Non-Photochemical Quenching Involved in the Regulation of Photosynthesis of Rice Leaves under High Nitrogen Conditions
Abstract
1. Introduction
2. Results
2.1. Chlorophyll Content and Dry Matter Weight
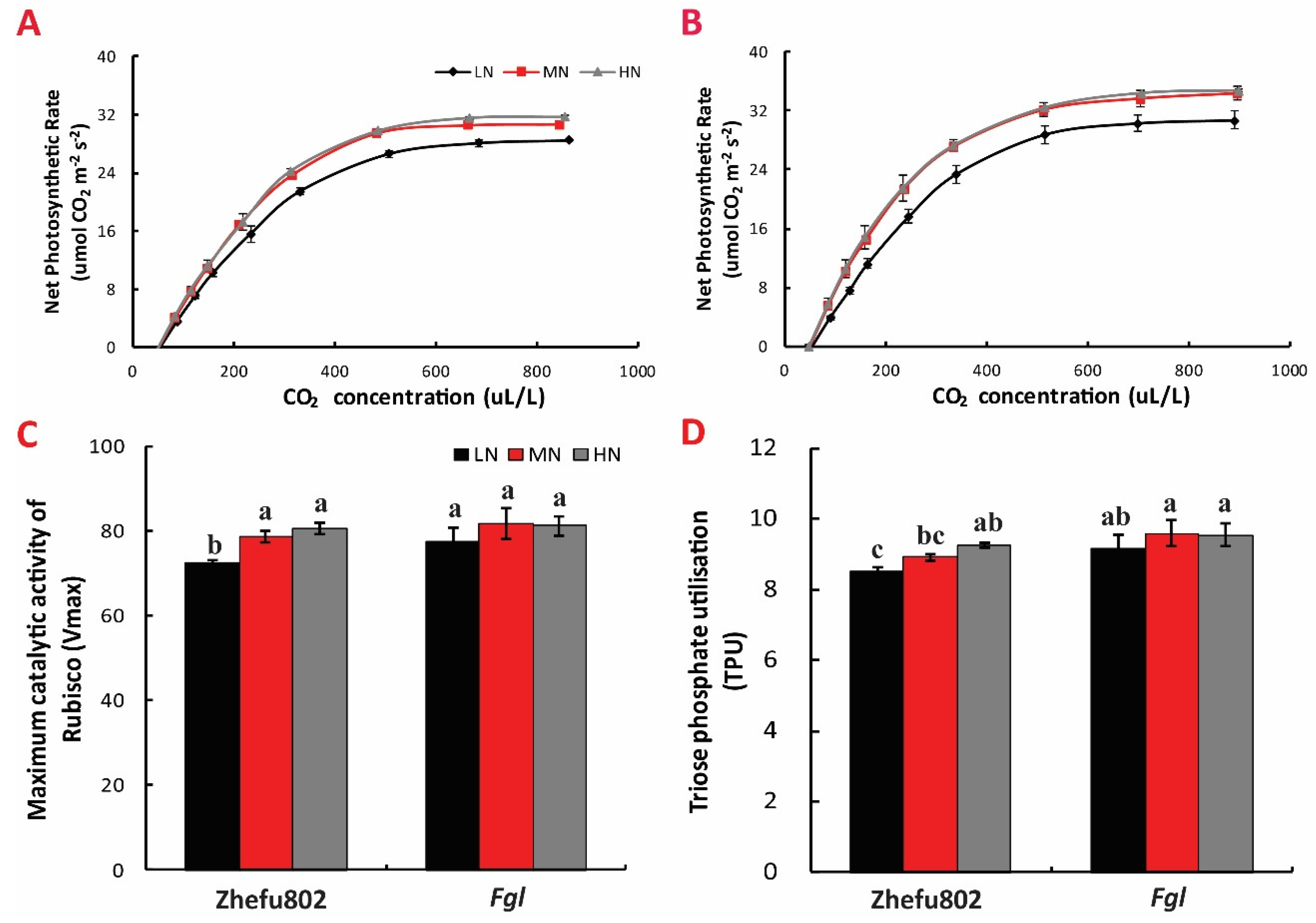
2.2. The A-Ci Curve
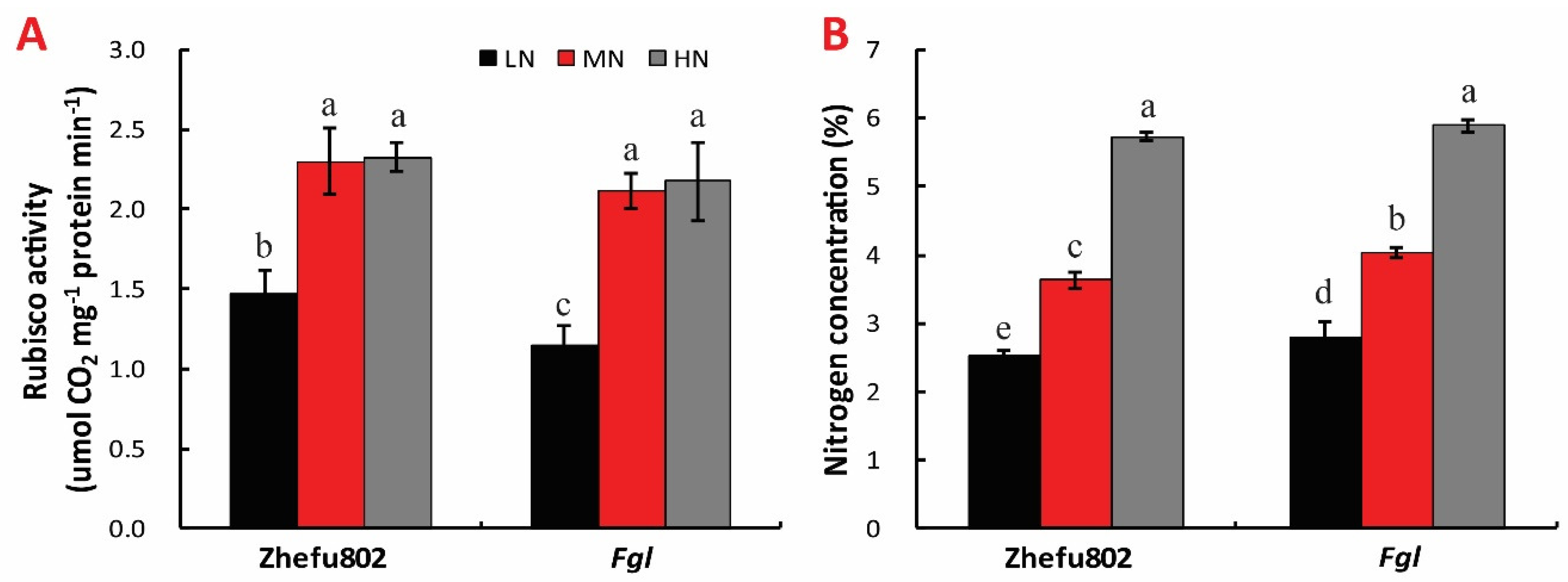
2.3. Rubisco Activity and N Content
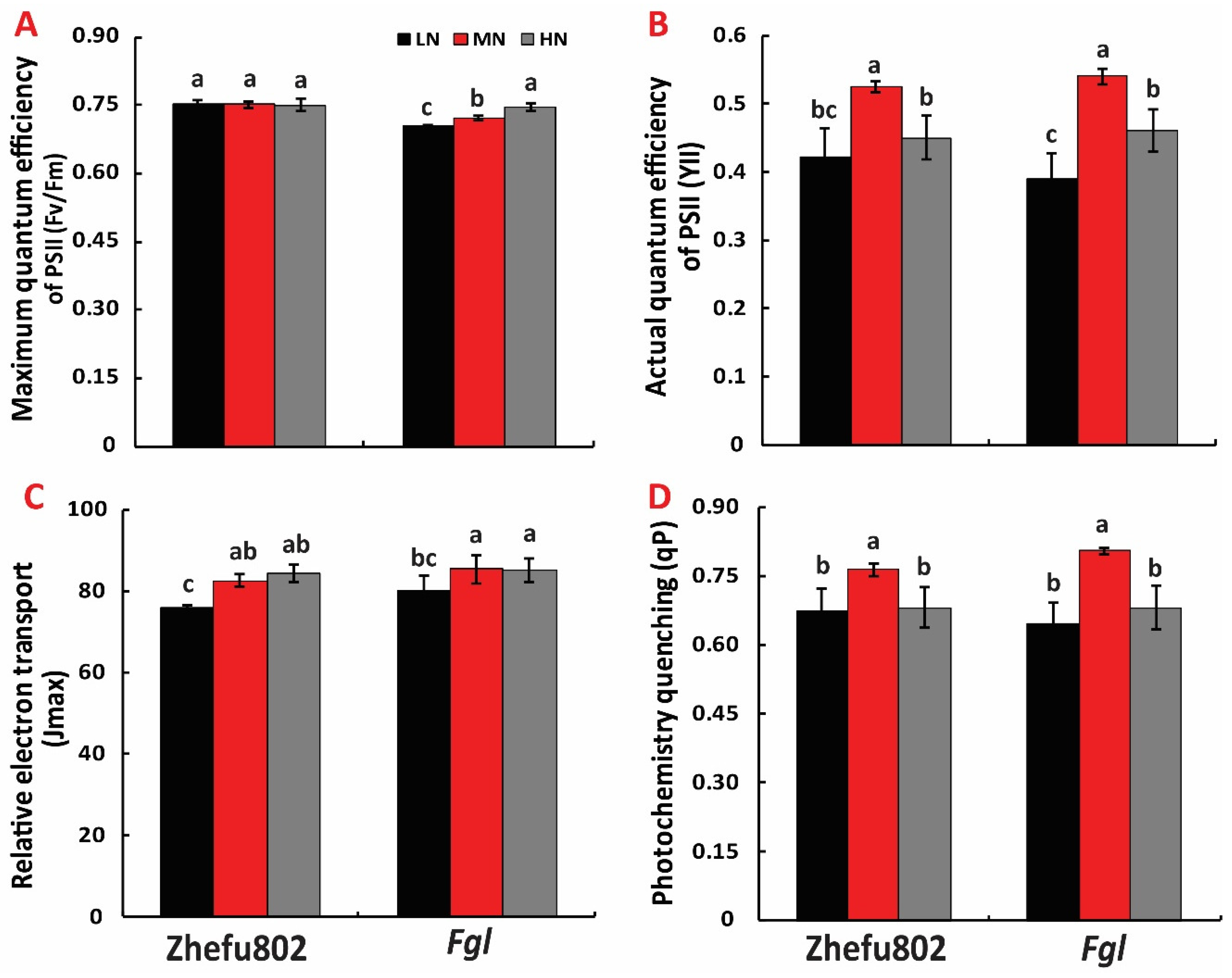
2.4. Chlorophyll Fluorescence
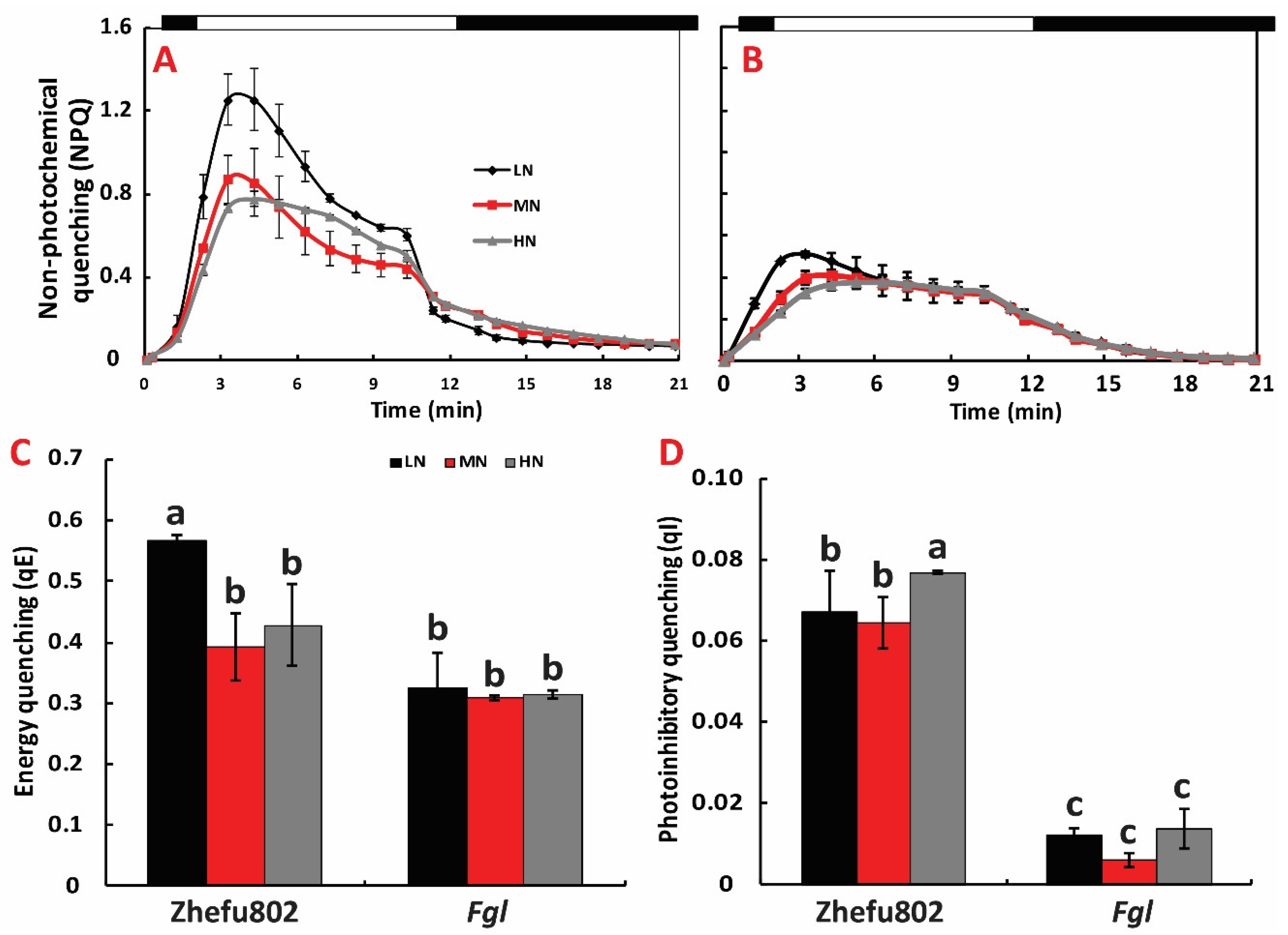
2.5. NPQ Induction and Relaxation Analysis
2.6. H2O2 and MDA Content
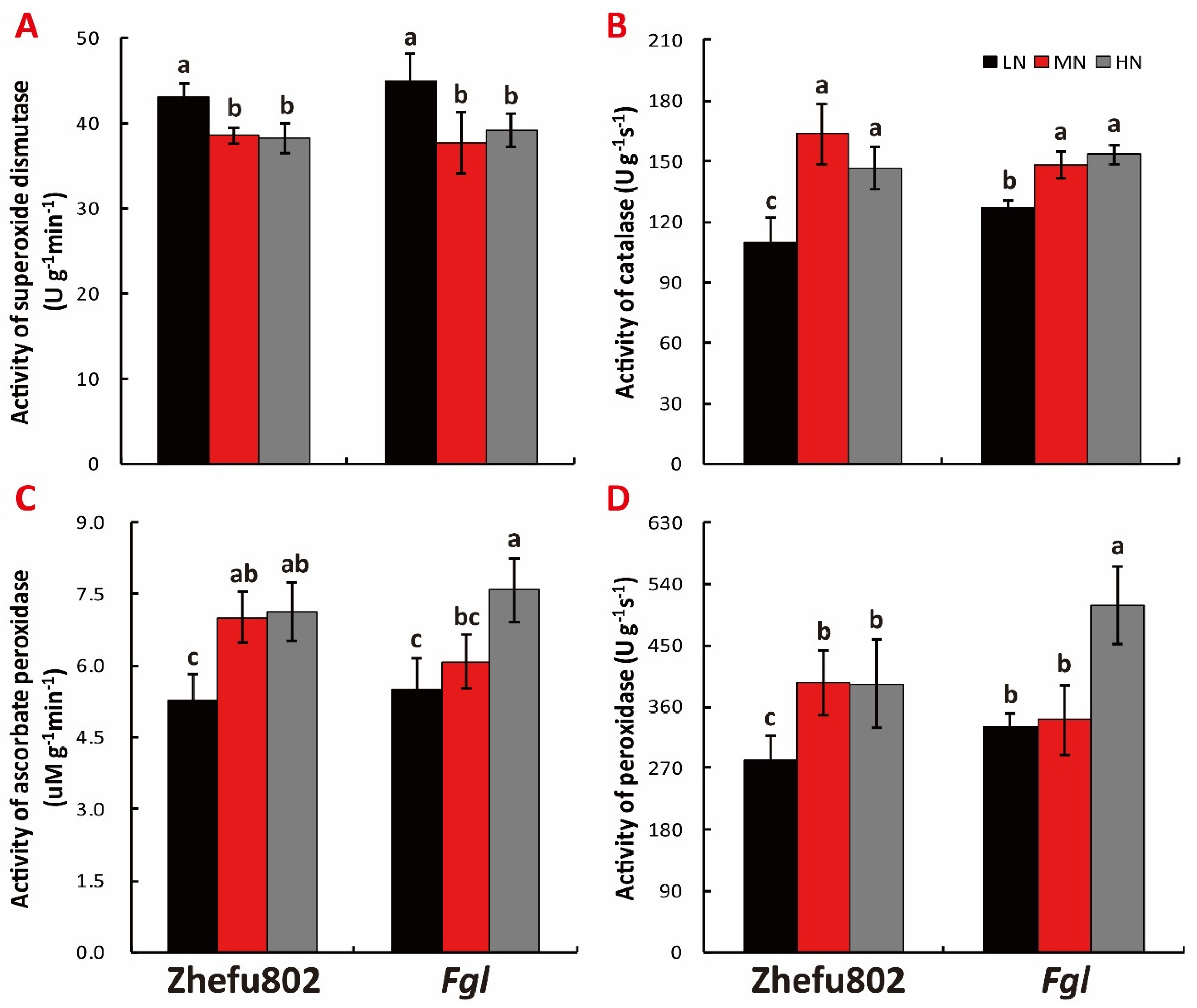
2.7. Antioxidant Activity
2.8. Pigments Involved in the Xanthophyll Cycle
2.9. Structure of Mesophyll Cell
3. Discussion
3.1. High N Supply does not Lead To Ideal Photosynthetic Rate and Raises Likelihood of Photoinhibition
3.2. Mechanism of Acclimation to N Insufficiency
4. Materials and Methods
4.1. Experimental-Set Up
4.2. Biomass Weight
4.3. Rubisco Activity Measurement
4.4. N Content Measurement
4.5. A-Ci Curve Determination
4.6. Chlorophyll Fluorescence Measurement and Gas Exchanges
4.7. Chlorophyll and Xanthophylls Measurement
4.8. Mesophyll Cell Structure Observation
4.9. H2O2 and MDA Content Measurement
4.10. Antioxidant Enzyme Activity Measurement
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Murayama, N. The importance of nitrogen for rice production. In Nitrogen and Rice; International Rice Research Institute: Los Banos, Philippines, 1978; pp. 5–25. [Google Scholar]
- Takahashi, J.; Wada, G.; Shoji, S. The fate of fertilizer nitrogen applied to the paddy field and its absorption by rice plants. VII. Relationships between the absorption of nitrogen by the rice plant and the growth of the plants. Pro. Crop Sci. Soc. Jpn. 1976, 45, 220–225. [Google Scholar] [CrossRef][Green Version]
- Li, H.D.; Gao, H.Y. Effects of different nitrogen application rate on allocation of photosynthetic electron flux in Rumex K-1 leaves. J. Plant Physiol. Mol. Biol. 2007, 33, 417–424. [Google Scholar]
