Bisphenol S Impaired Human Granulosa Cell Steroidogenesis in Vitro
Abstract
1. Introduction
2. Results
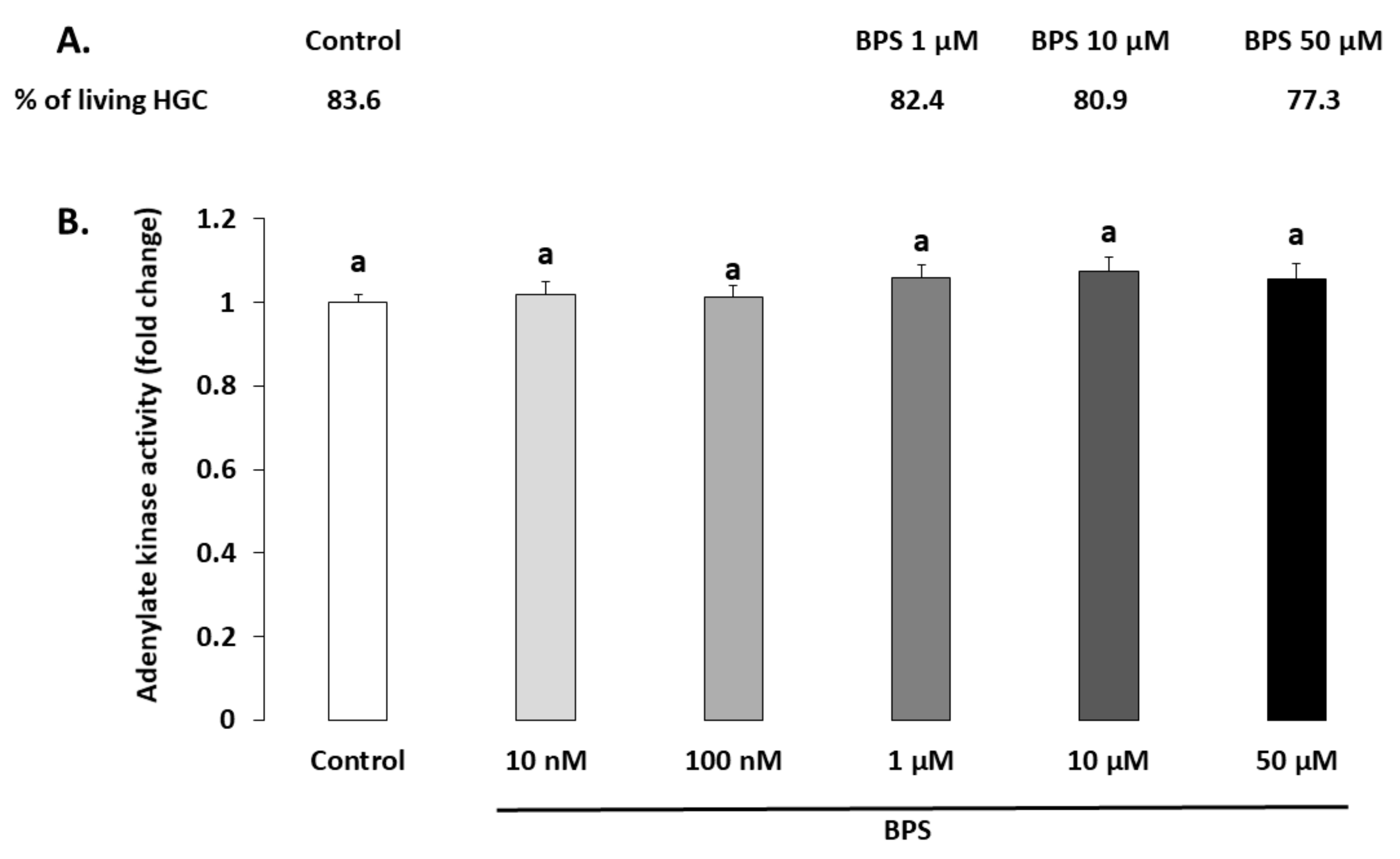
2.1. Cell Viability
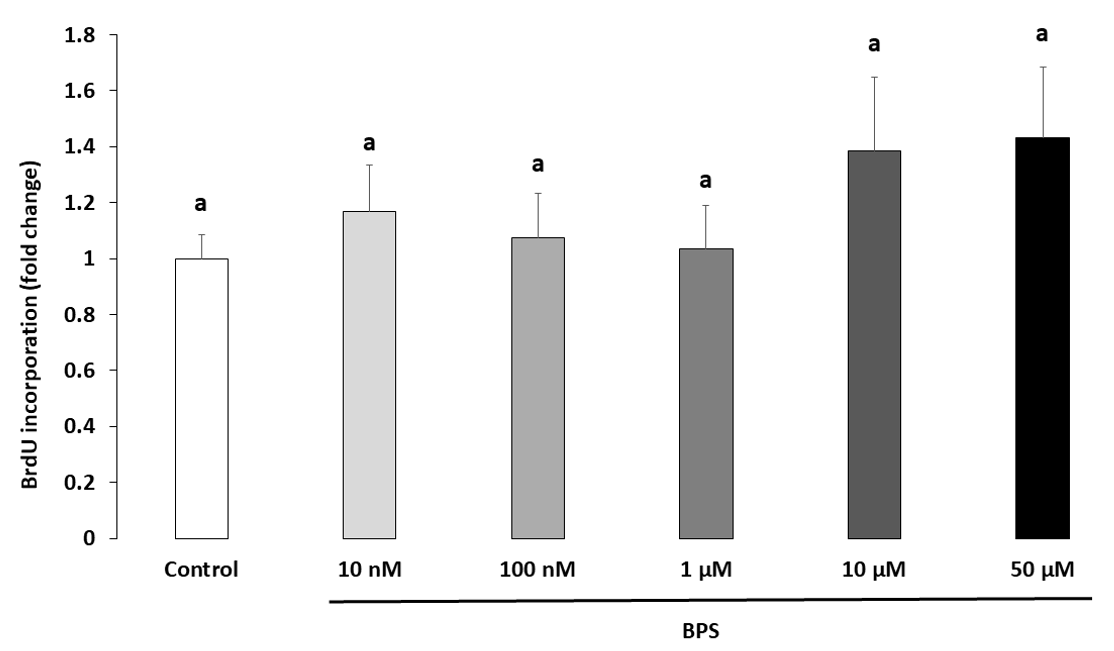
2.2. Cell Proliferation
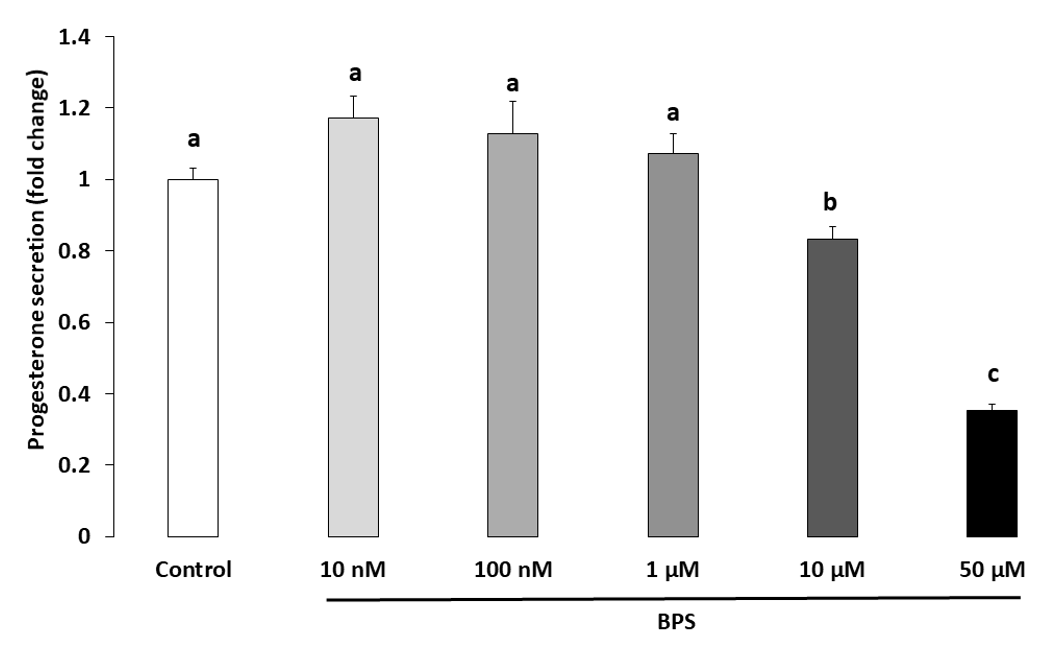
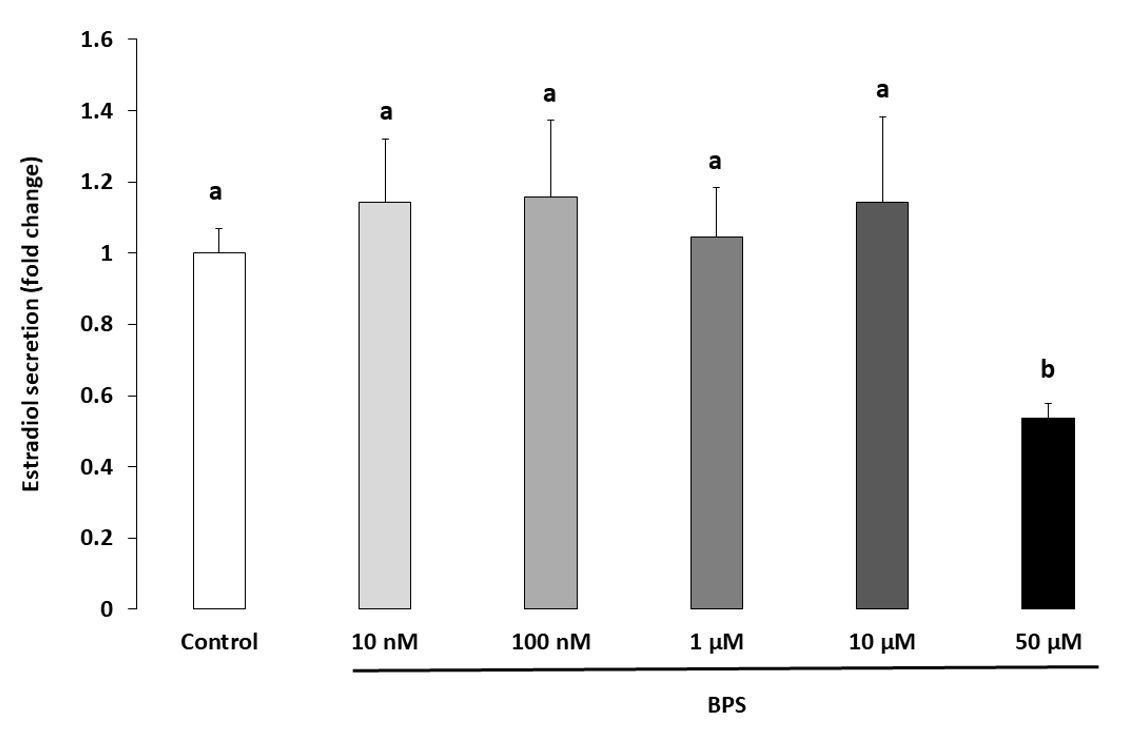
2.3. Steroidogenesis
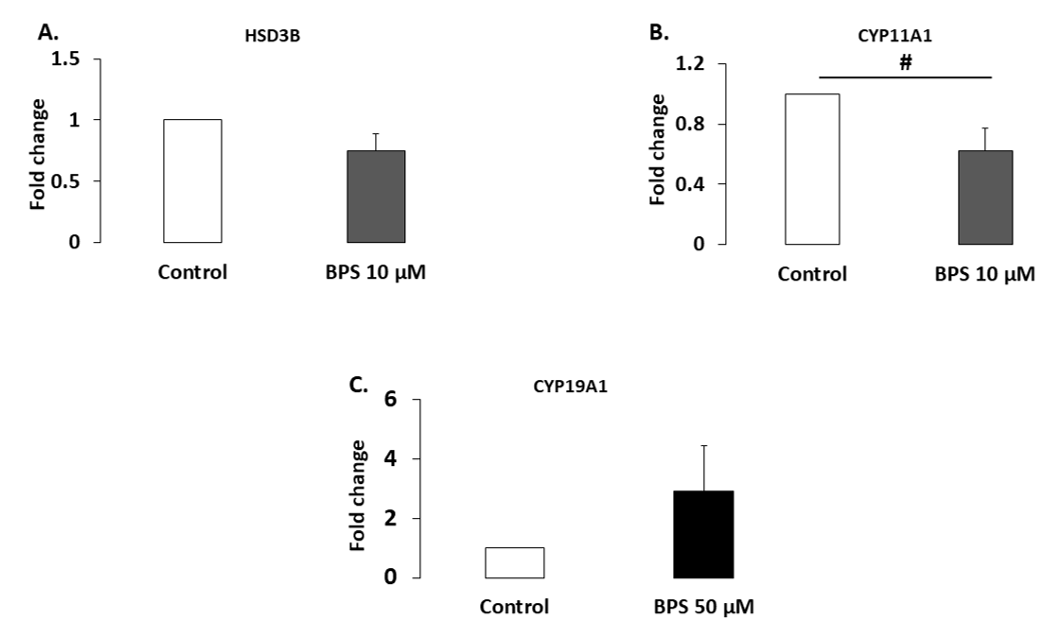
2.4. Protein Expression
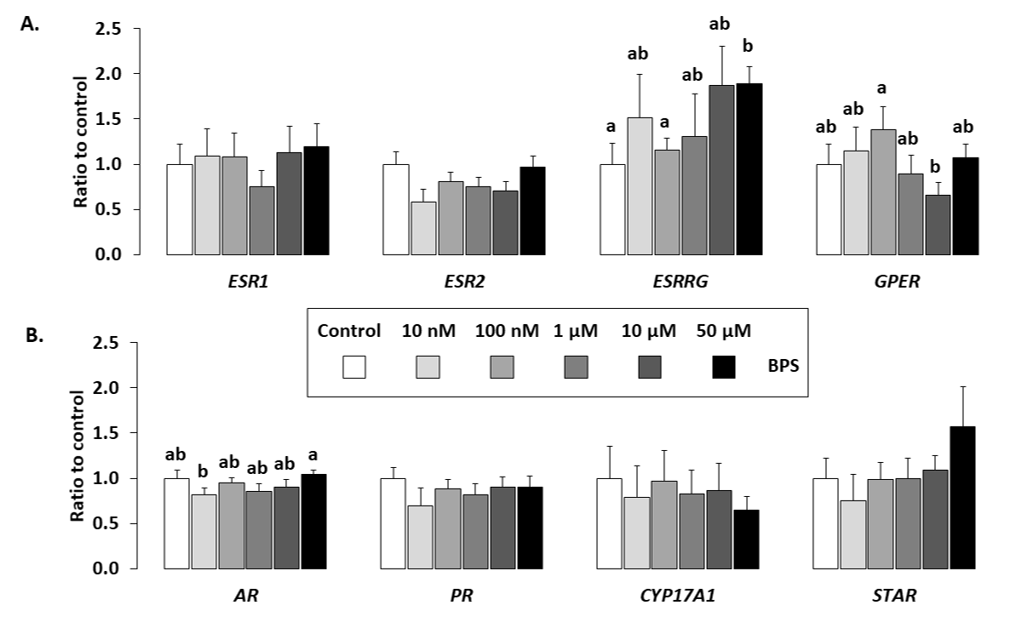
2.5. Gene Expression
2.6. Signalling Pathways
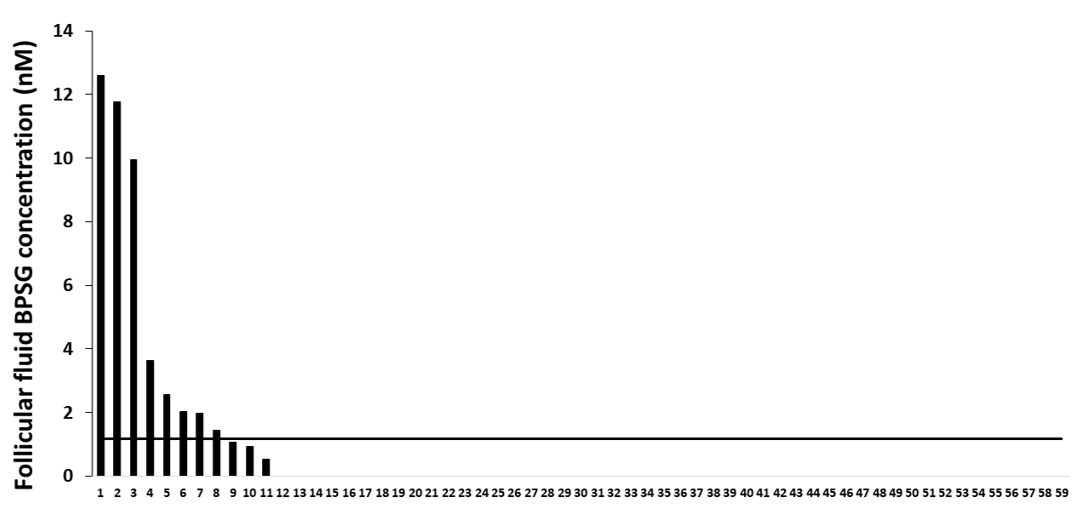
2.7. Female Follicular Fluid BPS Glucuronide Assays
3. Discussion
3.1. BPS Reduced HGC Progesterone and Oestradiol Secretion
3.2. BPS Mechanisms of Action
3.3. BPS in Follicular Fluid
4. Materials and Methods
4.1. Participants, Biological Material and Ethical Approval
4.2. Chemicals and Antibodies
4.3. Isolation and Culture of HGC
4.4. Cell Viability
4.5. HGC Proliferation
4.6. Steroidogenesis Measurement
4.7. Protein Extraction and Quantification
4.8. Western Blot Analysis
4.9. Gene Analysis
4.10. Follicular Fluid Sample Collection and Analysis
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al-Saleh, I.; El-Doush, I.; Grisellhi, B.; Coskun, S. The effect of caffeine consumption on the success rate of pregnancy as well various performance parameters of in-vitro fertilization treatment. Med. Sci. Monit. 2010, 16, CR598–CR605. [Google Scholar]
- Jirsova, S.; Masata, J.; Jech, L.; Zvarova, J. Effect of polychlorinated biphenyls (PCBs) and 1,1,1-trichloro-2,2,-bis (4-chlorophenyl)-ethane (DDT) in follicular fluid on the results of in vitro fertilization-embryo transfer (IVF-ET) programs. Fertil. Steril. 2010, 93, 1831–1836. [Google Scholar] [CrossRef]
- Giulivo, M.; Lopez de Alda, M.; Capri, E.; Barceló, D. Human exposure to endocrine disrupting compounds: Their role in reproductive systems, metabolic syndrome and breast cancer. A review. Environ. Res. 2016, 151, 251–264. [Google Scholar] [CrossRef]
- Eladak, S.; Grisin, T.; Moison, D.; Guerquin, M.J.; N’Tumba-Byn, T.; Pozzi-Gaudin, S.; Benachi, A.; Livera, G.; Rouiller-Fabre, V.; Habert, R. A new chapter in the bisphenol A story: Bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil. Steril. 2015, 103, 11–21. [Google Scholar] [CrossRef]
