Role of Oxytocin/Vasopressin-Like Peptide and Its Receptor in Vitellogenesis of Mud Crab
Abstract
1. Introduction
2. Results
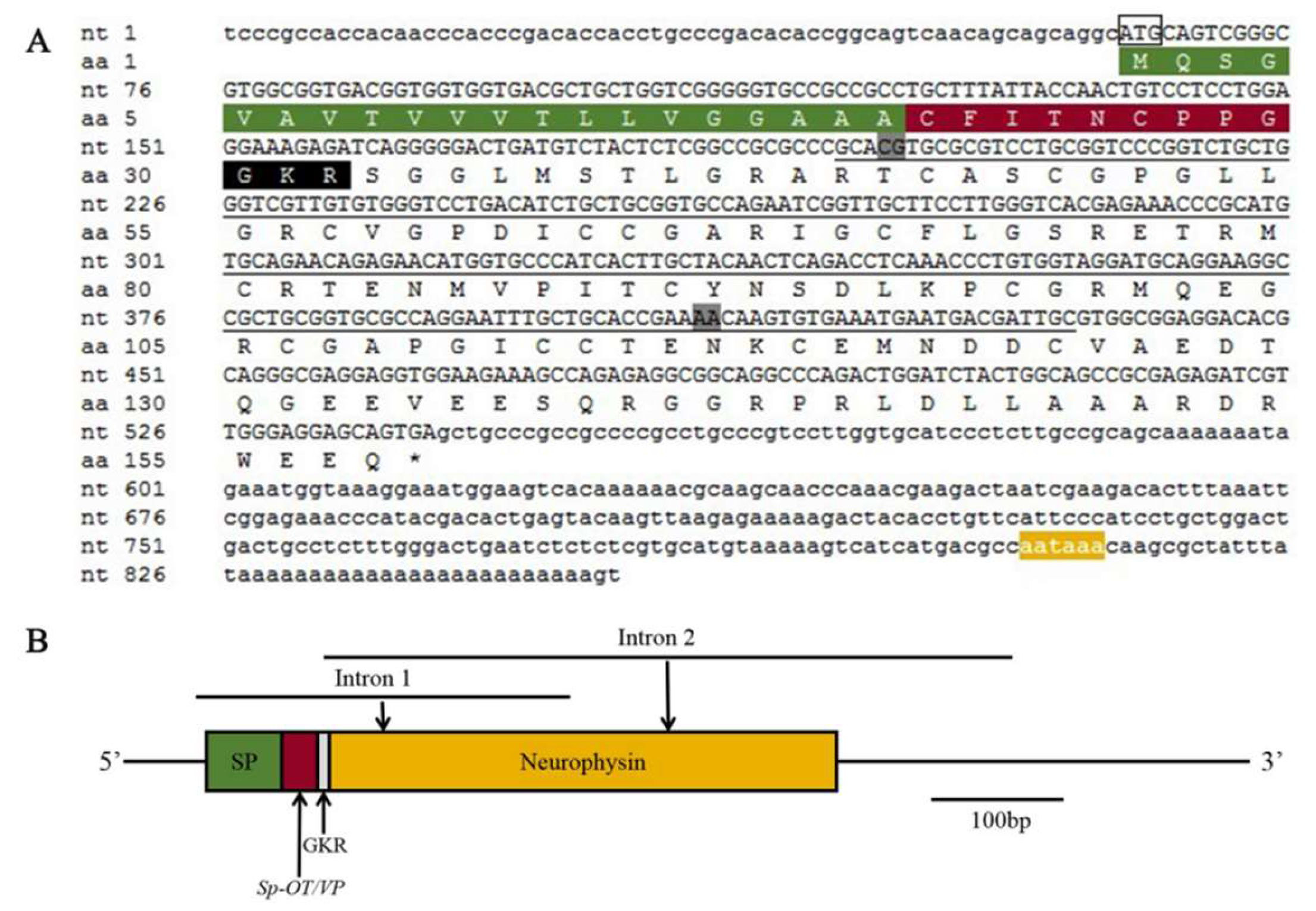
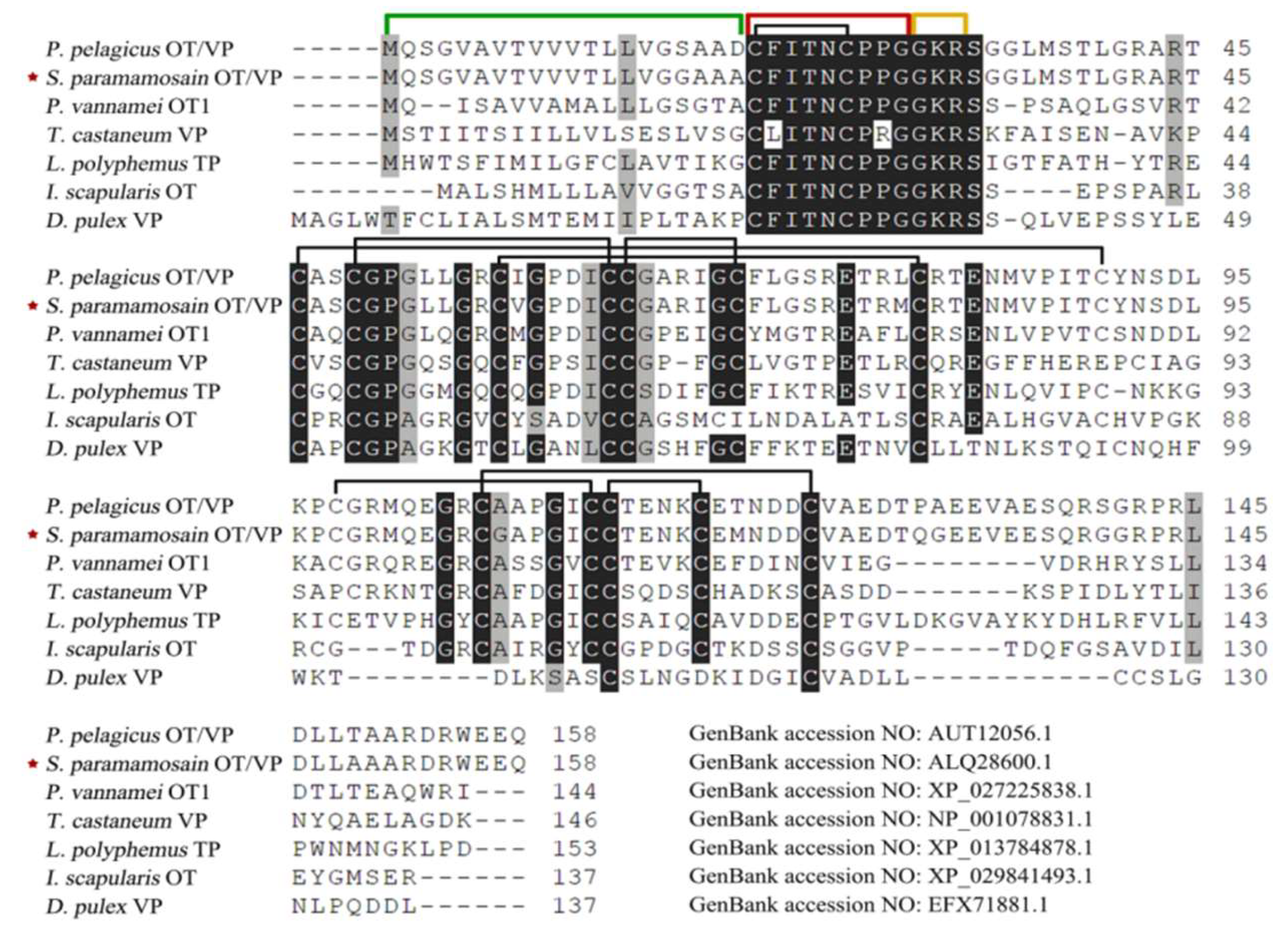
2.1. Molecular Cloning and Bioinformatics of SpOT/VP-Like Preprohormone
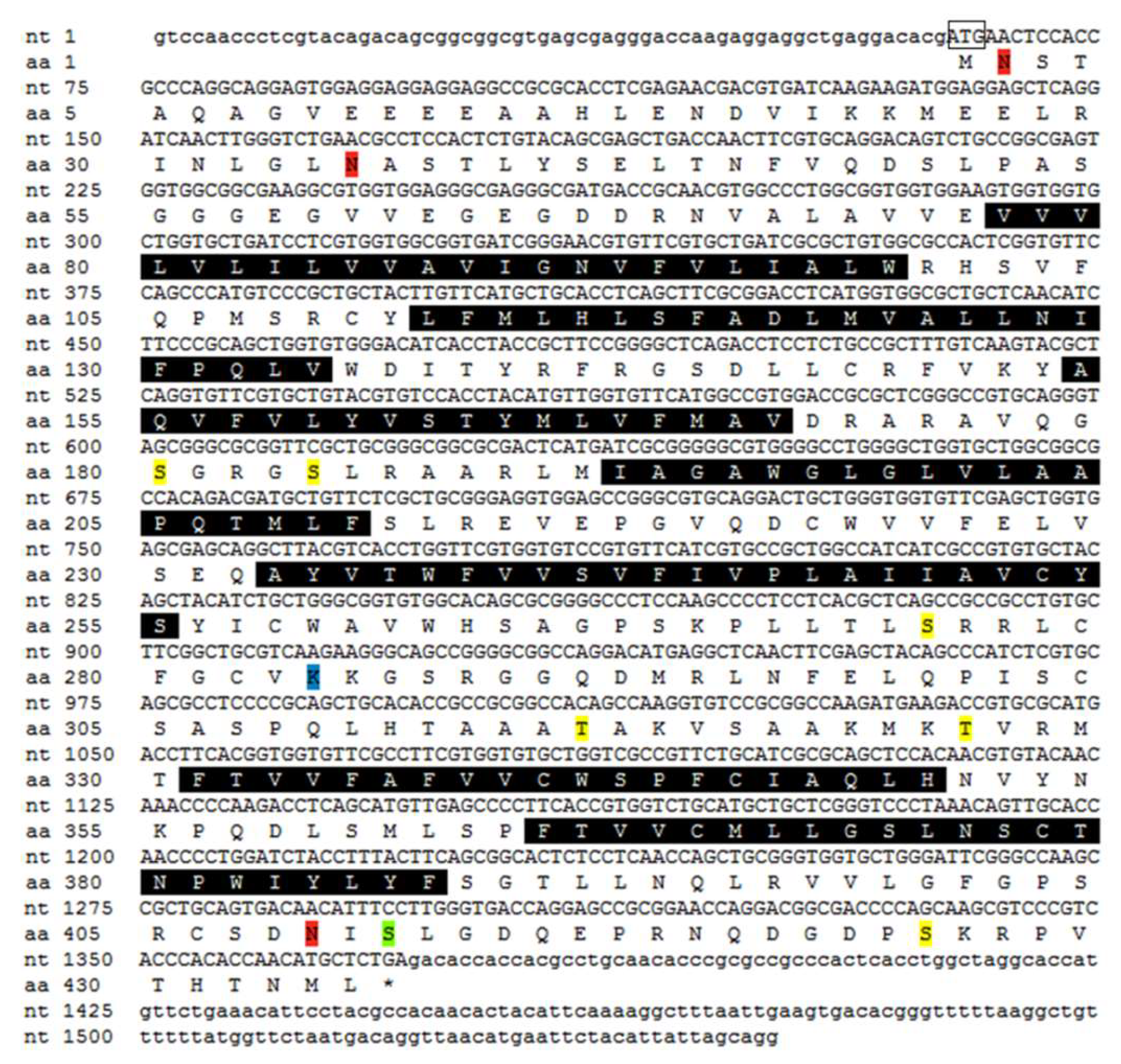
2.2. Molecular Cloning and Bioinformatics of SpOT/VPR-Like Preprohormone
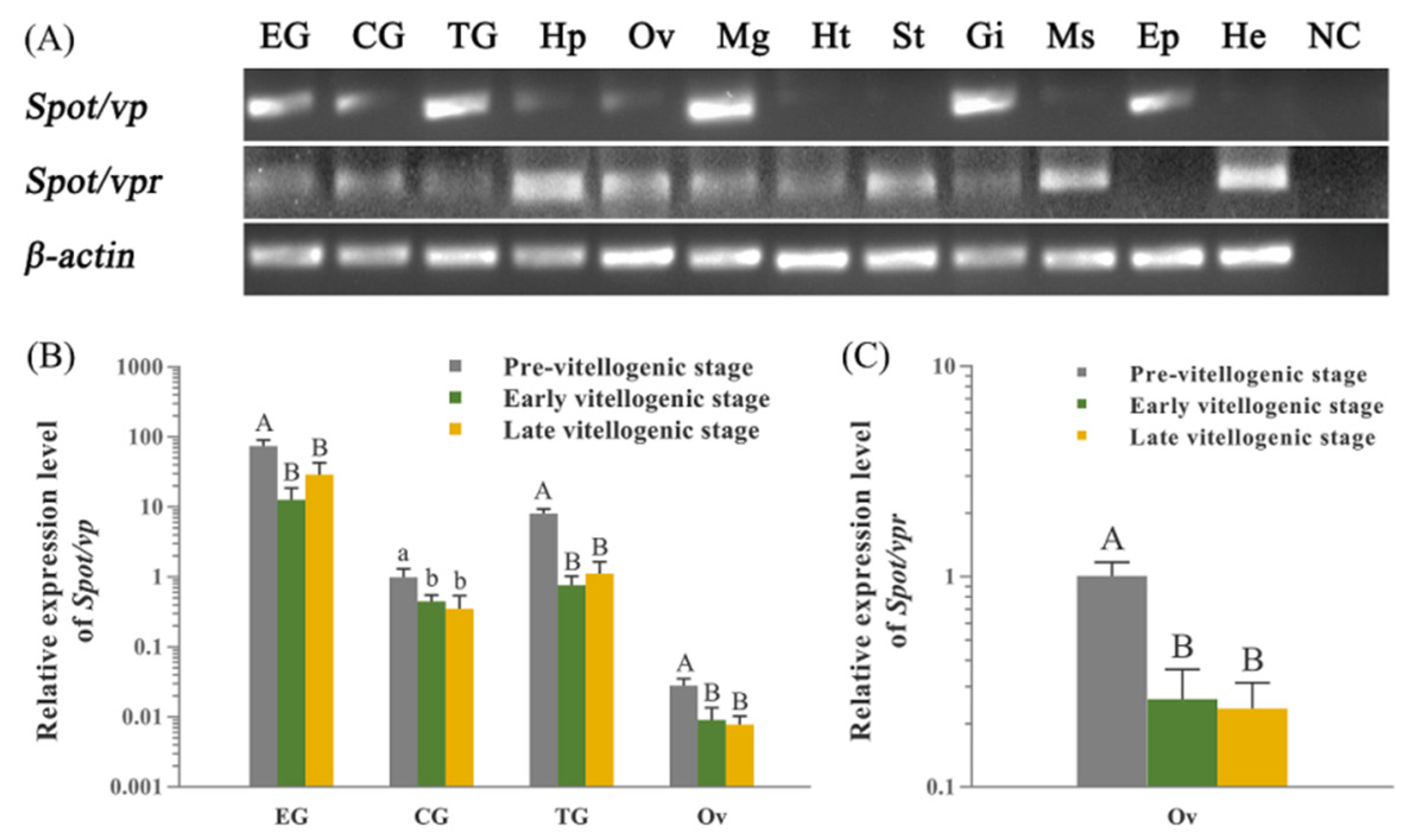
2.3. Expression Profiles of Spot/vp and Spot/vpr-Like mRNA
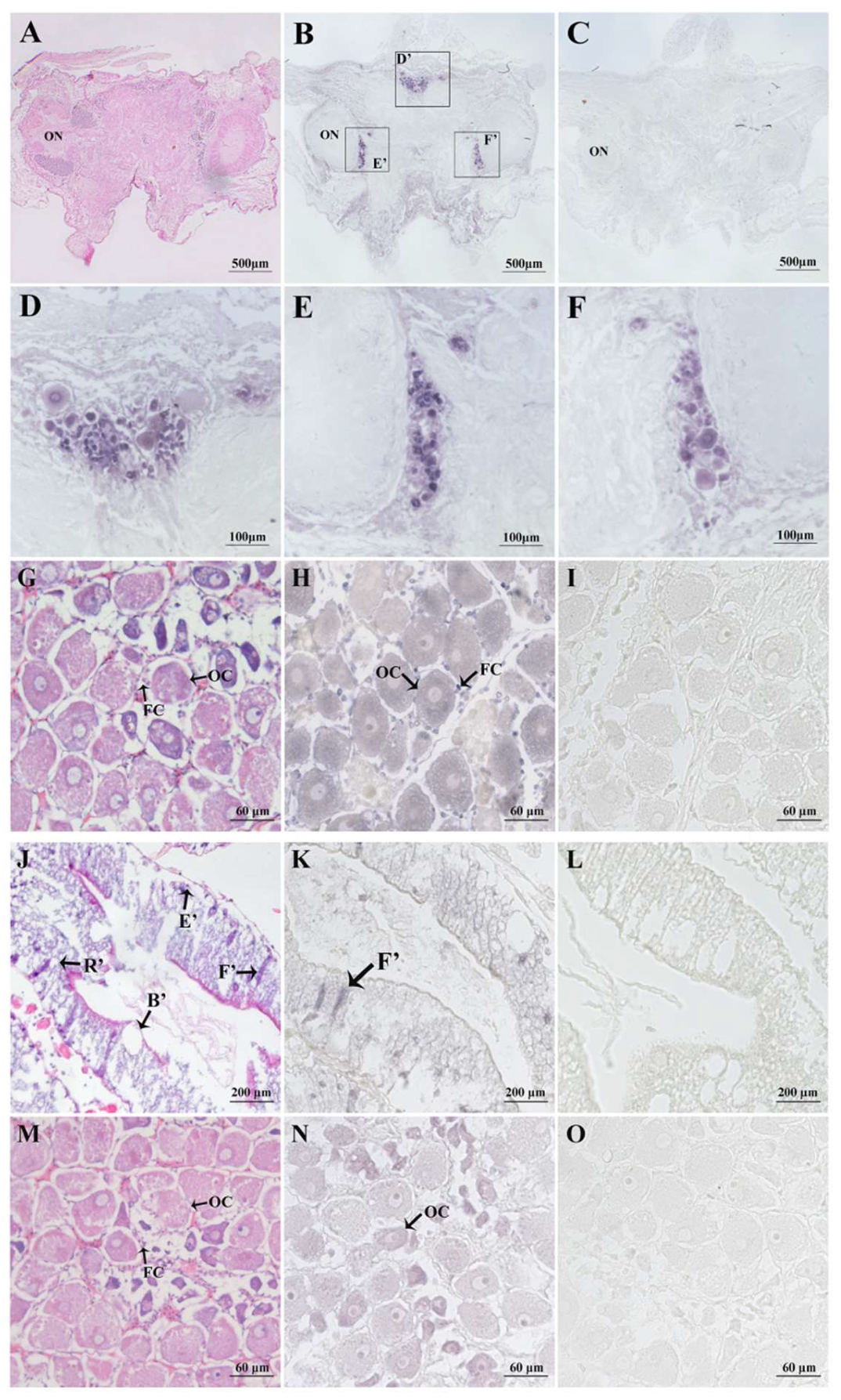
2.4. In Situ Hybridisation of Spot/vp and Spot/vpr-Like mRNA
2.5. Synthetic SpOT/VP-Like Peptide Treatment In Vitro
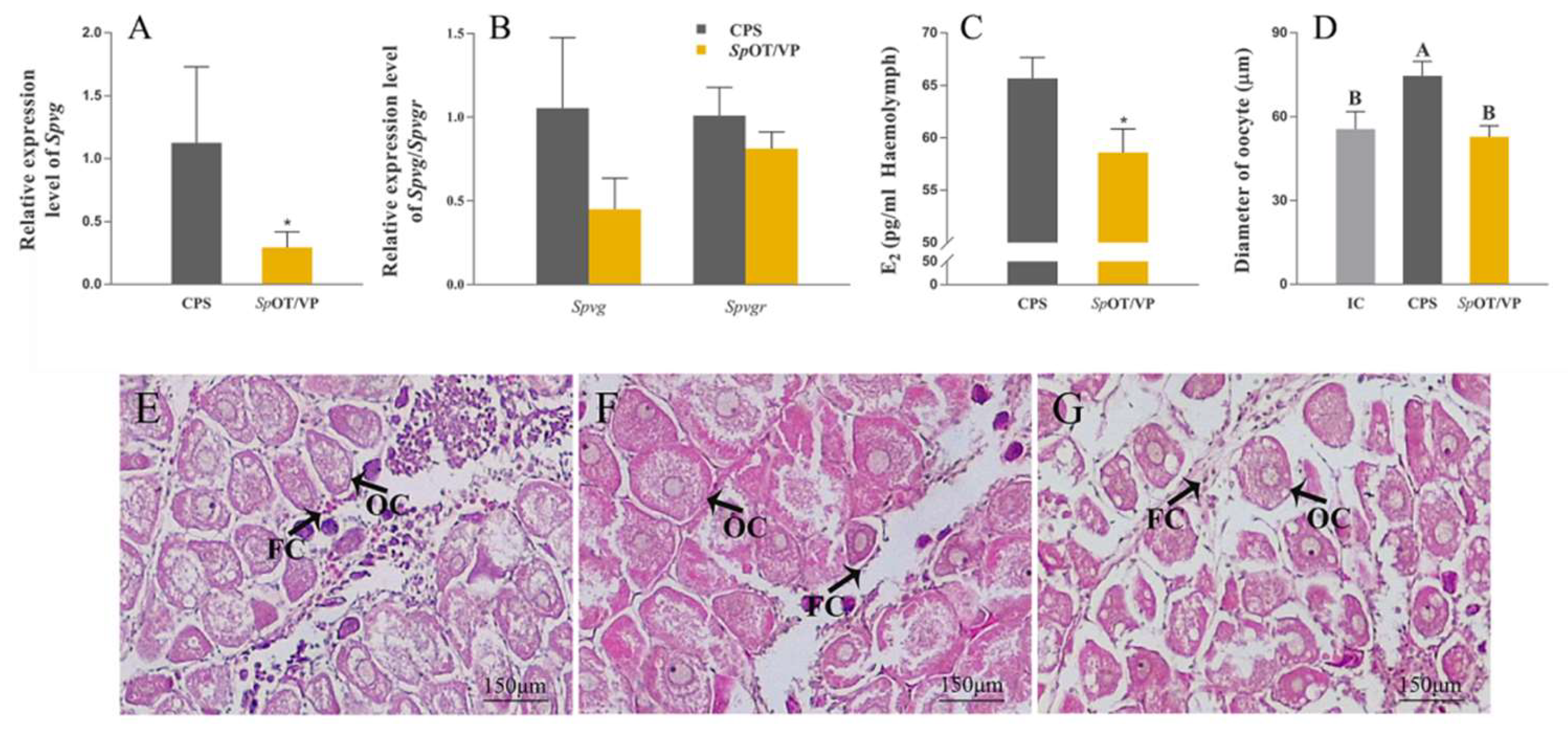
2.6. Synthetic SpOT/VP-Like Peptide Treatment In Vivo
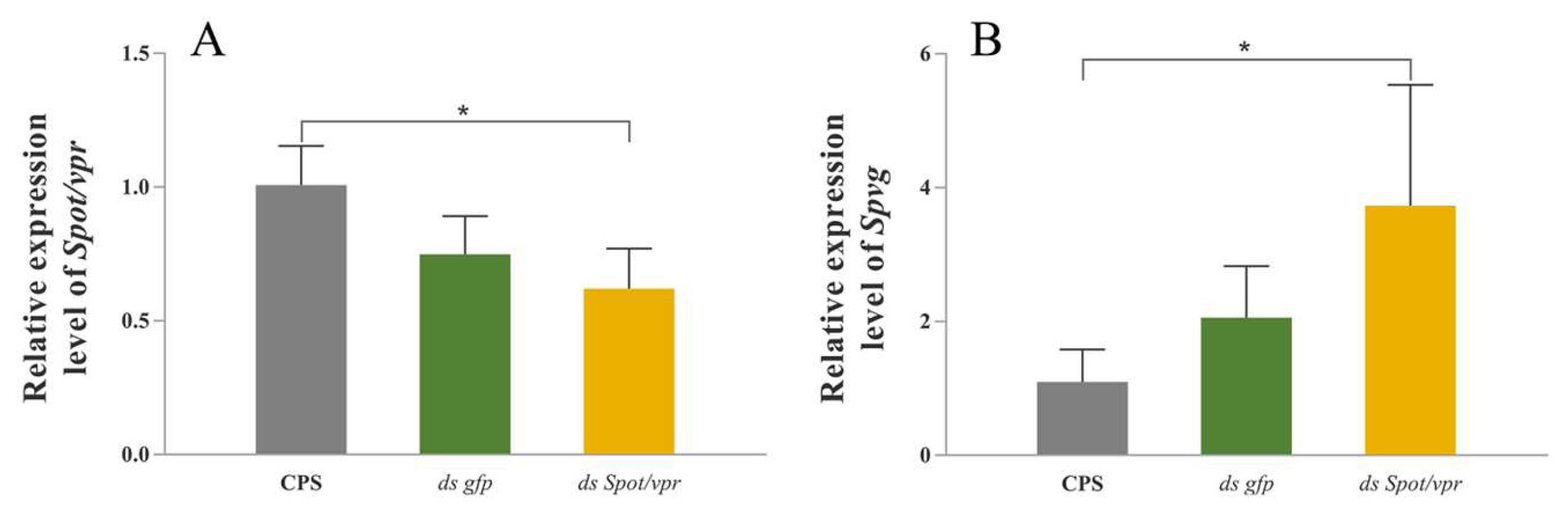
2.7. RNA Interference in the Hepatopancreas Explants In Vitro
3. Discussion
4. Materials and Methods
4.1. Animals
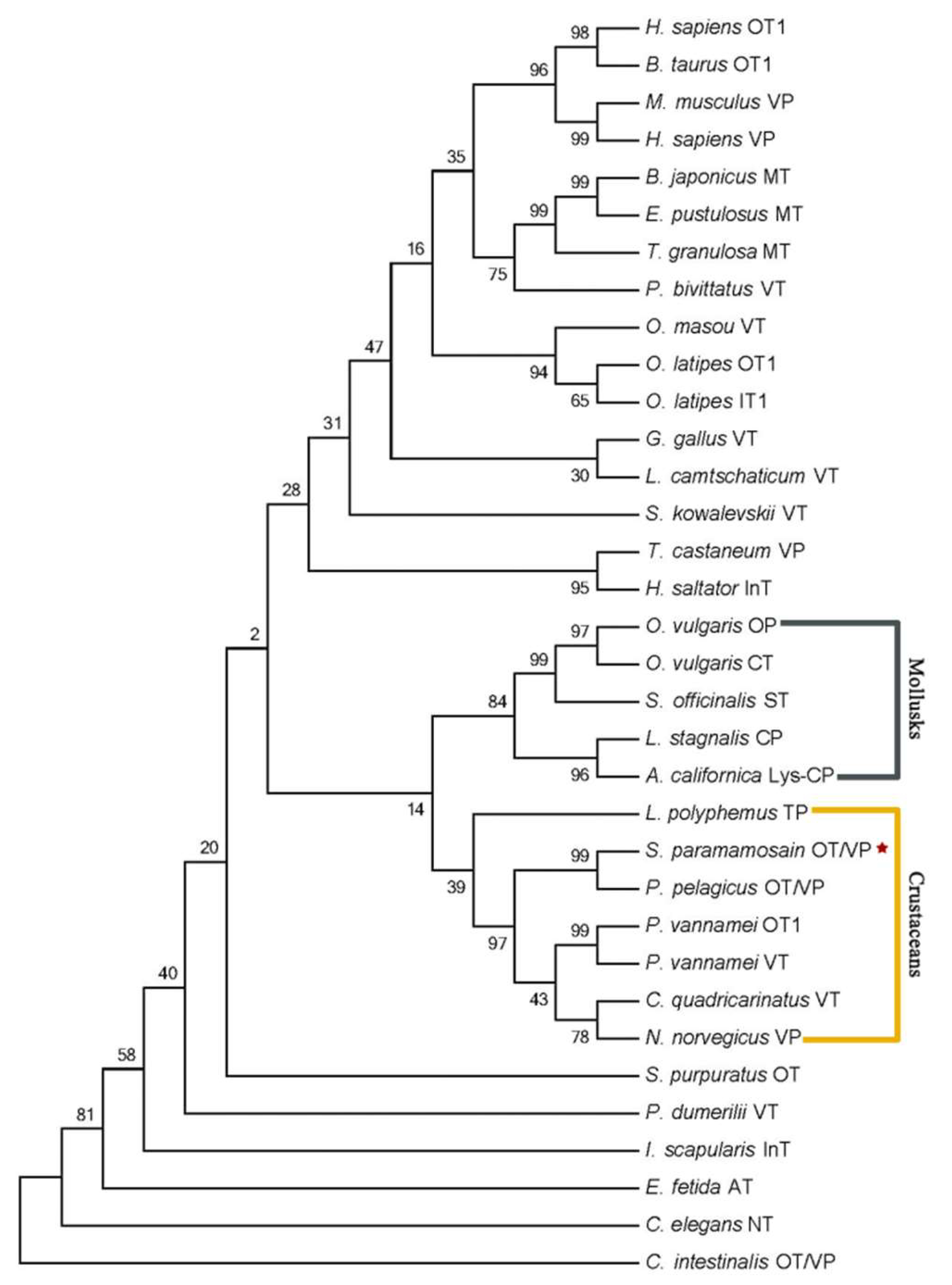
4.2. Molecular Cloning, Bioinformatics and Phylogenetics of SpOT/VP-Like Peptide and Its Receptor
4.3. Tissue Distributions of Spot/vp and Spot/vpr-Like mRNA
4.4. In Situ Hybridisation of Spot/vp and Spot/vpr-Like mRNA
4.5. Temporal Expression Profiles of Spot/vp and Spot/vpr-Like mRNA
4.6. Synthetic SpOT/VP-Like Peptide Treatment In Vitro
4.7. Synthetic SpOT/VP-Like Peptide Injection
4.8. RNA Interference In Vitro
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Primer | Sequence (5′-3′) | Application |
|---|---|---|
| OT/VP-F | CCGCCTGCTTTATTACCAACT | Fragment validation |
| OT/VP-R | CCACGCAATCGTCATTCATTT | |
| OT/VPR-F1 | GGTGGTGGAAGTGGTGGTGC | |
| OT/VPR-R1 | TACTTGACAAAGCGGCAGAGGA | |
| OT/VPR-F2 | TTCGTGGTGTCCGTGTTCAT | |
| OT/VPR-R2 | GGAGAGTGCCGCTGAAGTAA | |
| OT/VPR-F3 | TGCTCGGGTCCCTAAACAGT | |
| OT/VPR-R3 | TTGAATGTAGTGTTGTGGCGTAG | |
| OT/VP-3′-1 | GCGAGGAGGTGGAAGAAAGC | 3′ RACE |
| OT/VP-3′-2 | TCGTTGGGAGGAGCAGTGAG | |
| OT/VP-5′-1 | GCACACCACGAAGGCGAACACTACC | 5′ RACE |
| OT/VP-5′-2 | CTGAGCGTGAGGAGGGGCTTGGA | |
| OT/VP-GF | CAGTCAACAGCAGCAGGCA | cDNA/gDNA validation |
| OT/VP-GR | ACTTCCATTTCCTTTACCATTTCTA | |
| OT/VPR-OF | GTGAGCGAGGGACCAAGAGG | ORF validation |
| OT/VPR-OR | TGTAGTGTTGTGGCGTAGGAATGTTT | |
| OT/VP-QF | AATCGGTTGCTTCCTTGGG | RT-PCR/qRT-PCR |
| OT/VP-QR | CCTTCCTGCATCCTACCACA | |
| OT/VPR-QF | GTGAGCGAGGGACCAAGAGG | |
| OT/VPR-QR | GGAGGCGTTCAGACCCAAGT | |
