Group 2 Innate Lymphoid Cells: Central Players in a Recurring Theme of Repair and Regeneration
Abstract
1. ILCs: A Brief Overview
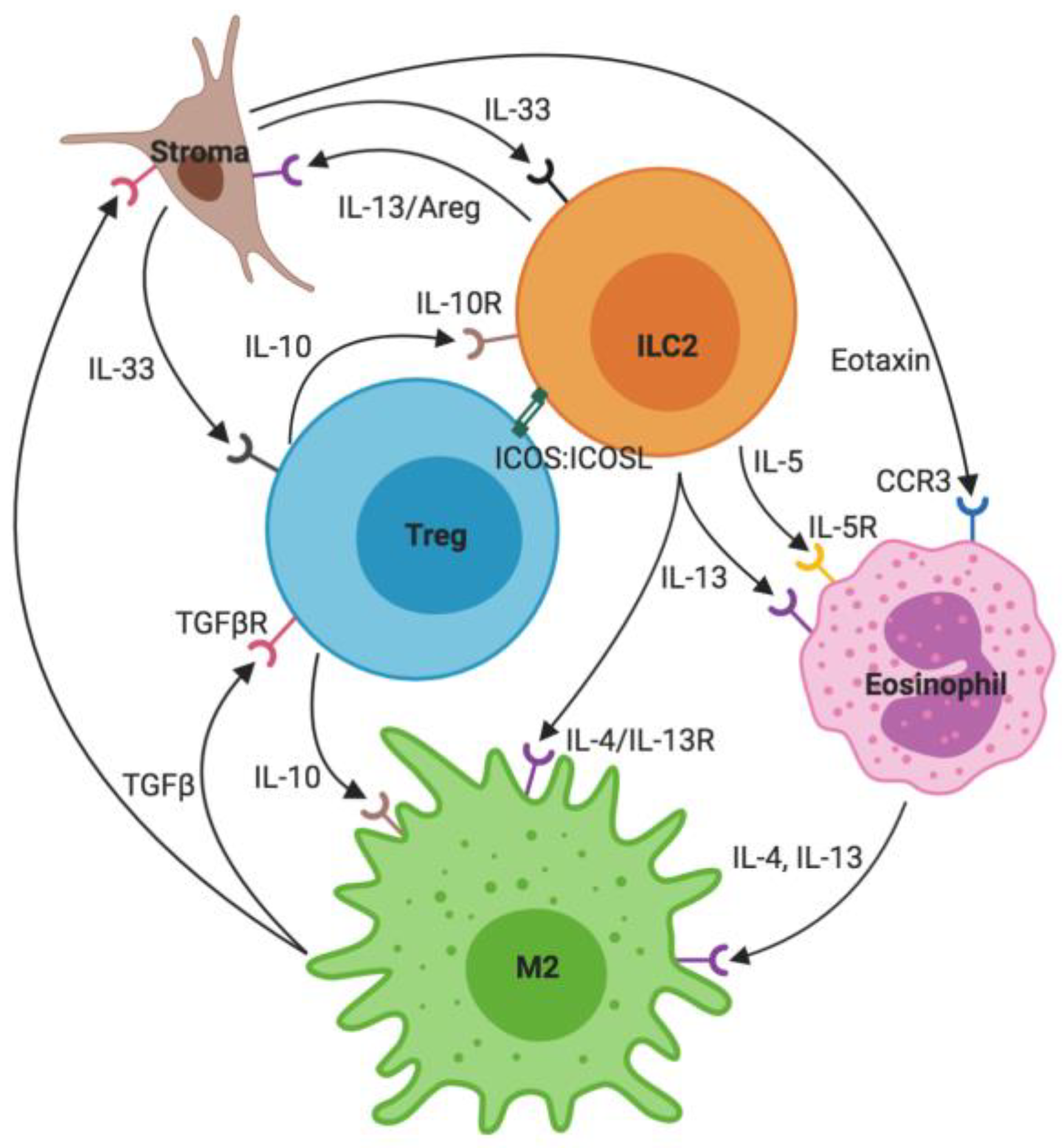
2. ILC2s: Key Interactions
2.1. Stroma and IL-33
2.2. Eosinophils
2.3. M2 Macrophages
2.4. T Regulatory Cells
3. ILC2s and Type-2 Immunity: Same Players, Different Settings
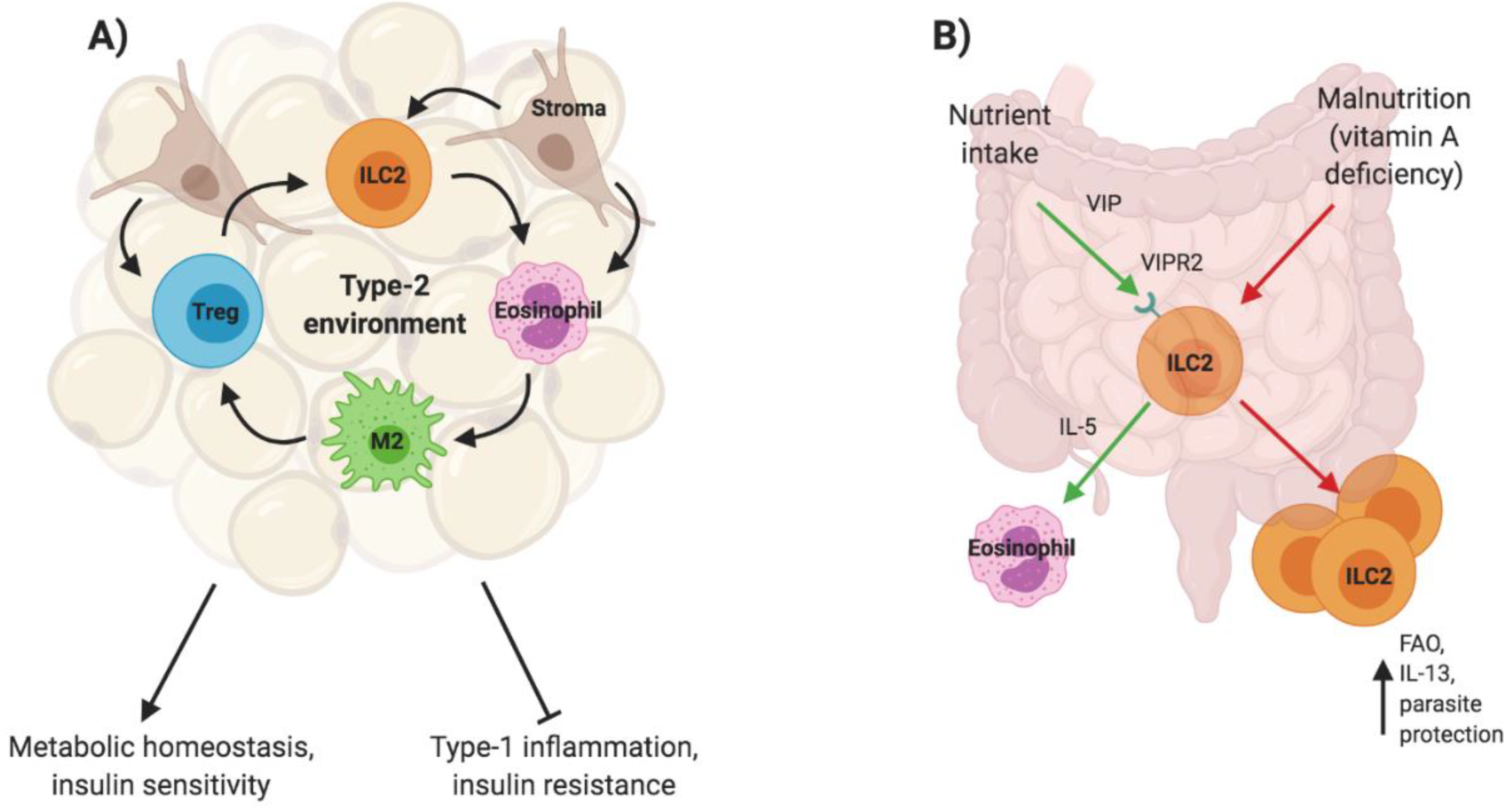
3.1. ILC2s in Metabolic Homeostasis
3.2. ILC2s in Neonatal Immunity
3.3. ILC2s in Allergic Lung Inflammation
3.4. ILC2s in tissue fibrosis
3.4.1. Lung Fibrosis
3.4.2. Liver Fibrosis
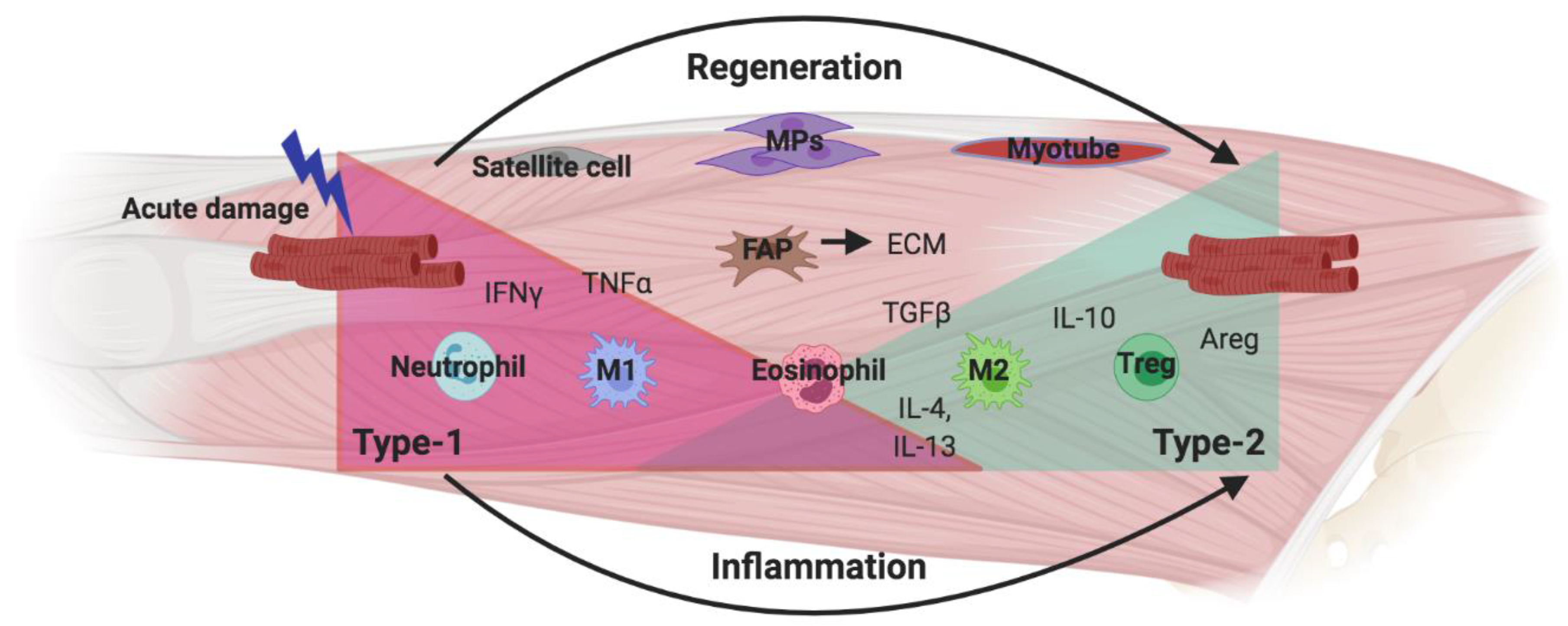
3.5. New Avenues: ILC2s in Tissue Regeneration
4. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Spits, H.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate Lymphoid Cells-a Proposal for Uniform Nomenclature. Nat. Rev. Immunol. 2013, 13, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, A. ILC1s in Tissue Inflammation and Infection. Front. Immunol. 2016, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Mesa, N.; Schroeder, J.H.; Stolarczyk, E.; Gallagher, A.L.; Lo, J.W.; Bailey, C.; Campbell, L.; Sexl, V.; MacDonald, T.T.; Howard, J.K.; et al. T-Bet Controls Intestinal Mucosa Immune Responses via Repression of Type 2 Innate Lymphoid Cell Function. Mucosal Immunol. 2019, 12, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Licona-Limón, P.; Kim, L.K.; Palm, N.W.; Flavell, R.A. TH2, Allergy and Group 2 Innate Lymphoid Cells. Nat. Immunol. 2013, 14, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Yagi, R.; Zhong, C.; Northrup, D.L.; Yu, F.; Bouladoux, N.; Spencer, S.; Hu, G.; Barron, L.; Sharma, S.; Nakayama, T.; et al. The Transcription Factor GATA3 Is Critical for the Development of All IL-7Rα-Expressing Innate Lymphoid Cells. Immunity 2014, 40, 378–388. [Google Scholar] [CrossRef]
- Monticelli, L.A.; Sonnenberg, G.F.; Abt, M.C.; Alenghat, T.; Ziegler, C.G.K.; Doering, T.A.; Angelosanto, J.M.; Laidlaw, B.J.; Yang, C.Y.; Sathaliyawala, T.; et al. Innate Lymphoid Cells Promote Lung-Tissue Homeostasis after Infection with Influenza Virus. Nat. Immunol. 2011, 12, 1045–1054. [Google Scholar] [CrossRef]
- Monticelli, L.A.; Osborne, L.C.; Noti, M.; Tran, S.V.; Zaiss, D.M.W.; Artis, D. IL-33 Promotes an Innate Immune Pathway of Intestinal Tissue Protection Dependent on Amphiregulin-EGFR Interactions. Proc. Natl. Acad. Sci. USA 2015, 112, 10762–10767. [Google Scholar] [CrossRef]
- Montaldo, E.; Juelke, K.; Romagnani, C. Group 3 Innate Lymphoid Cells (ILC3s): Origin, Differentiation, and Plasticity in Humans and Mice. Eur. J. Immunol. 2015, 45, 2171–2182. [Google Scholar] [CrossRef]
- Sanos, S.L.; Bui, V.L.; Mortha, A.; Oberle, K.; Heners, C.; Johner, C.; Diefenbach, A. RORγt and Commensal Microflora Are Required for the Differentiation of Mucosal Interleukin 22-Producing NKp46+ Cells. Nat. Immunol. 2009, 10, 83–91. [Google Scholar] [CrossRef]
- Lo, B.C.; Gold, M.J.; Hughes, M.R.; Antignano, F.; Valdez, Y.; Zaph, C.; Harder, K.W.; McNagny, K.M. The Orphan Nuclear Receptor RORa and Group 3 Innate Lymphoid Cells Drive Fibrosis in a Mouse Model of Crohn’s Disease. Sci. Immunol. 2016, 1. [Google Scholar] [CrossRef]
- Song, C.; Lee, J.S.; Gilfillan, S.; Robinette, M.L.; Newberry, R.D.; Stappenbeck, T.S.; Mack, M.; Cella, M.; Colonna, M. Unique and Redundant Functions of NKp46+ ILC3s in Models of Intestinal Inflammation. J. Exp. Med. 2015, 212, 1869–1882. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.D.; Arora, J.; Diehl, K.; Bora, S.A.; Cantorna, M.T. Vitamin D Is Required for ILC3 Derived IL-22 and Protection from Citrobacter Rodentium Infection. Front. Immunol. 2019, 10, 1. [Google Scholar] [CrossRef]
