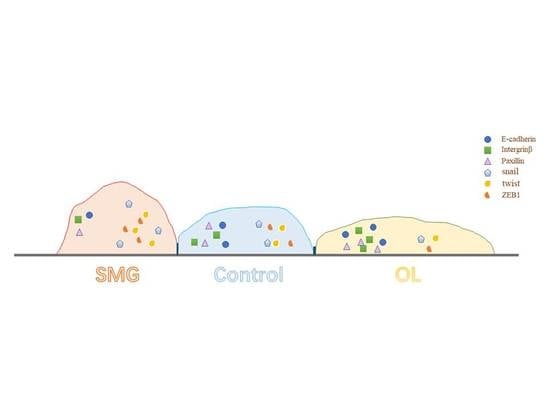
EMT Transcription Factors Are Involved in the Altered Cell Adhesion under Simulated Microgravity Effect or Overloading by Regulation of E-cadherin
Abstract
1. Introduction
2. Results
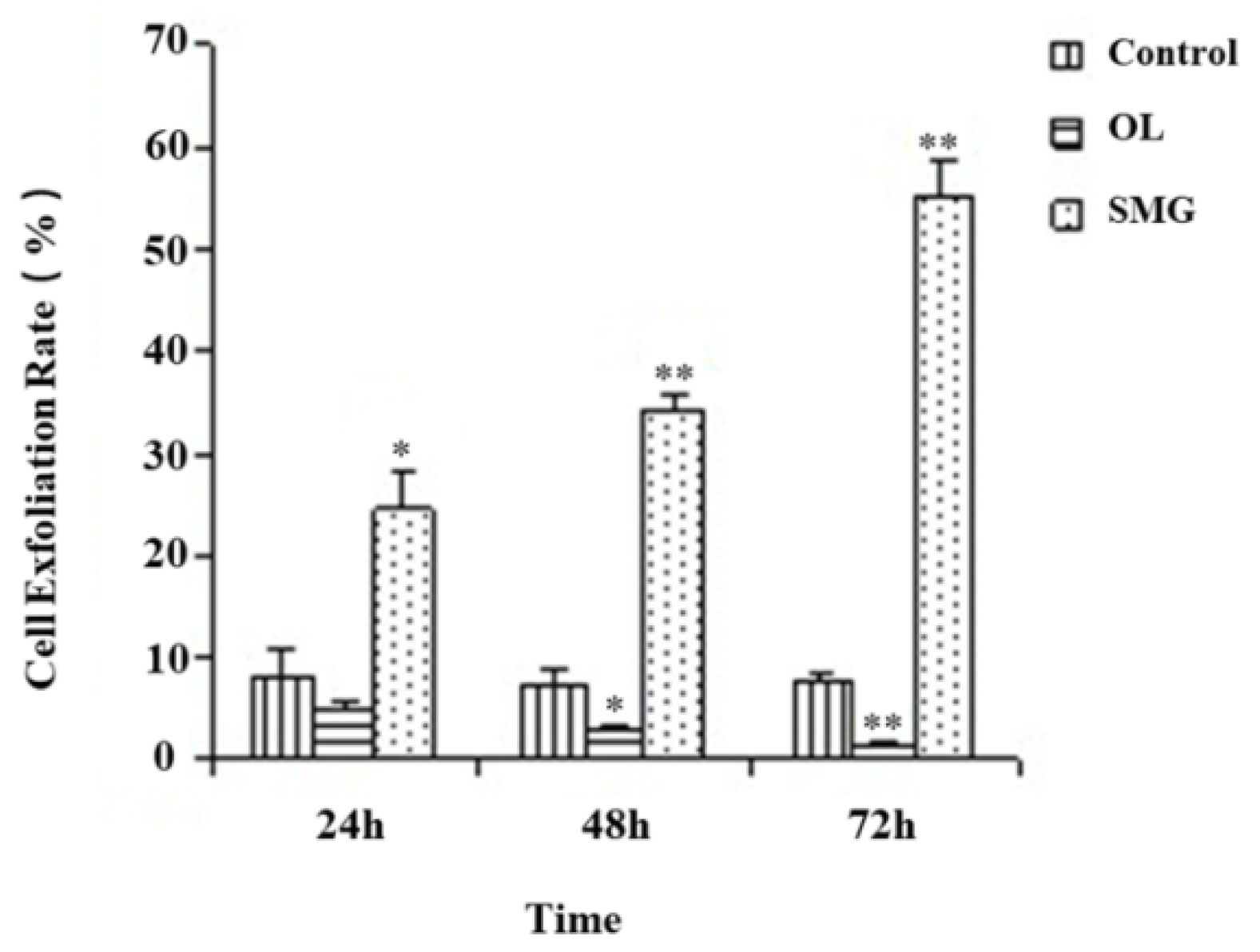
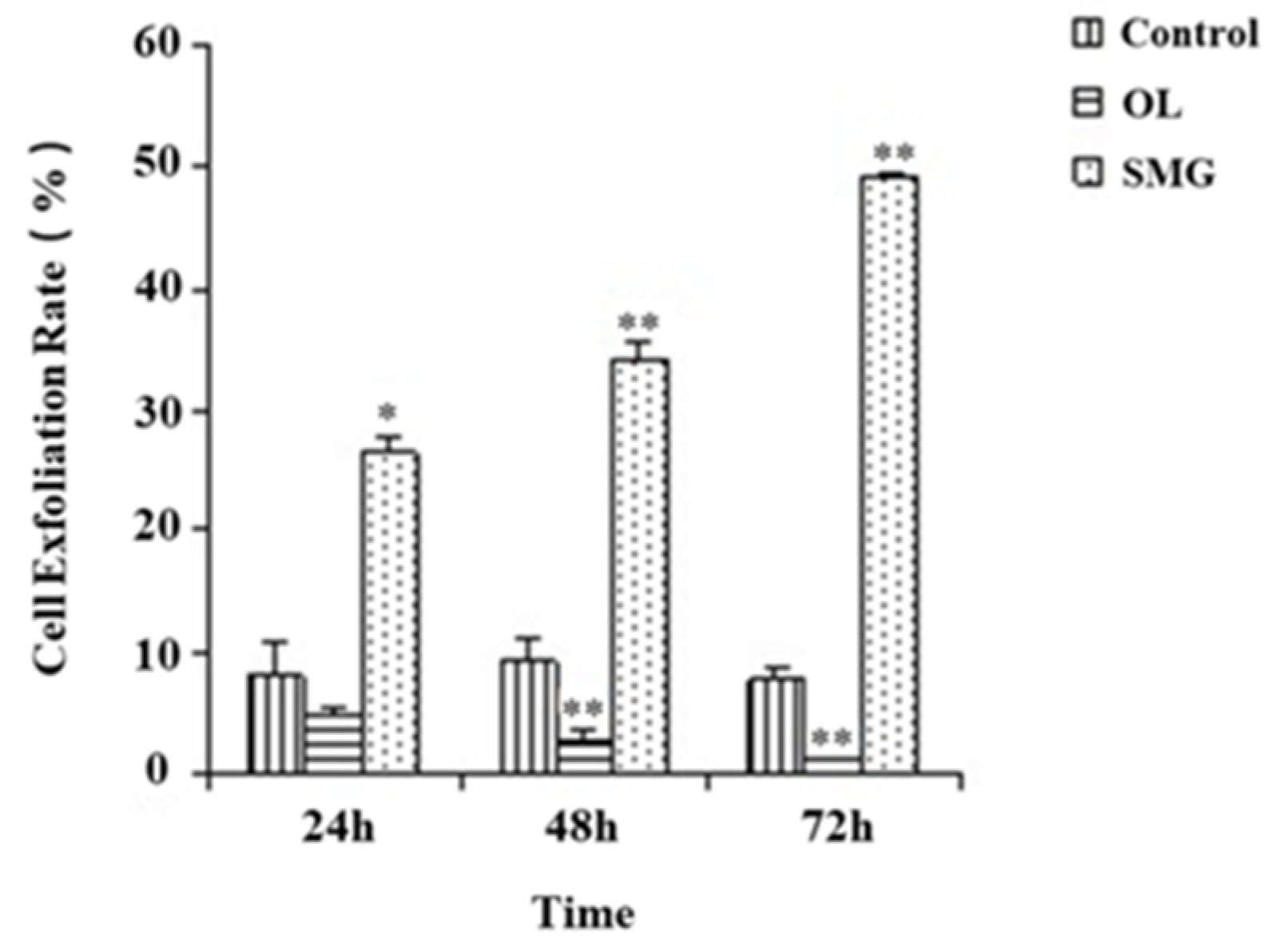
2.1. Cell Exfoliation Rate Analysis after Mechanical Treatment
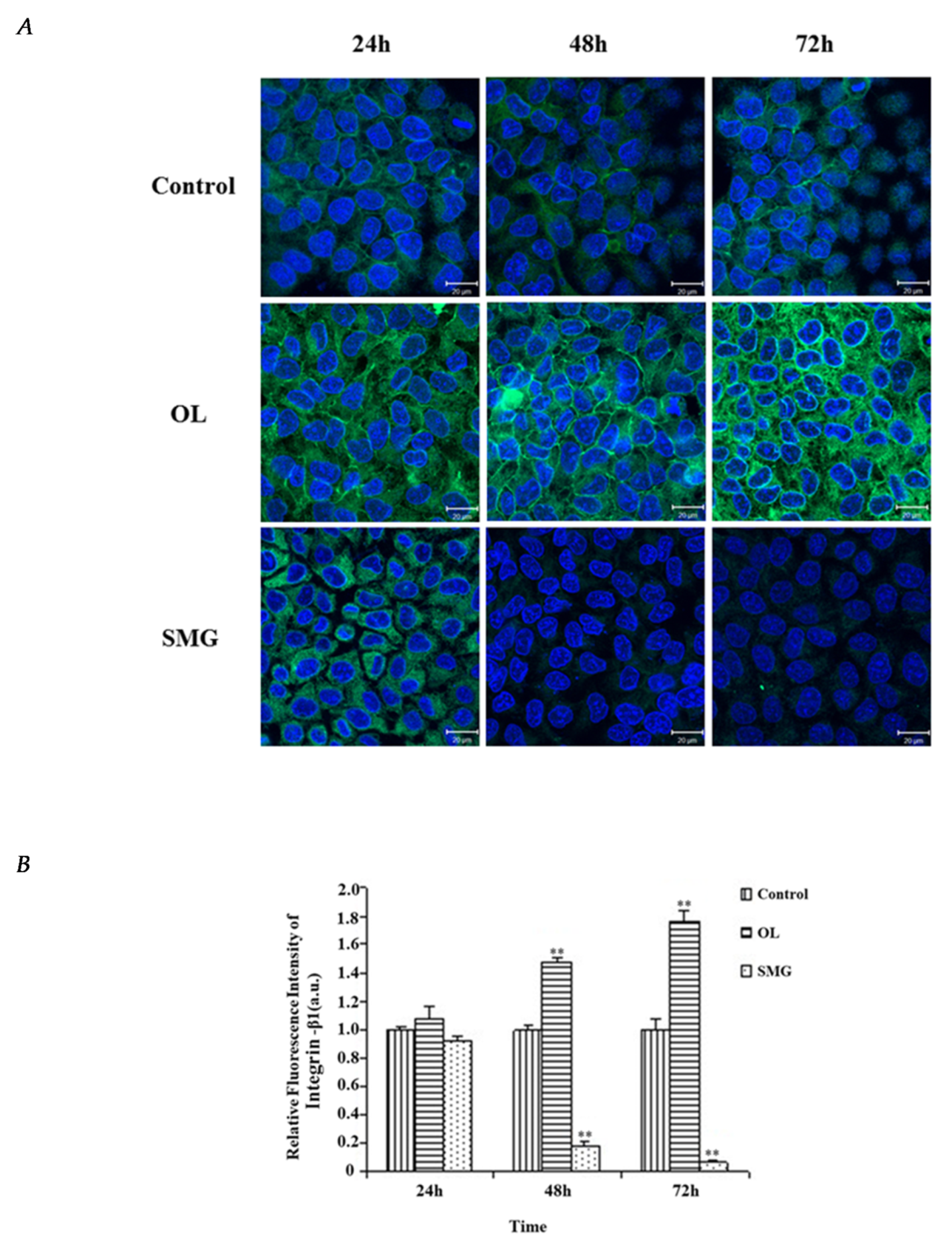
2.2. Localization and Semi-Quantitative Analysis of Adhesion Proteins by Immunofluorescence Analysis
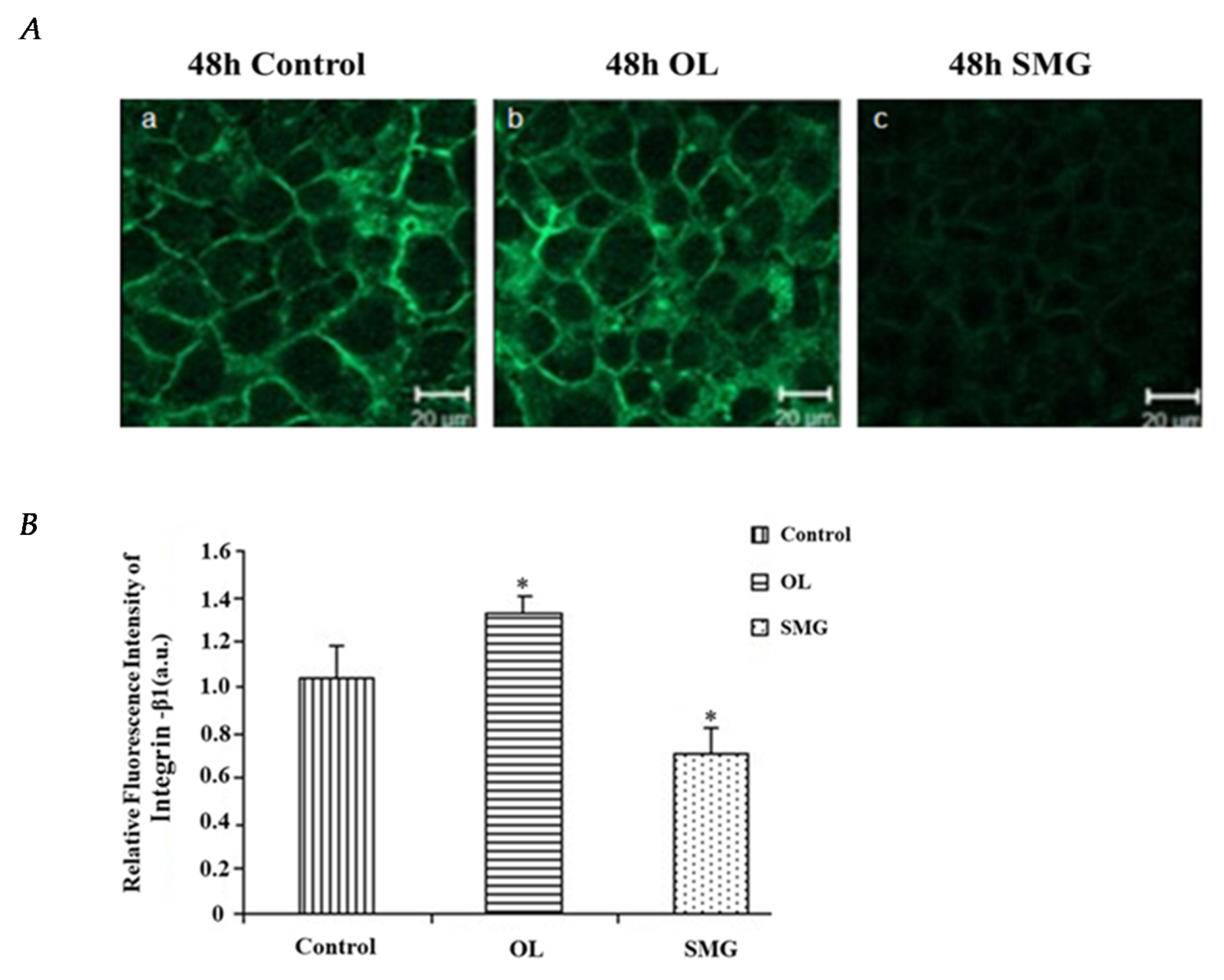
2.2.1. Integrin β1 Changed in HUVEC and MCF-7 Cells
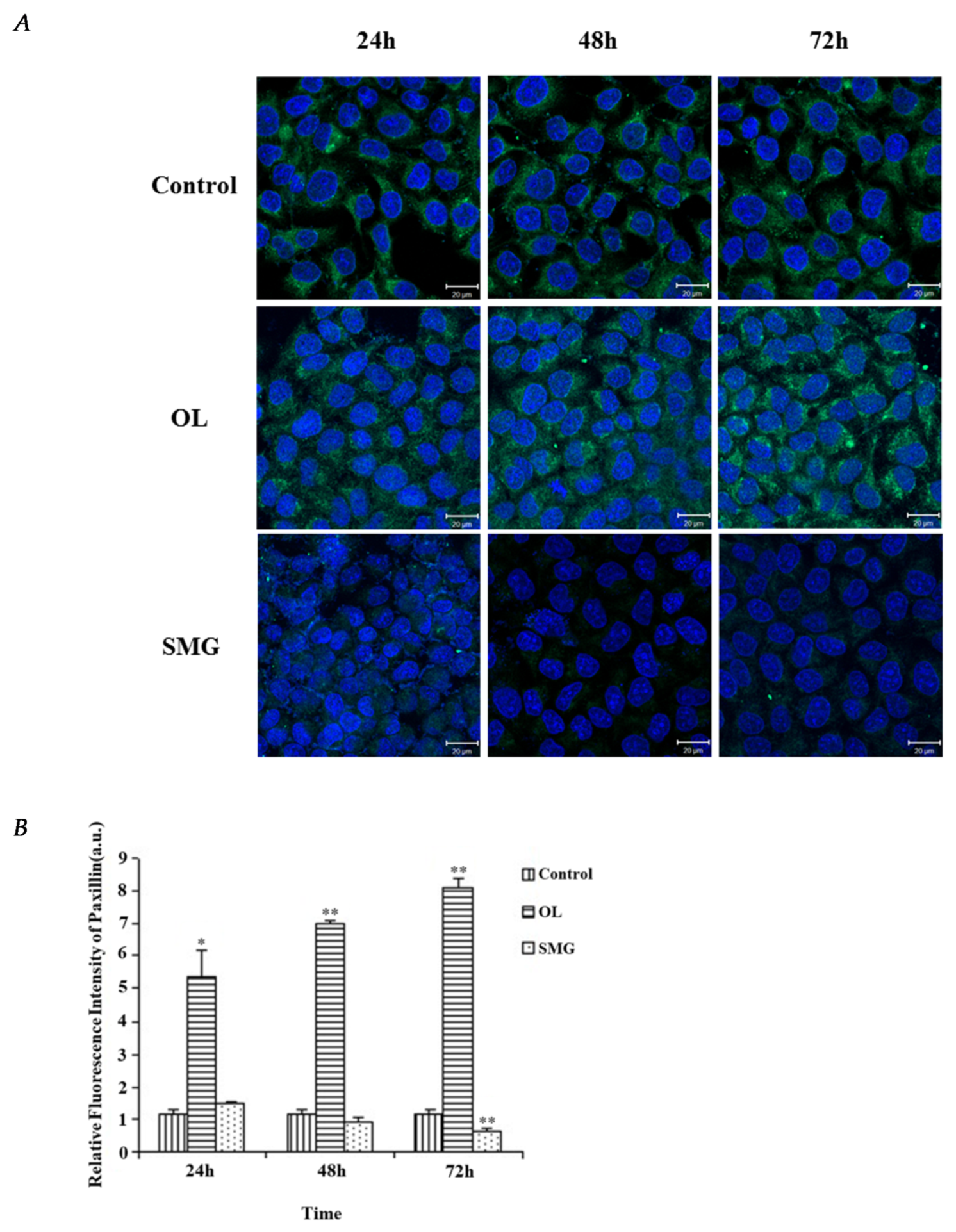
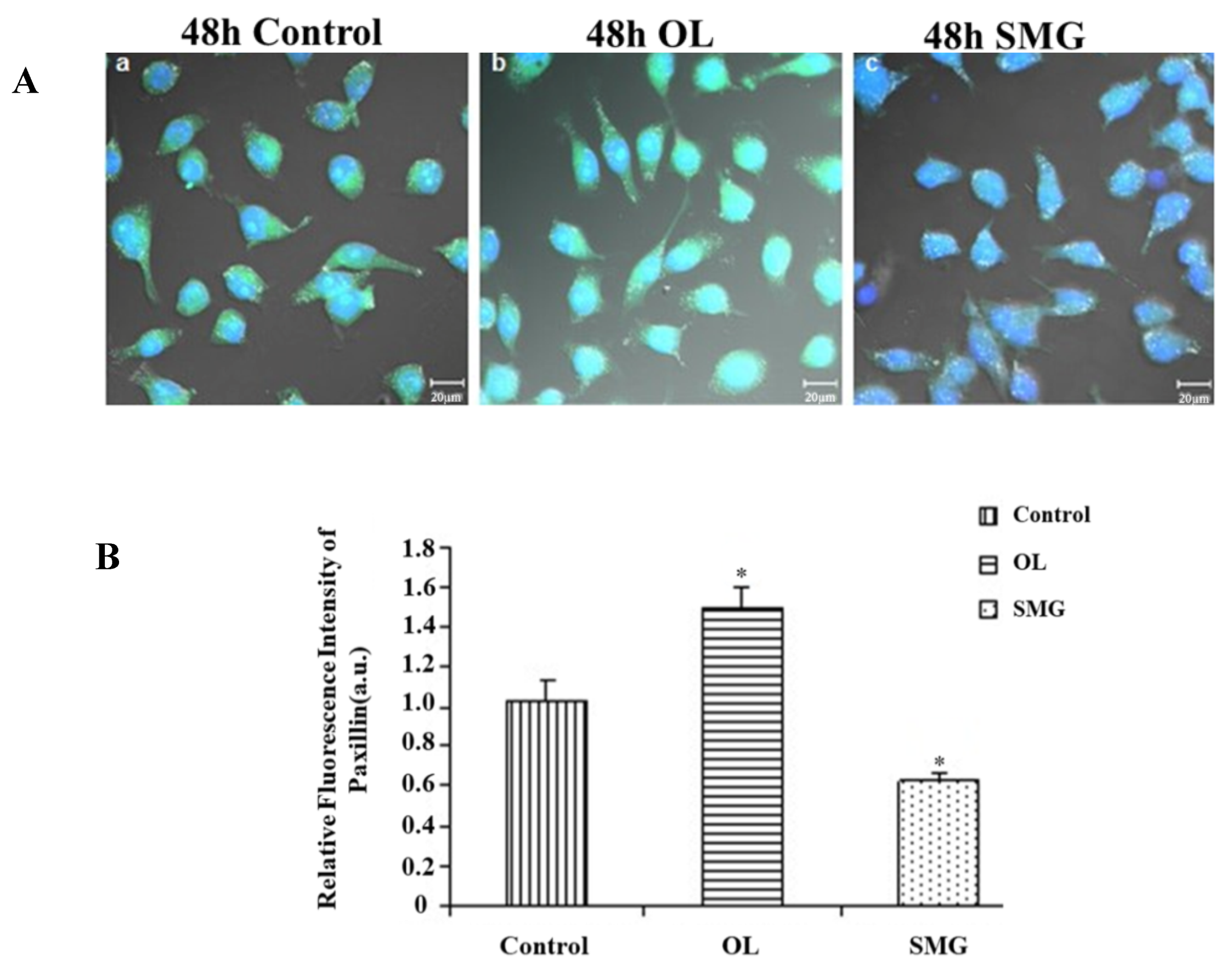
2.2.2. Paxillin Changed in HUVEC and MCF-7 Cells
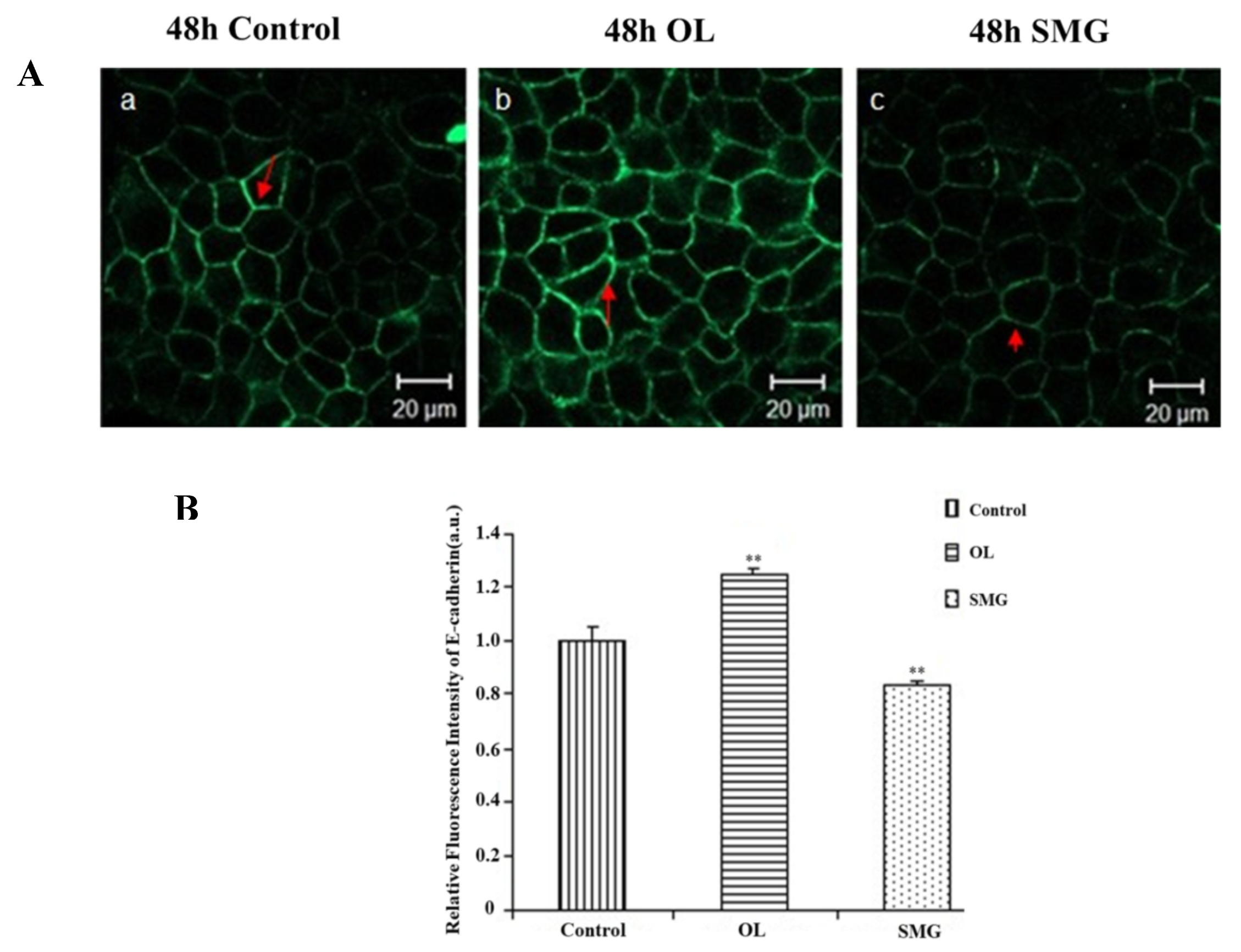
2.2.3. E-Cadherin Changed in MCF-7 Cells
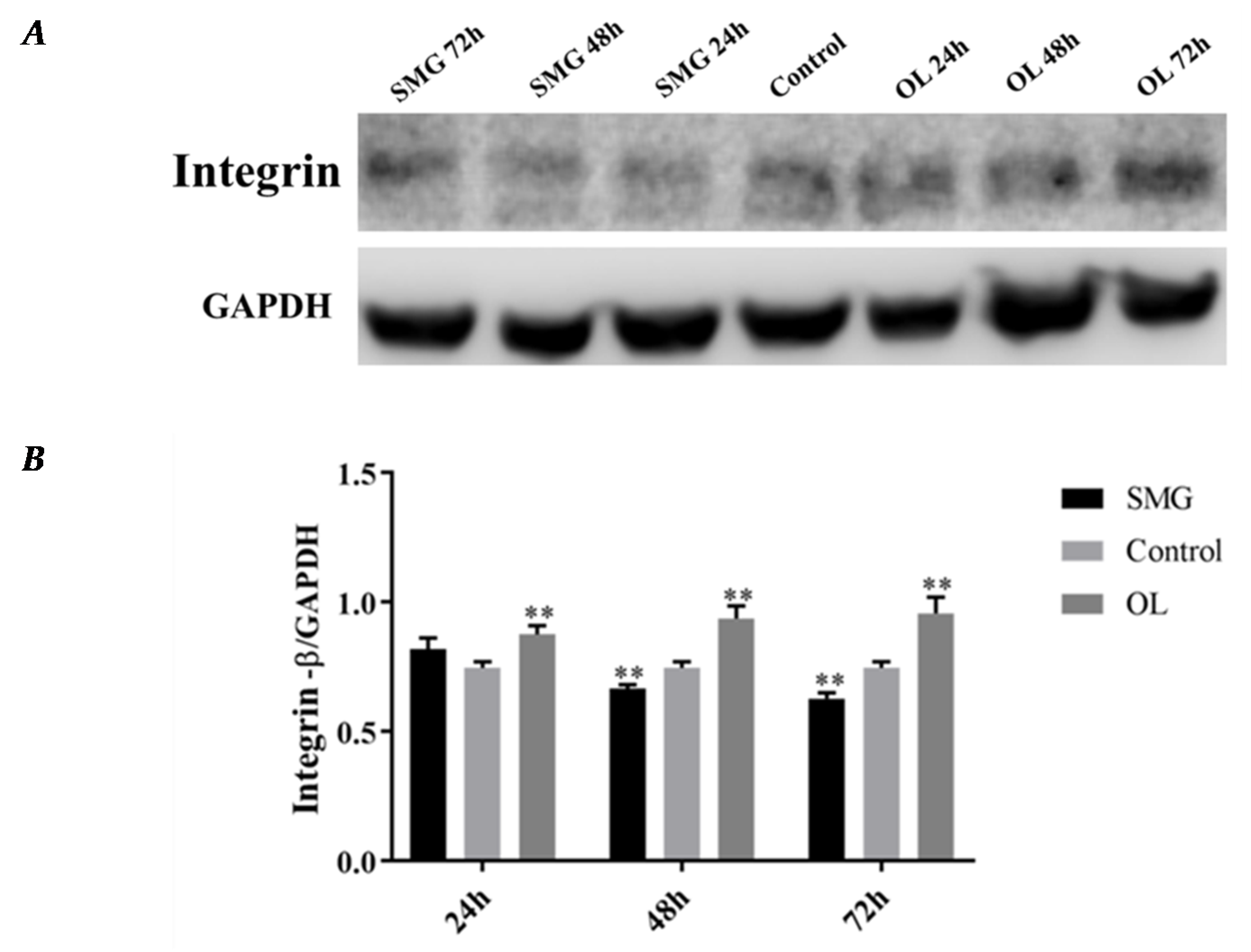
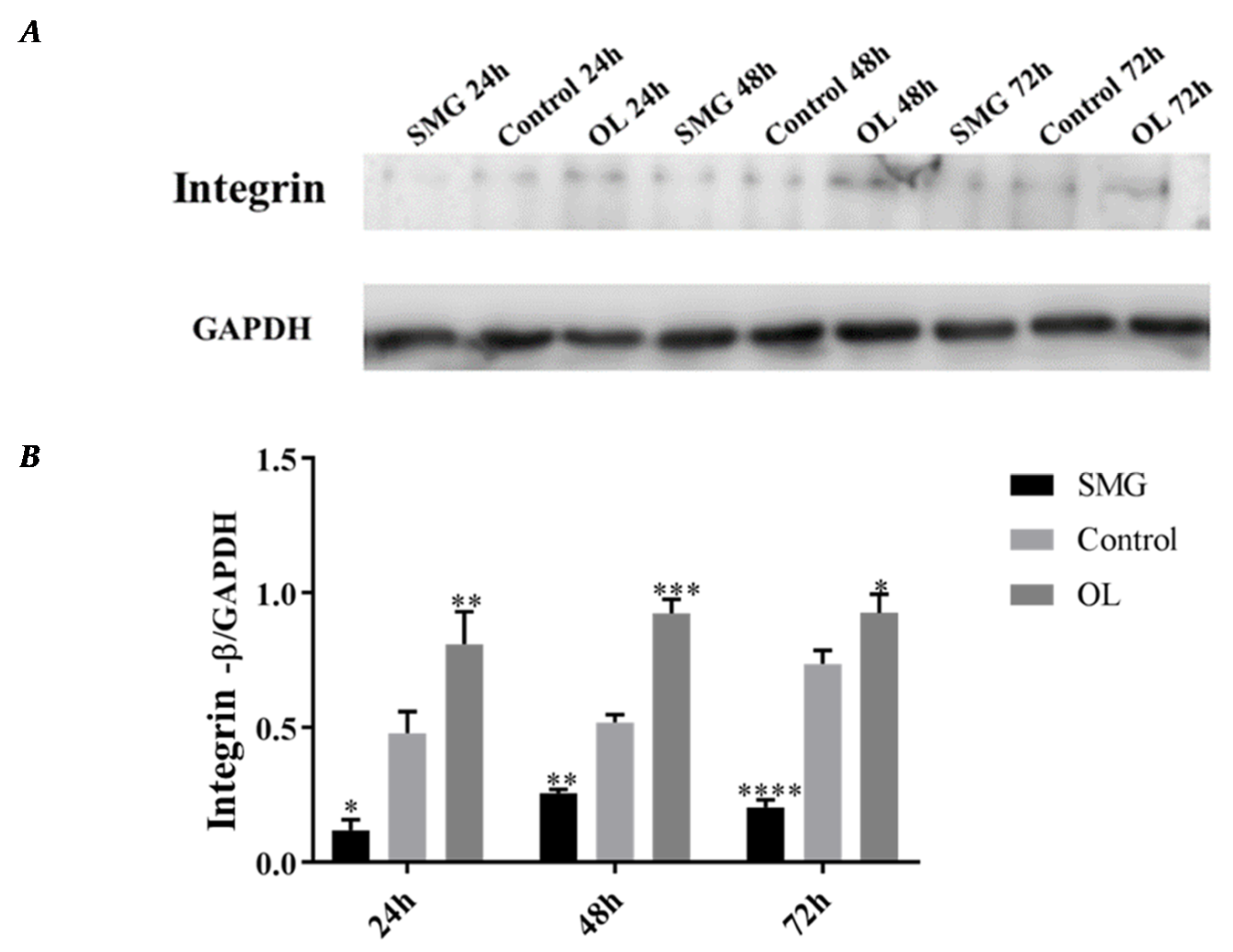
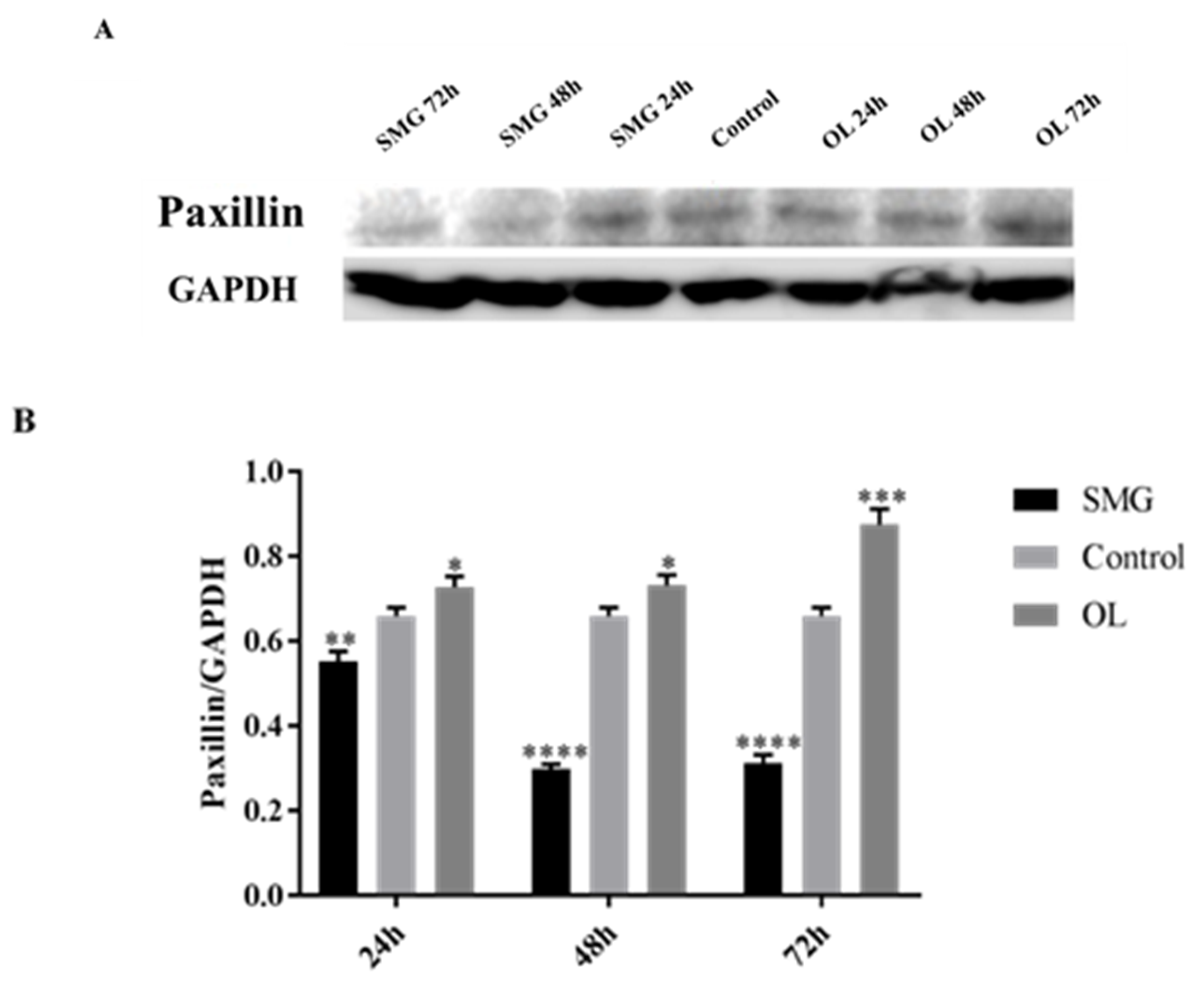
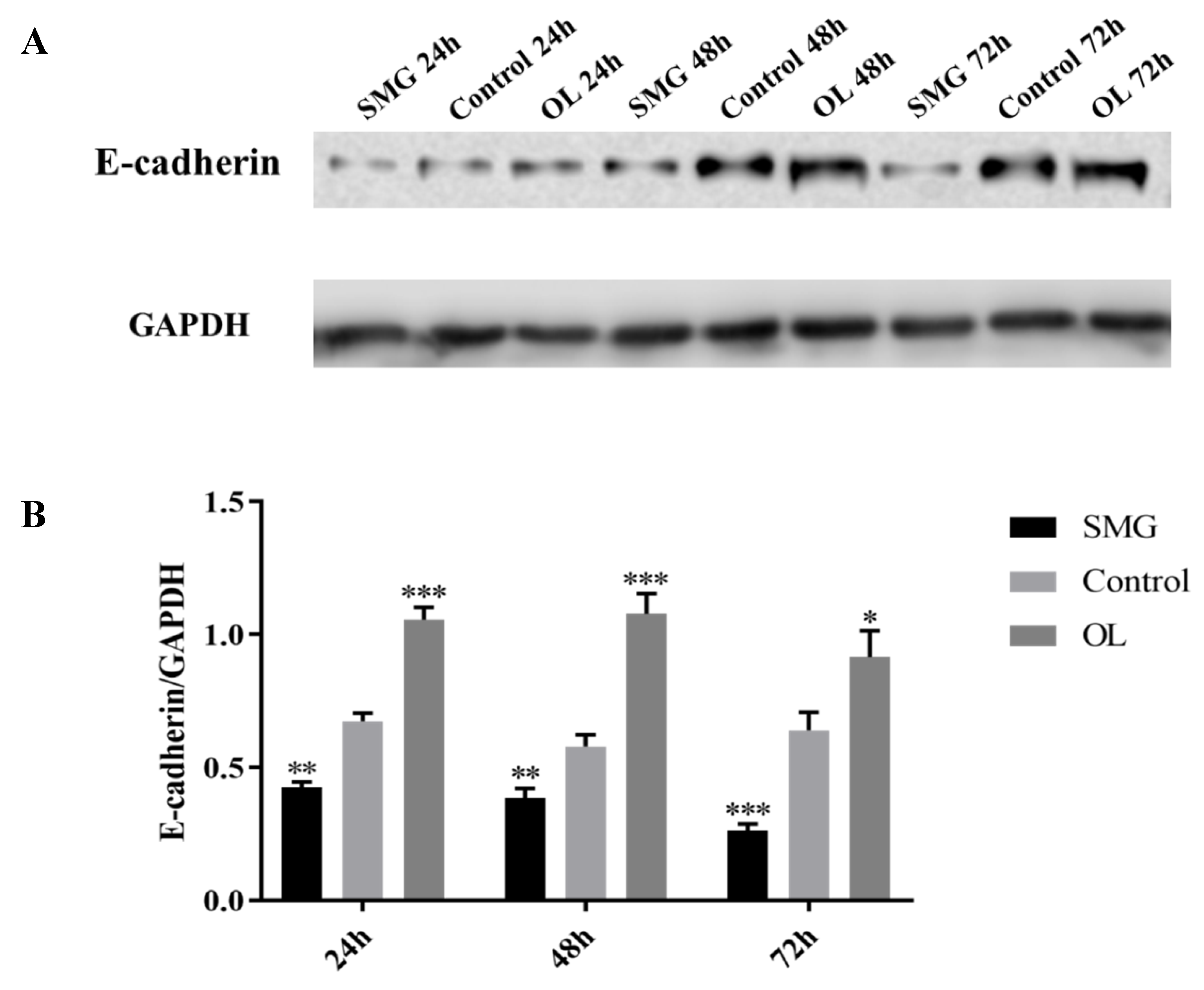
2.3. Quantitative Analysis of Adhesion Proteins by Western Blot
2.3.1. Integrin β1 Expression Analyzed by Western Blot
2.3.2. Paxillin Expression Analyzed by Western Blot
2.3.3. E-Cadherin Expression Analyzed by Western Blot
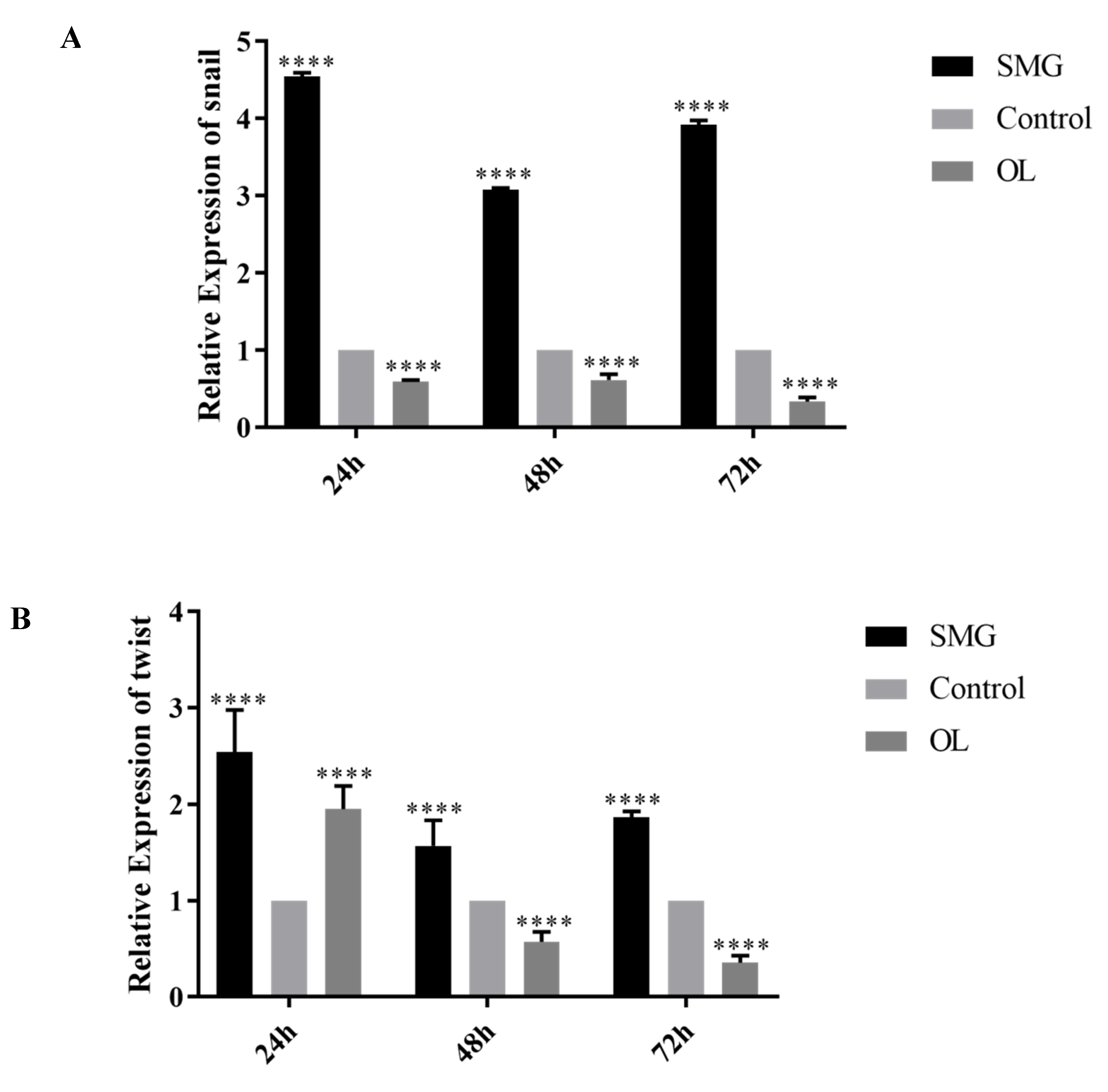
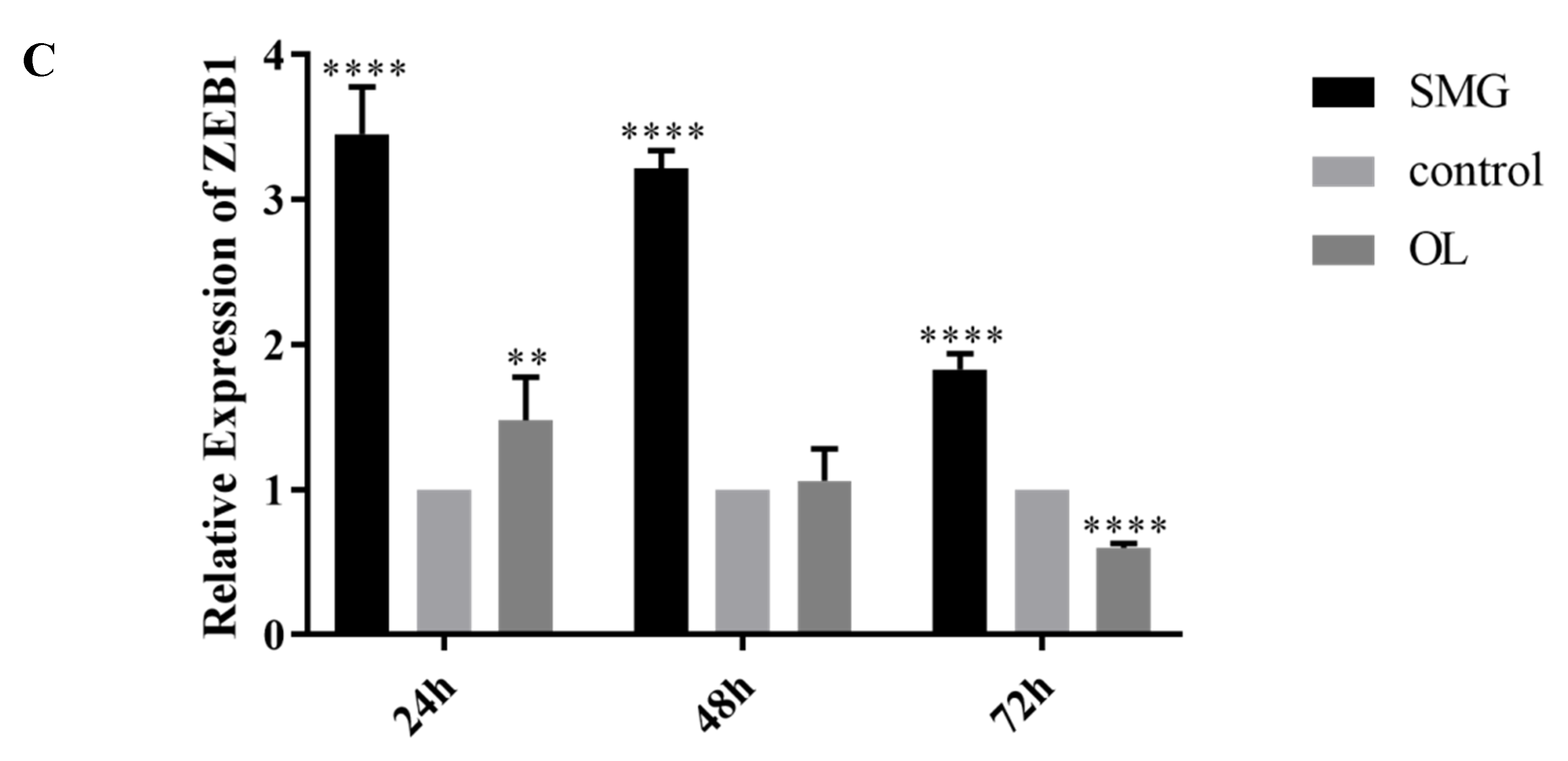
2.4. Snail, Twist and ZEB1 Expression Analyzed by qRT-PCR
3. Discussion
4. Material and Methods
4.1. Cultures of HUVEC and MCF-7 Cells
4.2. Cell Culture in Rotating Wall Vessel Bioreactor
4.3. Analysis of Cell Adhesion by Trypsin Digestion and Cell Counting
4.4. Immunofluorescence Analysis
4.5. Analysis of Cell Adhesion Proteins Expression by Western Blot
