Chronic Exposure to Low Concentration Lead Chloride-Induced Anxiety and Loss of Aggression and Memory in Zebrafish
Abstract
1. Introduction
2. Results
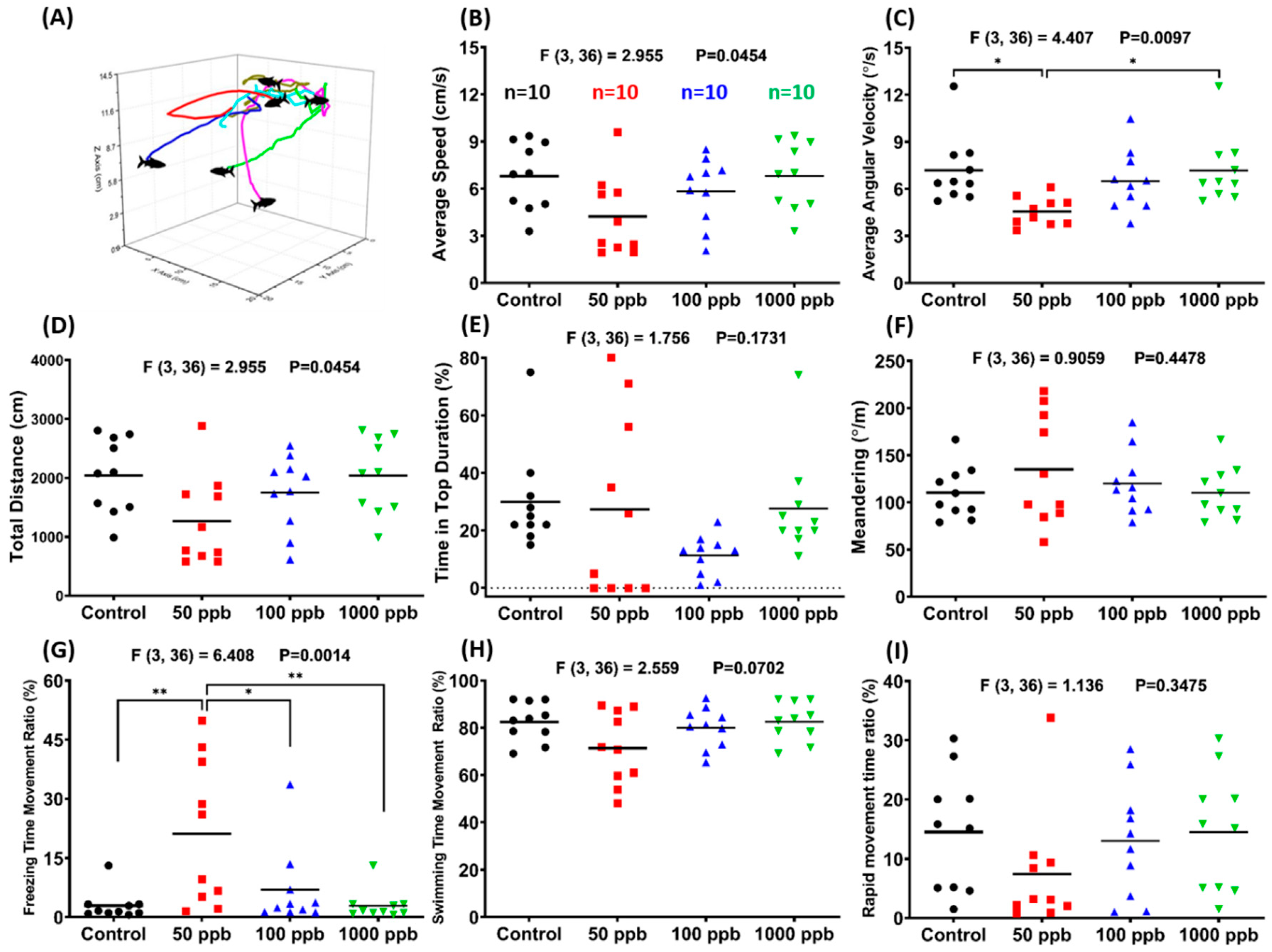
2.1. Lead Exposure Induced Anxiety-Like Behavior in Zebrafish
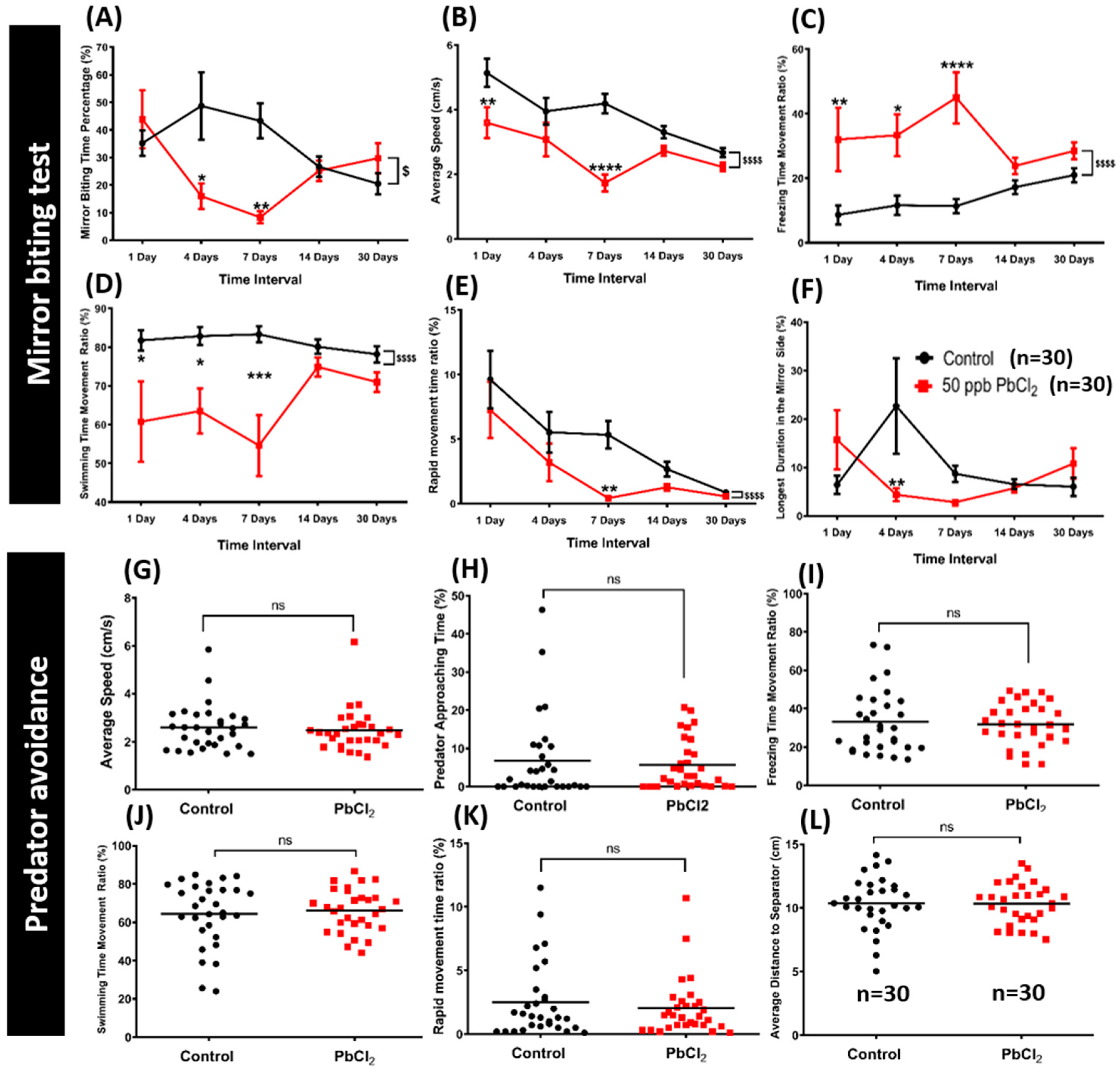
2.2. Lead Exposure Decreased Aggressiveness in Zebrafish
2.3. Lead Exposure Did Not Alter Fear Response in Zebrafish
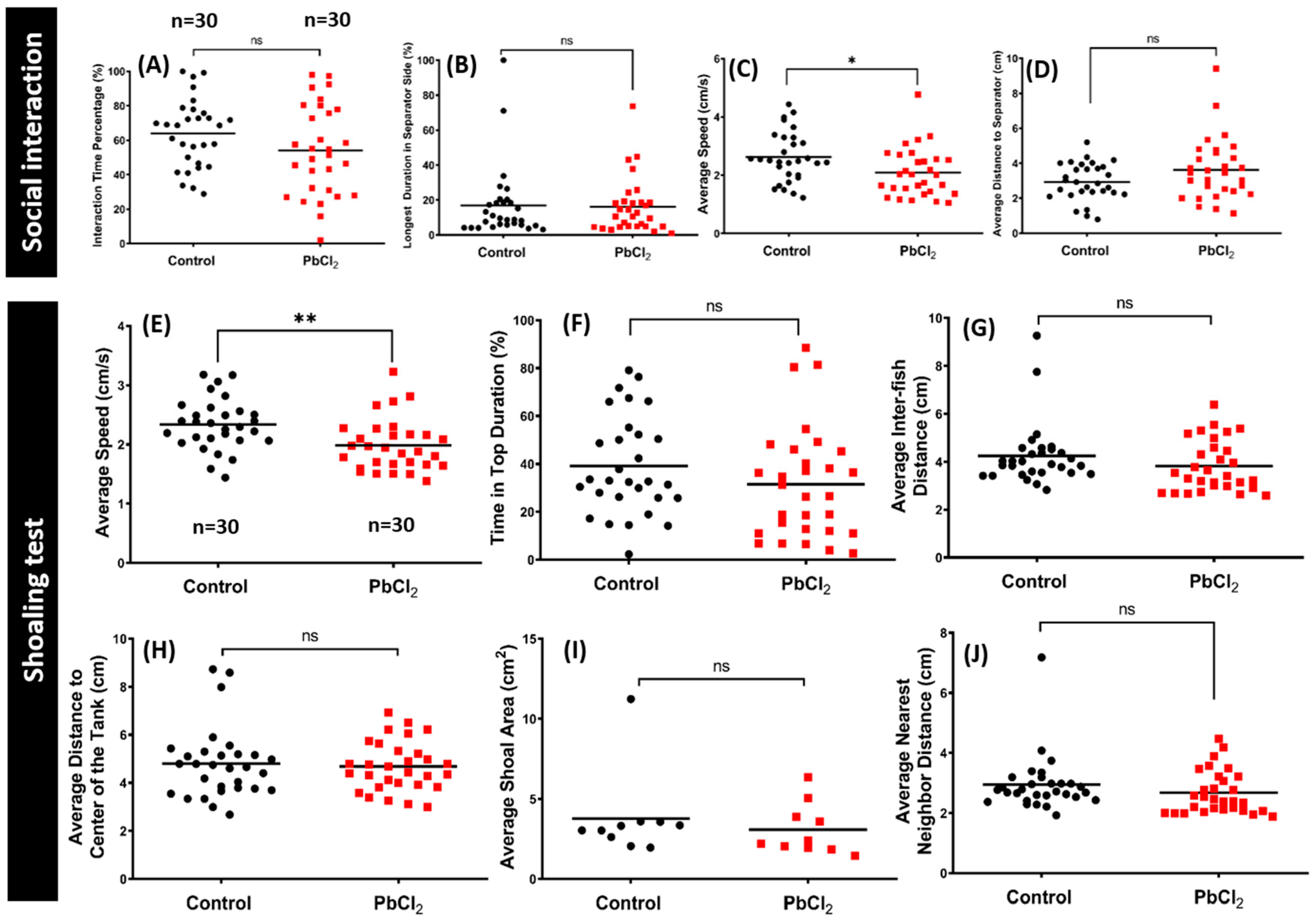
2.4. Lead Exposure Did Not Alter Conspecific Interaction and Shoaling Behaviors
2.5. Lead Exposure Altered Zebrafish Circadian Rhythm Locomotor Activity
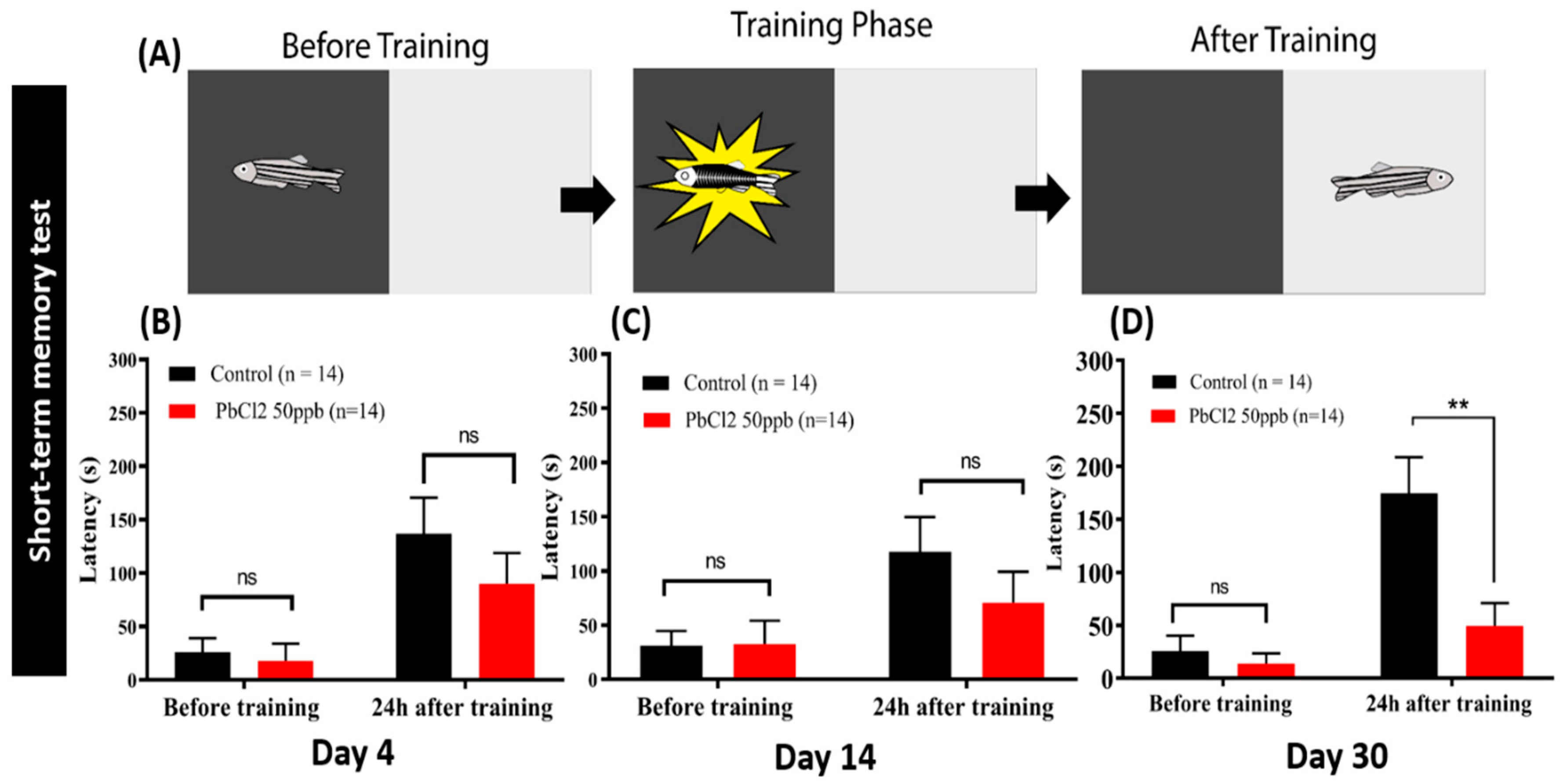
2.6. Lead Exposure Induced Short-Term Memory Loss in Zebrafish
2.7. Lead Exposure Did Not Alter Color Preference Sequence
2.8. Comparison of Biomarkers in the Brain Tissue of the Control and Lead-Treated Zebrafish
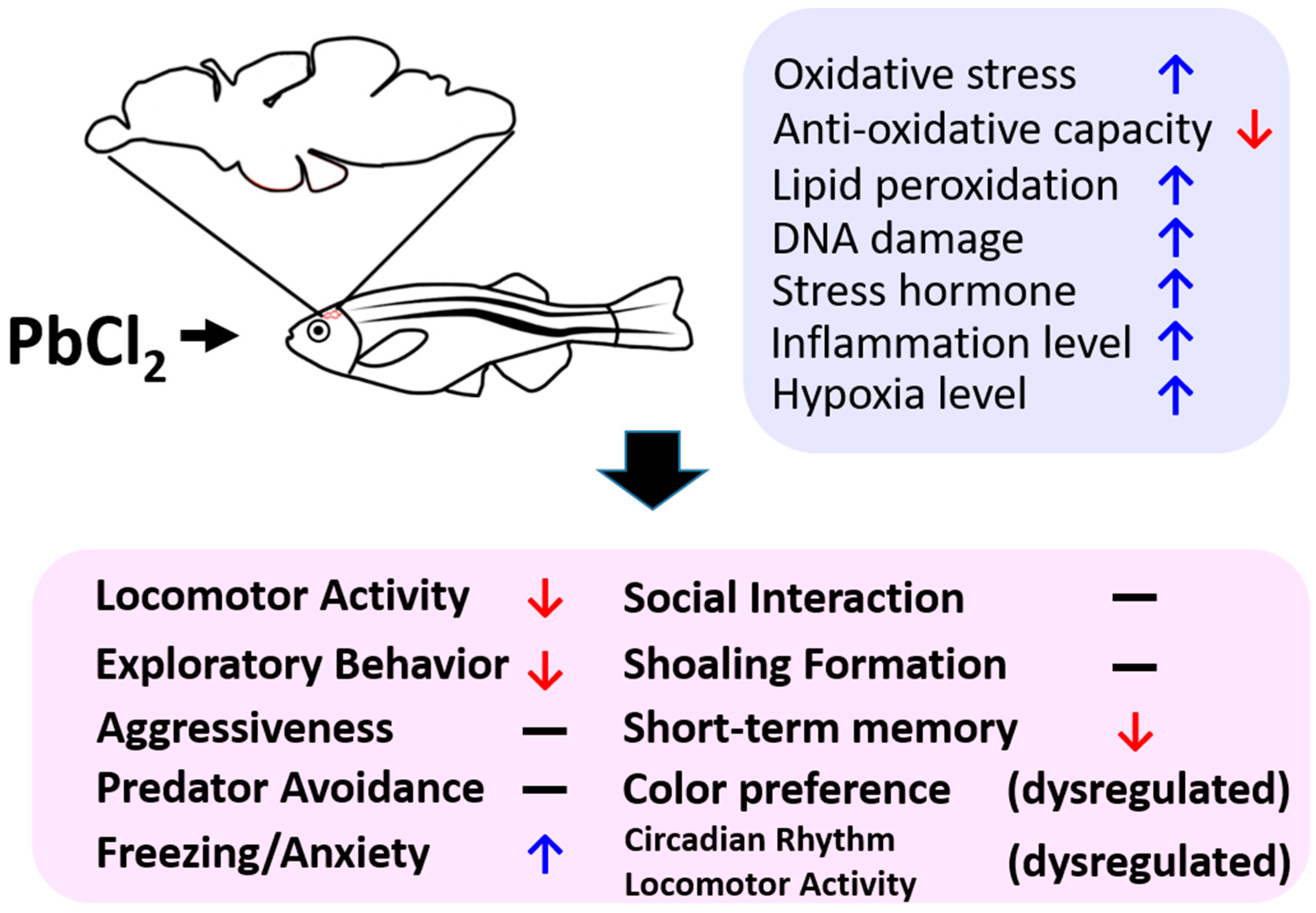
3. Discussion
4. Conclusions
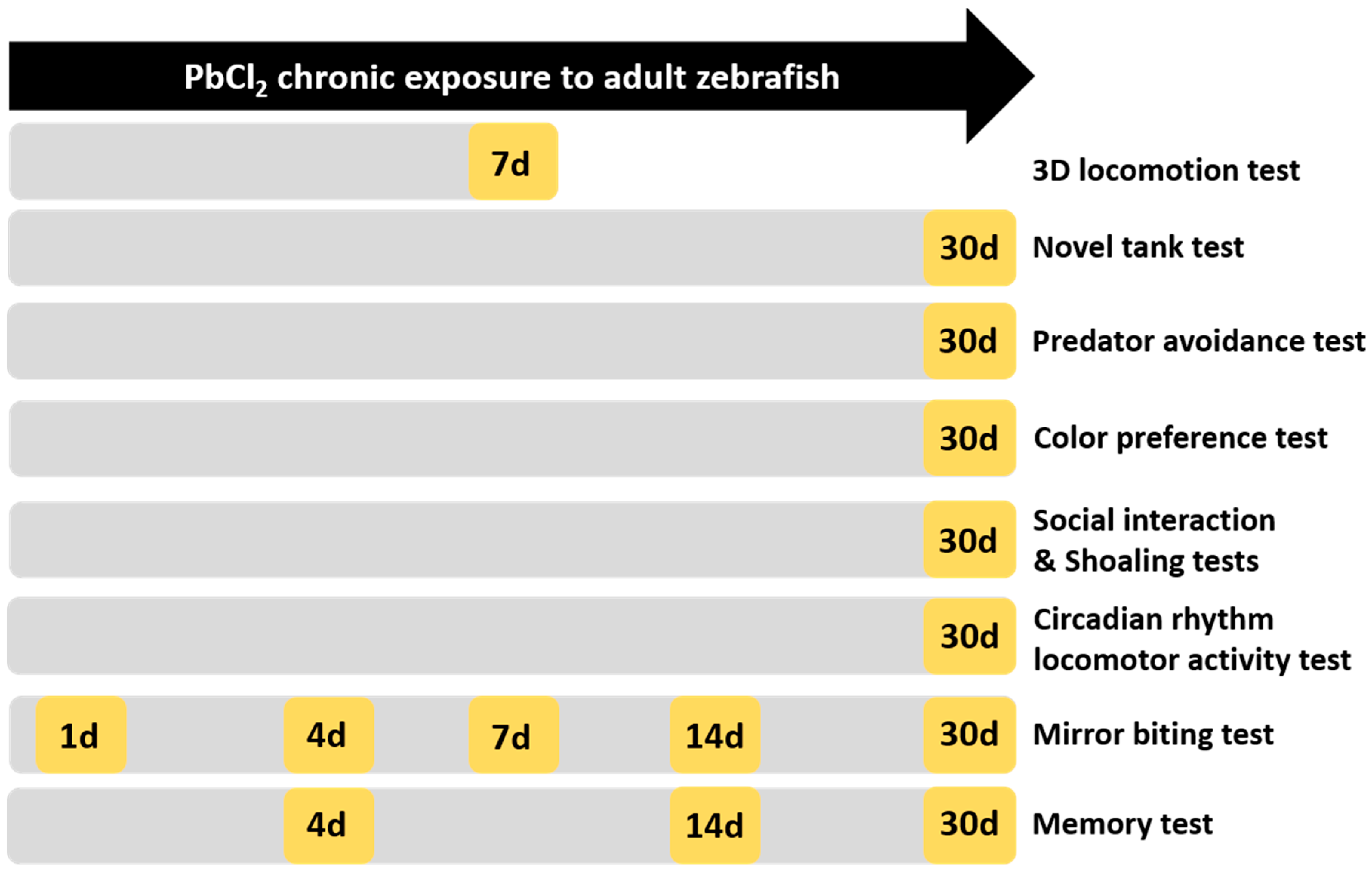
5. Materials and Methods
5.1. Animal Ethics
5.1.1. Lead Chloride Exposure
5.1.2. Adult Neurobehavioral Analysis
5.1.3. Zebrafish 3D Locomotor Activity Test
5.1.4. Novel Tank Test
5.1.5. Aggressiveness Test
5.1.6. Predator Avoidance Test
5.1.7. Social Interaction Test
5.1.8. Shoaling Test
5.1.9. Circadian Rhythm Locomotor Activity Test
5.1.10. Passive Avoidance Test (Short-Term Memory Test)
5.1.11. Color Preference Assay
5.1.12. Determination of Neurotransmitters, Neuroendocrine Markers, and Oxidative Stress Parameters
5.1.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gupta, S.; Jena, V.; Jena, S.; Davić, N.; Matić, N.; Radojević, D.; Solanki, J. Assessment of heavy metal contents of green leafy vegetables. Croat. J. Food Sci. Technol. 2013, 5, 53–60. [Google Scholar]
- Xia, J.; Jin, C.; Pan, Z.; Sun, L.; Fu, Z.; Jin, Y. Chronic exposure to low concentrations of lead induces metabolic disorder and dysbiosis of the gut microbiota in mice. Sci. Total Environ. 2018, 631, 439–448. [Google Scholar] [CrossRef] [PubMed]
- DeForest, D.K.; Santore, R.C.; Ryan, A.C.; Church, B.G.; Chowdhury, M.J.; Brix, K.V. Development of biotic ligand model–Based freshwater aquatic life criteria for lead following US Environmental Protection Agency guidelines. Environ. Toxicol. Chem. 2017, 36, 2965–2973. [Google Scholar] [CrossRef] [PubMed]