- Makino, A.; Sato, T.; Nakano, H.; Mae, T. Leaf photosynthesis, plant growth and nitrogen allocation in rice under different irradiances. Planta 1997, 203, 390–398. [Google Scholar] [CrossRef]
- Xu, G.H.; Fan, X.R.; Miller, A.J. Plant Nitrogen Assimilation and Use Efficiency. Ann. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef]
- Gu, J.; Yin, X.; Stomph, T.J.; Struik, P.C. Can exploiting natural genetic variation in leaf photosynthesis contribute to increasing rice productivity? A simulation analysis. Plant Cell Environ. 2014, 37, 22–34. [Google Scholar] [CrossRef]
- Li, Z.; Wakao, S.; Fischer, B.B.; Niyogi, K.K. Sensing and responding to excess light. Annu. Rev. Plant Biol. 2009, 60, 239–260. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhao, H.; Jiang, J.; Xu, J.; Xie, W.; Fu, X.; Liu, C.; He, Y.; Wang, G. Genetic architecture of natural variation in rice nonphotochemical quenching capacity revealed by genome-wide association study. Front. Plant Sci. 2017, 8, 1773. [Google Scholar] [CrossRef] [PubMed]
- Long, S.P.; Zhu, X.; Naidu, S.L.; Ort, D.R. Can improvement in photosynthesis increase crop yields? Plant Cell Environ. 2006, 29, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Yamori, W.; Shikanai, T. Physiological functions of cyclic electron transport around photosystem I in sustaining photosynthesis and plant growth. Annu. Rev. Plant Biol. 2016, 67, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Hideg, E.; Kálai, T.; Hideg, K.; Vass, I. Photoinhibition of photosynthesis in vivo results in singlet oxygen production detection via nitroxide-induced fluorescence quenching in broad bean leaves. Biochemistry 1998, 37, 11405–11411. [Google Scholar] [CrossRef] [PubMed]
- Vass, I.; Cser, K. Janus-faced charge recombinations in photosystem II photoinhibition. Trends Plant Sci. 2009, 14, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Krieger-Liszkay, A.; Fufezan, C.; Trebst, A. Singlet oxygen production in photosystem II and related protection mechanism. Photosynth. Res. 2008, 98, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Li, X.; Niyogi, K.K. Non-photochemical quenching: A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Müller-Moulé, P.; Gilmore, A.M.; Niyogi, K.K. PsbS dependent enhancement of feedback de-excitation protects photosystem II from photoinhibition. Proc. Natl. Acad. Sci. USA 2002, 99, 15222–15227. [Google Scholar] [CrossRef] [PubMed]
