Mechanism of Allium Crops Bulb Enlargement in Response to Photoperiod: A Review
Abstract
1. Introduction
2. Photoperiodic Control in Bulb Enlargement
3. Phytohormonal Control of Bulb Enlargement
3.1. Gibberellic Acid
3.2. Abscisic Acid
3.3. Indole Acetic Acid
3.4. Zeatin Riboside
3.5. Jasmonic Acid
3.6. Salicylic Acid
4. Genetic Regulation of Photoperiod
5. Gene Expression and Bulb Enlargement
6. FT Gene Regulates Bulb Formation
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jackson, S.D. Plant responses to photoperiod. New Phytol. 2009, 181, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Atif, M.J.; Amin, B.; Ghani, M.I.; Hayat, S.; Ali, M.; Zhang, Y.; Cheng, Z. Influence of different photoperiod and temperature regimes on growth and bulb quality of garlic (Allium sativum L.) cultivars. Agronomy 2019, 9, 879. [Google Scholar] [CrossRef]
- Lock, M.; Grubben, G.J.H.; Denton, O.A. Plant Resources of Tropical Africa 2. Vegetables; Kew Bull; Prota Foundation: Wageningen, The Netherlands, 2004; Volume 59, p. 650. ISBN 9057821478. [Google Scholar]
- Brewster, J.L. Onions and other vegetable alliums. In Horticulture Research International, 2nd ed.; CABI: Wellesbourne, UK, 2008; p. 448. ISBN 9781845933999. [Google Scholar]
- King, R.W.; Moritz, T.; Evans, L.T.; Martin, J.; Andersen, C.H.; Blundell, C.; Kardailsky, I.; Chandler, P.M. Regulation of flowering in the long-day grass Lolium temulentum by gibberellins and the FLOWERING LOCUS T gene. Plant Physiol. 2006, 141, 498–507. [Google Scholar] [CrossRef]
- Waterer, D.; Schmitz, D. Influence of variety and cultural practices on garlic yields in Saskatchewan. Can. J. Plant Sci. 1994, 74, 611–614. [Google Scholar] [CrossRef]
- Mathew, D.; Forer, Y.; Rabinowitch, H.D.; Kamenetsky, R. Effect of long photoperiod on the reproductive and bulbing processes in garlic (Allium sativum L.) genotypes. Environ. Exp. Bot. 2011, 71, 166–173. [Google Scholar] [CrossRef]
- Brewster, J.L. Environmental physiology of the onion: Towards quantitative models for the effects of photoperiod, temperature and irradiance on bulbing, flowering and growth. Acta Hortic. 1997, 433, 347–374. [Google Scholar] [CrossRef]
- Khokhar, K.M. Effect of temperature and photoperiod on the incidence of bulbing and bolting in seedlings of onion cultivars of diverse origin. J. Hortic. Sci. Biotechnol. 2008, 83, 488–496. [Google Scholar] [CrossRef]
- Brewster, J.L. Cultural systems and agronomic practices in temperate climates. In Onions and Allied Crops; CRC Press: Boca Raton, FL, USA, 2018; pp. 1–30. ISBN 9781351075152. [Google Scholar]
- Wickramasinghe, U.L.; Wright, C.J.; Currah, L. Bulbing responses of two cultivars of red tropical onions to photoperiod, light integral and temperature under controlled growth conditions. J. Hortic. Sci. Biotechnol. 2000, 75, 304–311. [Google Scholar] [CrossRef]
- Lee, R.; Baldwin, S.; Kenel, F.; McCallum, J.; Macknight, R. FLOWERING LOCUS T genes control onion bulb formation and flowering. Nat. Commun. 2013, 4, 2884. [Google Scholar] [CrossRef]
- Eviatar-Ribak, T.; Shalit-Kaneh, A.; Chappell-Maor, L.; Amsellem, Z.; Eshed, Y.; Lifschitz, E. A cytokinin-activating enzyme promotes tuber formation in tomato. Curr. Biol. 2013, 23, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, M.; Dong, Y.; Cheng, Z.; Meng, H. Growth, bolting and yield of garlic (Allium sativum L.) in response to clove chilling treatment. Sci. Hortic. 2015, 194, 43–52. [Google Scholar] [CrossRef]
- Wu, C.; Wang, M.; Dong, Y.; Cheng, Z.; Meng, H. Effect of plant age and vernalization on bolting, plant growth and enzyme activity of garlic (Allium sativum L.). Sci. Hortic. 2016, 201, 295–305. [Google Scholar] [CrossRef]
- Wu, C.; Wang, M.; Cheng, Z.; Meng, H. Response of garlic (Allium sativum L.) bolting and bulbing to temperature and photoperiod treatments. Biol. Open 2016, 5, 507–518. [Google Scholar] [CrossRef]
- Bradley, K.; Rieger, M.; Collins, G. Classification of australian garlic cultivars by DNA fingerprinting. Aust. J. Exp. Agric. 1996, 36, 613. [Google Scholar] [CrossRef]
- Bandara, M.S.; Krieger, K.; Slinkard, A.E.; Tanino, K.K. Pre-plant chilling requirements for cloving of spring-planted garlic. Can. J. Plant Sci. 2000, 80, 379–384. [Google Scholar] [CrossRef]
- Etoh, T.; Simon, P.W. Diversity, fertility and seed production of garlic. In Allium Crop Science: Recent Advances; CABI: Wallingford, UK, 2009; pp. 101–117. ISBN 0851995101. [Google Scholar]
- Lancaster, J. Bulbing in Onions: Photoperiod and temperature requirements and prediction of bulb size and maturity. Ann. Bot. 1996, 78, 423–430. [Google Scholar] [CrossRef]
- Damte, T.; Tabor, G.; Haile, M.; Mitiku, G.; Lulseged, T. Determination of beginning of bulb enlargement time in shallot, Allium cepa var aggregatum for managing onion thrips (Thrips tabaci). Sci. Hortic. 2017, 220, 154–159. [Google Scholar] [CrossRef]
- Suesada, T.; Usuki, K.; Muro, T.; Higashino, Y.; Kawashiro, H.; Morita, N.; Morinaga, Y. Effect of seeding time and phosphate fertilizer using the method of local application below the seeds on yield in direct-sown seeds of onions (Allium cepa L.) in Central Japan. Hortic. Res. 2018, 17, 49–54. [Google Scholar] [CrossRef][Green Version]
- Ikeda, H.; Kinoshita, T.; Yamamoto, T.; Yamasaki, A. Sowing time and temperature influence bulb development in spring-sown onion (Allium cepa L.). Sci. Hortic. 2019, 244, 242–248. [Google Scholar] [CrossRef]
- Kamenetsky, R.; Rabinowitch, H.D. Florogenesis. In Allium Crop Science: Recent Advances; CABI: Wallingford, UK, 2009; pp. 31–57. ISBN 0851995101. [Google Scholar]
- Lercari, B. Action spectrum for the photoperiodic induction of bulb formation in Allium cepa L. Photochem. Photobiol. 1983, 38, 219–222. [Google Scholar] [CrossRef]
- Lercari, B.; Deitzer, G. Time-dependent effectiveness of far-red light on the photoperiodic induction of bulb formation in Allium cepa L. Photochem. Photobiol. 1987, 45, 831–835. [Google Scholar] [CrossRef]
- Lercari, B. Changes in invertase activities during the photoperiodically induced bulb formation of onion (Allium cepa L.). Physiol. Plant. 1982, 54, 480–484. [Google Scholar] [CrossRef]
- Lercari, B. The promoting effect of far-red light on bulb formation in the long day plant Allium cepa L. Plant Sci. Lett. 1982, 27, 243–254. [Google Scholar] [CrossRef]