| Vg-QF | CGCAACCGCCACTGAAGAT | |
| Vg-QR | CCACCATGCTGCTCACGACT | |
| VgR-QF | TTCTATACCAGGCCACTACC | |
| VgR-QR | TTTTCACTCCAAGCACACTC | |
| OT/VP-IF | AGTCAACAGCAGCAGGCAT | ISH |
| OT/VP-IR | CAGGGTTTGAGGTCTGAGTTG | |
| OT/VPR-IF | TGCTCGGGTCCCTAAACAGT | |
| OT/VPR-IR | TTGAATGTAGTGTTGTGGCGTAG | |
| OT/VPR-SF | CAGGCTTACGTCACCTGGTT | dsRNA |
| OT/VPR-SR | GAGTGCCGCTGAAGTAAAGG |
Appendix B
References
- Wathes, D.C. Possible actions of gonadal oxytocin and vasopressin. J. Reprod. Fertil. 1984, 71, 315–345. [Google Scholar] [CrossRef]
- Vigneaud, V.D.; Ressler, C.; Trippett, S. The sequence of amino acids in oxytocin, with a proposal for the structure of oxytocin. J. Biol. Chem. 1953, 205, 949–957. [Google Scholar]
- Vigneaud, V.D.; Lawler, H.C.; Popenoe, E.A. Enzymatic cleavage of glycinamide from vasopressin and a proposed structure for this pressor-antidiuretic hormone of the posterior pituitary. J. Am. Chem. Soc. 1953, 75, 4880–4881. [Google Scholar] [CrossRef]
- Ivell, R.; Schmale, H.; Richter, D. Vasopressin and oxytocin precursors as model preprohormones. Neuroendocrinology 1983, 37, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, H.K.; Young, W.S. Oxytocin and vasopressin: Genetics and behavioral implications. In Handbook of Neurochemistry and Molecular Neurobiology; Springer: Berlin, Germany, 2006; pp. 573–607. [Google Scholar]
- Acher, R.; Chauvet, J.; Chauvet, M.T. Man and the chimaera: Selective versus neutral oxytocin evolution. Adv. Exp. Med. Biol. 1995, 395, 615–627. [Google Scholar] [PubMed]
- De Bree, F.M.; Van Der Kleij, A.A.; Nijenhuis, M.; Zalm, R.; Murphy, D.; Burbach, J.P. The hormone domain of the vasopressin prohormone is required for the correct prohormone trafficking through the secretory pathway. J. Neuroendocrinol. 2003, 15, 1156–1163. [Google Scholar] [CrossRef]
- MacDonald, K.; MacDonald, T.M. The peptide that binds: A systematic review of oxytocin and its prosocial effects in humans. Harv. Rev. Psychiatry 2010, 18, 1–21. [Google Scholar] [CrossRef]
- Donaldson, Z.R.; Young, L.J. Oxytocin, vasopressin, and the neurogenetics of sociality. Science 2008, 322, 900–904. [Google Scholar] [CrossRef]
- Buhimschi, C.S. Endocrinology of lactation. Obstet. Gynecol. Clin. N. Am. 2004, 31, 963–979. [Google Scholar] [CrossRef]