- Church, B.G.; Van Sprang, P.A.; Chowdhury, M.J.; DeForest, D.K. Updated species sensitivity distribution evaluations for acute and chronic lead toxicity to saltwater aquatic life. Environ. Toxicol. Chem. 2017, 36, 2974–2980. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, A.C.; McGlothan, J.L.; Guilarte, T.R. Developmental lead exposure causes spatial learning deficits in adult rats. Neurosci. Lett. 1997, 233, 101–104. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.; Liu, W.; Bai, C.; Liu, X.; Liu, K.; Li, R.; Zhu, J.-H.; Huang, C. Developmental lead acetate exposure induces embryonic toxicity and memory deficit in adult zebrafish. Neurotoxicol. Teratol. 2012, 34, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, Y.; Markowitz, M.E.; Rosen, J.F. Low-Level lead-Induced neurotoxicity in children: An update on central nervous system effects. Brain Res. Rev. 1998, 27, 168–176. [Google Scholar] [CrossRef]
- Costa, L.G.; Aschner, M.; Vitalone, A.; Syversen, T.; Soldin, O.P. Developmental neuropathology of environmental agents. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 87–110. [Google Scholar] [CrossRef]
- Roy, N.M.; DeWolf, S.; Carneiro, B. Evaluation of the developmental toxicity of lead in the Danio rerio body. Aquat. Toxicol. 2015, 158, 138–148. [Google Scholar] [CrossRef]
- Eubig, P.A.; Aguiar, A.; Schantz, S.L. Lead and PCBs as risk factors for attention deficit/hyperactivity disorder. Environ. Health Perspect. 2010, 118, 1654–1667. [Google Scholar] [CrossRef]
- Lee, J.; Freeman, J.L. Zebrafish as a model for investigating developmental lead (Pb) neurotoxicity as a risk factor in adult neurodegenerative disease: A mini-Review. Neurotoxicology 2014, 43, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Mason, L.H.; Harp, J.P.; Han, D.Y. Pb neurotoxicity: Neuropsychological effects of lead toxicity. BioMed Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Jain, S.; Aggarwal, C.; Ahuja, G. Encephalopthy due to Inorganic Lead Exposure in an Adult. Jpn. J. Med. 1987, 26, 253–254. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.C. Behavioral effects of lead: Commonalities between experimental and epidemiologic data. Environ. Health Perspect. 1996, 104, 337–351. [Google Scholar] [PubMed]