- Andra, S.S.; Charisiadis, P.; Arora, M.; van Vliet-Ostaptchouk, J.V.; Makris, K.C. Biomonitoring of human exposures to chlorinated derivatives and structural analogs of bisphenol A. Environ. Int. 2015, 85, 352–379. [Google Scholar] [CrossRef] [PubMed]
- Kubwabo, C.; Kosarac, I.; Stewart, B.; Gauthier, B.R.; Lalonde, K.; Lalonde, P.J. Migration of bisphenol A from plastic baby bottles, baby bottle liners and reusable polycarbonate drinking bottles. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 2009, 26, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Kondo, F.; Katayama, Y. Human exposure to bisphenol A. Toxicology 2006, 226, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Liu, F.; Guo, Y.; Moon, H.-B.; Nakata, H.; Wu, Q.; Kannan, K. Occurrence of Eight Bisphenol Analogues in Indoor Dust from the United States and Several Asian Countries: Implications for Human Exposure. Environ. Sci. Technol. 2012, 46, 9138–9145. [Google Scholar] [CrossRef]
- Thayer, K.A.; Taylor, K.W.; Garantziotis, S.; Schurman, S.H.; Kissling, G.E.; Hunt, D.; Herbert, B.; Church, R.; Jankowich, R.; Churchwell, M.I.; et al. Bisphenol A, Bisphenol S, and 4-Hydroxyphenyl 4-Isoprooxyphenylsulfone (BPSIP) in Urine and Blood of Cashiers. Environ. Health Perspect. 2016, 124, 437–444. [Google Scholar] [CrossRef]
- Calafat, A.M.; Kuklenyik, Z.; Reidy, J.A.; Caudill, S.P.; Ekong, J.; Needham, L.L. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ. Health Perspect. 2005, 113, 391–395. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Chahoud, I.; Heindel, J.J.; Padmanabhan, V.; Paumgartten, F.J.; Schoenfelder, G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ. Health Perspect. 2010, 118, 1055–1070. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.A.; Birnbaum, L.S.; Farabollini, F.; Newbold, R.R.; Rubin, B.S.; Talsness, C.E.; Vandenbergh, J.G.; Walser-Kuntz, D.R.; vom Saal, F.S. In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 2007, 24, 199–224. [Google Scholar] [CrossRef] [PubMed]
- Ikezuki, Y.; Tsutsumi, O.; Takai, Y.; Kamei, Y.; Taketani, Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum. Reprod. 2002, 17, 2839–2841. [Google Scholar] [CrossRef]
