Longitudinal Cognitive Decline in a Novel Rodent Model of Cerebral Amyloid Angiopathy Type-1
Abstract
1. Introduction
2. Results
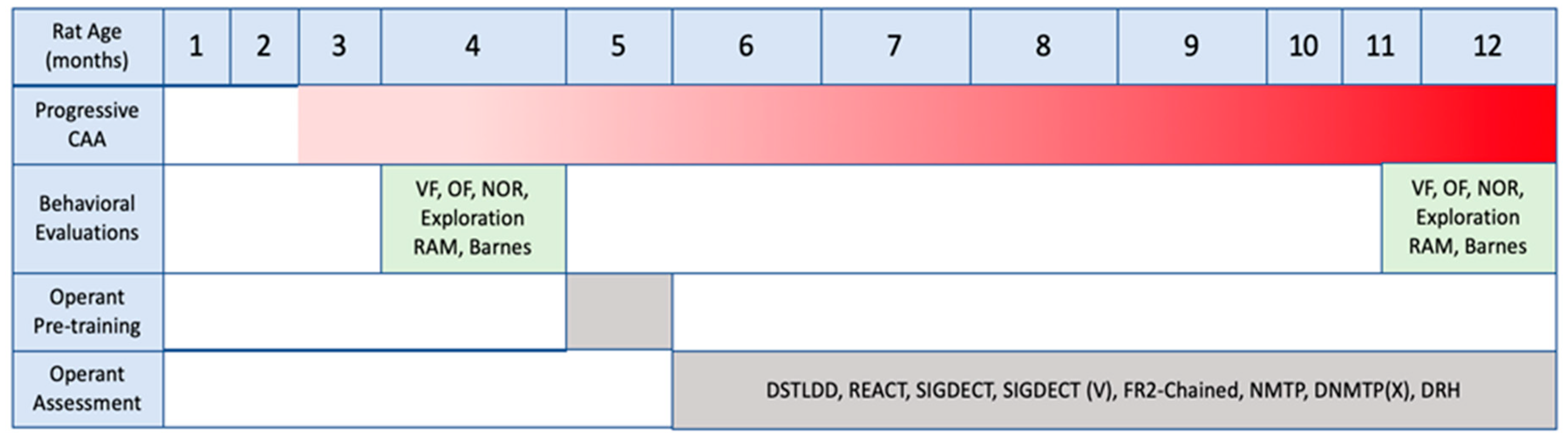
2.1. Overall Study Design
2.2. rTg-DI Rats
2.3. Health and Development of Rats
2.4. Operant Schedule Assessment
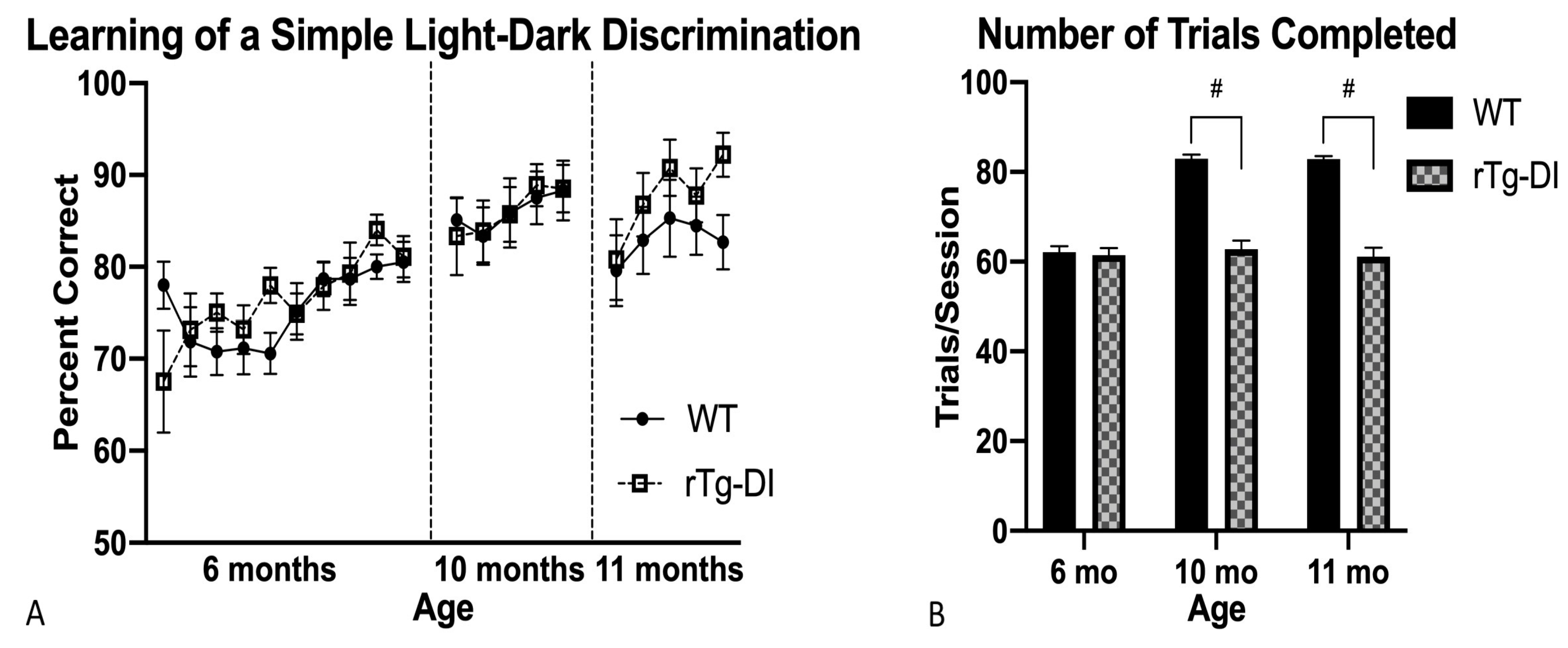
2.4.1. Light–Dark Discrimination
2.4.2. Reaction Time
2.4.3. Signal Detection
2.4.4. Signal Detection, Variable Pre-Stimulus Interval
2.4.5. FR2-Chained Responding
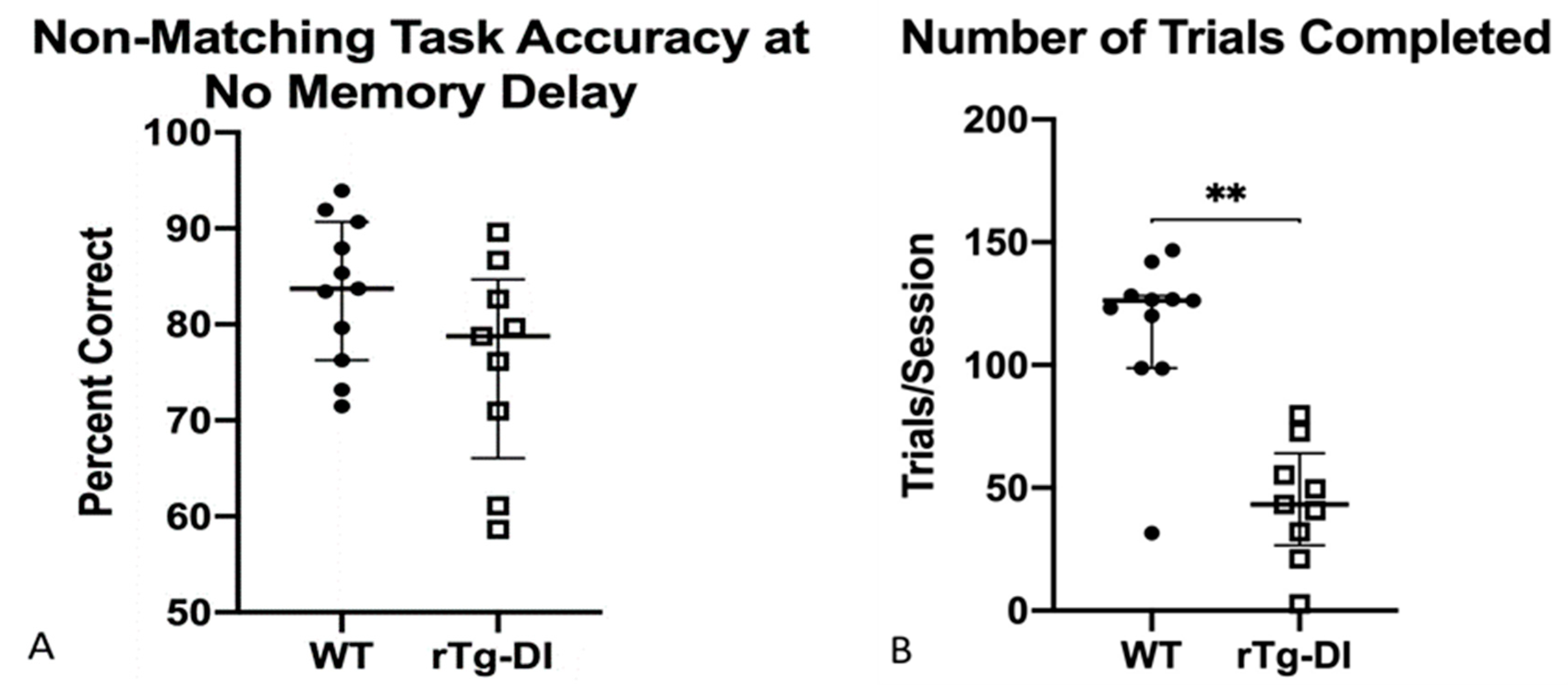
2.4.6. Non-Matching to Position (NMTP)
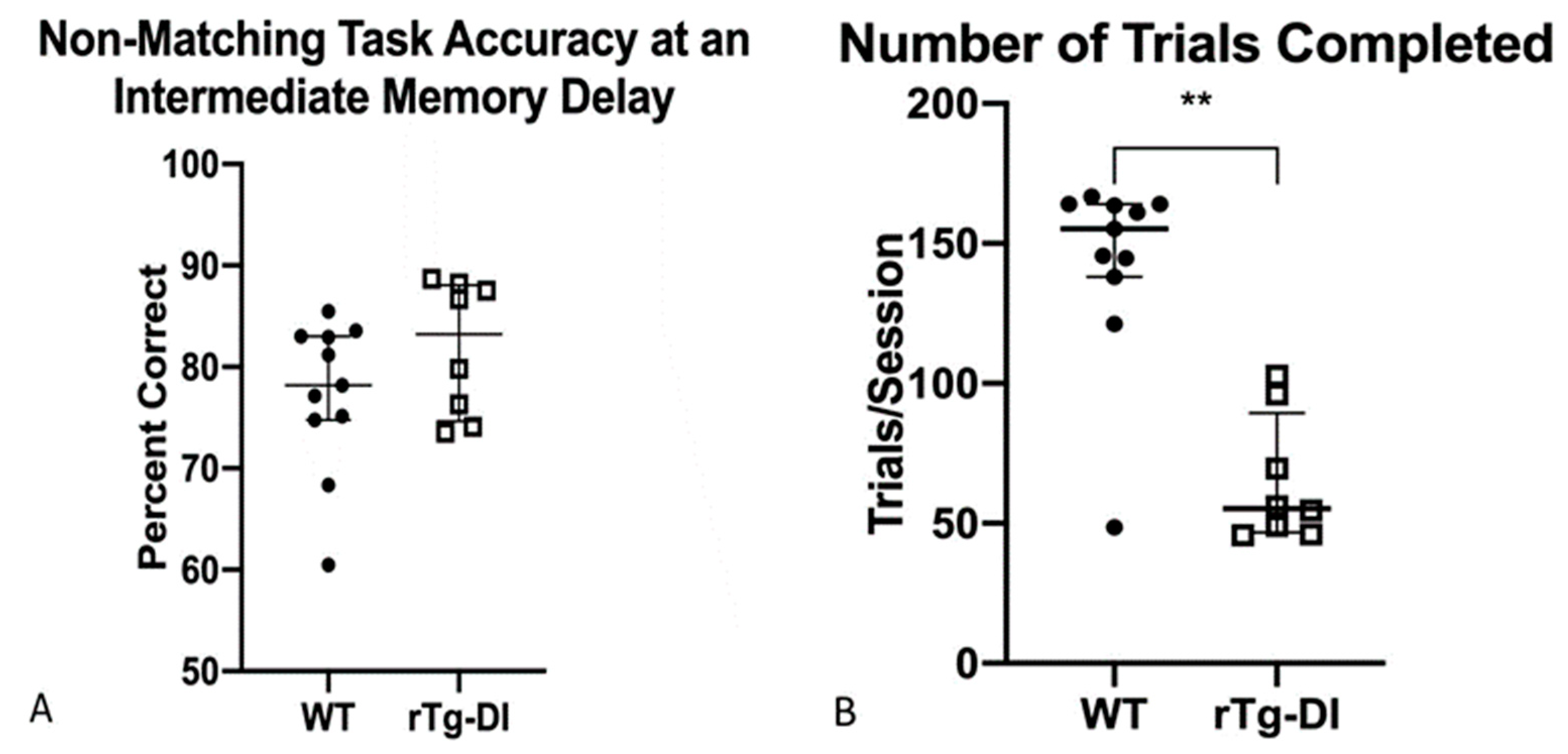
2.4.7. Delayed Non-Matching to Position: 5 s Delay Time Point, DNMTP (5)
2.4.8. Differential Reinforcement: High Responding (DRH)
2.5. Non-Operant Behavioral Evaluation
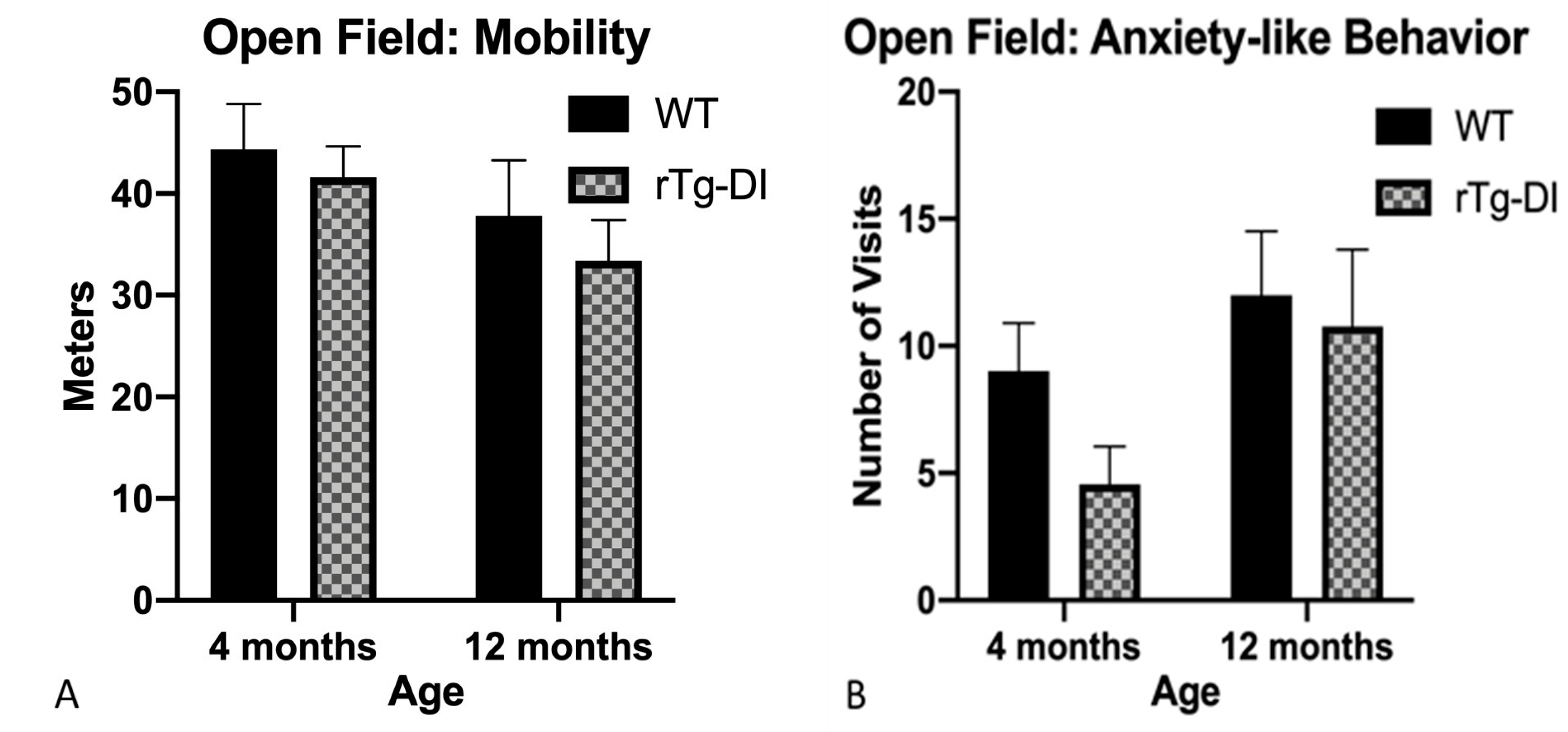
2.5.1. Open Field
2.5.2. Radial Arm Maze
2.5.3. Novel Object Recognition (NOR)
2.5.4. Barnes Maze
2.5.5. Von Frey Hairs
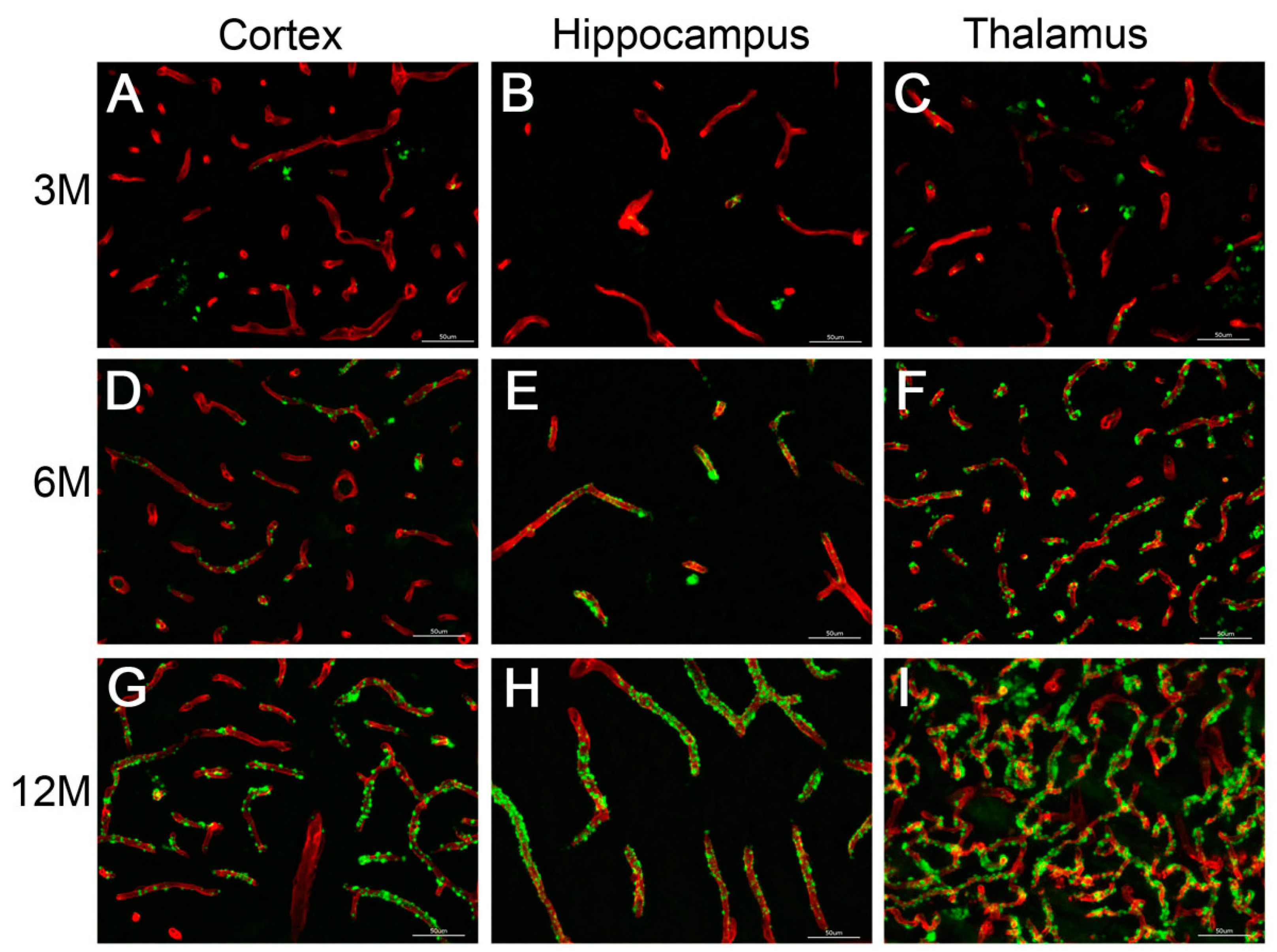
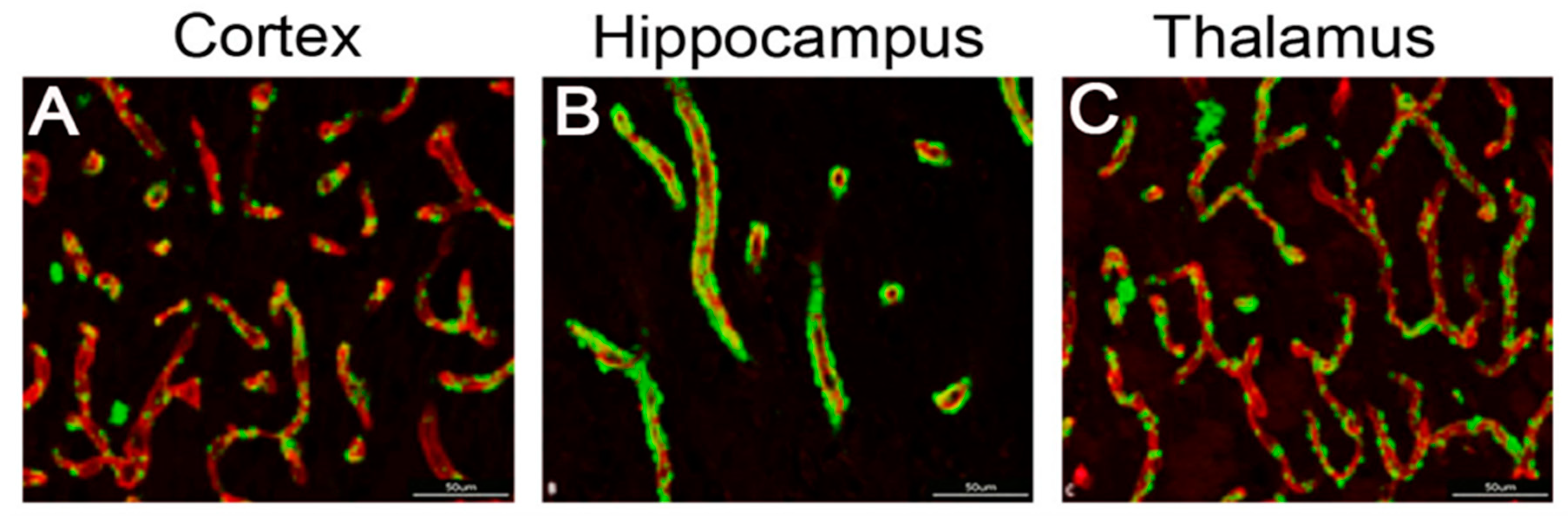
2.6. CAA Pathology
3. Discussion
Progression and Nature of the Behavioral Impairment
4. Materials and Methods
4.1. Subjects
4.2. Apparatus and Testing Procedures
4.3. Operant Schedule Training
4.4. Testing Environment Habituation and Pre-Training
4.5. Operant Schedule Assessment Procedures
4.5.1. Light–Dark Discrimination (DSTLDD)
4.5.2. Response Reaction Time (REACT)
4.5.3. Stimulus Detection (SIGDECT)
4.5.4. Signal Detection Varied Pre-Stimulus Interval (SIGDECTV)
4.5.5. FR2-Chained Response
4.5.6. Non-Matching to Position (NMTP)
4.5.7. Delayed Non-Match to Position Variation: DNMTP(X)
4.5.8. Differential Reinforcement High Rate of Response (DRH)
4.6. Physical and Cognitive Evaluation Procedures
4.6.1. Paw Withdrawal Reflex
4.6.2. Open Field
4.6.3. Radial Arm Maze (RAM)
4.6.4. Novel Object Recognition (NOR)
