Curcumin in Health and Diseases: Alzheimer’s Disease and Curcumin Analogues, Derivatives, and Hybrids
Abstract
1. Introduction
2. Curcumin Analogues and Derivatives for AD Therapy
2.1. Inhibition of (Aβ)Amyloid-β Aggregation
2.1.1. Mixed Curcuminoids and Amyloid-β Aggregation
2.1.2. Interaction of Curcumin Analogues and Derivatives with Aβ-Fibrils
2.2. Inhibition of Tau Aggregation
2.3. Anti-Neuroinflammatory Activity
2.4. Antioxidant Activity
2.4.1. Mitochondrial Dysfunction and Apoptosis
2.4.2. Metal Accumulation and Metal Ion Dyshomeostasis
2.5. Inhibition of AChE
3. Curcumin Hybrids for AD Therapy
4. Curcumin-Based Imaging Probes for Alzheimer’s Therapy and Diagnosis
4.1. Imaging Probes for Amyloid-β Plaques Detection
4.2. Imaging Probes for Tau Tangles Detection
5. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Aβplaque | β-amyloid plaques or senile plaques |
| AβPP | Amyloid-β precursor protein |
| Ach | Acetylcholine |
| AChE | Acetylcholinesterase |
| AChEI | Acetylcholinesterase inhibitors |
| AD | Alzheimer’s Disease |
| ADAM10 | A disintegrin and metalloproteinase domain-containing protein 10 |
| ADAM17 | A disintegrin and metalloproteinasedomain-containing protein 17 |
| (ADME)T | Absorption, distribution, metabolism, excretion, and toxicity prediction |
| AKT | Protein kinase B (PKB) |
| ApoE | Apolipoprotein E |
| APP IREs | APP internal ribosome entry site |
| ATF6 | Activating transcription factor 6 |
| ATP | Adenosine triphosphate |
| ATP5A | Allosteric α subunit of mATP synthase |
| BACE-1 | β-secretase or beta-site amyloid precursor protein cleaving enzyme 1 |
| Bax | Bcl-2-like protein 4 |
| BBB | Blood–brainbarrier |
| BchE | Butrylcholinesterase |
| BcL-XL | B-cell lymphoma-extra large |
| Bcl-2 | B-cell lymphoma 2 |
| BDE | O-H proton dissociation enthalpy |
| BMAC | Boronatedmonocarbonyl analogues of curcumin |
| BNCT | Boron neutron capture therapy |
| Brain P53 | Tumor proteinp53 |
| CAAs | Cerebral amyloid angiopathies |
| Caspases | Cysteine-aspartic proteases |
| CAT | Catalase |
| CCCCV | Test set using LOO cross validation |
| CCCtr | The concordance correlationcoefficient of the training set |
| CHOP | C/EBP homologous protein |
| CNS | Central nervous system |
| CoMFA | Comparative molecular field analysis |
| CoMSIA | Comparative molecular similarity indices analysis |
| Cyt-c | Cytochrome complex |
| DART TOMFS | Direct analysis in real-time mass spectrometry |
| DAVID | Database for Annotation, Visualization and Integrated Discovery NIAID, NIH |
| DFT | Density functional theory |
| DIPEA | Diisopropylethylamine salt |
| DNA | Deoxyribonucleic acid |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl radical scavenging |
| DTPA | Diethylenetriaminepentaacetic acid |
| EA | Calculated electronic affinity |
| elF2a | Eukaryotic translation initiation factor 2 alpha |
| ER | Endoplasmic reticulum |
| ERK1/2 | Extracellular signal-regulated kinase |
| [18F]FDG | Fluorodeoxyglucose(18F) |
| F | Fisher ratio |
| F01[C-N] | Frequency of C-N at topological distance of 01, a 2D frequency fingerprints descriptor |
| FRSA | Free radical scavenging activities |
| GA-MLR | Genetic algorithme multilinear regression |
| GFP | pCMV6-htau40-green fluorescent protein |
| GIAO | Gauge-Independent Atomic Orbital |
| Glu339 | Glutamate 339 |
| Gly230 | Glycine 230 |
| GRP78 | Glucose-regulated protein 78 |
| GSH | Glutathione |
| GSK3β | Glycogen synthase kinase 3 beta |
| HEWL | Hen egg-white lysozyme |
| HQSAR | Hologram quantitative structure–activity relationship |
| HO-1 | Heme oxygenase 1 |
| i.c.v. | Intracerebroventricular |
| IL-6 | Interleukin 6 |
| IL-1β | Interleukin 1β |
| iNOS | Inducible NO synthase |
| IP | Ionization potential |
| IRE1 | Inositol-requiring enzyme 1 |
| Keap1 | Kelch-like ECH-associated protein 1 |
| KLVFFA peptide | Aβ-binding motif |
| LPS | Lipopolysaccharide |
| mATP synthase | Mitochondrial adenosine triphosphate synthase |
| MCI | Mild cognitive impairment |
| MD | Molecular dynamic |
| MDEC-44 | Molecular distance edge between all quaternary carbons |
| MMPs | Matrix metallopeptidases |
| MRI | Magnetic resonance imaging |
| mRNA | Messenger RNA |
| MTDLs | Multitarget-directed Ligand |
| mTOR | Mammalian target of rapamycin |
| NEP | Neprilysin |
| Nex and Ntr | The numbers of samples in the training set and external set |
| NFTs | Neurofibrillary tangles |
| NIR(F) | Near infrared imaging (fluorescent) |
| NLs | Nanoliposomes |
| NMR | Nuclear magnetic resonance |
| 2D NMR | Two-dimensional nuclear magnetic resonance |
| NO | Nitric oxide |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NQO1 | NAD(P)H:quinone acceptor oxidoreductase 1 |
| OGD/R | Oxygen–glucose deprivation and re-oxygenation |
| ORAC-FL | Oxygen radical absorbance capacity assay using fluorescein |
| PEG | Polyethylene glycol |
| PERK | Protein kinase RNA like endoplasmic reticulum kinase |
| PET | Positron emission tomography |
| PI3K | Phosphoinositide 3-kinase |
| PKC | Protein kinase C |
| Presenilin | γ-secretase |
| PSD95 | Postsynaptic density protein 95 |
| PS1 | Presenilin-1 |
| PS2 | Presenilin-2 |
| p38 MAPK | p38 mitogen-activated protein kinase |
| QSAR | Quantitative structure–activity relationship |
| Q2 | Cross-validated R2 |
| Q2LOO | The square correlation coefficient for leave-one-out cross-validation |
| RDF090m | Radial distribution function 9.0/weighted by atomic masses, RDF descriptors |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| R2 | The square of correlation coefficient |
| R2adj | The adjusted determination coefficient |
| R2ex | Coefficient of determination |
| R2ncv | Non cross-validated correlation coefficient |
| R2pred | Predictive correlation coefficient |
| SAMP8 | Senescence-accelerated mouse prone 8 |
| sAPPα | Soluble amyloid precursor protein α |
| SAR | Structure–activity relationship |
| SEE | Standard errors of estimate |
| Skn-1 | The nematode ortholog of Nrf2 |
| SMD | Steered molecular dynamics |
| SOD | Superoxide dismutase |
| SOM | Site of metabolism |
| TBI | Traumatic brain injury |
| TC | Tetracycline |
| TERT | Telomerase reverse transcriptase |
| ThT assay | Thioflavin T fluorescence assay |
| TNF-α | Tumor necrosis factor –α |
| Trp63 | Tryptophan 63 |
| Tyr198 | Tyrosine 198 |
| WHIM | Weighted holistic invariant molecular |
| WK.eneg | Non-directional weighted holistic invariant molecular |
| XBP1 | X-box-binding protein-1 |
| 5-LOX | 5-lipoxygenase |
| 8-OHG | 8-hydroxyguanosine |
References
- Attitudes to dementia-World Alzheimer Report 2019. Available online: https://www.alz.co.uk/research/WorldAlzheimerReport2019.pdf (accessed on 26 January 2020).
- Potter, P.E. Curcumin: A natural substance with potential efficacy in Alzheimer’s disease. J. Exp. Pharmacol. 2013, 5, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Kandel, E.R.; Schwartz, J.H.; Jessell, T.M.; Siegelbaum, S.A.; Hudspeth, A.J. Principles of Neural Science, 5th ed.; McGraw-Hill Education: New York, NY, USA, 2013. [Google Scholar]
