Melatonin Relations with Energy Metabolism as Possibly Involved in Fatal Mountain Road Traffic Accidents
Abstract
1. Introduction
2. Rhythm Generation
3. Rhythms, an Information System
4. In the Beginning There Was Light
5. The Redox System, an Axis
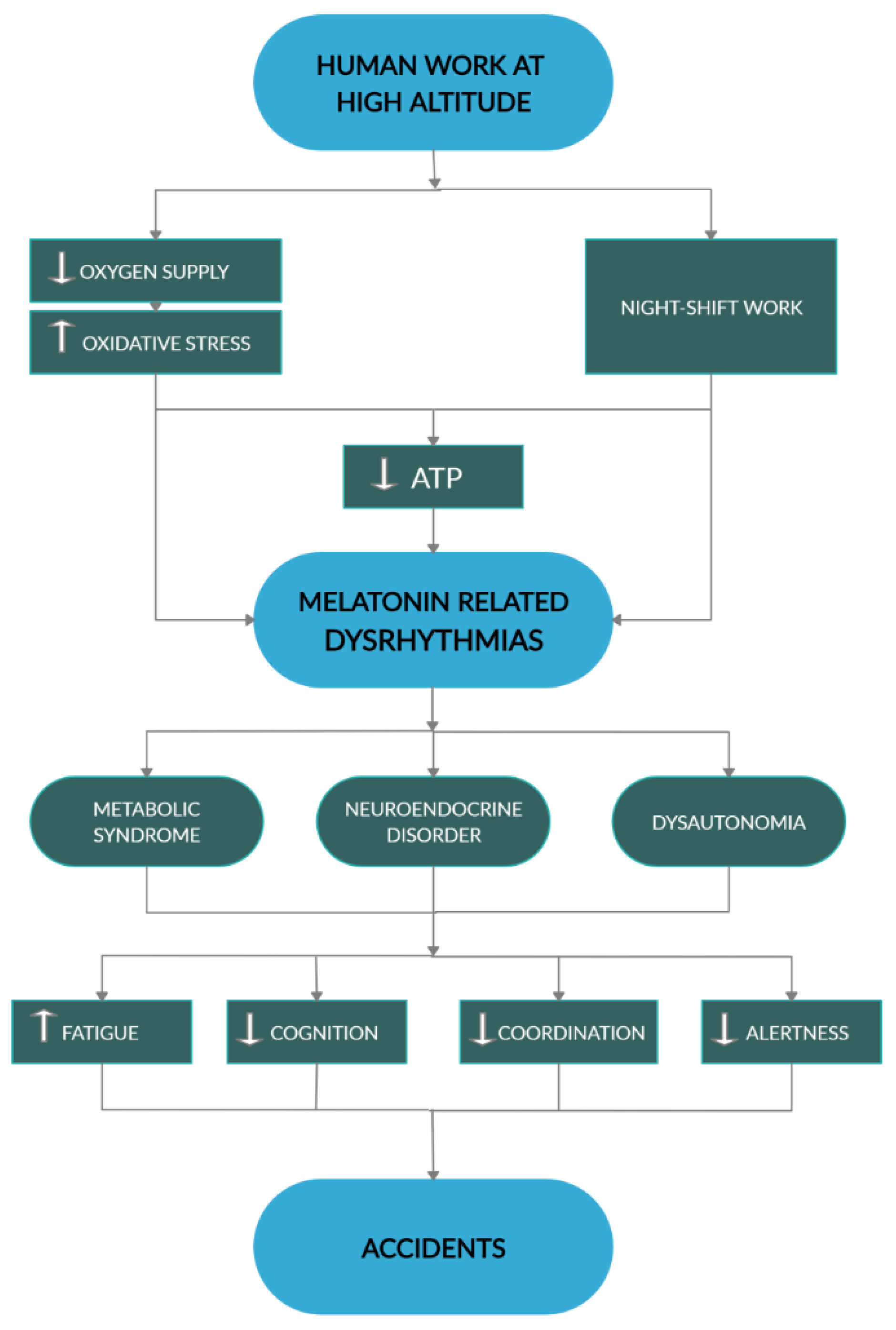
6. No Swing, No Pleasure, No Health
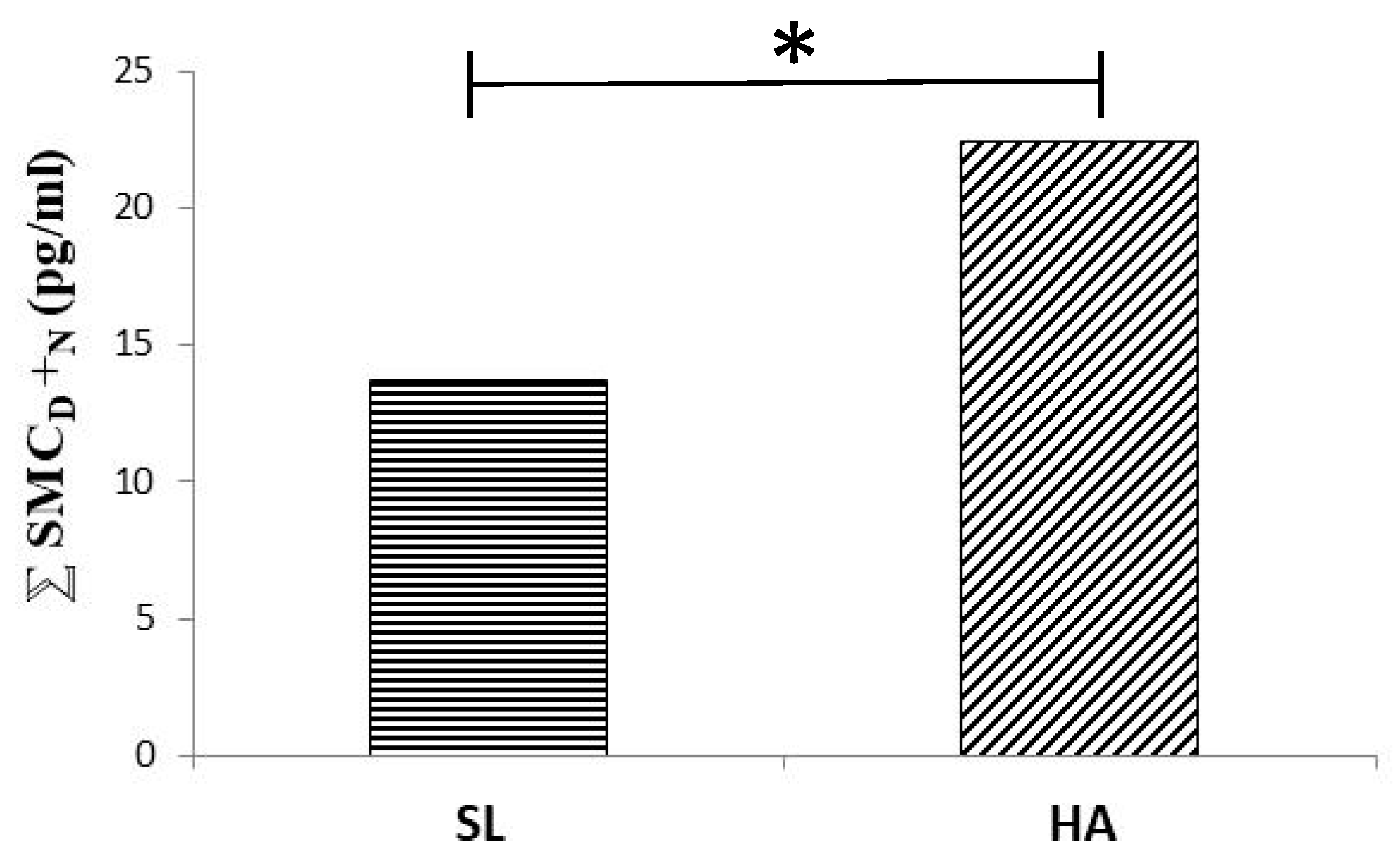
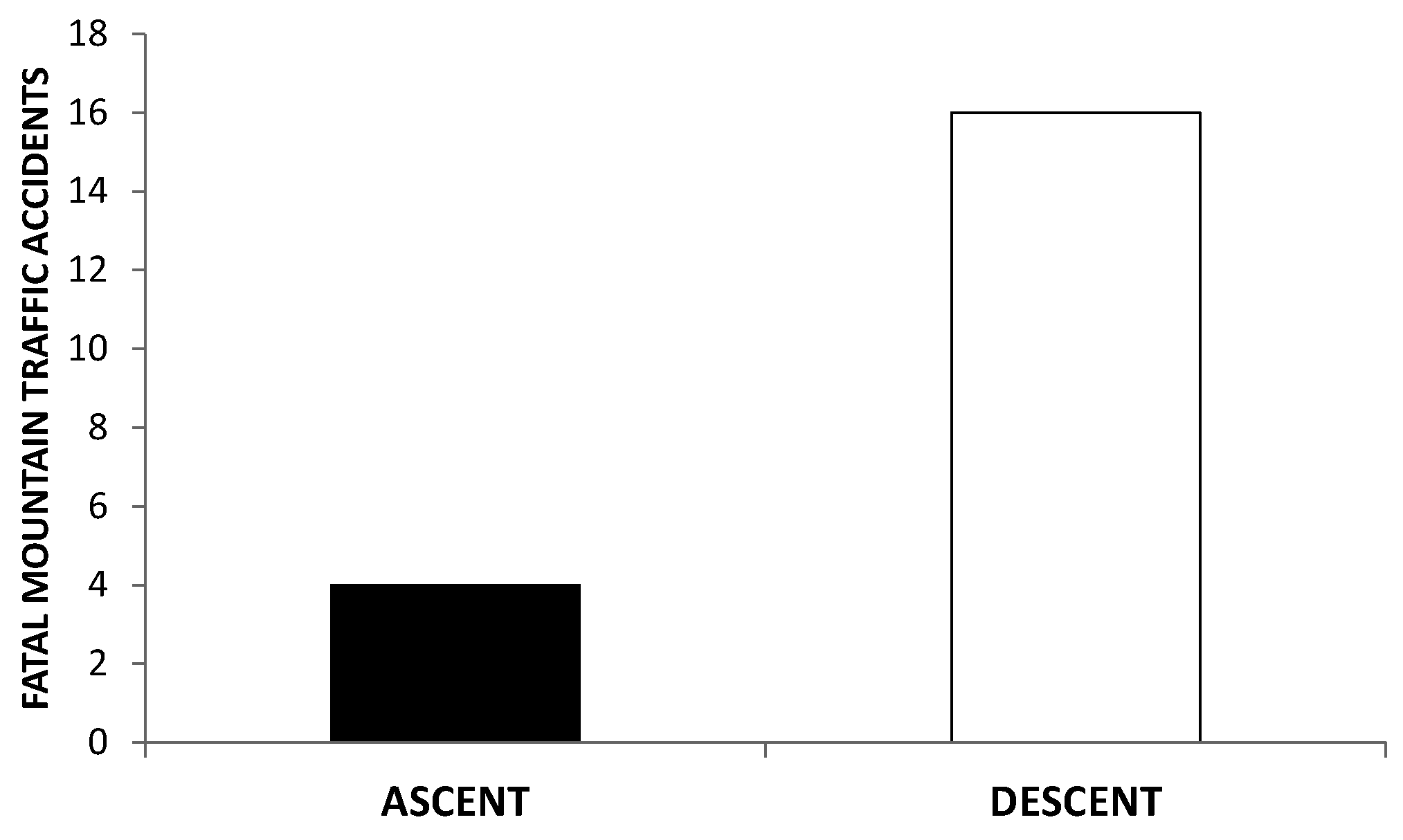
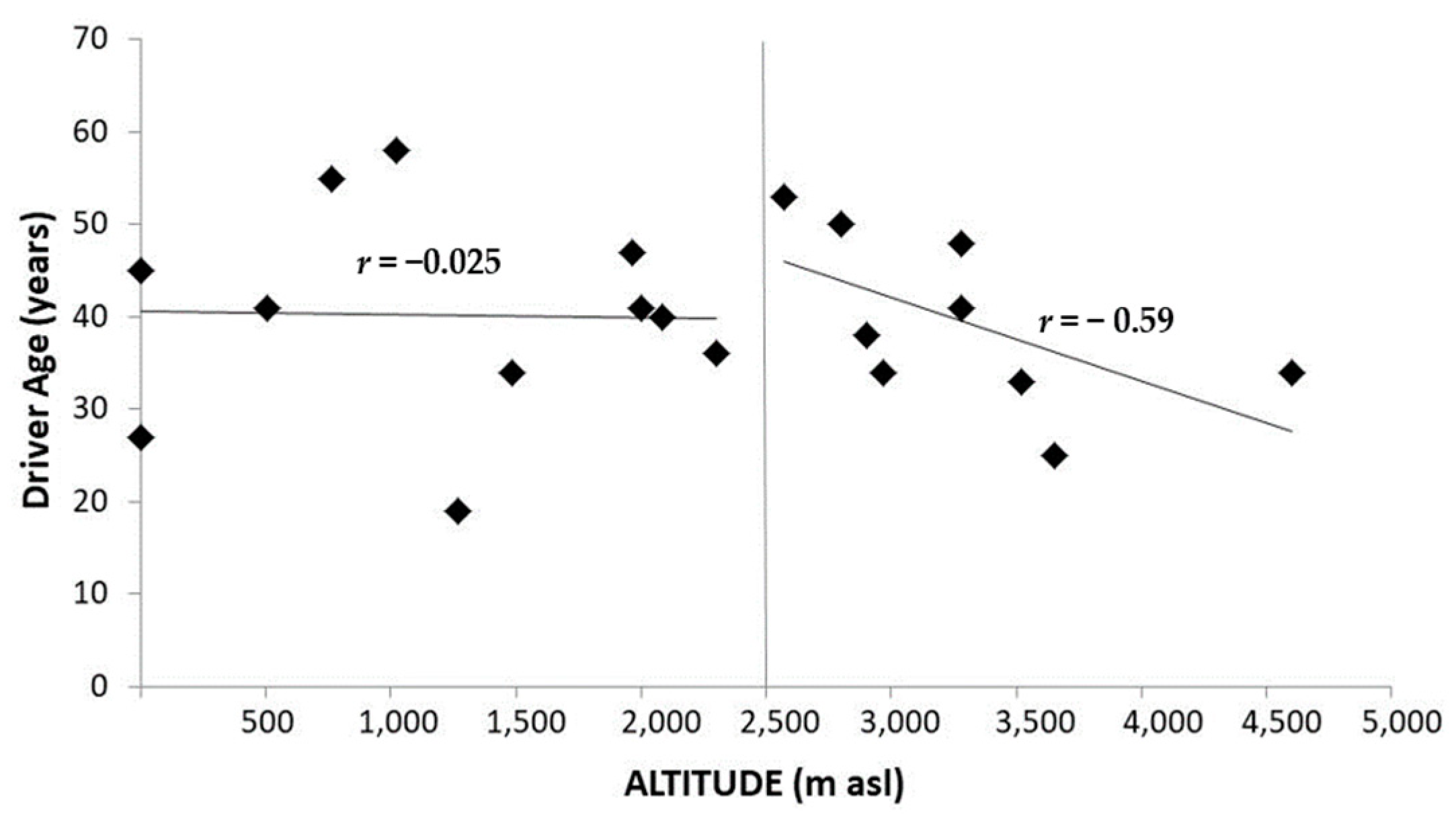
7. Death in the Mountain
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bünning, E. Die endogene Tagesrhythmik als Grundlage der Photoperiodischen Reaktion. Ber. Dtsch. Bot. Ges. 1936, 54, 590–607. [Google Scholar]
- Moore-Ede, M.C. Physiology of the circadian timing system: Predictive versus reactive homeostasis. Am. J. Physiol. 1986, 250, R737–R752. [Google Scholar] [CrossRef]
- Aschoff, J. Circadian timing. Ann. N. Y. Acad. Sci. 1984, 423, 442–468. [Google Scholar] [CrossRef] [PubMed]
- Pittendrigh, C. Temporal Organization: Reflections of a Darwinian Clock-Watcher. Annu. Rev. Physiol. 1993, 55, 17–54. [Google Scholar] [CrossRef] [PubMed]
- Sassone-Corsi, P. Molecular clocks: Mastering time by gene regulation. Nature 1998, 392, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Wilczek, A.M.; Burghardt, L.T.; Cobb, A.R.; Cooper, M.D.; Welch, S.M.; Schmitt, J. Genetic and physiological bases for phenological responses to current and predicted climates. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3129–3147. [Google Scholar] [CrossRef]
- Rijo-Ferreira, F.; Takahashi, J.S. Genomics of circadian rhythms in health and disease. Genome Med. 2019, 11, 82. [Google Scholar] [CrossRef]
- Kohsaka, A.; Bass, J. A sense of time: How molecular clocks organize metabolism. Trends Endocrinol. Metab. 2007, 18, 4–11. [Google Scholar] [CrossRef]
- Chua, E.C.P.; Shui, G.; Lee, I.T.G.; Lau, P.; Tan, L.C.; Yeo, S.C.; Lam, B.D.; Bulchand, S.; Summers, S.A.; Puvanendran, K.; et al. Extensive diversity in circadian regulation of plasma lipids and evidence for different circadian metabolic phenotypes in humans. Proc. Natl. Acad. Sci. USA 2013, 110, 14468–14473. [Google Scholar] [CrossRef]
- Brown, S.A.; Kunz, D.; Dumas, A.; Westermark, P.O.; Vanselow, K.; Tilmann-Wahnschaffe, A.; Herzel, H.; Kramer, A. Molecular insights into human daily behavior. Proc. Natl. Acad. Sci. USA 2008, 105, 1602–1607. [Google Scholar] [CrossRef]
- Facer-Childs, E.; Brandstaetter, R. The impact of circadian phenotype and time since awakening on diurnal performance in athletes. Curr. Biol. 2015, 25, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Rea, M.S.; Bierman, A.; Figueiro, M.G.; Bullough, J.D. A new approach to understanding the impact of circadian disruption on human health. J. Circadian Rhythm. 2008, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kervezee, L.; Kosmadopoulos, A.; Boivin, D.B. Metabolic and cardiovascular consequences of shift work: The role of circadian disruption and sleep disturbances. Eur. J. Neurosci. 2020, 51, 396–412. [Google Scholar] [CrossRef] [PubMed]
- Gorman, M.R. Temporal organization of pineal melatonin signaling in mammals. Mol. Cell. Endocrinol. 2020, 503, 110687. [Google Scholar] [CrossRef]
- McGinnis, G.R.; Young, M.E. Circadian regulation of metabolic homeostasis: Causes and consequences. Nat. Sci. Sleep 2016, 8, 163–180. [Google Scholar]
- Bray, M.S.; Ratcliffe, W.F.; Grennet, M.H.; Brewer, R.A.; Gamble, K.L.; Young, M.E. Metabolic Dyssynchrony in Mice. Int. J. Obes. 2013, 37, 843–852. [Google Scholar] [CrossRef]
- Bray, M.; Tsai, J.; Villegas-Montoya, C.; Boland, B.; Zackary, B.; Egbejimi, O.; Kueht, M.; Young, M.E. Time-of-Day-Dependent Dietary Fat Consumption Influences Multiple Cardiometabolic Syndrome Parameters in Mice. Int. J. Obes. 2010, 34, 1589–1598. [Google Scholar] [CrossRef]
- Vollmers, C.; Gill, S.; DiTacchio, L.; Pulivarthy, S.R.; Le, H.D.; Panda, S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc. Natl. Acad. Sci. USA 2009, 106, 21453–21458. [Google Scholar] [CrossRef]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; James, A.J.; et al. Time restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high fat diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef]
- Winget, C.; DeRoshia, C.; Markley, C.; Holley, D. A review of human physiological and performance changes associated with desynchronosis of biological rhythms. Aviat. Space Environ. Med. 1984, 55, 1085–1096. [Google Scholar]
- Burch, J.; Yost, M.; Johnson, W.; Allen, E. Melatonin, sleep, and shift work adaptation. J. Occup. Environ. Med. 2005, 47, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Gerstner, J.R.; Lyons, L.C.; Wright, K.P.; Loh, D.H.; Rawashdeh, O.; Eckel-Mahan, K.L.; Roman, G.W. Cycling behavior and memory formation. J. Neurosci. 2009, 29, 12824–12830. [Google Scholar] [CrossRef] [PubMed]
- Harrison, E.M.; Gorman, M.R. Changing the waveform of circadian rhythms: Considerations for shift-work. Front. Neurol. 2012, 3, 72. [Google Scholar] [CrossRef] [PubMed]
- Zaldívar, M. Estadísticas de Accidentabilidad; SUSESO. Gobierno de Chile: Santiago, Chile, 2013. [Google Scholar]
- Panda, S. The arrival of circadian medicine. Nat. Rev. Endocrinol. 2019, 15, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger, E. What is Life? The Physical Aspect of the Living Cell; Cambridge Univ. Press: Cambridge, UK, 1944. [Google Scholar]
- Krebs, H. Reminiscences and Reflections; Oxford University Press: Oxford, UK, 1981. [Google Scholar]
- Semenov, S.N.; Kraft, L.J.; Ainla, A.; Zhao, M.; Baghbanzadeh, M.; Campbell, V.E.; Kang, K.; Fox, J.M.; Whitesides, G.M. Autocatalytic, bistable, oscillatory networks of biologically relevant organic reactions. Nature 2016, 537, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Hess, B. The glycolytic oscillator. J. Exp. Biol. 1979, 81, 7–14. [Google Scholar]
- Rapp, P. Why are so many biological systems periodic? Prog. Neurobiol. 1987, 29, 261–273. [Google Scholar] [CrossRef]
- Cedernaes, J.; Waldeck, N.; Bass, J. Neurogenetic basis for circadian regulation of metabolism by the hypothalamus. Genes Dev. 2019, 33, 1136–1158. [Google Scholar] [CrossRef]
- Hatanaka, F.; Matsubara, C.; Myung, J.; Yoritaka, T.; Kamimura, N.; Tsutsumi, S.; Kanai, A.; Suzuki, Y.; Sassone-Corsi, P.; Aburatani, H.; et al. Genome-Wide Profiling of the Core Clock Protein BMAL1 Targets Reveals a Strict Relationship with Metabolism. Mol. Cell. Biol. 2010, 30, 5636–5648. [Google Scholar] [CrossRef]
- Archer, S.; Viola, A.; Kyriakopoulou, V.; Von Schantz, M.; Dijk, D. Inter-Individual Differences in Habitual Sleep Timing and Entrained Phase of Endogenous Circadian Rhythms of BMAL1, PER2 and PER3 mRNA in Human Leukocytes. Sleep 2008, 31, 608–617. [Google Scholar] [CrossRef]
- Zheng, X.; Sehgal, A. Probing the relative importance of molecular oscillations in the circadian clock. Genetics 2008, 178, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, D.T.; Hegde, R.S. Regulation of basal cellular physiology by the homeostatic unfolded protein response. J. Cell Biol. 2010, 189, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Golombek, D.A.; Rosenstein, R.E. Physiology of circadian entrainment. Physiol. Rev. 2010, 90, 1063–1102. [Google Scholar] [CrossRef]
