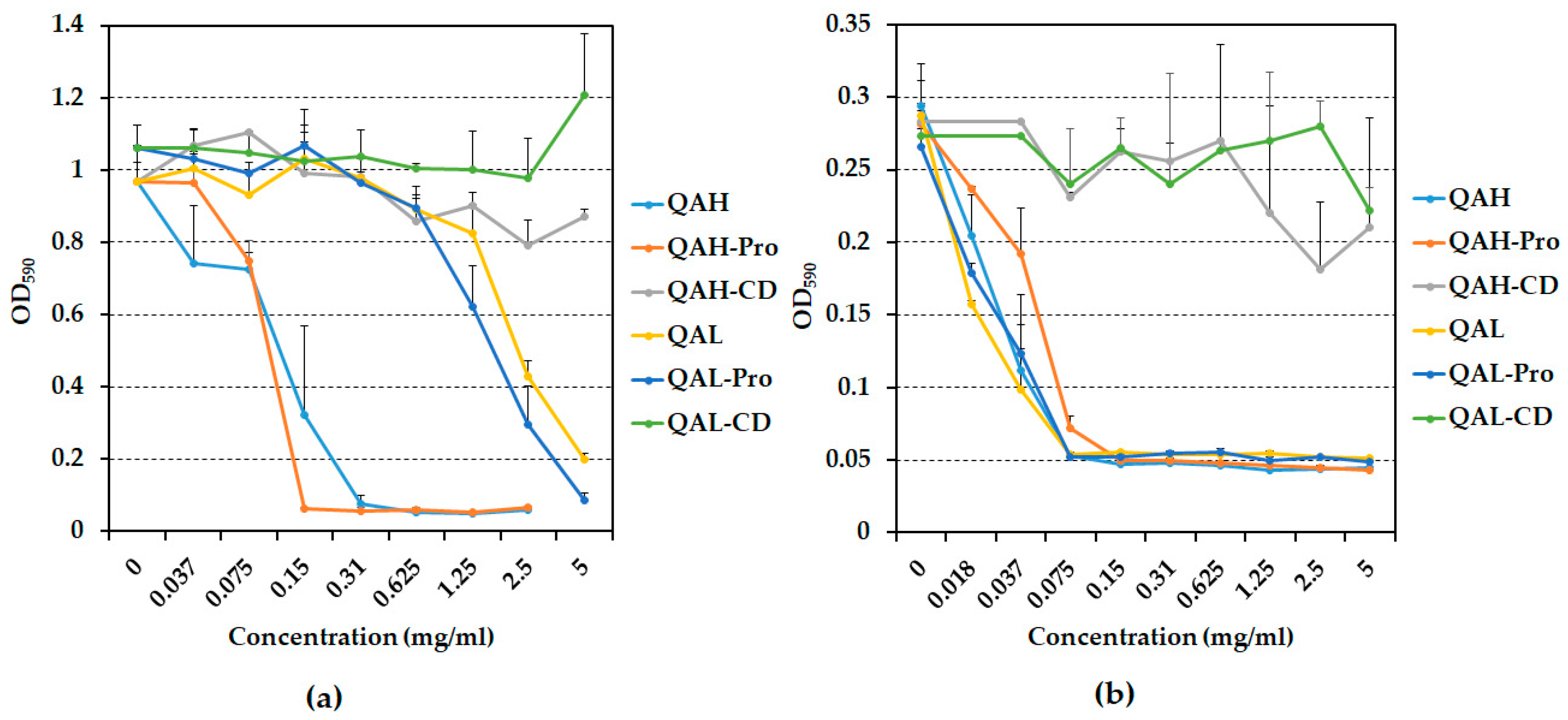
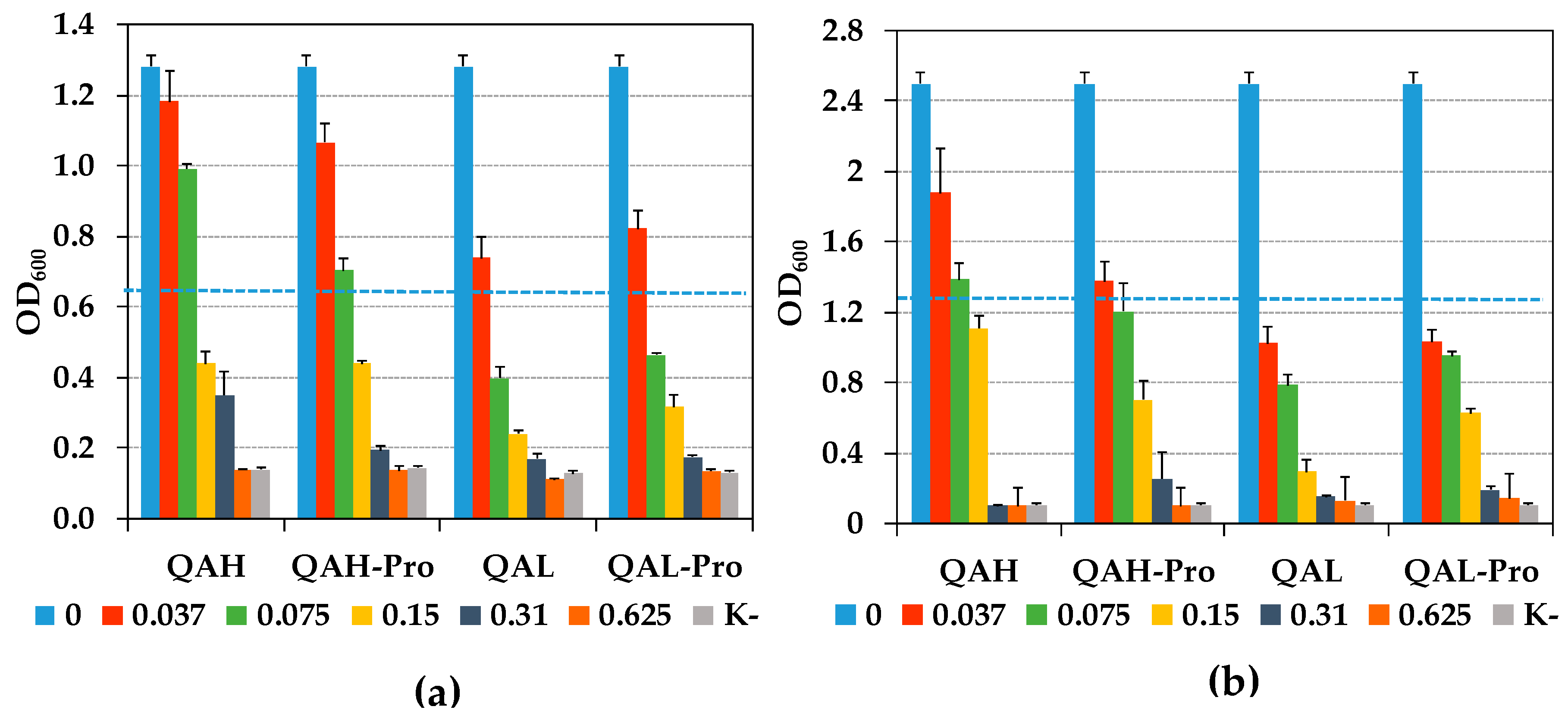
2.2. Ability of Quaternized Ch-Derivatives to Prevent Biofilm Formation by P. aeruginosa and S. epidermidis
2.3. Functionalization of Titanium Oxide Surfaces
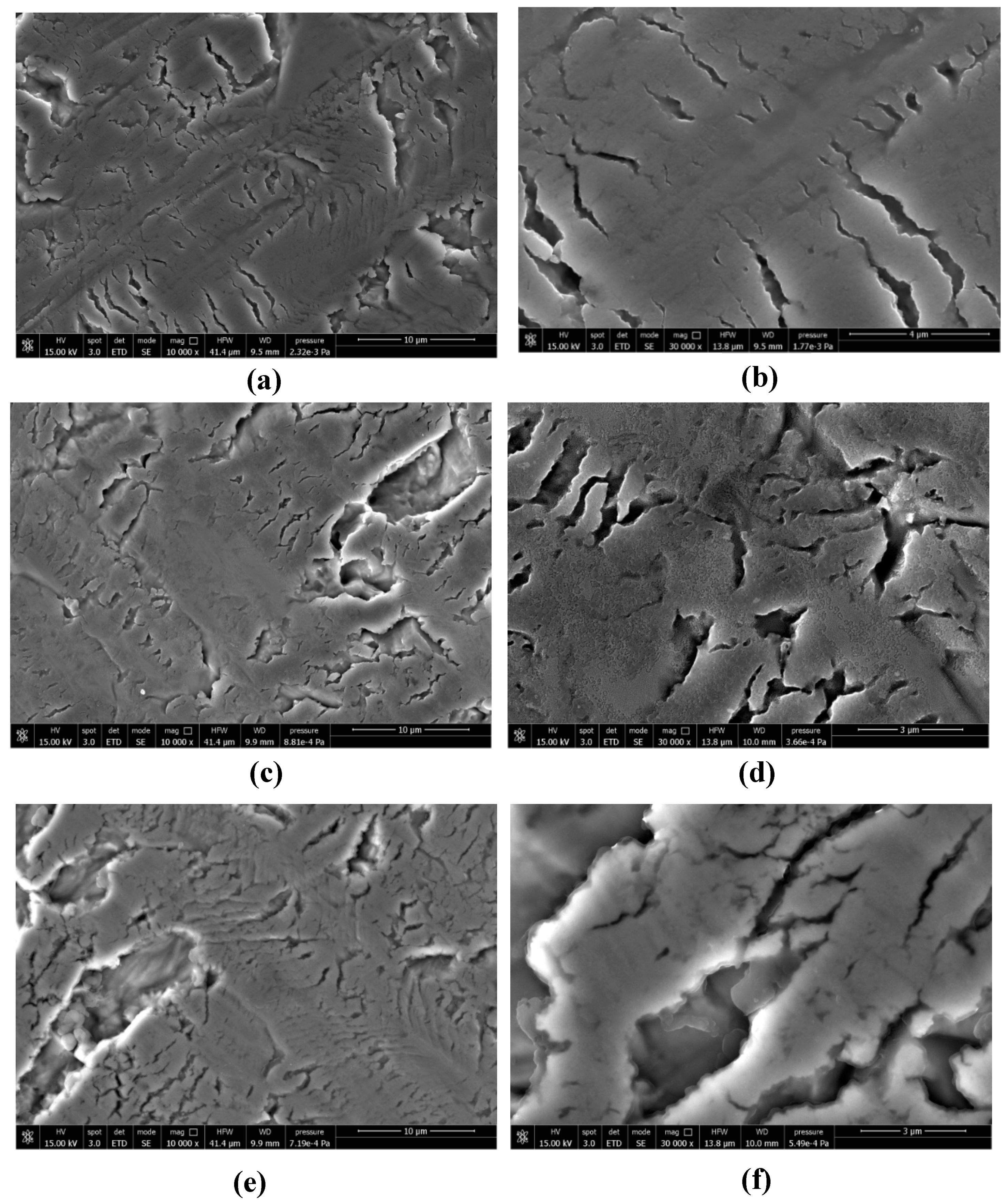
2.4. Surface Features of Polymer Grafted Titanium Surface
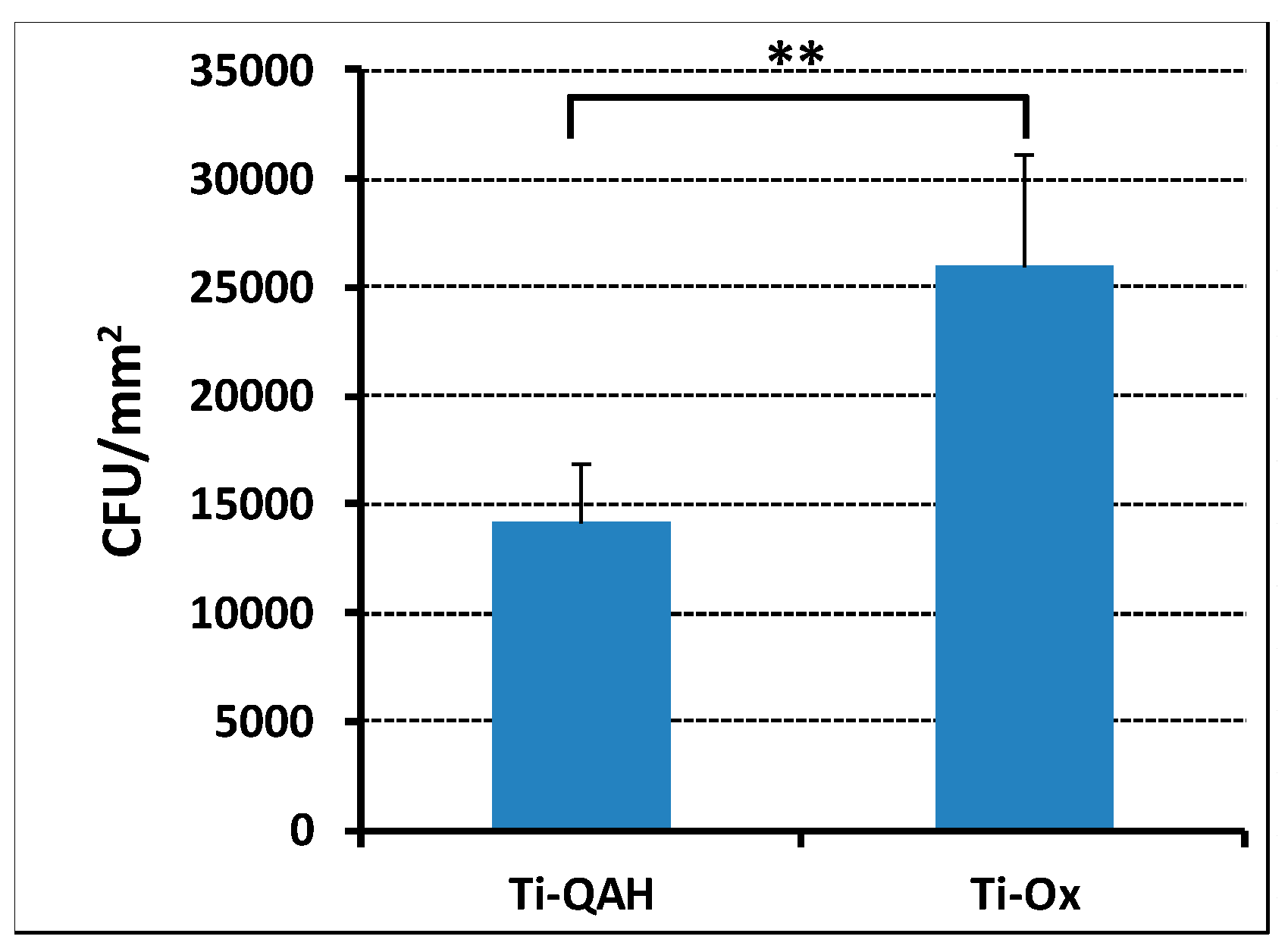
2.5. Ability of Ti-QAH to Prevent Adhesion of S. epidermidis
3. Discussion
3.1. Antibacterial Activity Toward P. aeruginosa and S. epidermidis
3.2. Prevention of Biofilm Formation
3.3. Titanium Functionalization and Prevention of Bacteria Adhesion
4. Materials and Methods
4.1. Polymeric Materials
4.2. Bacterials Strains
4.3. Determination of Minimal Inhibitory Concentrations (MICs) and Minimal Bactericidal Concentrations (MBCs)
4.4. Biofilm Inhibition Assays
4.5. Functionalization of Titanium Surface