- Mondal, M.F.; Brewster, J.L.; Morris, G.E.L.; Butler, H.A. Bulb development in onion (Allium cepa L.) II. The influence of red: Far-red spectral ratio and of photon flux density. Ann. Bot. 1986, 58, 197–206. [Google Scholar] [CrossRef]
- Lercari, B. Role of phytochrome in photoperiodic regulation of bulbing and growth in the long day plant Allium cepa. Physiol. Plant. 1984, 60, 433–436. [Google Scholar] [CrossRef]
- Randle, W.M. Onion Flavor Chemistry and Factors Influencing Flavor Intensity. ACS Symp. Ser. Washington, DC, USA. 1997, 660, 41–52. [Google Scholar]
- Veatch-Blohm, M.E.; Roche, B.M.; Sweeney, T. The effect of bulb weight on salinity tolerance of three common narcissus cultivars. Sci. Hortic. 2019, 49, 1158–1164. [Google Scholar] [CrossRef]
- Yoo, K.S.; Leskovar, D.; Patil, B.S.; Lee, E.J. Effects of leaf cutting on bulb weight and pungency of short-day onions after lifting the plants. Sci. Hortic. 2019, 110, 144–149. [Google Scholar] [CrossRef]
- Randle, W.M.; Lancaster, J.E. Sulphur compounds in alliums in relation to flavour quality. In Allium Crop Science: Recent Advances; CABI: Wallingford, UK, 2009; pp. 329–356. ISBN 0851995101. [Google Scholar]
- Maftuch, M.; Suprastyani, H.; Sanoesi, E.; Farida, N.; Fransira, I.; Habibah, N.; Fatmawati, D.R.; Rinaldi, R.; Nisyak, I.K.; Ardiansyah, D.; et al. Effect of dayak onion (Eleutherine palmifolia L.) Merr. crude extract on histopatology of gills, kidney, liver and muscle of aeromonas hydrophila- infected carp (Cyprinus carpio). Indones. Green Technol. J. 2018, 7, 35–39. [Google Scholar] [CrossRef]
- Pandey, A.; Belwal, T.; Sekar, K.C.; Bhatt, I.D.; Rawal, R.S. Optimization of ultrasonic-assisted extraction (UAE) of phenolics and antioxidant compounds from rhizomes of Rheum moorcroftianum using response surface methodology (RSM). Ind. Crop. Prod. 2018, 119, 218–225. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Martí-Quijal, F.; Cilla, A.; Munekata, P.; Lorenzo, J.; Remize, F.; Barba, F. Influence of temperature, solvent and ph on the selective extraction of phenolic compounds from tiger nuts by-products: Triple-TOF-LC-MS-MS characterization. Molecules 2019, 24, 797. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Du, W.; Wang, Y.; Teng, X.; Chen, X.; Ye, L. Total phenolic, flavonoid content, and antioxidant activity of bulbs, leaves, and flowers made from Eleutherine bulbosa (Mill.) Urb. Food Sci. Nutr. 2019, 7, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Kamarudin, A.A.; Esa, N.M.; Saad, N.; Sayuti, N.H.; Razak, N.A. Heat assisted extraction of phenolic compounds from Eleutherine bulbosa (Mill.) bulb and its bioactive profiles using response surface methodology. Ind. Crop. Prod. 2020, 144, 112064. [Google Scholar] [CrossRef]
- Lazare, S.; Bechar, D.; Fernie, A.R.; Brotman, Y.; Zaccai, M. The proof is in the bulb: Glycerol influences key stages of lily development. Plant J. 2019, 18, 577–584. [Google Scholar] [CrossRef]
- Islam, M.N.; Nielsen, G.; Stærke, S.; Kjær, A.; Jørgensen, B.; Edelenbos, M. Noninvasive determination of firmness and dry matter content of stored onion bulbs using shortwave infrared imaging with whole spectra and selected wavelengths. Appl. Spectrosc. 2018, 72, 1467–1478. [Google Scholar] [CrossRef]
- Teshika, J.D.; Zakariyyah, A.M.; Zaynab, T.; Zengin, G.; Rengasamy, K.R.; Pandian, S.K.; Fawzi, M.M. Traditional and modern uses of onion bulb (Allium cepa L.): A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 59, S39–S70. [Google Scholar] [CrossRef]
- Kahane, R.; Schweisguth, B.; Rancillac, M. Trophic versus environmental factors controlling in vitro bulb formation in onion and garlic micro propagated plants. Acta Hortic. 1997, 433, 435–443. [Google Scholar] [CrossRef]
- Nagakubo, T.; Nagasawa, A.; Ohkawa, H. Micropropagation of garlic through in vitro bulblet formation. Plant Cell. Tissue Organ Cult. 1993, 433, 435–443. [Google Scholar] [CrossRef]
- Wiles, G.C. The effect of different photoperiods and temperatures following bulb initiation on bulb development in tropical onion cultivars. Acta Hortic. 1994, 358, 419–427. [Google Scholar] [CrossRef]
- González, M.I. Effect of sowing date on the production of three storage varieties of onion in the eight region of Chile. Acta Hortic. 1997, 433, 549–554. [Google Scholar] [CrossRef]
- Tomer, N.; McGlone, A.; Künnemeyer, R. Validated multi-wavelength simulations of light transport in healthy onion. Comput. Electron. Agric. 2018, 146, 22–30. [Google Scholar] [CrossRef]
- Rohkin Shalom, S.; Gillett, D.; Zemach, H.; Kimhi, S.; Forer, I.; Zutahy, Y.; Tam, Y.; Teper-Bamnolker, P.; Kamenetsky, R.; Eshel, D. Storage temperature controls the timing of garlic bulb formation via shoot apical meristem termination. Planta 2015, 242, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.J. The plant hormones: Their nature, occurrence, and functions. In Plant Hormones; Springer: Dordrecht, The Netherlands, 2010; pp. 1–15. ISBN 9781402026867. [Google Scholar]
- Depuydt, S.; Hardtke, C.S. Hormone signalling crosstalk in plant growth regulation. Curr. Biol. 2011, 21, R365–R373. [Google Scholar] [CrossRef] [PubMed]
- Arguello, J.; Ledesma, A.; Bottini, R. Hormonal regulation of dormancy in garlic (Allium sativum L.) cv Rosado Paraguayo. Agriscientia 1991, 8, 9–14. [Google Scholar]
- Rahman, M.H.; Haque, M.S.; Karim, M.A.; Ahmed, M. Effects of gibberellic acid (GA3) on breaking dormancy in garlic (Allium sativum L.). Int. J. Agric. Biol. 2006, 8, 63–65. [Google Scholar]
- Kamenetsky, R.; Okubo, H. Ornamental Geophytes: From Basic Science to Sustainable Production, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781138198616. [Google Scholar]
- Guo, D.-P.; Ali Shah, G.; Zeng, G.-W.; Zheng, S.-J. The Interaction of plant growth regulators and vernalization on the growth and flowering of cauliflower (Brassica oleracea var. botrytis). Plant Growth Regul. 2004, 43, 163–171. [Google Scholar] [CrossRef]
- George, N.; Alderson, P.G.; Craigon, J.; Sparkes, D.L. Induction and generation of flowering in cabbage plants by seed vernalisation, gibberellic acid treatment and ratooning. J. Hortic. Sci. Biotechnol. 2007, 82, 346–350. [Google Scholar]
- Xu, X.; van Lammeren, A.A.M.; Vermeer, E.; Vreugdenhil, D. The role of gibberellin, abscisic acid, and sucrose in the regulation of potato tuber formation in vitro. Plant Physiol. 1998, 117, 575–584. [Google Scholar] [CrossRef]