4.6.5. Novel Exploration
4.6.6. Barnes Circular Maze
4.7. Tissues Oreparation and Histopathology
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CAA | Cerebral amyloid angiopathy |
| Aβ | Amyloid β-protein |
| APP | Amyloid precursor protein |
| AD | Alzheimer’s disease |
| WT | Wild-type |
| VCID | Vascular-mediated cognitive impairment and dementia |
| VF | Von Frey hairs |
| OF | Open Field |
| NOR | Novel Object Recognition |
| RAM | Radial Arm Maze |
| NE | Novel Exploration |
| DSTLDD | Light–dark discrimination |
| REACT | Reaction time |
| SIGDECT | Stimulus detection |
| NMTP/DNMTP | Non-matching to position/delayed non-matching to position |
| DRH | Differential Reinforcement: high responding |
| ITI | Inter-trial interval |
| ANOVA | Analysis of variance |
References
- Biffi, A.; Greenberg, S.M. Cerebral amyloid angiopathy: A systematic review. J. Clin. Neurol. 2011, 7, 1–9. [Google Scholar] [CrossRef]
- Banerjee, G.; Carare, R.; Cordonnier, C.; Greenberg, S.M.; Schneider, J.A.; Smith, E.E.; Buchem, M.V.; Grond, J.V.; Verbeek, M.M.; Werring, D.J. The increasing impact of cerebral amyloid angiopathy: Essential new insights for clinical practice. J. Neurol. Neurosurg. Psychiatry 2017, 88, 982–994. [Google Scholar] [CrossRef]
- Charidimou, A.; Gang. Q.; Werring, D.J. Sporadic cerebral amyloid angiopathy revisited: Recent insights into pathophysiology and clinical spectrum. J. Neurol. Neurosurg. Psychiatry 2011, 83, 124–137. [Google Scholar] [CrossRef]
- Thal, D.R.; Ghebremedhin, E.; Rub, U.; Yamaguchi, H.; Del Tredici, K.; Braak, H. Two types of sporadic cerebral amyloid angiopathy. J. Neuropathol. Exp. Neurol. 2002, 61, 282–293. [Google Scholar] [CrossRef]
- Vinters, H.V.; Gilbert, J.J. Cerebral amyloid angiopathy: Incidence and complications in the aging brain. II. The distribution of amyloid vascular changes. Stroke 1983, 14, 924–928. [Google Scholar] [CrossRef]