4.6. Total RNA Extraction and Real-Time PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SMG | simulated microgravity effect |
| OL | overloading |
| ECM | extracellular matrix |
| kDA | kilodalton |
| DAPI | 4′,6-diamidine-2-phenylindol |
| RWVS | Rotating Wall Vessel |
| PFA | paraformaldehyde |
| PBS | phosphate buffer saline |
| RIPA | Radio Immunoprecipitation Assay |
| HRP | Horseradish Peroxidase |
| HUVEC | Human Umbilical Vein Endothelial Cells |
| MCF-7 | Michigan Cancer Foundation - 7 |
References
- Oganov, V.S.; Bogomolov, V.V. Human bone system in microgravity: Review of research data, hypotheses and predictability of musculoskeletal system state in extended (exploration) missions. Aviakosm Ekolog Med. 2009, 43, 3–12. [Google Scholar]
- Lacquaniti, F.; Bosco, G.; Gravano, S.; Indovina, I.; La Scaleia, B.; Maffei, V.; Zago, M. Gravity in the Brain as a Reference for Space and Time Perception. Multisens. Res. 2015, 28, 397–426. [Google Scholar] [CrossRef] [PubMed]
- Blaber, E.; Marçal, H.; Burns, B.P. Bioastronautics: The Influence of Microgravity on Astronaut Health. Astrobiology 2010, 10, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Wade, C.E. Responses across the gravity continuum: Hypergravity to microgravity. Adv. Space Biol. Med. 2005, 10, 225–245. [Google Scholar] [PubMed]
- Ding, B.; Wang, G.; Zhang, X.; Yue, M.; Zeng, Y.; Tan, Y. Effects of simulated weightlessness and overweight on the growth of osteoblast cultured in vitro. Space Med. Med. Eng. (Beijing) 1997, 10, 104–107. [Google Scholar] [PubMed]
