Abstract
Post-breeding endometritis (i.e., inflammation/infection of the endometrium), is a physiological reaction taking place in the endometrium of mares within 48 h post-breeding, aimed to clear seminal plasma, excess sperm, microorganisms, and debris from the uterine lumen in preparation for the arrival of an embryo. Mares are classified as susceptible or resistant to persistent breeding-induced endometritis (PBIE) based on their ability to clear this inflammation/infection by 48 h post-breeding. Mares susceptible to PBIE, or those with difficulty clearing infection/inflammation, have a deficient immune response and compromised physical mechanisms of defense against infection. Molecular pathways of the innate immune response known to be involved in PBIE are discussed herein. The role of the adaptive uterine immune response on PBIE remains to be elucidated in horses. Advances in the pathobiology of microbes involved in PBIE are also revised here. Traditional and non-traditional therapeutic modalities for endometritis are contrasted and described in the context of clinical and molecular aspects. In recent years, the lack of efficacy of traditional therapeutic modalities, alongside the ever-increasing incidence of antibiotic-resistant microorganisms, has enforced the development of non-traditional therapies. Novel biological products capable of modulating the endometrial inflammatory response are also discussed here as part of the non-traditional therapies for endometritis.
1. Introduction
Endometritis, infection and/or inflammation of the endometrium, is the number one cause of subfertility and the third most common disease affecting horses [1,2]. Clinically, mares are classified as susceptible or resistant to persistent breeding-induced endometritis (PBIE) based on their ability to clear this inflammation/infection by 48 h post-breeding [3,4]. Susceptible mares, or those with difficulty clearing inflammation, may have a poor vulvar conformation in addition to a pendulous uterus [5]. Additionally, susceptible mares are typically older [6]. In a normal breeding program, as many as 25% of broodmares are ≥ 16 years old, which is when the fertility of mares starts to decline [7]. It is estimated that 10–15% of mares are susceptible to developing PBIE [8].
A normal phenomenon, post-breeding inflammation can be caused by infectious agents (i.e., bacteria and fungus) and or by non-infectious agents such as sperm [9]. All mares display this transient uterine inflammatory response within 30 minutes following natural mating or artificial insemination [10,11,12]. This physiological reaction occurs to eliminate seminal plasma, excess sperm, microorganisms, and debris from the uterine lumen in preparation for the arrival of an embryo [9]. However, mares deemed susceptible to PBIE have a delayed onset followed by a prolonged uterine inflammatory response [12,13,14], which culminates with excessive accumulation of polymorphonuclear neutrophil (PMNs) and intrauterine fluid accumulation in the uterus up to 96 h and beyond after breeding, impairing embryonic survival and the establishment of pregnancy [8,15,16]. Additionally, due to impaired innate immune response activation, microbes introduced into the uterus at the time of breeding are not eliminated efficiently, leading to potential infection.
The management of mares susceptible to PBIE is a daunting task. The equine embryo migrates from the uterine tube to the uterine lumen between 144 and 168 h after ovulation [17]. This occurs concomitantly with the increasing production of progesterone and cervical tone, leading to minimal time to resolve inflammation. Persistent neutrophilia, excess intraluminal fluid accumulation, and prolonged pro-inflammatory cytokine production are all embryotoxic and lead to decreased fertility potential of these mares in either natural mating or artificial insemination [18]. Additionally, embryo donor mares are bred and flushed multiple times per season, for cumulative breeding seasons, making them particularly prone to endometritis [19]. The persistent endometrial inflammation creates a hostile uterine environment for the embryo entering the uterus, compromising embryo recovery and pregnancy rates [12,13].
Traditionally, endometritis has been treated with multi-modal therapeutics such as a combination of uterine lavage, ecbolic agents, anti-inflammatories, and antibiotics. Unfortunately, a subset of mares fails to respond to traditional therapeutics [20,21]. The lack of efficacy to traditional therapeutics, alongside the ever-increasing incidence of antibiotic-resistant microorganisms, has led to the development of alternative therapies for mares suffering from PBIE [21].
While equine endometritis has been a widely studied disease in the past 40 years and elegant reviews have been published on the subject [22,23,24,25,26,27], much progress has been made in the last decade towards the understanding of the molecular aspects involved in PBIE and pathways down- or up-regulated in response to experimental induction and treatment [17,28,29,30,31,32,33,34,35]. Therefore, this manuscript aims to revise the clinical, molecular, and microbiological aspects of the pathogenesis, diagnosis, and treatment of endometritis in mares.
2. Etiology and Pathogenesis of Endometritis
Endometritis can be divided into both infectious and non-infectious causes, and yet in clinical practice, they often occur in association with another. The clinical signs for these two types of endometritis can be indistinguishable, except that the first type has microorganism(s) involved. In addition, to date, there are limited controlled studies comparing infectious vs. non-infectious endometritis in mares [36,37]. Mares with defective reproductive anatomy (e.g., poor vulvar conformation, torn vestibulovaginal sphincter, ventral sacculation of the uterus, impaired uterine contractility, cervix incompetence, and atrophied endometrium folds) are more prone to aspirate air or accumulate fluid or urine in the vagina and uterus, which make the mare simultaneously prone to infectious and non-infectious endometritis [20,38]. Additionally, mares with competent immune response and functional anatomy of the reproductive tract are able to clear infections spontaneously (i.e., mares resistant to endometritis), whereas mares with a deficient immune response may be unable to combat the development of an infection or may have persistent inflammation [31,32,33,39].
2.1. Infectious Endometritis
Infectious endometritis plays a major role in equine subfertility [40,41]. Microorganisms, including pathogenic or opportunistic bacteria and fungi, may gain access to the uterus during breeding. While the resistant mare should rapidly respond to the presence of microorganisms, inadequate immune response, and impaired uterine fluid drainage (e.g., due to pendulous uterus, tight cervix, or impaired myometrial contractility) may lead to infection [42,43]. Endometritis is mostly associated with aerobic bacteria [40]; however, anaerobes may also invade the uterus [44]. Of interest, retrospective reports identified that 25-60% of mares failing to become pregnant have bacterial uterine infection [45,46]. In clinical cases, the most commonly isolated bacteria linked with endometritis are the Streptococcus species, followed by Coliforms, Pseudomonas aeruginosa, and Staphylococcus aureus [20,42,47,48,49] (Table 1). Among all, Streptococcus equi subspecies zooepidemicus (Streptococcus zooepidemicus) and Escherichia coli predominate as causes of acute and chronic endometritis, respectively [50,51]. It is worth noting that Streptococcus zooepidemicus has also been shown to cause dormant, deep-seeded infections in the endometrium of mares, making them resistant to traditional therapy [52].

Table 1.
Common bacteria and fungi isolated from the uteruses of mares suffering from endometritis. G+: Gram-positive; G-: Gram-negative.
Fungi are less commonly associated with endometritis (1–5%), and they may occur alone or in association with bacteria [54]. Aspergillus and Candida are the most common genera, but other species have also been less commonly identified (e.g., Mucor sp) [49,53,54] (Table 1). It should be noted that fungal endometritis typically occurs as an opportunistic infection and has been identified after repeated use of intrauterine antimicrobials [53,55,56].
Mares susceptible to PBIE are prone to developing chronic infections, and some of those infections are due to bacteria and fungi capabilities to produce biofilm [57,58,59]. Biofilm is a complex aggregate of microorganisms and their secretions (i.e., extracellular matrix of polymeric substances) [60], which confers the ability to microorganisms to evade the immune system [61,62,63,64]. Biofilm works as a barrier for the diffusion of antimicrobials, and this limited penetration results in resistance to antimicrobial therapy, particularly when compared with planktonic bacterial infection (bacteria without biofilm) [65,66,67].
The shift of bacteria from the planktonic to the biofilm stage is attained via bacterial cell signaling molecule cyclic di-GMP [68,69], which regulates the production of exopolysaccharides alginate, Pel and Psl [70,71,72]. It is worth noting that both Pel and Psl are involved in the attachment of bacteria to a cellular or a noncellular substrate and in the attachment of a microcolony to a substrate and stabilization of extracellular DNA to support the biofilm [73,74,75,76,77]. Approximately 80% of bacteria isolated from the equine uterus is capable of producing a biofilm [58,59,78,79]. The host immunity and microenvironment are known to play a role in biofilm formation in other body systems such as the oral cavity [80,81]; however, it remains to be determined how these factors contribute to biofilm formation and pathogenesis of endometritis in the equine uterus [58,78,82].
Previously, it was believed that the mammalian female uterine environment was sterile [83,84,85]; however, this claim was challenged after the publication of the Human Microbiome Project (2007), which showed that the uterine cavity harbors a unique microbiome [83,85,86]. In horses, the uterus supports a moderately diverse microbiome, and its composition appears largely shared with microbial populations found on the external cervical os [87]. This communality of microbe populations between the cranial vagina and uterus in mares can be explained by the open cervix and close communication between the uterine lumen and cranial vagina during estrus [88]. The uterine microbiome changes according to the stage of the estrous cycle and across studies [89,90]. In one study [87], Proteobacteria-driven microbiota was the primary population, while a more diverse microbiome, including Proteobacteria, Firmicutes, Bacterioidetes, and Actinobacteria, was reported in a different study [91].
The uterine microbiome of women and cows suffering from endometritis differs from that of healthy females [92], suggesting that endometritis is associated with commensal microbiome dysbiosis [88]. Invasion of the uterine cavity in mares and other mammals mostly occurs via ascending migration from the vagina [86,93], in association with one or more reproductive problems as described above. The role of the resident uterine microbiome in preventing infection and its potential interactions with the embryo and potential role on pregnancy loss have not been elucidated in horses. Similarly, the role of therapies for uterine infections in restoring a healthy balance in the uterine microbiome has not been studied in horses.
2.2. Non-Infectious Endometritis
In the past, endometritis was believed to be caused solely by a bacterial or fungal infection. Yet, pioneer studies found a similar neutrophil response regardless of if the mare was challenged with sperm, saline, or bacteria [12,37,94]. The immune system of the mucosal reproductive tract consists of two branches: the innate and the adaptive. Adaptive immunity responds selectively and gradually to the detection of antigens, and this is mediated by the T lymphocytes [95]. In contrast, the response to breeding is dictated by the innate immune response [28,30]. This consists of a non-specific, rapid, and transient response [96]. Initiated by Toll-like receptors (TLRs), immunoglobulins, and complement, it leads to the leukocytic digestion and elimination of foreign material regardless of pathogenicity [97,98,99]. The predominantly innate immune response allows for repeat spermatozoic and embryonic “challenge” to the immune system without the development of anti-sperm or anti-embryo antibodies. More specifically, regulatory T cells (Treg cells) recognize male antigens and develop a tolerogenic immune environment by suppression of inflammation and immune rejection responses [100,101]. Treg cells are a population of T lymphocyte with immune suppressive properties that comprise both CD4+ and CD8+ subtypes [102]. Treg cells generally act to suppress cytokine synthesis and effector function in macrophages, T-, B-, natural killer (NK), and dendritic cells [103,104].
In addition, Treg cells play a paramount role in mediating the immune tolerance required for embryo implantation by increasing circulating CD4+ CD25+ cells highly enriched with the signature Treg transcription factor forkhead box protein P3 (FoxP3) [100,105,106]. It is worth noting that FoxP3 was identified as a potent marker for Tregs in mice; it is also essential for the immunosuppressive action of Treg cells [107,108]. During early normal pregnancy, there is a decrease in CD4+/CD8+ ratio and an increment of CD4+CD25+FoxP3 in the mouse model [107,108,109]. In addition, Treg-related transcripts are increased during pregnancy in comparison with non-pregnant diestrus mares, and FoxP3-positive cells in the fetoplacental unit are associated with pregnancy and gestational age in mares [110]. However, it remains to be determined if susceptibility to PBIE is also associated with a disruption of these physiological mechanisms in mares and consequent compromise the establishment of a pregnancy. Soluble factors, including transforming growth factor-β, prostaglandin E, and TLR4 ligands, which are present in male seminal fluid, also contribute to an immune-deviating activity that drives to the development of this tolerogenic immune environment in vivo mice and in vitro human models [100,111,112]. Anecdotally, it has been long speculated in clinical practice that mares bred to the same stallion repeatedly multiple times per season, for cumulative breeding seasons, particularly in embryo transfer programs, are prone to develop more profound PBIE or not to become pregnant; however, satisfactory pregnant rates can be achieved when these mares are bred to males not previously used for breeding. The disruption of some of these physiological pathways may alter the uterine immune response after repeated exposure to the same male; however, these hypotheses have not been critically addressed in horses.
2.3. Innate Immune Response to Endometritis
The local innate immune response is immediately activated after antigen recognition and signaling by mucosal epithelial cells in the endometrium [28,30]. The detection of foreign particles induces the activation of the innate immune system, which is the first line of defense. The major functions of the innate immune system are to (1) recruit immune cells to sites of infection through the activation of various cytokines including chemokines, (2) activate the complement cascade to promote the clearance of dead cells, (3) induce the activation of the adaptive immune system through antigen presentation, and (4) act as a physical barrier to the invading organisms and particles. Many of these functions have been studied for their involvement in the disorder of endometritis, a review of which is provided here.
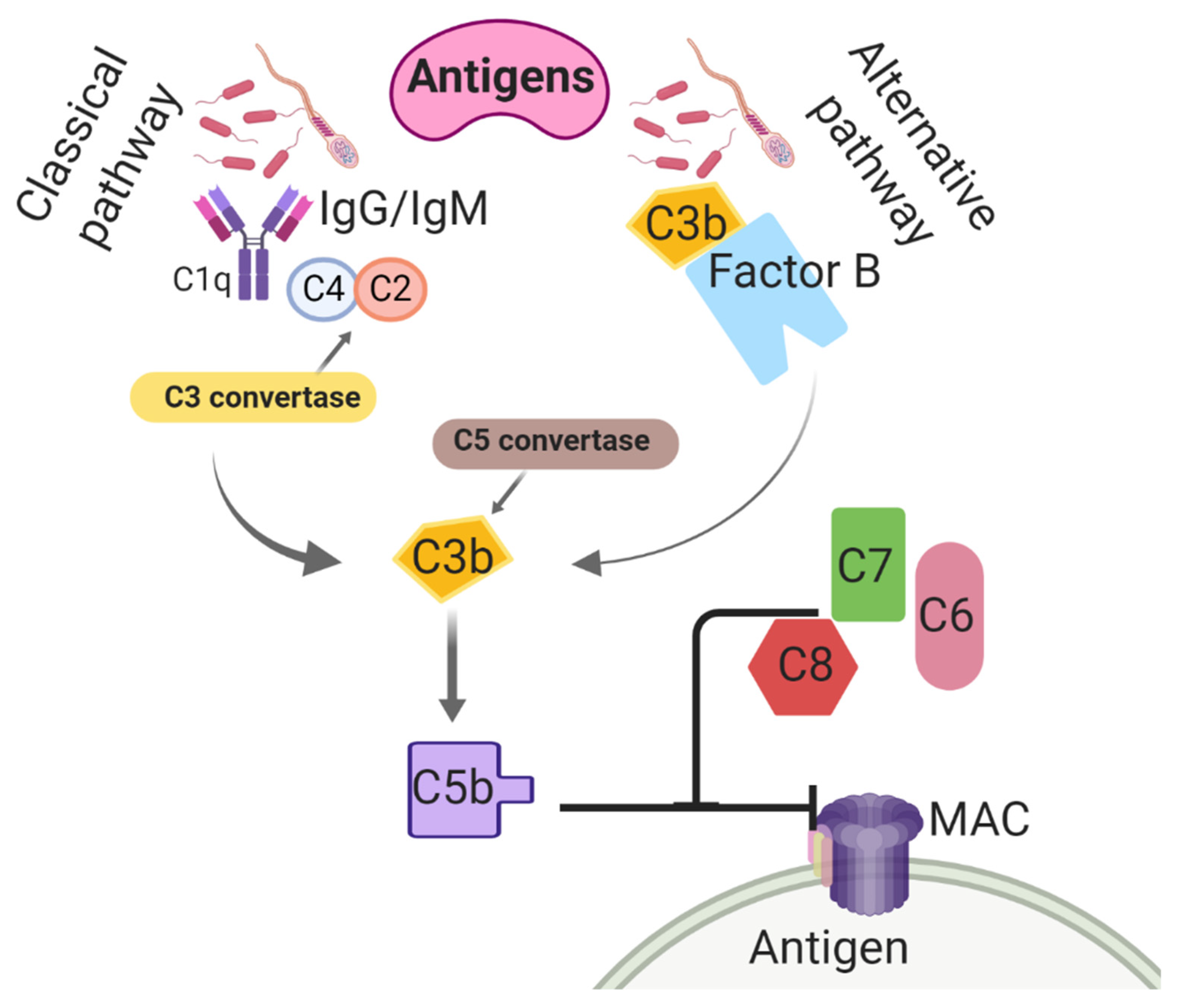
Believed to be the most ancient arm of the immune system, the complement system is utilized to nonspecifically enhance the phagocytic clearance of damaged cells and microbes [113]. Complement is an important contributor to opsonization activity in uterine secretions and inflammatory cell chemotaxis, preceding an inflammatory response [114]. In the classical complement pathway, the C4 subunit binds to IgM/IgG-associated C1q, initiating the enzymatic cleavages of C4 into C4a and C4b and C2 into C2a and C2b [115] (Figure 1). The association of C4b with C2b activates C3 convertase. Thereafter, C3 convertase cleaves C3 into C3a, and C3b [116] and then C3a contributes to leukocyte recruitment and further complement activation [117,118]. In addition, C3b binding to C4b activates convertase [119,120]. Both C4b and C3b are then able to bind to microbe surfaces or additional immunoglobulins [121]. The C5 convertase breaks C5 into C5a and C5b, and this results in conformational changes and activation of the terminal pathway, which promotes the formation of the membrane attack complex (MAC) [122]. The MAC is synthesized by interactions between C5b and other terminal components (e.g., C6, C7, C8, and C9) and lyses the target cell(s) by creating pores in the plasma membrane [123]. The complement cascade can also be triggered by an alternative pathway, which entails C3b protein binding directly to antigens [124]. Interestingly, the activation of this system has been found in response to breeding in the horse. Equine spermatozoa were found to induce the complement cascade, resulting in an increase of C3b and C5a, leukotrienes, and prostaglandins, all of which result in the chemotaxis of PMNs to the uterus [125,126,127].
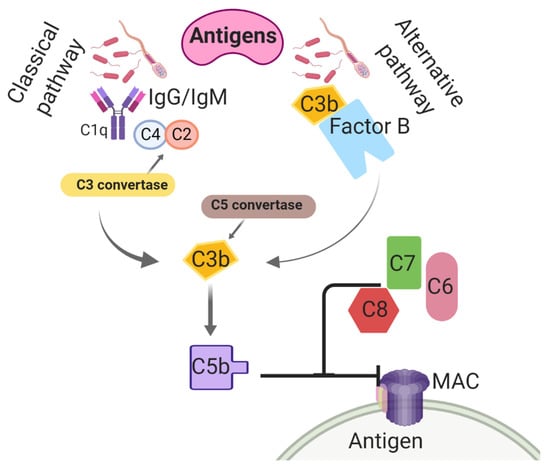
Figure 1.
Classical and alternative complement activation pathways taking place in the uterine lumen of mares post-breeding. C1q: Complement component 1; C2: Complement 2; C3: Complement 3; C4: Complement 4; C5: Complement 5; C6: Complement 6; C7: Complement 7; C8: Complement 8; IgG: immunoglobulin G; IgM: immunoglobulin M; MAC: membrane attack complex.
Additionally, complement cleavage factor C3b has been found in uterine secretions in both resistant and susceptible mare populations [128,129,130]. The various classes of immunoglobulins (e.g., IgA, IgG, and IgM) have also been identified in the uterine secretions of the mare. In addition to their relationship to complement, these molecules are thought to play an important role in antigen presentation to T cells [131,132,133,134,135]. Interestingly, no differences have been found when comparing the resistant mare to the susceptible mare regarding the expression or production of the various complement subunits or immunoglobulins, suggesting that they play a limited role in the pathogenesis of susceptibility to PBIE [2,136].
Microbes and foreign particles (such as sperm and seminal plasma proteins) are also detected via their antigen presentation to pattern recognition receptors (PRRs) located in the endometrial epithelial cells [29,137,138]. These cells, alongside the immune cells (e.g., tissue macrophages, NK cells, and neutrophils) that they recruit, produce various types of cytokines, including chemokines. Chemokines further recruit leukocytes to the site of inflammation, while other cytokines enable the differentiation and activation of other chemotaxed immune cells [139,140]. Collectively, these cells form a physical and immunological barrier at the uterine mucosa [141].
Toll-like receptors, a family of transmembrane proteins expressed in mammal cells [142,143,144,145], play a major role in that antigen recognition [146]. In this role, antigen-presenting cells (ACP), mainly dendritic, macrophages, and NK cells, express molecules to recognize these patterns [97,147]. Pathogen-associated molecular patterns (PAMPs) are recognized by the TLRs of endometrial and sentinel cells to begin the inflammatory reaction [148]. The exposure of Gram-negative bacteria or lipopolysaccharides (LPS) to these cells has been shown to enhance the expression of TLRs types 2 and 4 in mice, rabbits, and cattle [61,62,63,64]. The expression of TLRs by the endometrium after interaction with sperm is still not fully elucidated, particularly because semen is not sterile, and inherent bacterial contamination can be responsible for conflicting results in the literature. Toll-like receptor type 4 primarily recognizes LPS produced by Gram-negative bacteria, while TLR2 reacts to lipopeptides of Gram-positive bacteria, which may invade the uterus during breeding [149,150]. It is known that TLR2 and TLR4 are increased in the uteruses of mares resistant to PBIE after inoculation with Escherichia coli [29]. Additionally, work in other species indicates that human and murine sperm express TLR2 and TLR4, although this has not been confirmed in the horse [151]. In bovine, in vitro studies suggested that sperm can stimulate transcription of pro-inflammatory cytokines, including chemokines (TNFα, IL1β, CXCL8) and prostaglandin E and activate the local complement system (C3) mediated via TLR2/4 [152,153,154].