- Ciccia, F.; Guggino, G.; Rizzo, A.; Saieva, L.; Peralta, S.; Giardina, A.; Cannizzaro, A.; Sireci, G.; De Leo, G.; Alessandro, R.; et al. Type 3 Innate Lymphoid Cells Producing IL-17 and IL-22 Are Expanded in the Gut, in the Peripheral Blood, Synovial Fluid and Bone Marrow of Patients with Ankylosing Spondylitis. Ann. Rheum. Dis. 2015, 74, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Lee, H.J.; Chang, Y.J.; Pichavant, M.; Shore, S.A.; Fitzgerald, K.A.; Iwakura, Y.; Israel, E.; Bolger, K.; Faul, J.; et al. Interleukin-17-Producing Innate Lymphoid Cells and the NLRP3 Inflammasome Facilitate Obesity-Associated Airway Hyperreactivity. Nat. Med. 2014, 20, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Ebbo, M.; Crinier, A.; Vély, F.; Vivier, E. Innate Lymphoid Cells: Major Players in Inflammatory Diseases. Nat. Rev. Immunol. 2017, 17, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Salimi, M.; Panse, I.; Mjösberg, J.M.; McKenzie, A.N.J.; Spits, H.; Klenerman, P.; Ogg, G. Prostaglandin D 2 Activates Group 2 Innate Lymphoid Cells through Chemoattractant Receptor-Homologous Molecule Expressed on T H 2 Cells. J. Allergy Clin. Immunol. 2014, 133, 1184–1194. [Google Scholar] [CrossRef]
- Duerr, C.U.; Mccarthy, C.D.A.; Mindt, B.C.; Rubio, M.; Meli, A.P.; Pothlichet, J.; Eva, M.M.; Gauchat, J.F.; Qureshi, S.T.; Mazer, B.D.; et al. Type I Interferon Restricts Type 2 Immunopathology through the Regulation of Group 2 Innate Lymphoid Cells. Nat. Immunol. 2016, 17, 65–75. [Google Scholar] [CrossRef]
- Roozendaal, R.; Mebius, R.E. Stromal Cell–Immune Cell Interactions. Annu. Rev. Immunol. 2011, 29, 23–43. [Google Scholar] [CrossRef]
- Pichery, M.; Mirey, E.; Mercier, P.; Lefrancais, E.; Dujardin, A.; Ortega, N.; Girard, J.-P. Endogenous IL-33 Is Highly Expressed in Mouse Epithelial Barrier Tissues, Lymphoid Organs, Brain, Embryos, and Inflamed Tissues: In Situ Analysis Using a Novel Il-33-LacZ Gene Trap Reporter Strain. J. Immunol. 2012, 188, 3488–3495. [Google Scholar] [CrossRef]
- Cayrol, C.; Girard, J.P. Interleukin-33 (IL-33): A Nuclear Cytokine from the IL-1 Family. Immunol. Rev. 2018, 281, 154–168. [Google Scholar] [CrossRef]
- Molofsky, A.B.; Savage, A.K.; Locksley, R.M. Interleukin-33 in Tissue Homeostasis, Injury, and Inflammation. Immunity. 2015, 42, 1005–1019. [Google Scholar] [CrossRef] [PubMed]
- Waddell, A.; Vallance, J.E.; Hummel, A.; Alenghat, T.; Rosen, M.J. IL-33 Induces Murine Intestinal Goblet Cell Differentiation Indirectly via Innate Lymphoid Cell IL-13 Secretion. J. Immunol. 2019, 202, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Von Moltke, J.; Ji, M.; Liang, H.E.; Locksley, R.M. Tuft-Cell-Derived IL-25 Regulates an Intestinal ILC2-Epithelial Response Circuit. Nature 2016, 529, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Brussino, L.; Heffler, E.; Bucca, C.; Nicola, S.; Rolla, G. Eosinophils Target Therapy for Severe Asthma: Critical Points. Biomed Res. Int. 2018, 2018, 7582057. [Google Scholar] [CrossRef] [PubMed]
- Pope, S.M.; Brandt, E.B.; Mishra, A.; Hogan, S.P.; Zimmermann, N.; Rothenberg, M.E.; Pope, S.M.; Hogan, S.P.; Matthaei, K.I.; Foster, P.S. IL-13 Induces Eosinophil Recruitment into the Lung by an IL-5- and Eotaxin-Dependent Mechanism. J. Allergy Clin. Immunol. 2001, 108, 594–601. [Google Scholar] [CrossRef]
- Specht, S.; Saeftel, M.; Arndt, M.; Endl, E.; Dubben, B.; Lee, N.A.; Lee, J.J.; Hoerauf, A. Lack of Eosinophil Peroxidase or Major Basic Protein Impairs Defense against Murine Filarial Infection. Infect. Immun. 2006, 74, 5236–5243. [Google Scholar] [CrossRef]
- Nussbaum, J.C.; Van Dyken, S.J.; Von Moltke, J.; Cheng, L.E.; Mohapatra, A.; Molofsky, A.B.; Thornton, E.E.; Krummel, M.F.; Chawla, A.; Liang, H.E.; et al. Type 2 Innate Lymphoid Cells Control Eosinophil Homeostasis. Nature 2013, 502, 245–248. [Google Scholar] [CrossRef]
- Wong, T.W.; Doyle, A.D.; Lee, J.J.; Jelinek, D.F. Eosinophils Regulate Peripheral B Cell Numbers in Both Mice and Humans. J. Immunol. 2014, 192, 3548–3558. [Google Scholar] [CrossRef]