- Wei, L.; Diao, Y.; Qi, J.; Khokhlov, A.; Feng, H.; Yan, X.; Li, Y. Effect of Change in Spindle Structure on Proliferation Inhibition of Osteosarcoma Cells and Osteoblast under Simulated Microgravity during Incubation in Rotating Bioreactor. PLoS ONE 2013, 8, e76710. [Google Scholar] [CrossRef]
- Jiang, J.X.; Siller-Jackson, A.J.; Burra, S. Roles of gap junctions and hemichannels in bone cell functions and in signal transmission of mechanical stress. Front. Biosci. 2007, 12, 1450–1462. [Google Scholar] [CrossRef]
- Buytaert, K.; MacDougall, H.; Moore, S.; Clement, G.; Pattyn, N.; Migeotte, P.-F.; Wuyts, F. Validation of centrifugation as a countermeasure for otolith deconditioning during spaceflight: Preliminary data of the ESA SPIN study. J. Vestib. Res. 2013, 23, 23–31. [Google Scholar] [CrossRef]
- Park, S.H.; Sim, W.Y.; Park, S.W.; Yang, S.S.; Choi, B.H.; Park, S.R.; Park, K.; Min, B.H. An electromagnetic compressive force by cell exciter stimulates chondrogenic differentiation of bone marrow-derived mesenchymal stem cells. Tissue Eng. 2006, 12, 3107–3117. [Google Scholar] [CrossRef]
- Pua, H.H.; Dzhagalov, I.; Chuck, M.; Mizushima, N.; He, Y.-W. A critical role for the autophagy gene Atg5 in T cell survival and proliferation. J. Exp. Med. 2007, 204, 25–31. [Google Scholar] [CrossRef]
- Baerga, R.; Zhang, Y.; Chen, P.-H.; Goldman, S.; Jin, S.V. Targeted deletion of autophagy-related 5 (atg5) impairs adipogenesis in a cellular model and in mice. Autophagy 2009, 5, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Xiang, Y.; Wang, Y.; Baikati, K.; Cuervo, A.M.; Luu, Y.K.; Tang, Y.; Pessin, J.E.; Schwartz, G.J.; Czaja, M.J. Autophagy regulates adipose mass and differentiation in mice. J. Clin. Investig. 2009, 119, 3329–3339. [Google Scholar] [CrossRef] [PubMed]