- Ratajczak, C.K.; Muglia, L.J. Insights into parturition biology from genetically altered mice. Pediatr. Res. 2008, 64, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Joy, K.P.; Chaube, R. Structural and functional diversity of nonapeptide hormone from an evolutionary perspective: A review. Gen. Comp. Endocrinol. 2017, 241, 4–23. [Google Scholar] [CrossRef] [PubMed]
- Jard, S.; Barberis, C.; Audigier, S.; Tribollet, E. Neurohypophyseal hormone receptor systems in brain and periphery. Prog. Brain Res. 1987, 72, 173–187. [Google Scholar] [PubMed]
- Thibonnier, M.; Auzan, C.; Madhun, Z.; Wilkins, P.; Berti-Mattera, L.; Clauser, E. Molecular cloning, sequencing, and functional expression of a cDNA encoding the human V1a vasopressin receptor. J. Biol. Chem. 1994, 269, 3304–3310. [Google Scholar]
- Sugimoto, T.; Saito, M.; Mochizuki, S.; Watanabe, Y.; Hashimoto, S.; Kawashima, H. Molecular cloning and functional expression of a cDNA encoding the human V1b vasopressin receptor. J. Biol. Chem. 1994, 269, 27088–27092. [Google Scholar]
- Kimura, T.; Tanizawa, O.; Mori, K.; Brownstein, M.J.; Okayama, H. Structure and expression of a human oxytocin receptor. Nature 1992, 356, 526–529. [Google Scholar] [CrossRef]
- Lolait, S.J.; O’ Carroll, A.M.; McBride, O.W.; Konig, M.; Morel, A.; Brownstein, M.J. Cloning and characterization of a vasopressin V2 receptor and possible link to nephrogenic diabetes insipidus. Nature 1992, 357, 336–339. [Google Scholar] [CrossRef]
- Stewart, M.J.; Harding, B.I.; Adamson, K.J.; Wang, T.; Storey, K.B.; Cummins, S.F. Characterisation of two conopressin precursor isoforms in the land snail, Theba pisana. Peptides 2015, 80, 32–39. [Google Scholar] [CrossRef]
- Saetan, J.; Kruangkum, T.; Phanthong, P.; Tipbunjong, C.; Udomuksorn, W.; Sobhon, P.; Sretarugsa, P. Molecular cloning and distribution of oxytocin/vasopressin-like mRNA in the blue swimming crab, Portunus pelagicus, and its inhibitory effect on ovarian steroid release. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2018, 218, 46–55. [Google Scholar] [CrossRef]
- Stafflinger, E.; Hansen, K.K.; Hauser, F.; Schneider, M.; Cazzamali, G.; Williamson, M.; Grimmelikhuijzen, C.J.P. Cloning and identification of an oxytocin/vasopressin-like receptor and its ligand from insects. Proc. Natl. Acad. Sci. USA 2008, 105, 3262–3267. [Google Scholar] [CrossRef]