- Hubbart, S.; Smillie, I.R.A.; Heatley, M.; Swarup, R.; Foo, C.C.; Zhao, L.; Murchie, E.H. Enhanced thylakoid photoprotection can increase yield and canopy radiation use efficiency in rice. Commun. Biol. 2018, 1, 22. [Google Scholar] [CrossRef] [PubMed]
- Meacham, K.; Sirault, X.; Quick, W.P.; Von Caemmerer, S.; Furbank, R. Diurnal solar energy conversion and photoprotection in rice canopies. Plant Physiol. 2017, 173, 495–508. [Google Scholar] [CrossRef]
- Jahns, P.; Holzwarth, A.R. The role of the xanthophyll cycle and of lutein in photoprotection of photo systemII. Biochim. Biophys. Acta 2012, 1817, 182–193. [Google Scholar] [CrossRef]
- Rochaix, J.D. Regulation and dynamics of the light-harvesting system. Annu. Rev. Plant Biol. 2014, 65, 287–309. [Google Scholar] [CrossRef]
- Külheim, C.; Ågren, J.; Jansson, S. Rapid regulation of light harvesting and plant fitness in the field. Science 2002, 297, 91–93. [Google Scholar] [CrossRef]
- Goss, R.; Lepetit, B. Biodiversity of NPQ. J. Plant Physiol. 2015, 172, 13–32. [Google Scholar] [CrossRef]
- Johnson, M.P.; Ruban, A.V. Restoration of rapidly reversible photoprotective energy dissipation in the absence of PsbS protein by enhanced ΔpH. J. Biol. Chem. 2011, 286, 19973–19981. [Google Scholar] [CrossRef] [PubMed]
- Goral, T.K.; Johnson, M.P.; Duffy, C.D.; Brain, A.P.; Ruban, A.V.; Mullineaux, C.W. Light-harvesting antenna composition controls the macrostructure and dynamics of thylakoid membranes in Arabidopsis. Plant J. 2012, 69, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.P.; Goral, T.K.; Duffy, C.D.; Brain, A.P.; Mullineaux, C.W.; Ruban, A.V. Photoprotective energy dissipation involves the reorganization of photosystem II light-harvesting complexes in the grana membranes of spinach chloroplasts. Plant Cell 2011, 23, 1468–1479. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.Z.; Zhou, G.S. Nitrogen metabolism and photosynthesis in Leymus chinensis in response to long-term soil drought. J. Plant Growth Regul. 2006, 25, 252–266. [Google Scholar] [CrossRef]
