At the Crossroads of the Adipocyte and Osteoclast Differentiation Programs: Future Therapeutic Perspectives
Abstract
1. Introduction
2. Regulation of Osteoclastogenesis Through the Adipogenic Program
3. Transcription Factors in the Intertwining of Adipogenesis and Osteoclastogenesis
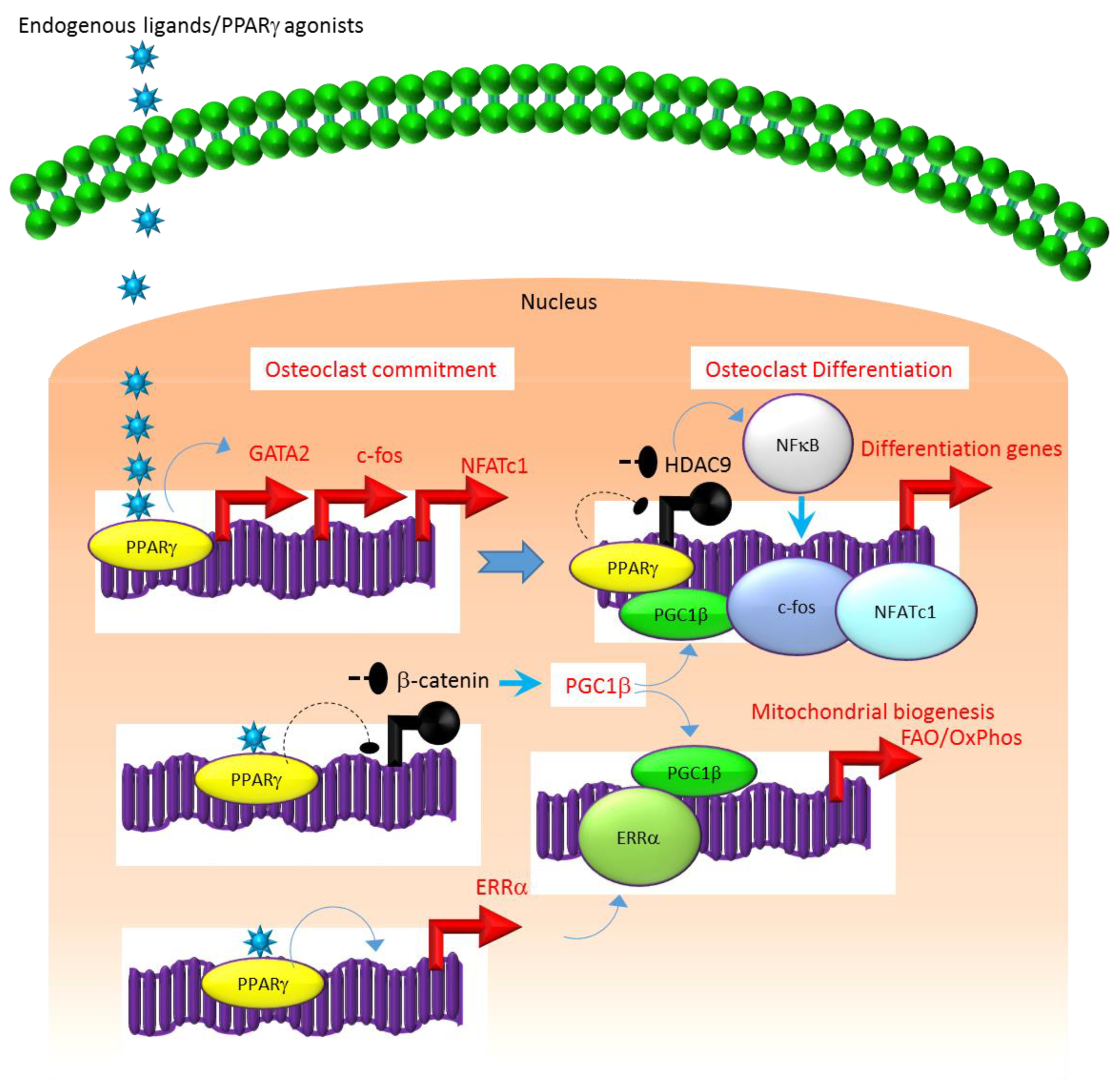
3.1. PPARγ: The Master Adipogenic Transcription Factor
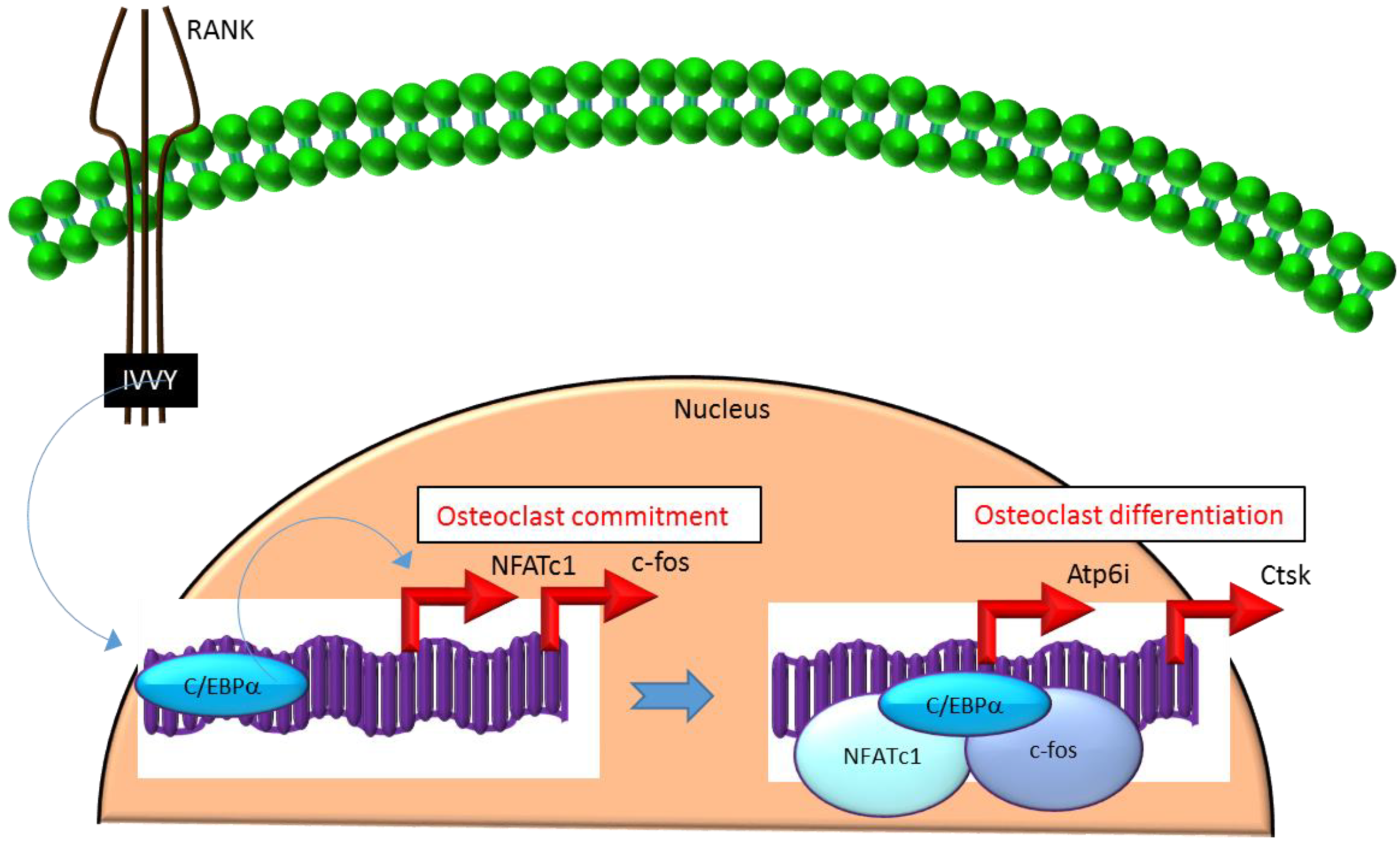
3.2. C/EBPα
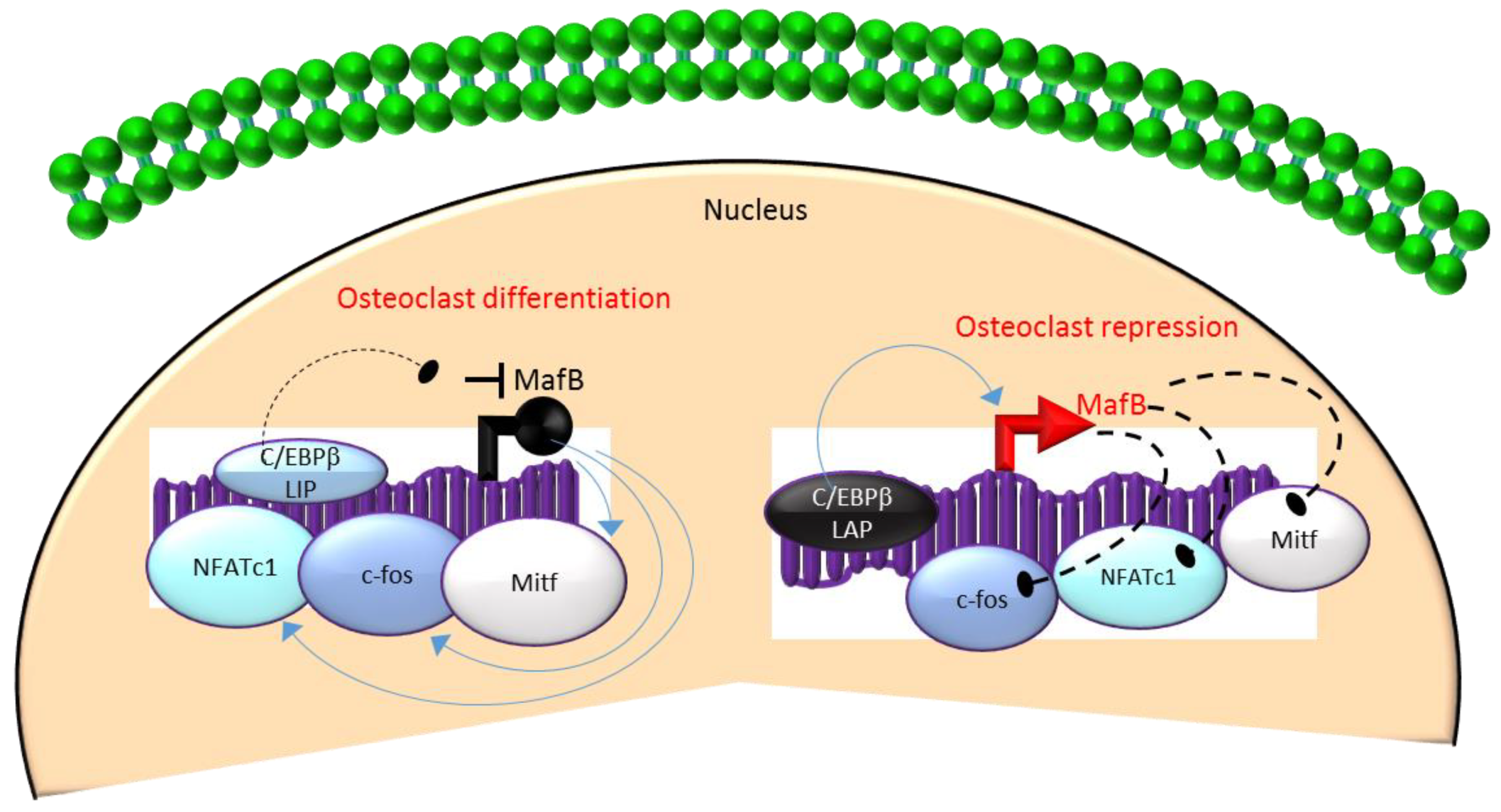
3.3. C/EBPβ
4. Bone Marrow Adipose Tissue (BMAT)-Secreted Molecules Regulating Osteoclast Differentiation
5. Future Perspective for Biomarkers and Therapeutic Targets
Author Contributions
Funding
Conflicts of Interest
References
- Zaidi, M. Skeletal remodeling in health and disease. Nat. Med. 2007, 13, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Sun, L.; Zaidi, M. Coupling bone degradation to formation. Nat. Med. 2009, 15, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Muruganandan, S.; Roman, A.A.; Sinal, C.J. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: Cross talk with the osteoblastogenic program. Cell. Mol. Life Sci. 2009, 66, 236–253. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wu, X.; Lei, W.; Pang, L.; Wan, C.; Shi, Z.; Zhao, L.; Nagy, T.R.; Peng, X.; Hu, J.; et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat. Med. 2009, 15, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Hayashi, M.; Fukunaga, T.; Kurata, K.; Oh-hora, M.; Feng, J.Q.; Bonewald, L.F.; Kodama, T.; Wutz, A.; Wagner, E.F.; et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 2011, 17, 1231–1234. [Google Scholar] [CrossRef]
- Muruganandan, S.; Sinal, C.J. The impact of bone marrow adipocytes on osteoblast and osteoclast differentiation. IUBMB Life 2014, 66, 147–155. [Google Scholar] [CrossRef]
- Muruganandan, S.; Govindarajan, R.; Sinal, C.J. Bone marrow adipose tissue and skeletal health. Curr. Osteoporos. Rep. 2018, 16, 434–442. [Google Scholar] [CrossRef]
- Fierro, F.A.; Nolta, J.A.; Adamopoulos, I.E. Concise review: Stem cells in osteoimmunology. Stem Cells 2017, 35, 1461–1467. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Duan, N.; Zhu, G.; Schwarz, E.M.; Xie, C. Osteoblast-osteoclast interactions. Connect Tissue Res. 2018, 59, 99–107. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xing, L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res. Ther. 2007, 9 (Suppl. 1), S1. [Google Scholar] [CrossRef]
- Xiong, J.; Cawley, K.; Piemontese, M.; Fujiwara, Y.; Zhao, H.; Goellner, J.J.; O’Brien, C.A. Soluble RANKL contributes to osteoclast formation in adult mice but not ovariectomy-induced bone loss. Nat. Commun. 2018, 9, 2909. [Google Scholar] [CrossRef] [PubMed]