- Song, J.-X.; Malampati, S.; Zeng, Y.; Durairajan, S.S.K.; Yang, C.-B.; Tong, B.C.-K.; Iyaswamy, A.; Shang, W.-B.; Sreenivasmurthy, S.G.; Zhu, Z.; et al. A small molecule transcription factor EB activator ameliorates beta-amyloid precursor protein and Tau pathology in Alzheimer’s disease models. Aging Cell 2020, 19, e13069. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.; Thakur, A.; Sharma, A.; Flora, S.J.S. Natural products and their derivatives as multifunctional ligands against Alzheimer’s disease. Drug Dev. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Pinkaew, D.; Changtam, C.; Tocharus, C.; Thummayot, S.; Suksamrarn, A.; Tocharus, J. Di-O-demethylcurcumin protects SK-N-SH cells against mitochondrial and endoplasmic reticulum-mediated apoptotic cell death induced by Aβ25-35. Neurochem. Int. 2015, 80, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Lakey-Beitia, J.; González, Y.; Doens, D.; Stephens, D.E.; Santamaría, R.; Murillo, E.; Gutiérrez, M.; Fernández, P.L.; Rao, K.S.; Larionov, O.V.; et al. Assessment of Novel Curcumin Derivatives as Potent Inhibitors of Inflammation and Amyloid-β Aggregation in Alzheimer’s Disease. J. Alzheimers Dis. 2017, 60, S59–S68. [Google Scholar] [CrossRef] [PubMed]
- Singulani, M.P.; Pereira, C.P.M.; Ferreira, A.F.F.; Garcia, P.C.; Ferrari, G.D.; Alberici, L.C.; Britto, L.R. Impairment of PGC-1α-mediated mitochondrial biogenesis precedes mitochondrial dysfunction and Alzheimer’s pathology in the 3xTg mouse model of Alzheimer’s disease. Exp. Gerontol. 2020, 133, 110882. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Chun-Li, X.; Jia-Heng, T.; Ding, L.; Tian-Miao, O.; Shi-Liang, H.; Lian-Quan, G.; Zhi-Shu, H. Syntheses and Evaluation of Asymmetric Curcumin Analogues as Potential Multifunctional Agents for the Treatment of Alzheimer’s Disease. Curr. Alzheimer Res. 2015, 12, 403–414. [Google Scholar] [CrossRef]
- Rosini, M.; Simoni, E.; Milelli, A.; Minarini, A.; Melchiorre, C. Oxidative Stress in Alzheimer’s Disease: Are We Connecting the Dots? J. Med. Chem. 2014, 57, 2821–2831. [Google Scholar] [CrossRef]
- Hareram, B.; Tarun, M.; Gaurav, K.; Anamika, M.; Sandeep Kumar, S. Role of Oxidative Stress and Metal Toxicity in The Progression of Alzheimer’s Disease. Curr. Neuropharmacol. 2020, 18. [Google Scholar] [CrossRef]
- Hulya, A.; İlhami, G. Potent Acetylcholinesterase Inhibitors: Potential Drugs for Alzheimer’s Disease. Mini-Rev. Med. Chem. 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Mesiti, F.; Chavarria, D.; Gaspar, A.; Alcaro, S.; Borges, F. The chemistry toolbox of multitarget-directed ligands for Alzheimer’s disease. Eur. J. Med. Chem. 2019, 181, 111572. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Liang, M.; Li, Q.; Zheng, X.; Zhang, C.; Wang, Q.; Tang, L.; Zhang, Z.; Wang, B.; Shen, Z. Development of the “hidden” multifunctional agents for Alzheimer’s disease. Eur. J. Med. Chem. 2019, 177, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, D.K.; Mishra, P.K. Curcumin and its analogues: Potential anticancer agents. Med. Res. Rev. 2010, 30, 818–860. [Google Scholar] [CrossRef]
- Fang, L.; Gou, S.; Liu, X.; Cao, F.; Cheng, L. Design, synthesis and anti-Alzheimer properties of dimethylaminomethyl-substituted curcumin derivatives. Bioorgan. Med. Chem. Lett. 2014, 24, 40–43. [Google Scholar] [CrossRef]
- Venigalla, M.; Sonego, S.; Gyengesi, E.; Sharman, M.J.; Münch, G. Novel promising therapeutics against chronic neuroinflammation and neurodegeneration in Alzheimer’s disease. Neurochem. Int. 2016, 95, 63–74. [Google Scholar] [CrossRef]
- Bisceglia, F.; Seghetti, F.; Serra, M.; Zusso, M.; Gervasoni, S.; Verga, L.; Vistoli, G.; Lanni, C.; Catanzaro, M.; De Lorenzi, E.; et al. Prenylated Curcumin Analogues as Multipotent Tools To Tackle Alzheimer’s Disease. Acs Chem. Neurosci. 2019, 10, 1420–1433. [Google Scholar] [CrossRef]
- Okuda, M.; Fujita, Y.; Hijikuro, I.; Wada, M.; Uemura, T.; Kobayashi, Y.; Waku, T.; Tanaka, N.; Nishimoto, T.; Izumi, Y.; et al. PE859, A Novel Curcumin Derivative, Inhibits Amyloid-β and Tau Aggregation, and Ameliorates Cognitive Dysfunction in Senescence-Accelerated Mouse Prone 8. J. Alzheimers Dis. 2017, 59, 313–328. [Google Scholar] [CrossRef]
- Khanna, S.; Park, H.-A.; Sen, C.K.; Golakoti, T.; Sengupta, K.; Venkateswarlu, S.; Roy, S. Neuroprotective and antiinflammatory properties of a novel demethylated curcuminoid. Antioxid Redox Signal 2009, 11, 449–468. [Google Scholar] [CrossRef]
- Chainoglou, E.; Hadjipavlou-Litina, D. Curcumin analogues and derivatives with anti-proliferative and anti-inflammatory activity: Structural characteristics and molecular targets. Expert Opin. Drug Discov. 2019, 14, 821–842. [Google Scholar] [CrossRef]
- Akaishi, T.; Abe, K. CNB-001, a synthetic pyrazole derivative of curcumin, suppresses lipopolysaccharide-induced nitric oxide production through the inhibition of NF-κB and p38 MAPK pathways in microglia. Eur. J. Pharmacol. 2018, 819, 190–197. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Gazioğlu, I.; Erim, F.B. Comparison of antioxidant, anticholinesterase, and antidiabetic activities of three curcuminoids isolated from Curcuma longa L. Nat. Prod. Res. 2017, 31, 2914–2917. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Hu, J.; Liu, A.; He, L.; Li, X.; Wei, H. Design, synthesis, and evaluation of multitarget-directed ligands against Alzheimer’s disease based on the fusion of donepezil and curcumin. Bioorgan. Med. Chem. 2017, 25, 2946–2955. [Google Scholar] [CrossRef] [PubMed]
- Konno, H.; Endo, H.; Ise, S.; Miyazaki, K.; Aoki, H.; Sanjoh, A.; Kobayashi, K.; Hattori, Y.; Akaji, K. Synthesis and evaluation of curcumin derivatives toward an inhibitor of beta-site amyloid precursor protein cleaving enzyme 1. Bioorgan. Med. Chem. Lett. 2014, 24, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, R.M.C.; De Simone, A.; Andrisano, V.; Bisignano, P.; Bisi, A.; Gobbi, S.; Rampa, A.; Fato, R.; Bergamini, C.; Perez, D.I.; et al. Versatility of the Curcumin Scaffold: Discovery of Potent and Balanced Dual BACE-1 and GSK-3β Inhibitors. J. Med. Chem. 2016, 59, 531–544. [Google Scholar] [CrossRef]
- Ferrari, E.; Benassi, R.; Sacchi, S.; Pignedoli, F.; Asti, M.; Saladini, M. Curcumin derivatives as metal-chelating agents with potential multifunctional activity for pharmaceutical applications. J. Inorg. Biochem. 2014, 139, 38–48. [Google Scholar] [CrossRef]
- Yanagisawa, D.; Shirai, N.; Amatsubo, T.; Taguchi, H.; Hirao, K.; Urushitani, M.; Morikawa, S.; Inubushi, T.; Kato, M.; Kato, F.; et al. Relationship between the tautomeric structures of curcumin derivatives and their Aβ-binding activities in the context of therapies for Alzheimer’s disease. Biomaterials 2010, 31, 4179–4185. [Google Scholar] [CrossRef]
- Akiko, K.; Hyuck Jin, L.; Sashiprabha, M.V.; Vediappen, P.; Matthew, J.A.; Mi Hee, L. Inhibitory Activity of Curcumin Derivatives Towards Metal-Free and Metal-Induced Amyloid-β aggregation. Curr. Alzheimer Res. 2015, 12, 415–423. [Google Scholar] [CrossRef]
- Yanagisawa, D.; Taguchi, H.; Morikawa, S.; Kato, T.; Hirao, K.; Shirai, N.; Tooyama, I. Novel curcumin derivatives as potent inhibitors of amyloid β aggregation. Biochem. Biophys. Rep. 2015, 4, 357–368. [Google Scholar] [CrossRef]
- Amit Chaudhary, P.K.M.; Yadav, B.S.; Singh, S.; Mani, A. Current Therapeutic Targets for Alzheimer’s Disease. J. Biomed. 2018, 3, 74–84. [Google Scholar] [CrossRef]
- Orteca, G.; Tavanti, F.; Bednarikova, Z.; Gazova, Z.; Rigillo, G.; Imbriano, C.; Basile, V.; Asti, M.; Rigamonti, L.; Saladini, M.; et al. Curcumin derivatives and Aβ-fibrillar aggregates: An interactions’ study for diagnostic/therapeutic purposes in neurodegenerative diseases. Bioorgan. Med. Chem. 2018, 26, 4288–4300. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, Y.; Zhang, C.; Tian, X.; Ross, A.W.; Moir, R.D.; Sun, H.; Tanzi, R.E.; Moore, A.; Ran, C. Near-infrared fluorescence molecular imaging of amyloid beta species and monitoring therapy in animal models of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2015, 112, 9734–9739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tian, Y.; Li, Z.; Tian, X.; Sun, H.; Liu, H.; Moore, A.; Ran, C. Design and synthesis of curcumin analogues for in vivo fluorescence imaging and inhibiting copper-induced cross-linking of amyloid beta species in Alzheimer’s disease. J. Am. Chem. Soc. 2013, 135, 16397–16409. [Google Scholar] [CrossRef] [PubMed]