- Rice, D.C. Parallels between attention deficit hyperactivity disorder and behavioral deficits produced by neurotoxic exposure in monkeys. Environ. Health Perspect. 2000, 108 (Suppl. 3), 405–408. [Google Scholar] [CrossRef]
- Flores-Montoya, M.G.; Sobin, C. Early chronic lead exposure reduces exploratory activity in young C57BL/6J mice. J. Appl. Toxicol. JAT 2015, 35, 759–765. [Google Scholar] [CrossRef]
- Engstrom, A.K.; Xia, Z. Lead exposure in late adolescence through adulthood impairs short-Term spatial memory and the neuronal differentiation of adult-Born cells in C57BL/6 male mice. Neurosci. Lett. 2017, 661, 108–113. [Google Scholar] [CrossRef]
- Faria, M.; Prats, E.; Padrós, F.; Soares, A.M.; Raldúa, D. Zebrafish is a predictive model for identifying compounds that protect against brain toxicity in severe acute organophosphorus intoxication. Arch. Toxicol. 2017, 91, 1891–1901. [Google Scholar] [CrossRef]
- Babin, P.J.; Goizet, C.; Raldua, D. Zebrafish models of human motor neuron diseases: Advantages and limitations. Prog. Neurobiol. 2014, 118, 36–58. [Google Scholar] [CrossRef]
- Xu, X.; Weber, D.; Burge, R.; VanAmberg, K. Neurobehavioral impairments produced by developmental lead exposure persisted for generations in zebrafish (Danio rerio). Neurotoxicology 2016, 52, 176–185. [Google Scholar] [CrossRef]
- Dooley, K.; Zon, L.I. Zebrafish: A model system for the study of human disease. Curr. Opin. Genet. Dev. 2000, 10, 252–256. [Google Scholar] [CrossRef]
- Roy, N.M.; DeWolf, S.; Schutt, A.; Wright, A.; Steele, L. Neural alterations from lead exposure in zebrafish. Neurotoxicol. Teratol. 2014, 46, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Peng, T.; Liu, J.; Chen, X.; Fan, C.; Huang, Z.; Zhang, Y.; Zou, F.; Meng, X. Role of neurexin2a in lead-Induced locomotor defect in developing zebrafish. Aquat. Toxicol. 2018, 194, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Audira, G.; Sampurna, B.; Juniardi, S.; Liang, S.-T.; Lai, Y.-H.; Hsiao, C.-D. A simple setup to perform 3D locomotion tracking in zebrafish by using a single camera. Inventions 2018, 3, 11. [Google Scholar] [CrossRef]