- Spanagel, R.; Pendyala, G.; Abarca, C.; Zghoul, T.; Sanchis-Segura, C.; Magnone, M.C.; Lascorz, J.; Depner, M.; Holzberg, D.; Soyka, M.; et al. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat. Med. 2005, 11, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, J.C.; Loros, J.J. Making Time: Conservation of Biological Clocks from Fungi to Animals. Microbiol. Spectr. 2017, 5, 1–19. [Google Scholar]
- Rijo-Ferreira, F.; Carvalho, T.; Afonso, C.; Sanches-Vaz, M.; Costa, R.M.; Figueiredo, L.M.; Takahashi, J.S. Sleeping sickness is a circadian disorder. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Henslee, E.A.; Crosby, P.; Kitcatt, S.J.; Parry, J.S.W.; Bernardini, A.; Abdallat, R.G.; Braun, G.; Fatoyinbo, H.O.; Harrison, E.J.; Edgar, R.S.; et al. Rhythmic potassium transport regulates the circadian clock in human red blood cells. Nat. Commun. 2017, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bass, J.T. The circadian clock system’s influence in health and disease. Genome Med. 2017, 9, 1–5. [Google Scholar] [CrossRef]
- Klevecz, R.R.; Li, C.M.; Marcus, I.; Frankel, P.H. Collective behavior in gene regulation: The cell is an oscillator, the cell cycle a developmental process. FEBS J. 2008, 275, 2372–2384. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Shi, K.; Shan, D.; Zhu, Y.; Wang, C.; Bai, Y.; Yan, T.; Zheng, X.; Kong, J. Apple tree flowering is mediated by low level of melatonin under the regulation of seasonal light signal. J. Pineal Res. 2019, 66, e12551. [Google Scholar] [CrossRef]
- Pilorz, V.; Astiz, M.; Heinen, K.O.; Rawashdeh, O.; Oster, H. The concept of coupling in the mammalian circadian clock-network. J. Mol. Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.R.; Doyle, F.J.; Petzold, L.R. Oscillator model reduction preserving the phase response: Application to the circadian clock. Biophys. J. 2008, 95, 1658–1673. [Google Scholar] [CrossRef] [PubMed]
- Weinert, D. Circadian temperature variation and ageing. Ageing Res. Rev. 2010, 9, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Greco, C.M.; Sassone–Corsi, P. Circadian blueprint of metabolic pathways in the brain. Nat. Rev. Neurosci. 2019, 20, 71–82. [Google Scholar] [CrossRef]
- Brown, L.A.; Fisk, A.S.; Pothecary, C.A.; Peirson, S.N. Telling the time with a broken clock: Quantifying circadian disruption in animal models. Biology 2019, 8, 18. [Google Scholar] [CrossRef]
- Hastings, M.H.; Reddy, A.B.; Maywood, E.S. A clockwork web: Circadian timing in brain and periphery, in health and disease. Nat. Rev. Neurosci. 2003, 4, 649–661. [Google Scholar] [CrossRef]
- Shellock, F.; Rubin, S.; Ellrodt, A.; Muchlinski, A.; Brown, H.; Swan, H. Unusual core temperature decrease in exercising heart-failure patients. J. Appl. Physiol. 1983, 54, 544–550. [Google Scholar] [CrossRef]
- Rubin, S. Core temperature regulation of heart rate during exercise in humans. J. Appl. Physiol. 1987, 62, 1997–2002. [Google Scholar] [CrossRef]
- Waterhouse, J.; Drust, B.; Weinert, D.; Edwards, B.; Gregson, W.; Atkinson, G.; Kao, S.; Aizawa, S.; Reilly, T. The circadian rhythm of core temperature: Origin and some implications for exercise performance. Chronobiol. Int. 2005, 22, 207–225. [Google Scholar] [CrossRef]
- Reilly, T.; Atkinson, G.; Edwards, B.; Waterhouse, J.; Farrelly, K.; Fairhurst, E. Diurnal variation in temperature, mental and physical performance, and tasks specifically related to football (soccer). Chronobiol. Int. 2007, 24, 207–519. [Google Scholar] [CrossRef]
- Shellock, F.G.; Riedinger, M.S.; Fishbein, M.C.; Shah, P.K. Prevalence of brown adipose tissue in chronic congestive heart failure secondary to coronary heart disease. Am. J. Cardiol. 1985, 56, 197–198. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Brown Adipose Tissue: Function and Physiological Significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Au-Yong, I.T.H.; Thorn, N.; Ganatra, R.; Perkins, A.C.; Symonds, M.E. Brown adipose tissue and seasonal variation in humans. Diabetes 2009, 58, 2583–2587. [Google Scholar] [CrossRef]
- Carrier, J.; Paquet, J.; Morettini, J.; Touchette, É. Phase advance of sleep and temperature circadian rhythms in the middle years of life in humans. Neurosci. Lett. 2002, 320, 1–4. [Google Scholar] [CrossRef]
- Van Someren, E. More than a marker: Interaction between the circadian regulation of temperature and sleep, age-related changes, and treatment possibilities. Chronobiol. Int. 2000, 17, 313–354. [Google Scholar] [CrossRef]
- Zulley, J.; Wever, R.; Aschoff, J. The dependence of onset and duration of sleep on th circadian rhythm of rectal temperature. Pflug. Arch. 1981, 391, 314–318. [Google Scholar] [CrossRef]
- Brown, S.A.; Zumbrunn, G.; Fleury-Olela, F.; Preitner, N.; Schibler, U. Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Curr. Biol. 2002, 12, 1574–1583. [Google Scholar] [CrossRef]
- Buhr, E.; Yoo, S.; Takahashi, J. Temperature as a universal resetting cue for mammalian circadian oscillators. Science 2010, 330, 379–385. [Google Scholar] [CrossRef]
- Young, M.; Bray, M. Potential Role for Peripheral Circadian Clock Dyssynchrony in the Pathogenesis of Cardiovascular Dysfunction. Sleep Med. Rev. 2007, 8, 656–657. [Google Scholar] [CrossRef]
- Terazono, H.; Mutoh, T.; Yamaguchi, S.; Kobayashi, M.; Akiyama, M.; Udo, R.; Ohdo, S.; Okamura, H.; Shibata, S. Adrenergic regulation of clock gene expression in mouse liver. Proc. Natl. Acad. Sci. USA 2003, 100, 6795–6800. [Google Scholar] [CrossRef]
- Serón-Ferré, M.; Torres, C.; Parraguez, V.H.; Vergara, M.; Valladares, L.; Forcelledo, M.L.; Constandil, L.; Valenzuela, G.J. Perinatal neuroendocrine regulation. Development of the circadian time-keeping system. Mol. Cell. Endocrinol. 2002, 186, 169–173. [Google Scholar] [CrossRef]
- Seron-Ferre, M.; Valenzuela, G.J.; Torres-Farfan, C. Circadian clocks during embryonic and fetal development. Birth Defects Res. Part C 2007, 81, 204–214. [Google Scholar] [CrossRef]
- Buxton, O.M.; Lee, C.W.; L’Hermite-Balériaux, M.; Turek, F.W.; Van Cauter, E. Exercise elicits phase shifts and acute alterations of melatonin that vary with circadian phase. Am. J. Physiol. 2003, 284, 714–724. [Google Scholar] [CrossRef]
- Mallis, M.; DeRoshia, C. Circadian rhythms, sleep, and performance in space. Aviat. Space Environ. Med. 2005, 76, B94–B107. [Google Scholar]
- Perreau-Lenz, S.; Pévet, P.; Buijs, R.; Kalsbeek, A. The biological clock: The bodyguard of temporal homeostasis. Chronobiol. Int. 2004, 21, 1–25. [Google Scholar] [CrossRef]
- Vandewalle, G.; Gais, S.; Schabus, M.; Balteau, E.; Carrier, J.; Darsaud, A.; Sterpenich, V.; Albouy, G.; Dijk, D.J.; Maquet, P. Wavelength-dependent modulation of brain responses to a working memory task by daytime light exposure. Cereb. Cortex 2007, 17, 2788–2795. [Google Scholar] [CrossRef]
- Mattson, M.P. Hormesis defined. Ageing Res. Rev. 2008, 7, 1–7. [Google Scholar] [CrossRef]
- Liu, A.; Welsh, D.; Ko, C.; Tran, H.; Zhang, E.; Priest, A.; Buhr, E.; Singer, O.; Meeker, K.; Verma, I.; et al. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell 2007, 129, 605–616. [Google Scholar] [CrossRef]
- Liu, C.; Li, S.; Liu, T.; Borjigin, J.; Lin, J. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature 2007, 447, 477–481. [Google Scholar] [CrossRef]
