Serum Levels and Removal by Haemodialysis and Haemodiafiltration of Tryptophan-Derived Uremic Toxins in ESKD Patients
Abstract
1. Introduction
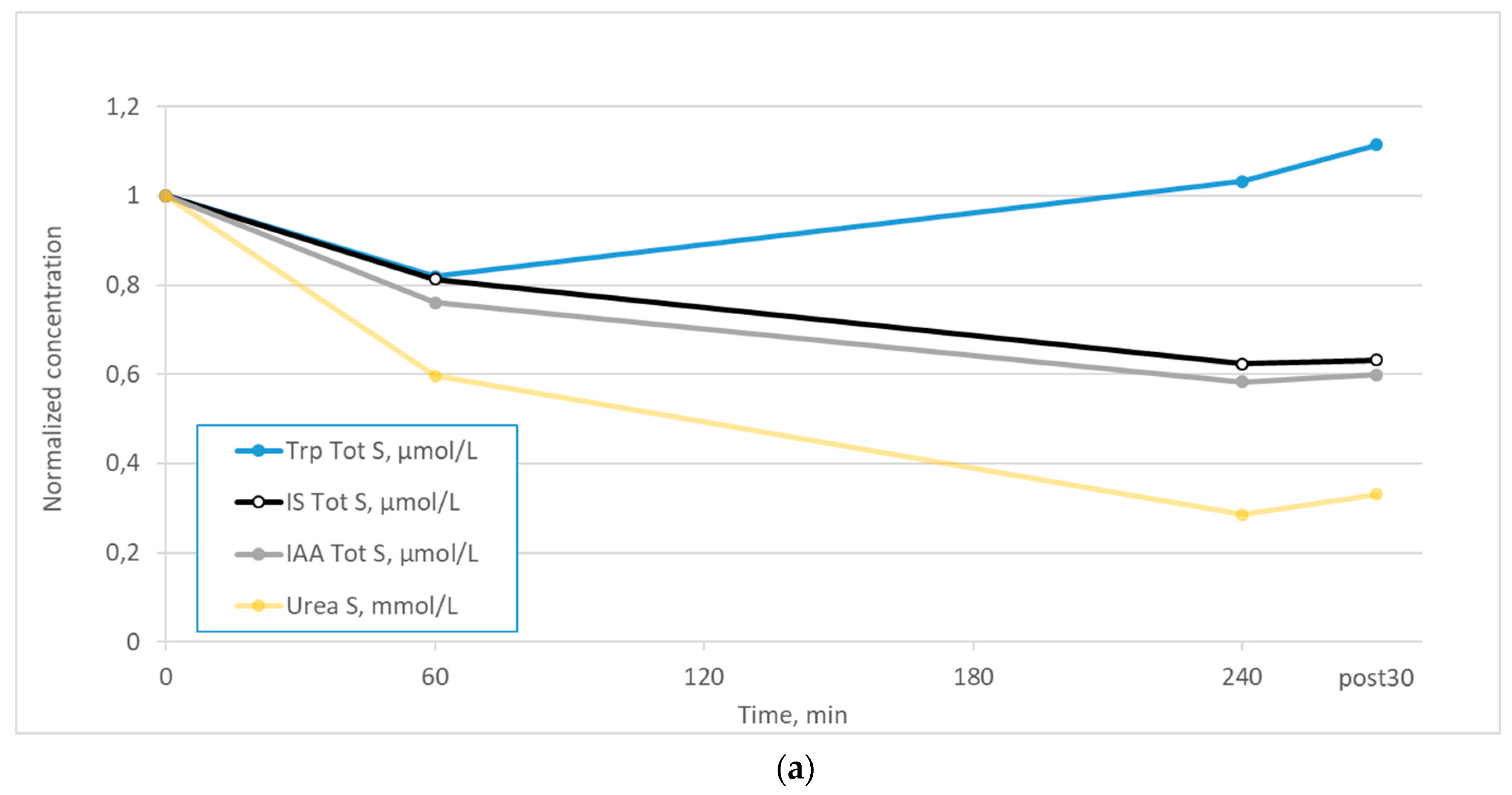
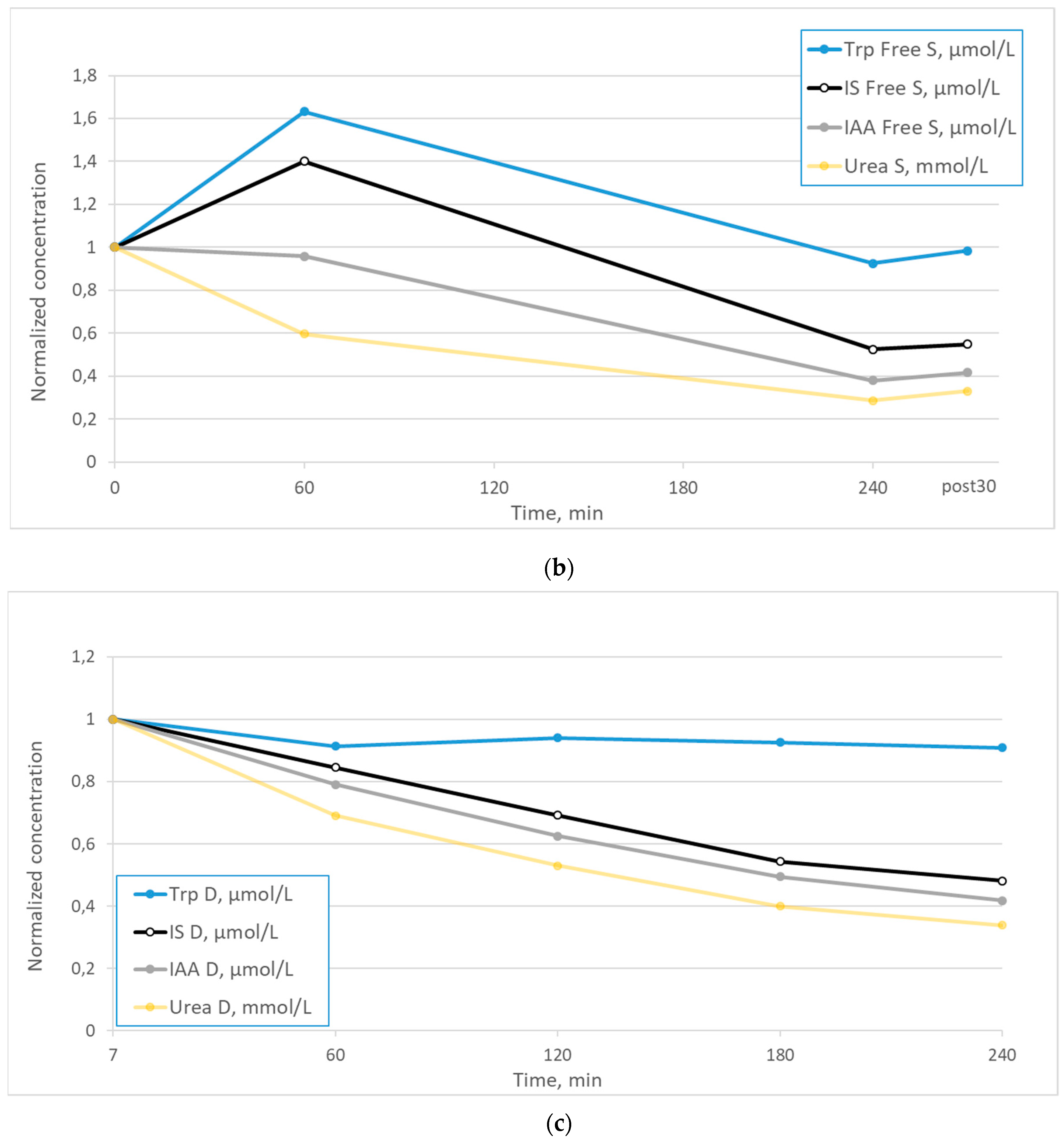
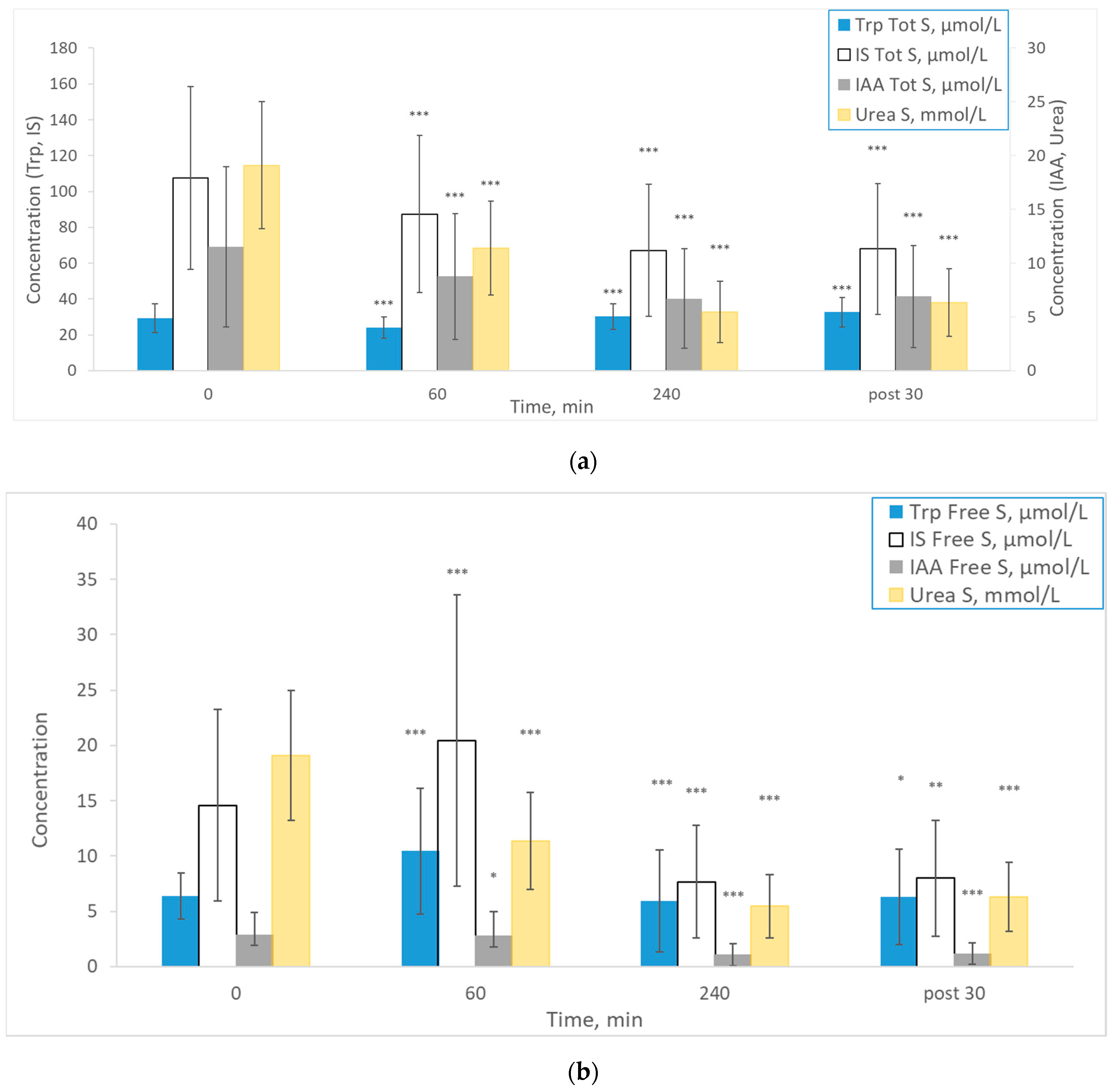
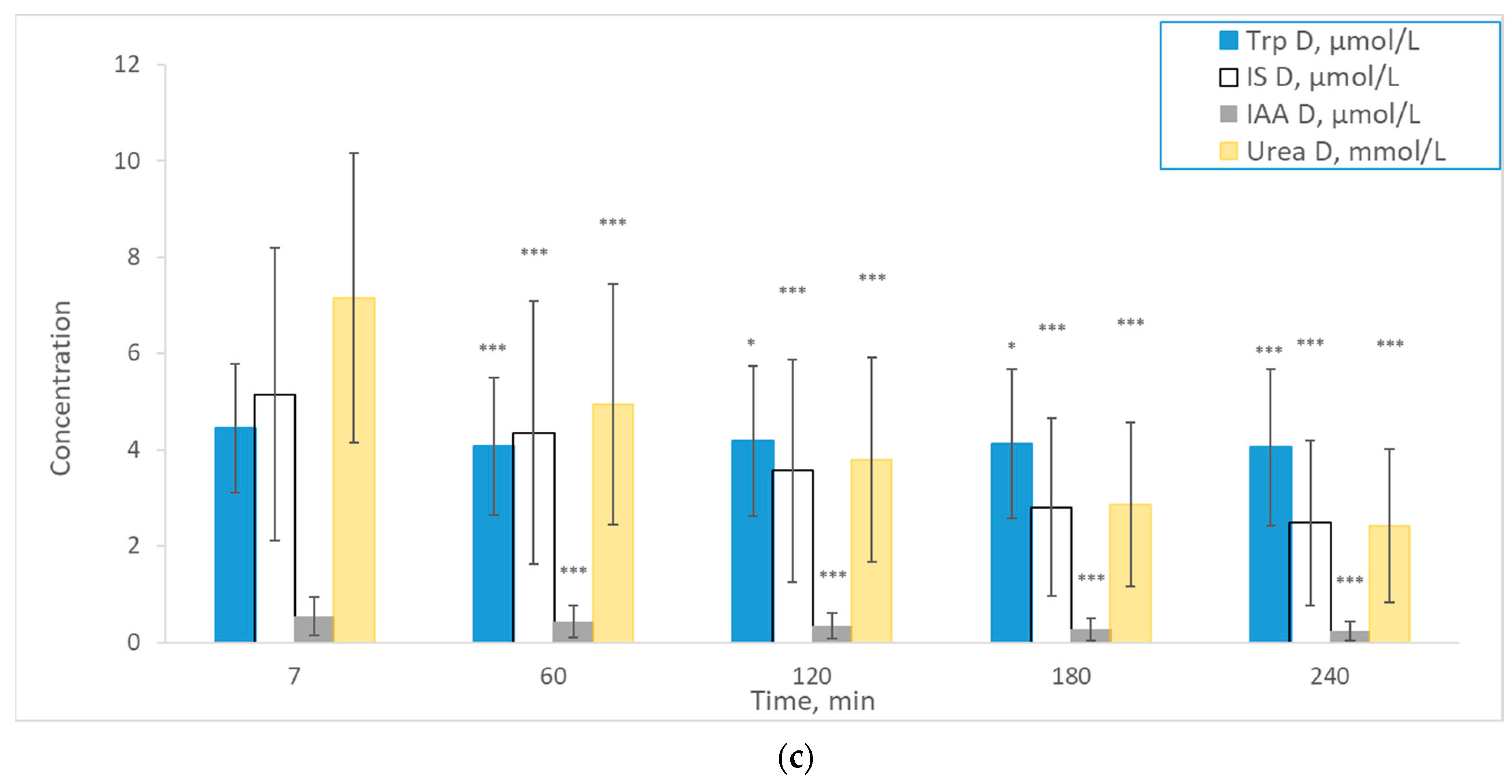
2. Results
3. Discussion
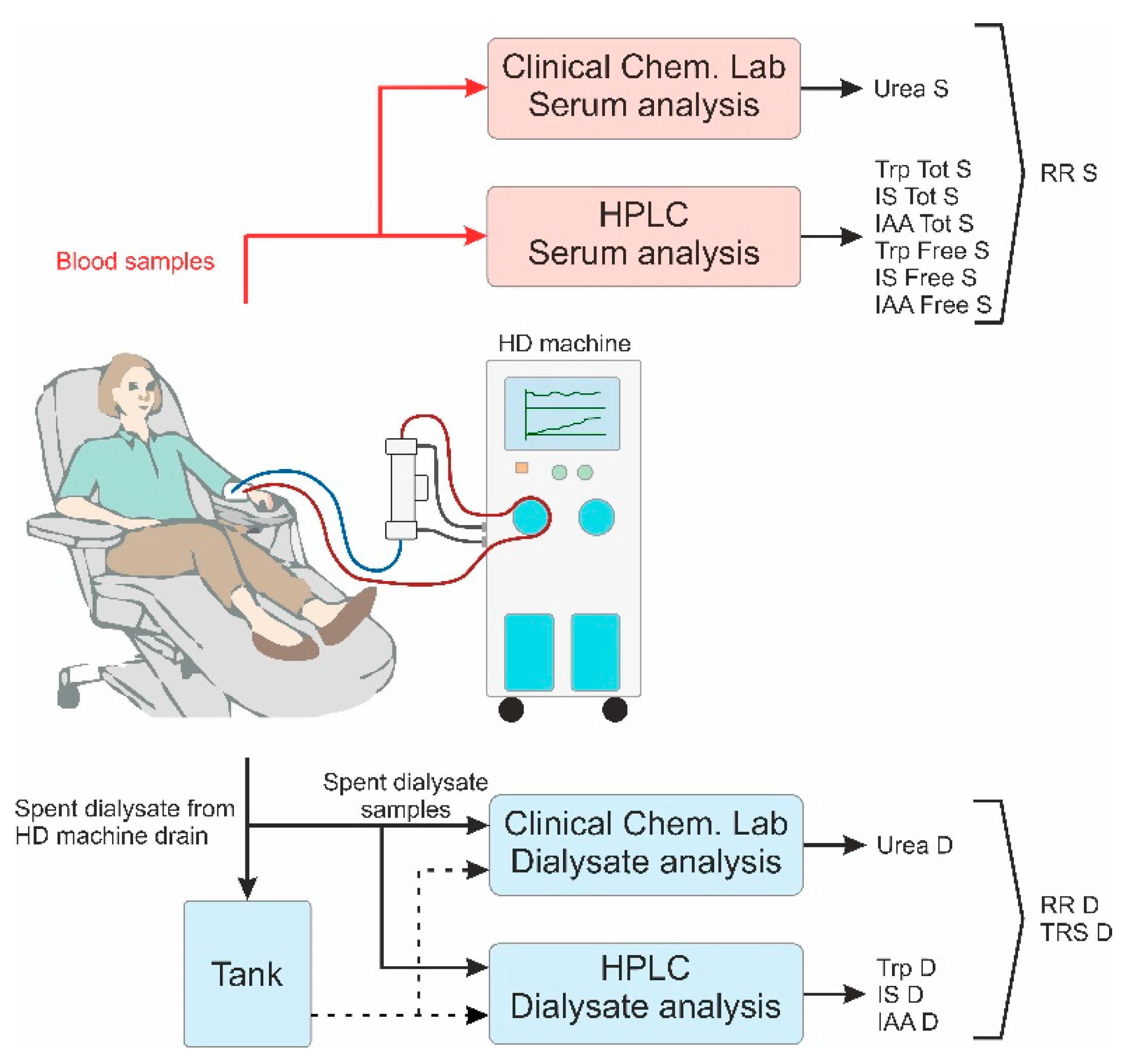
4. Materials and Methods
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ESKD | end-stage kidney disease |
| HD | haemodialysis (low flux) |
| HDF | haemodialfiltration |
| IAA | indole acetic acid |
| IS | indoxyl sulfate |
| RR | reduction ratio |
| TRS | total removed solute |
| Trp | tryptophan |
Appendix A
References
- Schmidt, J.A.; Rinaldi, S.; Scalbert, A.; Ferrari, P.; Achaintre, D.; Gunter, M.J.; Appleby, P.N.; Key, T.J.; Travis, R.C. Plasma concentrations and intakes of amino acids in male meat-eaters, fish-eaters, vegetarians and vegans: A cross-sectional analysis in the epic-oxford cohort. Eur. J. Clin. Nutr. 2016, 70, 306–312. [Google Scholar] [CrossRef]
- Cynober, L.A. Plasma amino acid levels with a note on membrane transport: Characteristics, regulation, and metabolic significance. Nutrition 2002, 18, 761–766. [Google Scholar] [CrossRef]
- McMenamy, R.H.; Lund, C.C.; Oncley, J.L. Unbound amino acid concentrations in human blood plasmas. J. Clin. Investig. 1957, 36, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Mingrone, G.; De Smet, R.; Greco, A.V.; Bertuzzi, A.; Gandolfi, A.; Ringoir, S.; Vanholder, R. Serum uremic toxins from patients with chronic renal failure displace the binding of l-tryptophan to human serum albumin. Clin. Chim. Acta 1997, 260, 27–34. [Google Scholar] [CrossRef]
- Richard, D.M.; Dawes, M.A.; Mathias, C.W.; Acheson, A.; Hill-Kapturczak, N.; Dougherty, D.M. L-tryptophan: Basic metabolic functions, behavioral research and therapeutic indications. Int. J. Tryptophan Res. 2009, 2, IJTR–S2129. [Google Scholar] [CrossRef]
- Møller, S.E. Pharmacokinetics of tryptophan, renal handling of kynurenine and the effect of nicotinamide on its appearance in plasma and urine following l-tryptophan loading of healthy subjects. Eur. J. Clin. Pharmacol. 1981, 21, 137–142. [Google Scholar] [CrossRef]
- Bender, D.A. Biochemistry of tryptophan in health and disease. Mol. Asp. Med. 1983, 6, 101–197. [Google Scholar] [CrossRef]
