Hericium Erinaceus Prevents DEHP-Induced Mitochondrial Dysfunction and Apoptosis in PC12 Cells
Abstract
1. Introduction
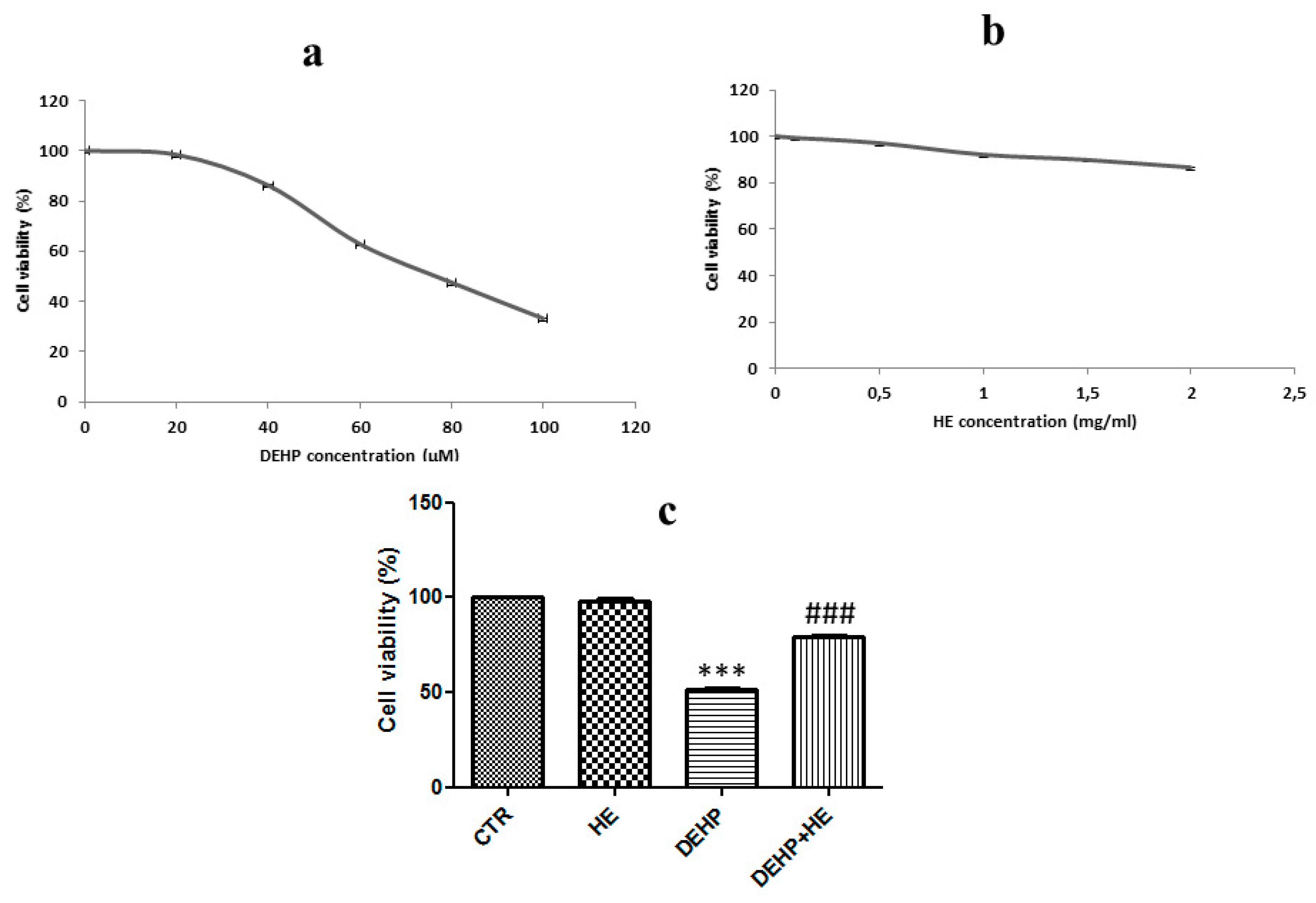
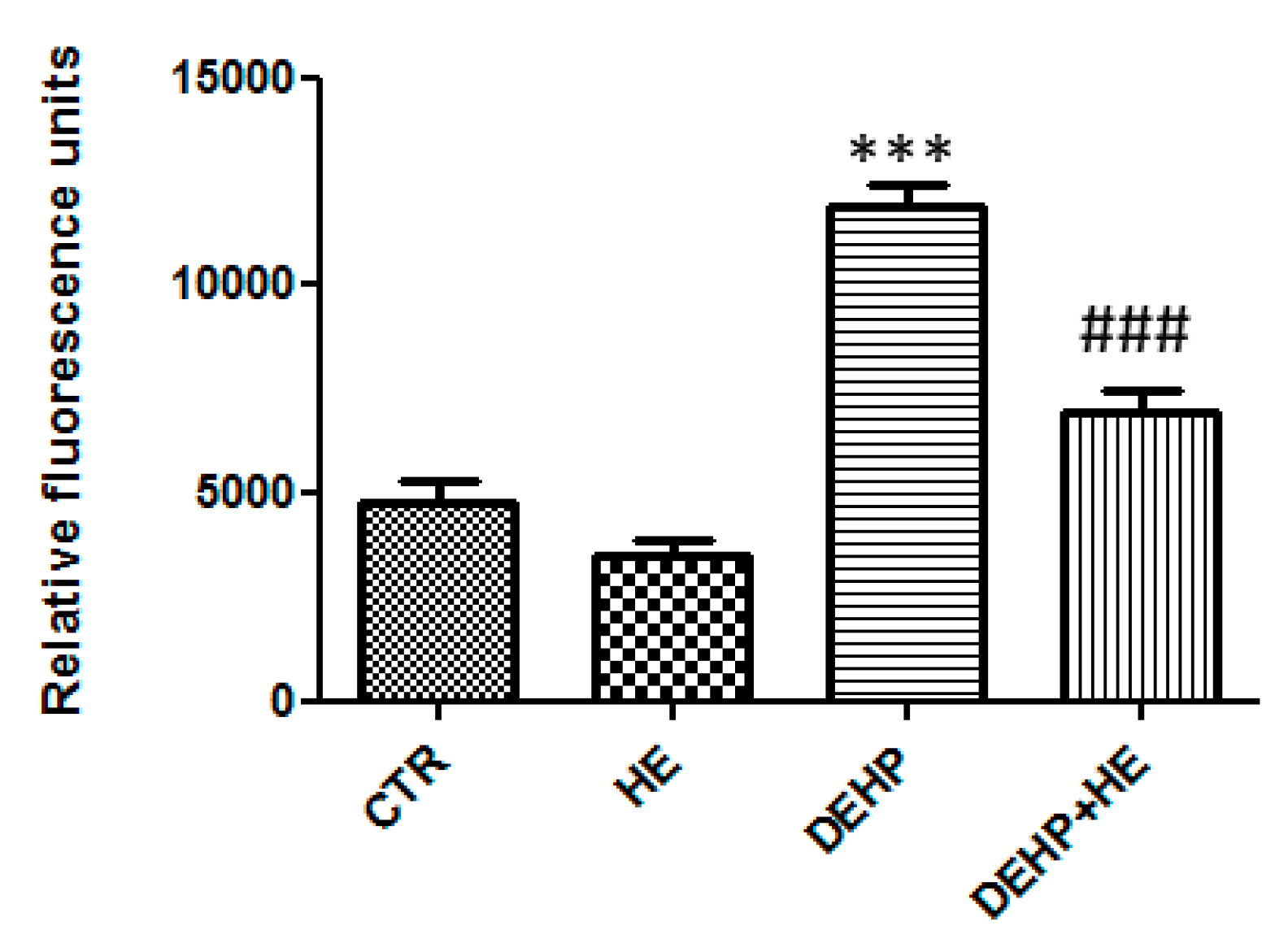
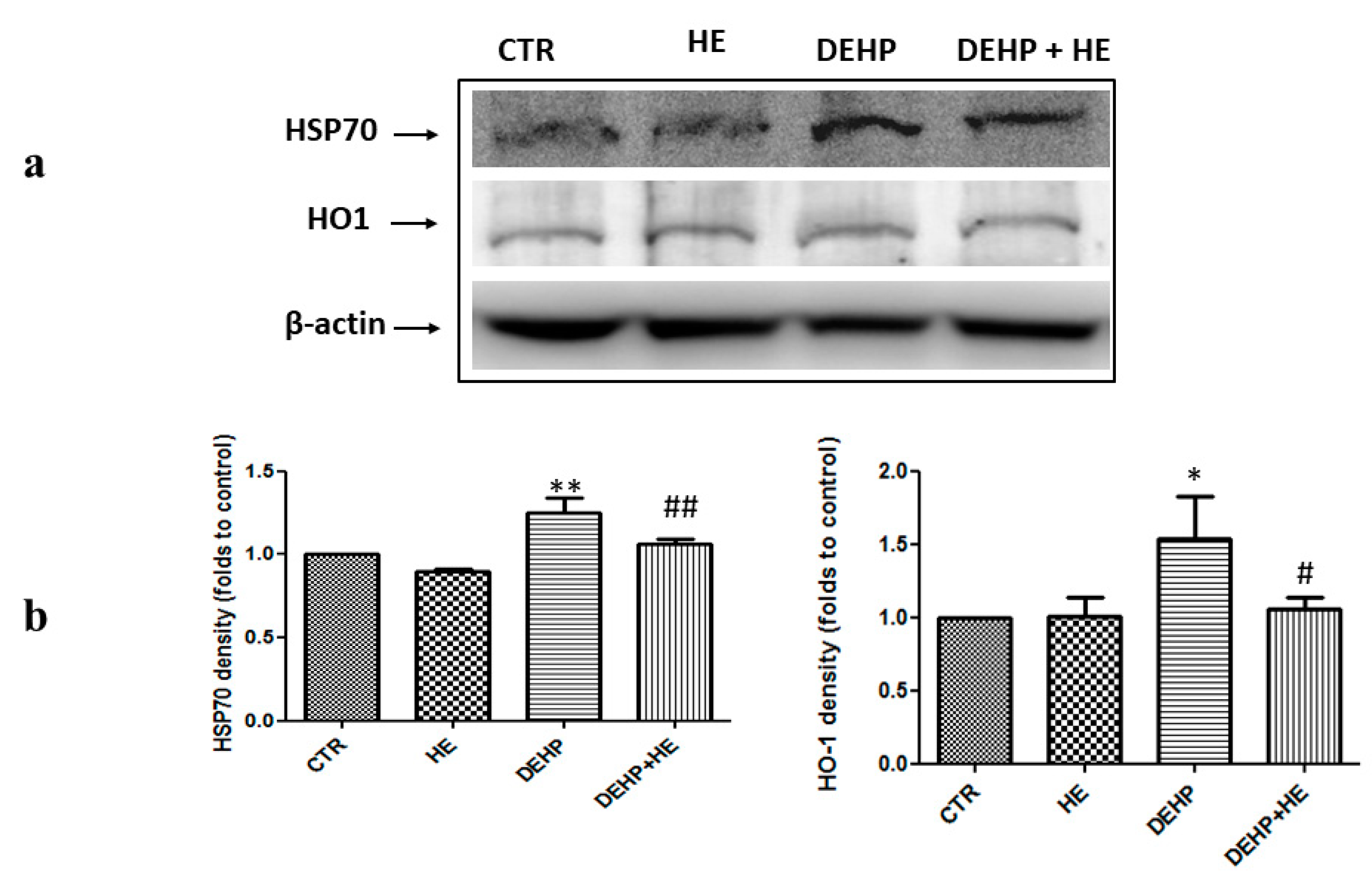
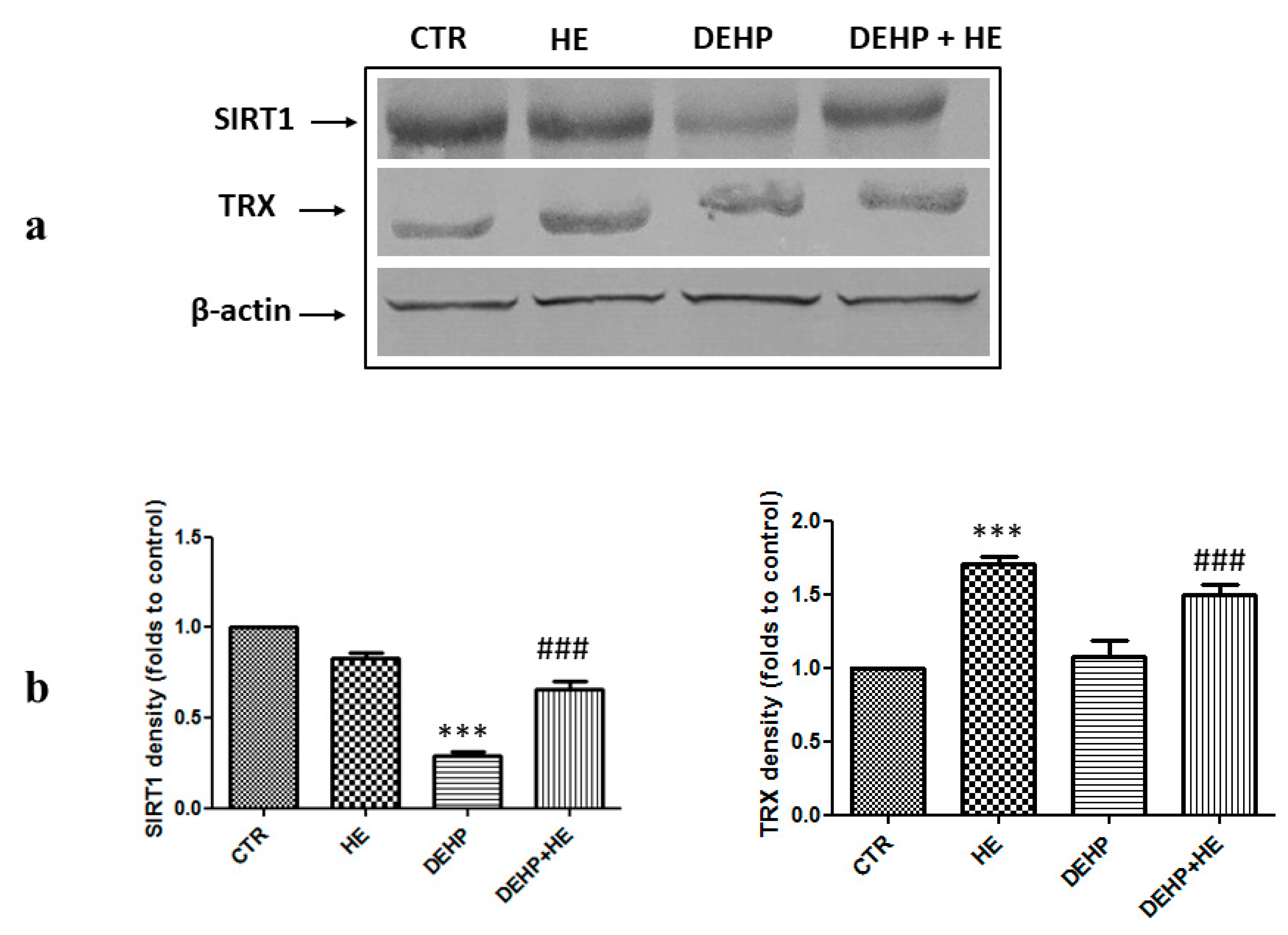
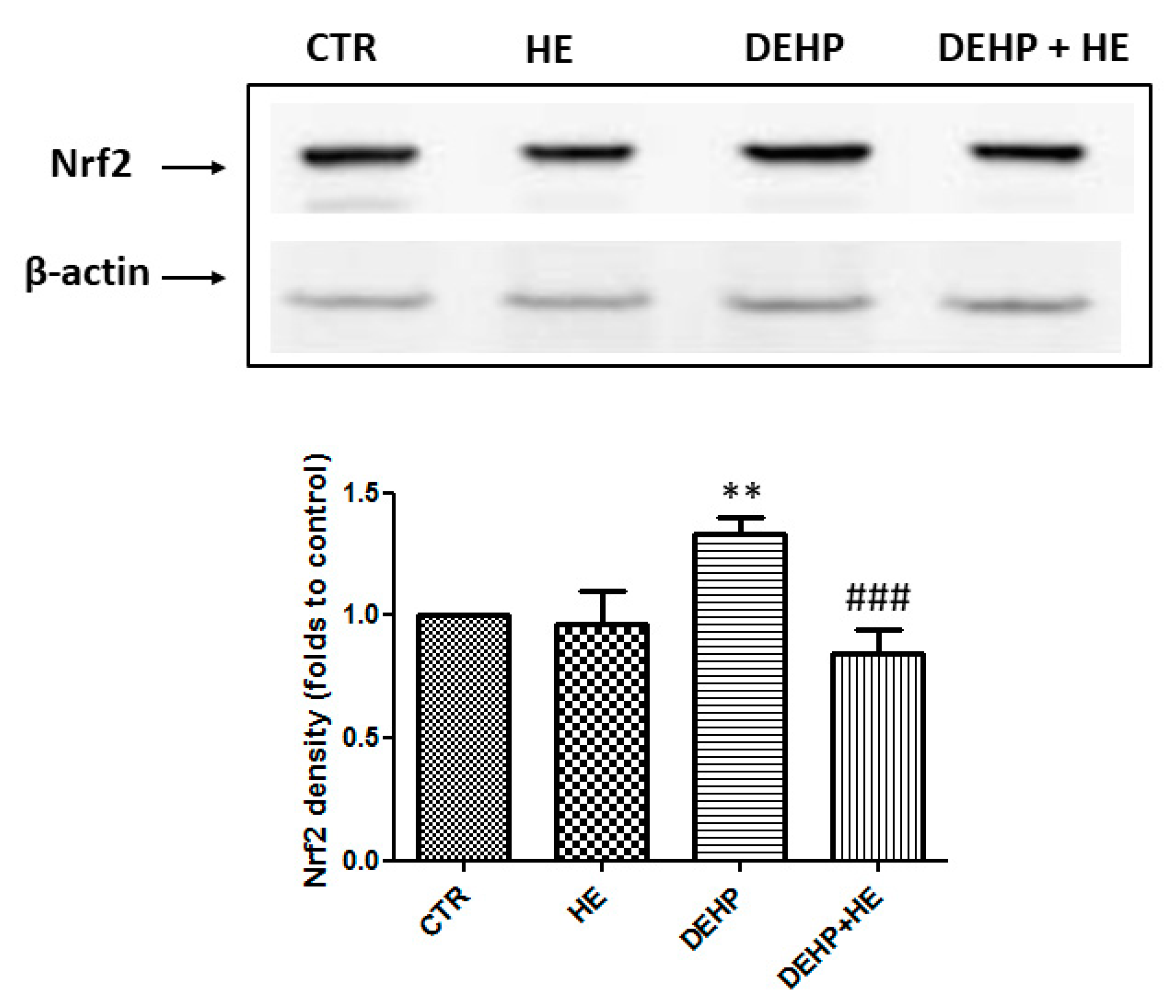
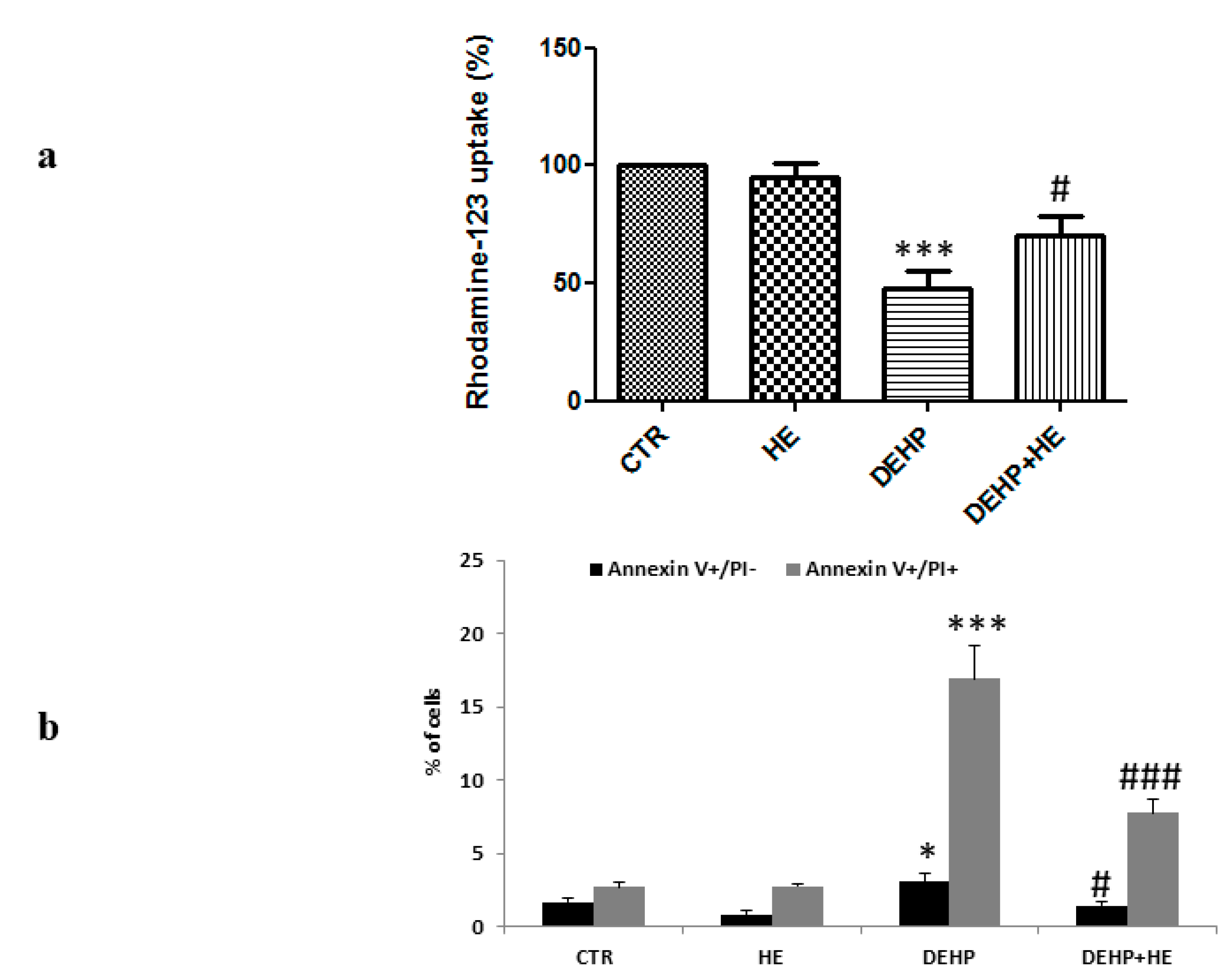
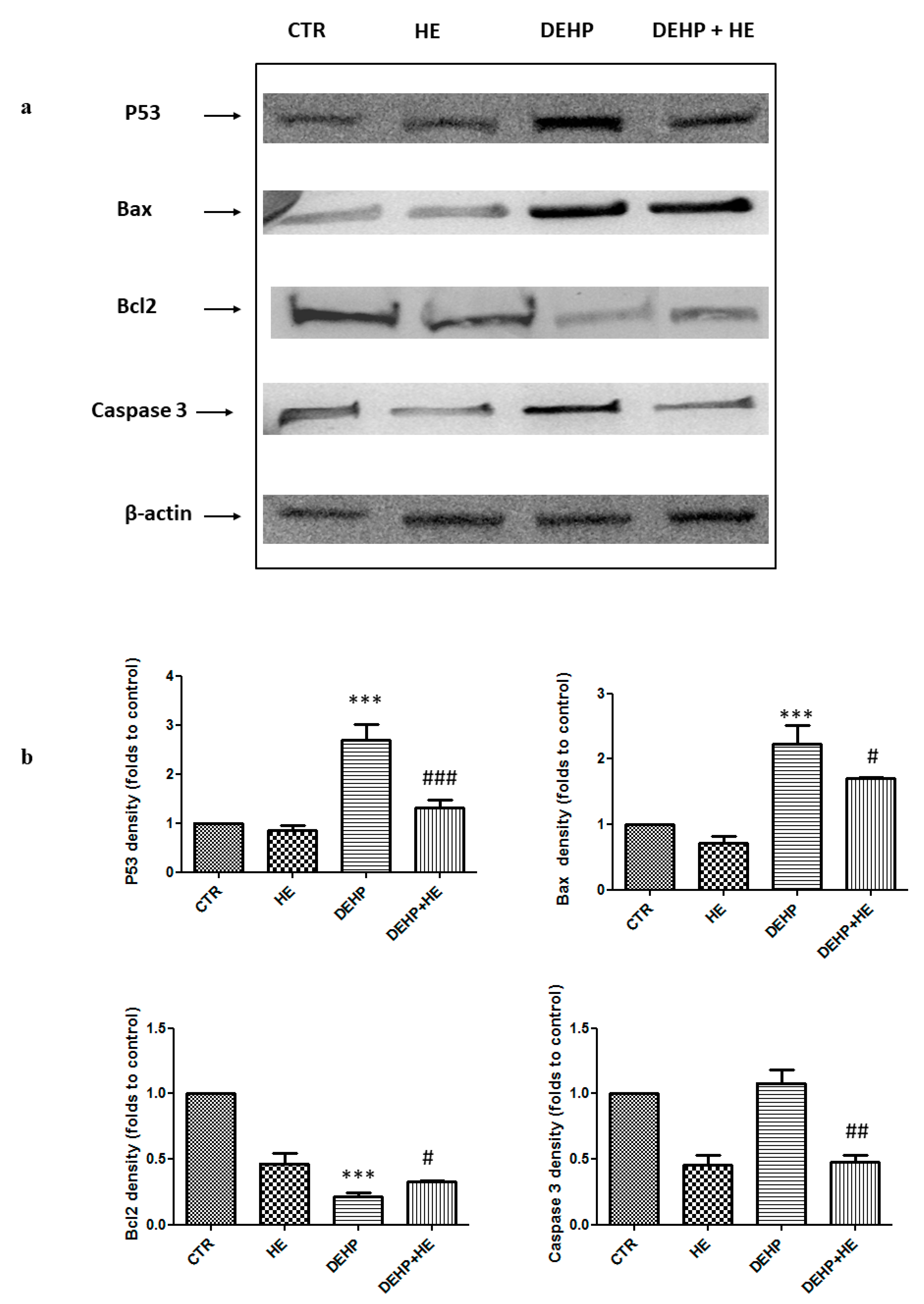
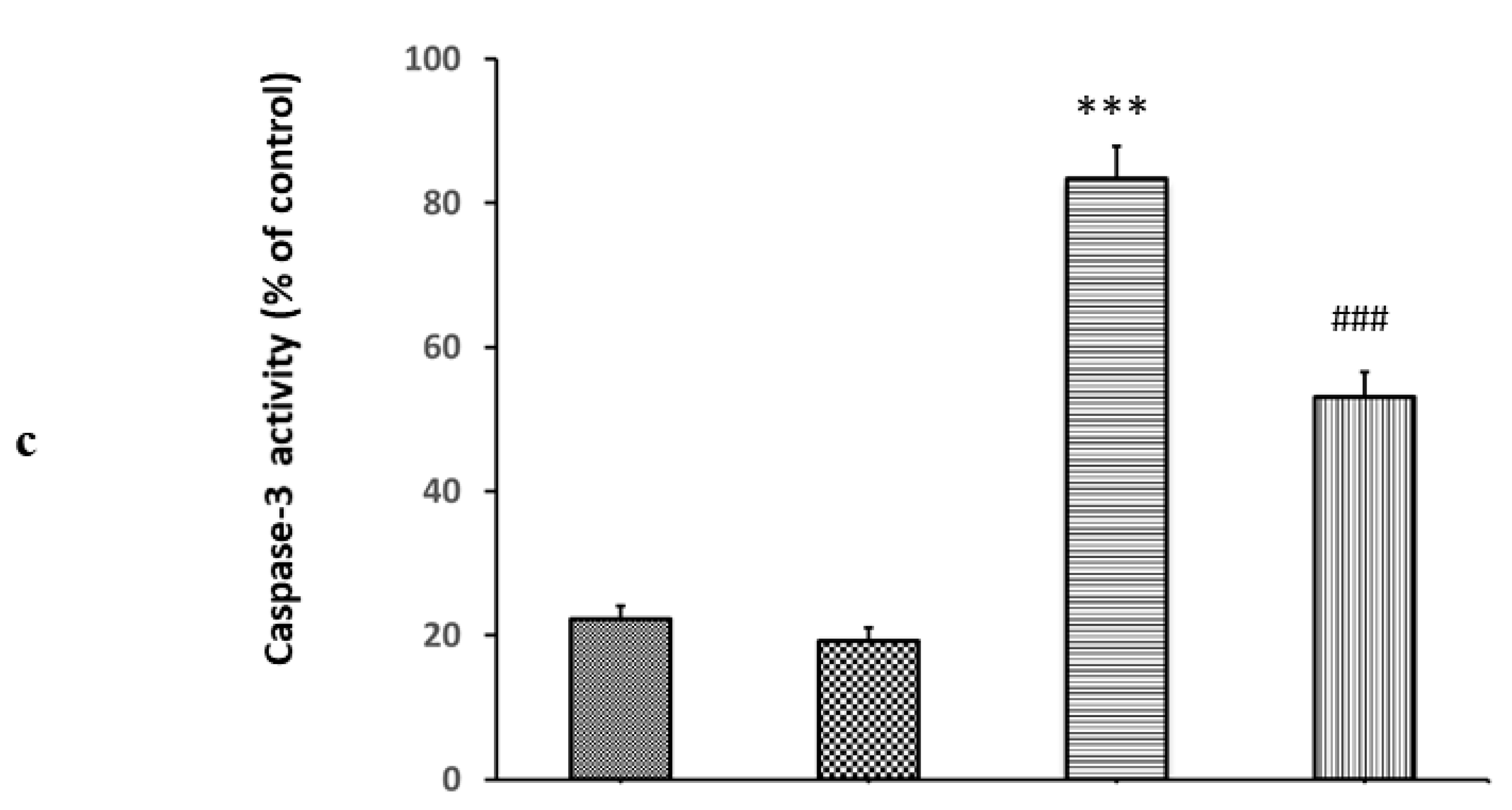
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Hericium Erinaceus (HE) Biomass Preparation
4.3. Cell Culture
4.4. Cell Viability Assay (MTT)
4.5. Determination of Reactive Oxygen Species (ROS) and Oxidative Stress Status
4.6. Measurement of Mitochondrial Membrane Potential (MMP)
4.7. Cell Death Induced by DEHP
4.8. Preparation of Mitoplasts
4.9. Mitochondrial Respiratory Complex Activity Assay
4.10. Protein Extraction and Western Blot Analysis
4.11. Caspase-3 Activation Assay
4.12. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mariana, M.; Feiteiro, J.; Verde Cairrao, E. The effects of phthalates in the cardiovascular and reproductive systems. Environ. Int. 2016, 94, 758–776. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.K. Endocrine disruptors epigenetic transgenerational inheritance. Nat. Rev. Endocrinol. 2015, 12, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Schlatter, C. Excretion and metabolism of di(2 ethylhexyl)phthalate in man. Xenobiotica 1985, 15, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.E.; Braun, J.; Trasande, L.; Dills, R.; Sathyanarayana, S. Phthalates and diet: A review of the food monitoring and epidemiology data. Environ. Health A Glob. Access Sci. Source 2014, 13. [Google Scholar] [CrossRef]
- Aung, K.H.; Win-Shwe, T.T.; Kanaya, M.; Takano, H.; Tsukahara, S. Involvement of hemeoxygenase-1 in di(2-ethylhexyl) phthalate (DEHP)-induced apoptosis of Neuro-2a cells. J. Toxicol. Sci. 2014, 39, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Dhanya, C.R.; Indu, A.R.; Deepadevi, K.V.; Kurup, P. Inhibition of membrane Na(+)-K+ Atpase of the brain, liver and RBC in rats administered di(2-ethyl hexyl) phthalate (DEHP) a plasticizer used in polyvinyl chloride (PVC) blood storage bags. Indian J. Exp. Biol. 2003, 41, 814–820. [Google Scholar]