- Gielen, S.; Schuler, G.; Adams, V. Cardiovascular effects of exercise training: Molecular mechanisms. Circulation 2010, 122, 1221–1238. [Google Scholar] [CrossRef]
- Jilg, A.; Bechstein, P.; Saade, A.; Dick, M.; Li, T.X.; Tosini, G.; Rami, A.; Zemmar, A.; Stehle, J.H. Melatonin modulates daytime-dependent synaptic plasticity and learning efficiency. J. Pineal Res. 2019, 66, 1–17. [Google Scholar] [CrossRef]
- Piazza, E.A.; Hasenfratz, L.; Hasson, U.; Lew-Williams, C. Infant and adult brains are coupled to the dynamics of natural communication. bioRxiv 2019, 359810. [Google Scholar] [CrossRef]
- Gwinner, E. Circadian and circannual programmes in avian migration. J. Exp. Biol. 1996, 199, 39–48. [Google Scholar]
- Dawson, D.; Gibbon, S.; Singh, P. The hypothermic effect of melatonin on core body temperature: Is more better? J. Pineal Res. 1996, 20, 192–197. [Google Scholar] [CrossRef]
- Cermakian, N.; Sassone-Corsi, P. Multilevel regulation of the circadian clock. Nat. Rev. Mol. Cell Biol. 2000, 1, 59–67. [Google Scholar] [CrossRef]
- Reppert, S.; Weaver, D. Molecular analysis of mammalian circadian rhythms. Annu. Rev. Physiol. 2001, 63, 647–676. [Google Scholar] [CrossRef]
- Richter, H.; Torres-Farfán, C.; Rojas-García, P.; Campino, C.; Torrealba, F.; Serón-Ferré, M. The circadian timing system: Making sense of day/night gene expression. Biol. Res. 2004, 37, 11–28. [Google Scholar] [CrossRef]
- Welsh, D.K.; Takahashi, J.S.; Kay, S.A. Suprachiasmatic Nucleus: Cell Autonomy and Network Properties. Annu. Rev. Physiol. 2010, 72, 551–577. [Google Scholar] [CrossRef]
- Erren, T.; Reiter, R. Melatonin: A universal time messenger. Neuroendocrinol. Lett. 2015, 36, 187–192. [Google Scholar]
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.H.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015, 59, 403–419. [Google Scholar] [CrossRef]
- Reiter, R.J.; Rosales-Corral, S.; Tan, D.X.; Jou, M.J.; Galano, A.; Xu, B. Melatonin as a mitochondria-targeted antioxidant: One of evolution’s best ideas. Cell. Mol. Life Sci. 2017, 74, 3863–3881. [Google Scholar] [CrossRef] [PubMed]
- Roopin, M.; Yacobi, Y.Z.; Levy, O. Occurrence, diel patterns, and the influence of melatonin on the photosynthetic performance of cultured HA-related Symbiodinium. J. Pineal Res. 2013, 55, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Dodd, A.; Salathia, N.; Hall, A.; Kévei, E.; Tóth, R.; Nagy, F.; Hibberd, J.; Millar, A.; Webb, A. Plant Circadian Clocks Increase Photosynthesis, Growth, Survival, and Competitive Advantage. Science 2005, 309, 630–633. [Google Scholar] [CrossRef]
- Andrés-Colás, N.; Perea-García, A.; Puig, S.; Peñarrubia, L. Deregulated copper transport affects Arabidopsis development especially in the absence of environmental cycles. Plant Physiol. 2010, 153, 170–184. [Google Scholar] [CrossRef]
- Mazars, C.; Thuleau, P.; Lamotte, O.; Bourque, S. Cross-talk between ROS and calcium in regulation of nuclear activities. Mol. Plant 2010, 3, 706–718. [Google Scholar] [CrossRef]
- Tan, X.; Long, W.; Zeng, L.; Ding, X.; Cheng, Y.; Zhang, X.; Zou, X. Melatonin-induced transcriptome variation of rapeseed seedlings under salt stress. Int. J. Mol. Sci. 2019, 20, 5355. [Google Scholar] [CrossRef]
- Suofu, Y.; Li, W.; Jean-Alphonse, F.G.; Jia, J.; Khattar, N.K.; Li, J.; Baranov, S.V.; Leronni, D.; Mihalik, A.C.; He, Y.; et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. USA 2017, 114, E7997–E8006. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and inflammation—Story of a double-edged blade. J. Pineal Res. 2018, 65, 1–23. [Google Scholar] [CrossRef]
- Hardeland, R. Aging, melatonin, and the pro-and anti-inflammatory networks. Int. J. Mol. Sci. 2019, 20, 1223. [Google Scholar] [CrossRef]
- Blask, D.E. Melatonin, sleep disturbance and cancer risk. Sleep Med. Rev. 2009, 13, 257–264. [Google Scholar] [CrossRef]
- Alvarez-Artime, A.; Cernuda-Cernuda, R.; Cepas, V.; Gonzalez-Menendez, P.; Fernadez-Vega, S.; Quiros-Gonzalez, I.; Sainz, R.M.; Mayo, J.C. Melatonin-Induced Cytoskeleton Reorganization Leads to Inhibition of Melanoma Cancer Cell Proliferation. Int. J. Mol. Sci. 2020, 21, 548. [Google Scholar] [CrossRef]
- Chen, Y.; Tjong, Y.W.; Ip, S.F.; Tipoe, G.L.; Fung, M.L. Melatonin enhances the hypoxic response of rat carotid body chemoreceptor. J. Pineal Res. 2005, 38, 157–163. [Google Scholar] [CrossRef]
- Gubin, D.; Gubin, G.; Waterhouse, J.; Weinert, D. The circadian body temperature rhythm in the elderly: Effect of single daily melatonin dosing. Chronobiol. Int. 2006, 23, 639–658. [Google Scholar] [CrossRef]
- Erden, S. Hypothermia Associated with Melatonin Ingestion in a Child with Autism. Clin. Neuropharmacol. 2019, 42, 179–180. [Google Scholar] [CrossRef]
- Weissová, K.; Škrabalová, J.; Skálová, K.; Bendová, Z.; Kopřivová, J. The Effect of a Common Daily Schedule on Human Circadian Rhythms during the Polar Day in Svalbard: A Field Study. J. Circadian Rhythm. 2019, 17, 1–8. [Google Scholar] [CrossRef]
- Kirsz, K.; Szczesna, M.; Molik, E.; Zieba, D.A. Effects of ghrelin on nocturnal melatonin secretion in sheep: An in vitro and in vivo approach. J. Anim. Sci. 2017, 95, 4101. [Google Scholar] [CrossRef]
- Kirsz, K.; Szczęsna, M.; Biernat, W.; Molik, E.; Zięba, D.A. Involvement of orexin A in nocturnal melatonin secretion into the cerebrospinal fluid and the blood plasma in seasonal sheep. Gen. Comp. Endocrinol. 2020, 286, 113304. [Google Scholar] [CrossRef]
- Pendergast, J.S.; Branecky, K.L.; Huang, R.; Niswender, K.D.; Yamazaki, S. Wheel-running activity modulates circadian organization and the daily rhythm of eating behavior. Front. Psychol. 2014, 5, 1–9. [Google Scholar] [CrossRef]
- Adamovich, Y.; Ladeuix, B.; Golik, M.; Koeners, M.P.; Asher, G. Rhythmic Oxygen Levels Reset Circadian Clocks through HIF1α. Cell Metab. 2017, 25, 93–101. [Google Scholar] [CrossRef]
- Figueiro, M. A proposed 24 h lighting scheme for older adults. Light. Res. Technol. 2008, 40, 153–160. [Google Scholar] [CrossRef]
- Figueiro, M.G.; Bierman, A.; Rea, M.S. Retinal mechanisms determine the subadditive response to polychromatic light by the human circadian system. Neurosci. Lett. 2008, 438, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Cajochen, C.; Jud, C.; Münch, M.; Kobialka, S.; Wirz-Justice, A.; Albrecht, U. Evening exposure to blue light stimulates the expression of the clock gene PER2 in humans. Eur. J. Neurosci. 2006, 23, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Touitou, Y.; Motohashi, Y.; Reinberg, A.; Touitou, C.; Bourdeleau, P.; Bogdan, A.; Auzéby, A. Effect of shift work on the night-time secretory patterns of melatonin, prolactin, cortisol and testosterone. Eur. J. Appl. Physiol. Occup. Physiol. 1990, 60, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Frank, A. Injuries related to shiftwork. Am. J. Prev. Med. 2000, 18, 33–36. [Google Scholar] [CrossRef]
- Knutsson, A. Health disorders of shift workers. Occup. Med. 2003, 53, 103–108. [Google Scholar] [CrossRef]
- Taheri, S.; Lin, L.; Austin, D.; Young, T.; Mignot, E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004, 1, 210–217. [Google Scholar] [CrossRef]
- James, F.O.; Cermakian, N.; Boivin, D.B. Circadian rhythms of melatonin, cortisol, and clock gene expression during simulated night shift work. Sleep 2007, 30, 1427–1436. [Google Scholar] [CrossRef]