- Honma, M.; Ikebuchi, Y.; Kariya, Y.; Hayashi, M.; Hayashi, N.; Aoki, S.; Suzuki, H. RANKL subcellular trafficking and regulatory mechanisms in osteocytes. J. Bone Miner. Res. 2013, 28, 1936–1949. [Google Scholar] [CrossRef]
- Ikebuchi, Y.; Aoki, S.; Honma, M.; Hayashi, M.; Sugamori, Y.; Khan, M.; Kariya, Y.; Kato, G.; Tabata, Y.; Penninger, J.M.; et al. Coupling of bone resorption and formation by RANKL reverse signalling. Nature 2018, 561, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Cao, X. RANKL-RANK signaling regulates osteoblast differentiation and bone formation. Bone Res. 2018, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, X.; Li, G.; Chong, Y.; Zhang, J.; Guo, X.; Li, B.; Bi, Z. Osteoclast regulation of osteoblasts via RANKRANKL reverse signal transduction in vitro. Mol. Med. Rep. 2017, 16, 3994–4000. [Google Scholar] [CrossRef] [PubMed]
- Weivoda, M.M.; Ruan, M.; Pederson, L.; Hachfeld, C.; Davey, R.A.; Zajac, J.D.; Westendorf, J.J.; Khosla, S.; Oursler, M.J. Osteoclast TGF-beta receptor signaling induces Wnt1 secretion and couples bone resorption to bone formation. J. Bone Miner. Res. 2016, 31, 76–85. [Google Scholar] [CrossRef]
- Tamma, R.; Zallone, A. Osteoblast and osteoclast crosstalks: From OAF to Ephrin. Inflamm. Allergy Drug Targets 2012, 11, 196–200. [Google Scholar] [CrossRef]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef]
- Li, J.; Zhang, N.; Huang, X.; Xu, J.; Fernandes, J.C.; Dai, K.; Zhang, X. Dexamethasone shifts bone marrow stromal cells from osteoblasts to adipocytes by C/EBPalpha promoter methylation. Cell Death Dis. 2013, 4, e832. [Google Scholar] [CrossRef]
- Fan, Q.; Tang, T.; Zhang, X.; Dai, K. The role of CCAAT/enhancer binding protein (C/EBP)-alpha in osteogenesis of C3H10T1/2 cells induced by BMP-2. J. Cell. Mol. Med. 2009, 13, 2489–2505. [Google Scholar] [CrossRef]
- Sun, H.; Kim, J.K.; Mortensen, R.; Mutyaba, L.P.; Hankenson, K.D.; Krebsbach, P.H. Osteoblast-targeted suppression of PPARgamma increases osteogenesis through activation of mTOR signaling. Stem Cells 2013, 31, 2183–2192. [Google Scholar] [CrossRef]
- Akune, T.; Ohba, S.; Kamekura, S.; Yamaguchi, M.; Chung, U.I.; Kubota, N.; Terauchi, Y.; Harada, Y.; Azuma, Y.; Nakamura, K.; et al. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J. Clin. Investig. 2004, 113, 846–855. [Google Scholar] [CrossRef]
- Muruganandan, S.; Govindarajan, R.; McMullen, N.M.; Sinal, C.J. Chemokine-Like Receptor 1 Is a Novel Wnt Target Gene that Regulates Mesenchymal Stem Cell Differentiation. Stem Cells 2017, 35, 711–724. [Google Scholar] [CrossRef]