Another group of PRRs is NOD-like receptors (NLR), which are responsible for the intracellular detection of pathogens [155]. NOD-like receptors are expressed in many cell types, including immune cells and epithelial cells, although certain NLR family members are expressed primarily in phagocytes, including macrophages and neutrophils. A genetic variation in these genes may predispose humans to several inflammatory diseases [155,156]; however, the role of NLRs on the pathogenesis of endometritis has not been elucidated in horses.
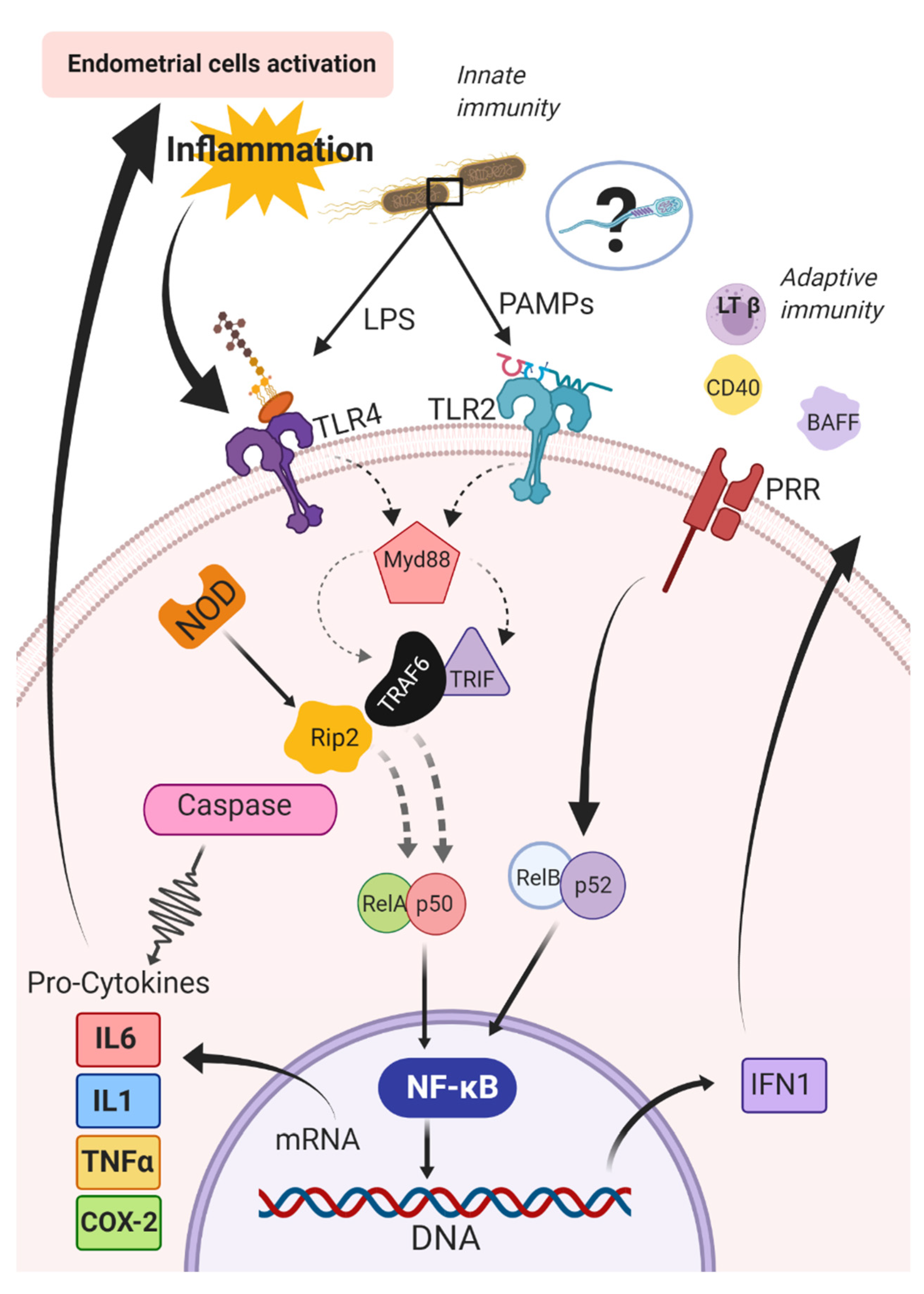
Pattern recognition receptors can respond to a diverse array of antigens and then activate pro-inflammatory cytokines [157,158,159] to regulate the immune response [160,161]. Activation of TLRs is a key event in the initiation of the inflammatory cascade [148], which stimulates nuclear factor kappa beta (NF-κB). The nuclear NF-κB is composed of five subunits, (RelA {p65}, RelB, ReL, p50, p52) and can be activated by innate (i.e., canonic) or adaptive (i.e., alternative) immune pathways [162]. The canonic pathway is triggered by microorganisms and pro-inflammatory cytokines (e.g., IL1 and TNFα) [162]. The triggering of this pathway entails the activation of RelA- or cRel- [163], which are regulated by IkB kinase β (IKKβ) through phosphorylation of inhibitors of kB (IkB) [164]. The IkB kinases are the key regulators of the NF-κB pathway [165]; conversely, in the alternative pathway, NF-κB is activated by other bioproducts such as lymphotoxin β, CD40 ligand, B cell-activating factor, and receptor activator of NF-κB [166,167,168,169,170,171]. The activation of RelB/p52 complex and IkB kinase α is required in this pathway for phosphorylation and processing of the precursors p52 p100 [166] (Figure 2).
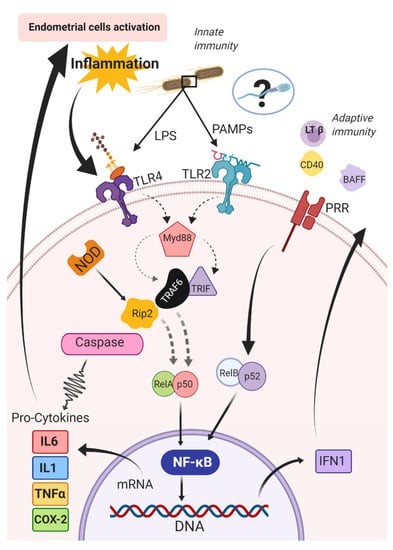
Figure 2.
Canonic and alternative pathways for activation of the nuclear factor kappa beta (NF-kB) in the endometrium of mares. BAFF: B cell activating Factor; CD40: Cluster of differentiation 40; COX-2: cyclooxygenase-2; IFN1: type 1 interferons; IL1: interleukin 1; IL6: interleukin 6; LPS: lipopolysaccharides; LTβ: lymphotoxin β; MyD88: Myeloid differentiation primary response 88; NF-κB: nuclear factor kappa beta; NOD: nucleotide-binding and oligomerization domain; PAMPs: pathogen-associated molecular patterns; PRR: pattern recognition receptors; RelA/p50 and RelB/p52: subunits of the NF-κB complex; Rip2: receptor-interacting protein 2; TLR2: Toll-like receptors type 2; TLR4: Toll-like receptors type 4; TNFα: tumor necrosis factor-alpha; TRIF: TIR-domain-containing adapter-inducing interferon-β; TRAF6: receptor-associated factor 6.
The NF-κB pathway then activates genes coding for pro-inflammatory cytokines, including chemokines and cyclooxygenase-2 (COX-2) [148,172]. Cytokines and COX-2 signal the immune cells modulating the acute inflammatory response [173]. Gene expression of members of both NLR and TLR families are upregulated after intrauterine inoculation with Escherichia coli in mares. In addition, several immune-related signal transduction pathways, including the mitogen-activated protein kinase, the NF-κB and TNFα signaling pathways, and the pathway for cytokine–cytokine receptor interaction, were also enriched after inoculation with Escherichia coli [29]. It should be noted that it remains to be determined if sperm induces a similar immune response to microorganisms.
A subset of these PRRs requires simultaneous binding to other endogenous cell surface receptors to be activated. As an example, when TLR4 binds to CD14, the subsequent detection of LPS alters downstream inflammatory responses [138,172]. After antigen recognition, the recruitment of adaptor proteins together with the MyD88-dependent cascade leads to the secretion of pro-inflammatory cytokines or the TIR-domain-containing adapter-inducing interferon-β (TRIF)-dependent cascade, which results in the production of type 1 interferons (IFN) in addition to inflammatory cytokines including chemokines [138,172]. The MyD88-dependent cascade induces the IL-1R-associated kinase family of kinases and consequently, activation of the ubiquitin-dependent kinase by TNFR-associated factor 6 [174,175]. The ubiquitin-dependent kinase induces NF-κB activation and the induction of the non-specific immune response by NF-κB transcription [176].
Initially, cytokines are synthesized as pro-molecules needing to be activated. Many types of molecules can activate cytokines (e.g., elastase, cathepsins, metalloproteinases, and trypsin). However, caspases, a large family of evolutionarily conserved proteases, play this role more broadly than other molecules [177]. For instance, the caspase-1 family (i.e., caspase-1, -4, -5, -11, -12, and -14) is primarily involved in the regulation of cytokines activation [177], and the caspase-3 family (e.g., caspases -3 and -7) can activate pro-inflammatory cytokines [178,179,180]. Specifically, caspase-1 activates IL1β, which is constitutively expressed and up-regulated in the endometrium of mares after experimental bacterial inoculation [29] and is also synthesized by NF-κB stimulation [173]. Under the action of prostaglandin-endoperoxide synthase, especially COX-2 during inflammation, prostaglandin synthesis occurs [181]. In the horse, an increase in COX-2 expression has been noted in the endometrium after exposure to seminal plasma or extender [182], as well as a local increase in prostaglandin F2 alpha (PGF2α) concentration in the uterus of normal mares 16 h after breeding [30].
From the synthesis of prostaglandins and pro-inflammatory cytokines, mainly interleukin 1 (IL1), interleukin 6 (IL6), and tumor necrosis factor-alpha (TNFα), vascular endothelial cell activation occurs. This leads to a constriction of the arterioles and dilation of the venules at the affected site, thereby increasing vascular permeability and exudate leakage to the interstitium, causing local edema [183]. The endometrial expression of various pro-inflammatory interleukins, including interleukin 1β (IL1β), chemokine ligand 8 (CXCL8, formerly known as IL8), and TNFα, is higher in susceptible mares to PBIE than in resistant mares, even before exposure to an antigen. This increase in susceptible mares is also noted following challenges with pathogens or sperm [31,182,184].
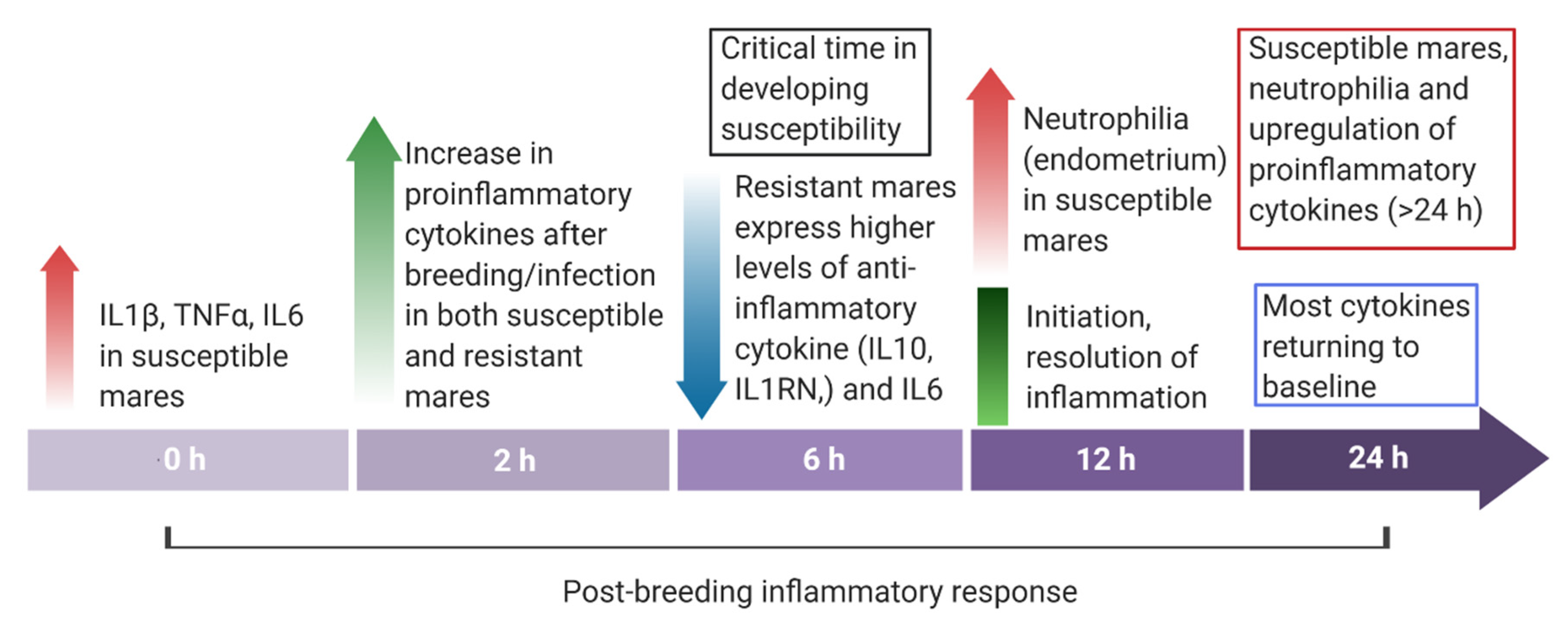
With alterations in the permeability of the vascular endothelium, cellular responses begin. Vascular endothelial cells increase in the expression of P-selectin via the inflammatory stimulus, which binds to L-selectin on the surface of neutrophils, inducing chemotaxis [185]. Then, neutrophils produce integrins to bind to adhesion molecules on endothelial cells until complete arrest and adherence to the walls of blood vessels [186]. Following the detection of foreign material, neutrophils migrate from the endometrium to the uterine lumen within 30 minutes [11] and have a peak inflammatory response between 6 and 12 h later [2] (Figure 3). Mares susceptible to PBIE experience increased neutrophilia at 2 and 12 h after breeding in comparison with resistant mares [16]. In addition to phagocytosis, neutrophils also secrete additional cytokines and chemotactic mediators, further inducing inflammation [186]. Leukocytes then release prostaglandins, which promote myometrial contractility and assist with the physical clearance of the uterus in the healthy mare [187].
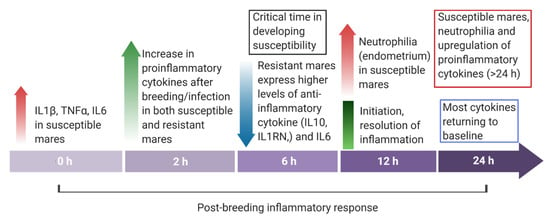
Figure 3.
Overview of the endometrial cytokine dynamics in mares resistant and susceptible to endometritis from immediately before (0 h) to 24 h post-breeding.
Neutrophils are the first immune cell line to respond after antigen recognition by innate immunity. In addition to phagocytosis and the release of lytic enzymes in response to antigens and pathogens, neutrophils form neutrophil extracellular traps (NETs). Neutrophil extracellular traps are DNA-associated molecules with antimicrobial and immunomodulating properties [188], which are induced by different inflammatory agents, such as reactive oxygen species [189], antibody-antigen complexes [190], CXCL8, lipopolysaccharide, and phorbol-myristate-acetate [191]. The antimicrobial activity of NETs, called NETosis, is due to neutrophils rupture and release of granules, allowing their chromatin to come in contact with antigens and other immune cells [192]. Several enzymes (e.g., elastase, protein 3, cathepsin G, and myeloperoxidase) and histones play a role in NETosis [193,194,195,196]. The formation of NETs is a complementary mechanism to eliminate bacteria, which may cause endometritis in mares [197].
When exacerbated, this increase in pro-inflammatory signals and the recruitment of immune cells can lead to tissue damage. Therefore, mechanisms are necessary to terminate the process for the resolution of the inflammatory process. An increase in anti-inflammatory or pleiotropic cytokines is noted as quickly as 2 to 6 h after breeding in the resistant mare [16] (Figure 3). They enact their properties by inhibiting the production of pro-inflammatory mediators, competing for pro-inflammatory receptors, or causing cell death [198]. Interleukin-10 (IL10), -1R antagonist (IL1RN), -4 (IL4), and -13 (IL13) are all considered anti-inflammatory and play an important role in the termination of this inflammatory response [14,199,200,201]. It has been well documented that IL1RN plays a role in balancing pro- and anti-inflammatory effects, because this cytokine competes with IL1 for binding to IL1 receptors, which prevents the binding of IL1α and IL1β [202]. Typically, IL10 is synthesized relatively late in the inflammatory response and acts as a generalized anti-inflammatory effector by reducing the transcription of pro-inflammatory cytokines by monocytes and macrophages [203,204].
Additionally, while IL6 is initially pro-inflammatory in response, its function is pleiotropic due to its ability to activate varying receptors and pathways to function as anti-inflammatories later in the inflammatory process. A study found the initiation of these anti-inflammatory cytokines to be defective in the susceptible mare [16]. At 6 h post-challenge with sperm, the endometrium of the resistant mare experienced an increase in IL10, IL1RN, and IL6 expression [16]. Susceptible mares had a significantly lower expression of these cytokines, indicating a failure to induce their response [184]. The authors hypothesized that this failure to mount an anti-inflammatory response leads to a prolonged endometrial inflammatory response in the mare susceptible to PBIE.
In addition to a defective anti-inflammatory cytokine production, susceptible mares have also been reported to have impaired myometrial contractility. A study found mares susceptible to PBIE to have a different myometrial response to the bacterial challenge when compared with the resistant mare, and this included the frequency, duration, and intensity of the contractions [205]. Interestingly, myometrial contractility is strongly connected to signaling within the immune system and particularly in the cytokine induction of nitric oxide (NO). Produced by inducible nitric oxide synthase, NO is a calcium-independent widespread signaling molecule that induces smooth muscle relaxation [206,207]. Interestingly, pro-inflammatory mediators, such as IL1 and IFNα, lead to increased transcription of this molecule [206]. This reduced smooth muscle activity is thought to interfere with the uterine clearance of mares susceptible to PBIE [10].
Numerous studies have investigated the role of NO in susceptibility to equine endometritis. Studies conducted with equine endometrial explants indicated a dose-dependent response to stimulation with NO, although a decreased response was noted in samples with lower endometrial quality [208]. In the susceptible mare, both NO expression and activity are upregulated [10,35,209]. The prolonged and undeterred increase in pro-inflammatory cytokines, such as IL1β, may be the cause of this increase in NO activity, leading to smooth muscle relaxation and decreased myometrial activity, all of which contribute to the pathophysiology of PBIE.
The stage of the estrous cycle (i.e., high vs. low progesterone concentrations) also plays a role in the uterine immune response in mares [210]. During progesterone dominance, the equine uterus is highly susceptible to infection, whereas, under estrogen dominance, the uterus is more capable of clearing infections [210]. For instance, expression of serum amyloid A (SAA) and the anti-inflammatory IL10 increased 3 h after bacterial inoculation in diestrus, but it did not in estrus [30,211].
Interestingly, matrix metalloproteinases (MMP) types 2 and 9 are significantly up-regulated 5 h after inoculation with Streptococcus zooepidemicus in both estrus and diestrus [212]. Matrix metalloproteinases are involved in extracellular matrix (ECM) remodeling [213] and modulated by tissue inhibitors of MMPs (TIMPs), which also have been identified in the endometrium of mares and women [212,214]. The activity of TIMP-2 decreases during induced endometritis in mares [212]. The fine balance between TIMPs and MMPs has been suggested to play a role in the development of endometrial fibrosis and degeneration in mares [212], but this is still controversial [215]. It is worth noting that mares susceptible to PBIE typically display endometrial fibrosis and other degenerative changes such as modifications in the type of collagen deposition [216].
The pathogenesis and etiology of endometrial fibrosis in mares remain poorly understood. It is known that MMPs and TIMPs regulate collagen deposition and other components of the ECM associated with fibrosis [217,218,219,220]. Also, NET components (e.g., myeloperoxidase, elastase, and cathepsin G) also up-regulate collagen types 1 and 3 and transforming growth factor-β1 [221,222,223]. In addition, endometrial expression of IL1β and IL6 is up-regulated during the progression of endometrial fibrosis in mares [224]. In vitro studies have shown the profibrotic effects of IL1β in the endometrium and other tissues [220,225,226]. A recent in vitro study suggested that IL1β and IL6 modulate ECM, MMP, and TIMP production in equine endometrium cells and might be important regulators in the pathogenesis of fibrosis [227]. Apparently, there might be an association between inflammation and development of endometrial fibrosis, in which IL1β and IL6 increase expression of ECM components, such as MMPs [227].
Additionally, endometrial epithelial cells produce antimicrobial peptides (e.g., defensins, elafin, cathelicidin, lactoferrin, and lysozyme), allowing for the nonspecific degradation of microbes and sperm alike [228]. In addition to their microbiocidal activity, these proteins affect cytokine induction, chemotaxis, and cell proliferation and modulate both innate and acquired immunity [211]. Antimicrobial peptides are modulated by the presence of bacteria, stage of the estrous cycle, and inflammation. These include factors that destabilize bacterial cell walls, such as defensins, lysozyme, and secreted phospholipase A2 [229,230] and those that inhibit bacterial enzymes, such as secretory leukoprotease inhibitor, which is also known as equine neutrophil antimicrobial peptide 2 [231,232,233]. Many of these antimicrobial proteins are increased in the endometrium of the susceptible mare population, including secretory leukoprotease inhibitor, equine β-defensin, lactoferrin, and lysozyme [28,234]. As many of these proteins are also produced within neutrophil granules, it is unknown if this increase is due to a defense mechanism within the endometrial glands of the susceptible mare or the increased neutrophilia caused by prolonged inflammatory processes.
Acute-phase proteins have also been investigated for their involvement and diagnostic capabilities in endometritis, as they are systemic markers of inflammation in the horse [235,236,237]. Serum amyloid A, haptoglobin, and fibrinogen have all been investigated [238]. The histologically normal endometrium of the mare has been shown to constitutively express mRNA for SAA at moderate levels [239]. Conflicting information exists regarding a detectable systemic SAA response to endometritis. One study found circulating SAA and fibrinogen to increase at 3- and 12-h post-inoculation with Escherichia coli, and this increase correlated with the endometrial expression of SAA [240]. In contrast, another study found no change in SAA concentrations following breeding with frozen/thawed semen [30]. Whether this difference was caused by the type of challenge used or due to the limited sample sizes remains to be determined. It should be noted that no difference in circulating SAA concentration or expression has been noted when comparing the susceptible to the resistant mare [199].