- Zhong, C.; Cao, X.; Bai, Z.; Zhang, J.; Zhu, L.; Huang, J.; Jin, Q.Y. Nitrogen metabolism correlates with the acclimation of photosynthesis to short-term water stress in rice (Oryza sativa L.). Plant Physiol. Biochem. 2018, 125, 52–62. [Google Scholar] [CrossRef]
- Chen, Y.; Murchie, E.H.; Hubbart, S.; Horton, P.; Peng, S. Effects of season-dependent irradiance levels and nitrogen-deficiency on photosynthesis and photoinhibition in field-grown rice (Oryza sativa L.). Physiol. Plantarum 2003, 117, 343–351. [Google Scholar] [CrossRef]
- Kumagai, E.; Araki, T.; Kubota, F. Characteristics of gas exchange and chlorophyll fluorescence during senescence of flag leaf in different rice (Oryza sativa L.) cultivars grown under nitrogen-deficient condition. Plant Prod. Sci. 2009, 12, 285–292. [Google Scholar] [CrossRef]
- Evans, J.; Poorter, H. Photosynthetic acclimation of plants to growth irradiance: The relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ. 2001, 24, 755–767. [Google Scholar] [CrossRef]
- Huang, Z.A.; Jiang, D.A.; Yang, Y.; Sun, J.W.; Jin, S.H. Effects of nitrogen deficiency on gas exchange, chlorophyll fluorescence, and antioxidant enzymes in leaves of rice plants. Photosynthetica 2004, 42, 357–364. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, Z.J.; Ma, X.D.; Wu, H.; Liu, Y.L.; Zhou, K.N.; Chen, Y.L.; Ma, W.W.; Bi, J.C.; Zhang, X.; et al. Both xanthophyll cycle-dependent thermal dissipation and the antioxidant system are up-regulated in grape (Vitis labrusca L. cv. Concord) leaves in response to N limitation. J. Exp. Bot. 2003, 54, 2165–2175. [Google Scholar] [CrossRef]