- Astudillo, P.; Rios, S.; Pastenes, L.; Pino, A.M.; Rodriguez, J.P. Increased adipogenesis of osteoporotic human-mesenchymal stem cells (MSCs) characterizes by impaired leptin action. J. Cell. Biochem. 2008, 103, 1054–1065. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, S.Y.; Kim, A.Y.; Lee, E.J.; Choi, J.Y.; Kim, J.B. Adiponectin stimulates osteoblast differentiation through induction of COX2 in mesenchymal progenitor cells. Stem Cells 2009, 27, 2254–2262. [Google Scholar] [CrossRef]
- Wu, S.S.; Liang, Q.H.; Liu, Y.; Cui, R.R.; Yuan, L.Q.; Liao, E.Y. Omentin-1 Stimulates Human Osteoblast Proliferation through PI3K/Akt Signal Pathway. Int. J. Endocrinol. 2013, 2013, 368970. [Google Scholar] [CrossRef]
- Wan, Y.; Chong, L.W.; Evans, R.M. PPAR-gamma regulates osteoclastogenesis in mice. Nat. Med. 2007, 13, 1496–1503. [Google Scholar] [CrossRef]
- Wei, W.; Wang, X.; Yang, M.; Smith, L.C.; Dechow, P.C.; Sonoda, J.; Evans, R.M.; Wan, Y. PGC1beta mediates PPARgamma activation of osteoclastogenesis and rosiglitazone-induced bone loss. Cell Metab. 2010, 11, 503–516. [Google Scholar] [CrossRef]
- Wei, W.; Zeve, D.; Wang, X.; Du, Y.; Tang, W.; Dechow, P.C.; Graff, J.M.; Wan, Y. Osteoclast progenitors reside in the peroxisome proliferator-activated receptor gamma-expressing bone marrow cell population. Mol. Cell. Biol. 2011, 31, 4692–4705. [Google Scholar] [CrossRef]
- Jules, J.; Chen, W.; Feng, X.; Li, Y.P. CCAAT/Enhancer-binding protein alpha (C/EBPalpha) is important for osteoclast differentiation and activity. J. Biol. Chem. 2016, 291, 16390–16403. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, G.; Hao, L.; Wu, M.; Ci, H.; Li, Y.P. C/EBPalpha regulates osteoclast lineage commitment. Proc. Natl. Acad. Sci. USA 2013, 110, 7294–7299. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhu, G.; Tang, J.; Zhou, H.D.; Li, Y.P. C/ebpalpha controls osteoclast terminal differentiation, activation, function, and postnatal bone homeostasis through direct regulation of Nfatc1. J. Pathol. 2018, 244, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Smink, J.J.; Leutz, A. Rapamycin and the transcription factor C/EBPbeta as a switch in osteoclast differentiation: Implications for lytic bone diseases. J. Mol. Med. 2010, 88, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Smink, J.J.; Begay, V.; Schoenmaker, T.; Sterneck, E.; de Vries, T.J.; Leutz, A. Transcription factor C/EBPbeta isoform ratio regulates osteoclastogenesis through MafB. EMBO J. 2009, 28, 1769–1781. [Google Scholar] [CrossRef]
- Jules, J.; Chen, W.; Feng, X.; Li, Y.P. C/EBPalpha transcription factor is regulated by the RANK cytoplasmic (535)IVVY(538) motif and stimulates osteoclastogenesis more strongly than c-Fos. J. Biol. Chem. 2018, 293, 1480–1492. [Google Scholar] [CrossRef]