- Levy, E.; Carman, M.D.; Fernandez-Madrid, I.J.; Power, M.D.; Lieberburg, I.; van Duinen, S.G.; Bots, G.T.; Luyendijk, W.; Frangione, B. Mutation of the Alzheimers disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science 1990, 248, 1124–1126. [Google Scholar] [CrossRef]
- Ghiso, J.; Tomidokoro, Y.; Revesz, T.; Frangione, B.; Rostagno, A. Cerebral amyloid angiopathy and Alzheimer’s disease. Hirosaki Igaku 2010, 61, S111–S124. [Google Scholar]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef]
- Rovelet-Lecrux, A.; Hannequin, D.; Raux, G.; Le Meur, N.; Laquerrière, A.; Vital, A.; Dumanchin, C.; Feuillette, S.; Brice, A.; Campion, D.; et al. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat. Genet. 2006, 38, 24–26. [Google Scholar] [CrossRef]
- Arvanitakis, Z.; Leurgans, S.E.; Wang, Z.; Wilson, R.S.; Bennett, D.A.; Schneider, J.A. Cerebral amyloid angiopathy pathology and cognitive domains in older persons. Ann. Neurol. 2010, 69, 320–327. [Google Scholar] [CrossRef]
- Rosand, J.; Greenberg, S.M. Cerebral amyloid angiopathy. Neurologist 2000, 6, 315–325. [Google Scholar] [CrossRef]
- Ikram, M.; Poels, M.; Lugt, A.V.; Hofman, A.; Krestin, G.; Breteler, M.; Vernooij, M. Cerebral microbleeds are associated with worse cognitive function: The rotterdam scan study. Neurology 2012, 78, 326–333. [Google Scholar] [CrossRef]
- Duyckaerts, C.; Potier, M.C.; Delatour, B. Alzheimer disease models and human neuropathology: Similarities and differences. Acta Neuropathol. 2008, 115, 5–38. [Google Scholar] [CrossRef]
- Blazquez-Llorca, L.; Valero-Freitag, S.; Rodrigues, E.F.; Merchán-Pérez, Á.; Rodríguez, J.R.; Dorostkar, M.M.; DeFelipe, J.; Herms, J. High plasticity of axonal pathology in Alzheimer’s disease mouse models. Acta Neuropathol. Commun. 2017, 5, 14. [Google Scholar] [CrossRef]