- Washing: titanium samples, each of 0.25 mm thickness, two faces of 7 × 7 mm2 were cut from a commercial titanium foil (Titanium foil, thickness 0.25 mm, Sigma Aldrich). The samples were cleaned by sonication at 40 MHz (Sonica Ultrasonic Cleanermod 2200 ETH, SOLTEC) in acetone, ethanol and distilled water, in sequence, 10 min at 25 °C and dried with filter paper by capillarity.
- Etching: Each sample was treated with 3 mL of a 2:1 30% H2SO4-H2O2 solution (piranha solution), vortexed 10 s and left to react in a shaker water bath 10 min, 80 rpm. Finally, the samples were washed 3 times with 20 mL of distilled water and wiped dry with filter paper by capillarity, subsequently the drying was completed under vacuum at 37 °C.
- Silanizing and curing: the deposition of 3-aminopropyltrietoxysilane (APTES, Sigma-Aldrich) was achieved by immersing each titanium sample in 5 mL of a 5% (v/v) APTES solution in toluene under inert N2 atmosphere and leaving the reaction proceeding 24 h in a shaker water bath (25 °C, 80 rpm). Next, the samples were washed with 20 mL each of toluene, ethanol and deionized water, dried by wiping with filter paper, then under vacuum, followed by heating to 110 °C in an oil bath for 20 min under an N2 stream (curing). Additional details are reported in the supporting info file.
- Spacer insertion: glutaraldehyde was used as the spacer between APTES and each polymer. For spacer insertion each sample was immersed in 5 mL of a 5% (v/v) glutaraldehyde solution in deionized water and the reaction was left to proceed 24 h in a shaker water bath (25 °C, 80 rpm). Thereafter, the samples were washed three times by dipping in 20 mL of deionized water in sequence and wiped dry by capillarity with filter paper. Additional details are reported in the supporting info file.