- Fernandez, M.F.; Arrebola, J.P.; Taoufiki, J.; Navalón, A.; Ballesteros, O.; Pulgar, R.; Vilchez, J.L.; Olea, N. Bisphenol-A and chlorinated derivatives in adipose tissue of women. Reprod. Toxicol. 2007, 24, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Wetherill, Y.B.; Akingbemi, B.T.; Kanno, J.; McLachlan, J.A.; Nadal, A.; Sonnenschein, C.; Watson, C.S.; Zoeller, R.T.; Belcher, S.M. In vitro molecular mechanisms of bisphenol A action. Reprod. Toxicol. 2007, 24, 178–198. [Google Scholar] [CrossRef] [PubMed]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef]
- Lang, I.A.; Galloway, T.S.; Scarlett, A.; Henley, W.E.; Depledge, M.; Wallace, R.B.; Melzer, D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA 2008, 300, 1303–1310. [Google Scholar] [CrossRef]
- Grun, F.; Blumberg, B. Perturbed nuclear receptor signaling by environmental obesogens as emerging factors in the obesity crisis. Rev. Endocr. Metab. Disord. 2007, 8, 161–171. [Google Scholar] [CrossRef]
- Peretz, J.; Vrooman, L.; Ricke, W.A.; Hunt, P.A.; Ehrlich, S.; Hauser, R.; Padmanabhan, V.; Taylor, H.S.; Swan, S.H.; VandeVoort, C.A.; et al. Bisphenol a and reproductive health: Update of experimental and human evidence, 2007–2013. Environ. Health Perspect. 2014, 122, 775–786. [Google Scholar] [CrossRef]
- Nadal, A.; Fuentes, E.; Ripoll, C.; Villar-Pazos, S.; Castellano-Munoz, M.; Soriano, S.; Martinez-Pinna, J.; Quesada, I.; Alonso-Magdalena, P. Extranuclear-initiated estrogenic actions of endocrine disrupting chemicals: Is there toxicology beyond paracelsus? J. Steroid Biochem. Mol. Biol. 2018, 176, 16–22. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef]
- Machtinger, R.; Orvieto, R. Bisphenol A, oocyte maturation, implantation, and IVF outcome: Review of animal and human data. Reprod. Biomed. Online 2014, 29, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, S.; Williams, P.L.; Missmer, S.A.; Flaws, J.A.; Ye, X.; Calafat, A.M.; Petrozza, J.C.; Wright, D.; Hauser, R. Urinary bisphenol A concentrations and early reproductive health outcomes among women undergoing IVF. Hum. Reprod. 2012, 27, 3583–3592. [Google Scholar] [CrossRef] [PubMed]
- Mok-Lin, E.; Ehrlich, S.; Williams, P.L.; Petrozza, J.; Wright, D.L.; Calafat, A.M.; Ye, X.; Hauser, R. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. Int. J. Androl. 2010, 33, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, V.Y.; Kim, D.; vom Saal, F.S.; Lamb, J.D.; Taylor, J.A.; Bloom, M.S. Serum unconjugated bisphenol A concentrations in women may adversely influence oocyte quality during in vitro fertilization. Fertil. Steril. 2011, 95, 1816–1819. [Google Scholar] [CrossRef] [PubMed]
