Alpha-Lipoic Acid Ameliorates Radiation-Induced Salivary Gland Injury by Preserving Parasympathetic Innervation in Rats
Abstract
:1. Introduction
2. Results
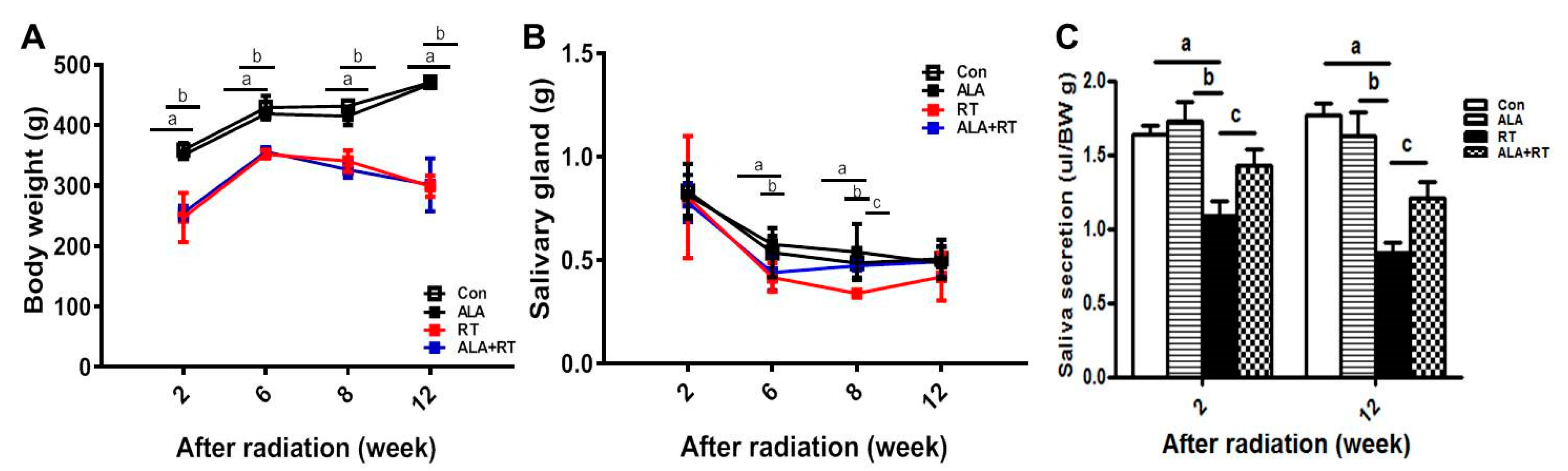
2.1. Radiation Significantly Induces Body Weight and SG Weight Loss, but ALA Ameliorates Radiation-Induced SG Weight Loss and Reduced Saliva Levels.