- Grafting of Ch-derivatives: the reaction between the aldehyde functional groups and the polymer derivatives was carried out by dipping each sample in 2 mL of a 0.5 mg/mL polymer solution and leaving the reaction proceed in a shaker water bath 24 h at 25 °C and 80 rpm. Then the samples were washed again with deionized water and vacuum dried. Additional details are reported in the supporting info file.
4.6. Characterization of Polymer Grafted Titanium Surfaces
4.6.1. Quantification of APTES Amino Moieties with Picric Acid
4.6.2. Quantification of Polymer Grafting by Size Exclusion Chromatography (SEC)
4.6.3. Surface Zeta Potential
4.6.4. Surface Morphology and Elemental Analysis
4.6.5. Sterilization of Titanium Samples
4.7. Bacterial Adhesion Inhibition Assay on Titanium Plates Functionalized with QAH
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| APTES | 3-aminopropyltrietoxysilane |
| CFU | Colony forming unit |
| Ch | Chitosan |
| CSH | High molecular weight Chitosan |
| CSL | Low molecular weight Chitosan |
| CV | Crystal violet |
| DEAE | 2-diethylaminoethyl chloride |
| EOFM | Electroosmotic flow mapping |
| FE-SEM | Field Emission-Scanning Electron Microscopy |
| FPA | Focal plane array |
| HACC | N-2-hydroxypropyltrimethylammonium Chitosan |
| HDMI | Hexamethylene diidocyanate |
| MBCs | Minimal Bactericidal concentrations |
| MHB | Mueller Hinton broth |
| MICs | Minimal Inhibitory concentrations |
| 6-MNA | 6-mercaptonicotinamide 6-MNA |
| MW | Molecular weight |
| PIA | Polysaccharide intercellular adhesin |
| PS | Physiological solution |
| QAH | High molecular weight quaternized chitosan |
| QAH-CD | High molecular weight quaternized chitosan grafted with methyl-β-cyclodextrin |
| QAH-Pro | High molecular weight quaternized chitosan with pendant protected thiols |
| QAL | Low molecular weight quaternized chitosan |
| QAL-CD | Low molecular weight quaternized chitosan grafted with methyl-β-cyclodextrin |
| QAL-Pro | Low molecular weight quaternized chitosan with pendant protected thiols |
| SEC | Size exclusion cromatography |
| SEM | Scanning electron microscopy |
| TEA | triethylamine |
| Ti | Untreated titanium |
| Ti-OX | Titanium subjected to etching |
| Ti-QAH | Titanium surface grafted with QAH |
| Ti-QAHcyan | Titanium surface grafted with QAH by using hexamethylene diisocyanate as crosslinker |
| Ti-QAH-Pro | Titanium surface grafted with QAH-Pro |
| Ti-QAH-Pro_ML | Titanium surface grafted with QAH-Pro by multi-layer grafting approach |
| Ti-QAL | Titanium surface grafted with QAL |
| Ti-QALcyan | Titanium surface grafted with QAL by using hexamethylene diisocyanate as crosslinker |
| TSA | Tryptone Soy agar |
| TSB | Tryptone Soy broth |
References
- Cooper, M.A.; Shlaes, D. Fix the antibiotics pipeline. Nature 2011, 472, 32. [Google Scholar] [CrossRef] [PubMed]
- Silver, L.L. Challenges of antibacterial discovery. Clin. Microbiol. Rev. 2011, 24, 71–109. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Costerton, W.; Veeh, R.; Shirtliff, M.; Pasmore, M.; Post, C.; Ehrlich, G. The application of biofilm science to the study and control of chronic bacterial infections. J. Clin. Investig. 2003, 112, 1466–1477. [Google Scholar] [CrossRef]
- Penesyan, A.; Gillings, M.; Paulsen, I.T. Antibiotic discovery: Combatting bacterial resistance in cells and in biofilm communities. Molecules 2015, 20, 5286–5298. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Francolini, I.; Donelli, G. Prevention and control of biofilm-based medical-device-related infections. FEMS Immunol. Med. Microbiol. 2010, 59, 227–238. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Priority Pathogens List for R&D of New Antibiotics (27 February 2017). Available online: https://www.who.int/en/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 30 June 2019).