- Narasingapa, R.B.; Jargaval, M.R.; Pullabhatla, S.; Htoo, H.H.; Rao, J.K.S.; Hernandez, J.-F.; Govitrapong, P.; Vincent, B. Activation of α-secretase by curcumin-aminoacid conjugates. Biochem. Biophys. Res. Commun. 2012, 424, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Otto, G.P.; Sharma, D.; Williams, R.S.B. Non-Catalytic Roles of Presenilin throughout Evolution. J Alzheimers Dis 2016, 52, 1177–1187. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z.; Qiu, D.; Gu, Q.; Lei, Q.; Mao, L. The inhibitory effects of different curcuminoids on β-amyloid protein, β-amyloid precursor protein and β-site amyloid precursor protein cleaving enzyme 1 in swAPP HEK293 cells. Neurosci. Lett. 2010, 485, 83–88. [Google Scholar] [CrossRef]
- Cui, L.; Wang, S.; Zhang, J.; Wang, M.; Gao, Y.; Bai, L.; Zhang, H.; Ma, G.; Ba, X. Effect of curcumin derivatives on hen egg white lysozyme amyloid fibrillation and their interaction study by spectroscopic methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 223, 117365. [Google Scholar] [CrossRef]
- Hotsumi, M.; Tajiri, M.; Nikaido, Y.; Sato, T.; Makabe, K.; Konno, H. Design, synthesis, and evaluation of a water soluble C5-monoketone type curcumin analogue as a potent amyloid β aggregation inhibitor. Bioorgan. Med. Chem. Lett. 2019, 29, 2157–2161. [Google Scholar] [CrossRef]
- Ferrari, E.; Benassi, R.; Saladini, M.; Orteca, G.; Gazova, Z.; Siposova, K. In vitro study on potential pharmacological activity of curcumin analogues and their copper complexes. Chem. Biol. Drug Des. 2017, 89, 411–419. [Google Scholar] [CrossRef]
- Okuda, M.; Hijikuro, I.; Fujita, Y.; Teruya, T.; Kawakami, H.; Takahashi, T.; Sugimoto, H. Design and synthesis of curcumin derivatives as tau and amyloid β dual aggregation inhibitors. Bioorgan. Med. Chem. Lett. 2016, 26, 5024–5028. [Google Scholar] [CrossRef]
- Chen, Q.; Prior, M.; Dargusch, R.; Roberts, A.; Riek, R.; Eichmann, C.; Chiruta, C.; Akaishi, T.; Abe, K.; Maher, P.; et al. A novel neurotrophic drug for cognitive enhancement and Alzheimer’s disease. PLoS ONE 2011, 6, e27865. [Google Scholar] [CrossRef]
- Clarkson, G.J.; Farran, M.Á.; Claramunt, R.M.; Alkorta, I.; Elguero, J. The structure of the anti-aging agent J147 used for treating Alzheimer’s disease. Acta Cryst. 2019, 75, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, D.; Ibrahim, N.F.; Taguchi, H.; Morikawa, S.; Hirao, K.; Shirai, N.; Sogabe, T.; Tooyama, I. Curcumin derivative with the substitution at C-4 position, but not curcumin, is effective against amyloid pathology in APP/PS1 mice. Neurobiol. Aging 2015, 36, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Villaflores, O.B.; Chen, Y.-J.; Chen, C.-P.; Yeh, J.-M.; Wu, T.-Y. Effects of curcumin and demethoxycurcumin on amyloid-β precursor and tau proteins through the internal ribosome entry sites: A potential therapeutic for Alzheimer’s disease. Taiwan J. Obstet. Gynecol. 2012, 51, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Orlando, R.A.; Gonzales, A.M.; Royer, R.E.; Deck, L.M.; Vander Jagt, D.L. A chemical analog of curcumin as an improved inhibitor of amyloid Abeta oligomerization. PLoS ONE 2012, 7, e31869. [Google Scholar] [CrossRef]
- Dolai, S.; Shi, W.; Corbo, C.; Sun, C.; Averick, S.; Obeysekera, D.; Farid, M.; Alonso, A.; Banerjee, P.; Raja, K. “Clicked” Sugar–Curcumin Conjugate: Modulator of Amyloid-β and Tau Peptide Aggregation at Ultralow Concentrations. ACS Chem. Neurosci. 2011, 2, 694–699. [Google Scholar] [CrossRef]
- Ramshini, H.; Mohammad-zadeh, M.; Ebrahim-Habibi, A. Inhibition of amyloid fibril formation and cytotoxicity by a chemical analog of Curcumin as a stable inhibitor. Int. J. Biol. Macromol. 2015, 78, 396–404. [Google Scholar] [CrossRef]
- Azzi, E.; Alberti, D.; Parisotto, S.; Oppedisano, A.; Protti, N.; Altieri, S.; Geninatti-Crich, S.; Deagostino, A. Design, synthesis and preliminary in-vitro studies of novel boronated monocarbonyl analogues of Curcumin (BMAC) for antitumor and β-amiloyd disaggregation activity. Bioorgan. Chem. 2019, 93, 103324. [Google Scholar] [CrossRef]
- Mohammadi, F.; Mahmudian, A.; Moeeni, M.; Hassani, L. Inhibition of amyloid fibrillation of hen egg-white lysozyme by the natural and synthetic curcuminoids. RSC Adv. 2016, 6, 23148–23160. [Google Scholar] [CrossRef]
- Mohammadi, F.; Moeeni, M.; Mahmudian, A.; Hassani, L. Inhibition of amyloid fibrillation of lysozyme by bisdemethoxycurcumin and diacetylbisdemethoxycurcumin. Biophys. Chem. 2018, 235, 56–65. [Google Scholar] [CrossRef]
- Wang, S.; Peng, X.; Cui, L.; Li, T.; Yu, B.; Ma, G.; Ba, X. Synthesis of water-soluble curcumin derivatives and their inhibition on lysozyme amyloid fibrillation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 190, 89–95. [Google Scholar] [CrossRef]
- Endo, H.; Nikaido, Y.; Nakadate, M.; Ise, S.; Konno, H. Structure activity relationship study of curcumin analogues toward the amyloid-beta aggregation inhibitor. Bioorgan. Med. Chem. Lett. 2014, 24, 5621–5626. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-T.; Chen, Z.-T.; Hou, W.-C.; Yu, L.-C.; Chen, R.P.Y. Polyhydroxycurcuminoids but not curcumin upregulate neprilysin and can be applied to the prevention of Alzheimer’s disease. Sci. Rep. 2016, 6, 29760. [Google Scholar] [CrossRef] [PubMed]
- Shilpa Mishra, M.M.; Seth, P.; Sharma, S.K. Tetrahydrocurcumin confers protection against amyloid b-induced toxicity. Neurochemistry 2011, 22, 23–27. [Google Scholar]
- Lee, E.H.-C.; Lim, S.S.-C.; Yuen, K.-H.; Lee, C.-Y. Curcumin and a hemi-analogue with improved blood–brain barrier permeability protect against amyloid-beta toxicity in Caenorhabditis elegans via SKN-1/Nrf activation. J. Pharm. Pharmacol. 2019, 71, 860–868. [Google Scholar] [CrossRef]
- Aswathy, L.; Jisha, R.S.; Masand, V.H.; Gajbhiye, J.M.; Shibi, I.G. Design of novel amyloid β aggregation inhibitors using QSAR, pharmacophore modeling, molecular docking and ADME prediction. Silico Pharmacol. 2018, 6, 12. [Google Scholar] [CrossRef]
- Bukhari, S.N.A.; Jantan, I.; Masand, V.H.; Mahajan, D.T.; Sher, M.; Naeem-ul-Hassan, M.; Amjad, M.W. Synthesis of α, β-unsaturated carbonyl based compounds as acetylcholinesterase and butyrylcholinesterase inhibitors: Characterization, molecular modeling, QSAR studies and effect against amyloid β-induced cytotoxicity. Eur. J. Med. Chem. 2014, 83, 355–365. [Google Scholar] [CrossRef]
- Kotani, R.; Urano, Y.; Sugimoto, H.; Noguchi, N. Decrease of Amyloid-β Levels by Curcumin Derivative via Modulation of Amyloid-β Protein Precursor Trafficking. J. Alzheimer’s Dis. JAD 2017, 56, 529–542. [Google Scholar] [CrossRef]
- Qi, Z.; Wu, M.; Fu, Y.; Huang, T.; Wang, T.; Sun, Y.; Feng, Z.; Li, C. Palmitic Acid Curcumin Ester Facilitates Protection of Neuroblastoma against Oligomeric Aβ40 Insult. Cell. Physiol. Biochem. 2017, 44, 618–633. [Google Scholar] [CrossRef]
- Ouberai, M.; Dumy, P.; Chierici, S.; Garcia, J. Synthesis and Biological Evaluation of Clicked Curcumin and Clicked KLVFFA Conjugates as Inhibitors of β-Amyloid Fibril Formation. Bioconjug. Chem. 2009, 20, 2123–2132. [Google Scholar] [CrossRef]