3. Diagnosis
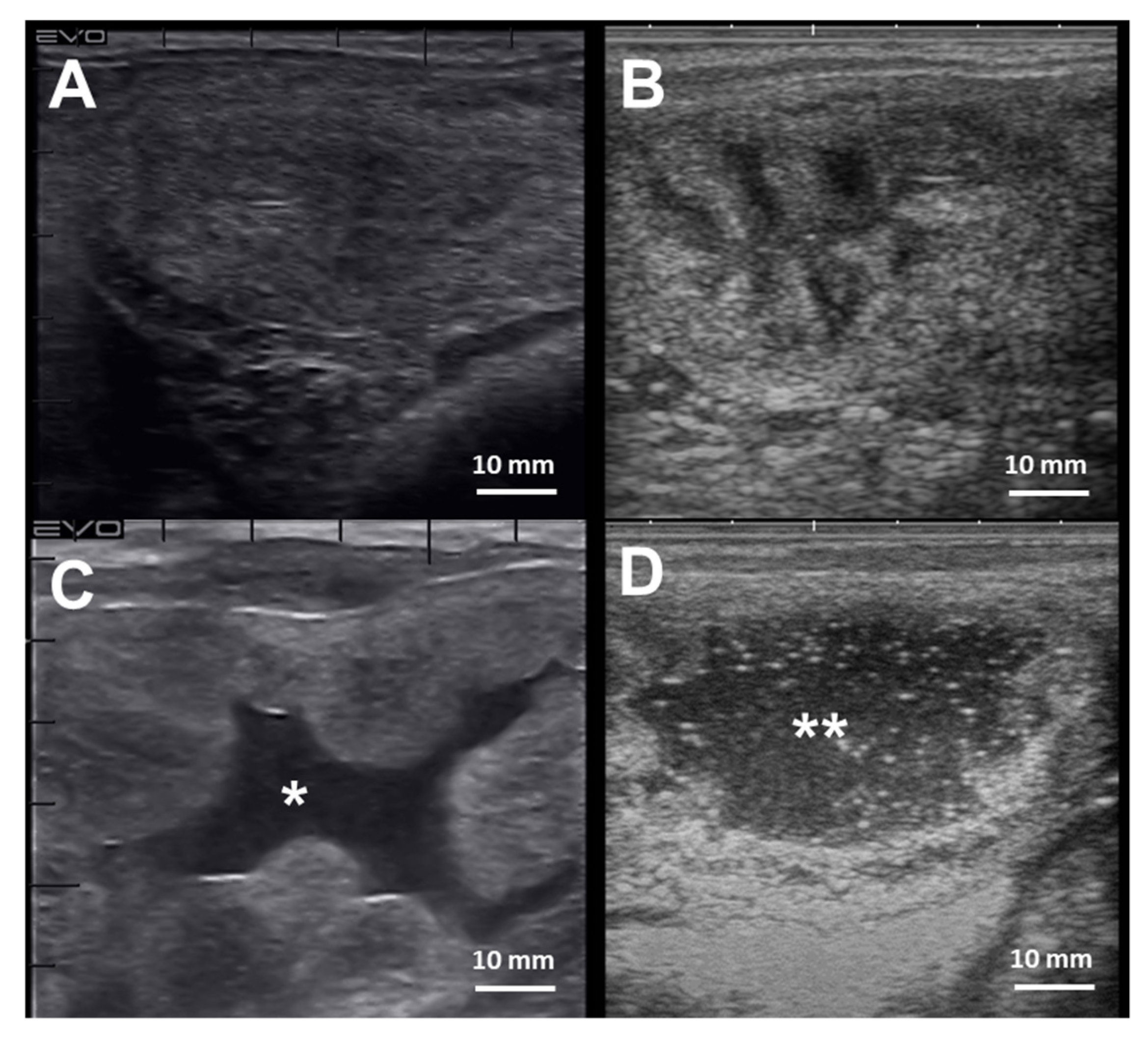
The diagnosis of endometritis entails a multi-modal approach coupled with a detailed clinical history. Endometrial culture, cytology, and biopsy are the most common tools employed to diagnose endometritis in mares [241] (Table 2). Mares susceptible to PBIE may have a history of accumulating intrauterine fluid before and after breeding (Figure 4), recurrent embryonic loss, early return to estrus, failure to become pregnant despite good breeding management, and presenting vulvar discharge.

Table 2.
Summary of common tools used to diagnose endometritis in mares.
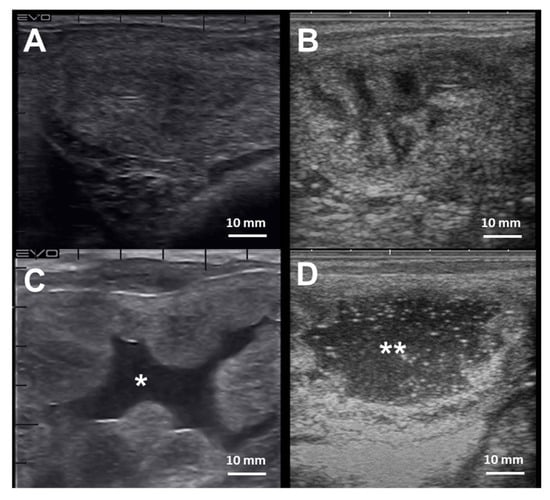
Figure 4.
Cross-section ultrasonographic images of the uterine horns in mares: (A) An image from the equine uterus with no endometrial edema, or intraluminal fluid accumulation, typically seen in mares during diestrus; (B) the uterine horn of a mare in estrus, characterized by the presence of edema in the lymphatic vessels surrounding the endometrium submucosa giving the “orange-slice” aspect, (C) Exacerbated endometrium edema with extravasation and intraluminal fluid accumulation (*) in a mare with endometritis, and (D) extensive hyperechogenic intraluminal fluid accumulation (**) in a mare with endometritis. Scale bars 10 mm (A–D)
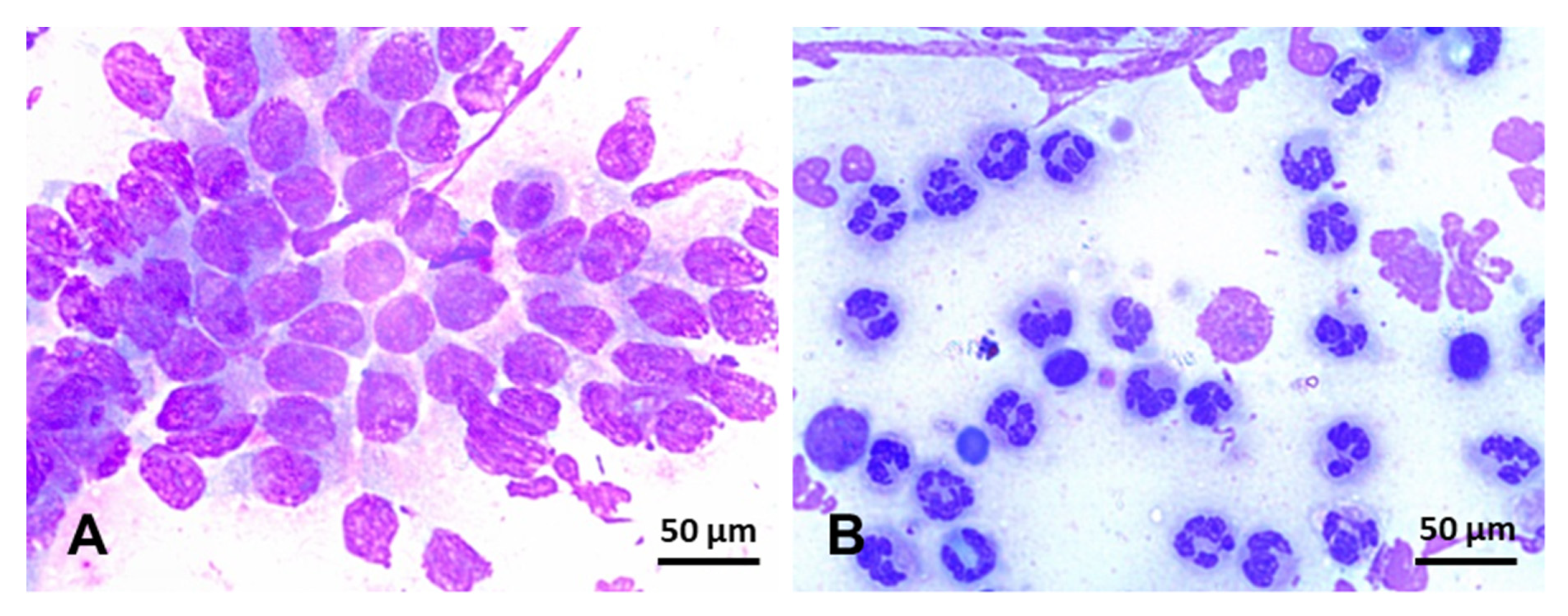
Endometrial cytology can be used to assess the type and proportion of inflammatory cells regarding endometrial epithelial cells present in the uterine lumen (Figure 5). In addition, cytology can occasionally detect the presence of bacterial colonies, hyphae, yeast, and urine crystals [242]. Specimens for endometrial cytology can be obtained with a simple or double-guarded cotton-tip swab, cytobrush, or low-volume uterine lavage [242,243,244]. Cytobrush and low-volume uterine lavages yield superior diagnostic samples than cotton-tip swabs [47,244] (Table 2). In addition, smears obtained with a cytobrush yield more cells (endometrial and PMNs) than cotton-tip swabs [47]. In addition, cytobrush smears had a positive association between the number of PMNs and the number of colonies of β-hemolytic streptococci [244] but not when Escherichia coli was the primary bacterial isolate [245]. After collection, endometrial smears are fixed and stained with Romanovsky-type stains for evaluation. Samples can be evaluated at 400x or 1000x magnification and quantified as the number of neutrophils for every 100 epithelial cells (EC) (Figure 5). The following categories may be used to define the endometrial inflammation: normal (no white blood cells (WBC) to rare WBC/100 EC), mild inflammation (1–2 WBC/EC), moderate inflammation (3–5 WBC/EC), and severe inflammation (>5 WBC/EC) [242]. As noninfectious endometritis will still lead to chemotaxis of neutrophils into the uterine lumen, cytologic examinations cannot be used singularly to rule out infectious endometritis. Therefore, samples for endometrial culture should always be harvested and interpreted in conjunction with cytology results.
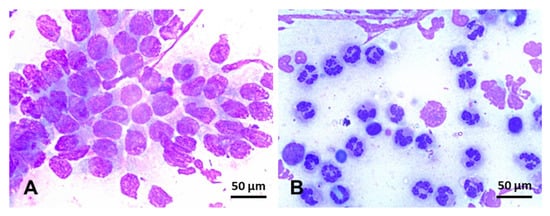
Figure 5.
Endometrial cytology in mares stained with Romanowsky-type stain (×100). (A) Negative endometrial cytology; in this slide, endometrium epithelium cells, can be seen in the absence of inflammatory cells. (B) Positive endometrial cytology; few endometrium epithelium cells can be seen in this slide, but, conversely, hypersegmented neutrophils are overly represented in this smear. Scale bars 50 µm (A and B).
Endometrial culture should always be collected before any uterine or vaginal procedure to avoid potential contamination. Endometrial culture can be done via double-guarded cotton-tip swab, low-volume uterine lavage, or biopsy [46,241,246,247,248]. Endometrial culture with double-guarded cotton-tip swab has lower sensitivity (0.34) in comparison with endometrial culture off a biopsy (0.82) [241] or low-volume uterine lavage (0.75) [245]. However, the specificity of low-volume uterine lavage (0.72) is reported to be lower than the other two techniques (swab—0.90; biopsy—0.90) [245]. After collection, the sample should be processed or placed in transport media (e.g., Ames or Steward) and kept refrigerated until arrival at the laboratory or streaked over culture media right way. In addition, to have microorganisms identified with biochemical tests or microbial proteins or genes, the growth can be grossly estimated by the number of colonies per plate as no growth (no colonies), very light (≤ 2 primary streak), light (3–5 primary streak), moderate (>5 into secondary streak), and heavy (>5 into tertiary streak) [33,249]. After bacterial or fungal isolation, an antimicrobial sensitivity test should be performed to determine the most suitable antimicrobial to treat the infection. In addition, PCR has been gaining popularity in clinical practice to identify bacteria and fungi in endometrium samples. Results can be available in 6 h, while the final culture and sensitivity results are pending [250].
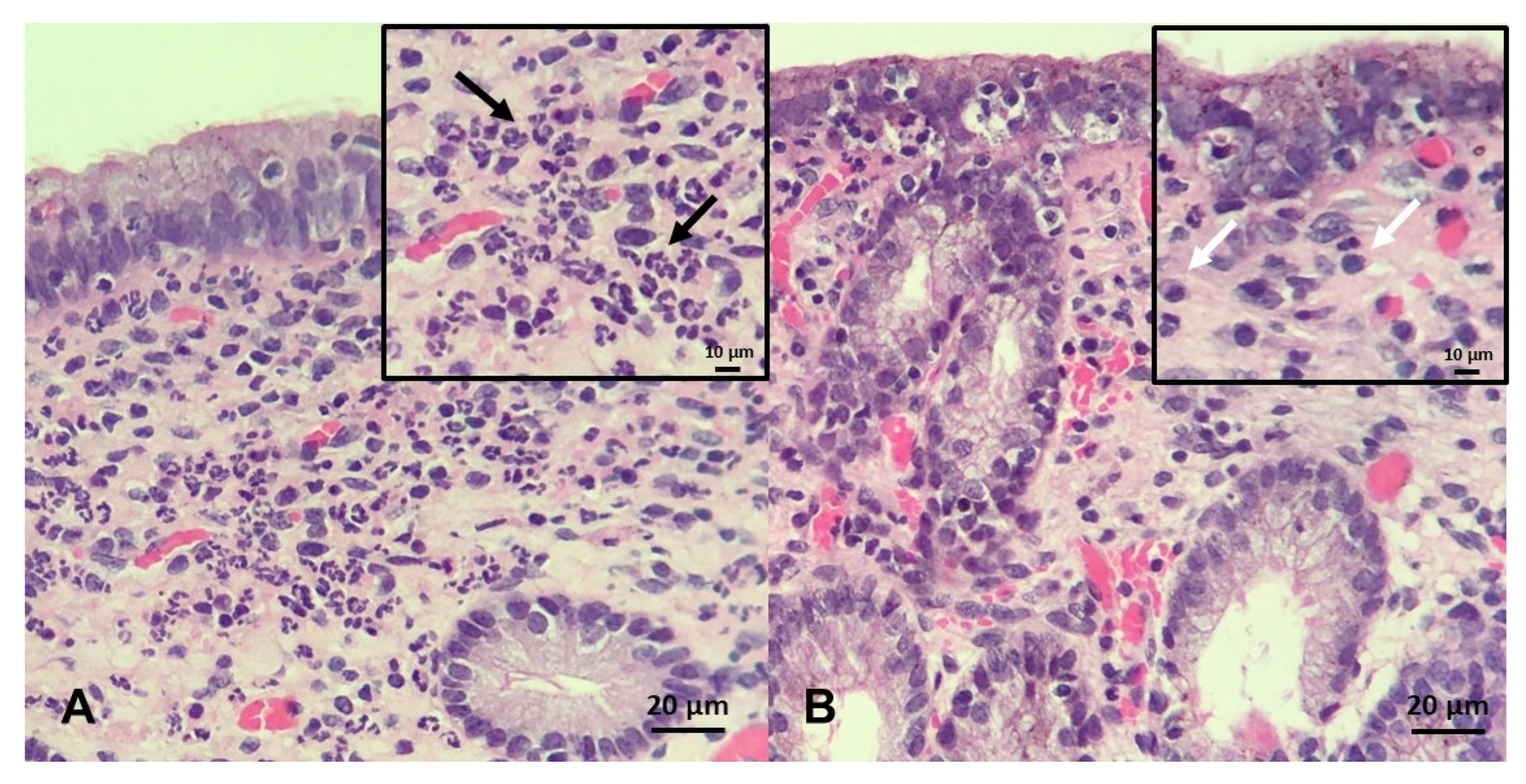
The endometrial biopsy also can be used as a diagnostic tool for endometritis, as well as for a proxy for putative ability of mares carrying a foal to term [246,251,252,253]. A grading system was developed by Kenney and Doig, where the endometrium is assessed for glandular distribution, inflammatory cells, lymphatic lacunae, and fibrosis and then graded on a scale of I–III [254] (Figure 6). As these aspects of endometrial degeneration are associated with a predisposition to endometritis, a biopsy score combined with the mare’s clinical history can be used to predict fertility [251,253]. In addition, as mentioned above, the culture of biopsy samples improves sensitivity (0.82) when compared with double-guarded cotton-tip swabs (0.34) [246]. Recently, it has been proposed that gene expression in endometrial biopsies, such as equine β-defensin 1, lysozyme, and secretory leukoprotease inhibitor, can be used as a diagnostic test to identify mares susceptible to PBIE with overall sensitivities of 78-94% [28]; however, to date, these genes have yet to be tested in clinical practice.
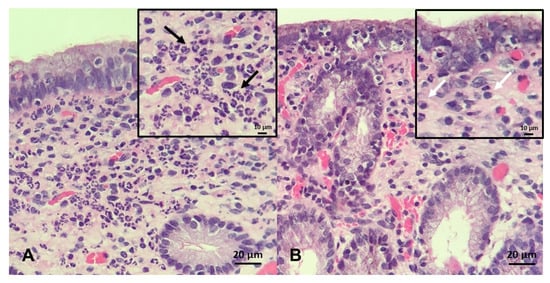
Figure 6.
Endometrial biopsy specimens stained with H&E (×20 magnification, insert ×40): (A) A mare with acute, persistent breeding-induced endometritis characterized by a massive neutrophilic infiltrate in the stratum compactum and spongiosum; black arrows indicate neutrophils (B) a mare with chronic persistent breeding-induced endometritis characterized by heavy lymphocytic infiltrates present in the stratum compactum of the endometrium; white arrows indicate lymphocytes. Scale bars 10 µm inserts (A and B); and 20 µm (A and B).
4. Treatment
Traditionally, endometritis has been treated with uterine lavage, ecbolics, anti-inflammatories, and antimicrobials [20]. In recent years, the lack of response to traditional therapy and the increasing prevalence of antimicrobial-resistant pathogens has led to the development of alternative therapies to treat mares suffering from chronic endometritis [21]. Traditional and alternative therapeutic modalities are discussed herein.
4.1. Ecbolics
Ecbolics, are a pharmacological class of drugs used to stimulate uterine contractions to eliminate intrauterine fluid accumulation via the cervix and lymphatic drainage [255,256,257]. Oxytocin, the most commonly used ecbolic, is typically administered any time before breeding (except, up to 1 hour before breeding) and then between 4 h post-breeding and 72 h after ovulation. It is believed that sperm transportation to the uterine tubes is complete by 4 h post-breeding; thus, this is the shortest amount of time in which mares can receive an ecbolic or uterine lavage without compromising pregnancy rates [258].
After ovulation, cervical closure occurs, preventing the elimination of uterine fluid through the cervix [12]; however, minimal amounts of fluid can still be eliminated via lymphatic vessels present in the uterus. Smaller doses of oxytocin (10-20 units) induce rapid uterine contractions of short duration, whereas higher doses (e.g., 40 units) result in tetanic, less effective contractions [256,259,260,261]. The effects of exogenous oxytocin are expected to last ~45 minutes and therefore, requiring repeated doses [187].
Cloprostenol, a PGF2α analog, is another ecbolic used in clinical practice to treat intrauterine fluid accumulation in mares [21]. This drug induces longer myometrial activity (5 h) when compared to oxytocin [262,263], benefiting uterine cleansing and lymphatic drainage in mares with a pendulous uterus [263]. However, colic-like signs (e.g., pawing, abdominal contractions, sweating, tachypnea) following PGF2α are undesirable features [264]. In addition, administration of PGF2α starting 18–24 h post-ovulation can compromise corpus luteum formation and function, thus resulting in suboptimal systemic progesterone concentrations and decreased pregnancy rates function [262,265].
Carbetocin, an oxytocin analog, is another ecbolic drug used to promote milder and longer uterine contractions when compared with oxytocin [266,267]. Carbetocin is a cyclic octapeptide with a substitution in the amino group by a hydrogen atom with a modification of the disulfide bond by a thioether bond and substitution of the hydroxyl group of tyrosine by a methoxyl group, which helps to avoid early metabolization following administration [266]. Carbetocin has a 2.5-fold longer half-life (17 minutes) than oxytocin (6 minutes) [266,267]. However, to date, there is no study comparing the ability of oxytocin and carbetocin to promote uterine clearance in mares.
4.2. Antibiotics
While antimicrobials are necessary to treat infectious endometritis in mares, these drugs are also often used with no clear medical indication, an example of which is the common single antibiotic dose intrauterine infusion following breeding [8]. The irrational use of antimicrobials has led to the rapid development of antimicrobial resistance. Therefore, the proper identification of microorganism(s), in addition to sensitivity to antimicrobials, is paramount to treat endometritis and prevent the development of antimicrobial resistance successfully.
Because the most common bacterial isolates in the mare’s reproductive tract are Streptococcus spp, Escherichia coli, Klebsiella sp, Pseudomonas sp, and Staphylococcus sp [20,42,47,48,49], the most common antimicrobials used to treat endometritis include β-lactam (e.g., ceftiofur, ampicillin, penicillin) and aminoglycosides (i.e., gentamicin and amikacin) [268] (Table 3). It is interesting that Streptococcus zooepidemicus and Escherichia coli, the two most common isolates from mares with endometritis, were reported to be highly resistant to common antimicrobials [269].

Table 3.
Common antimicrobials used to treat mares suffering from bacterial endometritis.
