Actin Depolymerizing Factor Modulates Rhizobial Infection and Nodule Organogenesis in Common Bean
Abstract
1. Introduction
2. Results
2.1. ADF Genes Constitute a Family of Nine Members in P. vulgaris
2.2. PvADF Genes Are Expressed in Roots and after Rhizobial Inoculation
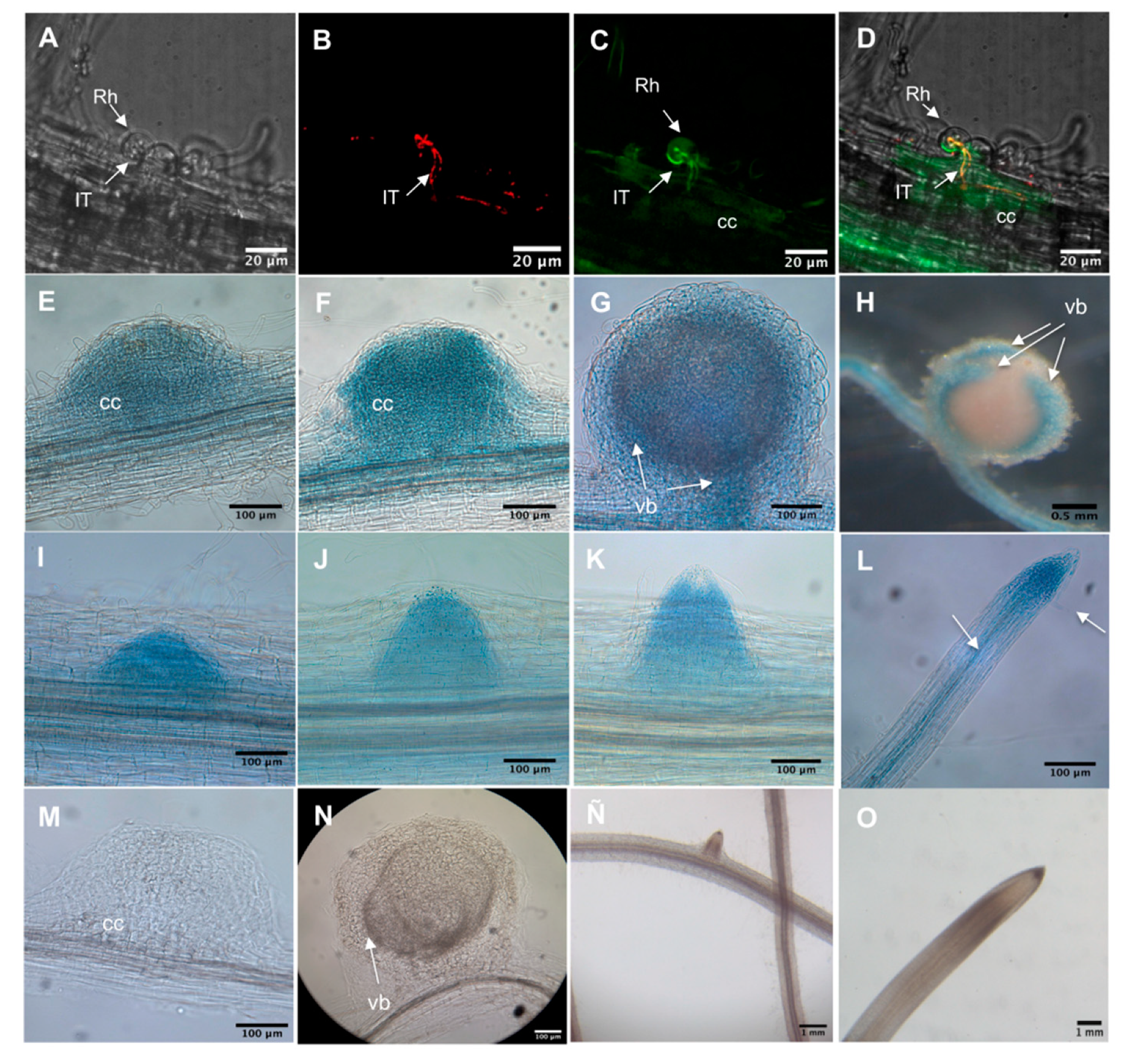
2.3. PvADFE Promoter Activity in Transgenic P. vulgaris after Rhizobial Inoculation
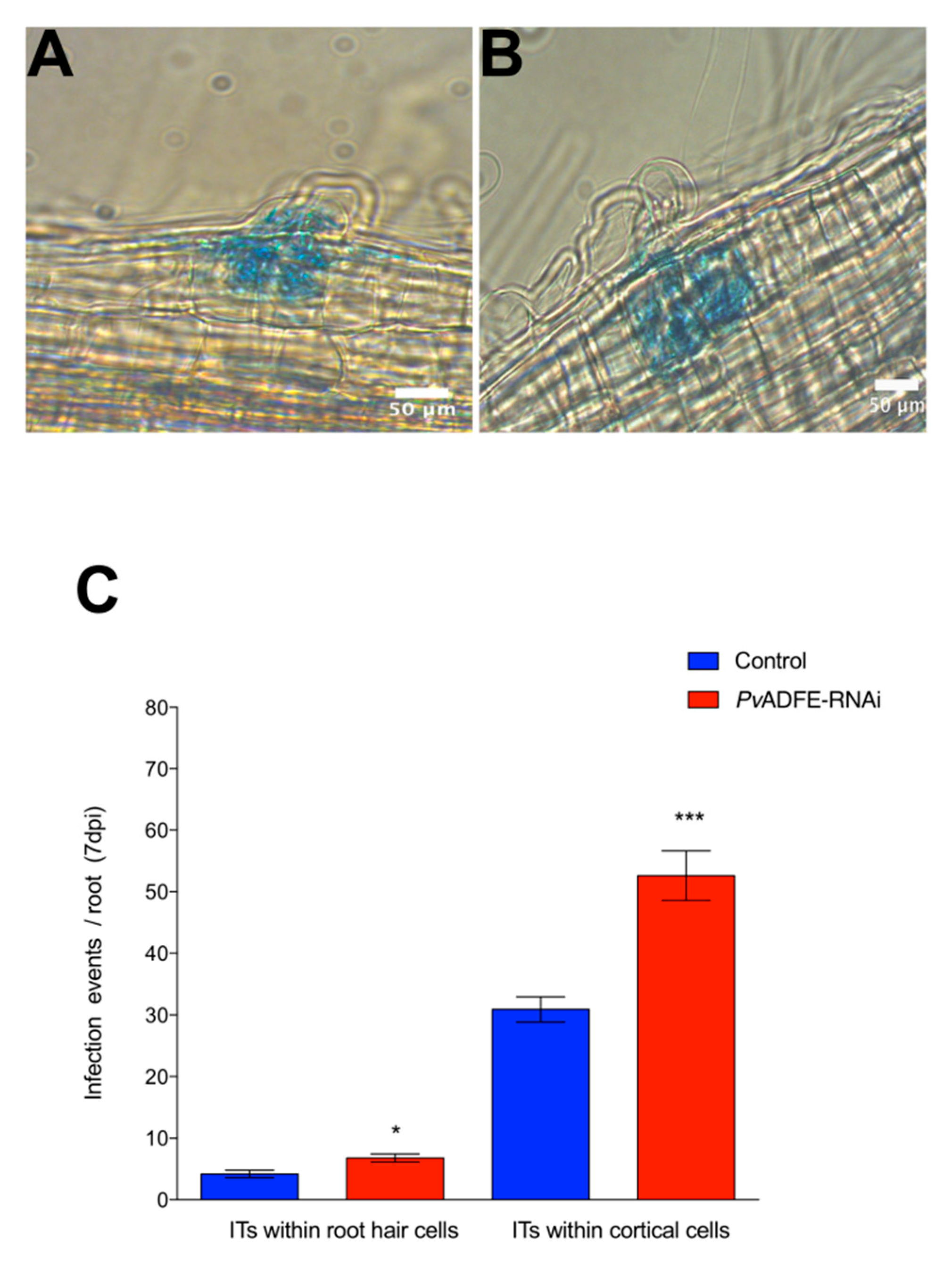
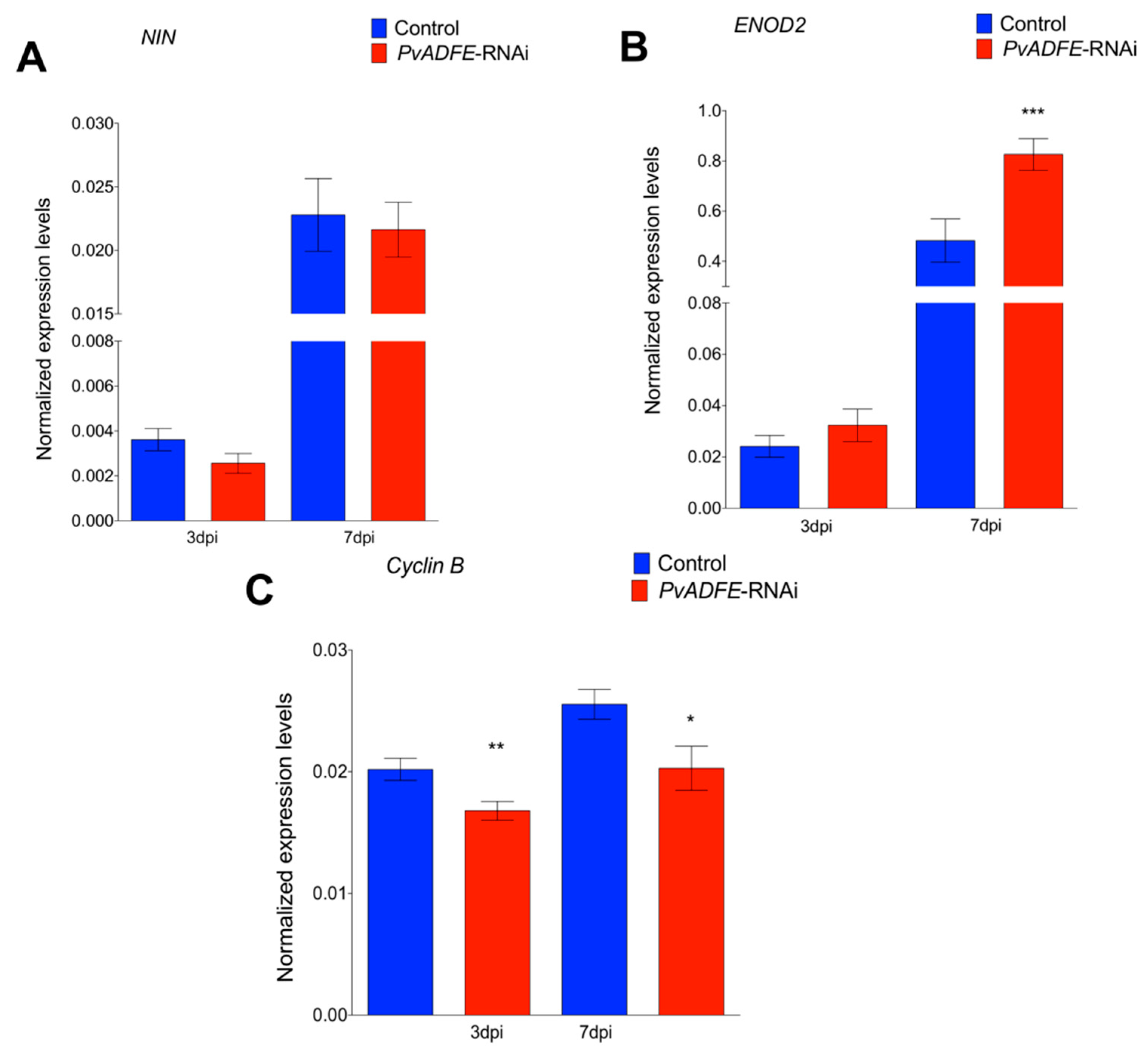
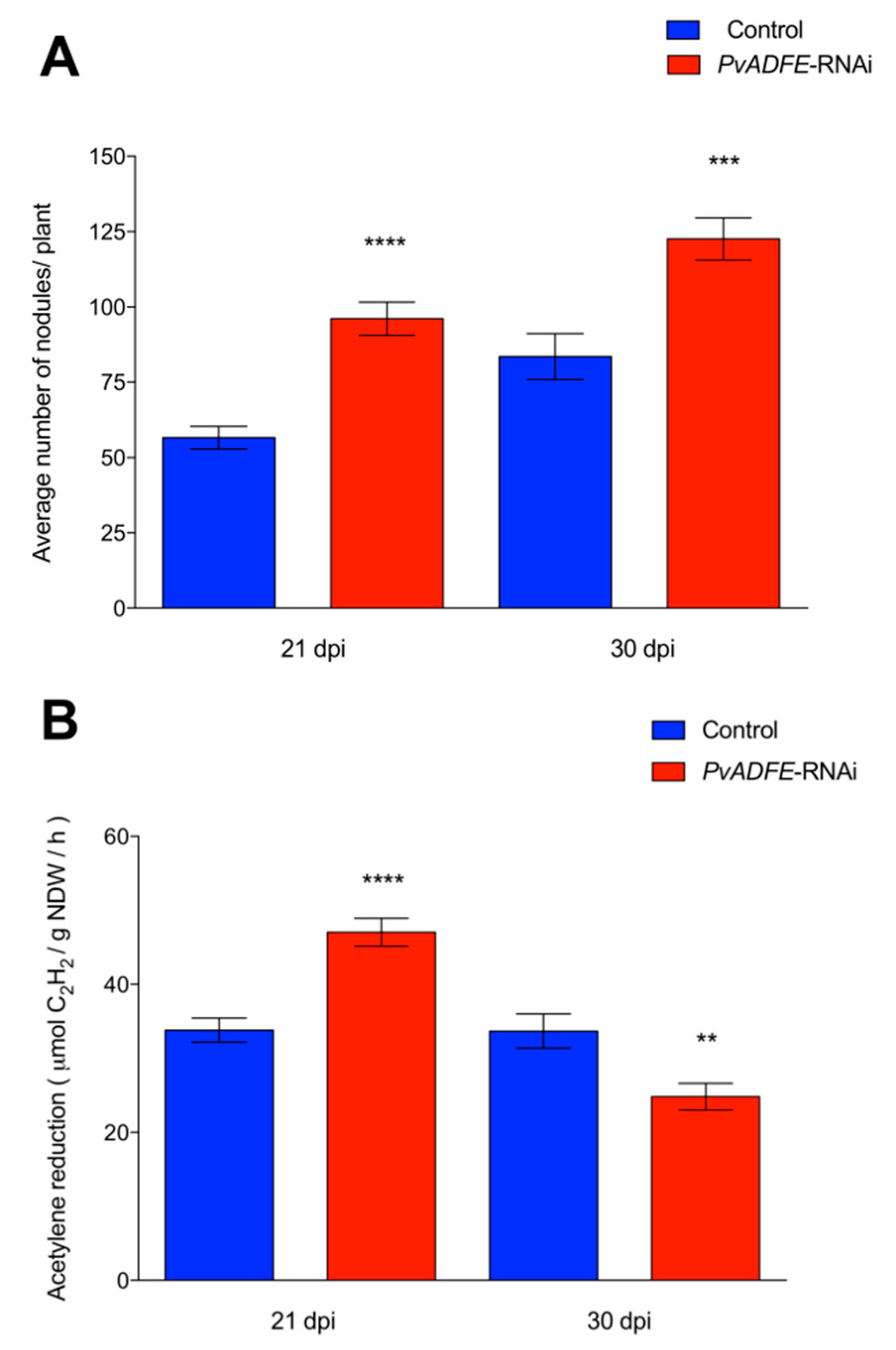
2.4. PvADFE Down-Regulation Increases the Number of Infection Events, Nodule Number, and Nitrogen Fixation in Rhizobium-Inoculated Transgenic Roots
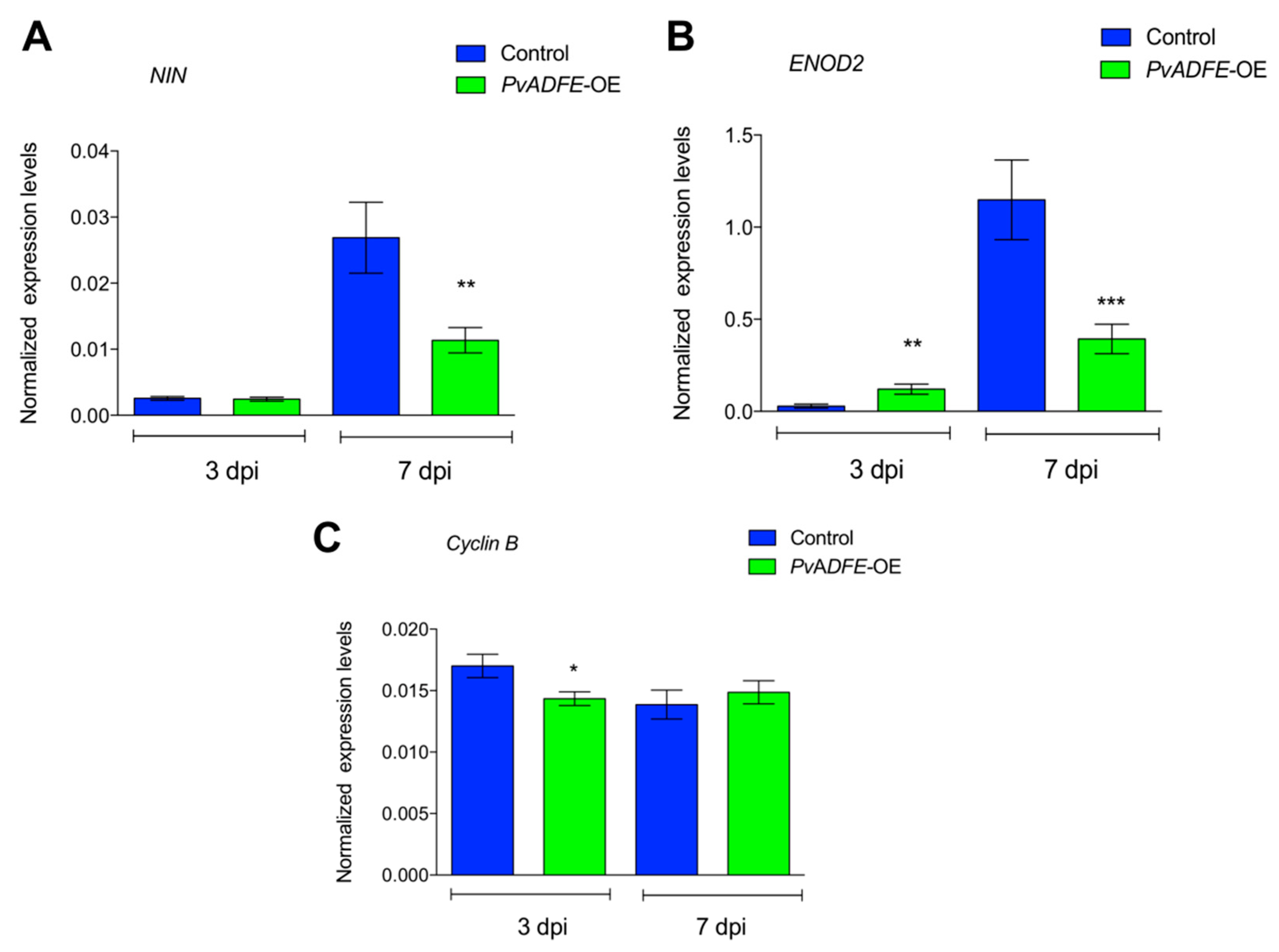
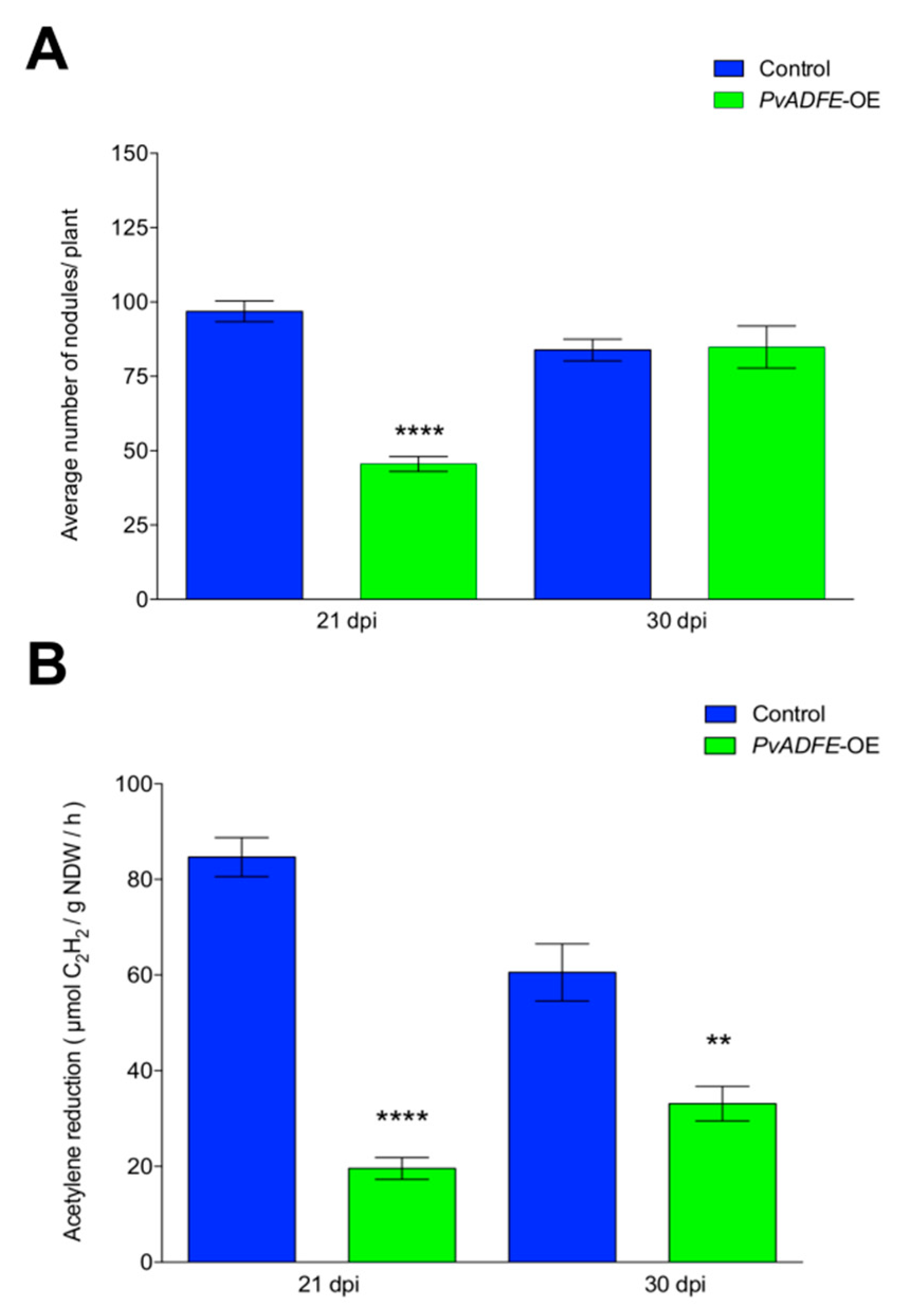
2.5. IT Progression, Nodule Number, and Nitrogen Fixation Are Impaired in PvADFE-Overexpressing Hairy Roots
3. Discussion
4. Materials and Methods
4.1. Plant Growth Conditions and Rhizobia Inoculation
4.2. Identification of PvADF Sequences, Three-Dimensional Structure Analysis, and Relative Transcript Accumulation in Different Organs and Tissues
4.3. RT-qPCR Assays
4.4. Plasmid Construction
4.5. Promoter Activity Analysis
4.6. Analysis of Infection Events, Nodule Number, and Nodule Diameter
4.7. Acetylene Reduction Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADFs | Actin Depolymerization Factors |
| NFs | Nod factors |
| IT | Infection Thread |
| ARP3 | Actin Related Protein 3 |
| SCARN | Suppressor of cAMP Receptor Refect-Nodulation |
| ABP | Actin-Binding |
| ADF-H | Proteins Actin-Depolymerizing Factor Homology |
| PIP2 | Phosphatidylinositol 4,5-bisphosphate |
| RPS5 | Disease Resistance Protein 5 |
| dpi | days post-inoculation |
| GFP | Green Fluorescent Protein |
| NDW | Nodule Dry Weight |
| GUS | β-glucuronidase |
| NIN | Nodule Inception |
| ENOD2 | Early Nodulin2 |
References
- Laranjo, M.; Alexandre, A.; Oliveira, S. Legume growth-promoting rhizobia: An overview on the Mesorhizobium genus. Microbiol. Res. 2014, 169, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, D.W.; Wais, R.; Long, S.R. Calcium spiking in plant root hairs responding to Rhizobium nodulation signals. Cell 1996, 85, 673–681. [Google Scholar] [CrossRef]
- Cárdenas, L.; Vidali, L.; Domínguez, J.; Pérez, H.; Sánchez, F.; Hepler, P.K.; Quinto, C. Rearrangement of actin microfilaments in plant root hairs responding to Rhizobium etli nodulation signals. Plant Physiol. 1998, 116, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Felle, H.H.; Kondorosi, É.; Kondorosi, A.; Schultze, M. Rapid alkalinization in alfalfa root hairs in response to rhizobial lipochitooligosaccharide signals. Plant J. 1996, 10, 295–301. [Google Scholar] [CrossRef]