- Davis, J.; Xu, F.; Deane, R.; Romanov, G.; Previti, M.L.; Zeigler, K.; Zlokovic, B.V.; Nostrand, W.E. Early-onset and robust cerebral microvascular accumulation of amyloid β-Protein in transgenic mice expressing low levels of a vasculotropic dutch/iowa mutant form of amyloid β-Protein precursor. J. Biol. Chem. 2004, 279, 20296–20306. [Google Scholar] [CrossRef]
- Xu, F.; Grande, A.M.; Robinson, J.K.; Previti, M.L.; Vasek, M.; Davis, J.; Nostrand, W.V. Early-onset subicular microvascular amyloid and neuroinflammation correlate with behavioral deficits in vasculotropic mutant amyloid β-protein precursor transgenic mice. Neuroscience 2007, 146, 98–107. [Google Scholar] [CrossRef]
- Xu, W.; Xu, F.; Anderson, M.E.; Kotarba, A.E.; Davis, J.; Robinson, J.K.; Nostrand, W.E. Cerebral microvascular rather than parenchymal amyloid-β protein pathology promotes early cognitive impairment in transgenic mice. J. Alzheimers Dis. 2014, 38, 621–632. [Google Scholar] [CrossRef]
- Blackshear, A.L.; Xu, W.; Anderson, M.E.; Xu, F.; Previti, M.L.; Van Nostrand, W.E.; Robinson, J.K. A novel operant testing regimen for multi-construct cognitive characterization of a murine model of alzheimer’s amyloid-related behavioral impairment. Neurobiol. Learn. Mem. 2011, 96, 443–451. [Google Scholar] [CrossRef]
- Adkins, R.M.; Gelke, E.L.; Rowe, D.; Honeycutt, R.L. Molecular phylogeny and divergence time estimates for major rodent groups: Evidence from multiple genes. Mol. Biol. Evol. 2001, 18, 777–791. [Google Scholar] [CrossRef]
- Steppan, S.J.; Adkins, R.M.; Anderson, J. Phylogeny and divergence-date estimates of rapid radiations in muroid rodents based on multiple nuclear genes. Syst. Biol. 2004, 53, 533–553. [Google Scholar] [CrossRef]