- Green, A.R.; Aronson, J.K.; Curzon, G.; Woods, H.F. Metabolism of an oral tryptophan load. I: Effects of dose and pretreatment with tryptophan. Br. J. Clin. Pharmacol. 1980, 10, 603–610. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Cosola, C.; Rocchetti, M.T.; Cupisti, A.; Gesualdo, L. Microbiota metabolites: Pivotal players of cardiovascular damage in chronic kidney disease. Pharmacol. Res. 2018, 130, 132–142. [Google Scholar] [CrossRef]
- Vanholder, R.C.; Eloot, S.; Glorieux, G.L.R.L. Future avenues to decrease uremic toxin concentration. Am. J. Kidney Dis. 2016, 67, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Adrian, P.; Marleen, H.; Enya, P.; Martijn van, F.; Ido, P.K.; Steffie, V.; Johanna, M.G.; Ralf, W.; Karin, J.R.I.; Stephan, J.L.B.; et al. Tryptophan intake and tryptophan losses in hemodialysis patients: A balance study. Nutrients 2019, 11. [Google Scholar] [CrossRef]
- Weng, C.O.; Bruno, M.; Mark, R.; Daniel, H.; Andrew, S.; Jennifer, C.A.; Ravi, M.; David, S.G.; Mark, A.J.D. Micronutrient and amino acid losses during renal replacement therapy for acute kidney injury. Kidney Int. Rep. 2019, 4, 1094–1108. [Google Scholar] [CrossRef]
- Stapel, S.N.; de Boer, R.J.; Thoral, P.J.; Vervloet, M.G.; Girbes, A.R.J.; Oudemans-van Straaten, H.M. Amino acid loss during continuous venovenous hemofiltration in critically ill patients. Blood Purif. 2019, 48, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Chua, H.R.; Baldwin, I.; Fealy, N.; Naka, T.; Bellomo, R. Amino acid balance with extended daily diafiltration in acute kidney injury. Blood Purif. 2012, 33, 292–299. [Google Scholar] [CrossRef]
- Davies, S.P.; Reaveley, D.A.; Brown, E.A.; Kox, W.J. Amino acid clearances and daily losses in patients with acute renal failure treated by continuous arteriovenous hemodialysis. Crit. Care Med. 1991, 19, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R.; Tan, H.K.; Bhonagiri, S.; Gopal, I.; Seacombe, J.; Daskalakis, M.; Boyce, N. High protein intake during continuous hemodiafiltration: Impact on amino acids and nitrogen balance. Int. J. Artif. Organs 2002, 25, 261–268. [Google Scholar] [CrossRef]
- Sainio, E.-L.; Pulkki, K.; Young, S.N. L-tryptophan: Biochemical, nutritional and pharmacological aspects. Amino Acids 1996, 10, 21–47. [Google Scholar] [CrossRef]
- Alp Ikizler, T.; Flakoll, P.J.; Parker, R.A.; Hakim, R.M. Amino acid and albumin losses during hemodialysis. Kidney Int. 1994, 46, 830–837. [Google Scholar] [CrossRef]
- Le Floc’h, N.; Otten, W.; Merlot, E. Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino Acids 2011, 41, 1195–1205. [Google Scholar] [CrossRef]