- Cárdenas, L.; Martínez, A.; Sánchez, F.; Quinto, C. Fast, transient and specific intracellular ROS changes in living root hair cells responding to Nod factors (NFs). Plant J. 2008, 56, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, L.; Holdaway-Clarke, T.L.; Sánchez, F.; Quinto, C.; Feijó, J.A.; Kunkel, J.G.; Hepler, P.K. Ion changes in legume root hairs responding to Nod factors. Plant Physiol. 2000, 123, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, D.W.; Atkinson, E.; Long, S.R. Depolarization of alfalfa root hair membrane potential by Rhizobium meliloti Nod factors. Science 1992, 256, 998–1000. [Google Scholar] [CrossRef]
- Oldroyd, G.E.D.; Murray, J.D.; Poole, P.S.; Downie, J.A. The rules of engagement in the legume-rhizobial symbiosis. Annu. Rev. Genet. 2011, 45, 119–144. [Google Scholar] [CrossRef]
- Oldroyd, G.E.D. Speak, friend, and enter: Signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol. 2013, 11, 252–263. [Google Scholar] [CrossRef]
- Fournier, J.; Teillet, A.; Chabaud, M.; Ivanov, S.; Genre, A.; Limpens, E.; de Carvalho-Niebel, F.; Barker, D.G. Remodeling of the Infection Chamber before Infection Thread Formation Reveals a Two-Step Mechanism for Rhizobial Entry into the Host Legume Root Hair. Plant Physiol. 2015, 167, 1233–1242. [Google Scholar] [CrossRef]
- Long, S.R. SnapShot: Signaling in Symbiosis. Cell 2016, 167, 582. [Google Scholar] [CrossRef] [PubMed]
- Gutjahr, C. Symbiosis: Plasmodesmata Link Root-Nodule Organogenesis with Infection. Curr. Biol. 2018, 28, R1400–R1403. [Google Scholar] [CrossRef] [PubMed]
- Gaudioso-Pedraza, R.; Beck, M.; Frances, L.; Kirk, P.; Ripodas, C.; Niebel, A.; Oldroyd, G.E.D.; Benitez-Alfonso, Y.; de Carvalho-Niebel, F. Callose-Regulated Symplastic Communication Coordinates Symbiotic Root Nodule Development. Curr. Biol. 2018, 28, 3562–3577. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.L.; Newcomb, W. Changes in actin microfilament arrays in developing pea root nodule cells. Can. J. Bot. 2001, 79, 767–776. [Google Scholar]
- Yokota, K.; Fukai, E.; Madsen, L.H.; Jurkiewicz, A.; Rueda, P.; Radutoiu, S.; Held, M.; Hossain, M.S.; Szczyglowski, K.; Morieri, G.; et al. Rearrangement of actin cytoskeleton mediates invasion of Lotus japonicus roots by Mesorhizobium loti. Plant Cell 2009, 21, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Timmers, A.C.J. The role of the plant cytoskeleton in the interaction between legumes and rhizobia. J. Microsc. 2008, 231, 247–256. [Google Scholar] [CrossRef]