- Liu, K.; Gandhi, R.; Chen, J.; Zhang, S. Bivalent ligands targeting multiple pathological factors involved in Alzheimer’s disease. ACS Med. Chem. Lett. 2012, 3, 942–946. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, A.; Lin, J.; Zheng, Z.; Shi, X.; Di, W.; Qi, W.; Zhu, Y.; Zhou, G.; Fang, Y. Telomerase: A Target for Therapeutic Effects of Curcumin and a Curcumin Derivative in Aβ1-42 Insult In Vitro. PLoS ONE 2014, 9, e101251. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Liang, Y.; Liang, F.; Shen, N.; Shinozuka, K.; Yu, J.T.; Ran, C.; Quan, Q.; Tanzi, R.E.; Zhang, C. A Curcumin Analog Reduces Levels of the Alzheimer’s Disease-Associated Amyloid-β Protein by Modulating AβPP Processing and Autophagy. J. Alzheimer’s Dis. JAD 2019, 72, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Chojnacki, J.E.; Liu, K.; Yan, X.; Toldo, S.; Selden, T.; Estrada, M.; Rodríguez-Franco, M.I.; Halquist, M.S.; Ye, D.; Zhang, S. Discovery of 5-(4-hydroxyphenyl)-3-oxo-pentanoic acid [2-(5-methoxy-1H-indol-3-yl)-ethyl]-amide as a neuroprotectant for Alzheimer’s disease by hybridization of curcumin and melatonin. ACS Chem. Neurosci. 2014, 5, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Gerenu, G.; Liu, K.; Chojnacki, J.E.; Saathoff, J.M.; Martínez-Martín, P.; Perry, G.; Zhu, X.; Lee, H.-G.; Zhang, S. Curcumin/melatonin hybrid 5-(4-hydroxy-phenyl)-3-oxo-pentanoic acid [2-(5-methoxy-1H-indol-3-yl)-ethyl]-amide ameliorates AD-like pathology in the APP/PS1 mouse model. ACS Chem. Neurosci. 2015, 6, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Mourtas, S.; Lazar, A.N.; Markoutsa, E.; Duyckaerts, C.; Antimisiaris, S.G. Multifunctional nanoliposomes with curcumin–lipid derivative and brain targeting functionality with potential applications for Alzheimer disease. Eur. J. Med. Chem. 2014, 80, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Meena, P.; Nemaysh, V.; Khatri, M.; Manral, A.; Luthra, P.M.; Tiwari, M. Synthesis, biological evaluation and molecular docking study of novel piperidine and piperazine derivatives as multi-targeted agents to treat Alzheimer’s disease. Bioorgan. Med. Chem. 2015, 23, 1135–1148. [Google Scholar] [CrossRef]
- Morroni, F.; Sita, G.; Graziosi, A.; Ravegnini, G.; Molteni, R.; Paladini, M.S.; Dias, K.S.T.; Dos Santos, A.F.; Viegas, C., Jr.; Camps, I.; et al. PQM130, a Novel Feruloyl-Donepezil Hybrid Compound, Effectively Ameliorates the Cognitive Impairments and Pathology in a Mouse Model of Alzheimer’s Disease. Front. Pharm. 2019, 10, 658. [Google Scholar] [CrossRef]
- Shytle, R.D.; Paula, C.B.; Kavon, R.-Z.; Hou, L.; Jin, Z.; Jun, T.; Paul, R.S.; Cyndy, D.S.; Bill, R., Jr.; Ryan, C.F.; et al. Optimized Turmeric Extracts have Potent Anti-Amyloidogenic Effects. Curr. Alzheimer Res. 2009, 6, 564–571. [Google Scholar] [CrossRef]
- Ahmed, T.; Enam, S.A.; Gilani, A.H. Curcuminoids enhance memory in an amyloid-infused rat model of Alzheimer’s disease. Neuroscience 2010, 169, 1296–1306. [Google Scholar] [CrossRef]
- Randino, R.; Grimaldi, M.; Persico, M.; De Santis, A.; Cini, E.; Cabri, W.; Riva, A.; D’Errico, G.; Fattorusso, C.; D’Ursi, A.M.; et al. Investigating the Neuroprotective Effects of Turmeric Extract: Structural Interactions of β-Amyloid Peptide with Single Curcuminoids. Sci. Rep. 2016, 6, 1–17. [Google Scholar] [CrossRef]
- Masuda, Y.; Fukuchi, M.; Yatagawa, T.; Tada, M.; Takeda, K.; Irie, K.; Akagi, K.-I.; Monobe, Y.; Imazawa, T.; Takegoshi, K. Solid-state NMR analysis of interaction sites of curcumin and 42-residue amyloid β-protein fibrils. Bioorgan. Med. Chem. 2011, 19, 5967–5974. [Google Scholar] [CrossRef] [PubMed]
- Mourtas, S.; Canovi, M.; Zona, C.; Aurilia, D.; Niarakis, A.; La Ferla, B.; Salmona, M.; Nicotra, F.; Gobbi, M.; Antimisiaris, S.G. Curcumin-decorated nanoliposomes with very high affinity for amyloid-β1-42 peptide. Biomaterials 2011, 32, 1635–1645. [Google Scholar] [CrossRef] [PubMed]
- Bulic, B.; Pickhardt, M.; Schmidt, B.; Mandelkow, E.-M.; Waldmann, H.; Mandelkow, E. Development of Tau Aggregation Inhibitors for Alzheimer’s Disease. Angew. Chem. Int. Ed. 2009, 48, 1740–1752. [Google Scholar] [CrossRef]
- Okuda, M.; Hijikuro, I.; Fujita, Y.; Wu, X.; Nakayama, S.; Sakata, Y.; Noguchi, Y.; Ogo, M.; Akasofu, S.; Ito, Y.; et al. PE859, a novel tau aggregation inhibitor, reduces aggregated tau and prevents onset and progression of neural dysfunction in vivo. PLoS ONE 2015, 10, e0117511. [Google Scholar] [CrossRef]
- Yanagisawa, D.; Hamezah, H.S.; Durani, L.W.; Taguchi, H.; Tooyama, I. Study of tau pathology in male rTg4510 mice fed with a curcumin derivative Shiga-Y5. PLoS ONE 2018, 13, e0208440. [Google Scholar] [CrossRef]
- Llorens-Martín, M.; Jurado, J.; Hernández, F.; Avila, J. GSK-3β, a pivotal kinase in Alzheimer disease. Front. Mol. Neurosci. 2014, 7, 46. [Google Scholar] [CrossRef]
- Jiraporn Tocharus, S.J.; Tocharus, C.; Changtam, C.; Suksamrarn, A. Curcuminoid analogs inhibit nitric oxide production from LPS-activated microglial cells. J. Nat. Med. 2012, 66, 400–405. [Google Scholar] [CrossRef]
- Gagliardi, S.; Franco, V.; Sorrentino, S.; Zucca, S.; Pandini, C.; Rota, P.; Bernuzzi, S.; Costa, A.; Sinforiani, E.; Pansarasa, O.; et al. Curcumin and Novel Synthetic Analogs in Cell-Based Studies of Alzheimer’s Disease. Fron.t Pharm. 2018, 9, 1404. [Google Scholar] [CrossRef]
- Deck, L.M.; Hunsaker, L.A.; Vander Jagt, T.A.; Whalen, L.J.; Royer, R.E.; Vander Jagt, D.L. Activation of anti-oxidant Nrf2 signaling by enone analogues of curcumin. Eur. J. Med. Chem. 2018, 143, 854–865. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, L.; Weng, Q.; Xiao, L.; Li, Q. Curcumin analogues attenuate Aβ25-35-induced oxidative stress in PC12 cells via Keap1/Nrf2/HO-1 signaling pathways. Chem. Biol. Interact. 2019, 305, 171–179. [Google Scholar] [CrossRef]
- Saharan, S.; Mandal, P.K. The Emerging Role of Glutathione in Alzheimer’s Disease. J. Alzheimers Dis. 2014, 40, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.-X.; Zhang, H.-Y.; Meng, X.; Guan, Y.; Wang, H.-Q. Role of oxidative stress and intracellular glutathione in the sensitivity to apoptosis induced by proteasome inhibitor in thyroid cancer cells. BMC Cancer 2009, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Wojsiat, J.; Zoltowska, K.M.; Laskowska-Kaszub, K.; Wojda, U. Oxidant/Antioxidant Imbalance in Alzheimer’s Disease: Therapeutic and Diagnostic Prospects. Oxid. Med. Cell. Longev. 2018, 2018, 6435861. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Elmegeed, G.A.; Ahmed, H.H.; Hashash, M.A.; Abd-Elhalim, M.M.; El-kady, D.S. Synthesis of novel steroidal curcumin derivatives as anti-Alzheimer’s disease candidates: Evidences-based on in vivo study. Steroids 2015, 101, 78–89. [Google Scholar] [CrossRef]
- Emmanuel, I.A.; Olotu, F.A.; Agoni, C.; Soliman, M.E.S. Deciphering the ‘Elixir of Life’: Dynamic Perspectives into the Allosteric Modulation of Mitochondrial ATP Synthase by J147, a Novel Drug in the Treatment of Alzheimer’s Disease. Chem. Biodivers. 2019, 16, e1900085. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, Q.; Peng, Y.; Zhang, X.; Wang, Y.; Shi, L. A natural diarylheptanoid protects cortical neurons against oxygen–glucose deprivation-induced autophagy and apoptosis. J. Pharm. Pharmacol. 2019, 71, 1110–1118. [Google Scholar] [CrossRef]
- Liu, K.; Chojnacki, J.E.; Wade, E.E.; Saathoff, J.M.; Lesnefsky, E.J.; Chen, Q.; Zhang, S. Bivalent Compound 17MN Exerts Neuroprotection through Interaction at Multiple Sites in a Cellular Model of Alzheimer’s Disease. J. Alzheimers Dis. 2015, 47, 1021–1033. [Google Scholar] [CrossRef]
- Gao, Y.; Li, J.; Wu, L.; Zhou, C.; Wang, Q.; Li, X.; Zhou, M.; Wang, H. Tetrahydrocurcumin provides neuroprotection in rats after traumatic brain injury: Autophagy and the PI3K/AKT pathways as a potential mechanism. J. Surg. Res. 2016, 206, 67–76. [Google Scholar] [CrossRef]