- Snyder, J.S.; Choe, J.S.; Clifford, M.A.; Jeurling, S.I.; Hurley, P.; Brown, A.; Kamhi, J.F.; Cameron, H.A. Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J. Neurosci. 2009, 29, 14484–14495. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.M.; Wang, B.S.; Pan, Q.; Zhang, J.B.; Kumar, S.; Sun, X.Q.; Liu, Z.J.; Pan, H.J.; Lin, Y.; Liu, G.J.; et al. Whole-genome sequencing of the snub-nosed monkey provides insights into folivory and evolutionary history. Nat. Genet. 2014, 46, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Ellenbroek, B.; Youn, J. Rodent models in neuroscience research: Is it a rat race? Dis. Model Mech. 2016, 9, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.; Xu, F.; Hatfield, J.; Lee, H.; Hoos, M.D.; Popescu, D.L.; Crooks, E.; Kim, R.; Smith, S.O.; Robinson, J.K.; et al. A novel transgenic rat model of robust cerebral microvascular amyloid with prominent vasculopathy. Am. J. Pathol. 2018, 188, 2877–2889. [Google Scholar] [CrossRef]
- Zhu, X.; Hatfield, J.; Sullivan, J.K.; Xu, F.; Van Nostrand, W.E. Robust neuroinflammation and perivascular pathology in rTg-DI rats, a novel model of microvascular cerebral amyloid angiopathy. J. Neuroinflamm. 2020, 17, 1–15. [Google Scholar] [CrossRef]
- Kritzer, M.F.; Brewer, A.; Montalmont, F.; Davenport, M.; Robinson, J.K. Effects of gonadectomy on performance of operant tasks measuring prefrontal cortical function in adult male rats. Horm. Behav. 2007, 51, 183–194. [Google Scholar] [CrossRef]
- Anderson, M.E.; Xu, F.; Ou-Yang, M.H.; Davis, J.; Van Nostrand, W.E.; Robinson, J.K. Intensive ‘Brain Training’ Intervention Fails to Reduce Amyloid Pathologies or Cognitive Deficits in Transgenic Mouse Models of Alzheimer’s Disease. J. Alzheimers Dis. 2017, 55, 1109–1121. [Google Scholar] [CrossRef]