- Muruganandan, S.; Dranse, H.J.; Rourke, J.L.; McMullen, N.M.; Sinal, C.J. Chemerin neutralization blocks hematopoietic stem cell osteoclastogenesis. Stem Cells 2013, 31, 2172–2182. [Google Scholar] [CrossRef]
- Thommesen, L.; Stunes, A.K.; Monjo, M.; Grosvik, K.; Tamburstuen, M.V.; Kjobli, E.; Lyngstadaas, S.P.; Reseland, J.E.; Syversen, U. Expression and regulation of resistin in osteoblasts and osteoclasts indicate a role in bone metabolism. J. Cell. Biochem. 2006, 99, 824–834. [Google Scholar] [CrossRef]
- Tu, Q.; Zhang, J.; Dong, L.Q.; Saunders, E.; Luo, E.; Tang, J.; Chen, J. Adiponectin inhibits osteoclastogenesis and bone resorption via APPL1-mediated suppression of Akt1. J. Biol. Chem. 2011, 286, 12542–12553. [Google Scholar] [CrossRef]
- Khosla, S. Minireview: The OPG/RANKL/RANK system. Endocrinology 2001, 142, 5050–5055. [Google Scholar] [CrossRef]
- Gravallese, E.M.; Galson, D.L.; Goldring, S.R.; Auron, P.E. The role of TNF-receptor family members and other TRAF-dependent receptors in bone resorption. Arthritis Res. 2001, 3, 6–12. [Google Scholar] [CrossRef]
- Takayanagi, H. Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007, 7, 292–304. [Google Scholar] [CrossRef]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef]
- Takeshita, S.; Fumoto, T.; Naoe, Y.; Ikeda, K. Age-related marrow adipogenesis is linked to increased expression of RANKL. J. Biol. Chem. 2014, 289, 16699–16710. [Google Scholar] [CrossRef]
- Goto, H.; Hozumi, A.; Osaki, M.; Fukushima, T.; Sakamoto, K.; Yonekura, A.; Tomita, M.; Furukawa, K.; Shindo, H.; Baba, H. Primary human bone marrow adipocytes support TNF-alpha-induced osteoclast differentiation and function through RANKL expression. Cytokine 2011, 56, 662–668. [Google Scholar] [CrossRef]
- Goto, H.; Osaki, M.; Fukushima, T.; Sakamoto, K.; Hozumi, A.; Baba, H.; Shindo, H. Human bone marrow adipocytes support dexamethasone-induced osteoclast differentiation and function through RANKL expression. Biomed. Res. 2011, 32, 37–44. [Google Scholar] [CrossRef]
- Fan, Y.; Hanai, J.I.; Le, P.T.; Bi, R.; Maridas, D.; DeMambro, V.; Figueroa, C.A.; Kir, S.; Zhou, X.; Mannstadt, M.; et al. Parathyroid hormone directs bone marrow mesenchymal cell fate. Cell Metab. 2017, 25, 661–672. [Google Scholar] [CrossRef]
- Song, B.Q.; Chi, Y.; Li, X.; Du, W.J.; Han, Z.B.; Tian, J.J.; Li, J.J.; Chen, F.; Wu, H.H.; Han, L.X.; et al. Inhibition of notch signaling promotes the adipogenic differentiation of mesenchymal stem cells through autophagy activation and PTEN-PI3K/AKT/mTOR pathway. Cell. Physiol. Biochem. 2015, 36, 1991–2002. [Google Scholar] [CrossRef]