- Moore, R.W.; Rudy, T.A.; Lin, T.M.; Ko, K.; Peterson, R.E. Abnormalities of sexual development in male rats with in utero and lactational exposure to the antiandrogenic plasticizer Di(2-ethylhexyl) phthalate. Environ. Health Perspect. 2001, 109, 229–237. [Google Scholar] [CrossRef]
- Lin, H.; Yuan, K.; Li, L.; Liu, S.; Li, S.; Hu, G.; Lian, Q.Q.; Ge, R.S. In Utero Exposure to Diethylhexyl Phthalate Affects Rat Brain Development: A Behavioral and Genomic Approach. Int. J. Environ. Res. Public Health 2015, 12, 13696–13710. [Google Scholar] [CrossRef]
- Komada, M.; Gendai, Y.; Kagawa, N.; Nagao, T. Prenatal exposure to di(2- ethylhexyl) phthalate impairs development of the mouse neoHEtex. Toxicol. Lett. 2016, 259, 69–79. [Google Scholar] [CrossRef]
- Yan, M.H.; Wang, X.; Zhu, X. Mitochondrial defects and oxidative stress in Alzheimer’s disease and Parkinson disease. Free Radic. Biol. Med. 2013, 62, 90–101. [Google Scholar] [CrossRef]
- Jacobson, M.D.; Weil, M.; Raff, M.C. Programmed cell death in animal development. Cell 1997, 88, 347–354. [Google Scholar] [CrossRef]
- Parsons, M.J.; Green, D.R. Mitochondria in cell death. Essays Biochem. 2010, 47, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, E.A.; El Enshasy, H.; Wadaan, M.A.; Aziz, R. Mushrooms: A potential natural source of anti-inflammatory compounds for medical applications. Mediat. Inflamm. 2014, 805841. [Google Scholar] [CrossRef] [PubMed]
- El Enshasy, H.; Elsayed, E.A.; Aziz, R.; Wadaan, M.A. Mushrooms and truffles: Historical biofactories for complementary medicine in Africa and in the Middle East. Evid. Based Complement. Alternat. Med. 2013, 620451. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Beelman, R.B.; Lambert, J.D. The cancer preventive effects of edible mushrooms. Anticancer Agents Med. Chem. 2012, 12, 1255–1263. [Google Scholar] [CrossRef]
- Paterson, R.R.; Lima, N. Biomedical effects of mushrooms with emphasis on pure compounds. Biomed. J. 2014, 37, 357–368. [Google Scholar] [CrossRef]
- Komura, D.L.; Ruthes, A.C.; Carbonero, E.R.; Gorin, P.A.; Iacomini, M. Water-soluble polysaccharides from Pleurotus ostreatus var. florida mycelial biomass. Int. J. Biol. Macromol. 2014, 70, 354–359. [Google Scholar] [CrossRef]
- Wasser, S.P. Medicinal mushroom science: Current perspectives, advances, evidences, and challenges. Biomed. J. 2014, 37, 345–356. [Google Scholar] [CrossRef]