- De Ruijter, N.C.A.; Bisseling, T.; Emons, A.M.C. Rhizobium Nod factors induce an increase in sub-apical fine bundles of actin filaments in Vicia sativa root hairs within minutes. Mol. Plant Microbe Interact. 1999, 12, 829–832. [Google Scholar] [CrossRef]
- Zepeda, I.; Sánchez-López, R.; Kunkel, J.G.; Bañuelos, L.A.; Hernández-Barrera, A.; Sánchez, F.; Quinto, C.; Cárdenas, L. Visualization of highly dynamic F-actin plus ends in growing Phaseolus vulgaris root hair cells and their responses to Rhizobium etli Nod factors. Plant Cell Physiol. 2014, 55, 580–592. [Google Scholar] [CrossRef]
- Zhang, X.; Han, L.; Wang, Q.; Zhang, C.; Yu, Y.; Tian, J.; Kong, Z. The host actin cytoskeleton channels rhizobia release and facilitates symbiosome accommodation during nodulation in Medicago truncatula. New Phytol. 2019, 221, 1049–1059. [Google Scholar] [CrossRef]
- Ketelaar, T. The actin cytoskeleton in root hairs: All is fine at the tip. Curr. Opin. Plant Biol. 2013, 16, 749–756. [Google Scholar] [CrossRef]
- McCurdy, D.W.; Kovar, D.R.; Staiger, C.J. Actin and actin-binding proteins in higher plants. Protoplasma 2001, 215, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Nagawa, S.; Xu, T.; Yang, Z. RHO GTPase in plants: Conservation and invention of regulators and effectors. Small GTPases 2010, 1, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Facette, M.R.; Park, Y.; Sutimantanapi, D.; Luo, A.; Cartwright, H.N.; Yang, B.; Bennett, E.J.; Sylvester, A.W.; Smith, L.G. The SCAR/WAVE complex polarizes PAN receptors and promotes division asymmetry in maize. Nat. Plants 2015, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gavrin, A.; Jansen, V.; Ivanov, S.; Bisseling, T.; Fedorova, E. ARP2/3-mediated actin nucleation associated with symbiosome membrane is essential for the development of symbiosomes in infected cells of Medicago truncatula root nodules. Mol. Plant Microbe Interact. 2015, 28, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Lin, J.-S.; Xu, J.; Sato, S.; Parniske, M.; Wang, T.L.; Downie, J.A.; Xie, F. SCARN a novel class of SCAR protein that is required for root-hair infection during legume Nodulation. PLoS Genet. 2015, 11, e1005623. [Google Scholar] [CrossRef]
- Poukkula, M.; Kremneva, E.; Serlachius, M.; Lappalainen, P. Actin-depolymerizing factor homology domain: A conserved fold performing diverse roles in cytoskeletal dynamics. Cytoskeleton 2011, 68, 471–490. [Google Scholar] [CrossRef]