- Kempenaers, B.; Borgström, P.; Loës, P.; Schlicht, E.; Valcu, M. Artificial night lighting affects dawn song, extra-pair siring success, and lay date in songbirds. Curr. Biol. 2010, 20, 1735–1739. [Google Scholar] [CrossRef]
- Do, M.T.H. Melanopsin and the Intrinsically Photosensitive Retinal Ganglion Cells: Biophysics to Behavior. Neuron 2019, 104, 205–226. [Google Scholar] [CrossRef]
- Hankins, M.W.; Peirson, S.N.; Foster, R.G. Melanopsin: An exciting photopigment. Trends Neurosci. 2008, 31, 27–36. [Google Scholar] [CrossRef]
- Mure, L.; Rieux, C.; Hattar, S.; Cooper, H. Melanopsin-dependent nonvisual responses: Evidence for photopigment bistability in vivo. J. Biol. Rhythm. 2007, 22, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Russell, B. A History of Western Philosophy; Unwin Paperbacks: London, UK; Sydney, Australia; Wellington, New Zealand, 1988. [Google Scholar]
- Damjanovic, A.; Milovanovic, S.D.; Trajanovic, N.N. Descartes and His Peculiar Sleep Pattern. J. Hist. Neurosci. 2015, 24, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Analysis, Nutrition, and Health Benefits of Tryptophan. Int. J. Tryptophan Res. 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Brainard, G.C.; Sliney, D.; Hanifin, J.P.; Glickman, G.; Byrne, B.; Greeson, J.M.; Jasser, S.; Gerner, E.; Rollag, M.D. Sensitivity of the human circadian system to short-wavelength (420-nm) light. J. Biol. Rhythm. 2008, 23, 379–386. [Google Scholar] [CrossRef]
- Kayumov, L.; Casper, R.F.; Hawa, R.J.; Perelman, B.; Chung, S.A.; Sokalsky, S.; Shapiro, C.M. Blocking low-wavelength light prevents nocturnal melatonin suppression with no adverse effect on performance during simulated shift work. J. Clin. Endocrinol. Metab. 2005, 90, 2755–2761. [Google Scholar] [CrossRef]
- Kayumov, L.; Lowe, A.; Rahman, S.; Casper, R.; Shapiro, C. Prevention of melatonin suppression by nocturnal lighting: Relevance to cancer. Eur. J. Cancer Prev. 2007, 16, 357–362. [Google Scholar] [CrossRef]
- Barger, L.; Lockley, S.; Rajaratnam, S.; Landrigan, C. Neurobehavioral, health, and safety consequences associated with shift work in safety-sensitive professions. Curr. Neurol. Neurosci. Rep. 2009, 9, 155–164. [Google Scholar] [CrossRef]
- Canazei, M.; Pohl, W.; Bliem, H.R.; Weiss, E.M. Acute effects of different light spectra on simulated night-shift work without circadian alignment. Chronobiol. Int. 2017, 34, 303–317. [Google Scholar] [CrossRef]
- Horowitz, T.S.; Cade, B.E.; Wolfe, J.M.; Czeisler, C.A. Efficacy of bright light and sleep/darkness scheduling in alleviating circadian maladaptation to night work. Am. J. Physiol. 2001, 281, 384–391. [Google Scholar] [CrossRef]
- Arendt, J. Melatonin and human rhythms. Chronobiol. Int. 2006, 23, 21–37. [Google Scholar] [CrossRef]
- Tapia, M.; Wulff-Zottele, C.; De Gregorio, N.; Lang, M.; Varela, H.; Serón-Ferré, M.J.; Vivaldi, E.A.; Araneda, O.F.; Silva-Urra, J.; Gunga, H.C.; et al. Melatonin relations with respiratory Quotient Weaken on acute exposure to high altitude. Front. Physiol. 2018, 9, 798. [Google Scholar] [CrossRef] [PubMed]
- Richalet, J.; Rutgers, V.; Bouchet, P.; Rymer, J.; Kéromès, A.; Duval-Arnould, G.; Rathat, C. Diurnal variations of acute mountain sickness, colour vision, and plasma cortisol and ACTH at high altitude. Aviat. Space Environ. Med. 1989, 60, 105–111. [Google Scholar] [PubMed]
- Tekavcic-Pompe, M.; Tekavcic, I. Color vision in the tritan axis is predominantly affected at high altitude. High Alt. Med. Biol. 2008, 9, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Willmann, G.; Ivanov, I.V.; Fischer, M.D.; Lahiri, S.; Pokharel, R.K.; Werner, A.; Khurana, T.S. Effects on colour discrimination during long term exposure to high altitudes on Mt Everest. Br. J. Ophthalmol. 2010, 94, 1393–1397. [Google Scholar] [CrossRef][Green Version]
- Davies, A.J.; Morris, D.S.; Kalson, N.S.; Wright, A.D.; Imray, C.H.E.; Hogg, C.R. Changes to colour vision on exposure to high altitude. J. R. Army Med. Corps 2011, 157, 107–109. [Google Scholar] [CrossRef]
- Tekavcic, B.; Milić, R.; Pompe, M. Does Physical Fatigue Affect Color Vision? Sport Med. Int. Open 2017, 1, E155–E159. [Google Scholar] [CrossRef][Green Version]
- Kaur, C.; Srinivasan, K.N.; Singh, J.; Peng, C.M.; Ling, E.A. Plasma melatonin, pinealocyte morphology, and surface receptors/antigen expression on macrophages/microglia in the pineal gland following a high-altitude exposure. J. Neurosci. Res. 2002, 67, 533–543. [Google Scholar] [CrossRef]
- Frisch, H.; Waldhauser, F.; Waldhör, T.; Müllner-Eidenböck, A.; Neupane, P.; Schweitzer, K. Increase in 6-hydroxymelatonin excretion in humans during ascent to high altitudes. J. Clin. Endocrinol. Metab. 2004, 89, 4388–4390. [Google Scholar] [CrossRef][Green Version]
- Klemm, P.; Hurst, J.; Dias Blak, M.; Herrmann, T.; Melchinger, M.; Bartz-Schmidt, K.U.; Zeck, G.; Schultheiss, M.; Spitzer, M.S.; Schnichels, S. Hypothermia protects retinal ganglion cells against hypoxia-induced cell death in a retina organ culture model. Clin. Exp. Ophthalmol. 2019, 47, 1043–1054. [Google Scholar] [CrossRef]
- Pires-Lapa, M.A.; Carvalho-Sousa, C.E.; Cecon, E.; Fernandes, P.A.; Markus, R.P. β-Adrenoceptors trigger melatonin synthesis in phagocytes. Int. J. Mol. Sci. 2018, 19, 2182. [Google Scholar] [CrossRef]
- Utrillas, M.P.; Marín, M.J.; Esteve, A.R.; Salazar, G.; Suarez, H.; Castillo, J.; Martínez-Lozano, J.A. UVER and UV index at high altitude in Northwestern Argentina. J. Photochem. Photobiol. B 2016, 163, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Yasukouchi, A.; Maeda, T.; Hara, K.; Furuune, H. Non-visual effects of diurnal exposure to an artificial skylight, including nocturnal melatonin suppression. J. Physiol. Anthropol. 2019, 38, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Moldavan, M.; Allen, C. Retinohypothalamic tract synapses in the rat suprachiasmatic nucleus demonstrate short-term synaptic plasticity. J. Neurophysiol. 2010, 103, 2390–2399. [Google Scholar] [CrossRef] [PubMed]
- Zucker, R.S.; Regehr, W.G. Short-Term Synaptic Plasticity. Annu. Rev. Physiol. 2002, 64, 355–405. [Google Scholar] [CrossRef]
- Himadri, P.; Kumari, S.S.; Chitharanjan, M.; Dhananjay, S. Role of oxidative stress and inflammation in hypoxia-induced cerebral edema: A molecular approach. High Alt. Med. Biol. 2010, 11, 231–244. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Zhou, X.J.; Xu, B. Mitochondria: Central organelles for melatonins antioxidant and anti-aging actions. Molecules 2018, 23, 509. [Google Scholar] [CrossRef]
- Jung-Hynes, B.; Ahmad, N. SIRT1 controls circadian clock circuitry and promotes cell survival: A connection with age-related neoplasms. FASEB J. 2009, 23, 2803–2809. [Google Scholar] [CrossRef]
- Vitaterna, M.H.; Ko, C.H.; Chang, A.M.; Buhr, E.D.; Fruechte, E.M.; Schook, A.; Antoch, M.P.; Turek, F.W.; Takahashi, J.S. The mouse Clock mutation reduces circadian pacemaker amplitude and enhances efficacy of resetting stimuli and phase-response curve amplitude. Proc. Natl. Acad. Sci. USA 2006, 103, 9327–9332. [Google Scholar] [CrossRef]