2.2. ALA Restores Radiation-Induced AQP5 Expression.
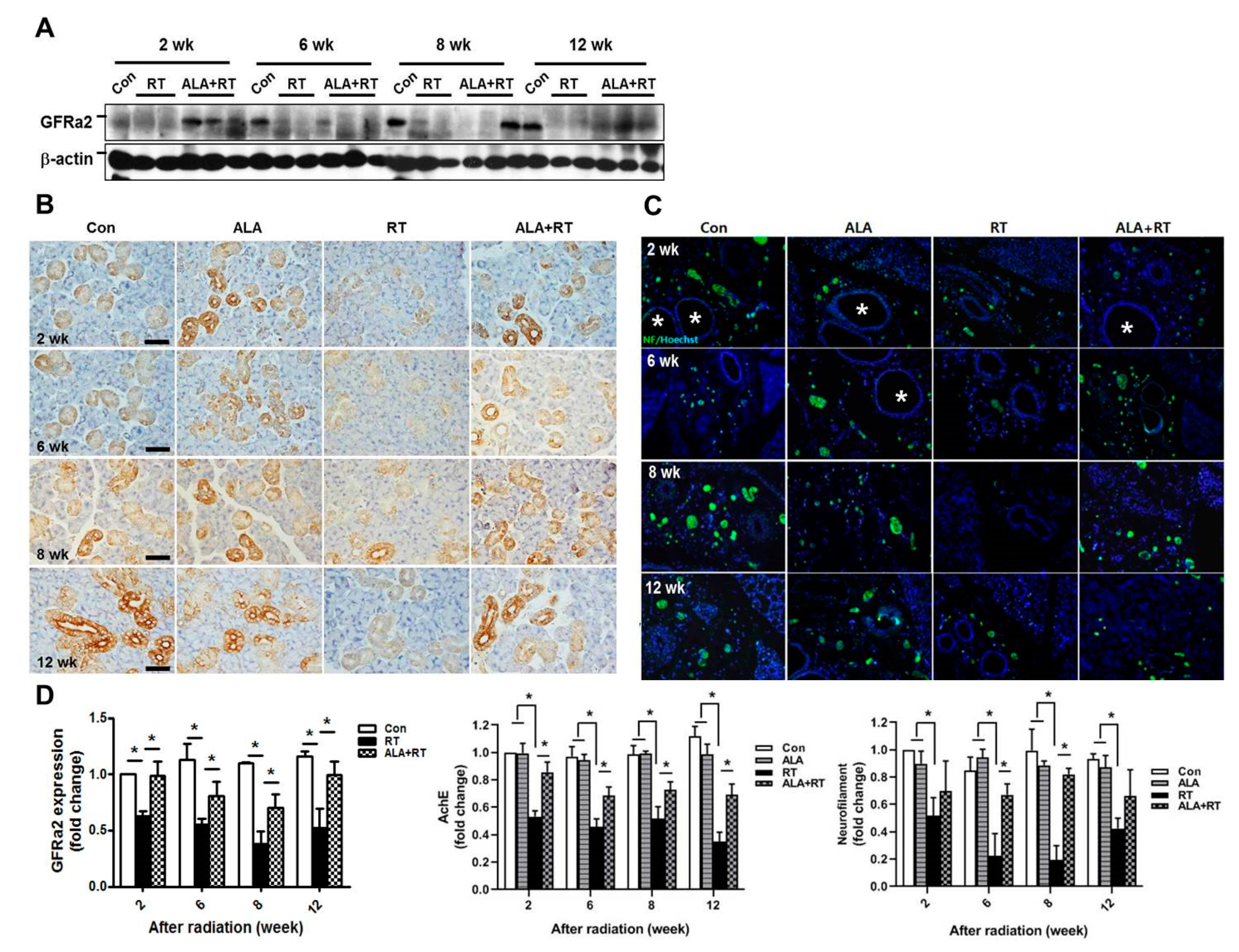
2.3. ALA Preserves Parasympathetic Innervation
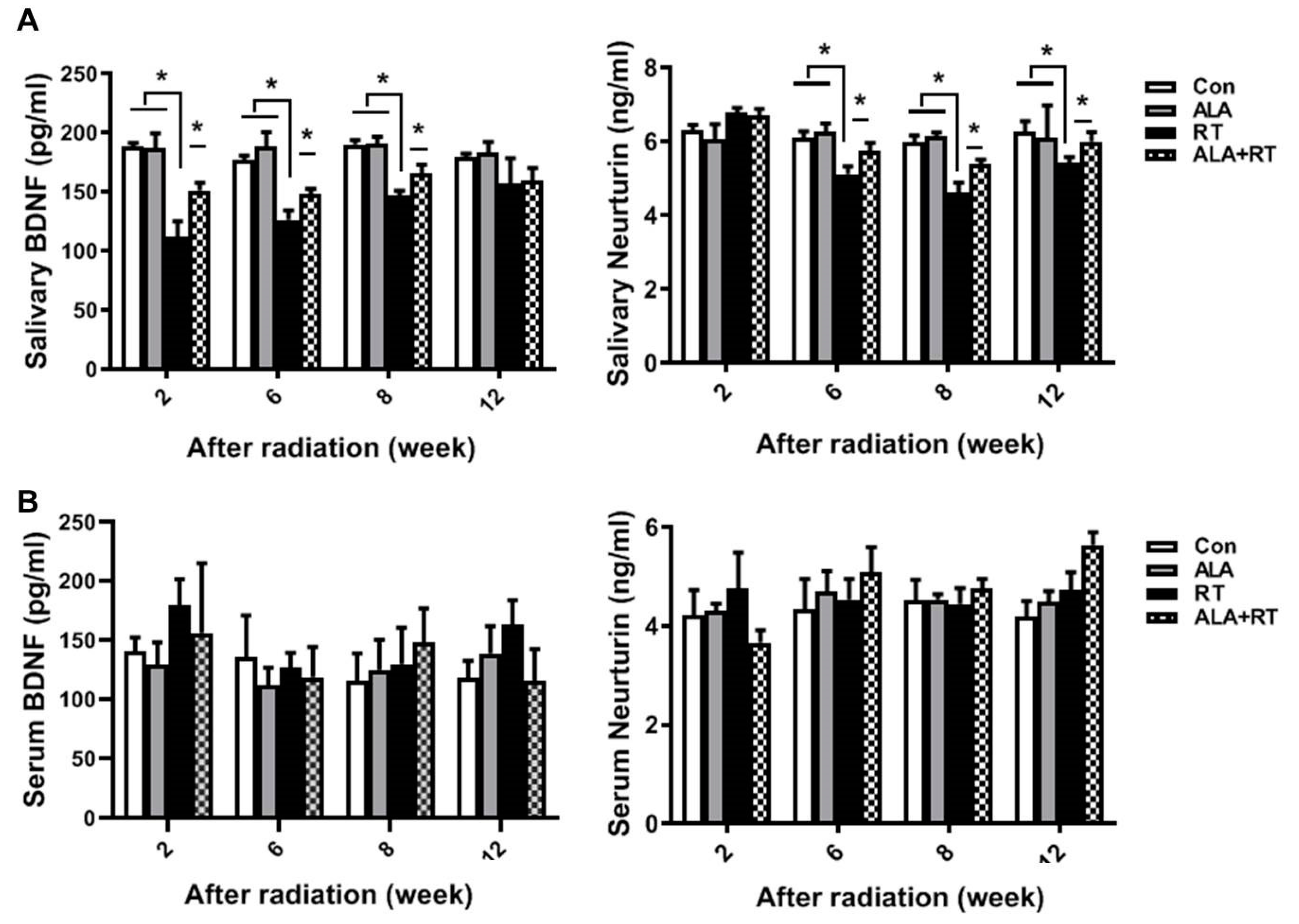
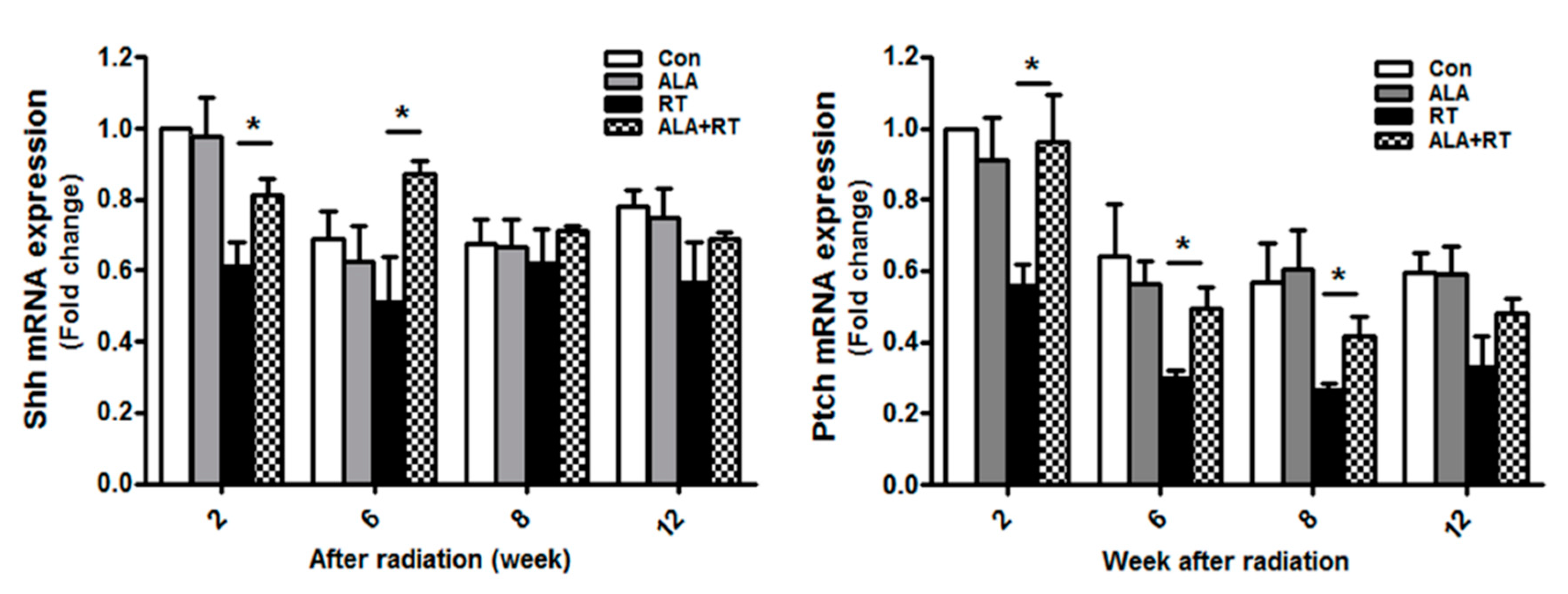
2.4. ALA Improves Neurotrophic Factor Levels in SGs.
2.5. ALA Increases SG Regeneration
2.6. ALA Rescues the Endogenous Resident Stem Cell Population.
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Radiation Exposure
4.3. Salivary Gland Function
4.4. Immunoblotting
4.5. Immunohistochemistry
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
4.8. Reverse-Transcription PCR
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Burlage, F.R.; Coppes, R.P.; Meertens, H.; Stokman, M.A.; Vissink, A. Parotid and submandibular/sublingual salivary flow during high dose radiotherapy. Radiother. Oncol. 2001, 61, 271–274. [Google Scholar] [CrossRef]
- Jellema, A.P.; Slotman, B.J.; Doornaert, P.; Leemans, C.R.; Langendijk, J.A. Impact of radiation-induced xerostomia on quality of life after primary radiotherapy among patients with head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Langendijk, J.A.; Doornaert, P.; Verdonck-de Leeuw, I.M. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J. Clin. Oncol. 2008, 26, 3770–3776. [Google Scholar] [CrossRef] [PubMed]
- Osailan, S.; Pramanik, R.; Shirodaria, S.; Challacombe, S.J.; Proctor, G.B. Investigating the relationship between hyposalivation and mucosal wetness. Oral Dis 2011, 17, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Dawes, C. Salivary flow patterns and the health of hard and soft oral tissues. J. Am. Dent. Assoc. 2008, 139, 18–24. [Google Scholar] [CrossRef]