- Osathanon, T.; Subbalekha, K.; Sastravaha, P.; Pavasant, P. Notch signalling inhibits the adipogenic differentiation of single-cell-derived mesenchymal stem cell clones isolated from human adipose tissue. Cell Biol. Int. 2012, 36, 1161–1170. [Google Scholar] [CrossRef]
- Ugarte, F.; Ryser, M.; Thieme, S.; Fierro, F.A.; Navratiel, K.; Bornhauser, M.; Brenner, S. Notch signaling enhances osteogenic differentiation while inhibiting adipogenesis in primary human bone marrow stromal cells. Exp. Hematol. 2009, 37, 867–875. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, X.; Wu, Y.; Jing, W.; Cai, X.; Tang, W.; Liu, L.; Liu, Y.; Grottkau, B.E.; Lin, Y. Gamma-secretase inhibitor induces adipogenesis of adipose-derived stem cells by regulation of Notch and PPAR-gamma. Cell Prolif. 2010, 43, 147–156. [Google Scholar] [CrossRef]
- Chatterjee, T.K.; Idelman, G.; Blanco, V.; Blomkalns, A.L.; Piegore, M.G.; Weintraub, D.S.; Kumar, S.; Rajsheker, S.; Manka, D.; Rudich, S.M.; et al. Histone deacetylase 9 is a negative regulator of adipogenic differentiation. J. Biol. Chem. 2011, 286, 27836–27847. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.N. Nuclear receptor corepressors and PPARgamma. Nucl. Recept. Signal. 2006, 4, e003. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Wei, W.; Huynh, H.; Wan, Y. HDAC9 Inhibits Osteoclastogenesis via Mutual Suppression of PPARgamma/RANKL Signaling. Mol. Endocrinol. 2015, 29, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Takada, I.; Kouzmenko, A.P.; Kato, S. Wnt and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat. Rev. Rheumatol. 2009, 5, 442–447. [Google Scholar] [CrossRef]
- Suh, H.C.; Gooya, J.; Renn, K.; Friedman, A.D.; Johnson, P.F.; Keller, J.R. C/EBPalpha determines hematopoietic cell fate in multipotential progenitor cells by inhibiting erythroid differentiation and inducing myeloid differentiation. Blood 2006, 107, 4308–4316. [Google Scholar] [CrossRef]
- Hasemann, M.S.; Lauridsen, F.K.; Waage, J.; Jakobsen, J.S.; Frank, A.K.; Schuster, M.B.; Rapin, N.; Bagger, F.O.; Hoppe, P.S.; Schroeder, T.; et al. C/EBPalpha is required for long-term self-renewal and lineage priming of hematopoietic stem cells and for the maintenance of epigenetic configurations in multipotent progenitors. PLoS Genet. 2014, 10, e1004079. [Google Scholar] [CrossRef]
- Huber, R.; Pietsch, D.; Panterodt, T.; Brand, K. Regulation of C/EBPbeta and resulting functions in cells of the monocytic lineage. Cell Signal. 2012, 24, 1287–1296. [Google Scholar] [CrossRef]