- Sandreschi, S.; Piras, A.M.; Batoni, G.; Chiellini, F. Perspectives on polymeric nanostructures for the therapeutic application of antimicrobial peptides. Nanomedicine 2016, 11, 1729–1744. [Google Scholar] [CrossRef]
- Raafat, D.; Sahl, H.G. Chitosan and its antimicrobial potential--a critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef]
- Martins, A.F.; Facchi, S.P.; Follmann, H.D.; Pereira, A.G.; Rubira, A.F.; Muniz, E.C. Antimicrobial activity of chitosan derivatives containing N-quaternized moieties in its backbone: A review. Int. J. Mol. Sci. 2014, 15, 20800–20832. [Google Scholar] [CrossRef] [PubMed]
- Zambito, Y.; Zaino, C.; Uccello-Barretta, G.; Balzano, F.; Di Colo, G. Improved synthesis of quaternary ammonium-chitosan conjugates (N+-Ch) for enhanced intestinal drug permeation. Eur. J. Pharm. Sci. 2008, 33, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Felice, F.; Zambito, Y.; Di Colo, G.; D’Onofrio, C.; Fausto, C.; Balbarini, A.; Di Stefano, R. Red grape skin and seeds polyphenols: Evidence of their protective effects on endothelial progenitor cells and improvement of their intestinal absorption. Eur. J. Pharm. Biopharm. 2012, 80, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Felice, F.; Zambito, Y.; Belardinelli, E.; Fabiano, A.; Santoni, T.; Di Stefano, R. Effect of different chitosan derivatives on in vitro scratch wound assay: A comparative study. Int. J. Biol. Macromol. 2015, 6, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Cesari, A.; Fabiano, A.; Piras, A.M.; Zambito, Y.; Uccello-Barretta, G.; Balzano, F. Binding and mucoadhesion of sulfurated derivatives of quaternary ammonium-chitosans and their nanoaggregates: An NMR investigation. J. Pharm. Biomed. Anal. 2019, 177, 112852. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Peng, Z.; Li, Q.; Xu, X.; Guo, S.; Tang, T. The use of quaternised chitosan-loaded PMMA to inhibit biofilm formation and downregulate the virulence-associated gene expression of antibiotic-resistant Staphylococcus. Biomaterials 2012, 33, 365–377. [Google Scholar] [CrossRef]
- Laskar, K.; Faisal, S.M.; Rauf, A.; Ahmed, A.; Owais, M. Undec-10-enoic acid functionalized chitosan based novel nano-conjugate: An enhanced anti-bacterial/biofilm and anti-cancer potential Carbohydr. Polym. 2017, 166, 14–23. [Google Scholar] [CrossRef]
- Piras, A.M.; Zambito, Y.; Burgalassi, S.; Monti, D.; Tampucci, S.; Terreni, E.; Fabiano, A.; Balzano, F.; Uccello-Barretta, G.; Chetoni, P. A water-soluble, mucoadhesive quaternary ammonium chitosan-methyl-β-cyclodextrin conjugate forming inclusion complexes with dexamethasone. J. Mater. Sci. Mater. Med. 2018, 29, 42. [Google Scholar] [CrossRef]
- Piras, A.M.; Fabiano, A.; Chiellini, F.; Zambito, Y. Methyl-β-cyclodextrin quaternary ammonium chitosan conjugate: Nanoparticles vs macromolecular soluble complex. Int. J. Nanomed. 2018, 13, 2531–2541. [Google Scholar] [CrossRef]
- Fabiano, A.; Bizzarri, R.; Zambito, Y. Thermosensitive hydrogel based on chitosan and its derivatives containing medicated nanoparticles for transcorneal administration of 5-fluorouracil. Int. J. Nanomed. 2017, 12, 633–643. [Google Scholar] [CrossRef]
- Batoni, G.; Maisetta, G.; Esin, S. Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. Biochim. Biophys. Acta 2016, 1858, 1044–1060. [Google Scholar] [CrossRef] [PubMed]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, I.; Mezzetta, A.; Guazzelli, L.; Sartini, S.; Pomelli, C.S.; Parker, W.O.; Chiappe, C. Chiral ionic liquids supported on natural sporopollenin microcapsules. RSC Adv. 2018, 8, 21174–21183. [Google Scholar] [CrossRef]