- Hablitz, L.M.; Molzof, H.E.; Abrahamsson, X.E.; Cooper, J.M.; Prosser, R.A.; Gamble, X.L. GIRK channels mediate the nonphotic effects of exogenous melatonin. J. Neurosci. 2015, 35, 14957–14965. [Google Scholar] [CrossRef]
- Passarella, S.; Duong, M. Diagnosis and treatment of insomnia. Am. J. Health Syst. Pharm. 2008, 65, 927–934. [Google Scholar] [CrossRef]
- Bonnefond, A.; Froguel, P. Disentangling the Role of Melatonin and its Receptor MTNR1B in Type 2 Diabetes: Still a Long Way to Go? Curr. Diabetes Rep. 2017, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Mukherjee, S.; Baluska, F.; Bhatla, S.C. Regulatory roles of serotonin and melatonin in abiotic stress tolerance in plants. Plant Signal. Behav. 2015, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Jin, H.; Sun, H.; Zhao, Y.; Liu, J.; Ma, H.; Sun, X.; Yang, Y. Antioxidant Melatonin: Potential Functions in Improving Cerebral Autoregulation After Subarachnoid Hemorrhage. Front. Physiol. 2018, 9, 1146. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, N.; Ishigaki, S.; Isobe, S. The pivotal role of melatonin in ameliorating chronic kidney disease by suppression of the renin–angiotensin system in the kidney. Hypertens. Res. 2019, 42, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Nishi, E.; Almeida, V.; Amaral, F.; Simon, K.; Futuro-Neto, H.; Pontes, R.; Cespedes, J.; Campos, R.; Bergamaschi, C. Melatonin attenuates renal sympathetic overactivity and reactive oxygen species in the brain in neurogenic hypertension. Hypertens. Res. 2019, 42, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; Qi, W. Biochemical reactivity of melatonin with reactive oxygen and nitrogen species: A review of the evidence. Cell Biochem. Biophys. 2001, 34, 237–256. [Google Scholar] [CrossRef]
- Vriend, J.; Reiter, R.J. Melatonin and the von Hippel-Lindau/HIF-1 oxygen sensing mechanism: A review. Biochim. Biophys. Acta 2016, 1865, 176–183. [Google Scholar] [CrossRef]
- Blanco, S.; Hernández, R.; Franchelli, G.; Ramos-Álvarez, M.M.; Peinado, M.Á. Melatonin influences NO/NOS pathway and reduces oxidative and nitrosative stress in a model of hypoxic-ischemic brain damage. Nitric Oxide 2017, 62, 32–43. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 2014, 19, 789–797. [Google Scholar] [CrossRef]
- Zhang, H.J.; Zhang, N.; Yang, R.C.; Wang, L.; Sun, Q.Q.; Li, D.B.; Cao, Y.Y.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis sativus L.). J. Pineal Res. 2014, 57, 269–279. [Google Scholar] [CrossRef]
- Cao, Y.Y.; Qi, C.D.; Li, S.; Wang, Z.; Wang, X.; Wang, J.; Ren, S.; Li, X.; Zhang, N.; Guo, Y.D. Melatonin Alleviates Copper Toxicity via Improving Copper Sequestration and ROS Scavenging in Cucumber. Plant Cell Physiol. 2019, 60, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Izon, G.; Zerkle, A.L.; Williford, K.H.; Farquhar, J.; Poulton, S.W.; Claire, M.W. Biological regulation of atmospheric chemistry en route to planetary oxygenation. Proc. Natl. Acad. Sci. USA 2017, 114, E2571–E2579. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.J.; Horscroft, J.A. Mitochondrial function at extreme high altitude. J. Physiol. 2016, 594, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, J.A.; Simoni, J.; Escudero, E.; Hurtado, M.E.; Swenson, E.R.; Wesson, D.E.; Schreiner, G.F.; Schoene, R.B.; Johnson, R.J.; Hurtado, A. Increased oxidative stress following acute and chronic high altitude exposure. High Alt. Med. Biol. 2004, 5, 61–69. [Google Scholar] [CrossRef]
- Behn, C.; Araneda, O.F.; Llanos, A.J.; Celedón, G.; González, G. Hypoxia-related lipid peroxidation: Evidences, implications and approaches. Respir. Physiol. Neurobiol. 2007, 158, 143–150. [Google Scholar] [CrossRef]
- Cable, N.T.; Drust, B.; Gregson, W.A. The impact of altered climatic conditions and altitude on circadian physiology. Physiol. Behav. 2007, 90, 267–273. [Google Scholar] [CrossRef]
- Mortola, J.P. Gender and the circadian pattern of body temperature in normoxia and hypoxia. Respir. Physiol. Neurobiol. 2017, 245, 4–12. [Google Scholar] [CrossRef]
- Pei, J.F.; Li, X.K.; Li, W.Q.; Gao, Q.; Zhang, Y.; Wang, X.M.; Fu, J.Q.; Cui, S.S.; Qu, J.H.; Zhao, X.; et al. Diurnal oscillations of endogenous H2O2 sustained by p66Shc regulate circadian clocks. Nat. Cell Biol. 2019, 21, 1–12. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Zhang, L.; Wu, Z.X.; Shan, T.T.; Xiong, C. Berberine Ameliorates Doxorubicin-Induced Cardiotoxicity via a SIRT1/p66Shc-Mediated Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 2150394. [Google Scholar] [CrossRef]
- Hao, C.; Wu, X.; Zhou, R.; Zhang, H.; Zhou, Y.; Wang, X.; Feng, Y.; Mei, L.; He, C.; Cai, X.; et al. Downregulation of p66Shc can reduce oxidative stress and apoptosis in oxidative stress model of marginal cells of stria vascularis in Sprague Dawley rats. Drug Des. Devel. Ther. 2019, 13, 3199–3206. [Google Scholar] [CrossRef]
- Del Olmo, M.; Kramer, A.; Herzel, H. A robust model for circadian redox oscillations. Int. J. Mol. Sci. 2019, 20, 2368. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Hammerling, U. The mitochondrial PKCδ/retinol signal complex exerts real-time control on energy homeostasis. Biochim. Biophys. Acta 2020. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Physical activity pacifies the problematic p66Shc. Eur. J. Prev. Cardiol. 2020, 27, 166–167. [Google Scholar] [CrossRef] [PubMed]
- Connett, R.J.; Honig, C.R.; Gayeski, T.E.J.; Brooks, G.A. Defining hypoxia: A systems view of VO2, glycolysis, energetics, and intracellular PO2. J. Appl. Physiol. 1990, 68, 833–842. [Google Scholar] [CrossRef]
- Ashkenazi, I.; Ribak, J.; Avgar, D.; Klepfish, A. Altitude and hypoxia as phase shift inducers. Aviat. Space Environ. Med 1982, 53, 342–346. [Google Scholar]
- Bishop, B.; Silva, G.; Krasney, J.; Salloum, A.; Roberts, A.; Nakano, H.; Shucard, D.; Rifkin, D.; Farkas, G. Circadian rhythms of body temperature and activity levels during 63 h of hypoxia in the rat. Am. J. Physiol. 2000, 279, 1378–1385. [Google Scholar] [CrossRef]
- Coste, O.; Beaumont, M.; Batéjat, D.; Van Beers, P.; Charbuy, H.; Touitou, Y. Hypoxic depression of melatonin secretion after simulated long duration flights in man. J. Pineal Res. 2004, 37, 1–10. [Google Scholar] [CrossRef]
- Mortola, J.P. Correlations between the circadian patterns of body temperature, metabolism and breathing in rats. Respir. Physiol. Neurobiol. 2007, 155, 137–146. [Google Scholar] [CrossRef]
- Vanlalhriatpuia, K.; Chhakchhuak, V.; Moses, S.K.; Iyyer, S.B.; Kasture, M.S.; Shivagaje, A.J.; Rajneesh, B.J.; Joshi, D.S. Effects of altitude on circadian rhythm of adult locomotor activity in Himalayan strains of Drosophila helvetica. J. Circadian Rhythm. 2007, 5, 1–11. [Google Scholar] [CrossRef]
- Saiki, C.; Mortola, J. Hypoxia abolishes the morning-night differences of metabolism and ventilation in 6-day-old rats. Can. J. Physiol. Pharmacol. 1995, 73, 159–164. [Google Scholar] [CrossRef]
- Mortola, J.P.; Seifert, E.L. Hypoxic depression of circadian rhythms in adult rats. J. Appl. Physiol. 2000, 88, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.; Jiménez, D.; León-Velarde, F.; Osorio, J.; Mortola, J. Circadian patterns in men acclimatized to intermittent hypoxia. Respir. Physiol. 2001, 126, 233–243. [Google Scholar] [CrossRef]