- Le Guen-Le Saos, F.; Hourmant, A.; Esnault, F.; Chauvin, J.E. In vitro bulb development in shallot (Allium cepa L. Aggregatum group): Effects of anti-gibberellins, sucrose and light. Ann. Bot. 2002, 89, 419–425. [Google Scholar] [CrossRef]
- Langens-Gerrits, M.M.; Miller, W.B.M.; Croes, A.F.; De Klerk, G.J. Effect of low temperature on dormancy breaking and growth after planting in lily bulblets regenerated in vitro. Plant Growth Regul. 2003, 40, 267–275. [Google Scholar] [CrossRef]
- Wang, G.-L.; Que, F.; Xu, Z.-S.; Wang, F.; Xiong, A.-S. Exogenous gibberellin altered morphology, anatomic and transcriptional regulatory networks of hormones in carrot root and shoot. BMC Plant Biol. 2015, 15, 290. [Google Scholar] [CrossRef] [PubMed]
- Passam, H.C.; Koutri, A.C.; Karapanos, I.C. The effect of chlormequat chloride (CCC) application at the bolting stage on the flowering and seed production of lettuce plants previously treated with water or gibberellic acid (GA3). Sci. Hortic. 2008, 116, 117–121. [Google Scholar] [CrossRef]
- Li, J.; Yu, X.; Lou, Y.; Wang, L.; Slovin, J.P.; Xu, W.; Wang, S.; Zhang, C. Proteomic analysis of the effects of gibberellin on increased fruit sink strength in Asian pear (Pyrus pyrifolia). Sci. Hortic. 2015, 195, 25–36. [Google Scholar] [CrossRef]
- Erogul, D.; Sen, F. Effects of gibberellic acid treatments on fruit thinning and fruit quality in Japanese plum (Prunus salicina Lindl.). Sci. Hortic. 2015, 202, 111–116. [Google Scholar] [CrossRef]
- Wakchaure, G.C.; Minhas, P.S.; Meena, K.K.; Singh, N.P.; Hegade, P.M.; Sorty, A.M. Growth, bulb yield, water productivity and quality of onion (Allium cepa L.) as affected by deficit irrigation regimes and exogenous application of plant bio–regulators. Agric. Water Manag. 2018, 199, 1–10. [Google Scholar] [CrossRef]
- Alexopoulos, A.A.; Akoumianakis, K.A.; Passam, H.C. The effect of the time and mode of application of gibberellic acid on the growth and yield of potato plants derived from true potato seed. J. Sci. Food Agric. 2006, 86, 2189–2195. [Google Scholar] [CrossRef]
- Pérez-Jiménez, M.; Pazos-Navarro, M.; López-Marín, J.; Gálvez, A.; Varó, P.; del Amor, F.M. Foliar application of plant growth regulators changes the nutrient composition of sweet pepper (Capsicum annuum L.). Sci. Hortic. 2015, 194, 188–193. [Google Scholar] [CrossRef]
- Domingos, S.; Nobrega, H.; Raposo, A.; Cardoso, V.; Soares, I.; Ramalho, J.C.; Leitão, A.E.; Oliveira, C.M.; Goulao, L.F. Light management and gibberellic acid spraying as thinning methods in seedless table grapes (Vitis vinifera L.): Cultivar responses and effects on the fruit quality. Sci. Hortic. 2016, 201, 68–77. [Google Scholar] [CrossRef]
- Canli, F.A.; Pektas, M. Improving fruit size and quality of low yielding and small fruited pear cultivars with benzyladenine and gibberellin applications. Eur. J. Hortic. Sci. 2015, 19, 231–239. [Google Scholar] [CrossRef]
- Alexopoulos, A.A.; Akoumianakis, K.A.; Passam, H.C. Effect of plant growth regulators on the tuberisation and physiological age of potato (Solanum tuberosum L.) tubers grown from true potato seed. Can. J. Plant Sci. 2006, 86, 1217–1225. [Google Scholar] [CrossRef]
- Yamazaki, H.; Shiraiwa, N.; Itai, A.; Honda, I. Involvement of gibberellins in the regulation of tillering in welsh onion (Allium fistulosum L.). Hortic. J. 2015, 84, 334–341. [Google Scholar] [CrossRef]
- Nishijima, T.; Sugii, H.; Fukino, N.; Mochizuki, T. Aerial tubers induced in turnip (Brassica rapa L. var. rapa (L.) Hartm.) by gibberellin treatment. Sci. Hortic. 2005, 105, 423–433. [Google Scholar] [CrossRef]
- Ni, J.; Gao, C.; Chen, M.-S.; Pan, B.-Z.; Ye, K.; Xu, Z.-F. Gibberellin promotes shoot branching in the perennial woody plant Jatropha curcas. Plant Cell Physiol. 2015, 56, 1655–1666. [Google Scholar] [CrossRef]
- Curry, E. Increase in epidermal planar cell density accompanies decreased russeting of ‘golden delicious’ apples treated with gibberellin A(4+7). HortScience 2012, 47, 232–237. [Google Scholar] [CrossRef]
- Huang, H.; Jing, G.; Wang, H.; Duan, X.; Qu, H.; Jiang, Y. The combined effects of phenylurea and gibberellins on quality maintenance and shelf life extension of banana fruit during storage. Sci. Hortic. 2014, 167, 36–42. [Google Scholar] [CrossRef]
- Li, W.; Yong, Y.; Zhang, Y.; Lyu, Y. Transcriptional regulatory network of GA floral induction pathway in LA hybrid lily. Int. J. Mol. Sci. 2019, 20, 2694. [Google Scholar] [CrossRef]
- Liu, H.; Deng, R.; Huang, C.; Cheng, Z.; Meng, H. Exogenous gibberellins alter morphology and nutritional traits of garlic (Allium sativum L.) bulb. Sci. Hortic. 2019, 246, 298–306. [Google Scholar] [CrossRef]
- Jokela, V.; Virkajärvi, P.; Tanskanen, J.; Seppänen, M.M. Vernalization, gibberellic acid and photo period are important signals of yield formation in timothy (Phleum pratense). Physiol. Plant. 2014, 152, 152–163. [Google Scholar] [CrossRef]
- Mauromicale, G.; Ierna, A.; Cavallaro, V. Effects of vernalization and gibberellic acid on bolting, harvest time and yield of seed-grown globe artichoke. Acta Hortic. 2005, 681, 243–250. [Google Scholar] [CrossRef]
- Claeys, H.; De Bodt, S.; Inzé, D. Gibberellins and DELLAs: Central nodes in growth regulatory networks. Trends Plant Sci. 2014, 19, 231–239. [Google Scholar] [CrossRef]
- Brian, P.W. Effects of gibberellins on plant growth and development. Biol. Rev. 1959, 34, 37–77. [Google Scholar] [CrossRef]
- Fernández, J.A.; Bañón, S.; Franco, J.A.; González, A.; Martínez, P.F. Effects of vernalization and exogenous gibberellins on curd induction and carbohydrate levels in the apex of cauliflower (Brassica oleracea var. botrytis). Sci. Hortic. 1997, 70, 223–230. [Google Scholar]
- Erogul, D.; Sen, F. The effect of preharvest gibberellic acid applications on fruit quality of ‘Angelino’ plums during storage. Sci. Hortic. 2016, 202, 111–116. [Google Scholar] [CrossRef]
- Alexopoulos, A.A.; Aivalakis, G.; Akoumianakis, K.A.; Passam, H.C. Effect of foliar applications of gibberellic acid or daminozide on plant growth, tuberisation, and carbohydrate accumulation in tubers grown from true potato seed. J. Hortic. Sci. Biotechnol. 2007, 82, 535–540. [Google Scholar] [CrossRef]