- Suwansa-Ard, S.; Thongbuakaew, T.; Wang, T.F.; Zhao, M.; Elizur, A.; Hanna, P.J.; Sretarugsa, P.; Cummins, S.F.; Sobhon, P. In silico neuropeptidome of female Macrobrachium rosenbergii based on transcriptome and peptide mining of eyestalk, central nervous system and ovary. PLoS ONE 2015, 10, e0123848. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.C.; Yang, Y.N.; Huang, H.Y.; Ye, H.H. Neuropeptides in the cerebral ganglia of the mud crab, Scylla paramamosain: Transcriptomic analysis and expression profiles during vitellogenesis. Sci. Rep. 2015, 5, 17055. [Google Scholar] [CrossRef] [PubMed]
- Garrison, J.L.; Macosko, E.Z.; Bernstein, S.; Pokala, N.; Albrecht, D.; Bargmann, C.I. Oxytocin/vasopressin-related peptides have an ancient role in reproductive behavior. Science 2012, 338, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Van Kesteren, R.E.; Tensen, C.P.; Smit, A.B.; van Minnen, J.; van Soest, P.F.; Kits, K.S.; Meyerhof, W.; Richter, D.; van Heerikhuizen, H.; Vreugdenhil, E. A novel G protein-coupled receptor mediating both vasopressin- and oxytocin-like functions of Lys-conopressin in Lymnaea stagnalis. Neuron 1995, 15, 897–908. [Google Scholar] [CrossRef]
- Van Kesteren, R.E.; Tensen, C.P.; Smit, A.B.; van Minnen, J.; Kolakowski, L.F.; Meyerhof, W.; Richter, D.; van Heerikhuizen, H.; Vreugdenhil, E.; Geraerts, W.P. Co-evolution of ligand–receptor pairs in the vasopressin/oxytocin superfamily of bioactive peptides. J. Biol. Chem. 1996, 271, 3619–3626. [Google Scholar] [CrossRef]
- Pateraki, L.E.; Stratakis, E. Synthesis and organization of vitellogenin and vitellin molecules from the land crab Potamon potamios. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2000, 125, 53–61. [Google Scholar] [CrossRef]
- Panouse, J.B. L’action de la glande du sinus sur I’ovaire chez crevette Leander serratus. C. R. Acad. Sci. Paris 1944, 218, 292–294. [Google Scholar]
- Soyez, D.; Van Deijnen, J.E.; Martin, M. Isolation and characterization of a vitellogenesis-inhibiting factor from sinus glands of the lobster, Homarus americanus. J. Exp. Zool. A Ecol. Gen. Physiol. 1987, 244, 479–484. [Google Scholar] [CrossRef]
- Zmora, N.; Chung, J.S. A novel hormone is required for the development of reproductive phenotypes in adult female crabs. Endocrinology 2014, 155, 230–239. [Google Scholar] [CrossRef]