- Pham, M.; Raymond, J.; Hester, J.; Kyzar, E.; Gaikwad, S.; Bruce, I.; Fryar, C.; Chanin, S.; Enriquez, J.; Bagawandoss, S. Assessing social behavior phenotypes in adult zebrafish: Shoaling, social preference, and mirror biting tests. In Zebrafish Protocols for Neurobehavioral Research; Springer: Berlin/Heidelberg, Germany, 2012; pp. 231–246. [Google Scholar]
- Yamamoto, M.; Shimizu, M. Effects of a new TRH analogue, YM-14673 on a passive avoidance test as a possible criterion of improvement in cognitive disturbance in rodents. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1988, 338, 262–267. [Google Scholar] [CrossRef]
- Dawson, G.; Bentley, G.; Draper, F.; Rycroft, W.; Iversen, S.; Pagella, P. The behavioral effects of heptyl physostigmine, a new cholinesterase inhibitor, in tests of long-Term and working memory in rodents. Pharmacol. Biochem. Behav. 1991, 39, 865–871. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, R.; Wang, Y.; Hu, Y.; Li, X.; Cheng, X.; Yin, Y.; Li, W.; Huang, H. Early-Life exposure to lead induces cognitive impairment in elder mice targeting SIRT1 phosphorylation and oxidative alterations. Front. Physiol. 2017, 8, 446. [Google Scholar] [CrossRef]
- Zhi-wei, Z.; Ru-Lai, Y.; Gui-juan, D.; Zheng-yan, Z. Study on the neurotoxic effects of low-Level lead exposure in rats. J. Zhejiang Univ. Sci. B 2005, 6, 686–692. [Google Scholar]
- Rice, D.C. Animal models of cognitive impairment produced by developmental lead exposure. In Animal Models of Cognitive Impairment; CRC Press: Boca Raton, FL, USA, 2006; pp. 83–177. [Google Scholar]
- Moreira, E.G.; Vassilieff, I.; Vassilieff, V.S.l. Developmental lead exposure: Behavioral alterations in the short and long term. Neurotoxicol. Teratol. 2001, 23, 489–495. [Google Scholar] [CrossRef]
- Reddy, G.R.; Basha, M.R.; Devi, C.B.; Suresh, A.; Baker, J.L.; Shafeek, A.; Heinz, J.; Chetty, C.S. Lead induced effects on acetylcholinesterase activity in cerebellum and hippocampus of developing rat. Int. J. Dev. Neurosci. 2003, 21, 347–352. [Google Scholar] [CrossRef]