- Lindequist, U.; Kim, H.W.; Tiralongo, E.; Van Griensven, L. Medicinal mushrooms. Evid. Based Complement. Alternat. Med 2014, 806180. [Google Scholar] [CrossRef]
- da Silva, A.F.; Sartori, D.; Macedo, F.C., Jr.; Ribeiro, L.R.; Fungaro, M.H.; Mantovani, M.S. Effects of beta-glucan extracted from Agaricus blazei on the expression of ERCC5, CASP9, and CYP1A1 genes and metabolic profile in HepG2 cells. Hum. Exp. Toxicol. 2013, 32647–32654. [Google Scholar] [CrossRef]
- Lai, C.L.; Lin, R.T.; Liou, L.M.; Liu, C.K. The role of event-related potentials in cognitive decline in Alzheimer’s disease. Clin. Neurophysiol. 2010, 121, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Trovato Salinaro, A.; Pennisi, M.; Di Paola, R.; Scuto, M.; Crupi, R.; Teresa Cambria, M.T.; Ontario, M.L.; Tomasello, M.; Uva, M.; Maiolino, L.; et al. Neuroinflammation and neurohormesis in the pathogenesis of Alzheimer’s disease and Alzheimer-linked pathologies: Modulation by nutritional mushrooms. Immun. Ageing 2018, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Scuto, M.C.; Mancuso, C.; Tomasello, B.; Laura Ontario, M.; Cavallaro, A.; Frasca, F.; Maiolino, L.; Trovato Salinaro, A.; Calabrese, E.J.; Calabrese, V. Curcumin, Hormesis and the Nervous System. Nutrients 2019, 11, 2417. [Google Scholar] [CrossRef]
- Calabrese, V.; Santoro, A.; Monti, D.; Crupi, R.; Di Paola, R.; Latteri, S.; Cuzzocrea, S.; Zappia, M.; Giordano, J.; Calabrese, E.J.; et al. Aging and Parkinson’s Disease: Inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesis. Free Radic. Biol. Med. 2018, 115, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Dhawan, G.; Kapoor, R.; Iavicoli, I.; Calabrese, V. HORMESIS: A Fundamental Concept with Widespread Biological and Biomedical Applications. Gerontology 2016, 62, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Dhawan, G.; Kapoor, R.; Iavicoli, I.; Calabrese, V. What is hormesis and its relevance to healthy aging and longevity? Biogerontology 2015, 16, 693–707. [Google Scholar] [CrossRef]
- Cornelius, C.; Graziano, A.; Calabrese, E.J.; Calabrese, V. Hormesis and vitagenes in aging and longevity: Mitochondrial control and hormonal regulation. Horm. Mol. Biol. Clin. Investig. 2013, 16, 73–89. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Calabrese, V.; Giordano, J. The role of hormesis in the functional performance and protection of neural systems. Brain Circ. 2017, 3, 1–13. [Google Scholar] [CrossRef]