- Ozkan, Y.; Ucar, M.; Yildiz, K.; Ozturk, B. Pre-harvest gibberellic acid (GA3) treatments play an important role on bioactive compounds and fruit quality of sweet cherry cultivars. Sci. Hortic. 2016, 211, 358–362. [Google Scholar] [CrossRef]
- Greb, T. Molecular analysis of the LATERAL SUPPRESSOR gene in Arabidopsis reveals a conserved control mechanism for axillary meristem formation. Genes Dev. 2003, 17, 1175–1187. [Google Scholar] [CrossRef]
- Schumacher, K.; Schmitt, T.; Rossberg, M.; Schmitz, G.; Theres, K. The Lateral suppressor (Ls) gene of tomato encodes a new member of the VHIID protein family. Proc. Natl. Acad. Sci. USA 1999, 96, 290–295. [Google Scholar] [CrossRef]
- Jiang, B.; Miao, H.; Chen, S.; Zhang, S.; Chen, F.; Fang, W. The Lateral suppressor-like gene, DgLsL, alternated the axillary branching in transgenic chrysanthemum (Chrysanthemum × morifolium) by modulating IAA and GA content. Plant Mol. Biol. Rep. 2010, 28, 144–151. [Google Scholar] [CrossRef]
- Yuan, L.-H.; Pan, J.-S.; Wang, G.; Zhu, J.; Zhang, W.-W.; Li, Z.; He, H.-L.; Yang, Z.-N.; Cai, R.; Zhu, L.-H. The Cucumber Lateral Suppressor gene (CLS) is functionally associated with axillary meristem initiation. Plant Mol. Biol. Rep. 2010, 28, 421–429. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Yang, F.; Qi, X.; Ahmad, H.; Wu, C.; Cheng, Z. Effect of the mode and time of gibberellic acid treatment on plant architecture and bulb structure in garlic (Allium sativum L.). Sci. Hortic. 2019, 257, 108723. [Google Scholar] [CrossRef]
- Mapelli, S.; Kinet, J.M. Plant growth regulator and graft control of axillary bud formation and development in the TO-2 mutant tomato. Plant Growth Regul. 1992, 11, 385–390. [Google Scholar] [CrossRef]
- Alexopoulos, A.A.; Akoumianakis, K.A.; Vemmos, S.N.; Passam, H.C. The effect of postharvest application of gibberellic acid and benzyl adenine on the duration of dormancy of potatoes produced by plants grown from TPS. Postharvest Biol. Technol. 2007, 46, 54–62. [Google Scholar] [CrossRef]
- Elfving, D.C.; Visser, D.B.; Henry, J.L. Gibberellins stimulate lateral branch development in young sweet cherry trees in the orchard. Int. J. Fruit Sci. 2011, 11, 41–54. [Google Scholar] [CrossRef]
- Guo, J.Y.; Jiang, F.L.; Tian, J.; Wu, Z. The dynamic changes of main endogenous hormone content in leaves during flower bud differentiation of bolting garlic cultivars. Acta Hortic. 2012, 938, 415–422. [Google Scholar] [CrossRef]
- Zhang, H.; Nie, Y.; Wang, D.; Xie, L. Effects of ETH and PP333 on the growth, florescence and physiological properties of bougainvillea spectabilis. Linye Kexue/Sci. Silvae Sin. 2018, 10, 46–55. [Google Scholar]
- Su, H.; Xu, K.; Liu, W. Changes of endogenous hormones during the process of flower bud differentiation of welsh onion (Allium fistulosum L.). Acta Hortic. Sin. 2007, 34, 671–676. [Google Scholar]
- Okubo, H.; Uemoto, S. Changes in the endogenous growth regulators in bulbous iris in bulb-forming and nonbulb-forming aspects. Plant Cell Physiol. 1981, 57, 2031–2035. [Google Scholar]
- Kamenetsky, R.; Gude, H.; Chastagner, G.A.; Okubo, H. Research challenges in geophyte science: From basic science to sustainable production. Acta Hortic. 2015, 119–130. [Google Scholar] [CrossRef]
- Galiba, G.; Vágújfalvi, A.; Li, C.; Soltész, A.; Dubcovsky, J. Regulatory genes involved in the determination of frost tolerance in temperate cereals. Plant Sci. 2009, 176, 12–19. [Google Scholar] [CrossRef]
- Kebrom, T.H.; Spielmeyer, W.; Finnegan, E.J. Grasses provide new insights into regulation of shoot branching. Trends Plant Sci. 2013, 18, 41–48. [Google Scholar] [CrossRef]
- Foley, M.E. Seed dormancy: An update on terminology, physiological genetics, and quantitative trait loci regulating germinability. Weed Sci. 2001, 49, 305–317. [Google Scholar] [CrossRef]
- Chope, G.A.; Cools, K.; Hammond, J.P.; Thompson, A.J.; Terry, L.A. Physiological, biochemical and transcriptional analysis of onion bulbs during storage. Ann. Bot. 2012, 109, 819–831. [Google Scholar] [CrossRef]
- Graeber, K.; Nakabayashi, K.; Miatton, E.; Leubner-Metzger, G.; Soppe, W.J.J. Molecular mechanisms of seed dormancy. Plant. Cell Environ. 2012, 35, 1769–1786. [Google Scholar] [CrossRef]
- Sonnewald, S.; Sonnewald, U. Regulation of potato tuber sprouting. Planta 2014, 239, 27–38. [Google Scholar] [CrossRef]
- Yamazaki, H.; Nishijima, T.; Yamato, Y.; Koshioka, M.; Miura, H. Involvement of abscisic acid (ABA) in bulb dormancy of Allium wakegi Araki I. Endogenous levels of ABA in relation to bulb dormancy and effects of exogenous ABA and fluridone. Plant Growth Regul. 1999, 29, 189–194. [Google Scholar] [CrossRef]
- Xue-Xuan, X.; Hong-Bo, S.; Yuan-Yuan, M.; Gang, X.; Jun-Na, S.; Dong-Gang, G.; Cheng-Jiang, R. Biotechnological implications from abscisic acid (ABA) roles in cold stress and leaf senescence as an important signal for improving plant sustainable survival under abiotic-stressed conditions. Crit. Rev. Biotechnol. 2010, 30, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Jacobo, M.F.; Steyaert, J.M.; Salazar-Badillo, F.B.; Nguyen, D.V.; Rostás, M.; Braithwaite, M.; De Souza, J.T.; Jimenez-Bremont, J.F.; Ohkura, M.; Stewart, A.; et al. Environmental growth conditions of Trichoderma spp. affects indole acetic acid derivatives, volatile organic compounds, and plant growth promotion. Front. Plant Sci. 2017, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Osugi, A.; Kojima, M.; Takebayashi, Y.; Ueda, N.; Kiba, T.; Sakakibara, H. Systemic transport of trans-zeatin and its precursor have differing roles in Arabidopsis shoots. Nat. Plants 2017, 3, 17112. [Google Scholar] [CrossRef] [PubMed]
- Koda, Y.; Okazawa, Y. Characteristic changes in the levels of endogenous plant hormones in relation to the onset of potato tuberization. Jpn. J. Crop Sci. 1983, 52, 592–597. [Google Scholar] [CrossRef]
- Wybouw, B.; De Rybel, B. Cytokinin–A developing story. Trends Plant Sci. 2019, 24, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Schaller, G.E.; Bishopp, A.; Kieber, J.J. The yin-yang of hormones: Cytokinin and auxin interactions in plant development. Plant Cell 2015, 27, 44–63. [Google Scholar] [CrossRef] [PubMed]