- An, M.R.; Hsieh, C.C.; Reisner, P.D.; Rabek, J.P.; Scott, S.G.; Kuninger, D.T.; Papaconstantinou, J. Evidence for posttranscriptional regulation of C/EBPalpha and C/EBPbeta isoform expression during the lipopolysaccharide-mediated acute-phase response. Mol. Cell. Biol. 1996, 16, 2295–2306. [Google Scholar] [CrossRef]
- Cao, Z.; Umek, R.M.; McKnight, S.L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991, 5, 1538–1552. [Google Scholar] [CrossRef]
- Lechner, S.; Mitterberger, M.C.; Mattesich, M.; Zwerschke, W. Role of C/EBPbeta-LAP and C/EBPbeta-LIP in early adipogenic differentiation of human white adipose-derived progenitors and at later stages in immature adipocytes. Differentiation 2013, 85, 20–31. [Google Scholar] [CrossRef]
- Hamm, J.K.; Park, B.H.; Farmer, S.R. A role for C/EBPbeta in regulating peroxisome proliferator-activated receptor gamma activity during adipogenesis in 3T3-L1 preadipocytes. J. Biol. Chem. 2001, 276, 18464–18471. [Google Scholar] [CrossRef] [PubMed]
- Zidek, L.M.; Ackermann, T.; Hartleben, G.; Eichwald, S.; Kortman, G.; Kiehntopf, M.; Leutz, A.; Sonenberg, N.; Wang, Z.Q.; von Maltzahn, J.; et al. Deficiency in mTORC1-controlled C/EBPbeta-mRNA translation improves metabolic health in mice. EMBO Rep. 2015, 16, 1022–1036. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Chen, J. Regulation of peroxisome proliferator-activated receptor-gamma activity by mammalian target of rapamycin and amino acids in adipogenesis. Diabetes 2004, 53, 2748–2756. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Horvat, S.; Festuccia, W.T.; Birsoy, K.; Prevorsek, Z.; Efeyan, A.; Sabatini, D.M. DEPTOR cell-autonomously promotes adipogenesis, and its expression is associated with obesity. Cell Metab. 2012, 16, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, J.H.; Lee, J.; Jin, H.M.; Kook, H.; Kim, K.K.; Lee, S.Y.; Kim, N. MafB negatively regulates RANKL-mediated osteoclast differentiation. Blood 2007, 109, 3253–3259. [Google Scholar] [CrossRef] [PubMed]
- Muruganandan, S.; Parlee, S.D.; Rourke, J.L.; Ernst, M.C.; Goralski, K.B.; Sinal, C.J. Chemerin, a novel peroxisome proliferator-activated receptor gamma (PPARgamma) target gene that promotes mesenchymal stem cell adipogenesis. J. Biol. Chem. 2011, 286, 23982–23995. [Google Scholar] [CrossRef] [PubMed]
- Goralski, K.B.; McCarthy, T.C.; Hanniman, E.A.; Zabel, B.A.; Butcher, E.C.; Parlee, S.D.; Muruganandan, S.; Sinal, C.J. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J. Biol. Chem. 2007, 282, 28175–28188. [Google Scholar] [CrossRef]
- Muruganandan, S.; Roman, A.A.; Sinal, C.J. Role of chemerin/CMKLR1 signaling in adipogenesis and osteoblastogenesis of bone marrow stem cells. J. Bone Miner. Res. 2010, 25, 222–234. [Google Scholar] [CrossRef]