- Yin, Y.; Li, S.; Liao, W.; Lu, Q.; Wen, X.; Lu, C. Photosystem II photochemistry, photoinhibition, and the xanthophyll cycle in heat-stressed rice leaves. J. Plant Physiol. 2010, 167, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Morales, F.; Abadía, J.; Abadía, A. Thermal Energy Dissipation in Plants under Unfavorable Soil Conditions. Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria; Springer: Dordrecht, The Netherlands, 2014; Volume 5, pp. 605–630. [Google Scholar]
- Makino, A.; Nakano, H.; Mac, T. Responses of ribulose-1, 5-bisphosphate carboxylase, cytochrome f, and sucrose synthesis enzymes in rice leaves to leaf nitrogen and their relationships to photosynthesis. Plant Physiol. 1994, 105, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Brueck, H.; Asch, F. Chlorophyll index, photochemical reflectance index and chlorophyll fluorescence measurements of rice leaves supplied with different N levels. J. Photoch. Photobiol. B 2012, 113, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Wada, S.; Hayashida, Y.; Izumi, M.; Kurusu, T.; Hanamata, S.; Kanno, K.; Kojima, S.; Yamaya, T.; Kuchitsu, K.; Makino, A. Autophagy supports biomass production and nitrogen use efficiency at the vegetative stage in rice. Plant Physiol. 2015, 168, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Kanemura, T.; Homma, K.; Ohsumi, A.; Shiraiwa, T.; Horie, T. Evaluation of genotypic variation in leaf photosynthetic rate and its associated factors by using rice diversity research set of germplasm. Photosynth. Res. 2007, 94, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Bassi, D.; Menossi, M.; Mattiello, L. Nitrogen supply influences photosynthesis establishment along the sugarcane leaf. Sci. Rep. 2018, 8, 2327. [Google Scholar] [CrossRef] [PubMed]
- Murchie, S.H.; Peng, S.; Horton, P. Acclimation of photosynthesis to high irradiance in rice: Gene expression and interactions with leaf development. J. Exp. Bot. 2005, 56, 449–460. [Google Scholar] [CrossRef]
- Kasajima, I.; Ebana, K.; Yamamoto, T.; Takahara, K.; Yano, M.; Kawai-Yamada, M. Molecular distinction in genetic regulation of nonphotochemical quenching in rice. Proc. Natl. Acad. Sci. USA 2011, 108, 13835–13840. [Google Scholar] [CrossRef]