- Leedy, M.R.; Martin, H.J.; Norowski, P.A.; Jennings, J.A.; Haggard, W.O.; Bumgardner, J.D. Use of Chitosan as a Bioactive Implant Coating for Bone-Implant Applications. In Chitosan for Biomaterials II; Jayakumar, R., Prabaharan, M., Muzzarelli, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Adv. Polym. Sci. 2011, 244, 129–165. [Google Scholar] [CrossRef]
- Sallem, F.; Boudon, J.; Heintz, O.; Séverin, I.; Megriche, A.; Millot, N. Synthesis and characterization of chitosan-coated titanate nanotubes: Towards a new safe nanocarrier. Dalton Trans. 2017, 46, 15386–15398. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.J.; Schulz, K.H.; Bumgardner, J.D.; Walters, K.B. XPS study on the use of 3-aminopropyltriethoxysilane to bond chitosan to a titanium surface. Langmuir 2007, 23, 6645–6651. [Google Scholar] [CrossRef]
- Wang, M.; Tang, T. Surface treatment strategies to combat implant-related infection from the beginning. J. Orthop. Transl. 2018, 17, 42–54. [Google Scholar] [CrossRef]
- Gomes, F.; Teixeira, P.; Oliveira, R. Mini-review: Staphylococcus epidermidis as the most frequent cause of nosocomial infections: Old and new fighting strategies. Biofouling 2014, 30, 131–141. [Google Scholar] [CrossRef]
- Tan, H.; Ma, R.; Lin, C.; Liu, Z.; Tang, T. Quaternized chitosan as an antimicrobial agent: Antimicrobial activity, mechanism of action and biomedical applications in orthopedics. Int. J. Mol. Sci. 2013, 14, 1854–1869. [Google Scholar] [CrossRef]
- Peng, Z.X.; Wang, L.; Du, L.; Guo, S.R.; Wang, X.Q.; Tang, T.T. Adjustment of the antibacterial activity and biocompatibility of hydroxypropyltrimethyl ammonium chloride chitosan by varying the degree of substitution of quaternary ammonium. Carbohydr. Polym. 2010, 81, 275–283. [Google Scholar] [CrossRef]
- Xu, T.; Xin, M.H.; Li, M.C.; Huang, H.L.; Zhou, S.Q.; Liu, J.Z. Synthesis, characterization and antibacterial activity of N,O-quaternary ammonium chitosan. Carbohydr. Res. 2011, 346, 2445–2450. [Google Scholar] [CrossRef]
- Kenawy, E.R.; Abdel-Hay, F.I.; El-Raheem, A.; El-Shanshoury, R.; El-Newehy, M.H. Biologically active polymers: Synthesis and antimicrobial activity of modified glycidyl methacrylate polymers having a quaternary ammonium and phosphonium groups. J. Control. Release 1998, 50, 145–152. [Google Scholar] [CrossRef]
- Chi, W.; Qin, C.; Zeng, L.; Li, W.; Wang, W. Microbiocidal activity of chitosan-N-2-hydroxypropyl trimethyl ammonium chloride. J. Appl. Polym. Sci. 2007, 103, 3851–3856. [Google Scholar] [CrossRef]
- Maisetta, G.; Grassi, L.; Esin, S.; Serra, I.; Scorciapino, M.A.; Rinaldi, A.C.; Batoni, G. The Semi-Synthetic Peptide Lin-SB056-1 in Combination with EDTA Exerts Strong Antimicrobial and Antibiofilm Activity against Pseudomonas aeruginosa in Conditions Mimicking Cystic Fibrosis Sputum. Int. J. Mol. Sci. 2017, 18, 1994. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Su, Y.P.; Chen, C.C.; Jia, G.; Wang, H.L.; Wu, J.C.; Lin, J.G. Relationship between antibacterial activity of chitosan and surface characteristics of cell wall. Acta Pharmacol. Sin. 2004, 25, 932–936. [Google Scholar] [PubMed]
- Lin, W.T.; Zhang, Y.Y.; Tan, H.L.; Ao, H.Y.; Duan, Z.L.; He, G.; Tang, T.T. Inhibited bacterial adhesion and biofilm formation on quaternized chitosan-loaded titania nanotubes with various diameters. Materials 2016, 9, 155. [Google Scholar] [CrossRef]
- Peng, Z.X.; Tu, B.; Shen, Y.; Du, L.; Wang, L.; Guo, S.R.; Tang, T.T. Quaternized chitosan inhibits icaA transcription and biofilm formation by Staphylococcus on a titanium surface. Antimicrob. Agents Chemother. 2011, 55, 860–866. [Google Scholar] [CrossRef]