- Bosco, G.; Ionadi, A.; Panico, S.; Faralli, F.; Gagliardi, R.; Data, P.; Mortola, J. Effects of hypoxia on the circadian patterns in men. High Alt. Med. Biol. 2003, 4, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Chilov, D.; Hofer, T.; Bauer, C.; Wenger, R.H.; Gassmann, M. Hypoxia affects expression of circadian genes PER1 and CLOCK in mouse brain. FASEB J. 2001, 15, 2613–2622. [Google Scholar] [CrossRef]
- Kwarecki, K.; Krawczyk, J. Comparison of the circadian rhythm in cell proliferation in corneal epithelium of male rats studied under normal and hypobaric (hypoxic) conditions. Chronobiol. Int. 1989, 6, 217–222. [Google Scholar] [CrossRef]
- Joseph, V.; Mamet, J.; Lee, F.; Dalmaz, Y.; Van Reeth, O. Prenatal hypoxia impairs circadian synchronisation and response of the biological clock to light in adult rats. J. Physiol. 2002, 543, 387–395. [Google Scholar] [CrossRef]
- Mortola, J.P.; Lanthier, C. Scaling the amplitudes of the circadian pattern of resting oxygen consumption, body temperature and heart rate in mammals. Comp. Biochem. Physiol. 2004, 139, 83–95. [Google Scholar] [CrossRef]
- Piccione, G.; Caola, G.; Mortola, J.P. Scaling the daily oscillations of breathing frequency and skin temperature in mammals. Comp. Biochem. Physiol. 2005, 140, 477–486. [Google Scholar] [CrossRef]
- De Goot, H.; Littauer, A. Hypoxia, reactive oxygen, and cell injury. Free Radic. Biol. Med. 1989, 6, 541–551. [Google Scholar] [CrossRef]
- Araneda, O.F.; García, C.; Lagos, N.; Quiroga, G.; Cajigal, J.; Salazar, M.P.; Behn, C. Lung oxidative stress as related to exercise and altitude. Lipid peroxidation evidence in exhaled breath condensate: A possible predictor of acute mountain sickness. Eur. J. Appl. Physiol. 2005, 95, 383–390. [Google Scholar] [CrossRef]
- Celedón, G.; González, G.; Sotomayor, C.P.; Behn, C. Membrane lipid diffusion and band 3 protein changes in human erythrocytes due to acute hypobaric hypoxia. Am. J. Physiol. 1998, 275, 5–7. [Google Scholar] [CrossRef] [PubMed]
- González, G.; Celedón, G.; Sandoval, M.; González, G.E.; Ferrer, V.; Astete, R.; Behn, C. Hypobaric hypoxia-reoxygenation diminishes band 3 protein functions in human erythrocytes. Pflug. Arch. Eur. J. Physiol. 2002, 445, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Hartman, P.; Ponder, R.; Lo, H.H.; Ishii, N. Mitochondrial oxidative stress can lead to nuclear hypermutability. Mech. Ageing Dev. 2004, 125, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.M.; Davies, B.; Young, I.S. Intermittent hypoxic training: Implications for lipid peroxidation induced by acute normoxic exercise in active men. Clin. Sci. 2001, 101, 465–475. [Google Scholar] [CrossRef]
- Richalet, J.P.; Hornych, A.; Rathat, C.; Aumont, J.; Larmignat, P.; Rémy, P. Plasma prostaglandins, leukotrienes and thromboxane in acute high altitude hypoxia. Respir. Physiol. 1991, 85, 205–215. [Google Scholar] [CrossRef]
- Gonzalez-Candia, A.; Veliz, M.; Carrasco-Pozo, C.; Castillo, R.L.; Cárdenas, J.C.; Ebensperger, G.; Reyes, R.V.; Llanos, A.J.; Herrera, E.A. Antenatal melatonin modulates an enhanced antioxidant/pro-oxidant ratio in pulmonary hypertensive newborn sheep. Redox Biol. 2019, 22, 101128. [Google Scholar] [CrossRef]
- Sagoo, R.S.; Hutchinson, C.E.; Wright, A.; Handford, C.; Parsons, H.; Sherwood, V.; Wayte, S.; Nagaraja, S.; Ng’Andwe, E.; Wilson, M.H.; et al. Magnetic Resonance investigation into the mechanisms involved in the development of high-altitude cerebral edema. J. Cereb. Blood Flow Metab. 2017, 37, 319–331. [Google Scholar] [CrossRef]
- Fan, C.; Zhao, Y.; Yu, Q.; Yin, W.; Liu, H.; Lin, J.; Yang, T.; Fan, M.; Gesang, L.; Zhang, J. Reversible Brain Abnormalities in People Without Signs of Mountain Sickness during High-Altitude Exposure. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Dwarakanath, R.S.; Sahar, S.; Reddy, M.A.; Castanotto, D.; Rossi, J.J.; Natarajan, R. Regulation of monocyte chemoattractant protein-1 by the oxidized lipid, 13-hydroperoxyoctadecadienoic acid, in vascular smooth muscle cells via nuclear factor-kappa B (NF-κB). J. Mol. Cell. Cardiol. 2004, 36, 585–595. [Google Scholar] [CrossRef]
- Cummins, E.P.; Berra, E.; Comerford, K.M.; Ginouves, A.; Fitzgerald, K.T.; Seeballuck, F.; Godson, C.; Nielsen, J.E.; Moynagh, P.; Pouyssegur, J.; et al. Prolyl hydroxylase-1 negatively regulates IκB kinase-β, giving insight into hypoxia-induced NFκB activity. Proc. Natl. Acad. Sci. USA 2006, 103, 18154–18159. [Google Scholar] [CrossRef]
- Napetschnig, J.; Wu, H. Molecular Basis of NF-κB Signaling. Annu. Rev. Biophys. 2013, 42, 443–468. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.; Taylor, C.T.; McNicholas, W.T. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 2005, 112, 2660–2667. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.; Taylor, C.T.; McNicholas, W.T. Predictors of elevated nuclear factor-κB-dependent genes in obstructive sleep apnea syndrome. Am. J. Respir. Crit. Care Med. 2006, 174, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, H.; Ye, X.; Wilson, D.; Htoo, A.K.; Hendersen, T.; Liu, S.F. Chronic intermittent hypoxia activates nuclear factor-κB in cardiovascular tissues in vivo. Biochem. Biophys. Res. Commun. 2006, 343, 591–596. [Google Scholar] [CrossRef]
- Ham, M.; Kaunitz, J.D. Gastroduodenal defense. Curr. Opin. Gastroenterol. 2007, 23, 607–616. [Google Scholar] [CrossRef]
- Fruehauf, H.; Vavricka, S.R.; Lutz, T.A.; Gassmann, M.; Wojtal, K.A.; Erb, A.; Maggiorini, M.; Schwizer, W.; Fried, M.; Fox, M.; et al. Evaluation of Acute Mountain Sickness by Unsedated Transnasal Esophagogastroduodenoscopy at High Altitude. Clin. Gastroenterol. Hepatol. 2019. [Google Scholar] [CrossRef]
- Cheng, J.; Yang, H.L.; Gu, C.J.; Liu, Y.K.; Shao, J.; Zhu, R.; He, Y.Y.; Zhu, X.Y.; Li, M.Q. Melatonin restricts the viability and angiogenesis of vascular endothelial cells by suppressing HIF-1α/ROS/VEGF. Int. J. Mol. Med. 2019, 43, 945–955. [Google Scholar] [CrossRef]
- Paick, S.; Choi, W.S. Varicocele and Testicular Pain: A Review. World J. Mens. Health 2019, 37, 4. [Google Scholar] [CrossRef]
- Zhu, S.M.; Rao, T.; Yang, X.; Ning, J.Z.; Yu, W.M.; Ruan, Y.; Yuan, R.; Li, C.L.; Jiang, K.; Hu, W.; et al. Autophagy may play an important role in varicocele. Mol. Med. Rep. 2017, 16, 5471–5479. [Google Scholar] [CrossRef]
- Goren, M.R.; Kilinc, F.; Kayaselcuk, F.; Ozer, C.; Oguzulgen, I.; Hasirci, E. Effects of experimental left varicocele repair on hypoxia-inducible factor-1α and vascular endothelial growth factor expressions and angiogenesis in rat testis. Andrologia 2017, 49, 1–6. [Google Scholar] [CrossRef]
- Ramsey, K.M.; Bass, J. Obeying the clock yields benefits for metabolism. Proc. Natl. Acad. Sci. USA 2009, 106, 4069–4070. [Google Scholar] [CrossRef] [PubMed]
- McClung, C. Circadian Genes, Rhythms and the Biology of Mood Disorders. Pharmacol. Ther. 2007, 114, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Roybal, K.; Theobold, D.; Graham, A.; DiNieri, J.A.; Russo, S.J.; Krishnan, V.; Chakravarty, S.; Peevey, J.; Oehrlein, N.; Birnbaum, S.; et al. Mania-like behavior induced by disruption of CLOCK. Proc. Natl. Acad. Sci. USA 2007, 104, 6406–6411. [Google Scholar] [CrossRef] [PubMed]