- Calabrese, V.; Santoro, A.; Trovato Salinaro, A.; Modafferi, S.; Scuto, M.; Albouchi, F.; Monti, D.; Giordano, J.; Zappia, M.; Franceschi, C.; et al. Hormetic approaches to the treatment of Parkinson’s disease: Perspectives and possibilities. J. Neurosci. Res. 2018, 96, 1641–1662. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Bhatia, T.N.; Calabrese, V.; Dhawan, G.; Giordano, J.; Hanekamp, Y.N.; Kapoor, R.; Kozumbo, W.J.; Leak, R.K. Cytotoxicity models of Huntington’s disease and relevance of hormetic mechanisms: A critical assessment of experimental approaches and strategies. Pharmacol. Res. 2019, 150, 104371. [Google Scholar] [CrossRef]
- Wang, D.; Calabrese, E.J.; Lian, B.; Lin, Z.; Calabrese, V. Hormesis as a mechanistic approach to understanding herbal treatments in traditional Chinese medicine. Pharmacol. Ther. 2018, 184, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Giordano, J.; Crupi, R.; Di Paola, R.; Ruggieri, M.; Bianchini, R.; Ontario, M.L.; Cuzzocrea, S.; Calabrese, E.J. Hormesis, cellular stress response and neuroinflammation in schizophrenia: Early onset versus late onset state. J. Neurosci. Res. 2017, 95, 1182–1193. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, M.; Crupi, R.; Di Paola, R.; Ontario, M.L.; Bella, R.; Calabrese, E.J.; Crea, R.; Cuzzocrea, S.; Calabrese, V. Inflammasomes, hormesis, and antioxidants in neuroinflammation: Role of NRLP3 in Alzheimer disease. J. Neurosci. Res. 2017, 95, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Giordano, J.; Signorile, A.; Laura Ontario, M.; Castorina, S.; De Pasquale, C.; Eckert, G.; Calabrese, E.J. Major pathogenic mechanisms in vascular dementia: Roles of cellular stress response and hormesis in neuroprotection. J. Neurosci. Res. 2016, 94, 1588–1603. [Google Scholar] [CrossRef]
- Calabrese, V.; Giordano, J.; Ruggieri, M.; Berritta, D.; Trovato, A.; Ontario, M.L.; Bianchini, R.; Calabrese, E.J. Hormesis, cellular stress response, and redox homeostasis in autism spectrum disorders. J. Neurosci. Res. 2016, 94, 1488–1498. [Google Scholar] [CrossRef]