- Liu, L.; Orozco, I.J.; Planel, E.; Wen, Y.; Bretteville, A.; Krishnamurthy, P.; Wang, L.; Herman, M.; Figueroa, H.; Yu, W.H.; et al. A transgenic rat that develops Alzheimer’s disease-like amyloid pathology, deficits in synaptic plasticity and cognitive impairment. Neurobiol. Dis. 2008, 31, 46–57. [Google Scholar] [CrossRef]
- Leon, W.C.; Canneva, F.; Partridge, V.; Allard, S.; Ferretti, M.T.; DeWilde, A.; Vercauteren, F.; Atifeh, R.; Ducatenzeiler, A.; Klein, W.; et al. A novel transgenic rat model with a full Alzheimer’s-like amyloid pathology displays pre-plaque intracellular amyloid-beta-associated cognitive impairment. J Alz Dis. 2010, 20, 113–126. [Google Scholar] [CrossRef]
- Cohen, R.M.; Rezai-Zadeh, K.; Weitz, T.M.; Rentsendorj, A.; Gate, D.; Spivak, I.; Bholat, Y.; Vasilevko, V.; Glabe, C.G.; Breunig, J.J.; et al. A transgenic Alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric aβ, and frank neuronal loss. J. Neurosci. 2013, 33, 6245–6256. [Google Scholar] [CrossRef]
- Nardone, R.; De Blasi, P.; Seidl, M.; Höller, Y.; Caleri, F.; Tezzon, F.; Ladurner, G.; Golaszewski, S.; Trinka, E. Cognitive function and cholinergic transmission in patients with subcortical vascular dementia and microbleeds: A TMS study. J. Neural. Transm. (Vienna) 2011, 118, 1349–1358. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martin-Rodriguez, J.F.; Mir, P. Short-afferent inhibition and cognitive impairment in Parkinson’s disease: A quantitative review and challenges. Neurosci. Lett. 2020, 719, 133679. [Google Scholar] [CrossRef] [PubMed]
- Schirinzi, T.; Di Lorenzo, F.; Sancesario, G.M.; Di Lazzaro, G.; Ponzo, V.; Pisani, A.; Mercuri, N.B.; Koch, G.; Martorana, A. Amyloid-mediated cholinergic dysfunction in motor impairment related to alzheimer’s disease. J. Alzheimers Dis. 2018, 64, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Bonnì, S.; Ponzo, V.; Di Lorenzo, F.; Caltagirone, C.; Koch, G. Real-time activation of central cholinergic circuits during recognition memory. Eur. J. Neurosci. 