- Moschen, A.R.; Geiger, S.; Gerner, R.; Tilg, H. Pre-B cell colony enhancing factor/NAMPT/visfatin and its role in inflammation-related bone disease. Mutat. Res. 2010, 690, 95–101. [Google Scholar] [CrossRef]
- Baek, J.M.; Ahn, S.J.; Cheon, Y.H.; Lee, M.S.; Oh, J.; Kim, J.Y. Nicotinamide phosphoribosyltransferase inhibits receptor activator of nuclear factor-kappaB ligand-induced osteoclast differentiation in vitro. Mol. Med. Rep. 2017, 15, 784–792. [Google Scholar] [CrossRef]
- Hassan, B.; Baroukh, B.; Llorens, A.; Lesieur, J.; Ribbes, S.; Chaussain, C.; Saffar, J.L.; Gosset, M. NAMPT expression in osteoblasts controls osteoclast recruitment in alveolar bone remodeling. J. Cell. Physiol. 2018, 233, 7402–7414. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, C.Y.; Wu, S.S.; Liang, Q.H.; Yuan, L.Q.; Liao, E.Y. Novel adipokines and bone metabolism. Int. J. Endocrinol. 2013, 2013, 895045. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Xie, P.L.; Luo, X.H.; Wu, X.P.; Zhou, H.D.; Tang, S.Y.; Liao, E.Y. Omentin-1 exerts bone-sparing effect in ovariectomized mice. Osteoporos. Int. 2012, 23, 1425–1436. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Li, T.; Li, W.; Chen, Y.; Xu, W.; Xu, L. Leptin can promote mineralization and up-regulate RANKL mRNA expression in osteoblasts from adult female SD rats. Int. J. Clin. Exp. Pathol. 2018, 11, 1610–1619. [Google Scholar]
- Hlaing, T.T.; Compston, J.E. Biochemical markers of bone turnover - uses and limitations. Ann. Clin. Biochem. 2014, 51 Pt 2, 189–202. [Google Scholar] [CrossRef]
- Greenblatt, M.B.; Tsai, J.N.; Wein, M.N. Bone turnover markers in the diagnosis and monitoring of metabolic bone disease. Clin. Chem. 2017, 63, 464–474. [Google Scholar] [CrossRef]
- Patsch, J.M.; Li, X.; Baum, T.; Yap, S.P.; Karampinos, D.C.; Schwartz, A.V.; Link, T.M. Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. J. Bone Miner. Res. 2013, 28, 1721–1728. [Google Scholar] [CrossRef]
- Grey, A.; Beckley, V.; Doyle, A.; Fenwick, S.; Horne, A.; Gamble, G.; Bolland, M. Pioglitazone increases bone marrow fat in type 2 diabetes: Results from a randomized controlled trial. Eur. J. Endocrinol. 2012, 166, 1087–1091. [Google Scholar] [CrossRef]
- Paccou, J.; Hardouin, P.; Cotten, A.; Penel, G.; Cortet, B. The role of bone marrow fat in skeletal health: Usefulness and perspectives for clinicians. J. Clin. Endocrinol. Metab. 2015, 100, 3613–3621. [Google Scholar] [CrossRef]
- Iwaniec, U.T.; Turner, R.T. Failure to generate bone marrow adipocytes does not protect mice from ovariectomy-induced osteopenia. Bone 2013, 53, 145–153. [Google Scholar] [CrossRef]