- Moradi, F.; Ismail, A.M. Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice. Ann. Bot. 2007, 99, 1161–1173. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Chen, L.J.; He, J.L.; Qian, L.S.; Wu, L.Q.; Wang, R.F. Characteristics of chlorophyll fluorescence and antioxidative system in super-hybrid rice and its parental cultivars under chilling stress. Biol. Plantarum 2010, 54, 164–168. [Google Scholar] [CrossRef]
- Zhu, S.Q.; Chen, M.W.; Ji, B.H.; Jiao, D.M. Roles of xanthophylls and exogenous ABA in protection against NaCl-induced photodamage in rice (Oryza sativa L) and cabbage (Brassica campestris). J. Exp. Bot. 2011, 62, 4617–4625. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ren, B.; Ding, L.; Shen, Q.; Peng, S.; Guo, S. Does chloroplast size influence photosynthetic nitrogen use efficiency? PLoS ONE 2013, 8, e62036. [Google Scholar] [CrossRef] [PubMed]
- Brestič, M.; Zivcak, M.; Kunderlikova, K.; Sytar, O.; Shao, H.; Kalaji, H.M.; Allakhverdiev, S.I. Low PSI content limits the photoprotection of PSI and PSII in early growth stages of chlorophyll b-deficient wheat mutant lines. Photosynth. Res. 2015, 125, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Zivcak, M.; Brestič, M.; Kunderlikova, K.; Sytar, O.; Allakhverdiev, S.I. Repetitive light pulse-induced photoinhibition of photosystem I severely affects CO2 assimilation and photoprotection in wheat leaves. Photosynth. Res. 2015, 126, 449–463. [Google Scholar] [CrossRef]
- Sun, J.; Ye, M.; Peng, S.; Li, Y. Nitrogen can improve the rapid response of photosynthesis to changing irradiance in rice (Oryza sativa L.) plants. Sci. Rep. 2016, 6, 31305. [Google Scholar] [CrossRef]
- Brestič, M.; Cornic, G.; Baker, F.N.R. Does photorespiration protect the photosynthetic apparatus in French bean leaves from photoinhibition during drought stress? Planta 1995, 196, 450–457. [Google Scholar] [CrossRef]
- Faseela, P.; Sinisha, A.K.; Brestič, M.; Puthur, J.T. Chlorophyll a fluorescence parameters as indicators of a particular abiotic stress in rice. Photosynthetica 2019, 57, 108–115. [Google Scholar]