- Meert, N.; Eloot, S.; Schepers, E.; Lemke, H.D.; Dhondt, A.; Glorieux, G.; Van Landschoot, M.; Waterloos, M.A.; Vanholder, R. Comparison of removal capacity of two consecutive generations of high-flux dialysers during different treatment modalities. Nephrol. Dial. Transplant. 2011, 26, 2624–2630. [Google Scholar] [CrossRef]
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A.; European Uremic Toxin Work Group. Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Ezawa, A.; Kikuchi, K.; Tsuruta, Y.; Niwa, T. Protein-bound uremic toxins in hemodialysis patients measured by liquid chromatography/tandem mass spectrometry and their effects on endothelial ros production. Anal. Bioanal. Chem. 2012, 403, 1841–1850. [Google Scholar] [CrossRef]
- Florens, N.; Yi, D.; Juillard, L.; Soulage, C.O. Using binding competitors of albumin to promote the removal of protein-bound uremic toxins in hemodialysis: Hope or pipe dream? Biochimie 2018, 144, 1–8. [Google Scholar] [CrossRef]
- Dou, L.; Sallée, M.; Cerini, C.; Poitevin, S.; Gondouin, B.; Jourde-Chiche, N.; Fallague, K.; Brunet, P.; Calaf, R.; Dussol, B.; et al. The cardiovascular effect of the uremic solute indole-3 acetic acid. J. Am. Soc. Nephrol. 2015, 26, 876–887. [Google Scholar] [CrossRef]
- Leong, S.C.; Sirich, T.L. Indoxyl sulfate-review of toxicity and therapeutic strategies. Toxins (Basel) 2016, 8, 358. [Google Scholar] [CrossRef] [PubMed]
- Meert, N.; Waterloos, M.-A.; Van Landschoot, M.; Dhondt, A.; Ledebo, I.; Glorieux, G.; Goeman, J.; Van der Eycken, J.; Vanholder, R. Prospective evaluation of the change of predialysis protein-bound uremic solute concentration with postdilution online hemodiafiltration. Artif. Organs 2010, 34, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Krieter, D.H.; Hackl, A.; Rodriguez, A.; Chenine, L.; Moragues, H.L.; Lemke, H.D.; Wanner, C.; Canaud, B. Protein-bound uraemic toxin removal in haemodialysis and post-dilution haemodiafiltration. Nephrol. Dial. Transplant. 2010, 25, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Deltombe, O.; Van Biesen, W.; Glorieux, G.; Massy, Z.; Dhondt, A.; Eloot, S. Exploring protein binding of uremic toxins in patients with different stages of chronic kidney disease and during hemodialysis. Toxins (Basel) 2015, 7, 3933–3946. [Google Scholar] [CrossRef]