- Na, S.; Trache, A.; Trzeciakowski, J.; Sun, Z.; Meininger, G.A.; Humphrey, J.D. Time-dependent Changes in Smooth Muscle Cell Stiffness and Focal Adhesion Area in Response to Cyclic Equibiaxial Stretch. Ann. Biomed. Eng. 2008, 36, 369–380. [Google Scholar] [CrossRef]
- Jeanes, A.I.; Wang, P.; Moreno-Layseca, P.; Paul, N.; Cheung, J.; Tsang, R.; Akhtar, N.; Foster, F.M.; Brennan, K.; Streuli, C.H. Specific beta-containing integrins exert differential control on proliferation and two-dimensional collective cell migration in mammary epithelial cells. J. Biol. Chem. 2012, 287, 24103–24112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Loyd, M.R.; Randall, M.S.; Waddell, M.B.; Kriwacki, R.W.; Ney, P.A. A short linear motif in BNIP3L (NIX) mediates mitochondrial clearance in reticulocytes. Autophagy 2012, 8, 1325–1332. [Google Scholar] [CrossRef]
- Xu, J.K.; Chen, H.J.; Li, X.D.; Huang, Z.L.; Xu, H.; Yang, H.L.; Hu, J. Optimal intensity shock wave promotes the adhesion and migration of rat osteoblasts via integrin beta1-mediated expression of phosphorylated focal adhesion kinase. J. Biol. Chem. 2012, 287, 26200–26212. [Google Scholar] [CrossRef]
- Perez-Moreno, M.; Jamora, C.; Fuchs, E. Sticky business: Orchestrating cellular signals at adherens junctions. Cell 2003, 112, 535–548. [Google Scholar] [CrossRef]
- Jimbo, K.; Tsuda, H.; Yoshida, M.; Miyagi-Maeshima, A.; Sasaki-Katsurada, Y.; Asaga, S.; Hojo, T.; Kitagawa, Y.; Kinoshita, T. Mucinous breast carcinoma with a lobular neoplasia component: A subset with aberrant expression of cell adhesion and polarity molecules and lack of neuroendocrine differentiation. Pathol. Int. 2014, 64, 217–223. [Google Scholar] [CrossRef]