- Bao, C.C.; Yang, Y.N.; Huang, H.Y.; Ye, H.H. Inhibitory role of mud crab short neuropeptide F in vitellogenesis and ovarian maturation via autocrine/paracrine signaling. Front. Endocrinol. 2018, 9, 390–401. [Google Scholar] [CrossRef]
- Liu, A.; Liu, F.; Shi, W.Y.; Huang, H.Y.; Wang, G.Z.; Ye, H.H. C-type allatostatin and its putative receptor from the mud crab serve an inhibitory role in ovarian development. J. Exp. Biol. 2019, 222, jeb207985. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, J.; Kjelsberg, M.A.; Caron, M.G.; Lefkowitz, R.J. Mutagenesis of the beta2-adrenergic receptor: How structure elucidates function. Annu. Rev. Pharmacol. Toxicol. 1992, 32, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.J.; De Santos, V.; Zafaralla, G.C.; Ramilo, C.A.; Zeikus, R.; Gray, W.R.; Olivera, B.M. Invertebrate vasopressin/oxytocin homologs. Characterization of peptides from Conus geographus and Conus striatus venoms. J. Biol. Chem. 1987, 262, 15821–15824. [Google Scholar] [PubMed]
- Venkatesh, B.; Si-Hoe, S.L.; Murphy, D.; Brenner, S. Transgenic rats reveal functional conservation of regulatory controls between the Fugu isotocin and rat oxytocin genes. Proc. Natl. Acad. Sci. USA 1997, 94, 12462–12466. [Google Scholar] [CrossRef]
- Gruber, C.W.; Koehbach, J.; Muttenthaler, M. Exploring bioactive peptides from natural sources for oxytocin and vasopressin drug discovery. Future Med. Chem. 2012, 4, 1791–1798. [Google Scholar] [CrossRef]
- Glenner, H.; Thomsen, P.F.; Hebsgaard, M.B.; Sørensen, M.V.; Willerslev, E. The origin of insects. Science 2006, 314, 1883–1884. [Google Scholar] [CrossRef]
- Dohlman, H.G.; Caron, M.G.; DeBlasi, A.; Frielle, T.; Lefkowitz, R.J. Role of extracellular disulphide bonded cysteines in the ligand binding function of the beta2-adrenergic receptor. Biochemistry 1990, 29, 2335–2342. [Google Scholar] [CrossRef]
- Holmgren, S.; Jensen, J. Evolution of vertebrate neuropeptides. Brain Res. Bull. 2001, 55, 723–735. [Google Scholar] [CrossRef]
- Mizuno, J.; Takeda, N. Phylogenetic study of the oxytocin-like immunoreactive system in invertebrates. Comp. Biochem. Physiol. A Physiol. 1988, 91, 733–738. [Google Scholar] [CrossRef]
- Mizuno, J.; Takeda, N. Phylogenetic study of the arginine-vasotocin/arginine-vasopressin-like immunoreactive system in invertebrates. Comp. Biochem. Physiol. A Physiol. 1988, 91, 739–747. [Google Scholar] [CrossRef]