- Jeong, B.K.; Song, J.H.; Jeong, H. Effect of alpha-lipoic acid on radiation-induced small intestine injury in mice. Oncotarget 2016, 7, 15105–15117. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.H.; Jung, J.; Kim, S.K.; Woo, S.H.; Kang, K.M.; Jeong, B.K.; Jung, M.H.; Kim, J.H.; Hahm, J.R. Alpha lipoic acid attenuates radiation-induced thyroid injury in rats. PLoS ONE 2014, 9, e112253. [Google Scholar] [CrossRef]
- Kim, J.H.; Jung, M.H.; Kim, J.P.; Kim, H.J.; Jung, J.H.; Hahm, J.R.; Kang, K.M.; Jeong, B.K.; Woo, S.H. Alpha lipoic acid attenuates radiation-induced oral mucositis in rats. Oncotarget 2017, 8, 72739–72747. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Kim, K.M.; Jung, M.H.; Jung, J.H.; Kang, K.M.; Jeong, B.K.; Kim, J.P.; Park, J.J.; Woo, S.H. Protective effects of alpha lipoic acid on radiation-induced salivary gland injury in rats. Oncotarget 2016, 7, 29143–29153. [Google Scholar] [CrossRef]
- Lombaert, I.; Movahednia, M.M.; Adine, C.; Ferreira, J.N. Concise Review: Salivary Gland Regeneration: Therapeutic Approaches from Stem Cells to Tissue Organoids. Stem Cells 2017, 35, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Bilska, A.; Wlodek, L. Lipoic acid - the drug of the future? Pharmacol Rep. 2005, 57, 570–577. [Google Scholar] [PubMed]
- Hai, B.; Qin, L.; Yang, Z.; Zhao, Q.; Shangguan, L.; Ti, X.; Zhao, Y.; Kim, S.; Rangaraj, D.; Liu, F. Transient activation of hedgehog pathway rescued irradiation-induced hyposalivation by preserving salivary stem/progenitor cells and parasympathetic innervation. Clin. Cancer Res. 2014, 20, 140–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knox, S.M.; Lombaert, I.M.; Haddox, C.L.; Abrams, S.R.; Cotrim, A.; Wilson, A.J.; Hoffman, M.P. Parasympathetic stimulation improves epithelial organ regeneration. Nat. Commun. 2013, 4, 1494. [Google Scholar] [CrossRef] [Green Version]