- Dattilo, S.; Mancuso, C.; Koverech, G.; Di Mauro, P.; Ontario, M.L.; Petralia, C.C.; Petralia, A.; Maiolino, L.; Serra, A.; Calabrese, E.J.; et al. Heat shock proteins and hormesis in the diagnosis and treatment of neurodegenerative diseases. Immun. Ageing 2015, 12, 20. [Google Scholar] [CrossRef]
- Trovato, A.; Siracusa, R.; Di Paola, R.; Scuto, M.; Fronte, V.; Koverech, G.; Luca, M.; Serra, A.; Toscano, M.A.; Petralia, A.; et al. Redox modulation of cellular stress response and lipoxin A4 expression by Coriolus versicolor in rat brain: Relevance to Alzheimer’s disease pathogenesis. Neurotoxicology 2016, 53, 350–358. [Google Scholar] [CrossRef]
- Scuto, M.; Di Mauro, P.; Ontario, M.L.; Amato, C.; Modafferi, S.; Ciavardelli, D.; Trovato Salinaro, A.; Maiolino, L.; Calabrese, V. Nutritional Mushroom Treatment in Meniere’s Disease with Coriolus versicolor: A Rationale for Therapeutic Intervention in Neuroinflammation and Antineurodegeneration. Int. J. Mol. Sci. 2019, 21, 284. [Google Scholar] [CrossRef]
- Trovato Salinaro, A.; Siracusa, R.; Di Paola, R.; Scuto, M.; Ontario, M.L.; Bua, O.; Serra, A. Redox modulation of cellular stress response and lipoxin A4 expression by Hericium Erinaceus in rat brain: Relevance to Alzheimer’s disease pathogenesis. Immun. Ageing 2016, 13, 23. [Google Scholar] [CrossRef]
- Hao, L.; Xie, Y.; Wu, G.; Cheng, A.; Liu, X.; Zheng, R.; Huo, H.; Zhang, J. Protective Effect of Hericium Erinaceus on Alcohol Induced Hepatotoxicity in Mice. Evid. Based Complement. Alternat. Med. 2015, 418023. [Google Scholar] [CrossRef]
- Kowaltowski, A.J.; de Souza-Pinto, N.C.; Castilho, R.F.; Vercesi, A.E. Mitochondria and reactive oxygen species. Free Radic. Biol. Med. 2009, 47, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Cornelius, C.; Leso, V.; Trovato-Salinaro, A.; Ventimiglia, B.; Cavallaro, M.; Scuto, M.; Rizza, S.; Zanoli, L.; Neri, S.; et al. Oxidative stress, glutathione status, sirtuin and cellular stress response in type 2 diabetes. Biochim. Biophys. Acta 2012, 1822, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Zoratti, M.; Szabò, I. The mitochondrial permeability transition. Biochim. Biophys. Acta 1995, 1241, 139–176. [Google Scholar] [CrossRef]
- Liao, B.; Zhou, C.; Liu, T.; Dai, Y.; Huang, H. A novel Hericium erinaceus polysaccharide: Structural characterization and prevention of H(2)O(2)-induced oxidative damage in GES-1 cells. Int. J. Biol. Macromol. 2019, 20. [Google Scholar] [CrossRef]