- Bassaganas, S.; Carvalho, S.; Dias, A.M.; Perez-Garay, M.; Ortiz, M.R.; Figueras, J.; Reis, C.A.; Pinho, S.S.; Peracaula, R. Pancreatic cancer cell glycosylation regulates cell adhesion and invasion through the modulation of alpha2beta1 integrin and E-cadherin function. PLoS ONE 2014, 9, e98595. [Google Scholar] [CrossRef]
- Zhu, Y.-T.; Chen, H.-C.; Chen, S.-Y.; Tseng, S.C.G. Nuclear p120 catenin unlocks mitotic block of contact-inhibited human corneal endothelial monolayers without disrupting adherent junctions. J. Cell Sci. 2012, 125 Pt 15, 3636–3648. [Google Scholar] [CrossRef]
- Yeaman, C.; Grindstaff, K.K.; Nelson, W.J. Mechanism of recruiting Sec6/8 (exocyst) complex to the apical junctional complex during polarization of epithelial cells. J. Cell Sci. 2004, 117 Pt 4, 559–570. [Google Scholar] [CrossRef]
- Lindley, L.E.; Briegel, K.J. Molecular characterization of TGFbeta-induced epithelial-mesenchymal transition in normal finite lifespan human mammary epithelial cells. Biochem. Biophys. Res. Commun. 2010, 399, 659–664. [Google Scholar] [CrossRef]
- Christoph, B.; Jayashree, S.; Thomas, J.; Daniela, M.; Johann, B.; Markus, W.; Marcus, K.; Silke, B.; Nauras, A. Manfred infanger and Daniela Grimm. Morphological and Molecular changes in Juvenile normal Human fibroblasts exposed to Simulated Microgravity. Sci. Rep. 2019, 9, 11882. [Google Scholar]