- Akiko, K.; Naoto, M.; Hiroki, T.; Sean, M.; Minoru, M.; Takayoshi, O.; Masayuki, M.; Laurent, K. Oxytocin/vasopressin-like peptide inotocin regulates cuticular hydrocarbon synthesis and water balancing in ants. Proc. Natl. Acad. Sci. USA 2019, 116, 5597–5606. [Google Scholar]
- Takuwa-Kuroda, K.; Iwakoshi-Ukena, E.; Kanda, A.; Minakata, H. Octopus, which owns the most advanced brain in invertebrates, has two members of vasopressin/oxytocin superfamily as in vertebrates. Regul. Pept. 2003, 115, 139–149. [Google Scholar] [CrossRef]
- Spanings-Pierrot, C.; Soyez, D.; Van Herp, F.; Gompel, M.; Skaret, G.; Grousset, E.; Charmantier, G. Involvement of crustacean hyperglycemic hormone in the control of gill ion transport in the crab Pachygrapsus marmoratus. Gen. Comp. Endocrinol. 2000, 119, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Serrano, L.; Blanvillain, G.; Soyez, D.; Charmantier, G.; Grousset, E.; Aujoulat, F.; Spanings-Pierrot, C. Putative involvement of crustacean hyperglycemic hormone isoforms in the neuroendocrine mediation of osmoregulation in the crayfish Astacus leptodactylus. J. Exp. Biol. 2003, 206, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Urlacher, E.; Soustelle, L.; Parmentier, M.L.; Verlinden, H.; Gherardi, M.J.; Fourmy, D.; Mercer, A.; Devaud, J.M.; Massou, I. Honey bee allatostatins target galanin/somatostatin-like receptors and modulate learning: A conserved function? PLoS ONE 2016, 11, e0146248. [Google Scholar] [CrossRef]
- Urlacher, E.; Devaud, J.M.; Mercer, A.R. C-type allatostatins mimic stress-related effects of alarm pheromone on honey bee learning and memory recall. PLoS ONE 2017, 12, e0174321. [Google Scholar] [CrossRef]
- Sandeman, D.; Sandeman, R.; Derby, C.; Schmidt, M. Morphology of the brain of crayfish, crabs, and spiny lobsters: A common nomenclature for homologous structures. Biol. Bull. 1992, 183, 304–326. [Google Scholar] [CrossRef]
- Shu, L.; Yang, Y.N.; Jiang, Q.L.; Huang, H.Y.; Ye, H.H. Does the bone morphogenetic protein 7 inhibit oocyte maturation by autocrine/paracrine in mud crab? Gen. Comp. Endocrinol. 2018, 266, 119–125. [Google Scholar]
- Sirotkin, A.V.; Gerasimova, G.G.; Golubev, A.K.; Dmitriev, V.B. The effect of arginine vasotocin on the production of steroid hormones by mouse, cow and chicken ovarian tissues in vitro. Biomed. Sci. 1990, 1, 308–310. [Google Scholar]