- Mlynarcikova, A.; Kolena, J.; Fickova, M.; Scsukova, S. Alterations in steroid hormone production by porcine ovarian granulosa cells caused by bisphenol A and bisphenol A dimethacrylate. Mol. Cell. Endocrinol. 2005, 244, 57–62. [Google Scholar] [CrossRef]
- Grasselli, F.; Baratta, L.; Baioni, L.; Bussolati, S.; Ramoni, R.; Grolli, S.; Basini, G. Bisphenol A disrupts granulosa cell function. Domest. Anim. Endocrinol. 2010, 39, 34–39. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, J.; Liao, L.; Han, S.; Liu, J. Effect of bisphenol A on steroid hormone production in rat ovarian theca-interstitial and granulosa cells. Mol. Cell. Endocrinol. 2008, 283, 12–18. [Google Scholar] [CrossRef]
- Bujnakova Mlynarcikova, A.; Scsukova, S. Simultaneous effects of endocrine disruptor bisphenol A and flavonoid fisetin on progesterone production by granulosa cells. Environ. Toxicol. Pharmacol. 2018, 59, 66–73. [Google Scholar] [CrossRef]
- Banerjee, O.; Singh, S.; Prasad, S.K.; Bhattacharjee, A.; Banerjee, A.; Banerjee, A.; Saha, A.; Maji, B.K.; Mukherjee, S. Inhibition of catalase activity with 3-amino-1,2,4-triazole intensifies bisphenol A (BPA)-induced toxicity in granulosa cells of female albino rats. Toxicol. Ind. Health 2018, 34, 787–797. [Google Scholar] [CrossRef]
- Samardzija, D.; Pogrmic-Majkic, K.; Fa, S.; Stanic, B.; Jasnic, J.; Andric, N. Bisphenol A decreases progesterone synthesis by disrupting cholesterol homeostasis in rat granulosa cells. Mol. Cell. Endocrinol. 2018, 461, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Mansur, A.; Adir, M.; Yerushalmi, G.; Hourvitz, A.; Gitman, H.; Yung, Y.; Orvieto, R.; Machtinger, R. Does BPA alter steroid hormone synthesis in human granulosa cells in vitro? Hum. Reprod. 2016, 31, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 2015, 13, 3978. [Google Scholar] [CrossRef]
- Usman, A.; Ahmad, M. From BPA to its analogues: Is it a safe journey? Chemosphere 2016, 158, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Wong, L.-Y.; Kramer, J.; Zhou, X.; Jia, T.; Calafat, A.M. Urinary Concentrations of Bisphenol A and Three Other Bisphenols in Convenience Samples of U.S. Adults during 2000–2014. Environ. Sci. Technol. 2015, 49, 11834–11839. [Google Scholar] [CrossRef]