2017, 45, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, G.; Summers, M.; Chan, E.; Wilson, D.; Charidimou, A.; Cipolotti, L.; Werring, D.J. Domain-specific characterisation of early cognitive impairment following spontaneous intracerebral haemorrhage. J. Neurol. Sci. 2018, 391, 25–30. [Google Scholar] [CrossRef]
- Case, N.F.; Charlton, A.; Zwiers, A.; Batool, S.; McCreary, C.R.; Hogan, D.B.; Ismail, Z.; Zerna, C.; Coutts, S.B.; Frayne, R.; et al. Cerebral amyloid angiopathy is associated with executive dysfunction and mild cognitive impairment. Stroke 2016, 47, 2010–2016. [Google Scholar] [CrossRef]
- Planton, M.; Raposo, N.; Albucher, J.F.; Pariente, J. Cerebral amyloid angiopathy-related cognitive impairment: The search for a specific neuropsychological pattern. Rev. Neurol. (Paris) 2017, 173, 562–565. [Google Scholar] [CrossRef]
- Dugger, B.N.; Davis, K.; Malek-Ahmadi, M.; Hentz, J.G.; Sandhu, S.; Beach, T.G.; Adler, C.H.; Caselli, R.J.; Johnson, T.A.; Serrano, G.E.; et al. Neuropathological comparisons of amnestic and nonamnestic mild cognitive impairment. BMC Neurol. 2015, 15, 146. [Google Scholar] [CrossRef]
- Ganz, A.B.; Beker, N.; Hulsman, M.; Sikkes, S.; Bank, N.B.; Scheltens, P.; Smit, A.B.; Rozemuller, A.J.; Hoozemans, J.J.; Holstege, H. Neuropathology and cognitive performance in self-reported cognitively healthy centenarians. Acta Neuropathol. Commun. 2018, 6, 64. [Google Scholar] [CrossRef]
- Valenti, R.; Charidimou, A.; Xiong, L.; Boulouis, G.; Fotiadis, P.; Ayres, A.; Riley, G.; Kuijf, H.J.; Reijmer, Y.D.; Pantoni, L.; et al. Visuospatial functioning in cerebral amyloid angiopathy: A pilot study. J. Alzheimers Dis. 2017, 56, 1223–1227. [Google Scholar] [CrossRef]