- Camacho, O.; Rosales, M.C.; Shafi, T.; Fullman, J.; Plummer, N.S.; Meyer, T.W.; Sirich, T.L. Effect of a sustained difference in hemodialytic clearance on the plasma levels of p-cresol sulfate and indoxyl sulfate. Nephrol. Dial. Transplant. 2016, 31, 1335–1341. [Google Scholar] [CrossRef]
- Madero, M.; Cano, K.B.; Campos, I.; Tao, X.; Maheshwari, V.; Brown, J.; Cornejo, B.; Handelman, G.; Thijssen, S.; Kotanko, P. Removal of protein-bound uremic toxins during hemodialysis using a binding competitor. Clin. J. Am. Soc. Nephrol. 2019, 14, 394–402. [Google Scholar] [CrossRef] [PubMed]
- de Brito, J.S.; Borges, N.A.; Dolenga, C.J.R.; Carraro-Eduardo, J.C.; Nakao, L.S.; Mafra, D. Is there a relationship between tryptophan dietary intake and plasma levels of indoxyl sulfate in chronic kidney disease patients on hemodialysis? Braz. J. Nephrol. 2016, 38, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Prado, R.; Esteras, R.; Perez-Gomez, M.V.; Gracia-Iguacel, C.; Gonzalez-Parra, E.; Sanz, A.B.; Ortiz, A.; Sanchez-Niño, M.D. Nutrients turned into toxins: Microbiota modulation of nutrient properties in chronic kidney disease. Nutrients 2017, 9, 489. [Google Scholar] [CrossRef] [PubMed]
- Snell, E.E. Tryptophanase: Structure, catalytic activities, and mechanism of action. Adv. Enzymol. Relat. Areas Mol. Biol. 1975, 42, 287–333. [Google Scholar] [CrossRef]
- Schütz, A.; Sandalova, T.; Ricagno, S.; Hübner, G.; König, S.; Schneider, G. Crystal structure of thiamindiphosphate-dependent indolepyruvate decarboxylase from enterobacter cloacae, an enzyme involved in the biosynthesis of the plant hormone indole-3-acetic acid. Eur. J. Biochem. 2003, 270, 2312–2321. [Google Scholar] [CrossRef]
- Hoshino, Y.; Yamamoto, T.; Kaneko, M.; Kumashiro, H. Plasma free tryptophan concentration in autistic children. Brain Dev. 1986, 8, 424–427. [Google Scholar] [CrossRef]
- Debnath, S.; Velagapudi, C.; Redus, L.; Thameem, F.; Kasinath, B.; Hura, C.E.; Lorenzo, C.; Abboud, H.E.; O’Connor, J.C. Tryptophan metabolism in patients with chronic kidney disease secondary to type 2 diabetes: Relationship to inflammatory markers. Int. J. Tryptophan Res. 2017, 10, 1178646917694600. [Google Scholar] [CrossRef]
- Murr, C.; Grammer, T.B.; Kleber, M.E.; Meinitzer, A.; März, W.; Fuchs, D. Low serum tryptophan predicts higher mortality in cardiovascular disease. Eur. J. Clin. Investig. 2015, 45, 247–254. [Google Scholar] [CrossRef]