- Liao, C.; Liu, F.; Alomirah, H.; Loi, V.D.; Mohd, M.A.; Moon, H.-B.; Nakata, H.; Kannan, K. Bisphenol S in Urine from the United States and Seven Asian Countries: Occurrence and Human Exposures. Environ. Sci. Technol. 2012, 46, 6860–6866. [Google Scholar] [CrossRef]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef]
- Naderi, M.; Wong, M.Y.L.; Gholami, F. Developmental exposure of zebrafish (Danio rerio) to bisphenol-S impairs subsequent reproduction potential and hormonal balance in adults. Aquat. Toxicol. 2014, 148, 195–203. [Google Scholar] [CrossRef]
- Ji, K.; Hong, S.; Kho, Y.; Choi, K. Effects of bisphenol s exposure on endocrine functions and reproduction of zebrafish. Environ. Sci. Technol. 2013, 47, 8793–8800. [Google Scholar] [CrossRef]
- Nevoral, J.; Kolinko, Y.; Moravec, J.; Zalmanova, T.; Hoskova, K.; Prokesova, S.; Klein, P.; Ghaibour, K.; Hosek, P.; Stiavnicka, M.; et al. Long-term exposure to very low doses of bisphenol S affects female reproduction. Reproduction 2018, 156, 47–57. [Google Scholar] [CrossRef]
- Ullah, H.; Jahan, S.; Ain, Q.U.; Shaheen, G.; Ahsan, N. Effect of bisphenol S exposure on male reproductive system of rats: A histological and biochemical study. Chemosphere 2016, 152, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Campen, K.A.; Lavallee, M.; Combelles, C. The impact of bisphenol S on bovine granulosa and theca cells. Reprod. Domest. Anim. 2018, 53, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, N.; Ullah, H.; Ullah, W.; Jahan, S. Comparative effects of Bisphenol S and Bisphenol A on the development of female reproductive system in rats; a neonatal exposure study. Chemosphere 2018, 197, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Teteau, O.; Jaubert, M.; Desmarchais, A.; Papillier, P.; Binet, A.; Maillard, V.; Elis, S. Bisphenol A and S impaired ovine granulosa cell steroidogenesis. Reproduction 2020. [Google Scholar] [CrossRef]
- Desmarchais, A.; Téteau, O.; Papillier, P.; Jaubert, M.; Druart, X.; Binet, A.; Maillard, V.; Elis, S. Bisphenol S Impaired In Vitro Ovine Early Developmental Oocyte Competence. Int. J. Mol. Sci. 2020, 21, 1238. [Google Scholar] [CrossRef]