- Lappalainen, P.; Kessels, M.M.; Cope, M.J.T.V.; Drubin, D.G. The ADF homology (ADF-H) domain: A highly exploited actin-binding module. Mol. Biol. Cell 1998, 9, 1951–1959. [Google Scholar] [CrossRef]
- Maciver, S.K.; Hussey, P.J. The ADF/cofilin family: Actin-remodeling proteins. Genome Biol. 2002, 3, reviews3007-1. [Google Scholar] [CrossRef]
- Hayakawa, K.; Sakakibara, S.; Sokabe, M.; Tatsumi, H. Single-molecule imaging and kinetic analysis of cooperative cofilin–actin filament interactions. Proc. Natl. Acad. Sci. USA 2014, 111, 9810–9815. [Google Scholar] [CrossRef]
- Augustine, R.C.; Vidali, L.; Kleinman, K.P.; Bezanilla, M. Actin depolymerizing factor is essential for viability in plants, and its phosphoregulation is important for tip growth. Plant J. 2008, 54, 863–875. [Google Scholar] [CrossRef]
- Gungabissoon, R.A.; Jiang, C.-J.; Drøbak, B.K.; Maciver, S.K.; Hussey, P.J. Interaction of maize actin-depolymerising factor with actin and phosphoinositides and its inhibition of plant phospholipase C. Plant J. 1998, 16, 689–696. [Google Scholar] [CrossRef]
- Allwood, E.G.; Smertenko, A.P.; Hussey, P.J. Phosphorylation of plant actin-depolymerising factor by calmodulin-like domain protein kinase. FEBS Lett. 2001, 499, 97–100. [Google Scholar] [CrossRef]
- Smertenko, A.P.; Jiang, C.-J.; Simmons, N.J.; Weeds, A.G.; Davies, D.R.; Hussey, P.J. Ser6 in the maize actin-depolymerizing factor, ZmADF3, is phosphorylated by a calcium-stimulated protein kinase and is essential for the control of functional activity. Plant J. 1998, 14, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Roy-Zokan, E.M.; Dyer, K.A.; Meagher, R.B. Phylogenetic patterns of codon evolution in the ACTIN-DEPOLYMERIZING FACTOR/COFILIN (ADF/CFL) gene family. PLoS ONE 2015, 10, e0145917. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, D.R.; Kandasamy, M.K.; McKinney, E.C.; Burgos-Rivera, B.; Meagher, R.B. The ancient subclasses of Arabidopsis ACTIN DEPOLYMERIZING FACTOR genes exhibit novel and differential expression. Plant J. 2007, 52, 460–472. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Chaudhry, F.; Ruzicka, D.R.; Meagher, R.B.; Staiger, C.J.; Day, B. Arabidopsis actin-depolymerizing factor AtADF4 mediates defense signal transduction triggered by the Pseudomonas syringae effector AvrPphB. Plant Physiol. 2009, 150, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Porter, K.; Shimono, M.; Tian, M.; Day, B. Arabidopsis actin-depolymerizing factor-4 links pathogen perception, defense activation and transcription to cytoskeletal dynamics. PLoS Pathog. 2012, 8, e1003006. [Google Scholar] [CrossRef]