- Signorile, A.; Micelli, L.; De Rasmo, D.; Santeramo, A.; Papa, F.; Ficarella, R.; Gattoni, G.; Scacco, S.; Papa, S. Regulation of the biogenesis of OXPHOS complexes in cell transition from replicating to quiescent state Involvement of PKA and effect of hydroxytyrosol. Biochim. et Biophys. Acta 2006, 4539–4543. [Google Scholar] [CrossRef]
- De Rasmo, D.; Micelli, L.; Santeramo, A.; Signorile, A.; Lattanzio, P.; Papa, S. cAMP regulates the functional activity, coupling efficiency and structural organization of mammalian FOF1 ATP synthase. Biochim. Biophys. Acta 2016, 1857, 350–358. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.T.; Calabrese, E.J.; Mattson, M.P. Cellular stress responses, the hormesis paradigm and vitagenes: Novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid. Redox Signal. 2010, 13, 1763–1811. [Google Scholar] [CrossRef]
- Choi, S.I.; Lim, K.H.; Seong, B.L. Chaperoning roles of macromolecules interacting with proteins in vivo. Int. J. Mol. Sci. 2011, 12, 1979–1990. [Google Scholar] [CrossRef]
- Mori, K.; Obara, Y.; Moriya, T.; Inatomi, S.; Nakahata, N. Effects of Hericium erinaceus on amyloid β(25-35) peptide-induced learning and memory deficits in mice. Biomed. Res. 2011, 32, 67–72. [Google Scholar] [CrossRef]
- Catino, S.; Paciello, F.; Miceli, F.; Rolesi, R.; Troiani, D.; Calabrese, V.; Santangelo, R.; Mancuso, C. Ferulic Acid Regulates the Nrf2/Heme Oxygenase-1 System and Counteracts Trimethyltin-Induced Neuronal Damage in the Human Neuroblastoma Cell Line SH-SY5Y. Front. Pharmacol. 2016, 6, 305. [Google Scholar] [CrossRef]
- Schuler, M.; Green, D.R. Mechanisms of P53-dependent apoptosis. Biochem. Soc. Trans. 2001, 29, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.-B. Kynurenine pathway of tryptophan metabolism: Regulatory and functional aspects. Int. J. Tryptophan Res. 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R. Role of the kynurenine metabolism pathway in inflammation-induced depression: Preclinical approaches. Curr. Top. Behav. Neurosci. 2017, 31, 117–138. [Google Scholar] [PubMed]
- Stone, T.W.; Mackay, G.M.; Forrest, C.M.; Clark, C.J.; Darlington, L.G. Tryptophan metabolites and brain disorders. Clin. Chem. Lab. Med. 2003, 41, 852–859. [Google Scholar] [CrossRef]