- Saathoff, J.M.; Liu, K.; Chojnacki, J.E.; He, L.; Chen, Q.; Lesnefsky, E.J.; Zhang, S. Mechanistic Insight of Bivalent Compound 21MO as Potential Neuroprotectant for Alzheimer’s Disease. Molecules 2016, 21, 412. [Google Scholar] [CrossRef]
- Sgarbossa, A.; Giacomazza, D.; di Carlo, M. Ferulic Acid: A Hope for Alzheimer’s Disease Therapy from Plants. Nutrients 2015, 7, 5764–5782. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fang, L.; Zhang, H.; Gou, S.; Chen, L. Design, synthesis and biological evaluation of multifunctional tacrine-curcumin hybrids as new cholinesterase inhibitors with metal ions-chelating and neuroprotective property. Bioorgan. Med. Chem. 2017, 25, 2387–2398. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Talalay, P. NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch. Biochem. Biophys. 2010, 501, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Shearer, J.; Callan, P.E.; Tran, T.; Szalai, V.A. Cu K-edge X-ray absorption spectroscopy reveals differential copper coordination within amyloid-β oligomers compared to amyloid-β monomers. Chem. Commun. 2010, 46, 9137–9139. [Google Scholar] [CrossRef][Green Version]
- Hureau, C.; Faller, P. Aβ-mediated ROS production by Cu ions: Structural insights, mechanisms and relevance to Alzheimer’s disease. Biochimie 2009, 91, 1212–1217. [Google Scholar] [CrossRef]
- Ahmed, T.; Gilani, A.-H. Inhibitory effect of curcuminoids on acetylcholinesterase activity and attenuation of scopolamine-induced amnesia may explain medicinal use of turmeric in Alzheimer’s disease. Pharmacol. Biochem. Behav. 2009, 91, 554–559. [Google Scholar] [CrossRef]
- Ahmed, T.; Gilani, A.-H. Therapeutic Potential of Turmeric in Alzheimer’s Disease: Curcumin or Curcuminoids? Phytother. Res. 2014, 28, 517–525. [Google Scholar] [CrossRef]
- Arunkhamkaew, S.; Athipornchai, A.; Apiratikul, N.; Suksamrarn, A.; Ajavakom, V. Novel racemic tetrahydrocurcuminoid dihydropyrimidinone analogues as potent acetylcholinesterase inhibitors. Bioorgan. Med. Chem. Lett. 2013, 23, 2880–2882. [Google Scholar] [CrossRef]
- Veronica, T.-F.; Maria Concepcion, L.-G.; Manuel, S.-G. Experimental and Computational Studies on the Inhibition of Acetylcholinesterase by Curcumin and Some of its Derivatives. Curr. Comput. Aided Drug Des. 2013, 9, 289–298. [Google Scholar] [CrossRef]
- Wilar, G.; Anggadiredja, K.; Shinoda, Y.; Fukunaga, K. Inhibition of Nicotine Dependence by Curcuminoid Is Associated with Reduced Acetylcholinesterase Activity in the Mouse Brain. Pharmacology 2018, 102, 223–232. [Google Scholar] [CrossRef]
- Li, Y.; Peng, P.; Tang, L.; Hu, Y.; Hu, Y.; Sheng, R. Design, synthesis and evaluation of rivastigmine and curcumin hybrids as site-activated multitarget-directed ligands for Alzheimer’s disease therapy. Bioorgan. Med. Chem. 2014, 22, 4717–4725. [Google Scholar] [CrossRef] [PubMed]
- Suppiah, S.; Didier, M.-A.; Vinjamuri, S. The Who, When, Why, and How of PET Amyloid Imaging in Management of Alzheimer’s Disease-Review of Literature and Interesting Images. Diagnostics 2019, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-F.; Xu, T.-H.; Yan, Y.; Zhou, Y.-R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharm. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-H.; Loo, C.-Y.; Bebawy, M.; Luk, F.; Mason, R.S.; Rohanizadeh, R. Curcumin and its derivatives: Their application in neuropharmacology and neuroscience in the 21st century. Curr. Neuropharmacol. 2013, 11, 338–378. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cheng, R.; Fu, H.; Yang, J.; Kumar, M.; Lu, J.; Xu, Y.; Liang, S.H.; Cui, M.; Ran, C. Half-curcumin analogues as PET imaging probes for amyloid beta species. Chem. Commun. 2019, 55, 3630–3633. [Google Scholar] [CrossRef] [PubMed]
- Ran, C.; Xu, X.; Raymond, S.B.; Ferrara, B.J.; Neal, K.; Bacskai, B.J.; Medarova, Z.; Moore, A. Design, synthesis, and testing of difluoroboron-derivatized curcumins as near-infrared probes for in vivo detection of amyloid-beta deposits. J. Am. Chem. Soc. 2009, 131, 15257–15261. [Google Scholar] [CrossRef]
- Lu, Y.; Prudent, M.; Qiao, L.; Mendez, M.A.; Girault, H.H. Copper(i) and copper(ii) binding to β-amyloid 16 (Aβ16) studied by electrospray ionization mass spectrometry. Metallomics 2010, 2, 474–479. [Google Scholar] [CrossRef]
- Liu, K.; Guo, T.L.; Chojnacki, J.; Lee, H.-G.; Wang, X.; Siedlak, S.L.; Rao, W.; Zhu, X.; Zhang, S. Bivalent ligand containing curcumin and cholesterol as fluorescence probe for Aβ plaques in Alzheimer’s disease. ACS Chem. Neurosci. 2012, 3, 141–146. [Google Scholar] [CrossRef]
- Gan, C.; Hu, J.; Nan, D.-D.; Wang, S.; Li, H. Synthesis and biological evaluation of curcumin analogs as β-amyloid imaging agents. Future Med. Chem. 2017, 9, 1587–1596. [Google Scholar] [CrossRef]
- Veldman, E.R.; Jia, Z.; Halldin, C.; Svedberg, M.M. Amyloid binding properties of curcumin analogues in Alzheimer’s disease postmortem brain tissue. Neurosci. Lett. 2016, 630, 183–188. [Google Scholar] [CrossRef]
- Rubagotti, S.; Croci, S.; Ferrari, E.; Iori, M.; Capponi, P.C.; Lorenzini, L.; Calzà, L.; Versari, A.; Asti, M. Affinity of (nat/68)Ga-Labelled Curcumin and Curcuminoid Complexes for β-Amyloid Plaques: Towards the Development of New Metal-Curcumin Based Radiotracers. Int. J. Mol. Sci. 2016, 17, 1480. [Google Scholar] [CrossRef] [PubMed]
- Asti, M.; Ferrari, E.; Croci, S.; Atti, G.; Rubagotti, S.; Iori, M.; Capponi, P.C.; Zerbini, A.; Saladini, M.; Versari, A. Synthesis and Characterization of 68Ga-Labeled Curcumin and Curcuminoid Complexes as Potential Radiotracers for Imaging of Cancer and Alzheimer’s Disease. Inorg. Chem. 2014, 53, 4922–4933. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Im, Y.H.; Ahn, J.; Yang, J.; Choi, J.Y.; Lee, K.-H.; Kim, B.-T.; Choe, Y.S. Synthesis and in vivo characterization of 18F-labeled difluoroboron-curcumin derivative for β-amyloid plaque imaging. Sci. Rep. 2019, 9, 6747. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Ono, M.; Kimura, H.; Liu, B.; Saji, H. Synthesis and Structure−Affinity Relationships of Novel Dibenzylideneacetone Derivatives as Probes for β-Amyloid Plaques. J. Med. Chem. 2011, 54, 2225–2240. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, D.; Amatsubo, T.; Morikawa, S.; Taguchi, H.; Urushitani, M.; Shirai, N.; Hirao, K.; Shiino, A.; Inubushi, T.; Tooyama, I. In vivo detection of amyloid β deposition using 19F magnetic resonance imaging with a 19F-containing curcumin derivative in a mouse model of Alzheimer’s disease. Neuroscience 2011, 184, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Rokka, J.; Snellman, A.; Zona, C.; La Ferla, B.; Nicotra, F.; Salmona, M.; Forloni, G.; Haaparanta-Solin, M.; Rinne, J.O.; Solin, O. Synthesis and evaluation of a 18F-curcumin derivate for β-amyloid plaque imaging. Bioorgan. Med. Chem. 2014, 22, 2753–2762. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-S.; Seo, Y.; Kim, M.K.; Kim, K.; Kim, Y.K.; Choo, H.; Chong, Y. A curcumin-based molecular probe for near-infrared fluorescence imaging of tau fibrils in Alzheimer’s disease. Org. Biomol. Chem. 2015, 13, 11194–11199. [Google Scholar] [CrossRef]
- Park, K.-S.; Kim, M.K.; Seo, Y.; Ha, T.; Yoo, K.; Hyeon, S.J.; Hwang, Y.J.; Lee, J.; Ryu, H.; Choo, H.; et al. A Difluoroboron β-Diketonate Probe Shows “Turn-on” Near-Infrared Fluorescence Specific for Tau Fibrils. ACS Chem. Neurosci. 2017, 8, 2124–2131. [Google Scholar] [CrossRef]