- Van Dyken, S.J.; Locksley, R.M. Interleukin-4- and Interleukin-13-Mediated Alternatively Activated Macrophages: Roles in Homeostasis and Disease. Annu. Rev. Immunol. 2013, 31, 317–343. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450. [Google Scholar] [CrossRef]
- Wu, D.; Molofsky, A.B.; Liang, H.E.; Ricardo-Gonzalez, R.R.; Jouihan, H.A.; Bando, J.K.; Chawla, A.; Locksley, R.M. Eosinophils Sustain Adipose Alternatively Activated Macrophages Associated with Glucose Homeostasis. Science 2011, 332, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 Programs the Development and Function of CD4+CD25+ Regulatory T Cells. J. Immunol. 2017, 198, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Josefowicz, S.Z.; Niec, R.E.; Kim, H.Y.; Treuting, P.; Chinen, T.; Zheng, Y.; Umetsu, D.T.; Rudensky, A.Y. Extrathymically Generated Regulatory T Cells Control Mucosal T H 2 Inflammation. Nature 2012, 482, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Rigas, D.; Lewis, G.; Aron, J.L.; Wang, B.; Banie, H.; Sankaranarayanan, I.; Galle-Treger, L.; Maazi, H.; Lo, R.; Freeman, G.J.; et al. Type 2 Innate Lymphoid Cell Suppression by Regulatory T Cells Attenuates Airway Hyperreactivity and Requires Inducible T-Cell Costimulator–Inducible T-Cell Costimulator Ligand Interaction. J. Allergy Clin. Immunol. 2017, 139, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Akbari, O.; Freeman, G.J.; Meyer, E.H.; Greenfield, E.A.; Chang, T.T.; Sharpe, A.H.; Berry, G.; Dekruyff, R.H.; Umetsu, D.T. Antigen-Specific Regulatory T Cells Develop via the ICOS-ICOS-Ligand Pathway and Inhibit Allergen-Induced Airway Hyperreactivity. Nat. Med. 2002, 8, 1024–1032. [Google Scholar] [CrossRef]
- Roediger, B.; Kyle, R.; Tay, S.S.; Mitchell, A.J.; Bolton, H.A.; Guy, T.V.; Tan, S.-Y.; Forbes-Blom, E.; Tong, P.L.; Shklovskaya, E.; et al. IL-2 Is a Critical Regulator of Group 2 Innate Lymphoid Cell Function during Pulmonary Inflammation. J. Allergy Clin. Immunol. 2015, 136, 1653–1663. [Google Scholar] [CrossRef]
- Han, J.M.; Wu, D.; Denroche, H.C.; Yao, Y.; Verchere, C.B.; Levings, M.K. IL-33 Reverses an Obesity-Induced Deficit in Visceral Adipose Tissue ST2+ T Regulatory Cells and Ameliorates Adipose Tissue Inflammation and Insulin Resistance. J. Immunol. 2015, 194, 4777–4783. [Google Scholar] [CrossRef]
- Schiering, C.; Krausgruber, T.; Chomka, A.; Fröhlich, A.; Adelmann, K.; Wohlfert, E.A.; Pott, J.; Griseri, T.; Bollrath, J.; Hegazy, A.N.; et al. The Alarmin IL-33 Promotes Regulatory T-Cell Function in the Intestine. Nature 2014, 513, 564–568. [Google Scholar] [CrossRef]
- Rana, B.M.J.; Jou, E.; Barlow, J.L.; Rodriguez-Rodriguez, N.; Walker, J.A.; Knox, C.; Jolin, H.E.; Hardman, C.S.; Sivasubramaniam, M.; Szeto, A.; et al. A Stromal Cell Niche Sustains ILC2-Mediated Type-2 Conditioning in Adipose Tissue. J. Exp. Med. 2019, 216, 1999–2009. [Google Scholar] [CrossRef]
- Molofsky, A.B.; Nussbaum, J.C.; Liang, H.E.; Dyken, S.J.V.; Cheng, L.E.; Mohapatra, A.; Chawla, A.; Locksley, R.M. Innate Lymphoid Type 2 Cells Sustain Visceral Adipose Tissue Eosinophils and Alternatively Activated Macrophages. J. Exp. Med. 2013, 210, 535–549. [Google Scholar] [CrossRef]
- Brestoff, J.R.; Kim, B.S.; Saenz, S.A.; Stine, R.R.; Monticelli, L.A.; Sonnenberg, G.F.; Thome, J.J.; Farber, D.L.; Lutfy, K.; Seale, P.; et al. Group 2 Innate Lymphoid Cells Promote Beiging of White Adipose Tissue and Limit Obesity. Nature 2015, 519, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Odegaard, J.I.; Ricardo-Gonzalez, R.R.; Goforth, M.H.; Morel, C.R.; Subramanian, V.; Mukundan, L.; Eagle, A.R.; Vats, D.; Brombacher, F.; Ferrante, A.W.; et al. Macrophage-Specific PPARγ Controls Alternative Activation and Improves Insulin Resistance. Nature 2007, 447, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Cipolletta, D.; Feuerer, M.; Li, A.; Kamei, N.; Lee, J.; Shoelson, S.E.; Benoist, C.; Mathis, D. PPAR-γ Is a Major Driver of the Accumulation and Phenotype of Adipose Tissue Treg Cells. Nature 2012, 486, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Spencer, S.P.; Wilhelm, C.; Yang, Q.; Hall, J.A.; Bouladoux, N.; Boyd, A.; Nutman, T.B.; Urban, J.F.; Wang, J.; Ramalingam, T.R.; et al. Adaptation of Innate Lymphoid Cells to a Micronutrient Deficiency Promotes Type 2 Barrier Immunity. Science 2014, 343, 432–437. [Google Scholar] [CrossRef]