- Xiong, D.; Liu, X.; Liu, L.M.; Douthe, C.; Li, Y.; Peng, S.; Huang, J. Rapid responses of mesophyll conductance to changes of CO2 concentration, temperature and irradiance are affected by N supplements in rice. Plant Cell Environ. 2015, 38, 2541–2550. [Google Scholar] [CrossRef]
- Yoshida, S.; Forno, D.; Cock, J.; Gomez, K. Laboratory Manual for Physiological Studies of Rice; International Rice Research Institute: Manila, Philippines, 1976. [Google Scholar]
- Lu, R. Soil Chemical Analysis Method for Agriculture; China Agriculture Science and Technique Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Sharkey, T.D.; Bernacchi, C.J.; Farquhar, G.D.; Singsaas, E.L. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ. 2007, 30, 1035–1040. [Google Scholar] [CrossRef]
- Ishida, S.; Morita, K.I.; KJshine, M.; Takabayashi, A.; Murakami, R.; Takeda, S. Allocation of absorbed light energy in PSII to thermal dissipations in the presence or absence of PsbS subunits of rice. Plant Cell Physiol. 2011, 52, 1822–1831. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence-a practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Krause, H.G.; Jahns, P. Pulse amplitude modulated chlorophyll fluorometry and its application in plant science. In Light-Harvesting Antennas in Photosynthesis; Springer: Dordrecht, The Netherland, 2003; pp. 373–399. [Google Scholar]
- Havaux, M.; Eymery, F.; Porfirova, S.; Rey, P.; Dörmarm, P. Vitamin E protects against photoinhibition and photooxidative stress in Arabidopsis thaliana. Plant Cell 2006, 17, 3451–3469. [Google Scholar] [CrossRef] [PubMed]
- Sartory, D.P.; Grobbelaar, J.U. Extraction of chlorophyll a from freshwater phytoplankton for spectrophotometric analysis. Hydrobiologia 1984, 114, 177–187. [Google Scholar] [CrossRef]
- Brennan, T.; Frenkel, C. Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol. 1977, 59, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases I. occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Maehly, A.; Chance, B. Catalases and peroxidases. Method Biochem. Annu. 1954, 1, 357–424. [Google Scholar]