The mechanisms through which bacteria become resistant to antibiotics have been characterized as modifications in the cell target that prevent antibiotic binding, synthesis of enzymes that digest the antimicrobial agent, activation of an alternate pathway that bypasses the mechanism of action of the antimicrobial drug, down-regulation or elimination of transmembrane porins from where drugs can access the cell, and elimination of drugs by efflux pumps [271,272]. Additionally, Streptococcus zooepidemicus can cause deep-seeded dormant infections (i.e., vegetative stage) in the uteruses of mares, making the bacteria resistant to conventional therapy [51]. One commercial product called b-Activate (Bojesen and Petersen Biotech, Copenhagen, Denmark) has been developed to stimulate dormant Streptococcus into an active proliferative stage, thus making the infection treatable with antimicrobials [52]. The cost of the product has limited its widespread use in clinical practice.
Antimicrobial usage for the treatment of endometritis can be administered either via intrauterine infusion or systemically. It should be noted that not all systemic antimicrobials can be administered in the uterus without some adjustment. An example of this is in fluoroquinolones such as enrofloxacin. The systemic formulation of enrofloxacin cannot be used in the uterus, as the vehicle causes endometrium necrosis [273]. However, if prepared with a different vehicle, enrofloxacin can be safely administered within the uterus [274]. In addition, aminoglycosides (e.g., gentamicin and amikacin) need to be buffered with sodium bicarbonate to counterbalance their acidic pH of 4 [20,275]. Infusion of incompatible antimicrobials into the uterus may precipitate and leave residues in the uterus, which could disturb embryo–endometrium interactions.
There is conflicting evidence regarding the most suitable route for antimicrobial administration to treat endometritis in mares. Intrauterine antimicrobial administration involves minimal systemic disturbances of the microbiome in other body systems, higher local endometrium concentrations, and a lower total amount of antimicrobial needed when compared with systemic administration [268,275]. It should be noted that systemic administration of antimicrobials, particularly for a prolonged period and when drugs need to be changed during treatment, can result in highly undesirable complications such as diarrhea, colitis, and systemic anaphylactic reactions [268]. In contrast, intrauterine administration may also irritate the endometrium and create noninfectious endometritis itself. Additionally, intrauterine antibiotics are presumed to lead to inconsistent tissue penetration, which favors the development of antimicrobial resistance. Presumably, deep-seeded and dormant endometrial infections, such as those caused by Streptococcus, may benefit from systemic administration, as intrauterine infusions may not result in deep penetration of antimicrobials.
Treatments for fungal endometritis are not well elucidated, as there is a lack of controlled studies on fungal endometritis in mares and the pharmacokinetic and pharmacodynamics of these drugs in the reproductive tract. The initial treatment should address the predisposing factors (e.g., poor perineal conformation, immunosuppression, discontinue intrauterine infusions with antibiotics) in combination with uterine lavages and ecbolic drugs. Elimination of predisposing factors for endometritis can sometimes restore the fertility of these mares [54,56]. However, when needed, three types of antifungal agents (polyenes, imidazoles, and triazoles) can be used to treat mares with fungal uterine infections [49,56]. The polyenes are considered fungicidal drugs, and the azoles (e.g., imidazoles and triazoles) are fungistatic [270]. Interestingly, polyenes and azoles have similar mechanisms of action, as they act in the fungal membrane by binding the ergosterol or inhibiting ergosterol biosynthesis [276], respectively (Table 4). In vitro results indicate that polyenes (e.g., amphotericin B, natamycin, and nystatin) are more effective in treating fungal endometritis than azoles (e.g., ketoconazole, fluconazole, and miconazole), mainly for molds with septated hyphae [49].

Table 4.
Common anti-fungal drugs used to treat mares suffering from fungal endometritis.
4.3. Uterine Lavage and Treatment for Biofilm
Uterine lavage is recommended in mares with excessive intrauterine fluid accumulation (e.g., >2 cm depth) and high ultrasonographic echogenicity [277]. Crystalloid solutions such as lactated Ringer’s solution (LRS) and 0.9% saline are most commonly used to lavage mares’ uteruses [278]. As studies have demonstrated that some bacteria, such as Escherichia coli, can utilize lactate (present in LRS) [279] and gluconate (present in Plasmalyte, a crystalloid solution less commonly used for uterine lavages) as substrates for growth [280], uterine lavage with crystalloids cannot be used alone to treat infectious endometritis. These solutions can be enriched with anti-septics (e.g., povidone-iodine and hydrogen peroxide), vinegar to change the uterine microbiome in cases of fungal endometritis, and additives to break biofilm such as mucolytics (e.g., N-acetylcysteine, dimethyl sulfoxide, ethylenediaminetetraacetic acid-2-amino-2-hydroxymethyl-propane-1,3-diol alone or in combination with Tris; disodium ethylenediaminetetraacetate dehydrate-2-amino-2-hydroxymethyl-1,3-propanediol). Despite the wide use of these products in the treatment of endometritis, it is unknown how they affect the resident uterine microbiome and how these agents can be used to restore the balance of microorganisms in utero.
Uterine lavage helps by physically removing microorganisms, debris, inflammatory cells and mediators, and dead sperm from the lumen, which can be detrimental for the sperm before breeding or the embryo after breeding [277,278,281]. It can be performed at any time before or starting four hours after breeding. Four hours after breeding is recognized as the minimal time necessary for sperm to reach the uterine tubes, not interfering with fertility [258,282,283]. Additionally, in a stagnant inflammatory state, the use of uterine lavage can reintroduce viable neutrophils to reinstate an active degradation of microbes.
4.4. Immunomodulatory Agents
The treatment of PBIE with non-steroidal anti-inflammatory drugs (NSAIDs) is still controversial, as inhibition of prostaglandin-endoperoxide synthase (types 1 and 2) and therefore the arachidonic acid cascade is a primary effect. This would decrease PGF2α production, potentially diminishing myometrial activity and leading to delayed uterine clearance. Two studies on phenylbutazone and flunixin meglumine (non-selective COX-2 NSAIDs) observed delayed uterine clearance and an increased inflammatory reaction in mares receiving treatment [284,285]. Additionally, the administration of high (>2X labeled dose) and sustained doses of NSAIDs in pre-ovulatory mares has been shown to increase the rate of anovulatory hemorrhagic follicles [286,287]. However, the labeled dose of NSAID did not interfere with ovulation [288]. In addition, the combination of NSAID with oxytocin has been shown to improve uterine clearance [255], reduce PMNs infiltration, and decrease endometrial expression of COX-2 [289] in susceptible mares.
Another alternative is the selective COX-2 NSAIDs, which do not act on the cyclooxygenase-1 (COX-1) pathway [290]. Firocoxib, a COX-2 selective NSAID, has been described to mitigate post-breeding inflammatory response in mares with a reduction in COX-2 in the endometrium of mares treated during the periovulatory period, while not affecting ovulation rates [291]. Vedaprofen, another selective COX-2 inhibitor, has been reported to affect fertility in mares with PBIE positively but without interfering with intrauterine fluid accumulation and the inflammatory score of the endometrium [292]. Based on these results, selective COX-2 NSAIDs may be an alternative treatment to mares suffering from PBIE.
Glucocorticoids are routinely used to modulate the post-breeding inflammatory response in mares. The seminal study used multiple prednisolone doses before ovulation for mares being bred with frozen semen [136,293]. A posterior study demonstrated that administration of dexamethasone before breeding reduced endometrial inflammation and improved pregnancy rates in barren mares with a history of or presenting predisposing signs for PBIE [15].
On a molecular level, dexamethasone affects the endometrial expression of cytokines and SAA in susceptible mares after intrauterine inoculation with Escherichia coli [33] or sperm [34,294]. A reduction of pro-inflammatory IL1β, CXCL8, and SAA and suppression of inflammatory mediators, such as COX-2, lipo-oxygenase 5, and NO, were reported after dexamethasone therapy, while an increase in the inflammatory modulating and anti-inflammatory IL6, IL10, and IL1RN was also observed after treatment [33]. Additionally, the administration of dexamethasone altered the production of acute-phase proteins following bacterial challenge [295] and did not alter the phagocytic function of blood-derived PMNs [296], which could predispose the mare to a secondary infection. This alteration in the acute phase protein profile has also been noted following treatment with prednisolone [297]. A study found that the administration of prednisolone following inoculation with bacteria to increase antitrypsin and transthyretin while reducing IgG [297]. It should be noted that while glucocorticoid therapy is associated with reduced endometrial edema following breeding, repeated administration of either dexamethasone and prednisolone may suppress the luteinizing hormone (LH) surge and increase the rate of ovulation failure [298]. In one study, prolonged administration (5 days) of dexamethasone tended to reduce (40%) ovulation rates in mares when compared with placebo (100%), while the prolonged administration of prednisolone did not alter these results (83%) [298]. In horses, dexamethasone may affect hypothalamic GnRH and pituitary LH secretion [299,300], and in other species, glucocorticoids may alter hypothalamic [301], anterior pituitary [302], and ovary [302,303] function. Therefore, a single and low-dose injection is recommended for the treatment of post-breeding endometritis in mares rather than repeated doses.
The use of bacterial extracts has also been found to alter the immune response to endometritis [31,32,33,184,304,305]. Mycobacterium phlei cell wall extract (MCWE) is a commercial immunomodulator (Settle, Bioniche Animal Health, Athens GA, USA) that is used for the treatment of equine endometritis caused by Streptococcus zooepidemicus. This immunomodulator acts by enhancing the innate humoral immune response. It has been shown to decrease the endometrial expression of pro-inflammatory cytokines IL1β, IL6, and TNFα in susceptible mares following both breeding and challenge with Gram-positive bacteria, while also increasing the expression of the anti-inflammatory IL10 [32]. In addition, mares treated with MCWE also show a decrease in NO [36]. Although MCWE did not affect endometrial cytokine expression following challenge with Escherichia coli, a pronounced reduction in bacterial growth was noted following treatment, in addition to a reduction in intrauterine fluid accumulation [33]. This was confirmed in the following study, and MCWE was found to be bactericidal regardless of being administered intravenously or in the uterus [304].
Another immunostimulant that has been described to be able to improve pregnancy rates in mares is the Propionibacterium acnes (EQStim, Neogen Corp, Lexington KY, USA) [305]. This therapy induces a non-specific cell-mediated response, predominantly by macrophage activation and cytokine release. In the single study investigating this immunostimulant, mares with clinical endometritis were treated with intravenous Propionibacterium acnes [305]. Repeated administration of this treatment as an adjunct to conventional therapies has improved pregnancy rates and increased live foaling rates in mares with a cytologic diagnosis of endometritis in comparison with a placebo. Unfortunately, the molecular mechanisms of using this therapy have yet to be investigated.
4.5. Lactoferrin
Lactoferrin is an 80kDa protein found promiscuously throughout the body, including in both the reproductive and immune systems [306]. It is believed to be bactericidal due to its ability to chelate free iron [307]. The endometrial expression of lactoferrin varies depending on the stage of the estrous cycle and is increased during estrus, indicating its endocrine dependency [234]. The administration of recombinant lactoferrin at the time of breeding in the normal mare has shown varying results, with one study finding no change in cytokine expression [308], while another found a significant decrease in endometrial IL6, alongside a trend towards a decrease in CXCL8, IL1β, and TNFα [309], indicating its anti-inflammatory properties.
Interestingly, this decrease in TNFα was also noted in mares susceptible to PBIE when lactoferrin was administered at the time of breeding [310]. When administered 6 h post-breeding in susceptible mares, lactoferrin decreased the expression of pro-inflammatory interferon gamma while increasing anti-inflammatory IL1RN [311]. This study also found varying concentrations of lactoferrin (50–500 µg) to decrease PMN infiltration in the susceptible mare, although it did not affect uterine fluid retention. The authors suggested that the recommended dose for human recombinant lactoferrin intrauterine infusion should be 1mL (50 μg/mL) diluted in 10 mL of LRS, which is comparable to the average concentration noted in the equine ejaculate. Interestingly, in other species, lactoferrin is administered for its bactericidal and anti-biofilm properties [312,313], but these findings have not been investigated in the horse.
4.6. Platelet-Rich Plasma
Platelet-rich plasma (PRP) is whole blood plasma with concentrated platelets (3-5-fold). It has been commonly used in equine clinical practice to treat joints, bursae, and soft tissue injuries (e.g., tendonitis, tenosynovitis, and skin wounds) [314,315,316,317]. Additionally, the combination of autologous plasma to antibiotic therapy has been reported to improve pregnancy rates for lactating and barren mares [318].
The biological mechanisms of PRP on the inflammatory response are not yet well elucidated. However, some studies [319,320,321,322] have demonstrated an anti-inflammatory action of PRP due to its ability to suppress the expression of COX-2, metalloproteinase-3 (MMP-3), TNFα, IL1, and vascular adhesion molecules [323]. Additionally, platelet granules contain antimicrobial peptides (RANTES, platelet factor 4, and thymosin beta-4), and these peptides may contribute to PRP’s known bactericidal activity against Staphylococcus aureus, Escherichia coli, and Klebsiella pneumoniae in the human [324,325,326,327,328,329,330,331]. All these bacteria are known causes of endometritis in mares [20].
For the treatment of endometritis in the horse, one study found that PRP administration at the time of breeding decreased the intrauterine inflammatory response in mares suffering from chronic endometritis, although it did not affect NO production [332]. This concurs with another study reporting that PRP decreased endometrial expression of COX-2, decreased PMN numbers in the uterine lumen, and increased pregnancy rates [333]. It has also been shown to act as an anti-inflammatory, and treatment of susceptible mares led to a down-regulation of endometrial IL1β, IL6, and CXCL8 expression [332,333,334,335].
4.7. Stem Cells
The use of mesenchymal stem cells (MSCs) has rapidly gained interest in human and veterinary medicine to modulate the inflammatory processes [336,337]. These cells can differentiate into skeletal myoblasts, renal parenchyma, hepatic epithelium, gut and skin epithelia, neuroectodermal cells [338], and even endometrial cells [339]. They can also signal residual cells through anti-apoptotic, chemotactic, and immune-modulatory properties and can be harvested from variable tissues with differential potency.
Injection [340] or infusion [341] of MSCs has been described as a possible alternative for the treatment of endometrial fibrosis. Therapy with MSCs can induce an early (7 days) and also a prolonged (60 days) remodeling of the endometrium of mares suffering from chronic-degenerative endometriosis by modulating the expression patterns (e.g., cytokeratin, vimentin, α-smooth muscle actin, and laminin) associated with the development of pathological fibrosis in the horse endometrium, as well as promoting glandular epithelial cells proliferation [341]. A report found that MSC administration decreases neutrophil numbers and increases anti-inflammatory ILRN expression in the endometrium of the normal mare [342]. Interestingly, while one study found that MSCs also attenuate markers of uterine inflammation following treatment, cells were unable to penetrate the endometrium and remained within the uterine lumen [343]. However, another study reported that intrauterine infusion of MSCs effectively engrafted MSCs in periglandular spaces [344]. In addition, it is known that MSCs can up-regulate anti-inflammatory cytokines (e.g., IL2, IL4, IL10 and basic fibroblast growth factor) and down-regulate pro-inflammatory cytokines (TNFα, IL1β and IL17) in animal disease models and rats within the endometrium [345,346,347]. Using rats as an experimental model, some authors indicated a regeneration of endometrial cells or a protective effect against cell damage in rat’s endometrium using an intrauterine MSCs therapy by the higher expression of cytokeratin and vimentin, a marker of endometrial cells [345]. In addition, in that study, intrauterine MSCs therapy was able to up-regulate markers for endometrial receptivity (integrin agb3 and leukemia inhibitory factor) [345], which are regulators of endometrial function and have an important role in embryo implantation [348]. To date, no studies have evaluated the efficacy of MSCs in mares susceptible to PBIE, so additional work is necessary. In addition, it is worth noting that primates and rodents undergo decidualization (i.e., morphological and functional changes of the endometrium in preparation for pregnancy), and horses do not; thus, findings obtained in these species cannot necessarily translate into similar findings in horses.
5. Conclusions
Endometritis is the most important cause of subfertility in mares. Mares susceptible to PBIE are frequently older, have an impaired uterine immune response, and deficient physical barriers and other mechanisms of defense against infection. An imbalance of pro- and anti-inflammatory mechanisms plays a pivotal role in the immunopathogenesis of PBIE in mares. Infections of the endometrium can be established if the natural mechanisms (physical and molecular) of defense are compromised. In addition, the pathobiology of microorganisms associated with endometritis in mares has been poorly understood, along with the role of the resident uterine microbiome in preventing infection and how diseases and treatment alter the composition of these microbe populations. Traditional and non-traditional therapies for endometritis are based on reestablishing the natural mechanisms of defense (e.g., fixing some reproductive seals of the reproductive tract or immunomodulate inflammation). The current trend of preventing indiscriminate use of antimicrobials in animals and humans will benefit from the continued attempts to develop alternative antibiotic-free therapies to treat endometritis.
Funding
This research received no external funding.