- Wilhelm, C.; Harrison, O.J.; Schmitt, V.; Pelletier, M.; Spencer, S.P.; Urban, J.F.; Ploch, M.; Ramalingam, T.R.; Siegel, R.M.; Belkaid, Y. Critical Role of Fatty Acid Metabolism in ILC2-Mediated Barrier Protection during Malnutrition and Helminth Infection. J. Exp. Med. 2016, 213, 1409–1418. [Google Scholar] [CrossRef]
- Mohr, E.; Siegrist, C.-A. Vaccination in Early Life: Standing up to the Challenges. Curr. Opin. Immunol. 2016, 41, 1–8. [Google Scholar] [CrossRef]
- Steer, C.A.; Martinez-Gonzalez, I.; Ghaedi, M.; Allinger, P.; Mathä, L.; Takei, F. Group 2 Innate Lymphoid Cell Activation in the Neonatal Lung Drives Type 2 Immunity and Allergen Sensitization. J. Allergy Clin. Immunol. 2017, 140, 593–595. [Google Scholar] [CrossRef]
- de Kleer, I.M.; Kool, M.; de Bruijn, M.J.W.; Willart, M.; van Moorleghem, J.; Schuijs, M.J.; Plantinga, M.; Beyaert, R.; Hams, E.; Fallon, P.G.; et al. Perinatal Activation of the Interleukin-33 Pathway Promotes Type 2 Immunity in the Developing Lung. Immunity 2016, 45, 1285–1298. [Google Scholar] [CrossRef]
- Halim, T.Y.F.; MacLaren, A.; Romanish, M.T.; Gold, M.J.; McNagny, K.M.; Takei, F. Retinoic-Acid-Receptor-Related Orphan Nuclear Receptor Alpha Is Required for Natural Helper Cell Development and Allergic Inflammation. Immunity 2012, 37, 463–474. [Google Scholar] [CrossRef]
- Kariyawasam, H.H.; Robinson, D.S. The Role of Eosinophils in Airway Tissue Remodelling in Asthma. Curr. Opin.Immunol. 2007, 19, 681–686. [Google Scholar] [CrossRef]
- Saluzzo, S.; Gorki, A.D.; Rana, B.M.J.; Martins, R.; Scanlon, S.; Starkl, P.; Lakovits, K.; Hladik, A.; Korosec, A.; Sharif, O.; et al. First-Breath-Induced Type 2 Pathways Shape the Lung Immune Environment. Cell Rep. 2017, 18, 1893–1905. [Google Scholar] [CrossRef] [PubMed]
- Gollwitzer, E.S.; Saglani, S.; Trompette, A.; Yadava, K.; Sherburn, R.; McCoy, K.D.; Nicod, L.P.; Lloyd, C.M.; Marsland, B.J. Lung Microbiota Promotes Tolerance to Allergens in Neonates via PD-L1. Nat. Med. 2014, 20, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Pawankar, R. Allergic Diseases and Asthma: A Global Public Health Concern and a Call to Action. World Allergy Organ. J. 2014, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Christianson, C.A.; Goplen, N.P.; Zafar, I.; Irvin, C.; Good, J.T.; Rollins, D.R.; Gorentla, B.; Liu, W.; Gorska, M.M.; Chu, H.W.; et al. Persistence of Asthma Requires Multiple Feedback Circuits Involving Type 2 Innate Lymphoid Cells and IL-33. J. Allergy Clin. Immunol. 2015, 136, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Bartemes, K.R.; Kephart, G.M.; Fox, S.J.; Kita, H. Enhanced Innate Type 2 Immune Response in Peripheral Blood from Patients with Asthma. J. Allergy Clin. Immunol. 2014, 134. [Google Scholar] [CrossRef]
- Smith, S.G.; Chen, R.; Kjarsgaard, M.; Huang, C.; Oliveria, J.P.; O’Byrne, P.M.; Gauvreau, G.M.; Boulet, L.P.; Lemiere, C.; Martin, J.; et al. Increased Numbers of Activated Group 2 Innate Lymphoid Cells in the Airways of Patients with Severe Asthma and Persistent Airway Eosinophilia. J. Allergy Clin. Immunol. 2016, 137, 75–86. [Google Scholar] [CrossRef]
- Halim, T.Y.F.; Krauß, R.H.; Sun, A.C.; Takei, F. Lung Natural Helper Cells Are a Critical Source of Th2 Cell-Type Cytokines in Protease Allergen-Induced Airway Inflammation. Immunity 2012, 36, 451–463. [Google Scholar] [CrossRef]