- Alexander, B.N.; Daniel, K.; Constantin, V.; Silvia, B.; Christian, S.N.; Steffen, B.; Tobias, B.; Jana, H.L.; Roland, H.-V.H.; Gerhard, M.; et al. Bis(arylvinyl)pyrazines, -pyrimidines, and -pyridazines As Imaging Agents for Tau Fibrils and β-Amyloid Plaques in Alzheimer’s Disease Models. J. Med. Chem. 2012. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, Y.; Yuan, P.; Li, Y.; Yaseen, M.A.; Grutzendler, J.; Moore, A.; Ran, C. A bifunctional curcumin analogue for two-photon imaging and inhibiting crosslinking of amyloid beta in Alzheimer’s disease. Chem. Commun. 2014, 50, 11550–11553. [Google Scholar] [CrossRef]
- Sato, T.; Hotsumi, M.; Makabe, K.; Konno, H. Design, synthesis and evaluation of curcumin-based fluorescent probes to detect Aβ fibrils. Bioorgan. Med. Chem. Lett. 2018, 28, 3520–3525. [Google Scholar] [CrossRef]
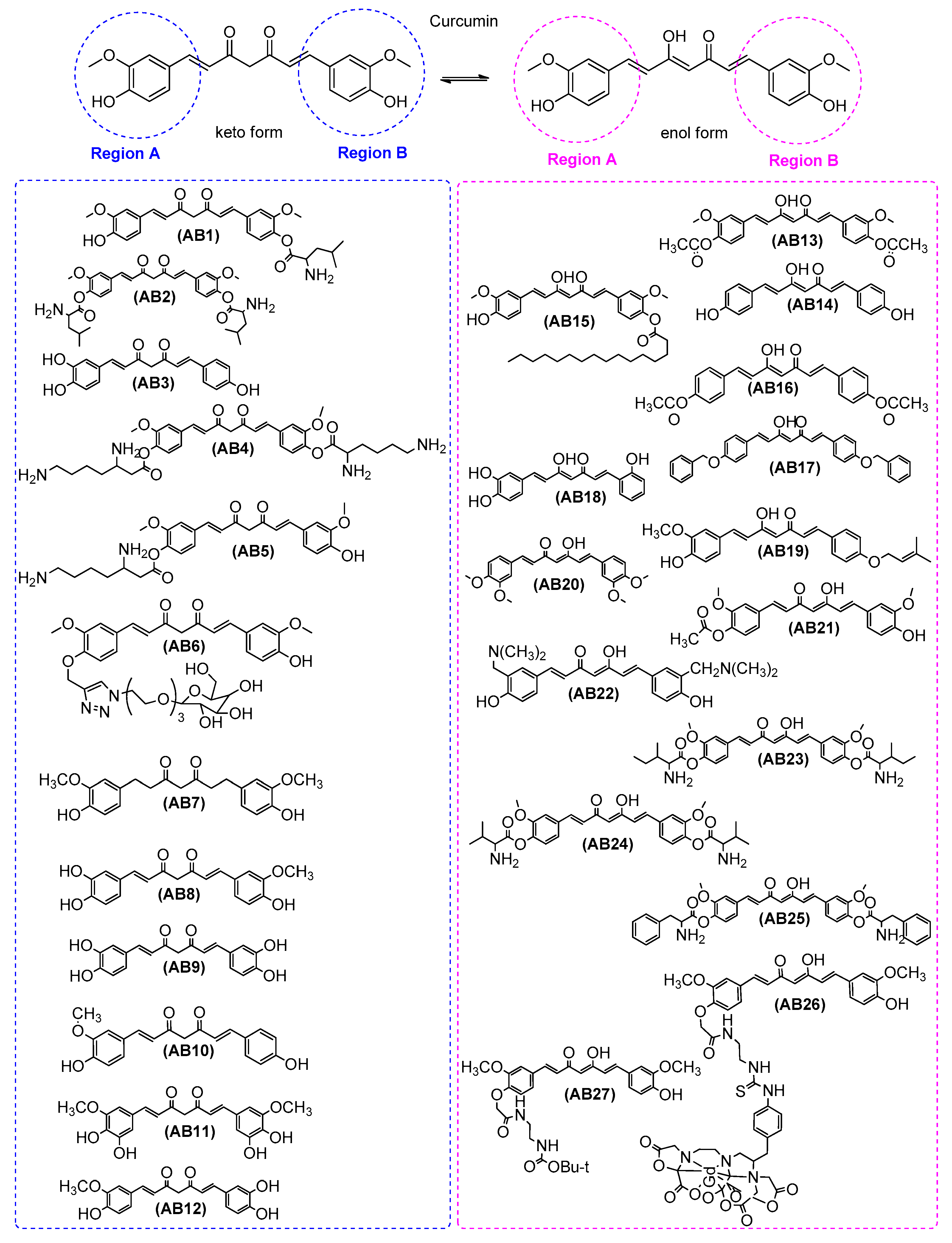

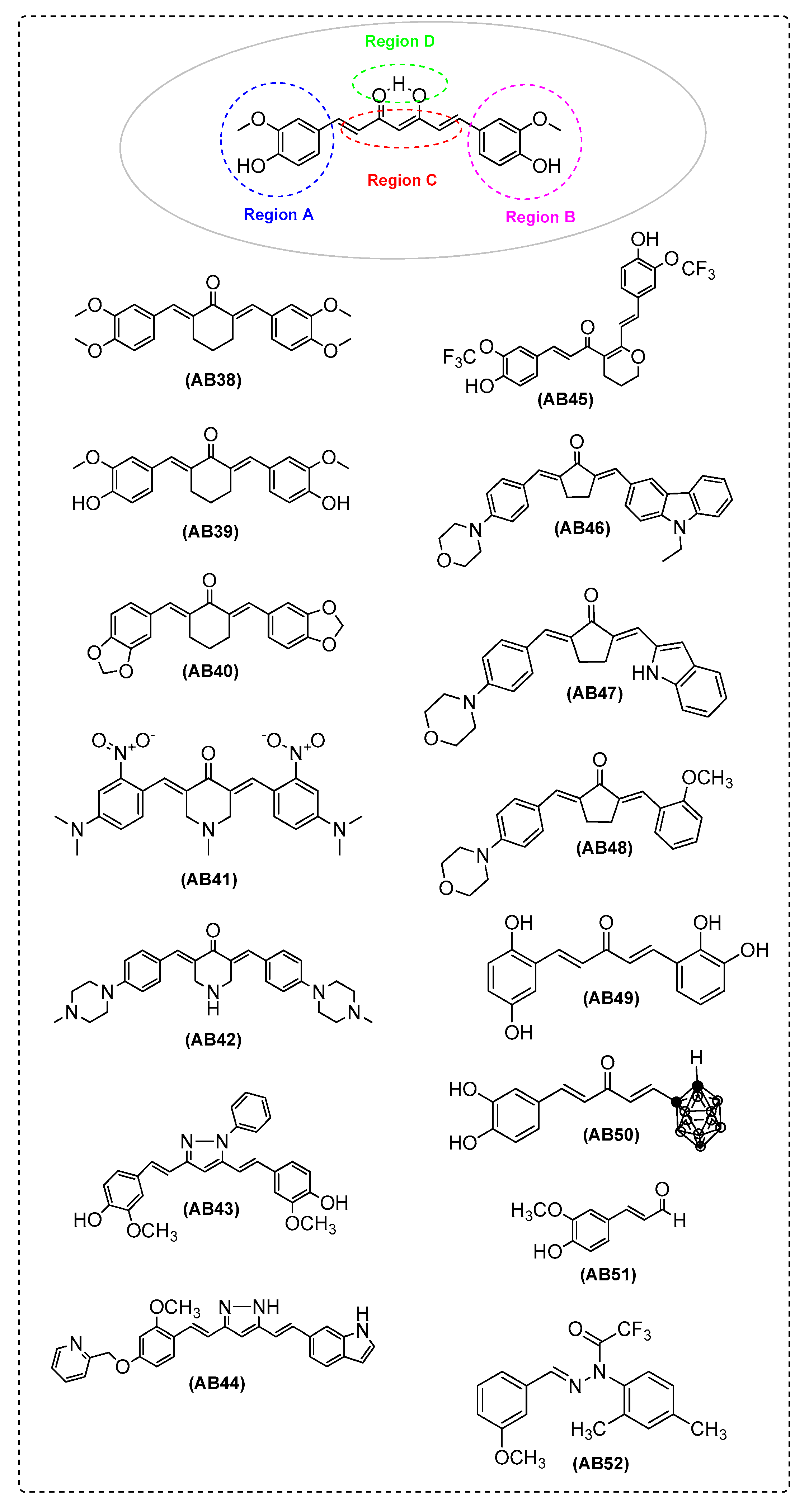
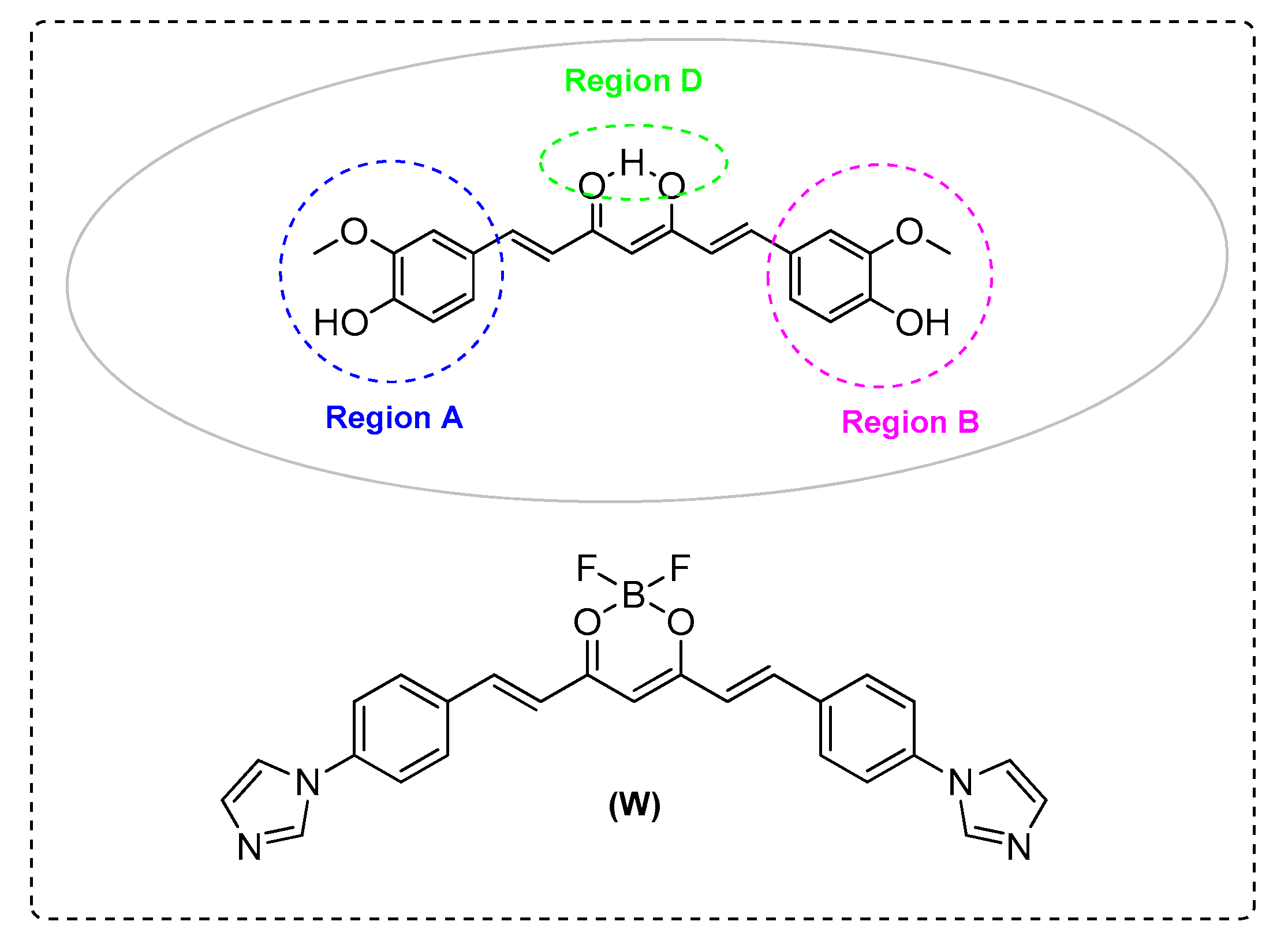
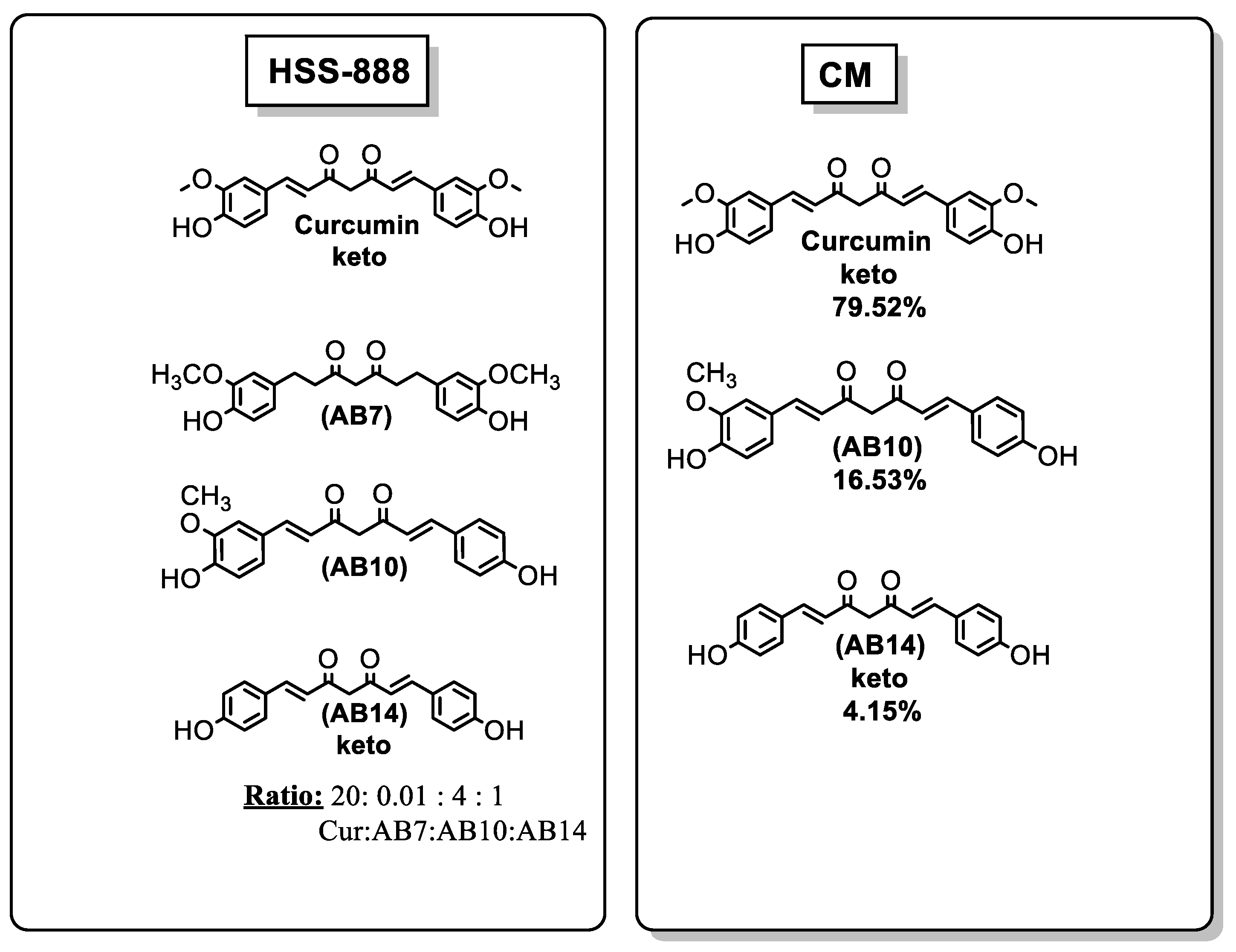
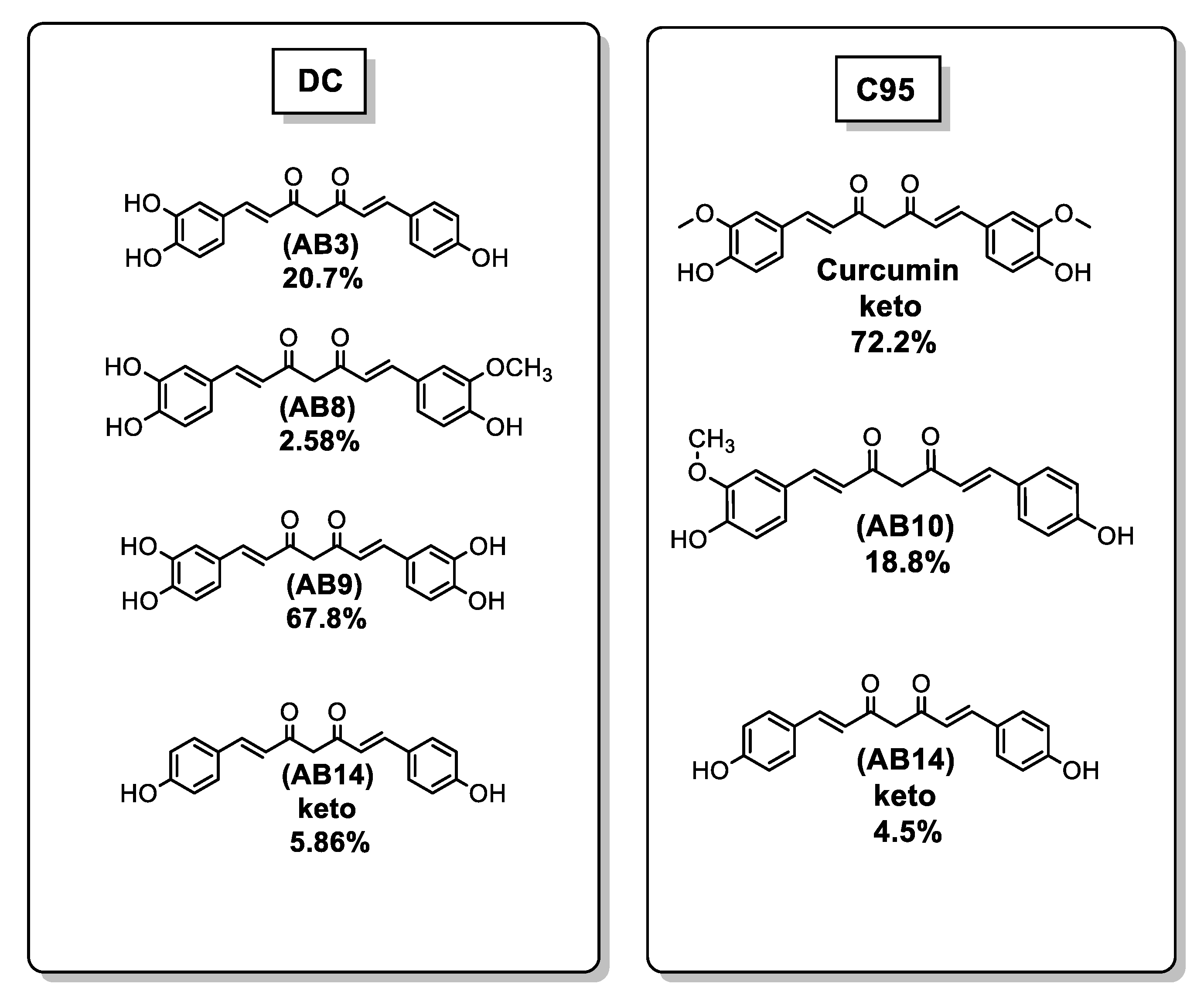


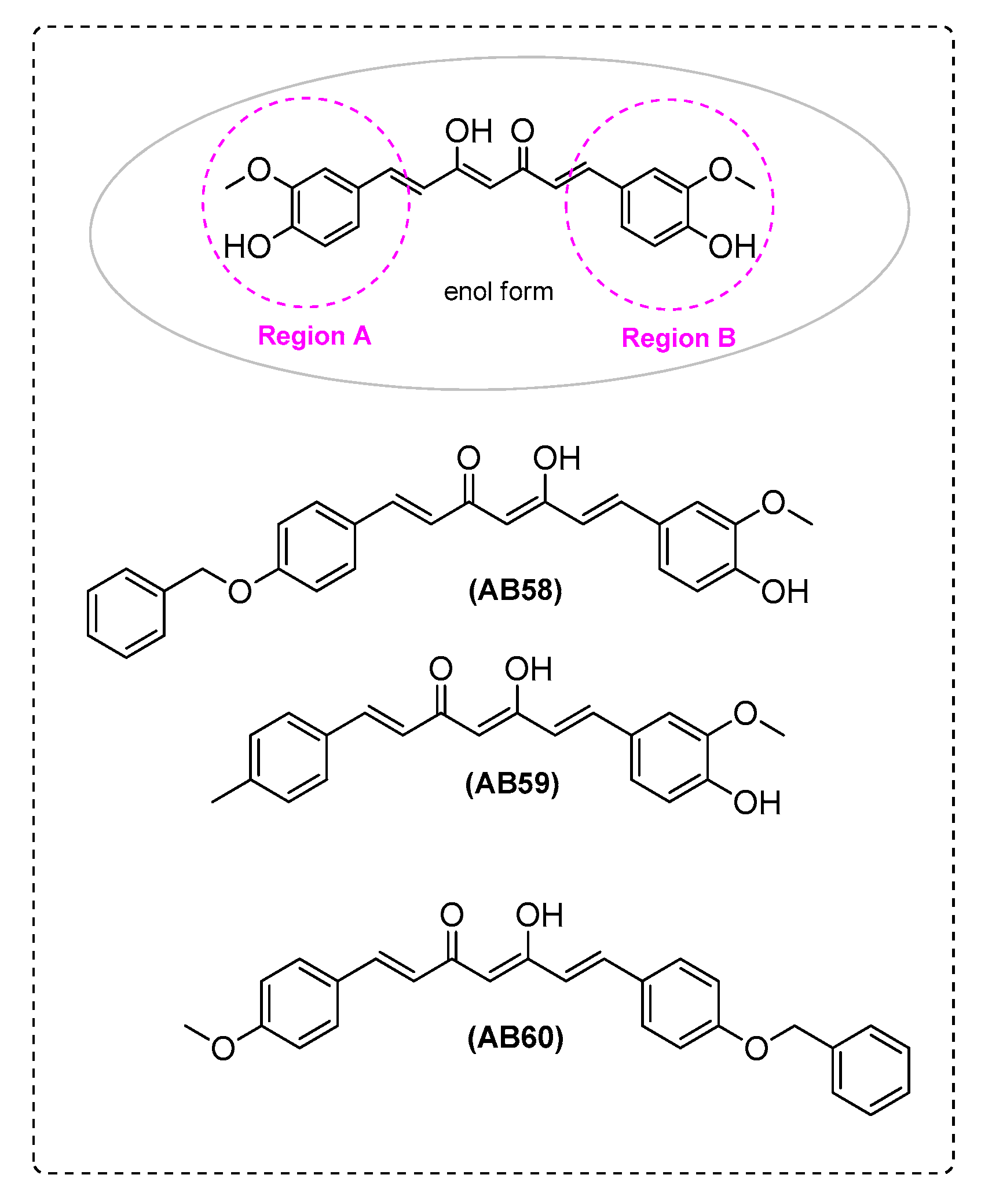
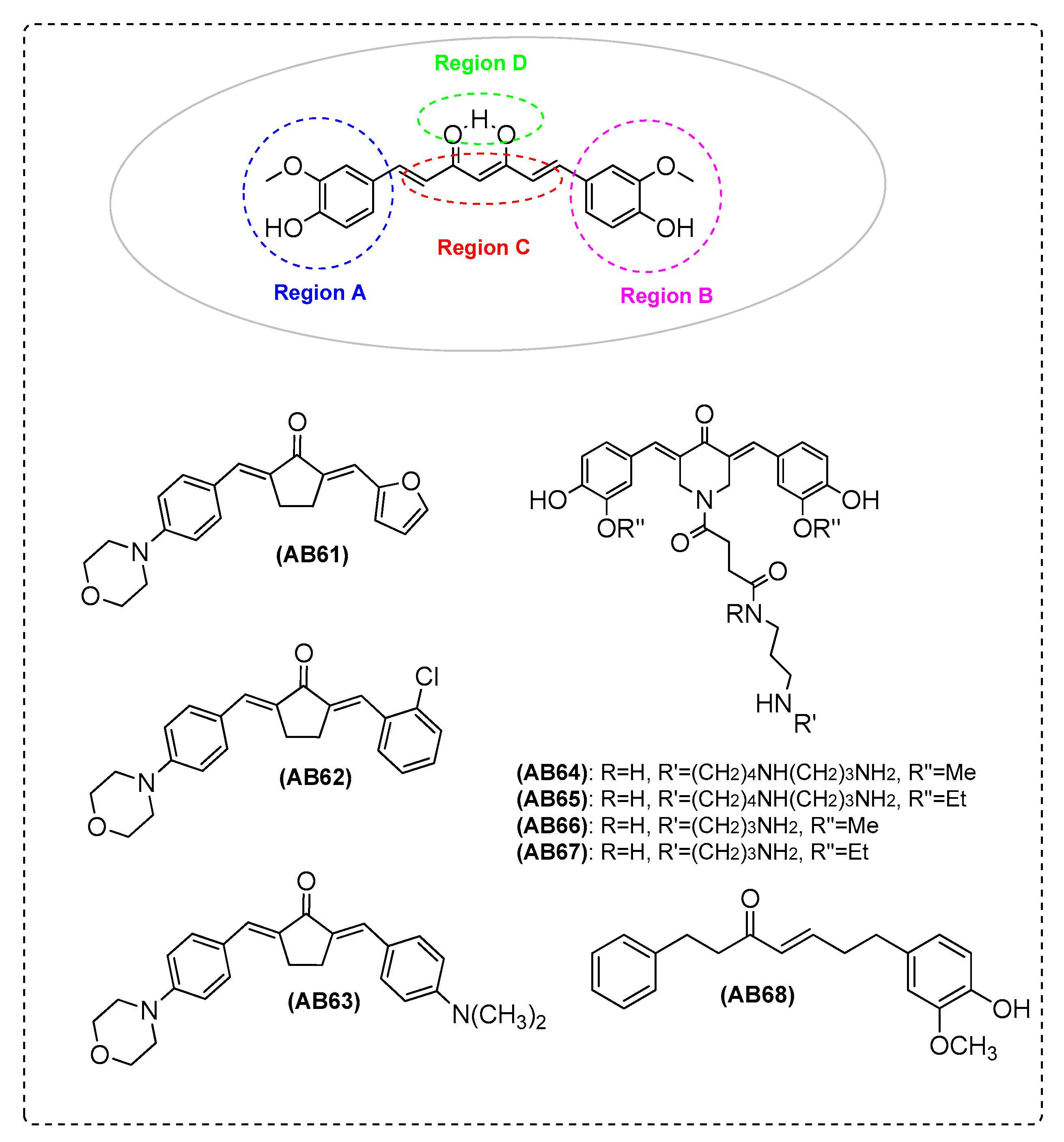
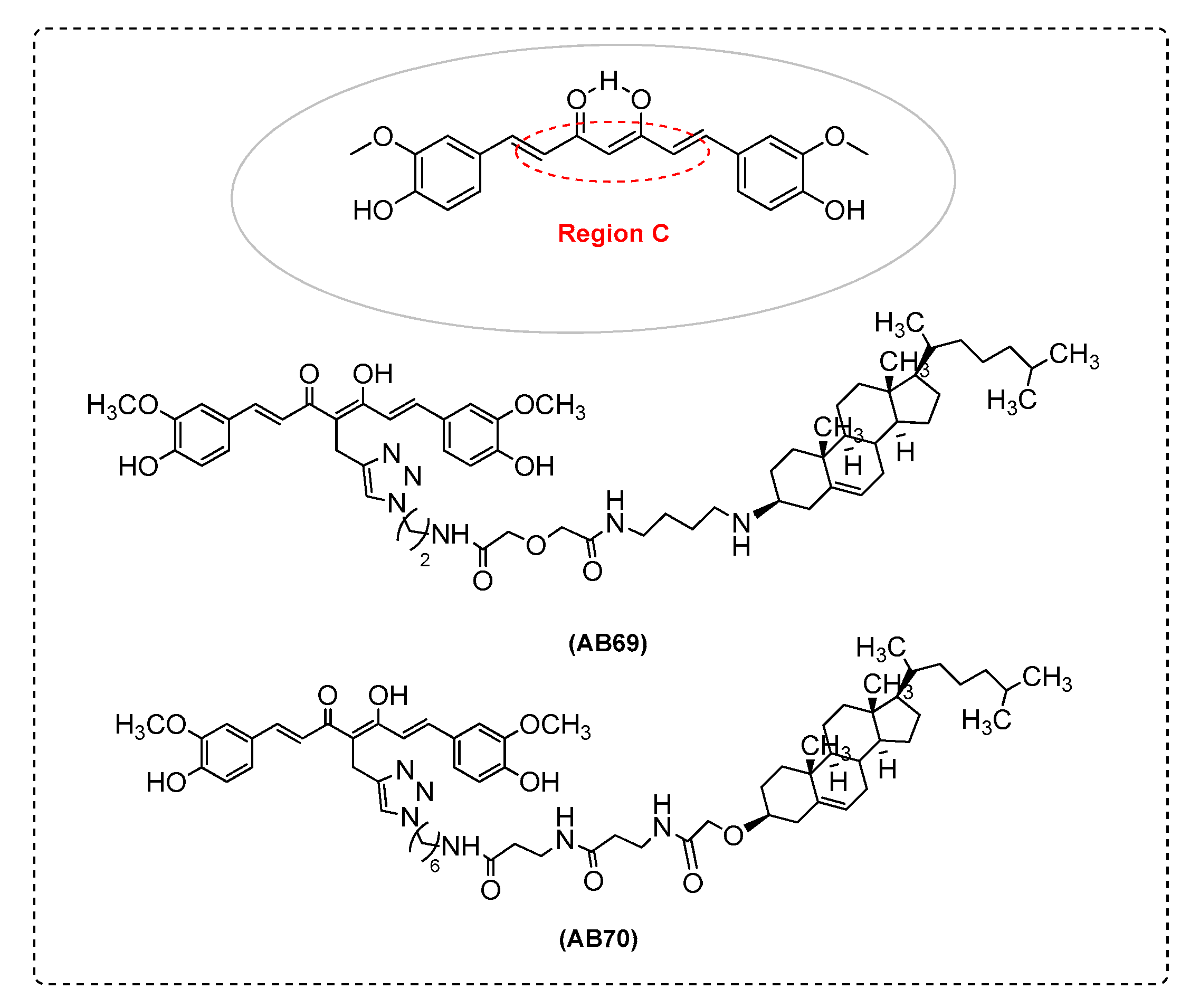
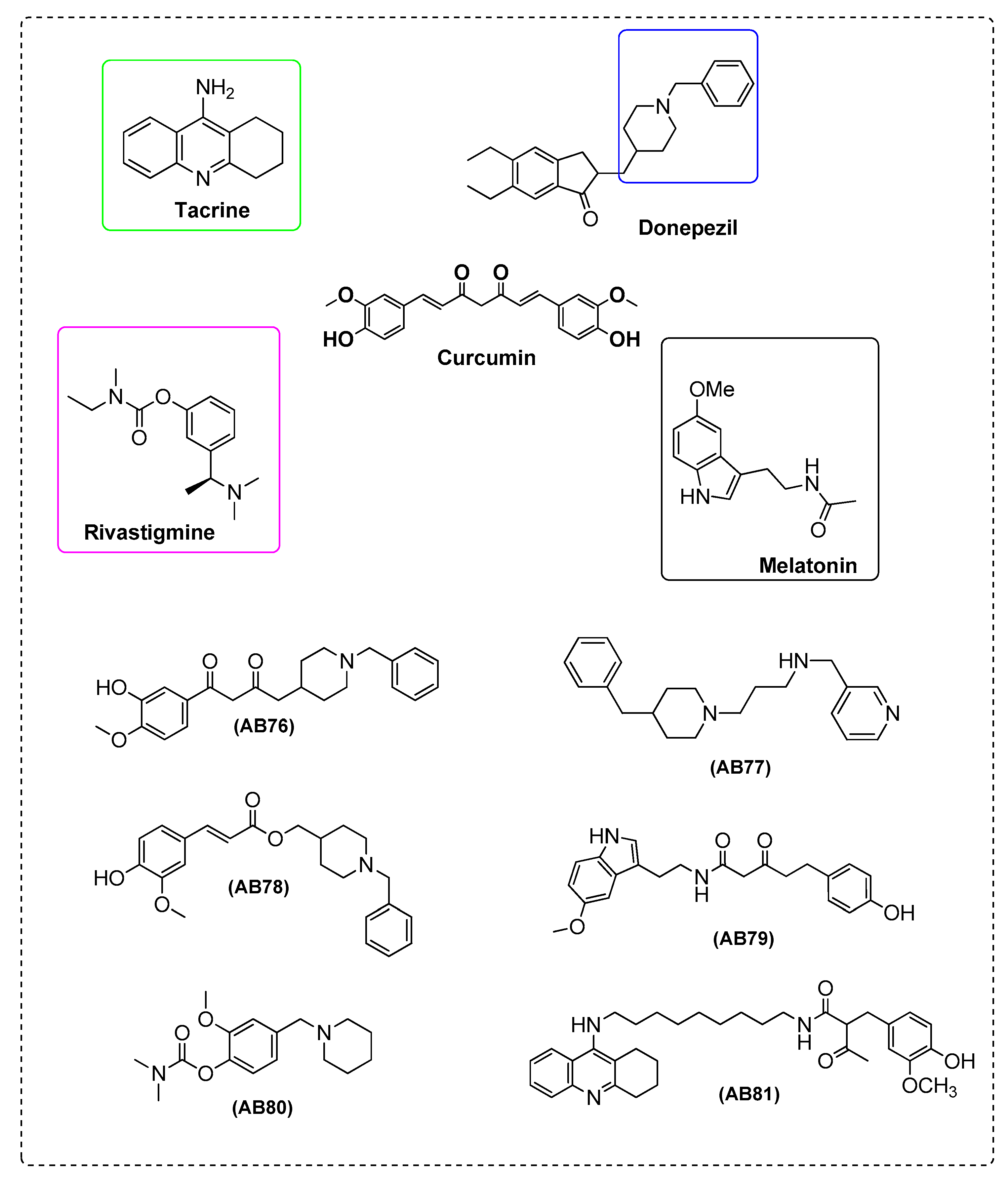
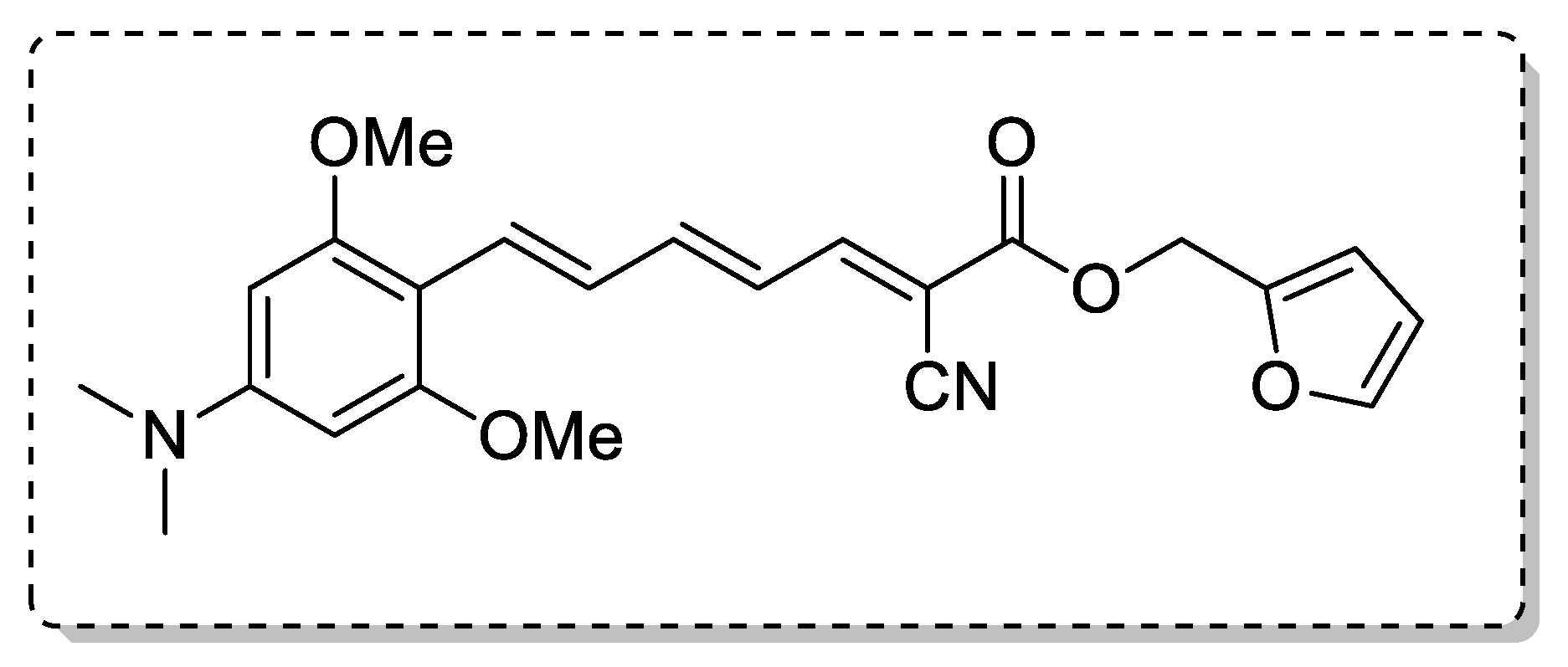
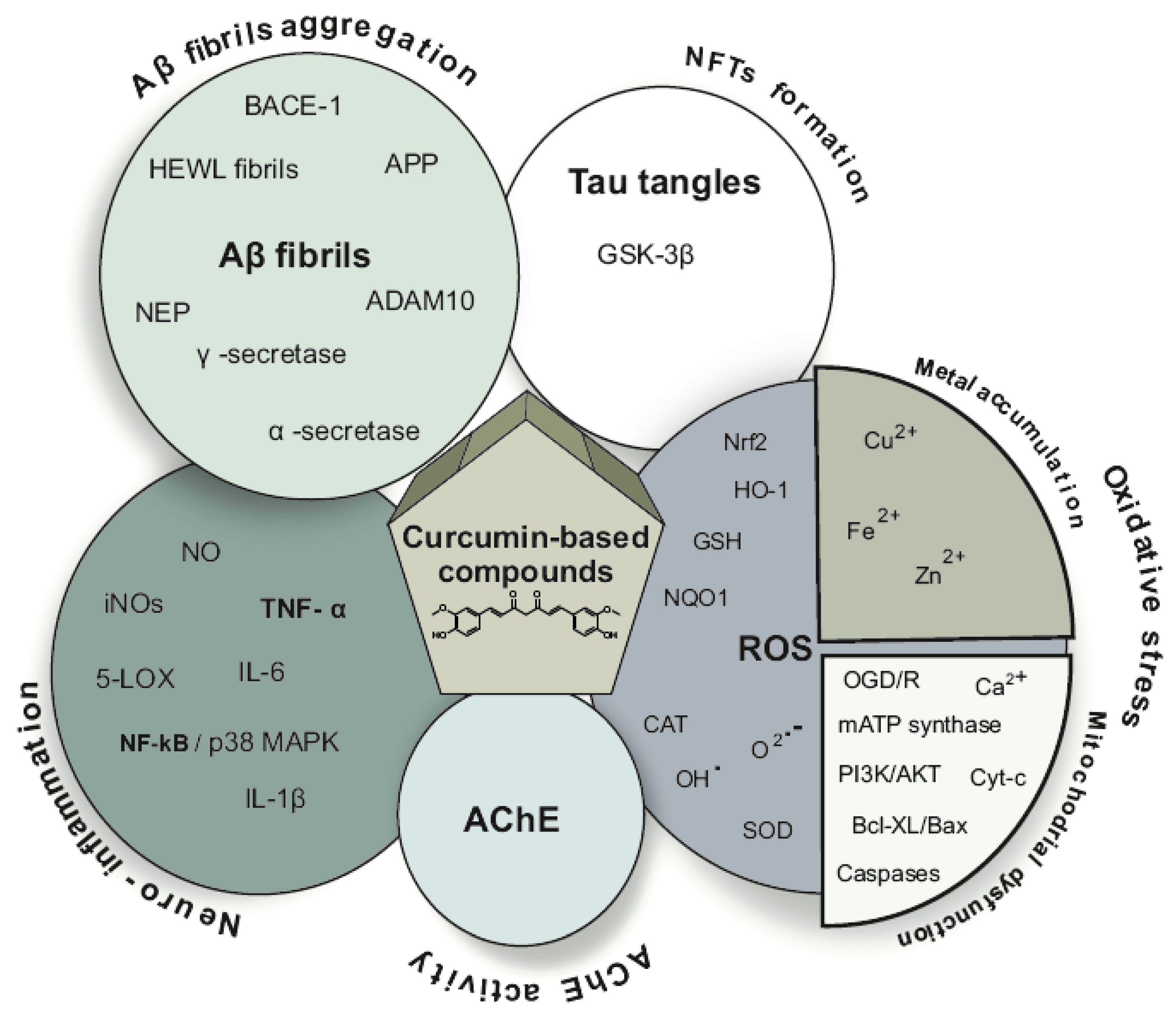
| Ref. | Modification of Curcumin | Action | In Vitro/In Vivo/Ex Vivo/In Silico | Blood-Brain-BARRIER (BBB) Permeation |
|---|---|---|---|---|
| Anti-Amyloidogenic Activity | ||||
| [39] | Optimization of the o-phenol and olefin spacer Incorporates a C5-monoketone spacer moiety and phenolic rings bearing hydroxy groups at positions 2,3,2′,5′ | potent anti-amyloid-β aggregation activity | In vitro docking | NI |
| [18] | Both the 4-hydroxy,3-methoxy and prenyloxy aryl substitution patterns are present | inhibit the formation of large toxic Aβ oligomers | In vitro docking | NI |
| [7] | Acetylation at only one side of the molecule | strong anti-aggregation effect | In vitro | NI |
| [40] | Phenolic group combined with methoxyl moiety in ortho position | inhibit Aβ1–40 aggregation | In vitro | NI |
| [19,41] | Replacement of diketone with a pyrazole | potent Aβ aggregation inhibitor | In vitro In vivo | Can pass BBB |
| [9] | Expansion of the aromatic rings An electron-releasing group to transfer electrons to the phenyl moiety Groups with large conjugated structure or electron-releasing groups | Aβ1–42 aggregation inhibition | In vitro | NI |
| [42,43] | Two aromatic rings connected by a nitrogen containing bridge | block extracellular amyloid toxicity | In vivo (rat hippocampal neurons) | Can pass BBB |
| [16] | Dimethylaminomethyl-substituted derivatives which have a large steric hindrance, to the ortho position of the hydroxy groups | inhibit the Aβ self-aggregation | In vitro | can pass BBB |
| [44] | Enol form of the compound Methoxycarbonylethyl group at the C-4 position | (i) high affinity for Aβ aggregation (ii) significantly attenuation of the cell toxicity of Aβ | In vitro In vivo | Can pass BBB |
| [30] | Hydroxyl groups on the aromatic ring Combination of 1,7-bis (4′-hydroxy-3′-trifluoromethoxyphenyl) groups and a suitable substituent at the C-4position | inhibit Aβ aggregation | In vitro | NI |
| [37] | Phenyl methoxy groups Hydroxyl groups | effect on Aβ42, APP and BACE1 | In vitro | NI |
| [45] | Demethoxycurcumin | effect on amyloid-β precursor protein through the internal ribosome entry sites | In vitro | can pass BBB |
| [35] | Aminoacid (isoleucine, phenylalanine and valine) | α-secretase activation | In vitro | NI |
| [46] | At least one enone group in the spacer between aryl rings An unsaturated carbon spacer between aryl rings Methoxyl and hydroxyl substitutions in the meta and para-positions on the aryl rings | anti-Aβ aggregation activity | In vitro In vivo | can pass BBB |
| [47] | Monogalactose group | inhibits Aβpeptide aggregation | In vitro | possibility to optimize the uptake across the BBB via the glucose transporter mechanism |
| [11] | The introduction of flexible moieties at the linker | inhibit β-sheet aggregation and fibril formation | In vitro | NI |
| [48] | Modifications in the spacer and the phenolic rings and diketone moiety of curcumin was replaced by cyclohexanone Methoxy substitution of hydroxyl groups Size of these compounds and presence of aromatic/cyclic components | inhibit HEWL aggregation and inhibit the cytotoxic activity of aggregated HEWL | In vitro docking | NI |
| [49] | The presence of a second –OH group | inhibition of the formation of amyloid-β aggregates | In vitro | NI |
| [38] | Phenolic hydroxyl group | inhibits the formation of amyloid fibrils | In vitro | NI |
| [50] | β-diketone moiety, phenolic OH groups, acetyl groups, benzene cycle, hepta-diene moiety and substitutions on the benzene cycle | HEWL aggregation | In vitro docking | NI |
| [51] | Bisdemethoxycurcumin Diacetylbisdemethoxycurcumin | HEWL aggregation | In vitro docking | NI |
| [52] | 2-methoxy-4-methylphenyl 3,7-diaminoheptanoate | HEWL aggregation | In vitro | NI |
| [25,53] | Motif Two polar phenolic hydroxy groups and an alkenyl spacer Ketones and double bonds in the spacer O-phenol motif Replacement of phenols with indole and pyrrole | inhibit Aβ aggregation inhibition of BACE-1 | In vitro docking | NI |
| [54] | Contain more hydrophilic hydroxyl groups | upregulating NEP | In vitro In vivo | can pass BBB |
| [55] | Tetrahydrocurcumin | protective effect against oligomeric amyloid-β induced toxicity | In vitro | NI |
| [26] | Side aryl rings 4-Hydroxy-3-methoxyphenyl as A ring 4-Benzyloxyphenyl or para-tolyl as B ring | inhibition of BACE-1 | In vitro docking | can pass BBB |
| [56] | Half side of curcumin’s structure | protection against Aβ toxicity through SKN-1/Nrf activation | In vitro | can pass BBB |
| [57] | Steric, electrostatic and hydrogen bond acceptor, play important role for interaction with the receptor site cavity Interactions between the amino acid residues at the catalytic site of the receptor and the ligands Methylene group (–CH2–) in between two carbonyl groups Methylene group attached to the –NH2 group The doublebonds of the benzene ring adjacent to the oxygen atom (vi) –CH3 of the methoxygroup | Aβ aggregation inhibitory activity | QSAR, pharmacophore modeling, molecular docking and ADME prediction | can pass BBB |
| [58] | Possessing 2-nitro and 4-dimethylamine groups Having a 4-piperidone Possessing N-methyl-4-piperidone | protective against Aβ-induced neuronal cell death | In vitro | NI |
| [59] | 4,4′-((1E,1′E)-(1-phenyl-1H-pyrazole-3,5-diyl)bis(ethene-2,1-diyl))bis(2-methoxyphenol) | (i) inhibits neither β- nor γ-secretase activity (ii) induces expression of the ER chaperone glucose-regulated protein 78 (GRP78) (iii) enhances formation of the AβPP/GRP78 complex | In vitro | NI |
| [60] | Palmitic Acid Curcumin Ester | neuroprotective effects against Aβ insult | In vitro | NI |
| [29] | Gd(III)(diethylenetriaminepentaacetate) tert-butyl (2-propionamidoethyl)carbamate | redirecting metaltriggered Aβ aggregation | In vitro | can pass BBB |
| [61] | KLVFFA peptide | strongly inhibit Aβ amyloid fibril formation | In vitro | NI |
| [62] | Cholesterylamine spacer (length of 17 atoms) | inhibits the formation of amyloid-β oligomers (AβOs) | In vitro | NI |
| [63] | 4-Hydroxyl group | protect from Aβ1–42 | In vitro | can pass BBB |
| [33,64] | 4,6-Bis((E)-4-(1H-imidazol-1-yl)styryl)-2,2-difluoro-2H-1,3,2-dioxaborinin-1-ium-2-uide | (i) lowers Aβ levels in conditioned media and reduces oligomeric amyloid levels in the cells (ii) attenuates the maturation of AβPPin the secretory pathway (iii) upregulates α-secretase processing of AβPP and inhibited β-secretase processing of AβPP by decreasing BACE1 protein levels | In vitro In vivo | can pass BBB |