- Kasten-Jolly, J.; Pabello, N.; Bolivar, V.J.; Lawrence, D.A. Developmental lead effects on behavior and brain gene expression in male and female BALB/cAnNTac mice. Neurotoxicology 2012, 33, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-H.; Shi, Y.-C.; Tseng, I.-L.; Liao, V.H.-C. Protective efficacy of selenite against lead-Induced neurotoxicity in Caenorhabditis elegans. PLoS ONE 2013, 8, e62387. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kong, H.; Ji, X.; Gao, Y.; Jin, M. Zebrafish behavioral phenomics applied for phenotyping aquatic neurotoxicity induced by lead contaminants of environmentally relevant level. Chemosphere 2019, 224, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Bhushan Singh, C.; Alam Ansari, B. Biochemical Markers of Oxidative Stress in Brain of Zebrafish Danio rerio Exposed to Different Heavy Metals Lead and Cobalt. Int. J. Life. Sci. Scienti. Res. 2017, 3, 1484–1494. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, Z.; Liu, F.; Ye, Y.; Peng, T.; Fu, Z. Embryonic exposure to cadmium (II) and chromium (VI) induce behavioral alterations, oxidative stress and immunotoxicity in zebrafish (Danio rerio). Neurotoxicol. Teratol. 2015, 48, 9–17. [Google Scholar] [CrossRef]
- White, L.; Cory-Slechta, D.; Gilbert, M.; Tiffany-Castiglioni, E.; Zawia, N.; Virgolini, M.; Rossi-George, A.; Lasley, S.; Qian, Y.; Basha, M.R. New and evolving concepts in the neurotoxicology of lead. Toxicol. Appl. Pharmacol. 2007, 225, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, N.C.; Hatzidaki, E.G.; Belivanis, S.; Tzanakakis, G.N.; Tsatsakis, A.M. Lead toxicity update. A brief review. Med Sci. Monit. 2005, 11, RA329–RA336. [Google Scholar]
- Istvan, J. Stress, anxiety, and birth outcomes: A critical review of the evidence. Psychol. Bull. 1986, 100, 331. [Google Scholar] [CrossRef]
- Dunn, A.J. Stress-Related changes in cerebral catecholamine and indoleamine metabolism: Lack of effect of adrenalectomy and corticosterone. J. Neurochem. 1988, 51, 406–412. [Google Scholar] [CrossRef]