- Tan, X.; Xu, A.; Zhao, T.; Zhao, Q.; Zhang, J.; Fan, C.; Deng, Y.; Freywald, A.; Genth, H.; Xiang, J. Simulated microgravity inhibits cell focal adhesions leading to reduced melanoma cell proliferation and metastasis via FAK/RhoA-regulated mTORC1 and AMPK pathways. Sci. Rep. 2018, 8, 3769. [Google Scholar] [CrossRef]
- Sahana, J.; Nassef, M.Z.; Wehland, M.; Kopp, S.; Krüger, M.; Corydon, T.J.; Infanger, M.; Bauer, J.; Grimm, D. E-Cadherin in MCF7 Human Breast Cancer Cells Forming Multicellular Spheroids Exposed to Simulated Microgravity. Proteomics 2018, 18, 1800015. [Google Scholar] [CrossRef] [PubMed]
- Nassef, M.Z.; Kopp, S.; Melnik, D.; Corydon, T.J.; Sahana, J.; Krüger, M.; Wehland, M.; Bauer, T.J.; Liemersdorf, C.; Hemmersbach, R.; et al. Short-Term Microgravity Influences Cell Adhesion in Human Breast Cancer Cells. Int. J. Mol. Sci. 2019, 20, 5730. [Google Scholar] [CrossRef] [PubMed]
- Plosa Erin, J.; Benjamin John, T.; Sucre Jennifer, M.; Gulleman Peter, M.; Gleaves Linda, A.; Wei, H.; Kook, S.; Polosukhin Vasiliy, V.; Haake Scott, M.; Guttentag Susan, H.; et al. β1 integrin regulates adult lung alveolar epithelial cell inflammation. JCI Insight. 2019. [Google Scholar] [CrossRef]
- Zanatta, G.; Rudisile, M.; Camassola, M.; Wendorff, J.; Nardi, N.; Gottfried, C.; Pranke, P.; Netto, C.A. Mesenchymal stem cell adherence on poly(D, L-lactide-co-glycolide) nanofibers scaffold is integrin-beta 1 receptor dependent. J. Biomed. Nanotechnol 2012, 8, 211–218. [Google Scholar] [CrossRef]
- Wu, C.-C.; Chao, Y.-C.; Chen, C.-N.; Chien, S.; Chen, Y.-C.; Chien, C.-C.; Chiu, J.-J.; Yen, B.L. Synergism of biochemical and mechanical stimuli in the differentiation of human placenta-derived multipotent cells into endothelial cells. J. Biomech. 2008, 41, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Hayden, J.A.; Pantalos, G.M.; Burgess, J.E.; Antaki, J.F. A Hermetically Sealed, Fluid-Filled Surgical Enclosure for Microgravity. Aviat. Space Environ. Med. 2013, 84, 1298–1303. [Google Scholar] [CrossRef]