- Mahonen, A.P. Cytokinin signaling and its inhibitor AHP6 regulate cell fate during vascular development. Science 2006, 311, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Bishopp, A.; Help, H.; El-Showk, S.; Weijers, D.; Scheres, B.; Friml, J.; Benková, E.; Mähönen, A.P.; Helariutta, Y. A mutually inhibitory interaction between auxin and cytokinin specifies vascular pattern in roots. Curr. Biol. 2011, 21, 917–926. [Google Scholar] [CrossRef] [PubMed]
- De Rybel, B.; Adibi, M.; Breda, A.S.; Wendrich, J.R.; Smit, M.E.; Novák, O.; Yamaguchi, N.; Yoshida, S.; Van Isterdael, G.; Palovaara, J.; et al. Integration of growth and patterning during vascular tissue formation in Arabidopsis. Science 2014, 345, 1255215. [Google Scholar] [CrossRef] [PubMed]
- Mellor, N.; Adibi, M.; El-Showk, S.; De Rybel, B.; King, J.; Mähönen, A.P.; Weijers, D.; Bishopp, A.; Etchells, P. Theoretical approaches to understanding root vascular patterning: A consensus between recent models. J. Exp. Bot. 2016, 68, 5–16. [Google Scholar] [CrossRef] [PubMed]
- El-Showk, S.; Help-Rinta-Rahko, H.; Blomster, T.; Siligato, R.; Marée, A.F.M.; Mähönen, A.P.; Grieneisen, V.A. Parsimonious model of vascular patterning links transverse hormone fluxes to lateral root initiation: Auxin leads the way, while cytokinin levels out. PLoS Comput. Biol. 2015, 11, e1004450. [Google Scholar] [CrossRef]
- Muraro, D.; Byrne, H.; King, J.; Bennett, M. The role of auxin and cytokinin signalling in specifying the root architecture of Arabidopsis thaliana. J. Theor. Biol. 2013, 317, 71–86. [Google Scholar] [CrossRef]
- Kang, J.; Lee, Y.; Sakakibara, H.; Martinoia, E. Cytokinin transporters: GO and STOP in signaling. Trends Plant Sci. 2017, 22, 455–461. [Google Scholar] [CrossRef]
- Ohashi-Ito, K.; Saegusa, M.; Iwamoto, K.; Oda, Y.; Katayama, H.; Kojima, M.; Sakakibara, H.; Fukuda, H. A bHLH complex activates vascular cell division via cytokinin action in root apical meristem. Curr. Biol. 2014, 24, 2053–2058. [Google Scholar] [CrossRef]
- De Rybel, B.; Möller, B.; Yoshida, S.; Grabowicz, I.; Barbier de Reuille, P.; Boeren, S.; Smith, R.S.; Borst, J.W.; Weijers, D. A bHLH complex controls embryonic vascular tissue establishment and indeterminate growth in Arabidopsis. Dev. Cell 2013, 24, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Ohashi-Ito, K.; Bergmann, D.C. Regulation of the Arabidopsis root vascular initial population by Lonesome highway. Development 2007, 24, 2053–2058. [Google Scholar] [CrossRef] [PubMed]
- Dello Ioio, R.; Nakamura, K.; Moubayidin, L.; Perilli, S.; Taniguchi, M.; Morita, M.T.; Aoyama, T.; Costantino, P.; Sabatini, S. A genetic framework for the control of cell division and differentiation in the root meristem. Science 2008, 322, 1380–1384. [Google Scholar] [CrossRef] [PubMed]
- Moubayidin, L.; Perilli, S.; Dello Ioio, R.; Di Mambro, R.; Costantino, P.; Sabatini, S. The rate of cell differentiation controls the arabidopsis root meristem growth phase. Curr. Biol. 2010, 20, 1138–1143. [Google Scholar] [CrossRef]
- Van Zeijl, A.; Op Den Camp, R.H.M.; Deinum, E.E.; Charnikhova, T.; Franssen, H.; Op Den Camp, H.J.M.; Bouwmeester, H.; Kohlen, W.; Bisseling, T.; Geurts, R. Rhizobium lipo-chitooligosaccharide signaling triggers accumulation of cytokinins in medicago truncatula roots. Mol. Plant 2015, 8, 1213–1226. [Google Scholar] [CrossRef]
- Lohar, D.P.; Schaff, J.E.; Laskey, J.G.; Kieber, J.J.; Bilyeu, K.D.; Bird, D.M.K. Cytokinins play opposite roles in lateral root formation, and nematode and rhizobial symbioses. Plant J. 2004, 38, 203–214. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, W.; Li, X.; Jiang, H.; Wu, P.; Xia, K.; Yang, Y.; Wu, G. Knockdown of LjIPT3 influences nodule development in Lotus japonicus. Plant Cell Physiol. 2014, 55, 183–193. [Google Scholar] [CrossRef]
- Reid, D.; Nadzieja, M.; Novák, O.; Heckmann, A.B.; Sandal, N.; Stougaard, J. Cytokinin biosynthesis promotes cortical cell responses during nodule development. Plant Physiol. 2017, 175, 361–375. [Google Scholar] [CrossRef]
- Faivre-Rampant, O.; Cardle, L.; Marshall, D.; Viola, R.; Taylor, M.A. Changes in gene expression during meristem activation processes in Solanum tuberosum with a focus on the regulation of an auxin response factor gene. J. Exp. Bot. 2004, 55, 613–622. [Google Scholar] [CrossRef]
- Herrera-Medina, M.J.; Tamayo, M.I.; Vierheilig, H.; Ocampo, J.A.; García-Garrido, J.M. The jasmonic acid signalling pathway restricts the development of the arbuscular mycorrhizal association in tomato. J. Plant Growth Regul. 2008, 27, 221–230. [Google Scholar] [CrossRef]
- Meyer, A.; Miersch, O.; Büttner, C.; Dathe, W.; Sembdner, G. Occurrence of the plant growth regulator jasmonic acid in plants. J. Plant Growth Regul. 1984, 3, 1–8. [Google Scholar] [CrossRef]
- Staswick, P.E. Novel regulation of vegetative storage protein genes. Plant Cell 1990, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Farmer, E.E.; Ryan, C.A. Interplant communication: Airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc. Natl. Acad. Sci. USA 1990, 87, 7713–7716. [Google Scholar] [CrossRef] [PubMed]
- Farmer, E.E.; Ryan, C.A. Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. Plant Cell 1992, 4, 129. [Google Scholar] [CrossRef]
- Koda, Y. Possible involvement of jasmonates in various morphogenic events. Physiol. Plant. 1997, 100, 639–646. [Google Scholar] [CrossRef]
- Regvar, M.; Gogala, N.; Zalar, P. Effects of jasmonic acid on mycorrhizal Allium sativum. New Phytol. 1996, 134, 703–707. [Google Scholar] [CrossRef]
- Žel, J.; Debeljak, N.; Ucman, R.; Ravnikar, M. The effect of jasmonic acid, sucrose and darkness on garlic (Allium sativum L. cv. Ptujski jesenski) bulb formation in vitro. Vitr. Cell. Dev. Biol. Plant 1997, 33, 231–235. [Google Scholar]
- Nojiri, H.; Toyomasu, T.; Yamane, H.; Shibaoka, H.; Murofushi, N. Qualitative and quantitative analysis of endogenous gibberellins in onion plants and their effects on bulb development. Biosci. Biotechnol. Biochem. 1993, 57, 2031–2035. [Google Scholar] [CrossRef]
- Hartmann, A.; Senning, M.; Hedden, P.; Sonnewald, U.; Sonnewald, S. Reactivation of meristem activity and sprout growth in potato tubers require both cytokinin and gibberellin. Plant Physiol. 2011, 155, 776–796. [Google Scholar] [CrossRef]
- Brewster, J.L. The genetics and plant breeding of allium crops. In Onions and Other Vegetable Alliums; CABI: Wallingford, UK, 2008; pp. 51–84. ISBN 9781845933999. [Google Scholar]