- Fu, Y.; Duan, X.; Tang, C.; Li, X.; Voegele, R.T.; Wang, X.; Wei, G.; Kang, Z. TaADF7, an actin-depolymerizing factor, contributes to wheat resistance against Puccinia striiformis f. sp. tritici. Plant J. 2014, 78, 16–30. [Google Scholar] [CrossRef]
- Tang, C.; Deng, L.; Chang, D.; Chen, S.; Wang, X.; Kang, Z. TaADF3, an actin-depolymerizing factor, negatively modulates wheat resistance against Puccinia striiformis. Front. Plant Sci. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Mondal, H.A.; Louis, J.; Archer, L.; Patel, M.; Nalam, V.J.; Sarowar, S.; Sivapalan, V.; Root, D.D.; Shah, J. Arabidopsis ACTIN-DEPOLYMERIZING FACTOR3 is required for controlling aphid feeding from the phloem. Plant Physiol. 2018, 176, 879–890. [Google Scholar] [CrossRef]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liu, Q.; Xue, Q. Comparative study of rice and Arabidopsis actin-depolymerizing factors gene families. J. Plant Physiol. 2006, 163, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Khatun, K.; Robin, A.H.K.; Park, J.-I.; Kim, C.K.; Lim, K.-B.; Kim, M.-B.; Lee, D.-J.; Nou, I.S.; Chung, M.-Y. Genome-wide identification, characterization and expression profiling of ADF family genes in Solanum lycopersicum L. Genes 2016, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Wong, E.I.; Vidali, L.; Estavillo, A.; Hepler, P.K.; Wu, H.-M.; Cheung, A.Y. The regulation of actin organization by actin-depolymerizing factor in elongating pollen tubes. Plant Cell 2002, 14, 2175–2190. [Google Scholar] [CrossRef] [PubMed]
- Lappalainen, P.; Fedorov, E.V.; Fedorov, A.A.; Almo, S.C.; Drubin, D.G. Essential functions and actin-binding surfaces of yeast cofilin revealed by systematic mutagenesis. EMBO J. 1997, 16, 5520–5530. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.-J.; Weeds, A.G.; Hussey, P.J. The maize actin-depolymerizing factor, ZmADF3, redistributes to the growing tip of elongating root hairs and can be induced to translocate into the nucleus with actin. Plant J. 1997, 12, 1035–1043. [Google Scholar] [CrossRef]
- Yonezawa, N.; Homma, Y.; Yahara, I.; Sakai, H.; Nishida, E. A short sequence responsible for both phosphoinositide binding and actin binding activities of cofilin. J. Biol. Chem. 1991, 266, 17218–17221. [Google Scholar]
- Bowman, G.D.; Nodelman, I.M.; Hong, Y.; Chua, N.-H.; Lindberg, U.; Schutt, C.E. A comparative structural analysis of the ADF/cofilin family. Proteins Struct. Funct. Bioinform. 2000, 41, 374–384. [Google Scholar] [CrossRef]