- Yu, E.; Ruiz-Canela, M.; Guasch-Ferré, M.; Zheng, Y.; Toledo, E.; Clish, C.B.; Salas-Salvadó, J.; Liang, L.; Wang, D.D.; Corella, D.; et al. Increases in plasma tryptophan are inversely associated with incident cardiovascular disease in the prevención con dieta mediterránea (predimed) study. J. Nutr. 2017, 147, 314–322. [Google Scholar] [CrossRef]
- Garibotto, G.; Bonanni, A.; Verzola, D. Effect of kidney failure and hemodialysis on protein and amino acid metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 78–84. [Google Scholar] [CrossRef]
- Raj, D.S.C.; Moseley, P.; Dominic, E.A.; Onime, A.; Tzamaloukas, A.H.; Boyd, A.; Shah, V.O.; Glew, R.; Wolfe, R.; Ferrando, A. Interleukin-6 modulates hepatic and muscle protein synthesis during hemodialysis. Kidney Int. 2008, 73, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Niwa, T.; Maeda, K.; Kobayashi, K.; Yamamoto, Y.; Ohta, K. Tryptophan and indolic tryptophan metabolites in chronic renal failure. Am. J. Clin. Nutr. 1980, 33, 1402–1406. [Google Scholar] [CrossRef] [PubMed]
- Dromgoole, S.H. The effect of haemodialysis on the binding capacity of albumin. Clin. Chim. Acta 1973, 46, 469–472. [Google Scholar] [CrossRef]
- Shibasaki, T.; Matsuda, H.; Ohno, I.; Gomi, H.; Nakano, H.; Misawa, T.; Abe, S.; Ishimoto, F.; Kisugi, R.; Ikeda, K.; et al. Significance of serum lipase in patients undergoing hemodialysis. Am. J. Nephrol. 1996, 16, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, T.; Johno, I.; Kitazawa, S.; Goto, M.; Hata, T. Plasma free fatty acids and protein binding of disopyramide during haemodialysis. Eur. J. Clin. Pharmacol. 1987, 33, 327–329. [Google Scholar] [CrossRef]
- Anguizola, J.; Debolt, E.; Suresh, D.; Hage, D.S. Chromatographic analysis of the effects of fatty acids and glycation on binding by probes for sudlow sites i and ii to human serum albumin. J. Chromatogr. B 2016, 1021, 175–181. [Google Scholar] [CrossRef]
- Tao, X.; Thijssen, S.; Kotanko, P.; Ho, C.H.; Henrie, M.; Stroup, E.; Handelman, G. Improved dialytic removal of protein-bound uraemic toxins with use of albumin binding competitors: An in vitro human whole blood study. Sci. Rep. 2016, 6, 23389. [Google Scholar] [CrossRef]
- Sakai, T.; Takadate, A.; Otagiri, M. Characterization of binding site of uremic toxins on human serum albumin. Biol. Pharm. Bull. 1995, 18, 1755–1761. [Google Scholar] [CrossRef]
- Cunningham, V.J.; Hay, L.; Stoner, H.B. The binding of l-tryptophan to serum albumins in the presence of non-esterified fatty acids. Biochem. J. 1975, 146, 653–658. [Google Scholar] [CrossRef]