Acknowledgments
FAPESP and USDA Animal Health Hatch Funds.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ACP | Antigen-presenting cells |
| BAFF | B cell-activating factor |
| C1q | Complement component 1 |
| C3a | Complement 3a |
| C3b | Complement 3b |
| C4a | Complement 4a |
| C4b | Complement 4b |
| C5a | Complement 5a |
| CD14 CD40 | Cluster of differentiation 14 Cluster of differentiation 40 |
| COX-1 | Cyclooxygenase-1 |
| COX-2 | Cyclooxygenase-2 |
| CXCL8 | Chemokine ligand 8 |
| EC | Epithelial cells |
| ECM | Extracellular matrix |
| EDTA | Ethylenediaminetetraacetic acid-2-amino-2-hydroxymethyl-propane-1,3-diol |
| FoxP3 | Forkhead box protein P3 |
| GnRH | Gonadotropin-releasing hormone |
| IFN | Interferon |
| IFNα | Interferon type I α |
| IgA | Immunoglobulin A |
| IgG | Immunoglobulin G |
| IgM | Immunoglobulin M |
| IL1 | Interleukin1 |
| IL1RN | Interleukin 1 receptor antagonist |
| IL10 | Interleukin 10 |
| IL13 | Interleukin 13 |
| IL17 | Interleukin 17 |
| IL1α | Interleukin 1alpha |
| IL1β | Interleukin 1Beta |
| IL4 | Interleukin 4 |
| IL6 | Interleukin 6 |
| LH | Luteinizing hormone |
| LPS LRS | Lipopolysaccharides Lactated Ringer’s Solution |
| MAC | Membrane attack complex |
| MCWE | Mycobacterium phlei cell wall extract |
| MMP-3 | Metalloproteinase-3 |
| MMPs | Matrix metalloproteinases |
| MSCs | Mesenchymal stem cells |
| MyD88 | Myeloid differentiation primary response 88 |
| NETs | Neutrophil extracellular traps |
| NF-κB | Nuclear factor kappa-B |
| NK | Natural killer cells |
| NLR | NOD-like receptors |
| NO | Nitric oxide |
| NOD | Nucleotide-binding and oligomerization domain |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| PABA PBIE | Para-aminobenzoic acid Persistent breeding-induced endometritis |
| PBPs PGF2α | Penicillin-binding proteins Prostaglandin 2α |
| PMNs | Polymorphonuclear neutrophils |
| PRP | Platelet-rich plasma |
| PRRs | Pattern recognition receptors |
| SAA | Serum amyloid A |
| TIMPs | Tissue inhibitors of MMPs |
| TLR2 | Toll-like receptors type 2 |
| TLR4 | Toll-like receptors type 4 |
| TLRs | Toll-like receptors |
| TNFα | Tumor necrosis factor-α |
| TRAF6 | Receptor-associated factor 6 |
| TRIF | TIR-domain-containing adapter-inducing interferon-β |
| WBC | White blood cells |
References
- Traub-Dargatz, J.L.; Salman, M.D.; Voss, J.L. Medical problems of adult horses, as ranked by equine practitioners. J. Am. Vet. Med. Assoc. 1991, 198, 1745–1747. [Google Scholar] [PubMed]
- Troedsson, M.H.T. Uterine clearance and resistance to persistent endometritis in the mare. Theriogenology 1999, 52, 461–471. [Google Scholar] [CrossRef]
- Leblanc, M.M.; Neuwirth, L.; Asbury, A.C.; Tran, T.; Mauragis, D.; Klapstein, E. Scintigraphic measurement of uterine clearance in normal mares and mares with recurrent endometritis. Equine Vet. J. 1994, 26, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Troedsson, M.H.T.; Desvousges, A.; Macpherson, M.L.; Pozor, M.P. Persistent breeding-induced endometritis. Pferdeheilkunde 1994, 24, 56–60. [Google Scholar] [CrossRef]
- Scoggin, C.F. Not just a number: Effect of age on fertility, pregnancy and offspring vigour in thoroughbred brood-mares. Reprod. Fertil. Dev. 2015, 27, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Woodward, E.M.; Christoffersen, M.; Campos, J.; Squires, E.L.; Troedsson, M.H.T. Susceptibility to persistent breeding-induced endometritis in the mare: Relationship to endometrial biopsy score and age, and variations between seasons. Theriogenology 2012, 78, 495–501. [Google Scholar] [CrossRef]
- Allen, W.; Pycock, J. Cyclical accumulation of uterine fluid in mares with lowered resistance to endometritis. Vet. Rec. 1988, 122, 489–490. [Google Scholar] [CrossRef]
- Zent, W.W.; Troedsson, M.H.T.; Xue, J.-L. Postbreeding uterine fluid accumulation in a normal population of Thoroughbred mares: A field study. In Proceedings of the 40th Annual Convention of the American Association of Equine Practitioners, Baltimore, MD, USA, 6 December 1998; Volume 44, pp. 64–65. [Google Scholar]
- Troedsson, M.H.; Liu, I.K.; Crabo, B. Sperm transport and survival in the mare. Theriogenology 1998, 49, 905–915. [Google Scholar] [CrossRef]
- Alghamdi, A.S.; Foster, D.N.; Carlson, C.S.; Troedsson, M.H.T. Nitric oxide levels and nitric oxide synthase expression in uterine samples from mares susceptible and resistant to persistent breeding-induced endometritis. Am. J. Reprod. Immunol. 2005, 53, 230–237. [Google Scholar] [CrossRef]
- Katila, T. Onset and duration of uterine inflammatory response of mares after insemination with fresh semen. Biol. Reprod. 1995, 52, 515–517. [Google Scholar] [CrossRef]
- Troedsson, M.H.T. Therapeutic considerations for mating-induced endometritis. Pferdeheilkunde 1997, 13, 516–520. [Google Scholar] [CrossRef]
- Troedsson, M.H.; Liu, I.K. Uterine clearance of non-antigenic markers (51Cr) in response to a bacterial challenge in mares potentially susceptible and resistant to chronic uterine infections. J. Reprod. Fertil. Suppl. 1991, 44, 283–288. [Google Scholar] [PubMed]
- Carnevale, E.M.; Ramirez, R.J.; Squires, E.L.; Alvarenga, M.A.; Vanderwall, D.K.; McCue, P.M. Factors affecting pregnancy rates and early embryonic death after equine embryo transfer. Theriogenology 2000, 54, 965–979. [Google Scholar] [CrossRef]
- Bucca, S.; Carli, A.; Buckley, T.; Dolci, G.; Fogarty, U. The use of dexamethasone administered to mares at breeding time in the modulation of persistent mating induced endometritis. Theriogenology 2008, 70, 1093–1100. [Google Scholar] [CrossRef]
- Woodward, E.M.; Christoffersen, M.; Campos, J.; Betancourt, A.; Horohov, D.; Scoggin, K.E.; Squires, E.L.; Troedsson, M.H.T. Endometrial inflammatory markers of the early immune response in mares susceptible or resistant to persistent breeding-induced endometritis. Reproduction 2013, 145, 289–296. [Google Scholar] [CrossRef]
- Freeman, D.A.; Weber, J.A.; Geary, R.T.; Woods, G.L. Time of embryo transport through the mare oviduct. Theriogenology 1991, 36, 823–830. [Google Scholar] [CrossRef]
- Robertson, S.A.; Chin, P.Y.; Femia, J.G.; Brown, H.M. Embryotoxic cytokines—Potential roles in embryo loss and fetal programming. J. Reprod. Immunol. 2018, 125, 80–88. [Google Scholar] [CrossRef]
- Squires, E.L. Embryo transfer challenges and perspectives. Rev. Bras. Reprod. Anim. 2013, 37, 105–107. [Google Scholar]
- Canisso, I.F.; Stewart, J.; Coutinho da Silva, M.A. Endometritis: Managing persistent post-breeding endometritis. Vet. Clin. N. Am. Equine Pract. 2016, 32, 465–480. [Google Scholar] [CrossRef]
- Scoggin, C.F. Endometritis: Nontraditional therapies. Vet. Clin. N. Am. Equine Pract. 2016, 32, 499–511. [Google Scholar] [CrossRef]
- Troedsson, M.H.T. Breeding-induced endometritis in mares. Vet. Clin. N. Am. Equine Pract. 2006, 22, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Liu, I.K.M.; Troedsson, M.H.T. The diagnosis and treatment of endometritis in the mare: Yesterday and today. Theriogenology 2008, 70, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Troedsson, M.H.T. Mating-induced endometritis: Physiology or pathology? Vet. J. 2014, 199, 9–10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Troedsson, M.H.T.; Woodward, E.M. Our current understanding of the pathophysiology of equine endometritis with an emphasis on breeding-induced endometritis. Reprod. Biol. 2016, 16, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Katila, T. Evaluation of diagnostic methods in equine endometritis. Reprod. Biol. 2016, 16, 189–196. [Google Scholar] [CrossRef]
- Woodward, E.M.; Troedsson, M.H.T. Inflammatory mechanisms of endometritis. Equine Vet. J. 2015, 47, 384–389. [Google Scholar] [CrossRef]
- Marth, C.D.; Firestone, S.M.; Hanlon, D.; Glenton, L.Y.; Browning, G.F.; Young, N.D.; Krekeler, N. Innate immune genes in persistent mating-induced endometritis in horses. Reprod. Fertil. Dev. 2018, 30, 533–545. [Google Scholar] [CrossRef]
- Marth, C.D.; Young, N.D.; Glenton, L.Y.; Noden, D.M.; Browning, G.F.; Krekeler, N. Deep sequencing of the uterine immune response to bacteria during the equine oestrous cycle. BMC Genom. 2015, 16, 1–19. [Google Scholar] [CrossRef]
- Nash, D.M.; Sheldon, I.M.; Herath, S.; Lane, E.A. Markers of the uterine innate immune response of the mare. Anim. Reprod. Sci. 2010, 119, 31–39. [Google Scholar] [CrossRef]
- Fumuso, E.A.; Aguilar, J.; Giguère, S.; Rivulgo, M.; Wade, J.; Rogan, D. Immune parameters in mares resistant and susceptible to persistent post-breeding endometritis: Effects of immunomodulation. Vet. Immunol. Immunopathol. 2007, 118, 30–39. [Google Scholar] [CrossRef]
- Fumuso, E.; Giguère, S.; Wade, J.; Rogan, D.; Videla-Dorna, I.; Bowden, R.A. Endometrial IL-1beta, IL-6 and TNF-alpha, mRNA expression in mares resistant or susceptible to post-breeding endometritis. Effects of estrous cycle, artificial insemination and immunomodulation. Vet. Immunol. Immunopathol. 2003, 96, 31–41. [Google Scholar] [CrossRef]
- Christoffersen, M.; Troedsson, M.H.T.; Woodward, E.M.; Lehn-Jensen, H.; Bojesen, A.M.; Squires, E.L.; Petersen, M.R. Effect of immunomodulatory therapy on the endometrial inflammatory response to induced infectious endometritis in susceptible mares. Theriogenology 2012, 78, 991–1004. [Google Scholar] [CrossRef] [PubMed]
- Woodward, E.M.; Christoffersen, M.; Horohov, D.; Squires, E.L.; Troedsson, M.H.T. The effect of treatment with immune modulators on endometrial cytokine expression in mares susceptible to persistent breeding-induced endometritis. Equine Vet. J. 2015, 47, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Woodward, E.; Christoffersen, M.; Campos, J.; Horohov, D.; Scoggin, K.; Squires, E.; Troedsson, M. An investigation of uterine nitric oxide production in mares susceptible and resistant to persistent breeding-induced endometritis and the effects of immunomodulation. Reprod. Domest. Anim. 2013, 48, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Troedsson, M.H.T.; Crabo, B.G.; Ibrahim, N.; Scott, M.; Ing, M. Mating-induced endometritis: Mechanisms, clinical importance and consequences. Proc. 40th Am. Assoc. Equine Pract. 1994, 41, 11–12. [Google Scholar]
- Kotilainen, T.; Huhtinen, M.; Katila, T. Sperm-induced leukocytosis in the equine uterus. Theriogenology 1994, 41, 629–636. [Google Scholar] [CrossRef]
- Trotter, G.W.; McKinnon, A.O. Surgery for abnormal vulvar and perineal conformation in the mare. Vet. Clin. N. Am. Equine Pract. 1988, 4, 389–405. [Google Scholar] [CrossRef]
- Christoffersen, M.; Söderlind, M.; Rudefalk, S.R.; Pedersen, H.G.; Allen, J.; Krekeler, N. Risk factors associated with uterine fluid after breeding caused by Streptococcus zooepidemicus. Theriogenology 2015, 84, 1283–1290. [Google Scholar] [CrossRef]
- Riddle, W.T.; LeBlanc, M.M.; Stromberg, A.J. Relationships between uterine culture, cytology and pregnancy rates in a Thoroughbred practice. Theriogenology 2007, 68, 395–402. [Google Scholar] [CrossRef]
- Wingfield Digby, N.J.; Ricketts, S.W. Results of concurrent bacteriological and cytological examinations of the endometrium of mares in routine stud farm practice 1978–1981. J. Reprod. Fertil. Suppl. 1982, 32, 181–185. [Google Scholar]
- Leblanc, M.; Causey, R. Clinical and subclinical endometritis in the mare: Both threats to fertility. Reprod. Domest. Anim. 2009, 44, 10–22. [Google Scholar] [CrossRef]
- Overbeck, W.; Witte, T.S.; Heuwieser, W. Comparison of three diagnostic methods to identify subclinical endometritis in mares. Theriogenology 2011, 75, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, S.W.; Mackintosh, M.E. Role of anaerobic bacteria in equine endometritis. J. Reprod. Fertil. Suppl. 1987, 35, 343–351. [Google Scholar] [PubMed]
- Collins, S. A study of the incidence of cervical and uterine infection in Thoroughbred mares in Ireland. Vet. Rec. 1964, 66, 673–676. [Google Scholar]
- Bain, A.M. The role of infection in infertility in the thoroughbred mare. Vet. Rec. 1966, 78, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.; Neuberg, K.P.; Failing, K.; Wehrend, A. Cytological diagnosis of endometritis in the mare: Investigations of sampling techniques and relation to bacteriological results. Anim. Reprod. Sci. 2012, 132, 178–186. [Google Scholar] [CrossRef]
- LeBlanc, M.M.; Magsig, J.; Stromberg, A.J. Use of a low-volume uterine flush for diagnosing endometritis in chronically infertile mares. Theriogenology 2007, 68, 403–412. [Google Scholar] [CrossRef]
- Beltaire, K.A.; Cheong, S.H.; Coutinho da Silva, M.A. Retrospective study on equine uterine fungal isolates and antifungal susceptibility patterns (1999–2011). Equine Vet. J. Suppl. 2012, 84–87. [Google Scholar] [CrossRef]
- Frontoso, R.; De Carlo, E.; Pasolini, M.P.; van der Meulen, K.; Pagnini, U.; Iovane, G.; De Martino, L. Retrospective study of bacterial isolates and their antimicrobial susceptibilities in equine uteri during fertility problems. Res. Vet. Sci. 2008, 84, 1–6. [Google Scholar] [CrossRef]
- Albihn, A.; Båverud, V.; Magnusson, U. Uterine microbiology and antimicrobial susceptibility in isolated bacteria from mares with fertility problems. Acta Vet. Scand. 2003, 44, 121–129. [Google Scholar] [CrossRef]
- Petersen, M.R.; Skive, B.; Christoffersen, M.; Lu, K.; Nielsen, J.M.; Troedsson, M.H.T.; Bojesen, A.M. Activation of persistent Streptococcus equi subspecies zooepidemicus in mares with subclinical endometritis. Vet. Microbiol. 2015, 179, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Coutinho da Silva, M.A.; Alvarenga, M.A. Fungal endometritis. In Equine Reproduction; McKinnon, A.O., Squires, E.L., Vaala, W.E., Varner, D.D., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2011; pp. 2643–2651. [Google Scholar]
- Dascanio, J.J.; Schweizer, C.; Ley, W.B. Equine fungal endometritis. Equine Vet. Educ. 2010, 13, 324–329. [Google Scholar] [CrossRef]
- Hinrichs, K.; Spensley, M.S.; McDonough, P.L. Evaluation of progesterone treatment to create a model for equine endometritis. Equine Vet. J. 1992, 24, 457–461. [Google Scholar] [CrossRef]
- Stout, T.A.E. Fungal endometritis in the mare. Pferdeheilkunde 2008, 24, 83–87. [Google Scholar] [CrossRef]
- Ferris, R.A. Current understanding of bacterial biofilms and latent infections: A clinical perspective. Rev. Bras. Reprod. Anim. 2017, 41, 74–80. [Google Scholar]
- Beehan, D.P.; Wolfsdorf, K.; Elam, J.; Krekeler, N.; Paccamonti, D.; Lyle, S.K. The evaluation of biofilm-forming potential of escherichia coli collected from the equine female reproductive tract. J. Equine Vet. Sci. 2015, 35, 935–939. [Google Scholar] [CrossRef]
- Ferris, R.A. Bacterial endometritis: A focus on biofilms. Clin. Theriogenol. 2014, 6, 315–319. [Google Scholar]
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as complex differentiated communities. Ann. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef]
- Shah, D.; Zhang, Z.; Khodursky, A.; Kaldalu, N.; Kurg, K.; Lewis, K. Persisters: A distinct physiological state of E. coli. BMC Microbiol. 2006, 6, 53. [Google Scholar] [CrossRef]
- Jensen, E.T.; Kharazmi, A.; Lam, K.; Costerton, J.W.; Høiby, N. Human polymorphonuclear leukocyte response to Pseudomonas aeruginosa grown in biofilms. Infect. Immun. 1990, 58, 2383–2385. [Google Scholar] [CrossRef]
- Thurlow, L.R.; Hanke, M.L.; Fritz, T.; Angle, A.; Aldrich, A.; Williams, S.H.; Engebretsen, I.L.; Bayles, K.W.; Horswill, A.R.; Kielian, T. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J. Immunol. 2011, 186, 6585–6596. [Google Scholar] [CrossRef] [PubMed]
- Mustoe, T. Understanding chronic wounds: A unifying hypothesis on their pathogenesis and implications for therapy. Am. J. Surg. 2004, 187, S65–S70. [Google Scholar] [CrossRef]
- Mah, T.F.C.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Brown, M.R.W.; Allison, D.G.; Gilbert, P. Resistance of bacterial biofilms to antibiotics a growth-rate related effect? J. Antimicrob. Chemother. 1988, 22, 777–780. [Google Scholar] [CrossRef]
- Anwar, H.; Strap, J.L.; Costerton, W. Establishment of aging biofilms: Possible mechanism of bacterial resistance to antimicrobial therapy. Antimicrob. Agents Chemother. 1992, 36, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Romling, U.; Galperin, M.Y.; Gomelsky, M. Cyclic di-GMP: The First 25 Years of a Universal Bacterial Second Messenger. Microbiol. Mol. Biol. Rev. 2013, 77, 1–52. [Google Scholar] [CrossRef]
- Hickman, J.W.; Tifrea, D.F.; Harwood, C.S. A chemosensory system that regulates biofilm formation through modulation of cyclic diguanylate levels. Proc. Natl. Acad. Sci. USA 2005, 102, 14422–14427. [Google Scholar] [CrossRef]
- Merighi, M.; Lee, V.T.; Hyodo, M.; Hayakawa, Y.; Lory, S. The second messenger bis-(3’-5’)-cyclic-GMP and its PilZ domain-containing receptor Alg44 are required for alginate biosynthesis in Pseudomonas aeruginosa. Mol. Microbiol. 2007, 65, 876–895. [Google Scholar] [CrossRef]
- Hay, I.D.; Remminghorst, U.; Rehm, B.H.A. MucR, a novel membrane-associated regulator of alginate biosynthesis in Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2009, 75, 1110–1120. [Google Scholar] [CrossRef]
- Kuchma, S.L.; Connolly, J.P.; O’Toole, G.A. A three-component regulatory system regulates biofilm maturation and type III secretion in Pseudomonas aeruginosa. J. Bacteriol. 2005, 187, 1441–1454. [Google Scholar] [CrossRef]
- Ma, L.; Jackson, K.D.; Landry, R.M.; Parsek, M.R.; Wozniak, D.J. Analysis of Pseudomonas aeruginosa conditional psl variants reveals roles for the psl polysaccharide in adhesion and maintaining biofilm structure postattachment. J. Bacteriol. 2006, 188, 8213–8221. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, A.; Hay, I.D.; Rehm, B.H.A. Role of exopolysaccharides in Pseudomonas aeruginosa biofilm formation and architecture. Appl. Environ. Microbiol. 2011, 77, 5238–5246. [Google Scholar] [CrossRef] [PubMed]
- Jennings, L.K.; Storek, K.M.; Ledvina, H.E.; Coulon, C.; Marmont, L.S.; Sadovskaya, I.; Secor, P.R.; Tseng, B.S.; Scian, M.; Filloux, A.; et al. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc. Natl. Acad. Sci. USA 2015, 112, 11353–11358. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.C.; Kundukad, B.; Seviour, T.; Van der Maarel, J.R.C.; Yang, L.; Rice, S.A.; Doyle, P.; Kjelleberg, S. Dynamic remodeling of microbial biofilms by functionally distinct exopolysaccharides. mBio 2014, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vasseur, P.; Vallet-Gely, I.; Soscia, C.; Genin, S.; Filloux, A. The pel genes of the Pseudomonas aeruginosa PAK strain are involved at early and late stages of biofilm formation. Microbiology 2005, 151, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.A.; Wittstock, S.M.; McCue, P.M.; Borlee, B.R. Evaluation of biofilms in gram-negative bacteria isolated from the equine uterus. J. Equine Vet. Sci. 2014, 34, 121. [Google Scholar] [CrossRef]
- Ferris, R.A.; McCue, P.M.; Borlee, G.I.; Loncar, K.D.; Hennet, M.L.; Borlee, B.R. In vitro efficacy of nonantibiotic treatments on biofilm disruption of gram-negative pathogens and an in vivo model of infectious endometritis utilizing isolates from the equine uterus. J. Clin. Microbiol. 2016, 54, 631–639. [Google Scholar] [CrossRef]
- Morse, D.J.; Wilson, M.J.; Wei, X.; Lewis, M.A.O.; Bradshaw, D.J.; Murdoch, C.; Williams, D.W. Denture-associated biofilm infection in three-dimensional oral mucosal tissue models. J. Med. Microbiol. 2018, 67, 364–375. [Google Scholar] [CrossRef]
- Berger, D.; Rakhamimova, A.; Pollack, A.; Loewy, Z. Oral biofilms: Development, control, and analysis. High Throughput 2018, 7, E24. [Google Scholar]
- Ferris, R.A.; McCue, P.M.; Borlee, G.I.; Glapa, K.E.; Martin, K.H.; Mangalea, M.R.; Hennet, M.L.; Wolfe, L.M.; Broeckling, C.D.; Borlee, B.R. Model of chronic equine endometritis involving a Pseudomonas aeruginosa biofilm. Infect. Immun. 2017, 85, e00332-17. [Google Scholar] [CrossRef]
- Moreno, I.; Franasiak, J.M. Endometrial microbiota—New player in town. Fertil. Steril. 2017, 108, 32–39. [Google Scholar] [CrossRef]
- Verstraelen, H.; Vilchez-Vargas, R.; Desimpel, F.; Jauregui, R.; Vankeirsbilck, N.; Weyers, S.; Verhelst, R.; De Sutter, P.; Pieper, D.H.; Van de Wiele, T. Characterisation of the human uterine microbiome in non-pregnant women through deep sequencing of the V1-2 region of the 16S rRNA gene. PeerJ 2016. [Google Scholar] [CrossRef]