- Sirotkin, A.V.; Nitray, J.; Tarasenko, L.W.; Uhrin, P.; Laurinčik, J.; Bulla, J. Effects of oxytocin, vasopressin and vasotocin and their agonists on steroid secretion by bovine granulosa cells. Anim. Reprod. Sci. 1995, 39, 81–87. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Schaeffer, H.J.; Mlyncek, M.; Missik, J.; Bulla, J. Oxytocin affects the release of steroids, insulin-like growth factor-I, prostaglandin F2a and cyclic nucleotides by human granulose cells in vitro. Hum. Reprod. 1996, 11, 152–155. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Singh, V.; Joy, K.P. Vasotocin induces final oocyte maturation and ovulation through production of a maturation-inducing steroid in the catfish Heteropneustes fossilis. Gen. Comp. Endocrinol. 2011, 174, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Chaube, R.; Joy, K.P. In situ localization of vasotocin and isotocin precursor mRNA in brain and ovary of the catfish Heteropneustes fossilis and estrogen regulation of the gene expression. J. Transl. Neurosci. 2016, 1, 1–10. [Google Scholar]
- Singh, V.; Joy, K.P. Relative in vitro seasonal effects of vasotocin and isotocin on ovarian steroid hormone levels in the catfish Heteropneustes fossilis. Gen. Comp. Endocrinol. 2009, 162, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Joy, K.P.; Singh, V. Functional interactions between vasotocin and prostaglandins during final oocyte maturation and ovulation in the catfish Heteropneustes fossilis. Gen. Comp. Endocrinol. 2013, 186, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Jeng, S.S.; Wan, W.C.; Chang, C.F. Existence of an estrogen-like compound in the ovary of the shrimp, Parapenaeus fissures. Gen. Comp. Endocrinol. 1978, 36, 211–214. [Google Scholar] [CrossRef]
- Thongbuakaew, T.; Siangcham, T.; Suwansa-ard, S.; Elizur, A.; Cummins, S.F.; Sobhon, P.; Sretarugsa, P. Steroids and genes related to steroid biosynthesis in the female giant freshwater prawn, Macrobrachium rosenbergii. Steroids 2016, 107, 149–160. [Google Scholar] [CrossRef]
- Fairs, N.J.; Quinlan, P.T.; Goad, L.J. Changes in ovarian unconjugated and conjugated steroid titers during vitellogenesis in Penaeus monodon. Aquaculture 1990, 89, 83–99. [Google Scholar] [CrossRef]
- Swevers, L.; Lambert, J.G.; de Loof, A. Synthesis and metabolism of vertebrate-type steroids by tissues of insects: A critical evaluation. Experientia 1991, 47, 687–698. [Google Scholar] [CrossRef]