- Hai, B.; Yang, Z.; Millar, S.E.; Choi, Y.S.; Taketo, M.M.; Nagy, A.; Liu, F. Wnt/beta-catenin signaling regulates postnatal development and regeneration of the salivary gland. Stem Cells Dev. 2010, 19, 1793–1801. [Google Scholar] [CrossRef] [Green Version]
- Pringle, S.; Maimets, M.; van der Zwaag, M.; Stokman, M.A.; van Gosliga, D.; Zwart, E.; Witjes, M.J.; de Haan, G.; van Os, R.; Coppes, R.P. Human Salivary Gland Stem Cells Functionally Restore Radiation Damaged Salivary Glands. Stem Cells 2016, 34, 640–652. [Google Scholar] [CrossRef] [Green Version]
- Nanduri, L.S.; Maimets, M.; Pringle, S.A.; van der Zwaag, M.; van Os, R.P.; Coppes, R.P. Regeneration of irradiated salivary glands with stem cell marker expressing cells. Radiother. Oncol. 2011, 99, 367–372. [Google Scholar] [CrossRef] [Green Version]
- Konings, A.W.; Coppes, R.P.; Vissink, A. On the mechanism of salivary gland radiosensitivity. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 1187–1194. [Google Scholar] [CrossRef]
- Proctor, G.B.; Carpenter, G.H. Regulation of salivary gland function by autonomic nerves. Auton. Neurosci. 2007, 133, 3–18. [Google Scholar] [CrossRef]
- Choi, J.S.; An, H.Y.; Park, I.S.; Kim, S.K.; Kim, Y.M.; Lim, J.Y. Radioprotective Effect of Epigallocatechin-3-Gallate on Salivary Gland Dysfunction After Radioiodine Ablation in a Murine Model. Clin. Exp. Otorhinolaryngol 2016, 9, 244–251. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.H.; Chung, W.K.; Jeong, J.U.; Cho, I.J.; Yoon, M.S.; Song, J.Y.; Nam, I.K.; Ahn, S.J.; Lee, D.H.; Yoon, T.M.; et al. Evaluation of Prognostic Factors for the Parotid Cancer Treated With Surgery and Postoperative Radiotherapy. Clin. Exp. Otorhinolaryngol 2020, 13, 69–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noh, J.M.; Ahn, Y.C.; Nam, H.; Park, W.; Baek, C.H.; Son, Y.I.; Jeong, H.S. Treatment Results of Major Salivary Gland Cancer by Surgery with or without Postoperative Radiation Therapy. Clin. Exp. Otorhinolaryngol 2010, 3, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Eguchi, T.; Skowronski, M.T.; Ishida, H. Acetylcholine acts on M3 muscarinic receptors and induces the translocation of aquaporin5 water channel via cytosolic Ca2+ elevation in rat parotid glands. Biochem. Biophys. Res. Commun. 1998, 245, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Hai, B.; Zhao, Q.; Qin, L.; Rangaraj, D.; Gutti, V.R.; Liu, F. Rescue Effects and Underlying Mechanisms of Intragland Shh Gene Delivery on Irradiation-Induced Hyposalivation. Hum. Gene Ther. 2016, 27, 390–399. [Google Scholar] [CrossRef] [Green Version]
- Cotroneo, E.; Proctor, G.B.; Carpenter, G.H. Regeneration of acinar cells following ligation of rat submandibular gland retraces the embryonic-perinatal pathway of cytodifferentiation. Differentiation 2010, 79, 120–130. [Google Scholar] [CrossRef] [Green Version]
- Cotroneo, E.; Proctor, G.B.; Paterson, K.L.; Carpenter, G.H. Early markers of regeneration following ductal ligation in rat submandibular gland. Cell Tissue Res. 2008, 332, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Katsumata, O.; Sato, Y.; Sakai, Y.; Yamashina, S. Intercalated duct cells in the rat parotid gland may behave as tissue stem cells. Anat. Sci. Int. 2009, 84, 148–154. [Google Scholar] [CrossRef]