- Baker, J.M.; Chase, D.M.; Herbst-Kralovetz, M.M. Uterine microbiota: Residents, tourists, or invaders? Front. Immunol. 2018, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Pelzer, E.; Gomez-Arango, L.F.; Barrett, H.L.; Nitert, M.D. Review: Maternal health and the placental microbiome. Placenta 2017, 54, 30–37. [Google Scholar] [CrossRef]
- Heil, B.A.; Thompson, S.K.; Kearns, T.A.; Davolli, G.M.; King, G.; Sones, J.L. Metagenetic characterization of the resident equine uterine microbiome using multiple techniques. J. Equine Vet. Sci. 2018, 66, 111. [Google Scholar] [CrossRef]
- Heil, B.A.; Paccamonti, D.L.; Sones, J.L. Role for the mammalian female reproductive tract microbiome in pregnancy outcomes. Physiol. Genom. 2019, 51, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Rock, K.; Love, B.; DeSilva, U.; Rezabek, G.; Carrington, S.; Holyoak, G.; Carroll, B.; Gragg, D. Detectable differences in the endometrial microbiome between normal and susceptible mares using metagenomic profiling and conventional bacterial culture. In Proceedings of the Society of Theriogenology, Milwaukee, WI, USA, 11 August 2011. [Google Scholar]
- Subramaniam, A.; Ptacek, T.; Lobashevsky, E.; Cliver, S.; Lefkowitz, E.; Morrow, C.; Biggio, J.; Edwards, R. Midtrimester Cervicovaginal Microbiota: Identification of Microbial Variations Associated with Puerperal Infection at Term. Am. J. Perinatol. 2016, 33, 1165–1175. [Google Scholar] [PubMed]
- Swartz, J.D.; Lachman, M.; Westveer, K.; O’Neill, T.; Geary, T.; Kott, R.W.; Berardinelli, J.G.; Hatfield, P.G.; Thomson, J.M.; Roberts, A.; et al. Characterization of the vaginal microbiota of ewes and cows reveals a unique microbiota with low levels of lactobacilli and near-neutral pH. Front. Vet. Sci. 2014, 1, 19. [Google Scholar] [CrossRef]
- Benner, M.; Ferwerda, G.; Joosten, I.; van der Molen, R.G. How uterine microbiota might be responsible for a receptive, fertile endometrium. Hum. Reprod. Update 2018, 24, 393–415. [Google Scholar] [CrossRef] [PubMed]
- Canisso, I.F.; Ball, B.A.; Cray, C.; Williams, N.M.; Scoggin, K.E.; Davolli, G.M.; Squires, E.L.; Troedsson, M.H. Serum amyloid A and haptoglobin concentrations are increased in plasma of mares with ascending placentitis in the absence of changes in peripheral leukocyte counts or fibrinogen concentration. Am. J. Reprod. Immunol. 2014, 72, 376–385. [Google Scholar] [CrossRef]
- Troedsson, M.H.T.; Loset, K.; Alghamdi, A.M.; Dahms, B.; Crabo, B.G. Interaction between equine semen and the endometrium: The inflammatory response to semen. Anim. Reprod. Sci. 2001, 68, 273–278. [Google Scholar] [CrossRef]
- Lieberman, J. The ABCs of granule-mediated cytotoxicity: New weapons in the arsenal. Nat. Rev. Immunol. 2003, 3, 361–370. [Google Scholar] [CrossRef]
- Muraille, E.; Goriely, S. The nonspecific face of adaptive immunity. Curr. Opin. Immunol. 2017, 48, 38–43. [Google Scholar] [CrossRef]
- Janeway, C.A.; Medzhitov, R. Innate immune recognition. Ann. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef]
- Medzhitov, R.; Janeway, C. Innate immune recognition: Mechanisms and pathways. Immunol. Rev. 2000, 173, 89–97. [Google Scholar] [CrossRef]
- Medzhitov, R.; Janeway, C.A. Innate immune recognition and control of adaptive immune responses. Semin. Immunol. 1998, 10, 351–353. [Google Scholar] [CrossRef]
- Moldenhauer, L.M.; Diener, K.R.; Thring, D.M.; Brown, M.P.; Hayball, J.D.; Robertson, S.A. Crosspresentation of male seminal fl uid antigens elicits T cell activation to initiate the female immune response to pregnancy. J. Immunol. 2009, 182, 8080–8093. [Google Scholar] [CrossRef] [PubMed]
- Schjenken, J.E.; Robertson, S.A. Seminal fluid signaling in the female reproductive tract: Implications for reproductive outcome and offspring health. Adv. Exp. Med. Biol. 2015, 868, 127–158. [Google Scholar] [PubMed]
- Jørgensen, N.; Persson, G.; Hviid, T.V.F. The tolerogenic function of regulatory T cells in pregnancy and cancer. Front. Immunol. 2019, 10, 911. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S. Regulatory T cells: Key controllers of immunologic self-tolerance. Cell 2000, 101, 455–458. [Google Scholar] [CrossRef]
- Shevach, E.M. CD4+ CD25+ suppressor T cells: More questions than answers. Nat. Rev. Immunol. 2002, 2, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Somerset, D.A.; Zheng, Y.; Kilby, M.D.; Sansom, D.M.; Drayson, M.T. Normal human pregnancy is associated with an elevation in the immune suppressive CD25 + CD4 + regulatory T-cell subset. Immunology 2004, 112, 38–43. [Google Scholar] [CrossRef]
- Aluvihare, V.R.; Kallikourdis, M.; Betz, A.G. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 2004, 5, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Hori, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Khattri, R.; Cox, T.; Yasayko, S.A.; Ramsdell, F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. J. Immunol. 2003, 198, 993–998. [Google Scholar] [CrossRef]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003, 4, 330–336. [Google Scholar] [CrossRef]
- Fedorka, C.E.; Loux, S.L.; Scoggin, K.E.; Adams, A.A.; Troedsson, M.H.T.; Ball, B.A. Alterations in T cell-related transcripts at the feto-maternal interface throughout equine gestation. Placenta 2019, 89, 78–87. [Google Scholar] [CrossRef]
- Baratelli, F.; Lin, Y.; Zhu, L.; Yang, S.-C.; Heuze-Vourc’h, N.; Zeng, G.; Reckamp, K.; Dohadwala, M.; Sharma, S.; Dubinett, S.M. Prostaglandin E2 induces FoxP3 gene expression and T regulatory cell function in human CD4+ T cells. J. Immunol. 2005, 175, 1483–1490. [Google Scholar] [CrossRef]
- Chen, W.; Jin, W.; Hardegen, N.; Lei, K.J.; Li, L.; Marinos, N.; McGrady, G.; Wahl, S.M. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003, 198, 1875–1886. [Google Scholar] [CrossRef]
- Kumar, V. The complement system, toll-like receptors and inflammasomes in host defense: Three musketeers’ one target. Int. Rev. Immunol. 2019, 38, 131–156. [Google Scholar] [CrossRef]
- Hakansson, A.; Albihn, A.; Magnusson, U. The contribution of complement to opsonic activity in the uterine secretions of mares free of endometritis. Theriogenology 1993, 39, 601–609. [Google Scholar] [CrossRef]
- Grossman, T.R.; Hettrick, L.A.; Johnson, R.B.; Hung, G.; Peralta, R.; Watt, A.; Henry, S.P.; Adamson, P.; Monia, B.P.; McCaleb, M.L. Inhibition of the alternative complement pathway by antisense oligonucleotides targeting complement factor B improves lupus nephritis in mice. Immunobiology 2016, 221, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Weiler, J.M.; Daha, M.R.; Austen, K.F.; Fearon, D.T. Control of the amplification convertase of complement by the plasma protein β1H. Proc. Natl. Acad. Sci. USA. 1976, 73, 3268–3272. [Google Scholar] [CrossRef] [PubMed]
- Conrad, D.H.; Carlos, J.R.; Ruddy, S. Interaction of β1H globulin with cell-bound C3b: Quantitative analysis of binding and influence of alternative pathway components on binding. J. Exp. Med. 1978, 147, 1792–1805. [Google Scholar] [CrossRef] [PubMed]
- Pangburn, M.K.; Schreiber, R.D.; Müller-Eberhard, H.J. Human complement C3b inactivator: Isolation, characterization, and demonstration of an absolute requirement for the serum protein β1H for cleavage of C3b and C4b in solution. J. Exp. Med. 1977, 146, 257–270. [Google Scholar] [CrossRef]
- Ahearn, J.M.; Fearon, D.T. Structure and Function of the Complement Receptors, CR1 (CD35) and CR2 (CD21). Adv. Immunol. 1989, 46, 183–219. [Google Scholar]
- Krych-Goldberg, M.; Atkinson, J.P. Structure-function relationships of complement receptor type 1. Immunol. Rev. 2001, 180, 112–122. [Google Scholar] [CrossRef]
- Nonaka, M. Evolution of the complement system. Subcell. Biochem. 2014, 80, 31–43. [Google Scholar]
- Walport, M.J. Complement. N. Engl. J. Med. 2001, 344, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Rus, H.; Cudrici, C.; Niculescu, F. The role of the complement system in innate immunity. Immunol. Res. 2005, 33, 103–112. [Google Scholar] [CrossRef]
- Meri, S. Self-nonself discrimination by the complement system. FEBS Lett. 2016, 590, 2418–2434. [Google Scholar] [CrossRef] [PubMed]
- Watson, E.D.; Stokes, C.R.; Bourne, F.J. Influence of arachidonic acid metabolites in vitro and in uterine washings on migration of equine neutrophils under agarose. Res. Vet. Sci. 1987, 43, 203–207. [Google Scholar] [CrossRef]
- Pycock, J.F.; Allen, W.E. Pre-chemotactic and chemotactic properties of uterine fluid from mares with experimentally induced endometritis. Vet. Rec. 1988, 123, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Pycock, J.F.; Allen, W.E. Inflammatory components in uterine fluid from mares with experimentally induced bacterial endometritis. Equine Vet. J. 1990, 22, 422–425. [Google Scholar] [CrossRef]
- Watson, E.D.; Stokes, C.R.; Bourne, F.J. Cellular and humoral defence mechanisms in mares susceptible and resistant to persistent endometritis. Vet. Immunol. Immunopathol. 1987, 16, 107–121. [Google Scholar] [CrossRef]
- Asbury, A.C.; Gorman, N.T.; Foster, G.W. Uterine defense mechanisms in the mare: Serum opsonins affecting phagocytosis of Streptococcus zooepidemicus by equine neutrophils. Theriogenology 1984, 21, 375–385. [Google Scholar] [CrossRef]
- Watson, E.D. Opsonins in uterine washings influencing in vitro activity of equine neutrophils. Equine Vet. J. 1988, 20, 435–437. [Google Scholar] [CrossRef]
- Asbury, A.C.; Halliwell, R.E.; Foster, G.W.; Longino, S.J. Immunoglobulins in uterine secretions of mares with differing resistance to endometritis. Theriogenology 1980, 14, 299–308. [Google Scholar] [CrossRef]
- Mitchell, G.; Liu, I.K.; Perryman, L.E.; Stabenfeldt, G.H.; Hughes, J.P. Preferential production and secretion of immunoglobulins by the equine endometrium—A mucosal immune system. J. Reprod. Fertil. Suppl. 1982, 32, 161–168. [Google Scholar]
- Troedsson, M.H.T.; Liu, I.K.M.; Thurmond, M. Immunoglobulin (IgG and IgA) and complement (C3) concentrations in uterine secretion following an intrauterine challenge of Streptococcus Zooepidemicus in mares susceptible to versus resistant to chronic uterine infection1. Biol. Reprod. 1993, 49, 502–506. [Google Scholar] [CrossRef]
- Widders, P.R.; Stokes, C.R.; David, J.S.; Bourne, F.J. Quantitation of the immunoglobulins in reproductive tract secretions of the mare. Res. Vet. Sci. 1984, 37, 324–330. [Google Scholar] [CrossRef]
- Williamson, P.; Dunning, A.; O’Connor, J.; Penhale, W.J. Immunoglobulin levels, protein concentrations and alkaline phosphatase activity in uterine flushings from mares with endometritis. Theriogenology 1983, 19, 441–448. [Google Scholar] [CrossRef]
- Dell’Aqua, J.A., Jr.; Papa, F.O.; Lopes, M.D.; Alvarenga, M.A.; Macedo, L.P.; Melo, C.M. Modulation of acute uterine inflammatory response after artificial insemination with equine frozen semen. Anim. Reprod. Sci. 2006, 94, 270–273. [Google Scholar]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Cronin, J.G.; Turner, M.L.; Goetze, L.; Bryant, C.E.; Sheldon, I.M. Toll-like receptor 4 and MYD88-dependent signaling mechanisms of the innate immune system are essential for the response to lipopolysaccharide by epithelial and stromal cells of the bovine endometrium. Biol. Reprod. 2012, 86, 51. [Google Scholar] [CrossRef]
- Kitaya, K.; Yamada, H. Pathophysiological Roles of Chemokines in Human Reproduction: An Overview. Am. J. Reprod. Immunol. 2011, 65, 449–459. [Google Scholar] [CrossRef]
- Wira, C.R.; Fahey, J.V.; Sentman, C.L.; Pioli, P.A.; Shen, L. Innate and adaptive immunity in female genital tract: Cellular responses and interactions. Immunol. Rev. 2005, 206, 306–335. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Gerberick, G.F.; Saito, F.H.; Ledger, W.J.; Witkin, S.S. Innate Immunity in the Lower Female Mucosal Tract. J. Steroids Horm. Sci. 2011, 2, 2. [Google Scholar] [CrossRef]
- An, H.; Yu, Y.; Zhang, M.; Xu, H.; Qi, R.; Yan, X.; Liu, S.; Wang, W.; Guo, Z.; Guo, J.; et al. Involvement of ERK, p38 and NF-κB signal transduction in regulation of TLR2, TLR4 and TLR9 gene expression induced by lipopolysaccharide in mouse dendritic cells. Immunology 2002, 106, 38–45. [Google Scholar] [CrossRef]
- Chen, C.; Zibiao, H.; Ming, Z.; Shiyi, C.; Ruixia, L.; Jie, W.; SongJia, L. Expression pattern of Toll-like receptors (TLRs) in different organs and effects of lipopolysaccharide on the expression of TLR 2 and 4 in reproductive organs of female rabbit. Dev. Comp. Immunol. 2014, 46, 341–348. [Google Scholar] [CrossRef]
- Silva, E.; Leitão, S.; Henriques, S.; Kowalewski, M.P.; Hoffmann, B.; Ferreira-Dias, G.; Lopes da Costa, L.; Mateus, L. Gene transcription of TLR2, TLR4, LPS ligands and prostaglandin synthesis enzymes are up-regulated in canine uteri with cystic endometrial hyperplasia-pyometra complex. J. Reprod. Immunol. 2010, 84, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Swangchan-Uthai, T.; Lavender, C.R.M.; Cheng, Z.; Fouladi-Nashta, A.A.; Wathes, D.C. Time course of defense mechanisms in bovine endometrium in response to lipopolysaccharide. Biol. Reprod. 2012, 87, 135. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.L.; Healey, G.D.; Sheldon, I.M. Immunity and inflammation in the uterus. Reprod. Domest. Anim. 2012, 47, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Souza-Fonseca-Guimaraes, F.; Adib-Conquy, M.; Cavaillon, J.M. Natural killer (NK) cells in antibacterial innate immunity: Angels or devils? Mol. Med. 2012, 18, 270–285. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Akira, S. TLR signaling pathways. Semin. Immunol. 2004, 16, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.; Young, D.W.; Golenbock, D.T.; Christ, W.J.; Gusovsky, F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 1999, 274, 10689–10692. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Cronin, J.; Goetze, L.; Donofrio, G.; Schuberth, H.J. Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biol. Reprod. 2009, 81, 1025–1032. [Google Scholar] [CrossRef]
- Fujita, Y.; Mihara, T.; Okazaki, T.; Shitanaka, M.; Kushino, R.; Ikeda, C.; Negishi, H.; Liu, Z.; Richards, J.S.; Shimada, M. Toll-like receptors (TLR) 2 and 4 on human sperm recognize bacterial endotoxins and mediate apoptosis. Hum. Reprod. 2011, 26, 2799–2806. [Google Scholar] [CrossRef]
- Akthar, I.; Suarez, S.; Morillo, V.A.; Sasaki, M.; Ezz, M.A.; Takahashi, K.I.; Shimada, M.; Marey, M.A.; Miyamoto, A. Sperm enter glands of preovulatory bovine endometrial explants and initiate inflammation. Reproduction 2019, 159, 181–192. [Google Scholar] [CrossRef]
- Ezz, M.A.; Marey, M.A.; Elweza, A.E.; Kawai, T.; Heppelmann, M.; Pfarrer, C.; Balboula, A.Z.; Montaser, A.; Imakawa, K.; Zaabel, S.M.; et al. TLR2/4 signaling pathway mediates sperm-induced inflammation in bovine endometrial epithelial cells in vitro. PLoS ONE 2019, 14. [Google Scholar] [CrossRef]
- Elweza, A.E.; Ezz, M.A.; Acosta, T.J.; Talukder, A.K.; Shimizu, T.; Hayakawa, H.; Shimada, M.; Imakawa, K.; Zaghloul, A.H.; Miyamoto, A. Aproinflammatory response of bovine endometrial epithelial cells to active sperm in vitro. Mol. Reprod. Dev. 2018, 85, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Franchi, L.; Warner, N.; Viani, K.; Nuñez, G. Function of Nod-like receptors in microbial recognition and host defense. Immunol. Rev. 2009, 227, 106–128. [Google Scholar] [CrossRef] [PubMed]
- Inohara, N.; Chamaillard, M.; McDonald, C.; Nuñez, G. NOD-LRR Proteins: Role in Host-Microbial Interactions and Inflammatory Disease. Ann. Rev. Biochem. 2005, 74, 355–383. [Google Scholar] [CrossRef] [PubMed]
- Mariathasan, S.; Weiss, D.S.; Newton, K.; McBride, J.; O’Rourke, K.; Roose-Girma, M.; Lee, W.P.; Weinrauch, Y.; Monack, D.M.; Dixit, V.M. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 2006, 440, 228–232. [Google Scholar] [CrossRef]
- Barbé, F.; Douglas, T.; Saleh, M. Advances in Nod-like receptors (NLR) biology. Cytokine Growth Factor Rev. 2014, 25, 681–697. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The Inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Benko, S.; Magalhaes, J.G.; Philpott, D.J.; Girardin, S.E. NLRC5 limits the activation of inflammatory pathways. J. Immunol. 2010, 185, 1681–1691. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, L.; Xia, X.; Wang, H.Y.; Legras, X.; Hong, J.; Ji, J.; Shen, P.; Zheng, S.; Chen, Z.J.; et al. NLRC5 negatively regulates the NF-κB and type I interferon signaling pathways. Cell 2010, 141, 483–496. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Karin, M.; Ben-Neriah, Y. Phosphorylation meets ubiquitination: The control of NF-κB activity. Ann. Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef]
- Zandi, E.; Rothwarf, D.M.; Delhase, M.; Hayakawa, M.; Karin, M. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for Iκb phosphorylation and NF-κB activation. Cell 1997, 91, 243–252. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Gaynor, R.B. IkB kinases: Key regulators of the NF-kB pathway. Trends Biochem. Sci. 2004, 29, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Senftleben, U.; Cao, Y.; Xiao, G.; Greten, F.R.; Krähn, G.; Bonizzi, G.; Chen, Y.; Hu, Y.; Fong, A.; Sun, S.C.; et al. Activation by IKKα of a second, evolutionary conserved, NF-κB signaling pathway. Science 2001, 293, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Dejardin, E.; Droin, N.M.; Delhase, M.; Haas, E.; Cao, Y.; Makris, C.; Li, Z.W.; Karin, M.; Ware, C.F.; Green, D.R. The lymphotoxin-β receptor induces different patterns of gene expression via two NF-κB pathways. Immunity 2002, 17, 525–535. [Google Scholar] [CrossRef]
- Bonizzi, G.; Bebien, M.; Otero, D.C.; Johnson-Vroom, K.E.; Cao, Y.; Vu, D.; Jegga, A.G.; Aronow, B.J.; Ghosh, G.; Rickert, R.C.; et al. Activation of IKKα target genes depends on recognition of specific κB binding sites by RelB:p52 dimers. EMBO J. 2004, 23, 4202–4210. [Google Scholar] [CrossRef]
- Novack, D.V.; Yin, L.; Hagen-Stapleton, A.; Schreiber, R.D.; Goeddel, D.V.; Ross, F.P.; Teitelbaum, S.L. The IκB function of NF-κB2 p100 controls stimulated osteoclastogenesis. J. Exp. Med. 2003, 198, 771–781. [Google Scholar] [CrossRef]
- Matsushima, A.; Kaisho, T.; Rennert, P.D.; Nakano, H.; Kurosawa, K.; Uchida, D.; Takeda, K.; Akira, S.; Matsumoto, M. Essential role of nuclear factor (NF)-κB-inducing kinase and inhibitor of κB (IκB) kinase α in NF-κB activation through lymphotoxin β receptor, but not through tumor necrosis factor receptor I. J. Exp. Med. 2001, 193, 631–636. [Google Scholar] [CrossRef]
- Bonizzi, G.; Karin, M. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004, 25, 280–288. [Google Scholar] [CrossRef]
- Girling, J.E.; Hedger, M.P. Toll-like receptors in the gonads and reproductive tract: Emerging roles in reproductive physiology and pathology. Immunol. Cell Biol. 2007, 85, 481–489. [Google Scholar] [CrossRef]
- Chandrasekharan, N.V.; Simmons, D.L. The cyclooxygenases. Genome Biol. 2004, 5, 241. [Google Scholar] [CrossRef]
- Gohda, J.; Matsumura, T.; Inoue, J. Cutting edge: TNFR-associated factor (TRAF) 6 is essential for MyD88-dependent pathway but not Toll/IL-1 receptor domain-containing adaptor-inducing IFN-β (TRIF)-dependent pathway in TLR signaling. J. Immunol. 2004, 173, 2913–2917. [Google Scholar] [CrossRef]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Sato, S.; Sanjo, H.; Takeda, K.; Ninomiya-Tsuji, J.; Yamamoto, M.; Kawai, T.; Matsumoto, K.; Takeuchi, O.; Akira, S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat. Immunol. 2005, 6, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Van de Craen, M.; Declercq, W.; Van den brande, I.; Fiers, W.; Vandenabeele, P. The proteolytic procaspase activation network: An in vitro analysis. Cell Death Differ. 1999, 6, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Hazuda, D.J.; Strickler, J.; Kueppers, F.; Simon, P.L.; Young, P.R. Processing of precursor interleukin1ß and inflammatory disease. J. Biol. Chem. 1990, 265, 6318–6322. [Google Scholar]
- Black, R.A.; Kronheim, S.R.; Cantrell, M.; Deeley, M.C.; March, C.J.; Prickett, K.S.; Wignall, J.; Conlon, P.J.; Cosman, D.; Hopp, T.P.; et al. Generation of biologically active interleukin 1b by proteolytic cleavage of the inactive precursor. J. Biol. Chem. 1988, 263, 9437–9442. [Google Scholar]