- Bou Daher, F.; van Oostende, C.; Geitmann, A. Spatial and temporal expression of actin depolymerizing factors ADF7 and ADF10 during male gametophyte development in Arabidopsis thaliana. Plant Cell Physiol. 2011, 52, 1177–1192. [Google Scholar] [CrossRef]
- Madsen, L.H.; Tirichine, L.; Jurkiewicz, A.; Sullivan, J.T.; Heckmann, A.B.; Bek, A.S.; Ronson, C.W.; James, E.K.; Stougaard, J. The molecular network governing nodule organogenesis and infection in the model legume Lotus japonicus. Nat. Commun. 2010, 1, 1–12. [Google Scholar] [CrossRef]
- Schauser, L.; Roussis, A.; Stiller, J.; Stougaard, J. A plant regulator controlling development of symbiotic root nodules. Nature 1999, 402, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Stougaard, J. Regulators and regulation of legume root nodule development. Plant Physiol. 2000, 124, 531–540. [Google Scholar] [CrossRef]
- Jeleńska, J.; Deckert, J.; Kondorosi, E.; Legocki, A.B. Mitotic B-type cyclins are differentially regulated by phytohormones and during yellow lupine nodule development. Plant Sci. 2000, 150, 29–39. [Google Scholar] [CrossRef]
- Murray, J.D. The Cell Cycle in Nodulation: Molecular Cell Biology of the Growth and Differentiation of Plant Cell; Rose, R.J., Ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Inada, N.; Higaki, T.; Hasezawa, S. Nuclear function of subclass I actin-depolymerizing factor contributes to susceptibility in Arabidopsis to an adapted powdery mildew fungus. Plant Physiol. 2016, 170, 1420–1434. [Google Scholar] [CrossRef] [PubMed]
- Henty-Ridilla, J.L.; Shimono, M.; Li, J.; Chang, J.H.; Day, B.; Staiger, C.J. The plant actin cytoskeleton responds to signals from microbe-associated molecular patterns. PLoS Pathog. 2013, 9, e1003290. [Google Scholar] [CrossRef]
- Day, B.; Henty, J.L.; Porter, K.J.; Staiger, C.J. The pathogen-actin connection: A platform for defense signaling in plants. Annu. Rev. Phytopathol. 2011, 49, 483–506. [Google Scholar] [CrossRef]
- Porter, K.; Day, B. From filaments to function: The role of the plant actin cytoskeleton in pathogen perception, signaling and immunity. J. Integr. Plant Biol. 2015, 58, 299–311. [Google Scholar] [CrossRef]
- Li, P.; Day, B. Battlefield Cytoskeleton: Turning the Tide on Plant Immunity. Mol. Plant Microbe Interact. 2019, 32, 25–34. [Google Scholar] [CrossRef]
- Takemoto, D.; Hardham, A.R. The cytoskeleton as a regulator and target of biotic interactions in plants. Plant Physiol. 2004, 136, 3864–3876. [Google Scholar] [CrossRef]