- Gold, M.J.; Antignano, F.; Halim, T.Y.F.; Hirota, J.A.; Blanchet, M.R.; Zaph, C.; Takei, F.; McNagny, K.M. Group 2 Innate Lymphoid Cells Facilitate Sensitization to Local, but Not Systemic, TH2-Inducing Allergen Exposures. J. Allergy Clin. Immunol. 2014, 133. [Google Scholar] [CrossRef]
- Halim, T.Y.F.; Steer, C.A.; Mathä, L.; Gold, M.J.; Martinez-Gonzalez, I.; McNagny, K.M.; McKenzie, A.N.J.; Takei, F. Group 2 Innate Lymphoid Cells Are Critical for the Initiation of Adaptive T Helper 2 Cell-Mediated Allergic Lung Inflammation. Immunity 2014, 40, 425–435. [Google Scholar] [CrossRef]
- Halim, T.Y.F.; Hwang, Y.Y.; Scanlon, S.T.; Zaghouani, H.; Garbi, N.; Fallon, P.G.; Mckenzie, A.N.J. Group 2 Innate Lymphoid Cells License Dendritic Cells to Potentiate Memory TH2 Cell Responses. Nat. Immunol. 2016, 17, 57–64. [Google Scholar] [CrossRef]
- Jacobsen, E.A.; LeSuer, W.E.; Nazaroff, C.D.; Ochkur, S.I.; Doyle, A.D.; Wright, B.L.; Rank, M.A.; Lee, J.J. Eosinophils Induce Recruitment and Activation of ILC2s. J. Allergy Clin. Immunol. 2019, 143, AB289. [Google Scholar] [CrossRef]
- Sugita, K.; Steer, C.A.; Martinez-Gonzalez, I.; Altunbulakli, C.; Morita, H.; Castro-Giner, F.; Kubo, T.; Wawrzyniak, P.; Rückert, B.; Sudo, K.; et al. Type 2 Innate Lymphoid Cells Disrupt Bronchial Epithelial Barrier Integrity by Targeting Tight Junctions through IL-13 in Asthmatic Patients. J. Allergy Clin. Immunol. 2018, 141, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Barlow, J.L.; Bellosi, A.; Hardman, C.S.; Drynan, L.F.; Wong, S.H.; Cruickshank, J.P.; McKenzie, A.N.J. Innate IL-13-Producing Nuocytes Arise during Allergic Lung Inflammation and Contribute to Airways Hyperreactivity. J. Allergy Clin. Immunol. 2012, 129. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, I.; Mathä, L.; Steer, C.A.; Ghaedi, M.; Poon, G.F.T.; Takei, F. Allergen-Experienced Group 2 Innate Lymphoid Cells Acquire Memory-like Properties and Enhance Allergic Lung Inflammation. Immunity 2016, 45, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Bando, J.K.; Nussbaum, J.C.; Liang, H.-E.; Locksley, R.M. Type 2 Innate Lymphoid Cells Constitutively Express Arginase-I in the Naïve and Inflamed Lung. J. Leukoc. Biol. 2013, 94, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Draijer, C.; Robbe, P.; Boorsma, C.E.; Hylkema, M.N.; Melgert, B.N. Dual Role of YM1+ M2 Macrophages in Allergic Lung Inflammation. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Krishnamoorthy, N.; Burkett, P.R.; Dalli, J.; Abdulnour, R.-E.E.; Colas, R.; Ramon, S.; Phipps, R.P.; Petasis, N.A.; Kuchroo, V.K.; Serhan, C.N.; et al. Cutting Edge: Maresin-1 Engages Regulatory T Cells To Limit Type 2 Innate Lymphoid Cell Activation and Promote Resolution of Lung Inflammation. J. Immunol. 2015, 194, 863–867. [Google Scholar] [CrossRef]
- Morita, H.; Arae, K.; Unno, H.; Miyauchi, K.; Toyama, S.; Nambu, A.; Oboki, K.; Ohno, T.; Motomura, K.; Matsuda, A.; et al. An Interleukin-33-Mast Cell-Interleukin-2 Axis Suppresses Papain-Induced Allergic Inflammation By Promoting Regulatory T Cell Numbers. Immunity 2015, 43, 175–186. [Google Scholar] [CrossRef]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of Fibrosis: Therapeutic Translation for Fibrotic Disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef]
- Collins, S.L.; Black, K.E.; Chan-Li, Y.; Ahn, Y.H.; Cole, P.A.; Powell, J.D.; Horton, M.R. Hyaluronan Fragments Promote Inflammation by Down-Regulating the Anti-Inflammatory A2a Receptor. Am. J. Respir. Cell Mol. Biol. 2011, 45, 675–683. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Fibroblast—Extracellular Matrix Interactions in Tissue Fibrosis. Curr. Pathobiol. Rep. 2016, 4, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Fichtner-Feigl, S.; Fuss, I.J.; Young, C.A.; Watanabe, T.; Geissler, E.K.; Schlitt, H.-J.; Kitani, A.; Strober, W. Induction of IL-13 Triggers TGF-β 1 -Dependent Tissue Fibrosis in Chronic 2,4,6-Trinitrobenzene Sulfonic Acid Colitis. J. Immunol. 2007, 178, 5859–5870. [Google Scholar] [CrossRef] [PubMed]