- Berni, M.; Gigante, P.; Bussolati, S.; Grasselli, F.; Grolli, S.; Ramoni, R.; Basini, G. Bisphenol S, a Bisphenol A alternative, impairs swine ovarian and adipose cell functions. Domest. Anim. Endocrinol. 2019, 66, 48–56. [Google Scholar] [CrossRef]
- Bloom, M.S.; Kim, D.; Vom Saal, F.S.; Taylor, J.A.; Cheng, G.; Lamb, J.D.; Fujimoto, V.Y. Bisphenol A exposure reduces the estradiol response to gonadotropin stimulation during in vitro fertilization. Fertil. Steril. 2011, 96, 672–677. [Google Scholar] [CrossRef]
- Padmanabhan, V.; Smith, P.; Veiga-Lopez, A. Developmental programming: Impact of prenatal testosterone treatment and postnatal obesity on ovarian follicular dynamics. J. Dev. Orig. Health Dis. 2012, 3, 276–286. [Google Scholar] [CrossRef][Green Version]
- Scaramuzzi, R.J.; Baird, D.T.; Campbell, B.K.; Driancourt, M.A.; Dupont, J.; Fortune, J.E. Regulation of folliculogenesis and the determination of ovulation rate in ruminants. Reprod. Fertil. Dev. 2011, 23, 444–467. [Google Scholar] [CrossRef]
- Monniaux, D.; Clement, F.; Dalbies-Tran, R.; Estienne, A.; Fabre, S.; Mansanet, C.; Monget, P. The ovarian reserve of primordial follicles and the dynamic reserve of antral growing follicles: What is the link? Biol. Reprod. 2014, 90, 85. [Google Scholar] [CrossRef]
- Lee, S.G.; Kim, J.Y.; Chung, J.-Y.; Kim, Y.-J.; Park, J.-E.; Oh, S.; Yoon, Y.-D.; Yoo, K.S.; Yoo, Y.H.; Kim, J.-M. Bisphenol A exposure during adulthood causes augmentation of follicular atresia and luteal regression by decreasing 17β-estradiol synthesis via downregulation of aromatase in rat ovary. Environ. Health Perspect. 2013, 121, 663–669. [Google Scholar] [CrossRef]
- Ptak, A.; Gregoraszczuk, E.L. Bisphenol A induces leptin receptor expression, creating more binding sites for leptin, and activates the JAK/Stat, MAPK/ERK and PI3K/Akt signalling pathways in human ovarian cancer cell. Toxicol. Lett. 2012, 210, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.W.; Yang, Z.J.; Huang, H.H.; Chang, A.A.; Cheng, Y.C.; Wu, G.J.; Lan, H.C. Low-dose bisphenol A activates the ERK signaling pathway and attenuates steroidogenic gene expression in human placental cells. Biol. Reprod. 2018, 98, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.-J.; Kang, S.K.; Tzeng, C.