- Kanellos, G.; Frame, M.C. Cellular functions of the ADF/cofilin family at a glance. J. Cell Sci. 2016, 129, 3211–3218. [Google Scholar] [CrossRef]
- Henty-Ridilla, J.L.; Li, J.; Day, B.; Staiger, C.J. ACTIN DEPOLYMERIZING FACTOR4 regulates actin dynamics during innate immune signaling in Arabidopsis. Plant Cell 2014, 26, 340–352. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Engler, J.; Van Poucke, K.; Karimi, M.; De Groodt, R.; Gheysen, G.; Engler, G.; Gheysen, G. Dynamic cytoskeleton rearrangements in giant cells and syncytia of nematode-infected roots. Plant J. 2004, 38, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Andrianantoandro, E.; Pollard, T.D. Mechanism of ACTIN filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol. Cell 2006, 24, 13–23. [Google Scholar] [CrossRef] [PubMed]
- De La Cruz, E.M. How cofilin severs an actin filament. Biophys. Rev. 2009, 1, 51–59. [Google Scholar] [CrossRef]
- Mun, J.-H.; Lee, S.-Y.; Yu, H.-J.; Jeong, Y.-M.; Shin, M.-Y.; Kim, H.; Lee, I.; Kim, S.-G. Petunia actin-depolymerizing factor is mainly accumulated in vascular tissue and its gene expression is enhanced by the first intron. Gene 2002, 292, 233–243. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Huang, W.-L.; Hong, C.-Y.; Lur, H.-S.; Chang, M.-C. Comprehensive analysis of differentially expressed rice actin depolymerizing factor gene family and heterologous overexpression of OsADF3 confers Arabidopsis Thaliana drought tolerance. Rice 2012, 5, 33. [Google Scholar] [CrossRef]
- Estrada-Navarrete, G.; Alvarado-Affantranger, X.; Olivares, J.E.; Guillen, G.; Diaz-Camino, C.; Campos, F.; Quinto, C.; Gresshoff, P.M.; Sánchez, F. Fast, efficient and reproducible genetic transformation of Phaseolus spp. by Agrobacterium rhizogenes. Nat. Protoc. 2007, 2, 1819–1824. [Google Scholar] [CrossRef]
- Young, J.M.; Kuykendall, L.D.; Martínez-Romero, E.; Kerr, A.; Sawada, H. A revision of Rhizobium Frank 1889, with an emended description of the genus, and the inclusion of all species of Agrobacterium Conn 1942 and Allorhizobium undicola de Lajudie et al. 1998 as new combinations: Rhizobium radiobacter, R. rhizogenes, R. rubi, R. undicola and R. vitis. Int. J. Syst. Evol. Microbiol. 2001, 51, 89–103. [Google Scholar]
- Broughton, W.J.; Dilworth, M.J. Control of leghaemoglobin synthesis in snake beans. Biochem. J. 1971, 125, 1075–1080. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2014, 32, 268–274. [Google Scholar] [CrossRef]