- Deltombe, O.; de Loor, H.; Glorieux, G.; Dhondt, A.; Van Biesen, W.; Meijers, B.; Eloot, S. Exploring binding characteristics and the related competition of different protein-bound uremic toxins. Biochimie 2017, 139, 20–26. [Google Scholar] [CrossRef]
- Lauri, K.; Arund, J.; Holmar, J.; Tanner, R.; Kalle, S.; Luman, M.; Fridolin, I.; Tanner, R.; Kalle, S.; Luman, M.; et al. Removal of urea, β2-microglobulin, and indoxyl sulfate assessed by absorbance and fluorescence in the spent dialysate during hemodialysis. ASAIO J. 2019. [Google Scholar] [CrossRef] [PubMed]
- Eloot, S.; Dhondt, A.; Van Landschoot, M.; Waterloos, M.A.; Vanholder, R. Removal of water-soluble and protein-bound solutes with reversed mid-dilution versus post-dilution haemodiafiltration. Nephrol. Dial. Transplant. 2012, 27, 3278–3283. [Google Scholar] [CrossRef] [PubMed]
- Arund, J.; Luman, M.; Uhlin, F.; Tanner, R.; Fridolin, I. Is fluorescence valid to monitor removal of protein bound uremic solutes in dialysis? PLoS ONE 2016, 11, e0156541. [Google Scholar] [CrossRef] [PubMed]
- Lesaffer, G.; De Smet, R.; Lameire, N.; Dhondt, A.; Duym, P.; Vanholder, R. Intradialytic removal of protein-bound uraemic toxins: Role of solute characteristics and of dialyser membrane. Nephrol. Dial. Transplant. 2000, 15, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Murtas, S.; Aquilani, R.; Deiana, M.L.; Iadarola, P.; Secci, R.; Cadeddu, M.; Salis, S.; Serpi, D.; Bolasco, P. Differences in amino acid loss between high-efficiency hemodialysis and postdilution and predilution hemodiafiltration using high convection volume exchange-a new metabolic scenario? A pilot study. J. Renal Nutr. 2019, 29, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, F.K.; Smeets, J.S.J.; Broers, N.J.H.; van Kranenburg, J.M.X.; van der Sande, F.M.; Kooman, J.P.; van Loon, L.J.C. End-stage renal disease patients lose a substantial amount of amino acids during hemodialysis. J. Nutr. 2020. [Google Scholar] [CrossRef]
- GBD 2017 DALYs and HALE, Collaborators. Global, regional, and national disability-adjusted life-years (dalys) for 359 diseases and injuries and healthy life expectancy (hale) for 195 countries and territories, 1990-2017: A systematic analysis for the global burden of disease study 2017. Lancet 2018, 392, 1859–1922. [Google Scholar] [CrossRef]
- Kramer, A.; Pippias, M.; Noordzij, M.; Stel, V.S.; Andrusev, A.M.; Aparicio-Madre, M.I.; Arribas Monzón, F.E.; Åsberg, A.; Barbullushi, M.; Beltrán, P.; et al. The european renal association – european dialysis and transplant association (era-edta) registry annual report 2016: A summary. Clin. Kidney J. 2019, 12, 702–720. [Google Scholar] [CrossRef]