- Ito, A.; Mukaiyama, A.; Itoh, Y.; Nagase, H.; Thogersen, I.B.; Enghild, J.J.; Sasaguri, Y.; Mori, Y. Degradation of interleukin 1beta by matrix metalloproteinases. J. Biol. Chem. 1996, 271, 14657–14660. [Google Scholar] [CrossRef]
- Boerboom, D.; Brown, K.A.; Vaillancourt, D.; Poitras, P.; Goff, A.K.; Watanabe, K.; Doré, M.; Sirois, J. Expression of key prostaglandin synthases in equine endometrium during late diestrus and early pregnancy. Biol. Reprod. 2004, 70, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Palm, F.; Walter, I.; Budik, S.; Kolodziejek, J.; Nowotny, N.; Aurich, C. Influence of different semen extenders and seminal plasma on PMN migration and on expression of IL-1β, IL-6, TNF-α and COX-2 mRNA in the equine endometrium. Theriogenology 2008, 70, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Contran, R.S.; Kumar, V.; Collins, T.; Robbins, S.L. Robbins Pathologic Basic of Disease, 6th ed.; W.B. Saunders: Philadelphia, PA, USA, 1999. [Google Scholar]
- Fumuso, E.; Aguilar, J.; Gigu, S. Interleukin-8 (IL-8) and 10 (IL-10) mRNA transcriptions in the endometrium of normal mares and mares susceptible to persistent post-breeding endometritis. Anim. Reprod. Sci. 2006, 94, 282–285. [Google Scholar]
- Doré, M.; Sirois, J. Regulation of P-selectin expression by inflammatory mediators in canine jugular endothelial cells. Vet. Pathol. 1996, 33, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Tizard, I.R. Innate immunity: The recognition of invaders. In Veterinary Immunology, 9th ed.; Elsevier Saunders: St. Louis, MI, USA, 2009; Volume 1, pp. 11–20. [Google Scholar]
- Troedsson, M.H.T.; Liu, I.K.M.; Ing, M.; Pascoe, J. Smooth muscle electrical activity in the oviduct, and the effect of oxytocin, prostaglandin F2α, and prostaglandin E2 on the myometrium and the oviduct of the cycling mare. Biol. Reprod. 1995, 52, 475–488. [Google Scholar] [CrossRef]
- Lögters, T.; Margraf, S.; Altrichter, J.; Cinatl, J.; Mitzner, S.; Windolf, J.; Scholz, M. The clinical value of neutrophil extracellular traps. Med. Microbiol. Immunol. 2009, 198, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef]
- Garcia-Romo, G.S.; Caielli, S.; Vega, B.; Connolly, J.; Allantaz, F.; Xu, Z.; Punaro, M.; Baisch, J.; Guiducci, C.; Coffman, R.L.; et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 2011, 3. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Brinkmann, V. Neutrophil extracellular traps in microbial infections. In Neutrophils Ininfectious Diseases; Tacchini-Cottier, F., Zandbergen, G., Eds.; Bentham eBooks: Dubais, UAE, 2011; pp. 3–10. [Google Scholar]
- Weinrauch, Y.; Drujan, D.; Shapiro, S.D.; Weiss, J.; Zychlinsky, A. Neutrophil elastase targets virulence factors of enterobacteria. Nature 2002, 417, 91–94. [Google Scholar] [CrossRef]
- Wartha, F.; Beiter, K.; Albiger, B.; Fernebro, J.; Zychlinsky, A.; Normark, S.; Henriques-Normark, B. Capsule and d-alanylated lipoteichoic acids protect Streptococcus pneumoniae against neutrophil extracellular traps. Cell. Microbiol. 2007, 9, 1162–1171. [Google Scholar] [CrossRef]
- Van der Windt, D.; Bootsma, H.J.; Burghout, P.; Van der Gaast-de Jongh, C.E.; Hermans, P.W.M.; Van der Flier, M. Nonencapsulated Streptococcus pneumoniae resists extracellular human neutrophil elastase- and cathepsin G-mediated killing. FEMS Immunol. Med. Microbiol. 2012, 66, 445–448. [Google Scholar] [CrossRef]
- Marin-Esteban, V.; Turbica, I.; Dufour, G.; Semiramoth, N.; Gleizes, A.; Gorges, R.; Beau, I.; Servin, A.L.; Lievin-Le, M.V.; Sandré, C.; et al. Afa/Dr diffusely adhering Escherichia coli strain C1845 induces neutrophil extracellular traps that kill bacteria and damage human enterocyte-like cells. Infect. Immun. 2012, 80, 1891–1899. [Google Scholar] [CrossRef]
- Rebordão, M.R.; Carneiro, C.; Alexandre-Pires, G.; Brito, P.; Pereira, C.; Nunes, T.; Galvão, A.; Leitão, A.; Vilela, C.; Ferreira-Dias, G. Neutrophil extracellular traps formation by bacteria causing endometritis in the mare. J. Reprod. Immunol. 2014, 106, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M.; DePalo, V.A. Anti-inflammatory cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Christoffersen, M.; Woodward, E.; Bojesen, A.M.; Jacobsen, S.; Petersen, M.R.; Troedsson, M.H.; Lehn-Jensen, H. Inflammatory responses to induced infectious endometritis in mares resistant or susceptible to persistent endometritis. BMC Vet. Res. 2012, 8, 41. [Google Scholar] [CrossRef]
- Arend, W.P.; Guthridge, C.J. Biological role of interleukin 1 receptor antagonist isoforms. Ann. Rheum. Dis. 2000, 59 (Suppl. S1), i60–i64. [Google Scholar] [CrossRef] [PubMed]
- Couper, K.N.; Blount, D.G.; Riley, E.M. IL-10: The master regulator of immunity to infection. J. Immunol. 2008, 180, 5771–5777. [Google Scholar] [CrossRef] [PubMed]
- Dripps, D.J.; Brandhuber, B.J.; Thompson, R.C.; Eisenberg, S.P. Interleukin-1 (IL-1) receptor antagonist binds to the 80-kDa IL-1 receptor but does not initiate IL-1 signal transduction. J. Biol. Chem. 1991, 266, 10331–10336. [Google Scholar] [PubMed]
- Cassatella, M.A.; Meda, L.; Gasperini, S.; Calzetti, F.; Bonora, S. Interleukin 10 (IL-10) upregulates IL-1 receptor antagonist production from lipopolysaccharide-stimulated human polymorphonuclear leukocytes by delaying mRNA degradation. J. Exp. Med. 1994, 179, 1695–1699. [Google Scholar] [CrossRef]
- Fiorentino, D.F.; Zlotnik, A.; Mosmann, T.R.; Howard, M.; O’Garra, A. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 1991, 147, 3815–3822. [Google Scholar]
- Troedsson, M.H.T.; Liu, I.K.M.; Ing, M.; Pascoe, J.; Thurmond, M. Multiple site electromyography recordings of uterine activity following an intrauterine bacterial challenge in mares susceptible and resistant to chronic uterine infection. J. Reprod. Fertil. 1993, 99, 307–313. [Google Scholar] [CrossRef]
- Griscavage, J.M.; Wilk, S.; Ignarro, L.J. Inhibitors of the proteasome pathway interfere with induction of nitric oxide synthase in macrophages by blocking activation of transcription factor NF-κB. Proc. Natl. Acad. Sci. USA 1996, 93, 3308–3312. [Google Scholar] [CrossRef]
- Liu, I.; Rakestraw, P.; Coit, C.; Harmon, F.; Snyder, J. An in vitro investigation of the mechanism of neuromuscular regulation in myometrial contractility. Pferdeheilkunde 1997, 13, 557. [Google Scholar]
- Khan, F.A.; Chenier, T.S.; Murrant, C.L.; Foster, R.A.; Hewson, J.; Scholtz, E.L. Dose-dependent inhibition of uterine contractility by nitric oxide: A potential mechanism underlying persistent breeding-induced endometritis in the mare. Theriogenology 2017, 90, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A.; Chenier, T.S.; Foster, R.A.; Hewson, J.; Scholtz, E.L. Endometrial nitric oxide synthase activity in mares susceptible or resistant to persistent breeding-induced endometritis and the effect of a specific iNOS inhibitor in vitro. Reprod. Domest. Anim. 2018, 53, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.J.; Hamer, J.M.; Gason, L.M.; Graham, C.S.; Asbury, A.C.; Irvine, C.H. Clearance of bacteria and non-antigenic markers following intra-uterine inoculation into maiden mares: Effect of steroid hormone environment. Theriogenology 1986, 26, 37–50. [Google Scholar] [CrossRef]
- Bowdish, D.M.E.; Davidson, D.J.; Hancock, R.E.W. Immunomodulatory properties of defensins and cathelicidins. Curr. Top. Microbiol. Immunol. 2006, 306, 27–66. [Google Scholar] [PubMed]
- Oddsdóttir, C.; Riley, S.C.; Leask, R.; Edwards, D.R. Activities of matrix metalloproteinases-9 and -2 in uterine fluid during induced equine endometritis. Pferdeheilkunde 2008, 24, 70–73. [Google Scholar]
- Chakraborti, S.; Mandal, M.; Das, S.; Mandal, A.; Chakraborti, T. Regulation of matrix metalloproteinases. An overview. Mol. Cell. Biochem. 2003, 253, 269–285. [Google Scholar] [CrossRef]
- Curry, T.E.; Osteen, K.G. The matrix metalloproteinase system: Changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr. Rev. 2003, 24, 428–465. [Google Scholar] [CrossRef]
- Rebordão, M.; Galvão, A.; Szóstek, A.; Amaral, A.; Mateus, L.; Skarzynski, D.; Ferreira-Dias, G. Physiopathologic mechanisms involved in mare endometrosis. Reprod. Domest. Anim. 2014, 49, 82–87. [Google Scholar] [CrossRef]
- Alpoim-Moreira, J.; Fernandes, C.; Rebordão, M.R.; Amaral, A.; Pinto-Bravo, P.; Bliebernicht, M.; Skarzynski, D.J.; Ferreira-Dias, G. Collagens and DNA methyltransferases in mare endometrosis. Reprod. Domest. Anim. 2019, 54, 46–52. [Google Scholar] [CrossRef]
- Hoffmann, C.; Ellenberger, C.; Mattos, R.C.; Aupperle, H.; Dhein, S.; Stief, B.; Schoon, H.-A. The equine endometrosis: New insights into the pathogenesis. Anim. Reprod. Sci. 2009, 111, 261–278. [Google Scholar] [CrossRef]
- Rawdanowicz, T.J.; Hampton, A.L.; Nagase, H.; Woolley, D.E.; Salamonsen, L.A. Matrix metalloproteinase production by cultured human endometrial stromal cells: Identification of interstitial collagenase, gelatinase-A, gelatinase-B, and stromelysin-1 and their differential regulation by interleukin-1 alpha and tumor necrosis factor-alpha. J. Clin. Endocrinol. Metab. 1994, 79, 530–536. [Google Scholar]
- Singer, C.F.; Marbaix, E.; Lemoine, P.; Courtoy, P.J.; Eeckhout, Y. Local cytokines induce differential expression of matrix metalloproteinases but not their tissue inhibitors in human endometrial fibroblasts. Eur. J. Biochem. 1999, 259, 40–45. [Google Scholar] [CrossRef]
- Braundmeier, A.G.; Nowak, R.A. Cytokines regulate matrix metalloproteinases in human uterine endometrial fibroblast cells through a mechanism that does not involve increases in extracellular matrix metalloproteinase inducer. Am. J. Reprod. Immunol. 2006, 56, 201–214. [Google Scholar] [CrossRef]
- Amaral, A.; Fernandes, C.; Lukasik, K.; Szóstek-Mioduchowska, A.; Baclawska, A.; Rebordão, M.R.; Aguiar-Silva, J.; Pinto-Bravo, P.; Skarzynski, D.J.; Ferreira-Dias, G. Elastase inhibition affects collagen transcription and prostaglandin secretion in mare endometrium during the estrous cycle. Reprod. Domest. Anim. 2018, 53, 66–69. [Google Scholar] [CrossRef]
- Rebordão, M.R.; Amaral, A.; Lukasik, K.; Szóstek-Mioduchowska, A.; Pinto-Bravo, P.; Galvão, A.; Skarzynski, D.J.; Ferreira-Dias, G. Constituents of neutrophil extracellular traps induce in vitro collagen formation in mare endometrium. Theriogenology 2018, 113, 8–18. [Google Scholar] [CrossRef]
- Szóstek-Mioduchowska, A.Z.; Lukasik, K.; Skarzynski, D.J.; Okuda, K. Effect of transforming growth factor -β1 on α-smooth muscle actin and collagen expression in equine endometrial fibroblasts. Theriogenology 2019, 124, 9–17. [Google Scholar] [CrossRef]
- Szóstek, A.Z.; Lukasik, K.; Galvão, A.M.; Ferreira-Dias, G.M.; Skarzynski, D.J. Impairment of the interleukin system in equine endometrium during the course of endometrosis. Biol. Reprod. 2013, 89, 79. [Google Scholar] [CrossRef]
- Vesey, D.A.; Cheung, C.; Cuttle, L.; Endre, Z.; Gobe, G.; Johnson, D.W. Interleukin-1 β stimulates human renal fibroblast proliferation and matrix protein production by means of a transforming growth factor-β-dependent mechanism. J. Lab. Clin. Med. 2002, 140, 342–350. [Google Scholar] [CrossRef]
- Xiao, H.; Ji, A.M.; Li, Z.L.; Song, X.D.; Su, D.; Chen, A.H. Interleukin-1β inhibits collagen synthesis and promotes its decomposition in cultured cardiac fibroblasts. Sheng Li Xue Bao 2008, 60, 355–361. [Google Scholar]
- Szóstek-Mioduchowska, A.Z.; Baclawska, A.; Okuda, K.; Skarzynski, D.J. Effect of proinflammatory cytokines on endometrial collagen and metallopeptidase expression during the course of equine endometrosis. Cytokine 2019, 123, 154767. [Google Scholar] [CrossRef]
- Wiesner, J.; Vilcinskas, A. Antimicrobial peptides: The ancient arm of the human immune system. Virulence 2010, 1, 440–464. [Google Scholar] [CrossRef]
- Linde, A.; Ross, C.R.; Davis, E.G.; Dib, L.; Blecha, F.; Melgarejo, T. Innate immunity and host defense peptides in veterinary medicine. J. Vet. Intern. Med. 2008, 22, 247–265. [Google Scholar] [CrossRef]
- Nevalainen, T.J.; Graham, G.G.; Scott, K.F. Antibacterial actions of secreted phospholipases A2. Review. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2008, 1781, 1–9. [Google Scholar] [CrossRef]
- Tomee, J.F.C.; Koëter, G.H.; Hiemstra, P.S.; Kauffman, H.F. Secretory leukoprotease inhibitor: A native antimicrobial protein presenting a new therapeutic option? Thorax 1998, 53, 114–116. [Google Scholar] [CrossRef]
- Couto, M.A.; Harwig, S.S.; Lehrer, R.I. Selective inhibition of microbial serine proteases by eNAP-2, an antimicrobial peptide from equine neutrophils. Infect. Immun. 1993, 61, 2991–2994. [Google Scholar] [CrossRef]
- Couto, M.A.; Harwig, S.S.; Cullor, J.S.; Hughes, J.P.; Lehrer, R.I. eNAP-2, a novel cysteine-rich bactericidal peptide from equine leukocytes. Infect. Immun. 1992, 60, 5042–5047. [Google Scholar] [CrossRef]
- Kolm, G.; Klein, D.; Knapp, E.; Watanabe, K.; Walter, I. Lactoferrin expression in the horse endometrium: Relevance in persisting mating-induced endometritis. Vet. Immunol. Immunopathol. 2006, 114, 159–167. [Google Scholar] [CrossRef]
- Hultén, C.; Grönlund, U.; Hirvonen, J.; Tulamo, R.-M.; Suominen, M.M.; Marhaug, G.; Forsberg, M. Dynamics in serum of the inflammatory markers serum amyloid A (SAA), haptoglobin, fibrinogen and α2-globulins during induced noninfectious arthritis in the horse. Equine Vet. J. 2010, 34, 699–704. [Google Scholar] [CrossRef]
- Hultén, C.; Sandgren, B.; Skiöldebrand, E.; Klingeborn, B.; Marhaug, G.; Forsberg, M. The acute phase protein serum amyloid A (SAA) as an inflammatory marker in equine influenza virus infection. Acta Vet. Scand. 1999, 40, 323–333. [Google Scholar]
- Nunokawa, Y.; Fujinaga, T.; Taira, T.; Okumura, M.; Yamashita, K.; Tsunoda, N.; Hagio, M. Evaluation of serum amyloid A protein as an acute-phase reactive protein in horses. J. Vet. Med. Sci. 1993, 55, 1011–1016. [Google Scholar] [CrossRef]
- Tuppits, U.; Orro, T.; Einarsson, S.; Kask, K.; Kavak, A. Influence of the uterine inflammatory response after insemination with frozen-thawed semen on serum concentrations of acute phase proteins in mares. Anim. Reprod. Sci. 2014, 146, 182–186. [Google Scholar] [CrossRef]
- Berg, L.C.; Thomsen, P.D.; Andersen, P.H.; Jensen, H.E.; Jacobsen, S. Serum amyloid A is expressed in histologically normal tissues from horses and cattle. Vet. Immunol. Immunopathol. 2011, 144, 155–159. [Google Scholar] [CrossRef]
- Mette, C.; Camilla Dooleweerdt, B.; Stine, J.; Anders Miki, B.; Morten Roenn, P.; Henrik, L.-J.; Bojesen, A.M.; Anders Miki, B.; Petersen, M.R.; Morten Roenn, P.; et al. Evaluation of the systemic acute phase response and endometrial gene expression of serum amyloid A and pro- and anti-inflammatory cytokines in mares with experimentally induced endometritis. Vet. Immunol. Immunopathol. 2010, 138, 95–105. [Google Scholar] [CrossRef]
- Nielsen, J.M. Endometritis in the mare: A diagnostic study comparing cultures from swab and biopsy. Theriogenology 2005, 64, 510–518. [Google Scholar] [CrossRef]
- Ferris, R.A.; Bohn, A.; McCue, P.M. Equine endometrial cytology: Collection techniques and interpretation. Equine Vet. Educ. 2015, 27, 316–322. [Google Scholar] [CrossRef]
- Bohn, A.A.; Ferris, R.A.; Mccue, P.M. Comparison of equine endometrial cytology samples collected with uterine swab, uterine brush, and low-volume lavage from healthy mares. Vet. Clin. Pathol. 2014, 43, 594–600. [Google Scholar] [CrossRef]
- Cocchia, N.; Paciello, O.; Auletta, L.; Uccello, V.; Silvestro, L.; Mallardo, K.; Paraggio, G.; Pasolini, M.P. Comparison of the cytobrush, cottonswab, and low-volume uterine flush techniques to evaluate endometrial cytology for diagnosing endometritis in chronically infertile mares. Theriogenology 2012, 77, 89–98. [Google Scholar] [CrossRef]
- Christoffersen, M.; Brandis, L.; Samuelsson, J.; Bojesen, A.M.; Troedsson, M.H.T.; Petersen, M.R. Diagnostic double-guarded low-volume uterine lavage in mares. Theriogenology 2015, 83, 222–227. [Google Scholar] [CrossRef]
- Nielsen, J.M.; Nielsen, F.H.; Petersen, M.R.; Dyrehospital, A. Diagnosis of equine endometritis-Microbiology, cytology and histology of endometrial biopsies and the correlation to fertility. Pferdeheilkunde 2012, 28, 8–13. [Google Scholar]
- Nielsen, J.M.; Troedsson, M.H.; Pedersen, M.R.; Bojesen, A.M.; Lehn-Jensen, H.; Zent, W.W. Diagnosis of endometritis in the mare based on bacteriological and cytological examinations of the endometrium: Comparison of results obtained by swabs and biopsies. J. Equine Vet. Sci. 2010, 30, 27–30. [Google Scholar] [CrossRef]
- Ball, B.A.; Shin, S.J.; Patten, V.H.; Lein, D.H.; Woods, G.L. Use of a low-volume uterine flush for microbiologic and cytologic examination of the mare’s endometrium. Theriogenology 1988, 29, 1269–1283. [Google Scholar] [CrossRef]
- Ferris, R.A. Endometritis: Diagnostic tools for infectious endometritis. Vet. Clin. N. Am. Equine Pract. 2016, 32, 481–498. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.A.; Dern, K.; Veir, J.K.; Hawley, J.R.; Lappin, M.R.; McCue, P.M. Development of a broad-range quantitative polymerase chain reaction assay to detect and identify fungal dna in equine endometrial samples. Am. J. Vet. Res. 2013, 74, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Kenney, R.M. Prognostic value of endometrial biopsy of the mare. J. Reprod. Fertil. Suppl. 1975, 23, 347–348. [Google Scholar]
- Doig, P.A.; McKnight, J.D.; Miller, R.B. The use of endometrial biopsy in the infertile mare. Can. Vet. J. 1981, 22, 72–76. [Google Scholar]
- Ricketts, S.W.; Alonso, S. Assessment of the breeding prognosis of mares using paired endometrial biopsy techniques. Equine Vet. J. 1991, 23, 185–188. [Google Scholar] [CrossRef]
- Kenney, R.M.; Doig, P.A. Equine endometrial biopsy. In Current Therapy in Theriogenology; Morrow, D.A., Ed.; W.B. Saunders Company: Philadelphia, PA, USA, 1986; pp. 723–729. [Google Scholar]
- Cadario, M.E.; Thatcher, M.J.D.; Leblanc, M.M. Relationship between prostaglandin and uterine clearance of radiocolloid in the mare. Biol. Reprod. 1995, 52, 495–500. [Google Scholar] [CrossRef]
- Rasch, K.; Schoon, H.A.; Sieme, H.; Klug, E. Histomorphological endometrial status and influence of oxytocin on the uterine drainage and pregnancy rate in mares. Equine Vet. J. 1996, 28, 455–460. [Google Scholar] [CrossRef]
- Allen, W.E. Investigations into the use of exogenous oxytocin for promoting uterine drainage in mares susceptible to endometritis. Vet. Rec. 1991, 128, 593–594. [Google Scholar] [CrossRef]
- Brinsko, S.P.; Varner, D.D.; Blanchard, T.L. The effect of uterine lavage performed four hours post insemination on pregnancy rate in mares. Theriogenology 1991, 35, 1111–1119. [Google Scholar] [CrossRef]
- Cadario, M.E.; Thatcher, W.W.; Klapstein, E.; Merrit, A.M.; Archbald, L.F.; Thatcher, M.J.; LeBlanc, M.M. Dynamics of prostaglandin secretion, intrauterine fluid and uterine clearance in reproductively normal mares and mares with delayed uterine clearance. Theriogenology 1999, 52, 1181–1192. [Google Scholar] [CrossRef]
- Madill, S.; Troedsson, M.H.T.; Santschi, E.M.; Malone, E.D. Dose-response effect of intramuscular oxytocin treatment on myometrial contraction of reproductively normal mares during estrus. Theriogenology 2002, 58, 479–481. [Google Scholar]
- Campbell, M.L.H.; England, G.C.W. A comparison of the ecbolic efficacy of intravenous and intrauterine oxytocin treatments. Theriogenology 2002, 58, 473–477. [Google Scholar]
- Brendemuehl, J.P. Effect of oxytocin and PGF2α on luteal formation, function and pregnancy rates in mares. Theriogenology 2002, 58, 623–626. [Google Scholar]
- LeBlanc, M.M. Persistent Mating-Induced Endometritis. In Current Therapy in Equine Medicine, 5th ed.; Sprayberry, K.A., Robinson, E., Eds.; Elsevier Saunders: St. Louis, MI, USA, 2003; pp. 234–237. [Google Scholar]
- Irvine, C.H.G.; Mckeough, V.-L.; Turner, J.E.; Alexander, S.L.; Taylor, T.B. Effectiveness of a two-dose regimen of prostaglandin administration in inducing luteolysis without adverse side effects in mares. Equine Vet. J. 2002, 34, 191–194. [Google Scholar] [CrossRef]
- Nie, G.J.; Johnson, K.E.; Wenzel, J.G.W.; Braden, T.D. Effect of periovulatory ecbolics on luteal function and fertility. Theriogenology 2002, 58, 461–463. [Google Scholar]