- Schaller, M.D. Paxillin: A focal adhesion-associated adaptor protein. Oncogene 2001, 20, 6459–6472. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, K.; Ikenouchi, J.; Izumi, Y.; Akashi, M.; Tsukita, S.; Furuse, M. Phosphorylation state regulates the localization of Scribble at adherens junctions and its association with E-cadherin–catenin complexes. Exp. Cell Res. 2011, 317, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Takeichi, M. Adherens Junction: Molecular Architecture and Regulation. Cold Spring Harb. Perspect. Boil. 2009, 1, a002899. [Google Scholar] [CrossRef] [PubMed]
- Le Bras, G.F.; Taubenslag, K.J.; Andl, C.D. The regulation of cell-cell adhesion during epithelial-mesenchymal transition, motility and tumor progression. Cell Adhes. Migr. 2012, 6, 365–373. [Google Scholar] [CrossRef]
- Antonio Garcia De, H.; Sandra, P.; Mayssaa, N.; Pierre, S. Snail family regulation and epithelial mesenchymal transitions in breast cancer progression. J. Mammary Gland Biol. Neoplasia 2010, 15, 135–147. [Google Scholar]
- O’Connor, J.W.; Mistry, K.; Detweiler, D.; Wang, C.; Gomez, E.W. Cell-cell contact and matrix adhesion promote αSMA expression during TGFβ1-induced epithelial-myofibroblast transition via Notch and MRTF-A. Sci. Rep. 2016, 6, 26226. [Google Scholar] [CrossRef]
- Ratushnyy, A.Y.; Buravkova, L.B. Expression of focal adhesion genes in mesenchymal stem cells under simulated microgravity. Biochem. Biophys. Mol. Biol. 2017, 477, 354–356. [Google Scholar] [CrossRef]