- Aebi, H. Methods of Enzymatic Analysis; Academic Press: New York, NY, USA, 1983; pp. 273–288. [Google Scholar]
- Bonnecarrère, V.; Borsani, O.; Díaz, P.; Capdevielle, F.; Blanco, P.; Monza, J. Response to photoxidative stress induced by cold in japonica rice is genotype dependent. Plant Sci. 2011, 180, 726–732. [Google Scholar] [CrossRef]



| Variety | Zhefu802 | Fgl | ||||
|---|---|---|---|---|---|---|
| Treatment | LN | MN | HN | LN | MN | HN |
| V (μmol/mmol Chl) | 47.05c | 42.71d | 41.42e | 57.36a | 53.55b | 52.93b |
| A (μmol/mmol Chl) | 30.40c | 23.73d | 16.64e | 40.13a | 35.32b | 29.42c |
| Z (μmol/mmol Chl) | 158.23a | 141.39b | 135.17c | 142.39b | 130.48d | 126.57e |
| β-Carotene (μmol/mmol Chl) | 438.21c | 431.95cd | 420.40d | 645.83a | 582.93b | 596.31b |
| Z + 1/2A | 173.44a | 153.26c | 143.49e | 162.46b | 148.14d | 141.28e |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cisse, A.; Zhao, X.; Fu, W.; Kim, R.E.R.; Chen, T.; Tao, L.; Feng, B. Non-Photochemical Quenching Involved in the Regulation of Photosynthesis of Rice Leaves under High Nitrogen Conditions. Int. J. Mol. Sci. 2020, 21, 2115. https://doi.org/10.3390/ijms21062115
Cisse A, Zhao X, Fu W, Kim RER, Chen T, Tao L, Feng B. Non-Photochemical Quenching Involved in the Regulation of Photosynthesis of Rice Leaves under High Nitrogen Conditions. International Journal of Molecular Sciences. 2020; 21(6):2115. https://doi.org/10.3390/ijms21062115
Chicago/Turabian StyleCisse, Amara, Xia Zhao, Weimeng Fu, Romesh Eric Romy Kim, Tingting Chen, Longxing Tao, and Baohua Feng. 2020. "Non-Photochemical Quenching Involved in the Regulation of Photosynthesis of Rice Leaves under High Nitrogen Conditions" International Journal of Molecular Sciences 21, no. 6: 2115. https://doi.org/10.3390/ijms21062115
APA StyleCisse, A., Zhao, X., Fu, W., Kim, R. E. R., Chen, T., Tao, L., & Feng, B. (2020). Non-Photochemical Quenching Involved in the Regulation of Photosynthesis of Rice Leaves under High Nitrogen Conditions. International Journal of Molecular Sciences, 21(6), 2115. https://doi.org/10.3390/ijms21062115