- Justesen, J.; Mosekilde, L.; Holmes, M.; Stenderup, K.; Gasser, J.; Mullins, J.J.; Seckl, J.R.; Kassem, M. Mice deficient in 11beta-hydroxysteroid dehydrogenase type 1 lack bone marrow adipocytes, but maintain normal bone formation. Endocrinology 2004, 145, 1916–1925. [Google Scholar] [CrossRef] [PubMed]
- Qaseem, A.; Forciea, M.A.; McLean, R.M.; Denberg, T.D. Treatment of low bone density or osteoporosis to prevent fractures in men and women: A clinical practice guideline update from the American College of Physicians. Ann. Intern. Med. 2017, 166, 818–839. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, T.H.; Scialdone, A.; Graja, A.; Gohlke, S.; Jank, A.M.; Bocian, C.; Woelk, L.; Fan, H.; Logan, D.W.; Schürmann, A.; et al. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell 2017, 20, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Duque, G.; Rivas, D. Alendronate has an anabolic effect on bone through the differentiation of mesenchymal stem cells. J. Bone Miner. Res. 2007, 10, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Duque, G.; Li, W.; Adams, M.; Xu, S.; Phipps, R. Effects of risedronate on bone marrow adipocytes in postmenopausal women. Osteoporos. Int. 2011, 22, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C. Bone: Risedronate and marrow adiposity. Nat. Rev. Endocrinol. 2010, 6, 597. [Google Scholar] [PubMed]
- Yang, Y.; Luo, X.; Xie, X.; Yan, F.; Chen, G.; Zhao, W.; Jiang, Z.; Fang, C.; Shen, J. Influences of teriparatide administration on marrow fat content in postmenopausal osteopenic women using MR spectroscopy. Climacteric 2016, 19, 285–291. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muruganandan, S.; Ionescu, A.M.; Sinal, C.J. At the Crossroads of the Adipocyte and Osteoclast Differentiation Programs: Future Therapeutic Perspectives. Int. J. Mol. Sci. 2020, 21, 2277. https://doi.org/10.3390/ijms21072277
Muruganandan S, Ionescu AM, Sinal CJ. At the Crossroads of the Adipocyte and Osteoclast Differentiation Programs: Future Therapeutic Perspectives. International Journal of Molecular Sciences. 2020; 21(7):2277. https://doi.org/10.3390/ijms21072277
Chicago/Turabian StyleMuruganandan, Shanmugam, Andreia M. Ionescu, and Christopher J. Sinal. 2020. "At the Crossroads of the Adipocyte and Osteoclast Differentiation Programs: Future Therapeutic Perspectives" International Journal of Molecular Sciences 21, no. 7: 2277. https://doi.org/10.3390/ijms21072277
APA StyleMuruganandan, S., Ionescu, A. M., & Sinal, C. J. (2020). At the Crossroads of the Adipocyte and Osteoclast Differentiation Programs: Future Therapeutic Perspectives. International Journal of Molecular Sciences, 21(7), 2277. https://doi.org/10.3390/ijms21072277