- Végh, A.M.D.; Duim, S.N.; Smits, A.M.; Poelmann, R.E.; Ten Harkel, A.D.J.; DeRuiter, M.C.; Goumans, M.J.; Jongbloed, M.R.M. Part and Parcel of the Cardiac Autonomic Nerve System: Unravelling Its Cellular Building Blocks during Development. J. Cardiovasc. Dev. Dis. 2016, 3, 28. [Google Scholar] [CrossRef]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Eto, K.; Mazilu-Brown, J.K.; Henderson-MacLennan, N.; Dipple, K.M.; McCabe, E.R. Development of catecholamine and cortisol stress responses in zebrafish. Mol. Genet. Metab. Rep. 2014, 1, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Cajochen, C.; Kräuchi, K.; Wirz-Justice, A. Role of melatonin in the regulation of human circadian rhythms and sleep. J. Neuroendocrinol. 2003, 15, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Chen, T.-M.; Zhong, Y. Possible molecular mechanism underlying cadmium-Induced circadian rhythms disruption in zebrafish. Biochem. Biophys. Res. Commun. 2016, 481, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Mackey, S.; Carew, T.J. Locomotion in Aplysia: Triggering by serotonin and modulation by bag cell extract. J. Neurosci. 1983, 3, 1469–1477. [Google Scholar] [CrossRef]
- Dernovici, S.; Starc, T.; Dent, J.A.; Ribeiro, P. The serotonin receptor SER-1 (5HT2ce) contributes to the regulation of locomotion in Caenorhabditis elegans. Dev. Neurobiol. 2007, 67, 189–204. [Google Scholar] [CrossRef]
- Gürel, G.; Gustafson, M.A.; Pepper, J.S.; Horvitz, H.R.; Koelle, M.R. Receptors and other signaling proteins required for serotonin control of locomotion in Caenorhabditis elegans. Genetics 2012, 192, 1359–1371. [Google Scholar] [CrossRef]
- Takahashi, H.; Takada, Y.; Nagai, N.; Urano, T.; Takada, A. Serotonergic neurons projecting to hippocampus activate locomotion. Brain Res. 2000, 869, 194–202. [Google Scholar] [CrossRef]
- Harris-Warrick, R.M.; Cohen, A.H. Serotonin modulates the central pattern generator for locomotion in the isolated lamprey spinal cord. J. Exp. Biol. 1985, 116, 27–46. [Google Scholar]
- Devi, M.; Fingerman, M. Inhibition of acetylcholinesterase activity in the central nervous system of the red swamp crayfish, Procambarus clarkii, by mercury, cadmium, and lead. Bull. Environ. Contam. Toxicol. 1995, 55, 746–750. [Google Scholar] [CrossRef]