- Boyhan, G.E.; Torrance, R.L.; Riner, C.M.; Cook, M.J.; Dollar, M.A.; Curry, D.S.; Hill, C.R.; Thigpen, D.R.; Bateman, A.G. Five-year evaluation of short-day onion varieties. Int. J. Veg. Sci. 2014, 20, 150–184. [Google Scholar] [CrossRef]
- Shrestha, R.; Gómez-Ariza, J.; Brambilla, V.; Fornara, F. Molecular control of seasonal flowering in rice, arabidopsis and temperate cereals. Ann. Bot. 2014, 114, 1445–1458. [Google Scholar] [CrossRef]
- Staiger, D.; Shin, J.; Johansson, M.; Davis, S.J. The circadian clock goes genomic. Genome Biol. 2013, 14, 208. [Google Scholar] [CrossRef]
- Taylor, A.; Massiah, A.J.; Thomas, B. Conservation of Arabidopsis thaliana photoperiodic flowering time genes in onion (Allium cepa L.). Plant Cell Physiol. 2010, 51, 1638–1647. [Google Scholar] [CrossRef]
- Pandey, A.; Pandey, R.; Negi, K.S.; Radhamani, J. Realizing value of genetic resources of Allium in India. Genet. Resour. Crop Evol. 2008, 55, 985–994. [Google Scholar] [CrossRef]
- Baldwin, S.; Revanna, R.; Pither-Joyce, M.; Shaw, M.; Wright, K.; Thomson, S.; Moya, L.; Lee, R.; Macknight, R.; McCallum, J. Genetic analyses of bolting in bulb onion (Allium cepa L.). Theor. Appl. Genet. 2014, 127, 535–547. [Google Scholar] [CrossRef]
- Noy-Porat, T.; Flaishman, M.A.; Eshel, A.; Sandler-Ziv, D.; Kamenetsky, R. Florogenesis of the Mediterranean geophyte Narcissus tazetta and temperature requirements for flower initiation and differentiation. Sci. Hortic. 2009, 120, 138–142. [Google Scholar] [CrossRef]
- Khokhar, K.M.; Hadley, P.; Pearson, S. Effect of photoperiod and temperature on inflorescence appearance and subsequent development towards flowering in onion raised from sets. Sci. Hortic. 2007, 112, 9–15. [Google Scholar] [CrossRef]
- Dong, Y.; Cheng, Z.; Meng, H.; Liu, H.; Wu, C.; Khan, A. The effect of cultivar, sowing date and transplant location in field on bolting of Welsh onion (Allium fistulosum L.). Bmc Plant Biol. 2013, 13, 154. [Google Scholar] [CrossRef]
- Benschop, M.; Kamenetsky, R.; Le Nard, M.; Okubo, H.; De Hertogh, A. The global flower bulb industry: Production, utilization, research. Hortic. Rev. 2010, 36, 1–115. [Google Scholar]
- Ramin, A.A.; Atherton, J.G. Manipulation of bolting and flowering in celery (Apium graveolens L. var. dulce ). I. Effects of chilling during germination and seed development. J. Hortic. Sci. 1991, 66, 709–717. [Google Scholar] [CrossRef]
- Kaul, S.; Koo, H.L.; Jenkins, J.; Rizzo, M.; Rooney, T.; Tallon, L.J.; Feldblyum, T.; Nierman, W.; Benito, M.I.; Lin, X.; et al. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 2000, 408, 796–815. [Google Scholar]
- Goff, S.A. A Draft Sequence of the Rice Genome (Oryza sativa L. ssp. japonica). Science 2002, 296, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, R.; Keim, P.; Vodkin, L.; Retzel, E.; Clifton, S.W.; Waterston, R.; Smoller, D.; Coryell, V.; Khanna, A.; Erpelding, J.; et al. A compilation of soybean ESTs: Generation and analysis. Genome 2002, 45, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Ware, D.H.; Jaiswal, P.; Ni, J.; Yap, I.V.; Pan, X.; Clark, K.Y.; Teytelman, L.; Schmidt, S.C.; Zhao, W.; Chang, K.; et al. Gramene, a tool for grass genomics. Plant Physiol. 2002, 130, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Hu, S.; Wang, J.; Wong, G.K.S.; Li, S.; Liu, B.; Deng, Y.; Dai, L.; Zhou, Y.; Zhang, X.; et al. A draft sequence of the rice genome (Oryza sativa L. ssp. indica). Science 2002, 296, 79–92. [Google Scholar] [CrossRef]
- Lunde, C.F.; Morrow, D.J.; Roy, L.M.; Walbot, V. Progress in maize gene discovery: A project update. Funct. Integr. Genom. 2003, 3, 25–32. [Google Scholar] [CrossRef]
- Young, N.D.; Mudge, J.; Ellis, T.H.N. Legume genomes: More than peas in a pod. Curr. Opin. Plant Biol. 2003, 6, 199–204. [Google Scholar] [CrossRef]
- McCallum, J.; Baldwin, S.; Shigyo, M.; Deng, Y.; van Heusden, S.; Pither-Joyce, M.; Kenel, F. AlliumMap-A comparative genomics resource for cultivated Allium vegetables. BMC Genom. 2012, 13, 168. [Google Scholar] [CrossRef]
- Galsurker, O.; Doron-Faigenboim, A.; Teper-Bamnolker, P.; Daus, A.; Fridman, Y.; Lers, A.; Eshel, D. Cellular and molecular changes associated with onion skin formation suggest involvement of programmed cell death. Front. Plant Sci. 2017, 7, 2031. [Google Scholar] [CrossRef]
- Ueno, K.; Sonoda, T.; Yoshida, M.; Shiomi, N.; Onodera, S. Purification, characterization, and functional analysis of a novel 6G&1-FEH mainly hydrolyzing neokestose from asparagus. J. Exp. Bot. 2018, 69, 4295–4308. [Google Scholar]
- Lazare, S.; Zaccai, M. Flowering pathway is regulated by bulb size in Lilium longiflorum (Easter lily). Plant Biol. 2016, 18, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Zhu, Z.; Guo, Q.; Zhu, Y.; Yang, X.; Sun, Y. Transcriptome analysis of differentially expressed genes provides insight into stolon formation in tulipa edulis. Front. Plant Sci. 2016, 7, 409. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Zhang, S.; Luo, C.; He, X.; Wei, S.; Jiang, W.; He, F.; Lin, Z.; Yan, M.; Dong, W. Transcriptome analysis of starch and sucrose metabolism across bulb development in Sagittaria sagittifolia. Gene 2018, 649, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Nielsen, G.; Stærke, S.; Kjær, A.; Jørgensen, B.; Edelenbos, M. Novel non-destructive quality assessment techniques of onion bulbs: A comparative study. J. Food Sci. Technol. 2018, 55, 3314–3324. [Google Scholar] [CrossRef]
- Mettananda, K.A.; Fordham, R. The effects of 12 and 16 h daylength treatments on the onset of bulbing in 21 onion cultivars (Allium cepa L) and its application to screening germplasm for use in the tropics. J. Hortic. Sci. 1997, 72, 981. [Google Scholar] [CrossRef]
- Fordham, R. Onions and other vegetable alliums. Sci. Hortic. 1995, 62, 145–146. [Google Scholar] [CrossRef]
- Shalit, A.; Rozman, A.; Goldshmidt, A.; Alvarez, J.P.; Bowman, J.L.; Eshed, Y.; Lifschitz, E. The flowering hormone florigen functions as a general systemic regulator of growth and termination. Proc. Natl. Acad. Sci. USA 2009, 106, 8392–8397. [Google Scholar] [CrossRef]
- Patil, H.B.; Chaurasia, A.K.; Azeez, A.; Krishna, B.; Subramaniam, V.R.; Sane, A.P.; Sane, P.V. Characterization of two TERMINAL FLOWER1 homologs PgTFL1 and PgCENa from pomegranate (Punica granatum L.). Tree Physiol. 2018, 38, 772–784. [Google Scholar] [CrossRef]