- Fichtner-Feigl, S.; Young, C.A.; Kitani, A.; Geissler, E.K.; Schlitt, H.J.; Strober, W. IL-13 Signaling via IL-13Rα2 Induces Major Downstream Fibrogenic Factors Mediating Fibrosis in Chronic TNBS Colitis. Gastroenterology 2008, 135, 2003–2013. [Google Scholar] [CrossRef] [PubMed]
- Kaviratne, M.; Hesse, M.; Leusink, M.; Cheever, A.W.; Davies, S.J.; McKerrow, J.H.; Wakefield, L.M.; Letterio, J.J.; Wynn, T.A. IL-13 Activates a Mechanism of Tissue Fibrosis That Is Completely TGF-β Independent. J. Immunol. 2004, 173, 4020–4029. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, J.; Tian, Z.; van Velkinburgh, J.C.; Song, J.; Wu, Y.; Ni, B. Innate Lymphoid Cells: A Promising New Regulator in Fibrotic Diseases. Int. Rev. Immunol. 2016, 35, 399–414. [Google Scholar] [CrossRef]
- Lukacs, N.W.; Hogaboam, C.; Chensue, S.W.; Blease, K.; Kunkel, S.L. Type 1/Type 2 Cytokine Paradigm and the Progression of Pulmonary Fibrosis. Chest 2001, 120 (Suppl. 1), 5S–8S. [Google Scholar] [CrossRef]
- Hams, E.; Armstrong, M.E.; Barlow, J.L.; Saunders, S.P.; Schwartz, C.; Cooke, G.; Fahy, R.J.; Crotty, T.B.; Hirani, N.; Flynn, R.J.; et al. IL-25 and Type 2 Innate Lymphoid Cells Induce Pulmonary Fibrosis. Proc. Natl. Acad. Sci. USA 2014, 111, 367–372. [Google Scholar] [CrossRef]
- Fanny, M.; Nascimento, M.; Baron, L.; Schricke, C.; Maillet, I.; Akbal, M.; Riteau, N.; Le Bert, M.; Quesniaux, V.; Ryffel, B.; et al. The IL-33 Receptor ST2 Regulates Pulmonary Inflammation and Fibrosis to Bleomycin. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Zhao, Y.; De Los Santos, F.G.; Wu, Z.; Liu, T.; Phan, S.H. An ST2-Dependent Role of Bone Marrow-Derived Group 2 Innate Lymphoid Cells in Pulmonary Fibrosis. J. Pathol. 2018, 245, 399–409. [Google Scholar] [CrossRef]
- Lee, J.U.; Chang, H.S.; Lee, H.J.; Jung, C.A.; Bae, D.J.; Song, H.J.; Park, J.S.; Uh, S.T.; Kim, Y.H.; Seo, K.H.; et al. Upregulation of Interleukin-33 and Thymic Stromal Lymphopoietin Levels in the Lungs of Idiopathic Pulmonary Fibrosis. BMC Pulm. Med. 2017, 17. [Google Scholar] [CrossRef]
- Li, D.; Guabiraba, R.; Besnard, A.G.; Komai-Koma, M.; Jabir, M.S.; Zhang, L.; Graham, G.J.; Kurowska-Stolarska, M.; Liew, F.Y.; McSharry, C.; et al. IL-33 Promotes ST2-Dependent Lung Fibrosis by the Induction of Alternatively Activated Macrophages and Innate Lymphoid Cells in Mice. J. Allergy Clin. Immunol. 2014, 134, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Hrusch, C.L.; Manns, S.T.; Bryazka, D.; Casaos, J.; Bonham, C.A.; Jaffery, M.R.; Blaine, K.M.; Mills, K.A.M.; Verhoef, P.A.; Adegunsoye, A.O.; et al. ICOS Protects against Mortality from Acute Lung Injury through Activation of IL-5 + ILC2s. Mucosal Immunol. 2018, 11, 61–70. [Google Scholar] [CrossRef]
- Lo, B.C.; Gold, M.J.; Scheer, S.; Hughes, M.R.; Cait, J.; Debruin, E.; Chu, F.S.F.; Walker, D.C.; Soliman, H.; Rossi, F.M.; et al. Loss of Vascular CD34 Results in Increased Sensitivity to Lung Injury. Am. J. Respir. Cell Mol. Biol. 2017, 57, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Cohen, D.A.; Jennings, C.D.; Bryson, J.S.; Kaplan, A.M. Bleomycin-Induced Pulmonary Fibrosis Is Independent of Eosinophils. J. Leukoc. Biol. 2000, 68, 515–521. [Google Scholar] [PubMed]
- Peterson, M.W.; Monick, M.; Hunninghake, G.W. Prognostic Role of Eosinophils in Pulmonary Fibrosis. Chest 1987, 92, 51–56. [Google Scholar] [CrossRef]
- Boyman, O.; Kovar, M.; Rubinstein, M.P.; Surh, C.D.; Sprent, J. Selective Stimulation of T Cell Subsets with Antibody-Cytokine Immune Complexes. Science 2006, 311, 1924–1927. [Google Scholar] [CrossRef]
- Birjandi, S.Z.; Palchevskiy, V.; Xue, Y.Y.; Nunez, S.; Kern, R.; Weigt, S.S.; Lynch, J.P.; Chatila, T.A.; Belperio, J.A. CD4+CD25hiFoxp3+ Cells Exacerbate Bleomycin-Induced Pulmonary Fibrosis. Am. J. Pathol. 2016, 186, 2008–2020. [Google Scholar] [CrossRef]