- Tan, Y.J.; Yu, J.R.; Nie, J.L.; Wang, C.Y.; Yang, F.; Ding, B.; Gu, Y.; Qu, L.N.; Wan, Y.M.; Li, Y.H. Development of twodirection multi-sample cell experimental device for microgravity effects simulation. Space Med. Med. Eng. 2011, 1, 13–16. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, S.; Li, Q.; Cao, Q.; Diao, Y.; Zhang, Y.; Yue, L.; Wei, L. EMT Transcription Factors Are Involved in the Altered Cell Adhesion under Simulated Microgravity Effect or Overloading by Regulation of E-cadherin. Int. J. Mol. Sci. 2020, 21, 1349. https://doi.org/10.3390/ijms21041349
Shi S, Li Q, Cao Q, Diao Y, Zhang Y, Yue L, Wei L. EMT Transcription Factors Are Involved in the Altered Cell Adhesion under Simulated Microgravity Effect or Overloading by Regulation of E-cadherin. International Journal of Molecular Sciences. 2020; 21(4):1349. https://doi.org/10.3390/ijms21041349
Chicago/Turabian StyleShi, Shuliang, Qiao Li, Qiuying Cao, Yan Diao, Yao Zhang, Lei Yue, and Lijun Wei. 2020. "EMT Transcription Factors Are Involved in the Altered Cell Adhesion under Simulated Microgravity Effect or Overloading by Regulation of E-cadherin" International Journal of Molecular Sciences 21, no. 4: 1349. https://doi.org/10.3390/ijms21041349
APA StyleShi, S., Li, Q., Cao, Q., Diao, Y., Zhang, Y., Yue, L., & Wei, L. (2020). EMT Transcription Factors Are Involved in the Altered Cell Adhesion under Simulated Microgravity Effect or Overloading by Regulation of E-cadherin. International Journal of Molecular Sciences, 21(4), 1349. https://doi.org/10.3390/ijms21041349