| Behavioral Task | Primary Measure | Operant Schedule | Primary Measure |
|---|---|---|---|
| Von Frey Hairs (VF) | Limb withdrawal reflex (somatosensory-motor) | Light/Dark Discrimination (DSTLDD) | Discrimination learning, association of secondary reinforcer |
| Open field (OF) | General mobility | Reaction Time (REACT) | Motor output ability |
| Novel object recognition (NOR) | Recognition memory | Stimulus detection (SIGDECT) | Attention and initiation of motor output |
| Novel exploration (NE) | Interaction with spatially-arrayed stimuli | Delayed non-matching to position (DNMTP) | Short term memory |
| Radial arm maze (RAM) | Working and reference memory | Differential reinforcement: high responding (DRH) | Sustained effort, motor output |
| Barnes maze | Spatial memory |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, D.L.; Van Nostrand, W.E.; Robinson, J.K. Longitudinal Cognitive Decline in a Novel Rodent Model of Cerebral Amyloid Angiopathy Type-1. Int. J. Mol. Sci. 2020, 21, 2348. https://doi.org/10.3390/ijms21072348
Popescu DL, Van Nostrand WE, Robinson JK. Longitudinal Cognitive Decline in a Novel Rodent Model of Cerebral Amyloid Angiopathy Type-1. International Journal of Molecular Sciences. 2020; 21(7):2348. https://doi.org/10.3390/ijms21072348
Chicago/Turabian StylePopescu, Dominique L., William E. Van Nostrand, and John K. Robinson. 2020. "Longitudinal Cognitive Decline in a Novel Rodent Model of Cerebral Amyloid Angiopathy Type-1" International Journal of Molecular Sciences 21, no. 7: 2348. https://doi.org/10.3390/ijms21072348
APA StylePopescu, D. L., Van Nostrand, W. E., & Robinson, J. K. (2020). Longitudinal Cognitive Decline in a Novel Rodent Model of Cerebral Amyloid Angiopathy Type-1. International Journal of Molecular Sciences, 21(7), 2348. https://doi.org/10.3390/ijms21072348