- Summavielle, T.; Monteiro, P.; Reis-Henriques, M.; Coimbra, J. In vitro metabolism of steroid hormones by ovary and hepatopancreas of the crustacean penaeid shrimp Marsupenaeus japonicus. Sci. Mar. 2003, 67, 299–306. [Google Scholar] [CrossRef]
- Lin, J.L.; Shi, X.; Fang, S.B.; Zhang, Y.; You, C.H.; Ma, H.Y.; Lin, F. Comparative transcriptome analysis combining SMRT and NGS sequencing provides novel insights into sex difffferentiation and development in mud crab (Scylla paramamosain). Aquaculture 2019, 513, 734447. [Google Scholar] [CrossRef]
- Warrier, S.R.; Tirumalai, R.; Subramoniam, T. Occurrence of vertebrate steroids, estradiol 17β and progesterone in the reproducing females of the mud crab Scylla serrata. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 130, 283–294. [Google Scholar] [CrossRef]
- Huang, X.S.; Ye, H.H.; Huang, H.Y.; Yang, Y.N.; Gong, J. An insulin-like androgenic gland hormone gene in the mud crab, Scylla paramamosain, extensively expressed and involved in the processes of growth and femalereproduction. Gen. Comp. Endocrinol. 2014, 204, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Chen, Y.D.; Han, K.H.; Zou, Z.H.; Zhang, Z.P. A vasa gene from green mud crab Scylla paramamosain and its expression during gonadal development and gametogenesis. Mol. Biol. Rep. 2012, 39, 4327–4335. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, D.; Wei, Y.; Ye, H. Role of Oxytocin/Vasopressin-Like Peptide and Its Receptor in Vitellogenesis of Mud Crab. Int. J. Mol. Sci. 2020, 21, 2297. https://doi.org/10.3390/ijms21072297
Lin D, Wei Y, Ye H. Role of Oxytocin/Vasopressin-Like Peptide and Its Receptor in Vitellogenesis of Mud Crab. International Journal of Molecular Sciences. 2020; 21(7):2297. https://doi.org/10.3390/ijms21072297
Chicago/Turabian StyleLin, Dongdong, Yujie Wei, and Haihui Ye. 2020. "Role of Oxytocin/Vasopressin-Like Peptide and Its Receptor in Vitellogenesis of Mud Crab" International Journal of Molecular Sciences 21, no. 7: 2297. https://doi.org/10.3390/ijms21072297
APA StyleLin, D., Wei, Y., & Ye, H. (2020). Role of Oxytocin/Vasopressin-Like Peptide and Its Receptor in Vitellogenesis of Mud Crab. International Journal of Molecular Sciences, 21(7), 2297. https://doi.org/10.3390/ijms21072297