- Sarin, S.K.; Choudhury, A. Acute-on-Chronic Liver Failure: Terminology, Mechanisms and Management. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 131–149, Nature Publishing Group March 1. [Google Scholar] [CrossRef]
- Tsuchida, T.; Friedman, S.L. Mechanisms of Hepatic Stellate Cell Activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef]
- Marvie, P.; Lisbonne, M.; L’helgoualc’h, A.; Rauch, M.; Turlin, B.; Preisser, L.; Bourd-Boittin, K.; Théret, N.; Gascan, H.; Piquet-Pellorce, C.; et al. Interleukin-33 Overexpression Is Associated with Liver Fibrosis in Mice and Humans. J. Cell. Mol. Med. 2010, 14, 1726–1739. [Google Scholar] [CrossRef]
- Mchedlidze, T.; Waldner, M.; Zopf, S.; Walker, J.; Rankin, A.L.; Schuchmann, M.; Voehringer, D.; McKenzie, A.N.J.; Neurath, M.F.; Pflanz, S.; et al. Interleukin-33-Dependent Innate Lymphoid Cells Mediate Hepatic Fibrosis. Immunity 2013, 39, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Frontera, W.R.; Ochala, J. Skeletal Muscle: A Brief Review of Structure and Function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Tidball, J.G.; Villalta, S.A. Regulatory Interactions between Muscle and the Immune System during Muscle Regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, 1173–1187. [Google Scholar] [CrossRef] [PubMed]
- Sciorati, C.; Rigamonti, E.; Manfredi, A.A.; Rovere-Querini, P. Cell Death, Clearance and Immunity in the Skeletal Muscle. Cell Death Differ. 2016, 23, 927–937. [Google Scholar] [CrossRef]
- Arnold, L.; Henry, A.; Poron, F.; Baba-Amer, Y.; Van Rooijen, N.; Plonquet, A.; Gherardi, R.K.; Chazaud, B. Inflammatory Monocytes Recruited after Skeletal Muscle Injury Switch into Antiinflammatory Macrophages to Support Myogenesis. J. Exp. Med. 2007, 204, 1057–1069. [Google Scholar] [CrossRef]
- Mounier, R.; Théret, M.; Arnold, L.; Cuvellier, S.; Bultot, L.; Göransson, O.; Sanz, N.; Ferry, A.; Sakamoto, K.; Foretz, M.; et al. AMPKα1 Regulates Macrophage Skewing at the Time of Resolution of Inflammation during Skeletal Muscle Regeneration. Cell Metab. 2013, 18, 251–264. [Google Scholar] [CrossRef]
- Lemos, D.R.; Babaeijandaghi, F.; Low, M.; Chang, C.K.; Lee, S.T.; Fiore, D.; Zhang, R.H.; Natarajan, A.; Nedospasov, S.A.; Rossi, F.M.V. Nilotinib Reduces Muscle Fibrosis in Chronic Muscle Injury by Promoting TNF-Mediated Apoptosis of Fibro/Adipogenic Progenitors. Nat. Med. 2015, 21, 786–794. [Google Scholar] [CrossRef]
- Varga, T.; Mounier, R.; Horvath, A.; Cuvellier, S.; Dumont, F.; Poliska, S.; Ardjoune, H.; Juban, G.; Nagy, L.; Chazaud, B. Highly Dynamic Transcriptional Signature of Distinct Macrophage Subsets during Sterile Inflammation, Resolution, and Tissue Repair. J. Immunol. 2016, 196, 4771–4782. [Google Scholar] [CrossRef]
- Giannakis, N.; Sansbury, B.E.; Patsalos, A.; Hays, T.T.; Riley, C.O.; Han, X.; Spite, M.; Nagy, L. Dynamic Changes to Lipid Mediators Support Transitions among Macrophage Subtypes during Muscle Regeneration. Nat. Immunol. 2019, 20, 626–636. [Google Scholar] [CrossRef]
- Heredia, J.E.; Mukundan, L.; Chen, F.M.; Mueller, A.A.; Deo, R.C.; Locksley, R.M.; Rando, T.A.; Chawla, A. Type 2 Innate Signals Stimulate Fibro/Adipogenic Progenitors to Facilitate Muscle Regeneration. Cell 2013, 153, 376–388. [Google Scholar] [CrossRef]
- Joe, A.W.B.; Yi, L.; Natarajan, A.; Le Grand, F.; So, L.; Wang, J.; Rudnicki, M.A.; Rossi, F.M.V. Muscle Injury Activates Resident Fibro/Adipogenic Progenitors That Facilitate Myogenesis. Nat. Cell Biol. 2010, 12, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Kuswanto, W.; Burzyn, D.; Panduro, M.; Wang, K.K.; Jang, Y.C.; Wagers, A.J.; Benoist, C.; Mathis, D. Poor Repair of Skeletal Muscle in Aging Mice Reflects a Defect in Local, Interleukin-33-Dependent Accumulation of Regulatory T Cells. Immunity 2016, 44, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, A.; Corna, G.; Rigamonti, E.; Basso, V.; Vezzoli, M.; Monno, A.; Almada, A.E.; Mondino, A.; Wagers, A.J.; Manfredi, A.A.; et al. FOXP3+ T Cells Recruited to Sites of Sterile Skeletal Muscle Injury Regulate the Fate of Satellite Cells and Guide Effective Tissue Regeneration. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Wehling-Henricks, M.; Villalta, S.A.; Wang, Y.; Tidball, J.G. IL-10 Triggers Changes in Macrophage Phenotype That Promote Muscle Growth and Regeneration. J. Immunol. 2012, 189, 3669–3680. [Google Scholar] [CrossRef] [PubMed]