- Defoirdt, T. Quorum-Sensing systems as targets for antivirulence therapy. Trends Microbiol. 2018, 26, 313–328. [Google Scholar] [CrossRef]
- Muslim, S.N.; Kadmy, I.M.S.A.; Ali, A.N.M.; Salman, B.K.; Ahmad, M.; Khazaal, S.S.; Hussein, N.H.; Muslim, S.N. Chitosan extracted from Aspergillus flavus shows synergistic effect, eases quorum sensing mediated virulence factors and biofilm against nosocomial pathogen Pseudomonas aeruginosa. Int. J. Biol. Macromol. 2018, 107, 52–58. [Google Scholar] [CrossRef]
- Rubini, D.; Banu, S.F.; Subramani, P.; Hari, B.N.V.; Gowrishankar, S.; Pandian, S.K.; Wilson, A.; Nithyanand, P. Extracted chitosan disrupts quorum sensing mediated virulence factors in urinary tract infection causing pathogens. Pathog. Dis. 2019, 77, ftz009. [Google Scholar] [CrossRef]
- Brancatisano, F.L.; Maisetta, G.; Di Luca, M.; Esin, S.; Bottai, D.; Bizzarri, R.; Campa, M.; Batoni, G. Inhibitory effect of the human liver-derived antimicrobial peptide hepcidin 20 on biofilms of polysaccharide intercellular adhesin (PIA)-positive and PIA-negative strains of Staphylococcus epidermidis. Biofouling 2014, 30, 435–446. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Kisch, J.; Albrektsson, T.; Wennerberg, A. A retrospective study on clinical and radiological outcomes of oral implants in patients followed up for a minimum of 20 years. Clin. Implant Dent. Relat. Res. 2018, 20, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.C.M.; Sordi, M.B.; Kanazawa, M.; Ravindran, S.; Henriques, B.; Silva, F.S.; Aparicio, C.; Cooper, L.F. Nano-scale modification of titanium implant surfaces to enhance osseointegration. Acta Biomater. 2019, 94, 112–131. [Google Scholar] [CrossRef] [PubMed]
- D’Almeida, M.; Attik, N.; Amalric, J.; Brunon, C.; Renaud, F.; Abouelleil, H.; Toury, B.; Grosgogeat, B. Chitosan coating as an antibacterial surface for biomedical applications. PLoS ONE 2017, 12, e0189537. [Google Scholar] [CrossRef] [PubMed]
- Foss, B.L.; Ghimire, N.; Tang, R.; Sun, Y.; Deng, Y. Bacteria and osteoblast adhesion to chitosan immobilized titanium surface: A race for the surface. Colloids Surf. B Biointerfaces 2015, 134, 370–376. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ghimire, N.; Luo, J.; Tang, R.; Sun, Y.; Deng, Y. Novel anti-infective activities of chitosan immobilized titanium surface with enhanced osteogenic properties. Colloids Surf. B Biointerfaces 2014, 122, 126–133. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nazarov, D.V.; Zemtsova, E.G.; Solokhin, A.Y.; Valiev, R.Z.; Smirnov, V.M. Modification of the surface topography and composition of ultrafine and coarse grained titanium by chemical etching. Nanomaterials 2017, 7, 15. [Google Scholar] [CrossRef]
- Variola, F.; Yi, J.H.; Richert, L.; Wuest, J.D.; Rosei, F.; Nanci, A. Tailoring the surface properties of Ti6Al4V by controlled chemical oxidation. Biomaterials 2008, 29, 1285–1298. [Google Scholar] [CrossRef]
- Vashist, S.K.; Lam, E.; Hrapovic, S.; Male, K.B.; Luong, J.H. Immobilization of antibodies and enzymes on 3-aminopropyltriethoxysilane-functionalized bioanalytical platforms for biosensors and diagnostics. Chem. Rev. 2014, 114, 11083–11130. [Google Scholar] [CrossRef]
- Renoud, P.; Toury, B.; Benayoun, S.; Attik, G.; Grosgogeat, B. Functionalization of titanium with chitosan via silanation: Evaluation of biological and mechanical performances. PLoS ONE 2012, 7, e39367. [Google Scholar] [CrossRef]
- Peng, Z.; Ao, H.; Wang, L.; Guo, S.; Tang, T. Quaternised Chitosan Coating on Titanium Provides a Self-protective Surface that Prevents Bacterial Colonisation and Implant-associated Infections. RSC Adv. 2015, 5, 54304–54311. [Google Scholar] [CrossRef]