- Partonen, T.; Treutlein, J.; Alpman, A.; Frank, J.; Johansson, C.; Depner, M.; Aron, L.; Rietschel, M.; Wellek, S.; Soronen, P.; et al. Three circadian clock genes Per2, Arntl, and Npas2 contribute to winter depression. Ann. Med. 2007, 39, 229–238. [Google Scholar] [CrossRef]
- McClung, C.A.; Sidiropoulou, K.; Vitaterna, M.; Takahashi, J.S.; White, F.J.; Cooper, D.C.; Nestler, E.J. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc. Natl. Acad. Sci. USA 2005, 102, 9377–9381. [Google Scholar] [CrossRef]
- Kovanen, L.; Saarikoski, S.T.; Haukka, J.; Pirkola, S.; Aromaa, A.; Lönnqvist, J.; Partonen, T. Circadian clock gene polymorphisms in alcohol use disorders and alcohol consumption. Alcohol. Alcohol. 2010, 45, 303–311. [Google Scholar] [CrossRef]
- Xu, Y.; Padiath, Q.S.; Shapiro, R.E.; Jones, C.R.; Wu, S.C.; Saigoh, N.; Saigoh, K.; Ptáček, L.J.; Fu, Y.H. Functional consequences of a CKIδ mutation causing familial advanced sleep phase syndrome. Nature 2005, 434, 640–644. [Google Scholar] [CrossRef]
- Haba-Rubio, J. Psychiatric aspects of organic sleep disorders. Dialogues Clin. Neurosci. 2005, 7, 335–346. [Google Scholar]
- Brown, G.M.; Pandi-Perumal, S.R.; Trakht, I.; Cardinali, D.P. Melatonin and its relevance to jet lag. Travel Med. Infect. Dis. 2009, 7, 69–81. [Google Scholar] [CrossRef]
- Monk, T.H.; Buysse, D.J.; Carrier, J.; Kupfer, D.J. Inducing jet-lag in older people: Directional asymmetry. J. Sleep Res. 2000, 9, 101–116. [Google Scholar] [CrossRef]
- Sookoian, S.; Gemma, C.; Fernández Gianotti, T.; Burgueño, A.; Alvarez, A.; González, C.D.; Pirola, C.J. Effects of rotating shift work on biomarkers of metabolic syndrome and inflammation. J. Intern. Med. 2007, 261, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, K.; Leproult, R.; Van Cauter, E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999, 354, 1435–1439. [Google Scholar] [CrossRef]
- Patel, S.R.; Ayas, N.T.; Malhotra, M.R.; White, D.P.; Schernhammer, E.S.; Speizer, F.E.; Stampler, M.J.; Hu, F.B. A prospective study of sleep duration and mortality risk in women. Sleep 2004, 27, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.J.; Sellix, M.T. Chronic jet- lag increases mortality in aged mice. Curr. Biol. 2006, 16, 914–916. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Abellán, P.; Hernández-Morante, J.; Luján, J.; Madrid, J.; Garaulet, M. Clock genes are implicated in the human metabolic syndrome. Int. J. Obes. 2008, 32, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.S.; Hong, H.K.; Ko, C.H.; McDearmon, E.L. Implications for Physiology and Disease. Nat. Rev. Genet. 2008, 9, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.J.L.; Hilton, M.F.; Mantzoros, C.S.; Shea, S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458. [Google Scholar] [CrossRef]
- Spiegel, K.; Tasali, E.; Penev, P.; Van Cauter, E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann. Intern. Med. 2004, 141, 846–850. [Google Scholar] [CrossRef]
- Turek, F.W.; Joshu, C.; Kohsaka, A.; Lin, E.; Ivanova, G.; McDearmon, E.; Laposky, A.; Losee-Olson, S.; Easton, A.; Jensen, D.R.; et al. Obesity and metabolic syndrome in circadian Clock mutant nice. Science 2005, 308, 1043–1045. [Google Scholar] [CrossRef]
- Guzmán-Ruiz, R.; Somoza, B.; Gil-Ortega, M.; Merino, B.; Cano, V.; Attané, C.; Castan-Laurell, I.; Valet, P.; Fernández-Alfonso, M.S.; Ruiz-Gayo, M. Sensitivity of cardiac carnitine palmitoyltransferase to malonyl-CoA is regulated by leptin: Similarities with a model of endogenous hyperleptinemia. Endocrinology 2010, 151, 1010–1018. [Google Scholar] [CrossRef][Green Version]
- Pavanello, S.; Stendardo, M.; Mastrangelo, G.; Casillo, V.; Nardini, M.; Mutti, A.; Campisi, M.; Andreoli, R.; Boschetto, P. Higher number of night shifts associates with good perception of work capacity and optimal lung function but correlates with increased oxidative damage and telomere attrition. BioMed Res. Int. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Maury, E.; Ramsey, K.; Bass, J. Circadian rhythms and metabolic syndrome: From experimental genetics to human disease. Circ. Res. 2010, 106, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Costa, G. Shift work and occupational medicine: An overview. Occup. Med. 2003, 53, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, C.H.; Spiegelman, D.; Manson, J.A.; Schernhammer, E.S.; Colditz, G.A.; Kawachi, I. Work characteristics and incidence of type 2 diabetes in women. Am. J. Epidemiol. 2007, 165, 175–183. [Google Scholar] [CrossRef]
- Coca, A. Circadian rhythm and blood pressure control: Physiological and pathophysiological factors. J. Hypertens. Suppl. 1994, 12, S13–S21. [Google Scholar]
- Goncharuk, V.; Van Heerikhuize, J.; Dai, J.; Swaab, D.; Buijs, R. Neuropeptide changes in the suprachiasmatic nucleus in primary hypertension indicate functional impairment of the biological clock. J. Comp. Neurol. 2001, 431, 320–330. [Google Scholar] [CrossRef]
- Gibson, M.; Williams, W., III; Kriegsfeld, L. Aging in the Circadian System: Considerations for Health, Disease Prevention, and Longevity. Exp. Gerontol. 2009, 44, 51–56. [Google Scholar] [CrossRef]
- Filipski, E.; Li, X.M.; Lévi, F. Disruption of circadian coordination and malignant growth. Cancer Causes Control 2006, 17, 509–514. [Google Scholar] [CrossRef]
- Stevens, R.G. Light-at-night, circadian disruption and breast cancer: Assessment of existing evidence. Int. J. Epidemiol. 2009, 38, 963–970. [Google Scholar] [CrossRef]
- Zhu, Y.; Stevens, R.; Hoffman, A.; FitzGerald, L.; Kwon, E.; Ostrander, E.; Davis, S.; Zheng, T.; Stanford, J. Testing the circadian gene hypothesis in prostate cancer: A population-based case-control study. Cancer Res. 2009, 69, 9315–9322. [Google Scholar] [CrossRef]
- Hoffman, E.; Zheng, T.; Yi, C.; Stevens, R.; Ba, Y.; Zhang, Y.; Leaderer, D.; Holford, T.; Hansen, J.; Zhu, Y. The core circadian gene cryptochrome 2 influences breast cancer risk, possibly by mediating hormone signaling. Cancer Prev. Res. 2010, 3, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.; Mirick, D.; Stevens, R. Night Shift Work, Light at Night, and Risk of Breast Cancer. J. Natl. Cancer Inst. 2001, 93, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J. Risk of breast cancer after night- and shift work: Current evidence and ongoing studies in Denmark. Cancer Causes Control 2006, 17, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Schernhammer, E.S.; Laden, F.; Speizer, F.E.; Willet, W.C.; Hunter, D.J.; Kawachi, I.; Fuchs, C.S.; Colditz, G.A. Night-shift work and risk of colorectal cancer in the Nurses’ Health Study. J. Natl. Cancer Inst. 2003, 95, 825–828. [Google Scholar] [CrossRef]
- Reppert, S.M.; Weaver, D.R. Coordination of circadian clocks in mammals. Nature 2002, 418, 935–941. [Google Scholar] [CrossRef]
- Erren, T.C.; Reiter, R.J. Defining chronodisruption. J. Pineal Res. 2009, 46, 245–247. [Google Scholar] [CrossRef]
- Preuss, F.; Tang, Y.; Laposky, A.; Arble, D.; Keshavarzian, A.; Turek, F. Adverse effects of chronic circadian desynchronization in animals in a “challenging” environment. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R2034–R2040. [Google Scholar] [CrossRef]
- Dallman, M.; Akana, S.; Pecoraro, N.; Warne, J.; La Fleur, S.; Foster, M. Glucocorticoids, the etiology of obesity and the metabolic syndrome. Curr. Alzheimer Res. 2007, 4, 199–204. [Google Scholar] [CrossRef]