- Dalvi, V.S.; Patil, Y.A.; Krishna, B.; Sane, P.V.; Sane, A.P. Indeterminate growth of the umbel inflorescence and bulb is associated with increased expression of the TFL1 homologue, AcTFL1, in onion. Plant Sci. 2019, 287, 110165. [Google Scholar] [CrossRef]
- Krieger, U.; Lippman, Z.B.; Zamir, D. The flowering gene SINGLE FLOWER TRUSS drives heterosis for yield in tomato. Nat. Genet. 2010, 42, 459–463. [Google Scholar] [CrossRef]
- Navarro, C.; Abelenda, J.A.; Cruz-Oró, E.; Cuéllar, C.A.; Tamaki, S.; Silva, J.; Shimamoto, K.; Prat, S. Control of flowering and storage organ formation in potato by FLOWERING LOCUS T. Nature 2011, 478, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Böhlenius, H.; Huang, T.; Charbonnel-Campaa, L.; Brunner, A.M.; Jansson, S.; Strauss, S.H.; Nilsson, O. CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science 2006, 312, 1040–1043. [Google Scholar] [CrossRef] [PubMed]
- Danilevskaya, O.N.; Meng, X.; McGonigle, B.; Muszynski, M.G. Beyond flowering time. Plant Signal. Behav. 2011, 6, 1267–1270. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Ono, N.; Hayashi, Y.; Morimoto, S.; Nakamura, S.; Soda, M.; Kato, Y.; Ohnishi, M.; Nakano, T.; Inoue, S.; et al. FLOWERING LOCUS T regulates stomatal opening. Curr. Biol. 2011, 21, 1232–1238. [Google Scholar] [CrossRef]
- Coelho, C.P.; Minow, M.A.A.; Chalfun-Júnior, A.; Colasanti, J. Putative sugarcane FT/TFL1 genes delay flowering time and alter reproductive architecture in Arabidopsis. Front. Plant Sci. 2014, 5, 1–12. [Google Scholar] [CrossRef]
- Andrés, F.; Coupland, G. The genetic basis of flowering responses to seasonal cues. Nat. Rev. Genet. 2012. [Google Scholar] [CrossRef]
- Abelenda, J.A.; Navarro, C.; Prat, S. Flowering and tuberization: A tale of two nightshades. Trends Plant Sci. 2014, 19, 115–122. [Google Scholar] [CrossRef]
- Sawa, M.; Nusinow, D.A.; Kay, S.A.; Imaizumi, T. FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science 2007, 318, 261–265. [Google Scholar] [CrossRef]
- Jung, J.-H.; Seo, Y.-H.; Seo, P.J.; Reyes, J.L.; Yun, J.; Chua, N.-H.; Park, C.-M. The GIGANTEA-regulated microRNA172 mediates photoperiodic flowering independent of CONSTANS in Arabidopsis. Plant Cell 2007, 19, 2736–2748. [Google Scholar] [CrossRef]
- Pnueli, L. Tomato SP-interacting proteins define a conserved signaling system that regulates shoot architecture and flowering. Plant Cell 2001, 13, 2687–2702. [Google Scholar] [CrossRef]
- Purwestri, Y.A.; Ogaki, Y.; Tamaki, S.; Tsuji, H.; Shimamoto, K. The 14-3-3 protein GF14c acts as a negative regulator of flowering in rice by interacting with the florigen Hd3a. Plant Cell Physiol. 2009, 50, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Abe, M. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 2005, 309, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Taoka, K.I.; Ohki, I.; Tsuji, H.; Furuita, K.; Hayashi, K.; Yanase, T.; Yamaguchi, M.; Nakashima, C.; Purwestri, Y.A.; Tamaki, S.; et al. 14-3-3 proteins act as intracellular receptors for rice Hd3a florigen. Nature 2011, 476, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Wigge, P.A. Integration of spatial and temporal information during floral induction in Arabidopsis. Science 2005, 309, 1056–1059. [Google Scholar] [CrossRef] [PubMed]
- Golembeski, G.S.; Imaizumi, T. Photoperiodic regulation of florigen function in Arabidopsis thaliana. Arab. B. 2015, 13, e0178. [Google Scholar] [CrossRef] [PubMed]
- Nakamichi, N. Molecular mechanisms underlying the Arabidopsis circadian clock. Plant Cell Physiol. 2011, 52, 1709–1718. [Google Scholar] [CrossRef]
- Yoo, S.K.; Chung, K.S.; Kim, J.; Lee, J.H.; Hong, S.M.; Yoo, S.J.; Yoo, S.Y.; Lee, J.S.; Ahn, J.H. Constans activates suppressor of overexpression of constans 1 through Flowering locus T to promote flowering in Arabidopsis. Plant Physiol. 2005, 139, 770–778. [Google Scholar] [CrossRef]
- Dalvi, V.S.; Patil, Y.A.; Krishna, B.; Sane, P.V.; Sane, A.P. Identification of bulbing related genes in short day, non vernalization requiring onion. Acta Hortic. 2016, 1143, 269–276. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Zhan, Z.; Cao, L.; Zeng, A.; Chang, G.; Liang, Y. Transcriptome sequencing and metabolism analysis reveals the role of cyanidin metabolism in dark-red onion (Allium cepa L.) bulbs. Sci. Rep. 2018, 8, 14109. [Google Scholar] [CrossRef]
- Sobeih, W.Y.; Wright, C.J. The photoperiodic regulation of bulbing in onions (Allium cepa L.) III. Response to red:far-red ratio and cyclic lighting. J. Hortic. Sci. 1987, 62, 379–389. [Google Scholar] [CrossRef]
- Rashid, M.H.A.; Cheng, W.; Thomas, B. Temporal and spatial expression of Arabidopsis gene homologs control daylength adaptation and bulb formation in onion (Allium cepa L.). Sci. Rep. 2019, 9, 14629. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B. Light signals and flowering. J. Exp. Bot. 2006, 57, 3387–3393. [Google Scholar] [CrossRef] [PubMed]
- Summerfield, R.J.; Roberts, E.H.; Ellis, R.H.; Lawn, R.J. Towards the reliable prediction of time to flowering in six annual crops. i. the development of simple models for fluctuating field environments. Exp. Agric. 1991, 27, 11–31. [Google Scholar] [CrossRef]
- Brewster, J.L.; Salter, P.J.; Darby, R.J. Analysis of the growth and yield of overwintered onions. J. Hortic. Sci. 1977, 52, 335–346. [Google Scholar] [CrossRef]
- Jack, T. Molecular and genetic mechanisms of floral control. Plant Cell 2004, 16, S1–S17. [Google Scholar] [CrossRef]
- Borém, A.; Doe, J.A.; Diola, V. Molecular biology and biotechnology. In Sugarcane: Agricultural Production, Bioenergy and Ethanol; Academic Press: Amsterdam, The Netherlands, 2015; pp. 257–274. ISBN 978-0-12-802239-9. [Google Scholar]
- Rabinowitch, H. Onions and Allied crops; CRC Press: Boca Raton, FL, USA, 1990; p. 287. [Google Scholar]
- Khokhar, K.M. Environmental and genotypic effects on bulb development in onion–A review. J. Hortic. Sci. Biotechnol. 2017, 92, 448–454. [Google Scholar] [CrossRef]
- Rashid, M.H.A.; Thomas, B. Diurnal expression of Arabidopsis gene homologs during daylength-regulated bulb formation in onion (Allium cepa L.). Sci. Hortic. 2020, 261, 108946. [Google Scholar] [CrossRef]
- Hayama, R.; Coupland, G. Shedding light on the circadian clock and the photoperiodic control of flowering. Curr. Opin. Plant Biol. 2003, 6, 13–19. [Google Scholar] [CrossRef]