| [24] | Hydroxyl group | Aβ self-aggregation inhibitory activity | In vitro | can pass BBB |
| [65,66] | 4-OH group | inhibitory effects on the production of amyloid-β oligomers (AβOs) | In vitro In vivo | can pass BBB |
| [67] | Ortho-methoxy carbamoyl moiety | inhibition of Aβ1-40 fibril formation | In vitro | NI |
| [68] | Presence of electron rich groups on benzyl ring and the terminal benzylpiperidine | inhibition of self-mediated Aβ1–42 aggregation | In vitro | possibility to pass BBB |
| [69] | Feruloyl-donepezil hybrid | ability to modify the kinetics of Aβ fibril formation | In vitro In vivo | can pass BBB |
| Ref. | Structure or Functional Groups | Antioxidant Activity | Metal Accumulation | Mitochondrial Dysfunction/Apoptosis | Techniques | In Vitro/In Vivo/Ex Vivo/In Silico | Blood–BrainBarrier (BBB) Permeation |
|---|---|---|---|---|---|---|---|
| [82] | Mono-ketone moiety Phenolic OH | (i) inhibit ROS accumulation (ii) inhibit oxidative stress via Keap1/Nrf2/HO-1 signaling pathways | - | (i) increase Bcl-2 expression level (ii) decrease the level of Bax and Cyt-c | ROS production assay (DCFH-DA probe) | In vitro (PC12 cells) | NI |
| [6] | 1,7-Bis(3,4-dihydroxyphenyl)-1,6-heptadiene-3,5-dione | reduce intracellular ROS | - | (i) increase the ratio of Bcl-XL/Bax protein level, cytochrome c protein expression (ii) cleave caspase-9 and caspase-3 protein expression | (i) determination of intracellular ROS by DCF assay (ii) Cell apoptosis analysis | In vitro (SK-N-SH cells) | NI |
| [63] | 4-Hydroxyl group | significant decrease in ROS generation and a significant increase in GSH level | - | - | (i) ROS production assay (DCFH-DA probe) (ii) determination of glutathione (GSH) | In vitro (SK-N-SH cells) | pass |
| [55] | Tetrahydro-curcumin (THC) | increases in the level of reactiveoxygen species | - | (i) decrease in mitochondrial membrane potential, (ii) caspase activation | (i) ROS production assay (DCFH-DA probe) (ii) mitochondrial membrane potential assay (iii) caspase-3 activity assay | In vitro (rat primary hippocampal and human neuron cultures) | NI |
| [20] | Demethylcurcumin | IncreasesGSH and reduceds reactive oxygen species (ROS) | - | - | (i) ROS production assay (DCFH-DA probe) (ii) glutathione (GSH) assay | In vitro (HT4 neuronal cells) | pass |
| [60] | Phenol groups | effect on Aβ-induced ROS production in vitro and in vivo | - | - | (i) ROS production assay (DCFH-DA probe) (ii) lipid peroxidation inhibition | In vitro (SH-SY5Y cells) In vivo | NI |
| [8] | Curcumin congeners with different polyamine motifs | decrease of ROS levels | - | an efficientintracellular uptake and mitochondria targeting | (i) ROS production assay (DCFH-DA probe) (ii) heme oxygenase-1 (HO-1) induction | In vitro (SH-SY5Y, hippocampal neuronal HT22 cell lines, T67 glioma cells) | NI |
| [42] | (E)-N-(2,4-dimethylphenyl)-2,2,2-trifluoro-N′-(3-methoxy-benzylidene)acetohydrazide | - | - | - | (ii) heme oxygenase-1 (HO-1) induction | In vivo (APP/PS1 transgenic mice) | pass |
| [18] | Vanillin moieties 4-Hydroxy,3-methoxy group | (i) reduction of H2O2-induced intracellular ROS production (ii) activation of antioxidant Nrf2 signaling | - | - | DCFH-fluorescence intensity in H2O2-treated cells | In vitro (SH-SY5Y cells) | NI |
| [9] | The styryl function and steric or electronic factors through the large aromatic structure A dimethylamino groupon benzene ring | reduction of oxidative stress | selectively chelating metal ions such as copper and iron | - | (i) ORAC (oxygen radical absorbance capacity) (ii) ORAC-FL | In vitro | NI |
| [11] | The styryl function and steric or electronic factors through the introduction of the piperazine groups | ability to counteract the formation of ROS | chelate metals such as iron and copper | - | (i) ROS production assay (DCFH-DA probe) (ii) ORAC | In vitro (SH-SY5Y cells) | NI |
| [16] | Phenolic hydroxy groups | - | - | - | DPPH | In vitro | pass |
| [27,40] | Hydroxyl substituent on the aromatic ring Keto-enolic group A phenolic group combined with methoxyl moiety in ortho position | complexes display possible superoxide dismutase (SOD)-like activity | ability to bind Cu(II) ion | - | (i) DPPH (ii) xanthine/xanthine oxidase assay | In vitro | NI |
| [87] | Phenolic OH group CH2 group of the β-diketone moiety Pyrazole ring andmethoxy groups | enhancing levels of: (i) GSH (mg/g brain tissue) (ii) paraxosnase (kl/mg protein) | - | decrease in brain 8-OHG level, caspase-3 level, and brain p53 level | - | In vitro In vivo (female albino rats) | pass |
| [22] | 4,4′-((1E,1′E)-(1-phenyl-1H- pyrazole-3,5-diyl)bis(ethene-2,1-diyl))bis(2-methoxyphenol) | (i) O2•- scavenging activity was much lower than that of curcumin (ii) OH• scavenging activity was similar to that of curcumin | - | - | (i) ORAC (O2•-) (ii) HORAC (OH•) | In vitro | pass |
| [39] | Incorporates a C5-monoketone spacer moiety and phenolic rings bearing hydroxy groups at positions 2,3,2′,5′ | - | - | - | DPPH | In vitro | |
| [23] | (1E,4Z,6E)-5-hydroxy-1,7-bis(4-hydroxyphenyl)hepta-1,4,6-trien-3-one | - | - | - | (i) DPPH (ii) FRAP assay | In vitro | NI |
| [43,88] | (E)-N-(2,4-dimethylphenyl)-2,2,2-trifluoro-N′-(3-methoxy-benzylidene)acetohydrazide | - | - | exhibits favorable bindingat the allosteric site of mATP synthase with considerable electrostatic energy contributionsfrom Gln215, Gly217, Thr219, Asp312, Asp313, Glu371, and Arg406 | - | (i)homology modeling, validation, and active site identification (ii) molecular dynamics (MD) simulation (ii) docking | NI |
| [89] | 7-(4-Hydroxy-3-methoxyphenyl)-1-phenyl-4E-hepten-3-one | - | - | (i)protects cortical neurons fromOGD/R-induced autophagic apoptosis (ii) suppresses OGD/R-induced autophagyand apoptosis in mTOR-dependent manner | OGD/Rinducedcell apoptosis was detected using the Annexin VFITC/PI (propidium iodide) Apoptosis detection kit | In vivo | pass |
| [81] | A 7-carbon dienone spacer A 5-carbon enone spacer with and without a ring A 3-carbon enone spacer | activation of antioxidant Nrf2 signaling | - | - | DPPH radical scavenger assay | In vitro (Nrf2-ARE reporter-HepG2 stable cell line) | NI |
| [90] | Bivalent compounds with varied spacer length and cell membrane anchor moiety | - | - | (i) can reverse the increased mitochondrial membrane potential (ii) can abolish the cytosolic Ca2+increase upon TC removal | (i) MC65 apoptosis assay (ii) MC65 mitochondrial membrane potential assay (iii) SH-SY5Y mitochondrial membrane potential assay | In vitro (SH-SY5Y andMC65 cells) | NI |
| [29] | Gd(III) (diethylenetriamine-penta-acetate)-linked-2-(4-((1E,4Z,6E)-5-hydroxy-7-(4-hydroxy-3-methoxy-phenyl)-3-oxohepta-1,4,6-trien-1-yl)-2-methoxy-phenoxy)-N-(2-(3-(ptolyl)thioureido)ethyl)acetamide | - | metal binding properties | - | trolox equivalent antioxidant capacity (TEAC) assay | In vitro (N2a cell line) | NI |
| [91] | Tetrahydrocurcumin | - | - | tetrahydrocurcumin treatment suppressed neuronal apoptosis in injured brains | - | In vivo | pass |
| [62] | Cholesterylamine spacer (length of 17 atoms) | antioxidant activity | metal-chelating properties (Cu, Fe, and Zn) | - | ROS production assay (DCFH-DA probe) | In vitro (MC65 cells) | NI |
| [92] | Bivalent compounds with varied spacer length and cell membrane anchor moiety | - | - | suppress the change of MMP, possibly via interaction with the mitochondrial complex I | (i) MC65 mitochondrial membrane potential assay (ii) SH-SY5Y mitochondrial membrane potential assay | In vitro (MC65 and SH-SY5Y cells) | NI |