- Schramme, A.R.; Pinto, C.R.F.; Davis, J.; Whisnant, C.S.; Whitacre, M.D. Pharmacokinetics of carbetocin, a long-acting oxytocin analogue, following intravenous administration in horses. Equine Vet. J. 2008, 40, 658–661. [Google Scholar] [CrossRef]
- Steckler, D.; Naidoo, V.; Gerber, D.; Kähn, W. Ex vivo influence of carbetocin on equine myometrial muscles and comparison with oxytocin. Theriogenology 2012, 78, 502–509. [Google Scholar] [CrossRef]
- Dascanio, J.J. How and When to Treat Endometritis With Systemic or Local Antibiotics. In Proceedings of the 57th American Association of Equine Practitioners, San Antonio, TX, USA, 1 January 2011; p. 57. [Google Scholar]
- Benko, T.; Boldizar, M.; Novotny, F.; Hura, V.; Valocky, I.; Dudrikova, K.; Karamanova, M.; Petrovic, V. Incidence of bacterial pathogens in equine uterine swabs, their antibiotic resistance patterns, and selected reproductive indices in English thoroughbred mares during the foal heat cycle. Vet. Med. 2015, 60, 613–620. [Google Scholar] [CrossRef]
- Giguère, S. Antimicrobial drug action and interaction: An introduction. In Antimicrobial Therapy in Veterinary Medicine, 5th ed.; Giguère, S., Prescott, J.F., Dowling, P.M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 3–10. [Google Scholar]
- Vila, J.; Martí, S.; Sánchez-Céspedes, J. Porins, efflux pumps and multidrug resistance in Acinetobacter baumannii. J. Antimicrob. Chemother. 2007, 59, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Alekshun, M.N.; Levy, S.B. Molecular mechanisms of antibacterial multidrug resistance. Cell 2007, 128, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.S.; Han, S.; Nielsen, S.; Pearson, L.K.; Gay, J.M.; Tibary, A. Consequences of intrauterine enrofloxacin infusion on mare endometrium. J. Equine Vet. Sci. 2012, 32, 106–111. [Google Scholar] [CrossRef]
- Schnobrich, M.R.; Pearson, L.K.; Barber, B.K.; Bradecamp, E.; Tibary, A. Effects of intrauterine infusion of a water-based suspension of enrofloxacin on mare endometrium. J. Equine Vet. Sci. 2015, 35, 662–667. [Google Scholar] [CrossRef]
- Leblanc, M. The current status of antibiotic use in equine reproduction. Equine Vet. Educ. 2009, 21, 156–167. [Google Scholar] [CrossRef]
- Giguère, S. Antifungal chemotherapy. In Antimicrobial Therapy in Veterinary Medicine; Giguère, S., Prescott, J.F., Dowling, P.M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 333–355. [Google Scholar]
- Brinsko, S.P.; Rigby, S.L.; Varner, D.; Blanchard, T.L. A practical method for recognizing mares susceptible to post-breeding endometritis. AAEP Proc. 2003, 49, 363–365. [Google Scholar]
- Vanderwall, D.K.; Woods, G.L. Effect on fertility of uterine lavage performed immediately prior to insemination in mares. J. Am. Vet. Med. Assoc. 2003, 222, 1108–1110. [Google Scholar] [CrossRef]
- Hua, Q.; Joyce, A.R.; Palsson, B.Ø.; Fong, S.S. Metabolic characterization of Escherichia coli strains adapted to growth on lactate. Appl. Environ. Microbiol. 2007, 73, 4639–4647. [Google Scholar] [CrossRef]
- Eisenberg, R.C.; Dobrogosz, W.J. Gluconate metabolism in Escherichia coli. J. Bacteriol. 1967, 93, 941–949. [Google Scholar] [CrossRef]
- Knutti, B.; Pycock, J.F.; Weijden, G.C.; Küpfer, U. The influence of early postbreeding uterine lavage on pregnancy rate in mares with intrauterine fluid accumulations after breeding. Equine Vet. Educ. 2010, 12, 267–270. [Google Scholar] [CrossRef]
- Brinsko, S.P.; Varner, D.D.; Blanchard, T.L.; Meyers, S.A. The effect of postbreeding uterine lavage on pregnancy rate in mares. Theriogenology 1990, 33, 465–475. [Google Scholar] [CrossRef]
- Fiala, S.M.; Pimentel, C.A.; Mattos, A.L.G.; Gregory, R.M.; Mattos, R.C. Effect of sperm numbers and concentration on sperm transport and uterine inflammatory response in the mare. Theriogenology 2007, 67, 556–562. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, M.M. Effects of oxytocin, prostaglandin and phenylbutazone on uterine clearance of radiocolloid. Pferdeheilkunde 1997, 13, 483–485. [Google Scholar] [CrossRef][Green Version]
- Reilas, T.; Risco, A.M.; Kareskoski, M.; Katila, T. Effect of flunixin meglumine and oxytocin on uterine response to insemination in mares. Anim. Reprod. Sci. 2006, 94, 252–253. [Google Scholar]
- Cuervo-Arango, J. The effect of treatment with flunixin meglumine at different times relative to hCG administration on ovulation failure and luteal function in mares. Anim. Reprod. Sci. 2011, 127, 84–90. [Google Scholar] [CrossRef]
- Armstrong, D.T. Prostaglandins and follicular functions. J. Reprod. Fertil. 1981, 62, 283–291. [Google Scholar] [CrossRef]
- Donnelly, C.G.; Sones, J.L.; Dockweiler, J.C.; Norberg, L.A.; Norberg, L.E.; Cheong, S.H.; Gilbert, R.O. Effects of flunixin meglumine on postponement of ovulation in mares. Am. J. Vet. Res. 2019, 80, 306–310. [Google Scholar] [CrossRef]
- Aurich, C.; Rojer, H.; Walter, I. Treatment of estrous mares with the non-steroidal anti-inflammatory drug vedaprofen reduces the inflammatory response of the endometrium to insemination. Anim. Reprod. Sci. 2010, 121, 104–106. [Google Scholar]
- Cook, V.L.; Blikslager, A.T. The use of nonsteroidal anti-inflammatory drugs in critically ill horses. J. Vet. Emerg. Crit. Care 2015, 25, 76–88. [Google Scholar] [CrossRef]
- Friso, A.M.; Segabinazzi, L.G.T.M.; Cyrino, M.; Correal, S.B.; Freitas-Dell’Aqua, C.P.; Teoro do Carmo, M.; Dell’Aqua, J.A.; Miró, J.; Papa, F.O.; Alvarenga, M.A. Periovulatory administration of firocoxib did not alter ovulation rates and mitigated post-breeding inflammatory response in mares. Theriogenology 2019, 138, 24–30. [Google Scholar] [CrossRef]
- Rojer, H.; Aurich, C. Treatment of persistent mating-induced endometritis in mares with the non-steroid anti-inflammatory drug vedaprofen. Reprod. Domest. Anim. 2010, 45, 2009–2011. [Google Scholar] [CrossRef] [PubMed]
- Papa, F.O.; Dell’Aqua, J.A., Jr.; Alvarenga, M.A.; Melo-Oña, C.M.; Zahn, F.; Lopes, M.D. Use of corticosteroid therapy on the modulation of uterine inflammatory response in mares after artificial insemination with frozen semen. Pferdeheilkunde 2007, 24, 79–82. [Google Scholar] [CrossRef]
- Ruijter-Villani, M.; de Grauw, J.C.; de Stout, T.A.E. Post-breeding endometritis: Effect of dexamethasone treatment on the expression of inflammatory markers. In Proceedings of the 7th International Conference on Equine Reproductive Medicine, Leipzig, Germany, 20–21 January 2012; Leipziger blaue Hefte Band 2: Leipzig, Germany; pp. 286–288. [Google Scholar]
- Arlas, T.R.; Wolf, C.A.; Petrucci, B.P.L.; Estanislau, J.F.; Gregory, R.M.; Jobim, M.I.M.; Mattos, R.C. Proteomics of endometrial fluid after dexamethasone treatment in mares susceptible to endometritis. Theriogenology 2015, 84, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Ohman, T.; Klein, C.; Doty, A.; Troedsson, M.H.T. The phagocytic function of blood-derived polymorphonuclear neutrophils after administration of dexamethasone for the modulation of post-breeding endometritis in the mare. Pferdeheilkunde 2011, 27, 267–270. [Google Scholar] [CrossRef][Green Version]
- Wolf, C.A.; Maslchitzky, E.; Gregory, R.M.; Jobim, M.I.M.; Mattos, R.C. Effect of corticotherapy on proteomics of endometrial fluid from mares susceptible to persistent postbreeding endometritis. Theriogenology 2012, 77, 1351–1359. [Google Scholar] [CrossRef]
- Ferris, R.A.; Mccue, P.M. The effects of dexamethasone and prednisolone on pituitary and ovarian function in the mare. Equine Vet. J. 2010, 42, 438–443. [Google Scholar] [CrossRef]
- McNeill-Wiest, D.R.; Thompson, D.L.; Wiest, J.J. Gonadotropin secretion in ovariectomized pony mares treated with dexamethasone or progesterone and subsequently with dihydrotestosterone. Domest. Anim. Endocrinol. 1988, 5, 149–155. [Google Scholar] [CrossRef]
- Thompson, D.L.; Garza, F.; St George, R.L.; Rabb, M.H.; Barry, B.E.; French, D.D. Relationships among LH, FSH and prolactin secretion, storage and response to secretagogue and hypothalamic GnRH content in ovariectomized pony mares administered testosterone, dihydrotestosterone, estradiol, progesterone, dexamethasone or follicular fluid. Domest. Anim. Endocrinol. 1991, 8, 189–199. [Google Scholar] [CrossRef]
- Andrews, R.V. Influence of the adrenal gland on gonadal function. Adv. Sex Horm. Res. 1977, 3, 197–215. [Google Scholar] [PubMed]
- Schreiber, J.R.; Nakamura, K.; Erickson, G.F. Rat ovary glucocorticoid receptor: Identification and characterization. Steroids 1982, 39, 569–584. [Google Scholar] [CrossRef]
- Hsueh, A.J.; Erickson, G.F. Glucocorticoid inhibition of FSH-induced estrogen production in cultured rat granulosa cells. Steroids 1978, 32, 639–648. [Google Scholar] [CrossRef]
- Rogan, D.; Fumuso, E.; Rodríguez, E.; Wade, J.; Sánchez Bruni, S.F. Use of a Mycobacterial cell wall extract (MCWE) in susceptible mares to clear experimentally induced endometritis with Streptococcus zooepidemicus. J. Equine Vet. Sci. 2007, 27, 112–117. [Google Scholar] [CrossRef]
- Rohrbach, B.W.; Sheerin, P.C.; Cantrell, C.K.; Matthews, P.M.; Steiner, J.V.; Dodds, L.E. Effect of adjunctive treatment with intravenously administered Propionibacterium acnes on reproductive performance in mares with persistent endometritis. J. Am. Vet. Med. Assoc. 2007, 231, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.A.; Lopez, V.; Lönnerdal, B. Lactoferrin. Cell. Mol. Life Sci. 2005, 62, 2560–2575. [Google Scholar] [CrossRef] [PubMed]
- Ammons, M.C.; Copié, V. Mini-review: Lactoferrin: A bioinspired, anti-biofilm therapeutic. Biofouling 2013, 29, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Fedorka, C.E.; Woodward, E.M.; Scoggin, K.E.; Esteller-Vico, A.; Squires, E.L.; Ball, B.A.; Troedsson, M.H.T. The effect of cysteine-rich secretory protein-3 and lactoferrin on endometrial cytokine mRNA expression after breeding in the horse. J. Equine Vet. Sci. 2017, 48, 136–142. [Google Scholar] [CrossRef]
- Coutinho da Silva, M.A.; Darr, C.R.; Moraes, L.E.; Forshey, B.S. Lactoferrin modulates uterine inflammation postbreeding in the mare. J. Equine Vet. Sci. 2017, 56, 63–67. [Google Scholar] [CrossRef]
- Fedorka, C.E.; Scoggin, K.E.; Woodward, E.M.; Squires, E.L.; Ball, B.A.; Troedsson, M.H.T. The effect of select seminal plasma proteins on endometrial mRNA cytokine expression in mares susceptible to persistent mating-induced endometritis. Reprod. Domest. Anim. 2017, 52, 89–96. [Google Scholar] [CrossRef]
- Fedorka, C.E.; Scoggin, K.E.; Boakari, Y.L.; Hoppe, N.E.; Squires, E.L.; Ball, B.A.; Troedsson, M.H.T. The anti-inflammatory effect of exogenous lactoferrin on breeding-induced endometritis when administered post-breeding in susceptible mares. Theriogenology 2018, 114, 63–69. [Google Scholar] [CrossRef]
- Ammons, M.C.B.; Ward, L.S.; Fisher, S.T.; Wolcott, R.D.; James, G.A. In vitro susceptibility of established biofilms composed of a clinical wound isolate of Pseudomonas aeruginosa treated with lactoferrin and xylitol. Int. J. Antimicrob. Agents 2009, 33, 230–236. [Google Scholar] [CrossRef]
- Ammons, M.C.B.; Ward, L.S.; James, G.A. Anti-biofilm efficacy of a lactoferrin/xylitol wound hydrogel used in combination with silver wound dressings. Int. Wound J. 2011, 8, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.C.D.F.; De La Côrte, F.D.; Brass, K.E.; da Silva Azevedo, M.; Gallio, M.; Cantarelli, C.; Dau, S.L.; Cezar, A.S.; Inkelmann, M.A. Evaluation of three methods of platelet-rich plasma for treatment of equine distal limb skin wounds. J. Equine Vet. Sci. 2019, 72, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Carmona, J.U.; Argüelles, D.; Climent, F.; Prades, M. Autologous platelet concentrates as a treatment of horses with osteoarthritis: A preliminary pilot clinical study. J. Equine Vet. Sci. 2007, 27, 167–170. [Google Scholar] [CrossRef]
- Argüelles, D.; Carmona, J.U.; Climent, F.; Muñoz, E.; Prades, M. Autologous platelet concentrates as a treatment for musculoskeletal lesions in five horses. Vet. Rec. 2008, 162, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Georg, R.; Maria, C.; Gisela, A.; Bianca, C. Autologous conditioned plasma as therapy of tendon and ligament lesions in seven horses. J. Vet. Sci. 2010, 11, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, D.R. Effect of Adding Autologous Plasma to an intrauterine antibiotic therapy after breeding on pregnancy rates in mares. Biol. Reprod. 1995, 52, 539–543. [Google Scholar] [CrossRef]
- Kim, H.-J.; Yeom, J.S.; Koh, Y.-G.; Yeo, J.-E.; Kang, K.-T.; Kang, Y.-M.; Chang, B.-S.; Lee, C.-K. Anti-inflammatory effect of platelet-rich plasma on nucleus pulposus cells with response of TNF-α and IL-1. J. Orthop. Res. 2014, 32, 551–556. [Google Scholar] [CrossRef]
- Sundman, E.A.; Cole, B.J.; Karas, V.; Della Valle, C.; Tetreault, M.W.; Mohammed, H.O.; Fortier, L.A. The anti-inflammatory and matrix restorative mechanisms of platelet-rich plasma in osteoarthritis. Am. J. Sports Med. 2014, 42, 35–41. [Google Scholar] [CrossRef]
- Wu, C.-C.; Chen, W.-H.; Zao, B.; Lai, P.-L.; Lin, T.-C.; Lo, H.-Y.; Shieh, Y.-H.; Wu, C.-H.; Deng, W.-P. Regenerative potentials of platelet-rich plasma enhanced by collagen in retrieving pro-inflammatory cytokine-inhibited chondrogenesis. Biomaterials 2011, 32, 5847–5854. [Google Scholar] [CrossRef]
- Woodell-May, J.; Matuska, A.; Oyster, M.; Welch, Z.; O’Shaughnessey, K.; Hoeppner, J. Autologous protein solution inhibits MMP-13 production by IL-1β and TNFα-stimulated human articular chondrocytes. J. Orthop. Res. 2011, 29, 1320–1326. [Google Scholar] [CrossRef]
- Mazzocca, A.D.; McCarthy, M.B.R.; Intravia, J.; Beitzel, K.; Apostolakos, J.; Cote, M.P.; Bradley, J.; Arciero, R.A. An in vitro evaluation of the anti-inflammatory effects of platelet-rich plasma, ketorolac, and methylprednisolone. Arthrosc. J. Arthrosc. Relat. Surg. 2013, 29, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Cieslik-Bielecka, A.; Bielecki, T.; Gazdzik, T.S.; Arendt, J.; Król, W.; Szczepanski, T. Autologous platelets and leukocytes can improve healing of infected high-energy soft tissue injury. Transfus. Apher. Sci. 2009, 41, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Trowbridge, C.C.; Stammers, A.H.; Woods, E.; Yen, B.R.; Klayman, M.; Gilbert, C. Use of platelet gel and its effects on infection in cardiac surgery. J. Extra Corpor. Technol. 2005, 37, 381–386. [Google Scholar] [PubMed]
- Yuan, T.; Zhang, C.; Zeng, B. Treatment of chronic femoral osteomyelitis with platelet-rich plasma (PRP): A case report. Transfus. Apher. Sci. 2008, 38, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Alonso, R.; Girbau, C.; Aguirre, J.J.; Muruzabal, F.; Orive, G. Antibacterial effect of plasma rich in growth factors (PRGF®-Endoret®) against Staphylococcus aureus and Staphylococcus epidermidis strains. Clin. Exp. Dermatol. 2012, 37, 652–657. [Google Scholar] [CrossRef]
- Álvarez, M.; López, C.; Giraldo, C.; Samudio, I.; Carmona, J. In vitro bactericidal activity of equine platelet concentrates, platelet poor plasma, and plasma against methicillin-resistant Staphylococcus aureus. Arch. Med. Vet. 2011, 43, 155–161. [Google Scholar] [CrossRef][Green Version]
- Bielecki, T.M.; Gazdzik, T.S.; Arendt, J.; Szczepanski, T.; Kròl, W.; Wielkoszynski, T. Antibacterial effect of autologous platelet gel enriched with growth factors and other active substances. J. Bone Joint Surg. Br. 2007, 89, 417–420. [Google Scholar] [CrossRef]
- Burnouf, T.; Chou, M.-L.; Wu, Y.-W.; Su, C.-Y.; Lee, L.-W. Antimicrobial activity of platelet (PLT)-poor plasma, PLT-rich plasma, PLT gel, and solvent/detergent-treated PLT lysate biomaterials against wound bacteria. Transfusion 2013, 53, 138–146. [Google Scholar] [CrossRef]
- Moojen, D.J.F.; Everts, P.A.M.; Schure, R.-M.; Overdevest, E.P.; van Zundert, A.; Knape, J.T.A.; Castelein, R.M.; Creemers, L.B.; Dhert, W.J.A. Antimicrobial activity of platelet-leukocyte gel against Staphylococcus aureus. J. Orthop. Res. 2008, 26, 404–410. [Google Scholar] [CrossRef]
- Reghini, M.F.S.; Ramires Neto, C.; Segabinazzi, L.G.; Castro Chaves, M.M.B.; Dell’Aqua, C.D.P.F.; Bussiere, M.C.C.; Dell’Aqua, J.A.; Papa, F.O.; Alvarenga, M.A. Inflammatory response in chronic degenerative endometritis mares treated with platelet-rich plasma. Theriogenology 2015, 86, 516–522. [Google Scholar] [CrossRef]
- Segabinazzi, L.G.; Friso, A.M.; Correal, S.B.; Crespilho, A.M.; Dell’Aqua, J.A.; Miró, J.; Papa, F.O.; Alvarenga, M.A. Uterine clinical findings, fertility rate, leucocyte migration, and COX-2 protein levels in the endometrial tissue of susceptible mares treated with platelet-rich plasma before and after AI. Theriogenology 2017, 104, 120–126. [Google Scholar] [CrossRef]
- Metcalf, E.S. The effect of Platelet-Rich Plasma (PRP) on intraluminal fluid and pregnancy rates in mares susceptible to persistent mating-induced endometritis (PMIE). J. Equine Vet. Sci. 2014, 34, 128. [Google Scholar] [CrossRef]
- Metcalf, E.S.; Scoggin, K.; Troedsson, M.H.T. The effect of platelet-rich plasma on endometrial pro-inflammatory cytokines in susceptible mares following semen deposition. J. Equine Vet. Sci. 2012, 32, 498. [Google Scholar] [CrossRef]
- Timmers, L.; Lim, S.K.; Hoefer, I.E.; Arslan, F.; Lai, R.C.; van Oorschot, A.A.M.; Goumans, M.J.; Strijder, C.; Sze, S.K.; Choo, A.; et al. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res. 2011, 6, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Barrachina, L.; Remacha, A.; Romero, A.; Vázquez, F.; Albareda, J.; Prades, M.; Ranera, B.; Zaragoza, P.; Martín-Burriel, I.; Rodellar, C. Effect of inflammatory environment on equine bone marrow derived mesenchymal stem cells immunogenicity and immunomodulatory properties. Vet. Immunol. Immunopathol. 2016, 171, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Grove, J.E.; Bruscia, E.; Krause, D.S. Plasticity of bone marrow-derived stem cells. Stem Cells 2004, 22, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Taylor, H.S. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells 2007, 25, 2082–2086. [Google Scholar] [CrossRef]
- Alvarenga, M.A.; do Carmo, M.T.; Segabinazzi, L.G.; Guastali, M.D.; Maia, L.; Landim-Alvarenga, F.C. Feasibility and safety of endometrial injection of autologous bone marrow mesenchymal stem cells in mares. J. Equine Vet. Sci. 2016, 42, 12–18. [Google Scholar] [CrossRef]
- Mambelli, L.I.; Mattos, R.C.; Winter, G.H.Z.; Madeiro, D.S.; Morais, B.P.; Malschitzky, E.; Miglino, M.A.; Kerkis, A.; Kerkis, I. Changes in expression pattern of selected endometrial proteins following mesenchymal stem cells infusion in mares with endometrosis. PLoS ONE 2014, 9, e97889. [Google Scholar] [CrossRef]
- Ferris, R.A.; Frisbie, D.D.; McCue, P.M. Use of mesenchymal stem cells or autologous conditioned serum to modulate the inflammatory response to spermatozoa in mares. Theriogenology 2014, 82, 36–42. [Google Scholar] [CrossRef]
- Rink, B.E.; Beyer, T.; French, H.M.; Watson, E.; Aurich, C.; Donadeu, F.X. The fate of autologous endometrial mesenchymal stromal cells after application in the healthy equine uterus. Stem Cells Dev. 2018, 27, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Mambelli, L.I.; Winter, G.H.Z.; Kerkis, A.; Malschitzky, E.; Mattos, R.C.; Kerkis, I. A novel strategy of mesenchymal stem cells delivery in the uterus of mares with endometrosis. Theriogenology 2013, 79, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Q.; Wang, Y.; Li, Y. Uterine infusion with bone marrow mesenchymal stem cells improves endometrium thickness in a rat model of thin endometrium. Reprod. Sci. 2015, 22, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Mao, F.; Xu, W.R.; Qian, H.; Zhu, W.; Yan, Y.M.; Shao, Q.X.; Xu, H.X. Immunosuppressive effects of mesenchymal stem cells in collagen-induced mouse arthritis. Inflamm. Res. 2010, 59, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Lennon, D.P.; Eaton, V.; Maier, K.; Caplan, A.I.; Miller, S.D.; Miller, R.H. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia 2009, 57, 1192–1203. [Google Scholar] [CrossRef]
- Achache, H.; Revel, A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum. Reprod. Update 2006, 12, 731–746. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).