- Tham, L.; Perumal, N.; Syed, M.; Shamaan, N.; Shukor, M. Assessment of Clarias batrachus as a source of acetylcholinesterase (AChE) for the detection of insecticides. J. Environ. Biol. 2009, 30, 135–138. [Google Scholar] [PubMed]
- Olson, D.; Christensen, G. Effects of water pollutants and other chemicals on fish acetylcholinesterase (in vitro). Environ. Res. 1980, 21, 327–335. [Google Scholar] [CrossRef]
- Romani, R.; Antognelli, C.; Baldracchini, F.; De Santis, A.; Isani, G.; Giovannini, E.; Rosi, G. Increased acetylcholinesterase activities in specimens of Sparus auratus exposed to sublethal copper concentrations. Chem.-Biol. Interact. 2003, 145, 321–329. [Google Scholar] [CrossRef]
- Jebali, J.; Banni, M.; Guerbej, H.; Almeida, E.; Bannaoui, A.; Boussetta, H. Effects of malathion and cadmium on acetylcholinesterase activity and metallothionein levels in the fish Seriola dumerilli. Fish Physiol. Biochem. 2006, 32, 93. [Google Scholar] [CrossRef] [PubMed]
- Sarasamma, S.; Audira, G.; Juniardi, S.; Sampurna, B.; Liang, S.-T.; Hao, E.; Lai, Y.-H.; Hsiao, C.-D. Zinc Chloride Exposure Inhibits Brain Acetylcholine Levels, Produces Neurotoxic Signatures, and Diminishes Memory and Motor Activities in Adult Zebrafish. Int. J. Mol. Sci. 2018, 19, 3195. [Google Scholar] [CrossRef]
- Westerfield, M. A Guide for the Laboratory Use of Zebrafish (Danio rerio); University of Oregon Press: Eugene, OR, USA, 2000. [Google Scholar]
- Audira, G.; Sampurna, B.; Juniardi, S.; Liang, S.-T.; Lai, Y.-H.; Hsiao, C.-D. A versatile setup for measuring multiple behavior endpoints in zebrafish. Inventions 2018, 3, 75. [Google Scholar] [CrossRef]
- Audira, G.; Sampurna, B.P.; Juniardi, S.; Liang, S.-T.; Lai, Y.-H.; Han, L.; Hsiao, C.-D. Establishing simple image-based methods and cost-Effective instrument for toxicity assessment on circadian rhythm dysregulation in fish. Biol. Open 2019, 8, bio041871. [Google Scholar] [CrossRef]
- Pérez-Escudero, A.; Vicente-Page, J.; Hinz, R.C.; Arganda, S.; De Polavieja, G.G. idTracker: Tracking individuals in a group by automatic identification of unmarked animals. Nat. Methods 2014, 11, 743. [Google Scholar] [CrossRef]
- Blank, M.; Guerim, L.D.; Cordeiro, R.F.; Vianna, M.R. A one-Trial inhibitory avoidance task to zebrafish: Rapid acquisition of an NMDA-Dependent long-Term memory. Neurobiol. Learn. Mem. 2009, 92, 529–534. [Google Scholar] [CrossRef]