-R.; Leung, P.C.K. Adenosine Triphosphate Activates Mitogen-Activated Protein Kinase in Human Granulosa-Luteal Cells*. Endocrinology 2001, 142, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Kang, J.-H. Bisphenol A (BPA) and cell signaling pathways. Biotechnol. Adv. 2018, 36, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Liu, X.; Sumiyoshi, M.; Matsushima, A.; Shimihigashi, M.; Shimiohigashi, Y. Placenta expressing the greatest quantity of bisphenol A receptor ERRg among the human reproductive tissues: Predominant expression of type-1 ERRg isoform. J. Biol. Chem. 2009, 146, 113–122. [Google Scholar] [CrossRef]
- Canepa, S.; Laine, A.B.; Bluteau, A.; Fagu, C.; Flon, C.; Monniaux, D. Validation d’une methode immunoenzymatique pour le dosage de la progesterone dans le plasma des ovins et des bovins. Cahier des Techniques de L’INRA 2008, 64, 19–30. [Google Scholar]
- Maillard, V.; Desmarchais, A.; Durcin, M.; Uzbekova, S.; Elis, S. Docosahexaenoic acid (DHA) effects on proliferation and steroidogenesis of bovine granulosa cells. Reprod. Biol. Endocrinol. 2018, 16, 40. [Google Scholar] [CrossRef]
- Elis, S.; Oseikria, M.; Vitorino Carvalho, A.; Bertevello, P.S.; Corbin, E.; Teixeira-Gomes, A.P.; Lecardonnel, J.; Archilla, C.; Duranthon, V.; Labas, V.; et al. Docosahexaenoic acid mechanisms of action on the bovine oocyte-cumulus complex. J. Ovarian Res. 2017, 10, 74. [Google Scholar] [CrossRef]

| Abbrev. | Name | Forward (5′→ 3′) | Reverse (5′ → 3′) | Size (bp) | E (%) |
|---|---|---|---|---|---|
| AR | Androgen receptor | CTCTGGTGGTTCCCTCTCTG | AGCATCCAAGTGGCTTATGG | 165 | 87.5 |
| CYP17A1 | Cytochrome P450 family 19 subfamily A member 1 | TGAGTTTGCTGTGGACAAGG | TCCGAAGGGCAAATAGCTTA | 163 | 100.4 |
| ESR1 | Oestrogen receptor 1 | TCCAACTGCATTTCCTTTCC | TTGGAACATGGCAGCATTTA | 201 | 82 |
| ESR2 | Oestrogen receptor 2 | GATGCTTTGGTTTGGGTGAT | ATCGTTGCTTCAGGCAAAAG | 175 | 98.9 |
| ESRRG | Oestrogen-related receptor γ | AATGCTATCCTGCAGCTGGT | GCAGCGCTTCATGTAAGACA | 159 | 94.7 |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | GTCAGTGGTGGACCTGACCT | TGCTGTAGCCAAATTCGTTG | 245 | 78.3 |
| GPER | G protein-coupled oestrogen receptor | GCTGGTCTTCTTCGTCTGCT | GGTTTAGGCAGCTGTTGGAG | 167 | 104.5 |
| PR | Progesterone receptor | TCGAGCTCACAGCGTTTCTA | ACCATCCCTGCCAATATCT | 183 | 76.5 |