- Pringle, S.; Van Os, R.; Coppes, R.P. Concise review: Adult salivary gland stem cells and a potential therapy for xerostomia. Stem Cells 2013, 31, 613–619. [Google Scholar] [CrossRef]
- Aure, M.H.; Konieczny, S.F.; Ovitt, C.E. Salivary gland homeostasis is maintained through acinar cell self-duplication. Dev. Cell 2015, 33, 231–237. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Jeong, B.K.; Jang, S.J.; Yun, J.W.; Jung, M.H.; Kang, K.M.; Kim, T.G.; Woo, S.H. Alpha-Lipoic Acid Ameliorates Radiation-Induced Salivary Gland Injury by Preserving Parasympathetic Innervation in Rats. Int. J. Mol. Sci. 2020, 21, 2260. https://doi.org/10.3390/ijms21072260
Kim JH, Jeong BK, Jang SJ, Yun JW, Jung MH, Kang KM, Kim TG, Woo SH. Alpha-Lipoic Acid Ameliorates Radiation-Induced Salivary Gland Injury by Preserving Parasympathetic Innervation in Rats. International Journal of Molecular Sciences. 2020; 21(7):2260. https://doi.org/10.3390/ijms21072260
Chicago/Turabian StyleKim, Jin Hyun, Bae Kwon Jeong, Si Jung Jang, Jeong Won Yun, Myeong Hee Jung, Ki Mun Kang, Tae Gyu Kim, and Seung Hoon Woo. 2020. "Alpha-Lipoic Acid Ameliorates Radiation-Induced Salivary Gland Injury by Preserving Parasympathetic Innervation in Rats" International Journal of Molecular Sciences 21, no. 7: 2260. https://doi.org/10.3390/ijms21072260
APA StyleKim, J. H., Jeong, B. K., Jang, S. J., Yun, J. W., Jung, M. H., Kang, K. M., Kim, T. G., & Woo, S. H. (2020). Alpha-Lipoic Acid Ameliorates Radiation-Induced Salivary Gland Injury by Preserving Parasympathetic Innervation in Rats. International Journal of Molecular Sciences, 21(7), 2260. https://doi.org/10.3390/ijms21072260