- Nabinger, D.D.; Altenhofen, S.; Bitencourt, P.E.R.; Nery, L.R.; Leite, C.E.; Vianna, M.R.M.R.; Bonan, C.D. Nickel exposure alters behavioral parameters in larval and adult zebrafish. Sci. Total Environ. 2018, 624, 1623–1633. [Google Scholar] [CrossRef]
- Avdesh, A.; Martin-Iverson, M.T.; Mondal, A.; Chen, M.; Askraba, S.; Morgan, N.; Lardelli, M.; Groth, D.M.; Verdile, G.; Martins, R.N. Evaluation of color preference in zebrafish for learning and memory. J. Alzheimer’s Dis. 2012, 28, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Raghupathy, R.K.; Albalawi, A.; Zhao, Z.; Reilly, J.; Xiao, Q.; Shu, X. A colour preference technique to evaluate acrylamide-Induced toxicity in zebrafish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 199, 11–19. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bui Thi, N.H.; Nguyen Thi, N.A.; Audira, G.; Siregar, P.; Liang, S.-T.; Huang, J.-C.; Hsiao, C.-D. Chronic Exposure to Low Concentration Lead Chloride-Induced Anxiety and Loss of Aggression and Memory in Zebrafish. Int. J. Mol. Sci. 2020, 21, 1844. https://doi.org/10.3390/ijms21051844
Bui Thi NH, Nguyen Thi NA, Audira G, Siregar P, Liang S-T, Huang J-C, Hsiao C-D. Chronic Exposure to Low Concentration Lead Chloride-Induced Anxiety and Loss of Aggression and Memory in Zebrafish. International Journal of Molecular Sciences. 2020; 21(5):1844. https://doi.org/10.3390/ijms21051844
Chicago/Turabian StyleBui Thi, Ngoc Hieu, Ngoc Anh Nguyen Thi, Gilbert Audira, Petrus Siregar, Sung-Tzu Liang, Jong-Chin Huang, and Chung-Der Hsiao. 2020. "Chronic Exposure to Low Concentration Lead Chloride-Induced Anxiety and Loss of Aggression and Memory in Zebrafish" International Journal of Molecular Sciences 21, no. 5: 1844. https://doi.org/10.3390/ijms21051844
APA StyleBui Thi, N. H., Nguyen Thi, N. A., Audira, G., Siregar, P., Liang, S.-T., Huang, J.-C., & Hsiao, C.-D. (2020). Chronic Exposure to Low Concentration Lead Chloride-Induced Anxiety and Loss of Aggression and Memory in Zebrafish. International Journal of Molecular Sciences, 21(5), 1844. https://doi.org/10.3390/ijms21051844