| RPL19 | Ribosomal protein L19 | CATGGAACACATCCACAAGC | TTGGTCTCTTCCTCCTTGGAT | 171 | 92.5 |
| STAR | Steroidogenic acute regulatory protein | CCTGAGCAGAAGGGTGTCAT | AGGACCTGGTTGATGATGCT | 151 | 88.9 |
| Name | Species Specificity | Source | Supplier (Distributor, Town, Country) | Catalogue Number | Primary Ab Dilution |
|---|---|---|---|---|---|
| MAPK3/1 (ERK1/2) | Rat | Rabbit polyclonal antibody | Cell Signaling Technology (Ozyme, Saint Quentin Yvelines, France) | 9102 | 1/1000 |
| Phospho-MAPK3/1 (ERK1/2; Thr202/Tyr204, D13.14.4E, XPTM) | Human | Rabbit monoclonal antibody | Cell Signaling Technology | 4370 | 1/2000 |
| CYP19A1 | Human | Rabbit polyclonal antibody | Sigma Aldrich (Saint Louis, Missouri, USA) | SAB1410280X | 1/500 |
| CYP11A1 | Human | Goat polyclonal antibody | Santa Cruz Biotechnology (Dallas, USA) | sc-18043 | 1/500 |
| HSD3B1 | Human | Rabbit polyclonal antibody | Abgent (San Diego, USA) | AP14585a | 1/500 |
| Vinculin | Human | Mouse monoclonal antibody | Sigma Aldrich (Saint Quentin Fallavier) | V9131 | 1/1000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amar, S.; Binet, A.; Téteau, O.; Desmarchais, A.; Papillier, P.; Lacroix, M.Z.; Maillard, V.; Guérif, F.; Elis, S. Bisphenol S Impaired Human Granulosa Cell Steroidogenesis in Vitro. Int. J. Mol. Sci. 2020, 21, 1821. https://doi.org/10.3390/ijms21051821
Amar S, Binet A, Téteau O, Desmarchais A, Papillier P, Lacroix MZ, Maillard V, Guérif F, Elis S. Bisphenol S Impaired Human Granulosa Cell Steroidogenesis in Vitro. International Journal of Molecular Sciences. 2020; 21(5):1821. https://doi.org/10.3390/ijms21051821
Chicago/Turabian StyleAmar, Sarah, Aurélien Binet, Ophélie Téteau, Alice Desmarchais, Pascal Papillier, Marlène Z. Lacroix, Virginie Maillard, Fabrice Guérif, and Sebastien Elis. 2020. "Bisphenol S Impaired Human Granulosa Cell Steroidogenesis in Vitro" International Journal of Molecular Sciences 21, no. 5: 1821. https://doi.org/10.3390/ijms21051821
APA StyleAmar, S., Binet, A., Téteau, O., Desmarchais, A., Papillier, P., Lacroix, M. Z., Maillard, V., Guérif, F., & Elis, S. (2020). Bisphenol S Impaired Human Granulosa Cell Steroidogenesis in Vitro. International Journal of Molecular Sciences, 21(5), 1821. https://doi.org/10.3390/ijms21051821