- Sorgdrager, F.J.H.; Naudé, P.J.W.; Kema, I.P.; Nollen, E.A.; Deyn, P.P. Tryptophan Metabolism in Inflammaging: From Biomarker to Therapeutic Target. Front. Immunol. 2019, 10, 2565. [Google Scholar] [CrossRef]
- Gostner, J.M.; Geisler, S.; Stonig, M.; Mair, L.; Sperner-Unterweger, B.; Fuchs, D. Tryptophan Metabolism and Related Pathways in Psychoneuroimmunology: The Impact of Nutrition and Lifestyle. Neuropsychobiology 2019, 26, 1–11. [Google Scholar] [CrossRef]
- Ragusa, N.; Sfogliano, L.; Calabrese, V.; Rizza, V. Effects of multivitamin treatment on the activity of rat liver tryptophan pyrrolase during ethanol administration. Acta Vitaminol. Enzymol. 1981, 3, 199–204. [Google Scholar]
- Calabrese, V.; Ragusa, N.; Rizza, V. Effect of pyrrolidone carboxylate (PCA) and pyridoxine on liver metabolism during chronic ethanol intake in rats. Int. J. Tissue React. 1995, 17, 15–20. [Google Scholar]
- Gao, K.; Mu, C.L.; Farzi, A.; Zhu, W.Y. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv. Nutr. 2019. [Google Scholar] [CrossRef]
- Diling, C.; Chaoqun, Z.; Jian, Y.; Jian, L.; Jiyan, S.; Yizhen, X.; Guoxiao, L. Immunomodulatory Activities of a Fungal Protein Extracted from Hericium erinaceus through Regulating the Gut Microbiota. Front. Immunol. 2017, 8, 666. [Google Scholar] [CrossRef]
- Ferreiro, E.; Pita, I.; Mota, S.; Valero, J.; Ferreira, N.; Fernandes, T.; Calabrase, V.; Fontes-Ribeiro, C.; Pereira, F.; Rego, A. Coriolus versicolor biomass increases dendritic arborization of newly-generated neurons in mouse hippocampal dentate gyrus. Oncotarget 2018, 8, 32929–32942. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, Y.; Yang, S.; Zhao, D.; Wang, M. A polysaccharide from cultured mycelium of Hericium erinaceus relieves ulcerative colitis by counteracting oxidative stress and improving mitochondrial function. Int. J. Biol. Macromol. 2019, 125, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Nagano, M.; Shimizu, K.; Kondo, R.; Hayashi, C.; Sato, D.; Kitagawa, K.; Ohnuki, K. Reduction of depression and anxiety by 4 weeks Hericium erinaceus intake. Biomed. Res. 2010, 31, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- McLennan, H.R.; Degli Esposti, M. The contribution of mitochondrial respiratory complexes to the production of reactive oxygen species. J. Bioenerg. Biomembr. 2000, 32, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Debbasch, C.; Brignole, F.; Pisella, P.J.; Warnet, J.M.; Rat, P.; Baudouin, C. Quaternary ammoniums and other preservatives’ contribution in oxidative stress and apoptosis on Chang conjunctival cells. Investig. Ophthalmol. Vis. Sci. 2001, 42, 642–652. [Google Scholar]