- Eloot, S.; Dhondt, A.; Vierendeels, J.; De Wachter, D.; Verdonck, P.; Vanholder, R. Temperature and concentration distribution within the genius® dialysate container. Nephrol. Dial. Transplant. 2007, 22, 2962–2969. [Google Scholar] [CrossRef]
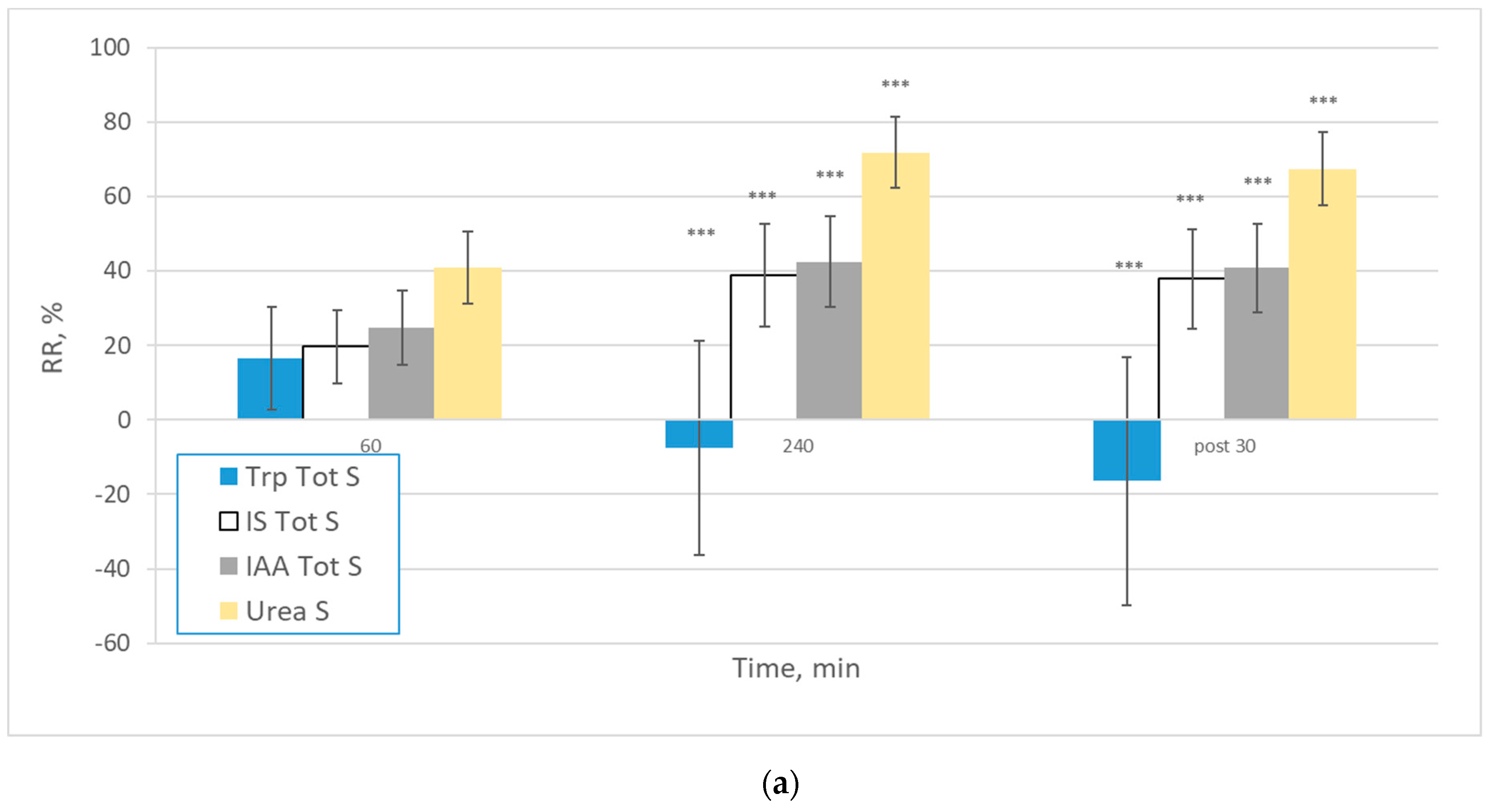


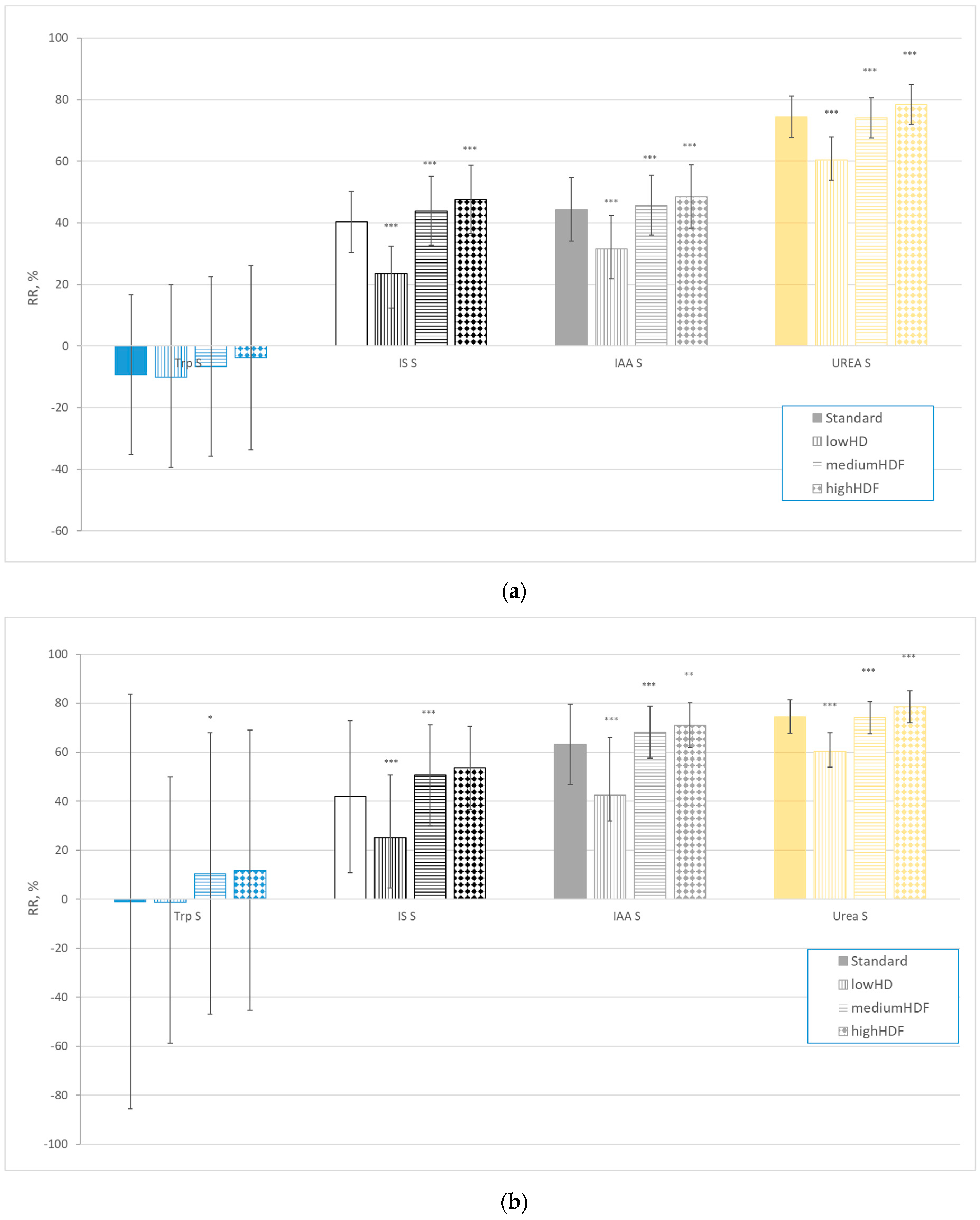
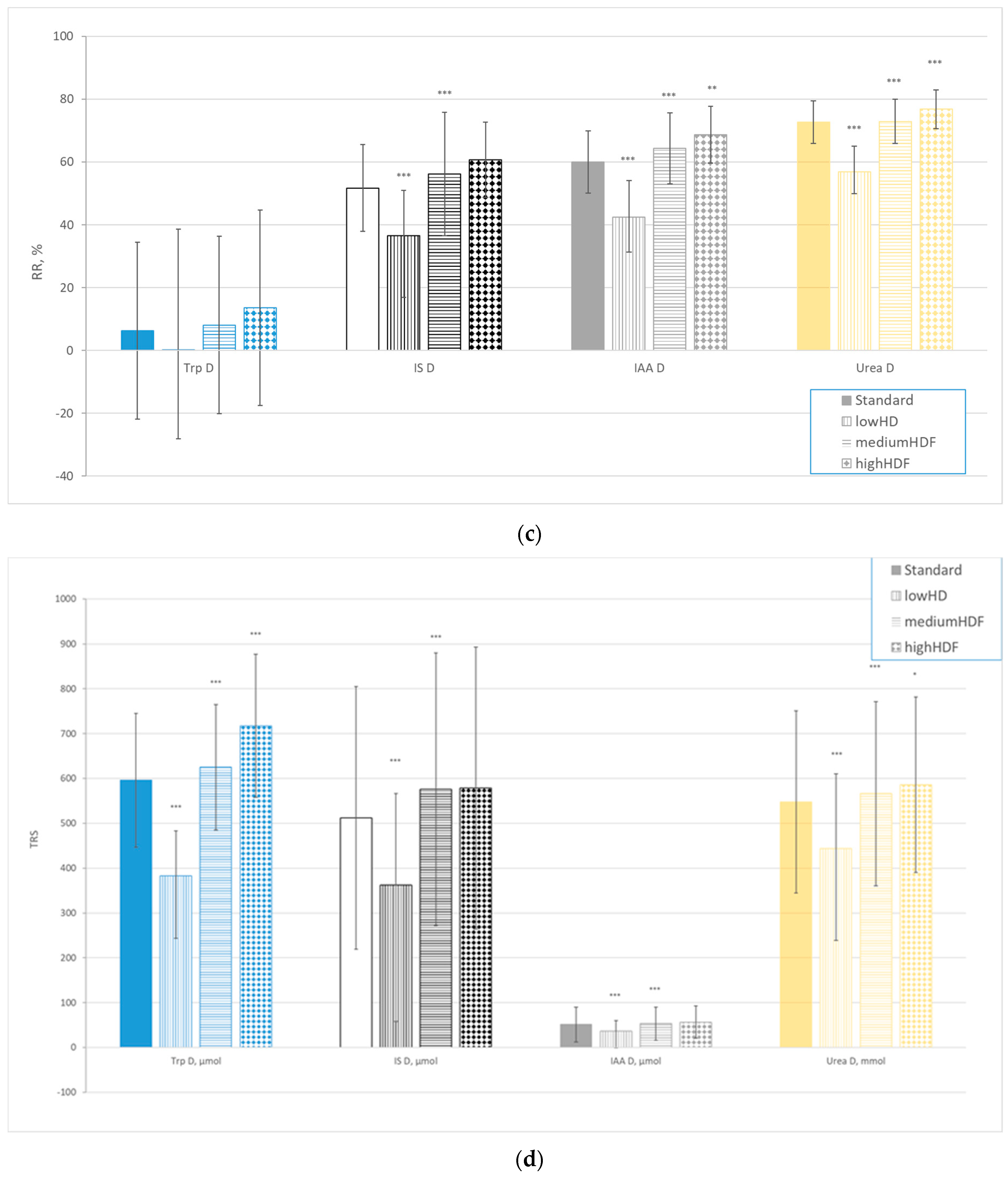
| Solutes | All (n = 78) | Centre 1 (n = 22) | Centre 2 (n = 21) | Centre 3 (n = 15) | Centre 4 (n = 20) | |
|---|---|---|---|---|---|---|
| Total | Trp | 29.3 ± 8.0 | 29.00 ± 6.28 | 32.6 ± 9.7 ** | 27.7 ± 8.8 °° | 27.3 ± 6.0 °°° |
| IS | 107.6 ± 51.2 | 94.8 ± 47.6 | 121.4 ± 57.0 ** | 98.5 ± 55.5 ° | 113.6 ± 40.0 ** | |
| IAA | 11.8 ± 9.2 | 13.5 ± 13.8 | 11.4 ± 5.9 | 11.3 ± 7.3 | 10.9 ± 6.7 | |
| Free | Trp | 6.41 ± 2.07 | 6.22 ± 2.02 | 6.30 ± 1.63 | 5.54 ± 1.57 */°° | 7.39 ± 2.50 **/°°/§ |
| IS | 14.7 ± 8.7 | 11.3 ± 5.8 | 15.0 ± 9.1 | 12.7 ± 7.8 | 19.4 ± 9.4 ***/°°/§ | |
| IAA | 2.91 ± 2.00 | 3.08 ± 2.55 | 2.72 ± 1.68 | 2.61 ± 1.30 | 3.18 ± 2.06 | |
| Urea | 19.1 ± 5.9 | 21.2 ± 7.3 | 16.8 ± 4.1 *** | 18.7 ± 6.5 */° | 19.6 ± 4.4 °°° |
| Patients | All (n = 78) | Centre 1 (n = 22) | Centre 2 (n = 21) | Centre 3 (n = 15) | Centre 4 (n = 20) |
|---|---|---|---|---|---|
| Diagnosis 1 | ADPKD (8); Diabetes (13); GN (16); Hypertension (12); Other (10); Renal; carcinoma (4); TIN (8); Unknown (7) | Diabetes (4); Hypertension (8); GN (3); TIN (3); Other (2); Renal carcinoma (2) | ADPKD (4); Diabetes (3); GN (4); Hypertension (2); Other (1); Renal carcinoma (2); TIN (1); Unknown (4) | ADPKD (2); Diabetes (2); GN (5); Hypertension (1); Other (4); TIN (1) | ADPKD (2); Diabetes (4); GN (4); Hypertension (1); Other (3); TIN (3); Unknown (3) |
| Age (years) | 63 ± 16 | 55 ± 17 | 71 ± 11 | 59 ± 15 | 68 ± 14 |
| Gender | M (63), F (15) | M (16), F (6) | M (16), F (5) | M (13), F (2) | M (15), F(5) |
| Race, Caucasian (%) | 94 | 100 | 90 | 93 | 90 |
| BMI, kg/m2 | 26.5 ± 5.5 | 26.8 ± 5.8 | 26.5 ± 3.7 | 26.2 ± 7.1 | 26.4 ± 5.4 |
| BW, kg | 78.3 ± 18.3 | 81.5 ± 21.3) | 77.1 ± 12.6 | 81.4 ± 19.7 | 73.8 ± 17.5 |
| Ultrafiltration volume, mL | 2250 ± 1049 | 2565 ± 1190 | 1746 ± 1031 | 2208 ± 807 | 2468 ± 833 |
| Residual diuresis, n (%) | 30 (38%) | 8 (35%) | 14 (67%) | 8 (53%) | 0 |
| Urinary volume, mL | 342 ± 607 | 227 ± 397 | 700 ± 754 | 457 ± 713 | 0 |