- Mao, S.; Shuai, X.; Unger, F.; Simon, M.; Bi, D.; Kissel, T. The depolymerization of chitosan: Effects on physicochemical and biological properties. Int. J. Pharm. 2004, 281, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Zambito, Y.; Uccello-Barretta, G.; Zaino, C.; Balzano, F.; Di Colo, G. Novel transmucosal absorption enhancers obtained by aminoalkylation of chitosan. Eur. J. Pharm. Sci. 2006, 29, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, A.; Piras, A.M.; Uccello-Barretta, G.; Balzano, F.; Cesari, A.; Testai, L.; Citi, V.; Zambito, Y. Impact of mucoadhesive polymeric nanoparticulate systems on oral bioavailability of a macromolecular model drug. Eur. J. Pharm. Biopharm. 2018, 130, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.M.; Sandreschi, S.; Maisetta, G.; Esin, S.; Batoni, G.; Chiellini, F. Chitosan nanoparticles for the linear release of model cationic Peptide. Pharm. Res. 2015, 32, 2259–2265. [Google Scholar] [CrossRef]
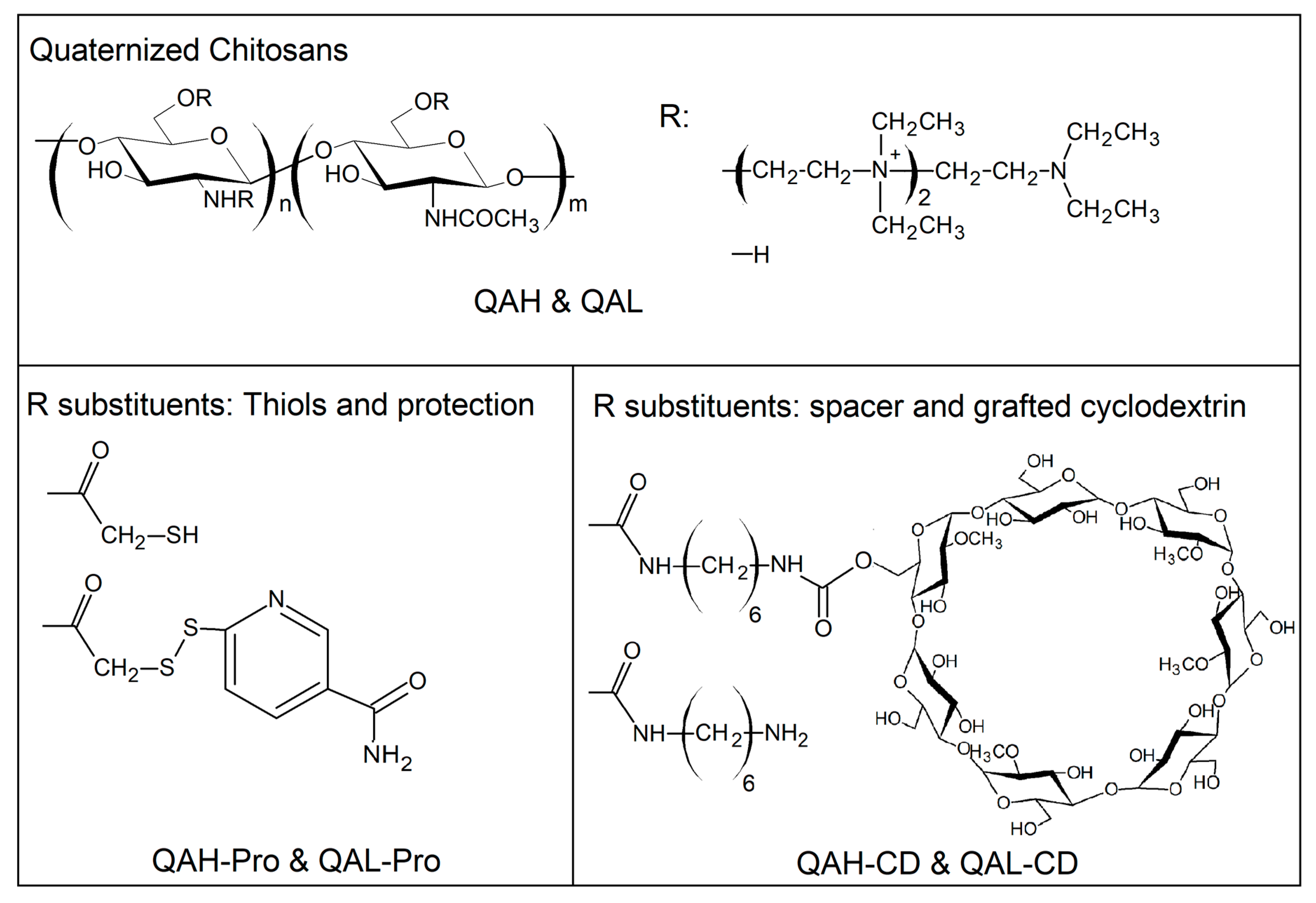


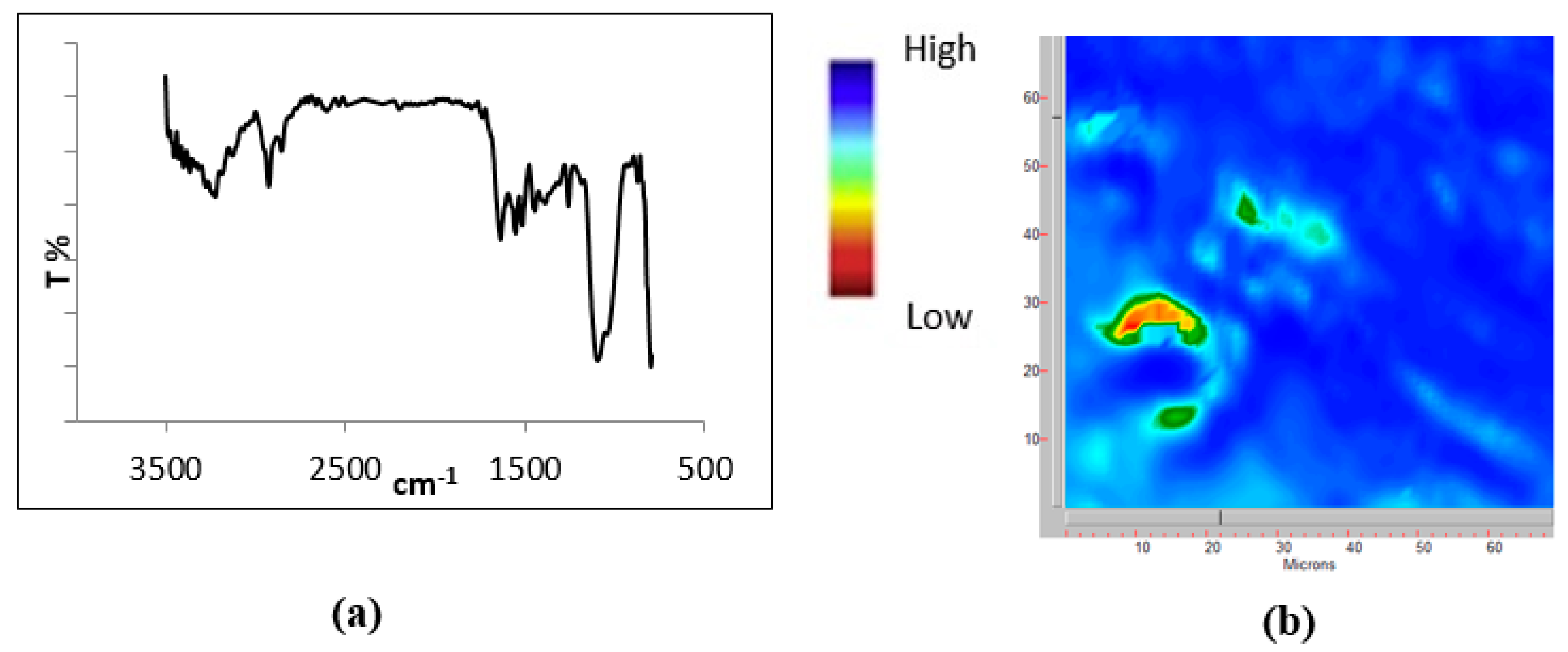
| Polymers | MW, kDa; A2 | AD a, % | QD b, %; n | TD c, % | DSD d, % | PD e, % | CDD f, % |
|---|---|---|---|---|---|---|---|
| CSH | 379; 0.0009 | − | − | − | − | − | |
| CSL | 131; 0.0008 | − | − | − | − | − | − |
| QAH | 428; 0.0001 | 7 | 85; 2 | − | − | − | − |
| QAL | 163; 0.0013 | 3 | 80; 2 | − | − | − | − |
| QAH-Pro | 460; 0.0001 | 7 | 85; 2 | 1.1 | 17.0 | 3.1 | − |
| QAL-Pro | 161; 0.0007 | 3 | 80; 2 | 1.2 | 14.9 | 2.3 | − |
| QAH-CD | 2344; 0.0001 | 7 | 85; 2 | − | − | − | 16 |
| QAL-CD | 633; 0.0001 | 3 | 80; 2 | − | − | − | 10 |
| Chitosan Derivatives | MICs | MBCs | ||
|---|---|---|---|---|
| P. aeruginosa | S. epidermidis | P. aeruginosa | S. epidermidis | |
| QAH | 0.31 | 0.075 | >2.5 | 0.075 |
| QAH-Pro | 0.15 | 0.075 | >2.5 | 0.31 |
| QAH-CD | >5 | >5 | − | − |
| QAL | 5 | 0.075 | >5 | 0.075 |
| QAL-Pro | 5 | 0.075 | >5 | 0.15 |
| QAL-CD | >5 | >5 | − | − |
| Sample | Linker | Polymer, µg/mm2 |
|---|---|---|
| Ti-QAH | Glutaraldehyde | 3.9 ± 0.02 |
| Ti-QAL | Glutaraldehyde | 0.7 ± 0.22 |
| Ti-QAHSPro | Glutaraldehyde | − |
| Ti-QAH-Pro_ML | Multi-layering Glutaraldehyde | 0.4 ± 0.20 |
| Ti-QAH_Cyan | HDMI | 4.5 ± 1.20 |
| Ti-QAL_Cyan | HDMI | 1.0 ± 0.81 |
| Element | Ti | Ti-Ox | Ti-QAH |
|---|---|---|---|
| Carbon | 1.5 | 1.5 | 25.2 |
| Oxygen | 3.9 | 8.9 | 25.5 |
| Titanium | 94.6 | 89.6 | 43.5 |
| Silicium | 3.1 | ||
| Natrium | 2.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piras, A.M.; Esin, S.; Benedetti, A.; Maisetta, G.; Fabiano, A.; Zambito, Y.; Batoni, G. Antibacterial, Antibiofilm, and Antiadhesive Properties of Different Quaternized Chitosan Derivatives. Int. J. Mol. Sci. 2019, 20, 6297. https://doi.org/10.3390/ijms20246297
Piras AM, Esin S, Benedetti A, Maisetta G, Fabiano A, Zambito Y, Batoni G. Antibacterial, Antibiofilm, and Antiadhesive Properties of Different Quaternized Chitosan Derivatives. International Journal of Molecular Sciences. 2019; 20(24):6297. https://doi.org/10.3390/ijms20246297
Chicago/Turabian StylePiras, Anna Maria, Semih Esin, Arianna Benedetti, Giuseppantonio Maisetta, Angela Fabiano, Ylenia Zambito, and Giovanna Batoni. 2019. "Antibacterial, Antibiofilm, and Antiadhesive Properties of Different Quaternized Chitosan Derivatives" International Journal of Molecular Sciences 20, no. 24: 6297. https://doi.org/10.3390/ijms20246297
APA StylePiras, A. M., Esin, S., Benedetti, A., Maisetta, G., Fabiano, A., Zambito, Y., & Batoni, G. (2019). Antibacterial, Antibiofilm, and Antiadhesive Properties of Different Quaternized Chitosan Derivatives. International Journal of Molecular Sciences, 20(24), 6297. https://doi.org/10.3390/ijms20246297