- Minh, B.Q.; Nguyen, M.A.T.; von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. I-TASSER server for protein 3D structure prediction. BMC Bioinform. 2008, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Valdés-López, O.; Arenas-Huertero, C.; Ramírez, M.; Girard, L.; Sánchez, F.; Vance, C.P.; Luis Reyes, J.; Hernández, G. Essential role of MYB transcription factor: PvPHR1 and microRNA: PvmiR399 in phosphorus-deficiency signalling in common bean roots. Plant Cell Environ. 2008, 31, 1834–1843. [Google Scholar] [CrossRef]
- Karimi, M.; Inzé, D.; Depicker, A. GATEWAY vectors for Agrobacterium mediated plant transformation. Trends Plant Sci. 2002, 7, 193–195. [Google Scholar] [CrossRef]
- Jefferson, R.A. Assaying chimeric genes in plants: The GUS gene fusion system. Plant Mol. Biol. Rep. 1987, 5, 387–405. [Google Scholar] [CrossRef]
- Ramírez, M.; Valderrama, B.; Arredondo-Peter, R.; Soberón, M.; Mora, J.; Hernández, G. Rhizobium etli genetically engineered for the heterologous expression of Vitreoscilla sp. hemoglobin: Effects on free-living and symbiosis. Mol. Plant Microbe Interact. 1999, 12, 1008–1015. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Ortega, Y.; Carrasco-Castilla, J.; Juárez-Verdayes, M.A.; Toscano-Morales, R.; Fonseca-García, C.; Nava, N.; Cárdenas, L.; Quinto, C. Actin Depolymerizing Factor Modulates Rhizobial Infection and Nodule Organogenesis in Common Bean. Int. J. Mol. Sci. 2020, 21, 1970. https://doi.org/10.3390/ijms21061970
Ortega-Ortega Y, Carrasco-Castilla J, Juárez-Verdayes MA, Toscano-Morales R, Fonseca-García C, Nava N, Cárdenas L, Quinto C. Actin Depolymerizing Factor Modulates Rhizobial Infection and Nodule Organogenesis in Common Bean. International Journal of Molecular Sciences. 2020; 21(6):1970. https://doi.org/10.3390/ijms21061970
Chicago/Turabian StyleOrtega-Ortega, Yolanda, Janet Carrasco-Castilla, Marco A. Juárez-Verdayes, Roberto Toscano-Morales, Citlali Fonseca-García, Noreide Nava, Luis Cárdenas, and Carmen Quinto. 2020. "Actin Depolymerizing Factor Modulates Rhizobial Infection and Nodule Organogenesis in Common Bean" International Journal of Molecular Sciences 21, no. 6: 1970. https://doi.org/10.3390/ijms21061970
APA StyleOrtega-Ortega, Y., Carrasco-Castilla, J., Juárez-Verdayes, M. A., Toscano-Morales, R., Fonseca-García, C., Nava, N., Cárdenas, L., & Quinto, C. (2020). Actin Depolymerizing Factor Modulates Rhizobial Infection and Nodule Organogenesis in Common Bean. International Journal of Molecular Sciences, 21(6), 1970. https://doi.org/10.3390/ijms21061970