| Serum total protein, g/L | 65.1 ± 5.8 | 62.8 ± 5.5 | 68.5 ± 4.6 | 65.2 ± 5.5 | 63.8 ± 5.9 |
| spKt/Vurea | 1.64 ± 0.34 | 1.48 ± 0.30 | 1.70 ± 0.23 | 1.51 ± 0.29 | 1.85 ± 0.37 |
| Dialysis access | native fistula (64); graft (11); catheter (3) | native fistula (15); graft (7) | native fistula (19); graft (2) | native fistula (12); catheter (3) | native fistula (18); graft (2) |
| Dialysis vintage, months | 55 ± 66 | 50 ± 50 | 86 ± 04 | 47 ± 31 | 35 ± 23 |
| Standard | LowHD | MediumHDF | HighHDF | |
|---|---|---|---|---|
| Modality | HD/HDF | HD | HDF | HDF |
| Vs, L | 23.0 ± 3.5 | 0 | 16.4 ± 3.0 | 24.6 ± 4.0 |
| Time, min | 240 | 240 | 240 | 240 |
| Qb, mL/min | 323 ± 40 | 200 ± 10 | 306 ± 62 | 378 ± 30 |
| Qd, mL/min | 458 ± 63 | 301 ± 11 | 793 ± 57 | 793 ± 47 |
| Filter area 1, m2 | 1.90 ± 0.20 | 1.62 ± 0.19 | 2.13 ± 0.13 | 2.13 ± 0.14 |
| Number of dialyses, n | 78 | 78 | 78 | 78 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paats, J.; Adoberg, A.; Arund, J.; Dhondt, A.; Fernström, A.; Fridolin, I.; Glorieux, G.; Leis, L.; Luman, M.; Gonzalez-Parra, E.; et al. Serum Levels and Removal by Haemodialysis and Haemodiafiltration of Tryptophan-Derived Uremic Toxins in ESKD Patients. Int. J. Mol. Sci. 2020, 21, 1522. https://doi.org/10.3390/ijms21041522
Paats J, Adoberg A, Arund J, Dhondt A, Fernström A, Fridolin I, Glorieux G, Leis L, Luman M, Gonzalez-Parra E, et al. Serum Levels and Removal by Haemodialysis and Haemodiafiltration of Tryptophan-Derived Uremic Toxins in ESKD Patients. International Journal of Molecular Sciences. 2020; 21(4):1522. https://doi.org/10.3390/ijms21041522
Chicago/Turabian StylePaats, Joosep, Annika Adoberg, Jürgen Arund, Annemieke Dhondt, Anders Fernström, Ivo Fridolin, Griet Glorieux, Liisi Leis, Merike Luman, Emilio Gonzalez-Parra, and et al. 2020. "Serum Levels and Removal by Haemodialysis and Haemodiafiltration of Tryptophan-Derived Uremic Toxins in ESKD Patients" International Journal of Molecular Sciences 21, no. 4: 1522. https://doi.org/10.3390/ijms21041522
APA StylePaats, J., Adoberg, A., Arund, J., Dhondt, A., Fernström, A., Fridolin, I., Glorieux, G., Leis, L., Luman, M., Gonzalez-Parra, E., Perez-Gomez, V. M., Pilt, K., Sanchez-Ospina, D., Segelmark, M., Uhlin, F., & Arduan Ortiz, A. (2020). Serum Levels and Removal by Haemodialysis and Haemodiafiltration of Tryptophan-Derived Uremic Toxins in ESKD Patients. International Journal of Molecular Sciences, 21(4), 1522. https://doi.org/10.3390/ijms21041522