- Scheer, F.A.J.L.; Ter Horst, G.J.; Van Der Vliet, J.; Buijs, R.M. Physiological and anatomic evidence for regulation of the heart by suprachiasmatic nucleus in rats. Am. J. Physiol. 2001, 280, 1391–1399. [Google Scholar] [CrossRef]
- Zhao, H.; Yin, Z.; Xiang, H.; Liao, Z.; Wang, Z. Preliminary study on alterations of altitude road traffic in China from 2006 to 2013. PLoS ONE 2017, 12, 1–12. [Google Scholar] [CrossRef][Green Version]
- Knott, M.; Classen, S.; Krasniuk, S.; Tippett, M.; Alvarez, L. Insufficient sleep and fitness to drive in shift workers: A systematic literature review. Accid. Anal. Prev. 2020, 134, 105234. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Gangwar, A.; Bhargava, K.; Khurana, P.; Ahmad, Y. Diagnosis and prophylaxis for high-altitude acclimatization: Adherence to molecular rationale to evade high-altitude illnesses. Life Sci. 2018, 203, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Tuttle, T.; Higgins, J.A. Altitude and the heart: Is going high safe for your cardiac patient? Am. Heart J. 2010, 159, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Behn, C.; Dinamarca, G.A.; De Gregorio, N.F.; Lips, V.; Vivaldi, E.A.; Soza, D.; Guerra, M.A.; Jiménez, R.F.; Lecannelier, E.A.; Varela, H.; et al. Age-related arrhythmogenesis on ascent and descent: “Autonomic conflicts” on hypoxia/reoxygenation at high altitude? High Alt. Med. Biol. 2014, 15, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Roche, E.; Romero-Alvira, D. Role of oxygen free radicals in altitude-related disorders. Med. Hypotheses 1994, 42, 105–109. [Google Scholar] [CrossRef]
- Vearrier, D.; Greenberg, M.I. Occupational health of miners at altitude: Adverse health effects, toxic exposures, pre-placement screening, acclimatization, and worker surveillance. Clin. Toxicol. 2011, 49, 629–640. [Google Scholar] [CrossRef]
- Firth, P.G.; Zheng, H.; Windsor, J.S.; Sutherland, A.I.; Imray, C.H.; Moore, G.W.K.; Semple, J.L.; Roach, R.C.; Salisbury, R.A. Christmas 2008: Sport: Mortality on Mount Everest, 1921-2006: Descriptive study. BMJ 2008, 337, 1–6. [Google Scholar] [CrossRef]
- Firth, P.; Zheng, H.; Windsor, J.; Sutherland, A.; Imray, C.; Moore, G.; Semple, J.; Roach, R.; Salisbury, R. Mortality on Mount Everest, 1921-2006: Descriptive study. BMJ 2008, 337, a2654. [Google Scholar] [CrossRef]
- Fornasiero, A.; Savoldelli, A.; Skafidas, S.; Stella, F.; Bortolan, L.; Boccia, G.; Zignoli, A.; Schena, F.; Mourot, L.; Pellegrini, B. Delayed parasympathetic reactivation and sympathetic withdrawal following maximal cardiopulmonary exercise testing (CPET) in hypoxia. Eur. J. Appl. Physiol. 2018, 118, 2189–2201. [Google Scholar] [CrossRef]
- Hamm, W.; Von Stülpnagel, L.; Klemm, M.; Baylacher, M.; Rizas, K.D.; Bauer, A.; Brunner, S. Deceleration Capacity of Heart Rate after Acute Altitude Exposure. High Alt. Med. Biol. 2018, 19, 299–302. [Google Scholar] [CrossRef]
- Gianfredi, V.; Albano, L.; Basnyat, B.; Ferrara, P.; Count, W. Does age have an impact on acute mountain sickness? A systematic review. J. Travel Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.B.J.; Li, J.Y.; Kuo, H.K.; Chern, C.M.; Yang, C.C.H. Differential changes and interactions of autonomic functioning and sleep architecture before and after 50 years of age. Age 2016, 38, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ray, C. Melatonin attenuates the sympathetic nerve responses to ortho-static stress in humans. J. Physiol. 2003, 551, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Pechanova, O.; Paulis, L.; Simko, F. Peripheral and central effects of melatonin on blood pressure regulation. Int. J. Mol. Sci. 2014, 15, 17920–17937. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.; Kimpinski, K. Role of melatonin in blood pressure regulation: An adjunct anti-hypertensive agent. Clin. Exp. Pharmacol. Physiol. 2018, 45, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Rauch, S.; Schenk, K.; Strapazzon, G.; Dal Cappello, T.; Gatterer, H.; Palma, M.; Erckert, M.; Oberhuber, L.; Bliemsrieder, B.; Brugger, H.; et al. Suspension syndrome: A potentially fatal vagally mediated circulatory collapse—An experimental randomized crossover trial. Eur. J. Appl. Physiol. 2019, 119, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Reno, C.M.; Bayles, J.; Huang, Y.; Oxspring, M.; Hirahara, A.M.; Dosdall, D.J.; Fisher, S.J. Severe hypoglycemia–induced fatal cardiac arrhythmias are mediated by the parasympathetic nervous system in rats. Diabetes 2019, 68, 2107–2119. [Google Scholar] [CrossRef]
- Nishiyama, K.; Yasue, H.; Moriyama, Y.; Tsunoda, R.; Ogawa, H.; Yoshimura, M.; Kugiyama, K. Acute effects of melatonin administration on cardiovascular autonomic regulation in healthy men. Am. Heart J. 2001, 141, 13A–17A. [Google Scholar] [CrossRef]
- Doolen, S.; Krause, D.N.; Dubocovich, M.L.; Duckles, S.P. Melatonin mediates two distinct responses in vascular smooth muscle. Eur. J. Pharmacol. 1998, 345, 67–69. [Google Scholar] [CrossRef]
- Masana, M.I.; Doolen, S.; Ersahin, C.; Al-Ghoul, W.M.; Duckles, S.P.; Dubocovich, M.L.; Krause, D.N. MT2 melatonin receptors are present and functional in rat caudal artery. J. Pharmacol. Exp. Ther. 2002, 302, 1295–1302. [Google Scholar] [CrossRef]
- Campos, L.A.; Pereira, V.L.; Muralikrishna, A.; Albarwani, S.; Brás, S.; Gouveia, S. Mathematical biomarkers for the autonomic regulation of cardiovascular system. Front. Physiol. 2013, 4, 279. [Google Scholar] [CrossRef] [PubMed]
- Imenshahidi, M.; Karimi, G.; Hosseinzadeh, H. Effects of melatonin on cardiovascular risk factors and metabolic syndrome: A comprehensive review. Naunyn. Schmiedebergs. Arch. Pharmacol. 2020, 393, 521–536. [Google Scholar] [CrossRef] [PubMed]
- Green, E.A.; Black, B.K.; Biaggioni, I.; Paranjape, S.Y.; Bagai, K.; Shibao, C.; Okoye, M.C.; Dupont, W.D.; Robertson, D.; Raj, S.R. Melatonin reduces tachycardia in postural tachycardia syndrome: A randomized, crossover trial. Cardiovasc. Ther. 2014, 32, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhai, X.; Li, S.; McCabe, M.F.; Wang, X.; Rong, P. Transcutaneous vagus nerve stimulation induces tidal melatonin secretion and has an antidiabetic effect in Zucker fatty rats. PLoS ONE 2015, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behn, C.; De Gregorio, N. Melatonin Relations with Energy Metabolism as Possibly Involved in Fatal Mountain Road Traffic Accidents. Int. J. Mol. Sci. 2020, 21, 2184. https://doi.org/10.3390/ijms21062184
Behn C, De Gregorio N. Melatonin Relations with Energy Metabolism as Possibly Involved in Fatal Mountain Road Traffic Accidents. International Journal of Molecular Sciences. 2020; 21(6):2184. https://doi.org/10.3390/ijms21062184
Chicago/Turabian StyleBehn, Claus, and Nicole De Gregorio. 2020. "Melatonin Relations with Energy Metabolism as Possibly Involved in Fatal Mountain Road Traffic Accidents" International Journal of Molecular Sciences 21, no. 6: 2184. https://doi.org/10.3390/ijms21062184
APA StyleBehn, C., & De Gregorio, N. (2020). Melatonin Relations with Energy Metabolism as Possibly Involved in Fatal Mountain Road Traffic Accidents. International Journal of Molecular Sciences, 21(6), 2184. https://doi.org/10.3390/ijms21062184