- Villalta, S.A.; Rinaldi, C.; Deng, B.; Liu, G.; Fedor, B.; Tidball, J.G. Interleukin-10 Reduces the Pathology of Mdx Muscular Dystrophy by Deactivating M1 Macrophages and Modulating Macrophage Phenotype. Hum. Mol. Genet. 2011, 20, 790–805. [Google Scholar] [CrossRef] [PubMed]
- Armando Villalta, S.; Rosenberg, A.S.; Bluestone, J.A. The Immune System in Duchenne Muscular Dystrophy: Friend or Foe. Rare Dis. 2015, 3, e1010966. [Google Scholar] [CrossRef]
- Wehling-Henricks, M.; Sokolow, S.; Lee, J.J.; Myung, K.H.; Villalta, S.A.; Tidball, J.G. Major Basic Protein-1 Promotes Fibrosis of Dystrophic Muscle and Attenuates the Cellular Immune Response in Muscular Dystrophy. Hum. Mol. Genet. 2008, 17, 2280–2292. [Google Scholar] [CrossRef]
- Sek, A.C.; Moore, I.N.; Smelkinson, M.G.; Pak, K.; Minai, M.; Smith, R.; Ma, M.; Percopo, C.M.; Rosenberg, H.F. Eosinophils Do Not Drive Acute Muscle Pathology in the Mdx Mouse Model of Duchenne Muscular Dystrophy. J. Immunol. 2019, 203, 476–484. [Google Scholar] [CrossRef]
- Villalta, S.A.; Rosenthal, W.; Martinez, L.; Kaur, A.; Sparwasser, T.; Tidball, J.G.; Margeta, M.; Spencer, M.J.; Bluestone, J.A. Regulatory T Cells Suppress Muscle Inflammation and Injury in Muscular Dystrophy. Sci. Transl. Med. 2014, 6. [Google Scholar] [CrossRef]
- Andreetta, F.; Bernasconi, P.; Baggi, F.; Ferro, P.; Oliva, L.; Arnoldi, E.; Cornelio, F.; Mantegazza, R.; Confalonieri, P. Immunomodulation of TGF-Beta1 in Mdx Mouse Inhibits Connective Tissue Proliferation in Diaphragm but Increases Inflammatory Response: Implications for Antifibrotic Therapy. J. Neuroimmunol. 2006, 175, 77–86. [Google Scholar] [CrossRef]
- Villalta, S.A.; Deng, B.; Rinaldi, C.; Wehling-Henricks, M.; Tidball, J.G. IFN-γ Promotes Muscle Damage in the Mdx Mouse Model of Duchenne Muscular Dystrophy by Suppressing M2 Macrophage Activation and Inhibiting Muscle Cell Proliferation. J. Immunol. 2011, 187, 5419–5428. [Google Scholar] [CrossRef] [PubMed]

| ILC Subsets | Stimuli | Cytokines | TFs | |
|---|---|---|---|---|
| ILC1 | ILC1 | Intracellular infections, IL-12, IL-18 | TNFα, IFNγ | Tbet |
| NKs | TNFα, IFNγ Perforin, Granzymes | Tbet, EOMES | ||
| ILC2 | Large parasites, tissue damage, IL-33, IL-25, TSLP | IL-4, IL-5, IL-9, IL-13, Areg | GATA3, RORα | |
| ILC3 | NCR+ | Extracellular infections, TGFβ, IL-1β, IL-23 | IL-22 | RORγt |
| NCR− | IL-22, IL-17 | |||
| LTi | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messing, M.; Jan-Abu, S.C.; McNagny, K. Group 2 Innate Lymphoid Cells: Central Players in a Recurring Theme of Repair and Regeneration. Int. J. Mol. Sci. 2020, 21, 1350. https://doi.org/10.3390/ijms21041350
Messing M, Jan-Abu SC, McNagny K. Group 2 Innate Lymphoid Cells: Central Players in a Recurring Theme of Repair and Regeneration. International Journal of Molecular Sciences. 2020; 21(4):1350. https://doi.org/10.3390/ijms21041350
Chicago/Turabian StyleMessing, Melina, Sia Cecilia Jan-Abu, and Kelly McNagny. 2020. "Group 2 Innate Lymphoid Cells: Central Players in a Recurring Theme of Repair and Regeneration" International Journal of Molecular Sciences 21, no. 4: 1350. https://doi.org/10.3390/ijms21041350
APA StyleMessing, M., Jan-Abu, S. C., & McNagny, K. (2020). Group 2 Innate Lymphoid Cells: Central Players in a Recurring Theme of Repair and Regeneration. International Journal of Molecular Sciences, 21(4), 1350. https://doi.org/10.3390/ijms21041350