| NADH-UQ Oxidoreductase Complex I µmol Oxidized NADH/min/mg Protein | Succinate-Cytochrome c Oxidoreductase Complex II-III µmol Reduced Cyt c/min/mg Protein | Cytochrome c Oxidase Complex IV µmol Reduced Cyt c/min/mg Protein | ATP Synthase Complex V µmol Oxidized NADH/min/mg Protein | |
|---|---|---|---|---|
| CTR | 0.053 ± 0.0036 | 0.050 ± 0.0013 | 0.1882 ± 0.0152 | 0.0066 ± 0.0008 |
| HE | 0.0452 ± 0.0036 | 0.043 ± 0.0015 | 0.1742 ± 0.0078 | 0.0075 ± 0.00049 |
| DEHP | 0.0182 ± 0.0007*** | 0.026 ± 0.0031*** | 0.0961 ± 0.0173*** | 0.0034 ± 0.00040** |
| DEHP+HE | 0.037 ± 0.0031# | 0.035 ± 0.0037# | 0.1218 ± 0.0067 | 0.0047 ± 0.00032 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amara, I.; Scuto, M.; Zappalà, A.; Ontario, M.L.; Petralia, A.; Abid-Essefi, S.; Maiolino, L.; Signorile, A.; Trovato Salinaro, A.; Calabrese, V. Hericium Erinaceus Prevents DEHP-Induced Mitochondrial Dysfunction and Apoptosis in PC12 Cells. Int. J. Mol. Sci. 2020, 21, 2138. https://doi.org/10.3390/ijms21062138
Amara I, Scuto M, Zappalà A, Ontario ML, Petralia A, Abid-Essefi S, Maiolino L, Signorile A, Trovato Salinaro A, Calabrese V. Hericium Erinaceus Prevents DEHP-Induced Mitochondrial Dysfunction and Apoptosis in PC12 Cells. International Journal of Molecular Sciences. 2020; 21(6):2138. https://doi.org/10.3390/ijms21062138
Chicago/Turabian StyleAmara, Ines, Maria Scuto, Agata Zappalà, Maria Laura Ontario, Antonio Petralia, Salwa Abid-Essefi, Luigi Maiolino, Anna Signorile, Angela Trovato Salinaro, and Vittorio Calabrese. 2020. "Hericium Erinaceus Prevents DEHP-Induced Mitochondrial Dysfunction and Apoptosis in PC12 Cells" International Journal of Molecular Sciences 21, no. 6: 2138. https://doi.org/10.3390/ijms21062138
APA StyleAmara, I., Scuto, M., Zappalà, A., Ontario, M. L., Petralia, A., Abid-Essefi, S., Maiolino, L., Signorile, A., Trovato Salinaro, A., & Calabrese, V. (2020). Hericium Erinaceus Prevents DEHP-Induced Mitochondrial Dysfunction and Apoptosis in PC12 Cells. International Journal of Molecular Sciences, 21(6), 2138. https://doi.org/10.3390/ijms21062138