| [26] | (1E,4Z,6E)-1-(4-(benzyloxy)phenyl)-5-hydroxy-7-(4-hydroxy-3-methoxyphenyl)hepta-1,4,6-trien-3-one (1E,4Z,6E)-5-hydroxy-7-(4-hydroxy-3-methoxyphenyl)-1-(p-tolyl)hepta-1,4,6-trien-3-one (1E,4Z,6E)-7-(4-(benzyloxy)phenyl)-5-hydroxy-1-(4-methoxyphenyl)hepta-1,4,6-trien-3-one | ROS NQO1 induction | - | - | (i) ROS production assay (DCFH-DA probe) (ii) assay for NQO1 induction | In vitro (T67 cells) | pass |
| [65,66] | 5-(4-Hydroxyphenyl)-N-(2-(5-methoxy-1H-indol-3-yl)ethyl)-3-oxopentanamide | possible function against ROS accumulation | - | increased the expression level of complexes I, II, and IV of the mitochondria electron transport chain in the brain tissue of APP/PS1 mice | (i) ROS production assay (DCFH-DA probe) (ii) hydrogen peroxide toxicity | In vitro (MC65 cells, HT22 cells) In vivo | pass |
| [24] | 4-(1-Benzylpiperidin-4-yl)-1-(3-hydroxy-4-methoxyphenyl)butane-1,3-dione | antioxidant activity | metal-chelating properties | - | ORAC-FL | In vitro (PC12 cells) | pass |
| [68] | Methoxy and hydroxyl groups | antioxidant activity | - | - | ORAC | In vitro | pass |
| [69,93] | (E)-(1-benzylpiperidin-4-yl)methyl 3-(4-hydroxy-3-methoxyphenyl)acrylate | (i) decreases ROS (ii) increases GSH levels | - | - | (i) ROS production assay (DCFH-DA probe) (ii) glutathione (GSH) assay | In vitro (human neuronal cells) In vivo | pass |
| [58] | 2-Methoxy-4-(piperidin-1-ylmethyl)phenyl dimethylcarbamate | antioxidant activity | metal-chelating properties | - | ABTS assay | In vitro | NI |
| [94] | Phenolic hydroxyl group | antioxidant activity | metal-chelating properties | - | ORAC | In vitro | NI |
| Targeting Amyloid-βPlaques | ||||
|---|---|---|---|---|
| Ref. | Structure’s Name | Imaging | Effects | Potential Application |
| [108] | CRANAD-2 | NIRF | high affinity for Aβ aggregates | potentially used as a tool for drug screening |
| [109] | Me-CUR 9 | NIRF | detectamyloid-β fibrils with high sensitivity | usefulin vitro amyloid fluorescence sensor |
| [33] | GRANAD-3 | NIRF | capable of detecting both soluble and insoluble Aβ species | potential to have a high impact on AD drug development. |
| [34] | CRANAD-17 | NIRF | capable of inhibiting Aβ42 crosslinking induced by copper | potential for AD diagnosis and theraphy |
| [67] | curcumin-derivative liposomes | NIRF | high affinity for the amyloid deposits, on post-mortembrains samples of AD patients | used asAD theragnostic nanoformulations, to carry therapeutic and/or imaging agents to amyloid deposits in the brain |
| [110] | BMAOI 14 | NIRF | ability to label and detect aggregated amyloid-β (Aβ) peptide as a fluorescent probe | Aβ imaging probes |
| [109] | CRANAD-28 | two-photon imaging | could inhibit the crosslinking of amyloid beta induced either by copper or by natural conditions | could contribute to AD diagnosis and therapy development in the future |
| [111] | [125I] 1,5-diphenyl-1,4-pentadien-3-one derivative | NIRF | high binding affinities with Aβ plaques | potential amyloid imaging agent for the detection of senile plaques in AD |
| [112] | [3H]AB14 | Autoradiography | significantly higher specific binding in cortical AD brain tissue | potential radioligands for Aβ plaque neuroimaging |
| [113] | 68Ga(CUR)2+, 68Ga(DAC)2+, 68Ga(bDHC)2+ | NIRF | high affinity for amyloid-β plaques | potentially directed to the diagnosis of AD |
| [114] | 68Ga(CUR)2+ and 68Ga(DAC)2+ | NIRF | affinityto synthetic amyloid-β fibrils | possibility of synthesizing a mixed radioactive/fluorescent pharmacophore that can beexploited as a dual-mode imaging tool. |
| [4] | [18F] 1-(4-fluoroethyl)-7-(4′-methyl)curcumin 1 | NIRF | high binding affinity for Aβ1–42 aggregates, suitablelipophilicity, specific binding to Aβ plaques in Tg APP/PS-1 mouse brain sections | may be a potential radioligandfor Aβ plaque imaging |
| [115] | [18F] 4′-dimethylamino-4″-(2-(2-fluoroethoxy)ethoxy)curcuminoid | NIR | affinity for senile plaques | for Aβ imaging |
| [116] | [125I] and [18F]dibenzylideneacetone derivatives | Autoradiography | affinity towardAβ1–42 aggregates | potential new scaffold for amyloid-β imaging probes |
| [117] | [19F] FMeC1 | MRI | affinity for senile plaques in human brain sections | detecting Aβ deposition in the brain |
| [118] | [18F]2-[3,5-bis (4-hydroxy-3-methoxystyryl)-1Hpyrazol-1-yl]-N-{1-[2-(2-(2-fluoroethoxy)ethoxy)ethyl)-1H-1,2,3-triazol-4-yl]methyl}acetamide | PET | high amyloid-β plaque binding | a promising tracer for Aβ imaging |
| [107] | [18F]-CRANAD-101 | PE | significant response to both soluble and insoluble Aβs | potentialfor detecting the early abnormality of the accumulation of Aβs |
| Targeting Tau-Fibrils | ||||
| [119] | (1E,4Z,6E)-1,7-bis(4-(dimethylamino)-2,6-dimethoxyphenyl)-5-hydroxyhepta-1,4,6-trien-3-one | NIRF | selectively detected tau fibrils | a promising NIRfluorescent probe for noninvasive imaging in patients with AD |
| [120] | Difluoroboron β–Diketonate Probe | NIRF | specific to tau fibrils | potential as a tau-specific fluorescent dye in bothin vitro and ex vivo settings |
| [121] | 4,4′-(1E,1′E)-2,2′-(pyrimidine-4,6-diyl)bis(ethene-2,1-diyl)bis(N,N-dimethylaniline) | NIRF | affinity for Tau aggregates | potential for an endoscopic diagnosis of AD in the olfactory system |
| Curcumin-Based Compounds | Aβ Aggregation | Tau Formation | Neuro-Inflammation | Oxidative Stress | AChE | BBB |
|---|---|---|---|---|---|---|
| AB3 | + | - | + | + | - | + |
| AB6 | + | + | - | - | - | ∆ |
| AB7 | + | - | - | + | - | + |
| AB8 | + | - | - | + | - | + |
| AB9 | + | - | + | + | - | + |
| AB10 | + | + | + | + | + | + |
| AB14 | + | - | + | + | + | + |
| AB15 | + | - | - | + | - | - |
| AB17 | + | + | - | + | - | + |
| AB19 | + | - | + | + | - | - |
| AB21 | + | - | + | - | - | - |
| AB22 | + | - | - | + | - | + |
| AB26 | + | - | - | + | - | + |
| AB29 | + | + | - | - | - | + |
| AB31 | + | - | - | + | - | - |
| AB41 | + | - | - | + | + | - |
| AB42 | + | - | - | + | - | - |
| AB43 | + | - | + | + | - | - |
| AB44 | + | + | - | - | - | + |
| AB46 | + | - | - | + | - | - |
| AB47 | + | - | - | + | - | - |
| AB48 | + | - | - | + | - | - |
| AB52 | + | - | + | + | - | + |
| AB56 | - | - | + | + | - | - |
| AB57 | - | - | + | + | - | - |
| AB69 | + | - | - | + | - | - |
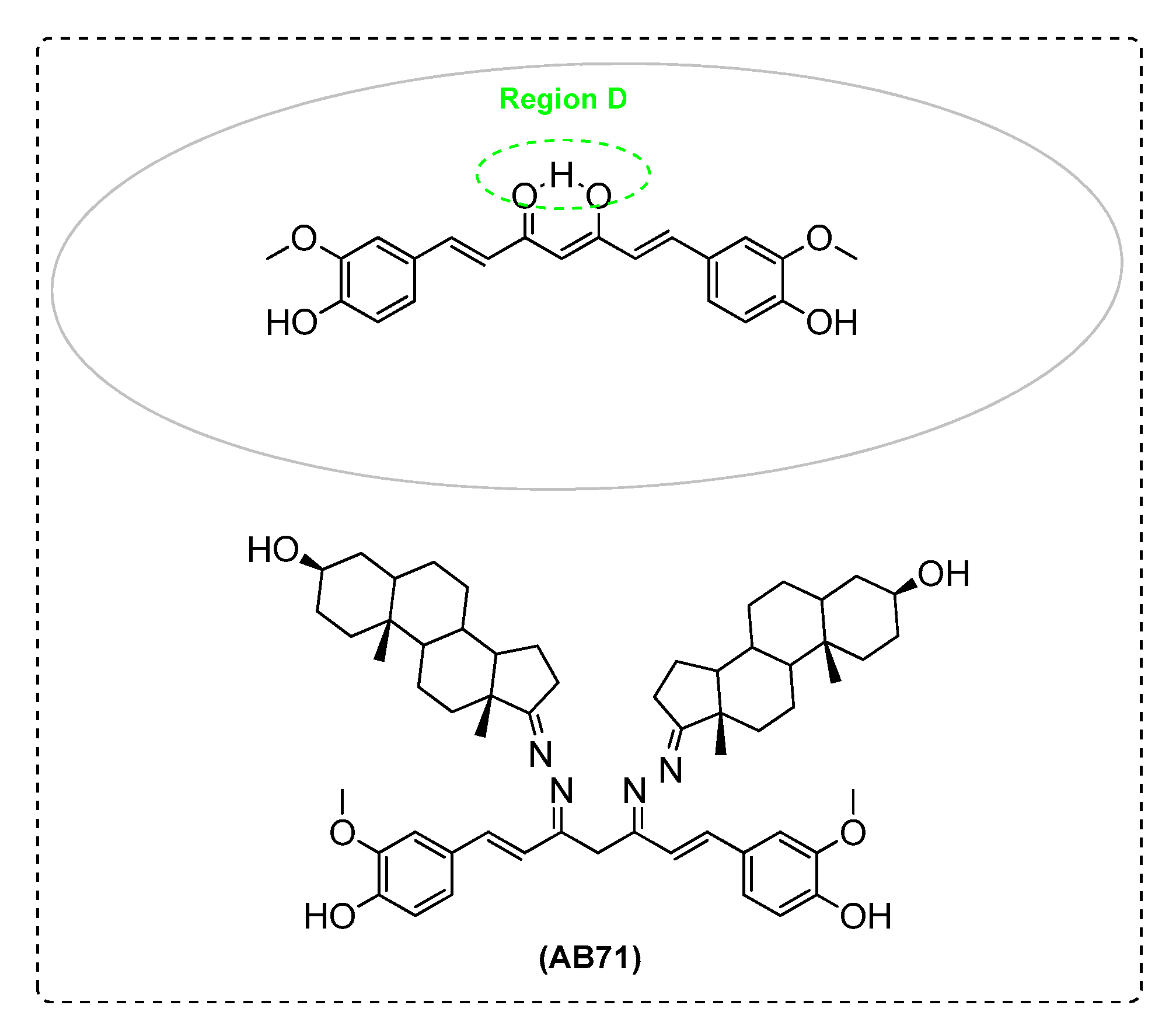
| AB71 | - | - | - | + | + | + |
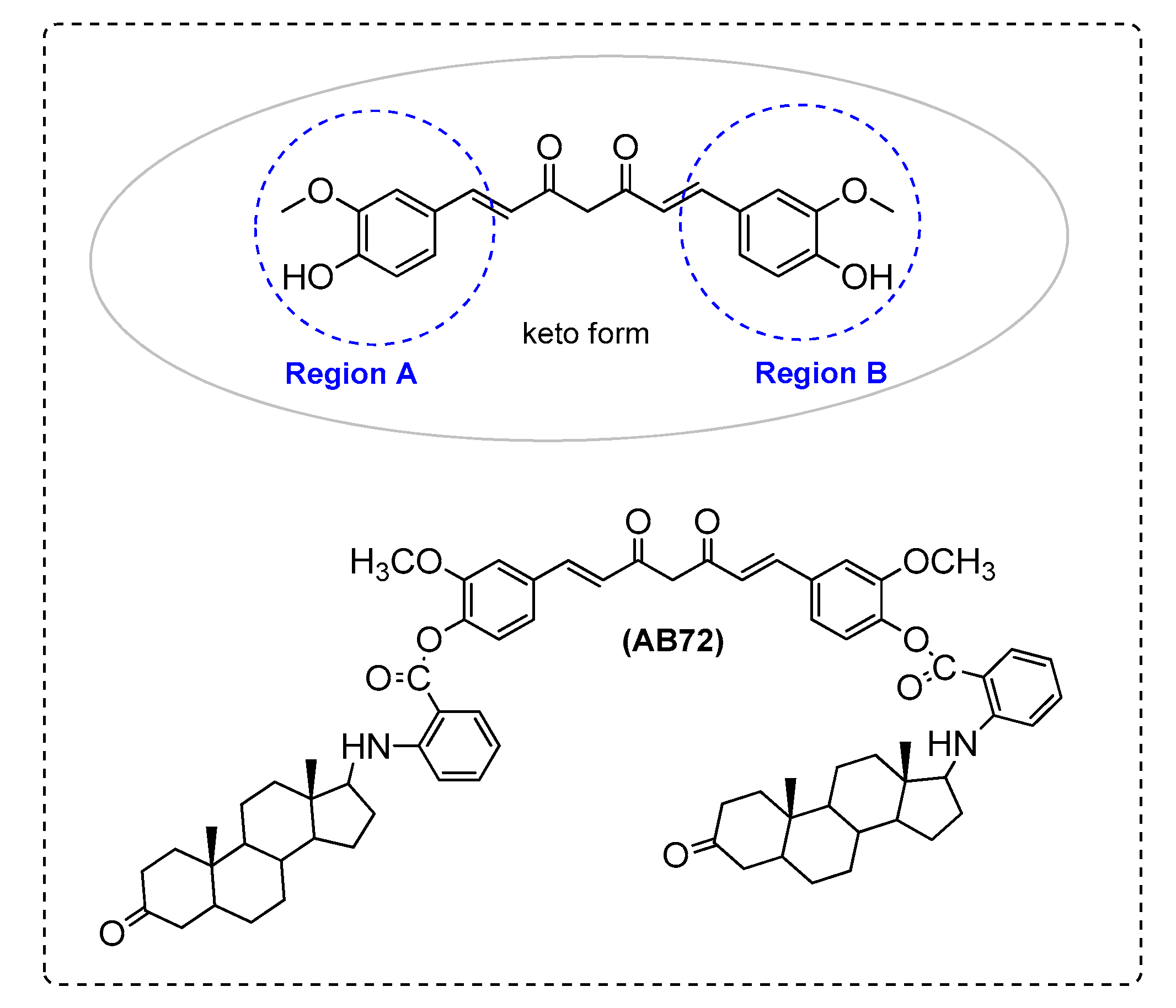
| AB72 | - | - | - | + | + | + |
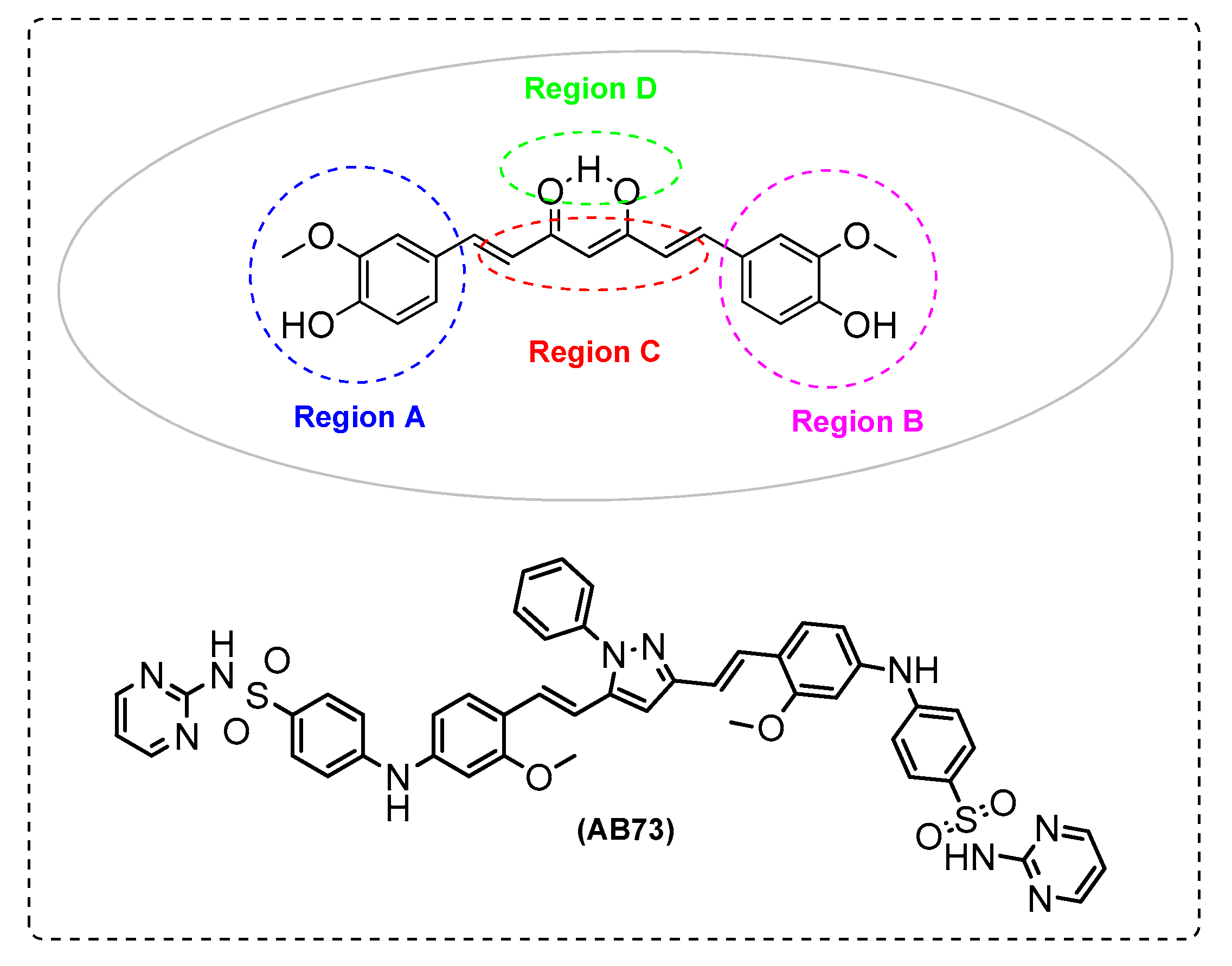
| AB73 | - | - | - | + | + | + |
| AB76 | + | - | - | + | + | + |
| AB77 | + | - | - | + | + | ∆ |
| AB78 | + | ∆ | + | + | - | + |
| AB79 | + | - | + | + | - | + |
| AB80 | + | - | - | + | + | - |
| AB81 | - | - | - | + | + | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chainoglou, E.; Hadjipavlou-Litina, D. Curcumin in Health and Diseases: Alzheimer’s Disease and Curcumin Analogues, Derivatives, and Hybrids. Int. J. Mol. Sci. 2020, 21, 1975. https://doi.org/10.3390/ijms21061975
Chainoglou E, Hadjipavlou-Litina D. Curcumin in Health and Diseases: Alzheimer’s Disease and Curcumin Analogues, Derivatives, and Hybrids. International Journal of Molecular Sciences. 2020; 21(6):1975. https://doi.org/10.3390/ijms21061975
Chicago/Turabian StyleChainoglou, Eirini, and Dimitra Hadjipavlou-Litina. 2020. "Curcumin in Health and Diseases: Alzheimer’s Disease and Curcumin Analogues, Derivatives, and Hybrids" International Journal of Molecular Sciences 21, no. 6: 1975. https://doi.org/10.3390/ijms21061975
APA StyleChainoglou, E., & Hadjipavlou-Litina, D. (2020). Curcumin in Health and Diseases: Alzheimer’s Disease and Curcumin Analogues, Derivatives, and Hybrids. International Journal of Molecular Sciences, 21(6), 1975. https://doi.org/10.3390/ijms21061975