- Michael, T.P. Enhanced fitness conferred by naturally occurring variation in the circadian clock. Science 2003, 302, 1049–1053. [Google Scholar] [CrossRef]
- Devlin, P.F.; Kay, S.A. Cryptochromes are required for phytochrome signaling to the circadian clock but not for rhythmicity. Plant Cell 2000, 12, 2499–2509. [Google Scholar] [CrossRef]
- Lin, C. Blue light receptors and signal transduction. Plant Cell 2002, 14, S207–S225. [Google Scholar] [CrossRef] [PubMed]
- Kardailsky, I. Activation tagging of the floral inducer FT. Science 1999, 286, 1962–1965. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y. A pair of related genes with antagonistic roles in mediating flowering signals. Science 1999, 286, 1960–1962. [Google Scholar] [CrossRef] [PubMed]
- Erskine, W.; Ellis, R.H.; Summerfield, R.J.; Roberts, E.H.; Hussain, A. Characterization of responses to temperature and photoperiod for time to flowering in a world lentil collection. Theor. Appl. Genet. 1990, 80, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Corbesier, L.; Vincent, C.; Jang, S.; Fornara, F.; Fan, Q.; Searle, I.; Giakountis, A.; Farrona, S.; Gissot, L.; Turnbull, C.; et al. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 2007, 316, 1030–1033. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Adams, J.P.; Kim, H.; No, K.; Ma, C.; Strauss, S.H.; Drnevich, J.; Vandervelde, L.; Ellis, J.D.; Rice, B.M.; et al. FLOWERING LOCUS T duplication coordinates reproductive and vegetative growth in perennial poplar. Proc. Natl. Acad. Sci. USA 2011, 108, 10756–10761. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Liu, Y.; Luthe, D.S.; Yuceer, C. Poplar FT2 shortens the juvenile phase and promotes seasonal flowering. Plant Cell 2006, 18, 1846–1861. [Google Scholar] [CrossRef]
- Danilevskaya, O.N.; Meng, X.; McGonigle, B.; Muszynski, M.G. Beyond flowering time: Pleiotropic function of the maize flowering hormone florigen. Plant Signal. Behav. 2011, 6, 267–270. [Google Scholar] [CrossRef]
- Lifschitz, E.; Eviatar, T.; Rozman, A.; Shalit, A.; Goldshmidt, A.; Amsellem, Z.; Alvarez, J.P.; Eshed, Y. The tomato FT ortholog triggers systemic signals that regulate growth and flowering and substitute for diverse environmental stimuli. Proc. Natl. Acad. Sci. USA 2006, 103, 6398–6403. [Google Scholar] [CrossRef]
- Samach, A.; Wigge, P.A. Ambient temperature perception in plants. Curr. Opin. Plant Biol. 2005, 5, 483–486. [Google Scholar] [CrossRef]
- Teper-Bamnolker, P.; Samach, A. The flowering integrator FT regulates SEPALLATA3 and FRUITFULL accumulation in Arabidopsis leaves. Plant Cell 2005, 17, 2661–2675. [Google Scholar] [CrossRef] [PubMed]
- Levy, Y.Y.; Dean, C. The transition to flowering. Plant Cell 1998, 10, 1973–1989. [Google Scholar] [CrossRef] [PubMed]
- Komeda, Y. Genetic regulation of time to flower in Arabidopsis thaliana. Annu. Rev. Plant Biol. 2004, 55, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, R.; Han, J.; Vijayakumar, H.; Subramani, B.; Thamilarasan, S.; Park, J.-I.; Nou, I.-S. Molecular and functional characterization of FLOWERING LOCUS T homologs in Allium cepa. Molecules 2016, 21, 217. [Google Scholar] [CrossRef] [PubMed]
- Brewster, J.L. Effects of photoperiod, nitrogen nutrition and temperature on inflorescence initiation and development in onion (Allium cepa L.). Ann. Bot. 1983, 51, 429–440. [Google Scholar] [CrossRef]
- Lin, M.K.; Belanger, H.; Lee, Y.J.; Varkonyi-Gasic, E.; Taoka, K.I.; Miura, E.; Xoconostle-Cázares, B.; Gendler, K.; Jorgensen, R.A.; Phinney, B.; et al. FLOWERING LOCUS T protein may act as the long-distance florigenic signal in the cucurbits. Plant Cell 2007, 19, 1488–1506. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, J.F.; García-Martínez, J.L.; Bou, J.; Prat, S. The interaction of gibberellins and photoperiod in the control of potato tuberization. J. Plant Growth Regul. 2001, 20, 377–386. [Google Scholar] [CrossRef]
- Kojima, S.; Takahashi, Y.; Kobayashi, Y.; Monna, L.; Sasaki, T.; Araki, T.; Yano, M. Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant Cell Physiol. 2002, 43, 1096–1105. [Google Scholar] [CrossRef]
- Pin, P.A.; Benlloch, R.; Bonnet, D.; Wremerth-Weich, E.; Kraft, T.; Gielen, J.J.L.; Nilsson, O. An antagonistic pair of FT homologs mediates the control of flowering time in sugar beet. Science 2010, 330, 1397–1400. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Liu, M.S.; Li, J.R.; Guan, C.M.; Zhang, X.S. The wheat TaGI1, involved in photoperiodic flowering, encodesan Arabidopsis GI ortholog. Plant Mol. Biol. 2005, 58, 53–64. [Google Scholar] [CrossRef]
- Pin, P.A.; Nilsson, O. The multifaceted roles of FLOWERING LOCUS T in plant development. PlantCell Environ. 2012, 35, 1742–1755. [Google Scholar] [CrossRef] [PubMed]
- Lyngkhoi, F.; Khar, A.; Mangal, M.; Gaikwad, A.B.; Thirunavukkarasu, N. Expression analysis and association of bulbing to FLOWERING LOCUS T (FT) gene in short day onion (Allium cepa L.). Indian J. Genet. Plant Breed. 2019, 79, 77–81. [Google Scholar] [CrossRef]
- Turner, A. The pseudo-response regulator Ppd-H1 provides adaptation to photoperiod in barley. Science 2005, 310, 1031–1034. [Google Scholar] [CrossRef] [PubMed]
- Faure, S.; Turner, A.S.; Gruszka, D.; Christodoulou, V.; Davis, S.J.; von Korff, M.; Laurie, D.A. Mutation at the circadian clock gene EARLY MATURITY 8 adapts domesticated barley (Hordeum vulgare) to short growing seasons. Proc. Natl. Acad. Sci. USA 2012, 109, 8328–8333. [Google Scholar] [CrossRef]
- Matsubara, K.; Ogiso-Tanaka, E.; Hori, K.; Ebana, K.; Ando, T.; Yano, M. Natural variation in Hd17, a homolog of arabidopsis ELF3 that is involved in rice photoperiodic flowering. Plant Cell Physiol. 2012, 53, 709–716. [Google Scholar] [CrossRef]
- Weller, J.L.; Liew, L.C.; Hecht, V.F.G.; Rajandran, V.; Laurie, R.E.; Ridge, S.; Wenden, B.; Schoor, J.K.V.; Jaminon, O.; Blassiau, C. A conserved molecular basis for photoperiod adaptation in two temperate legumes. Proc. Natl. Acad. Sci. USA 2012, 109, 21158–21163. [Google Scholar] [CrossRef]
- Tsuji, H.; Taoka, K.I.; Shimamoto, K. Regulation of flowering in rice: Two florigen genes, a complex gene network, and natural variation. Curr. Opin. Plant Biol. 2011, 14, 45–52. [Google Scholar] [CrossRef]
- Schwartz, C.; Balasubramanian, S.; Warthmann, N.; Michael, T.P.; Lempe, J.; Sureshkumar, S.; Kobayashi, Y.; Maloof, J.N.; Borevitz, J.O.; Chory, J.; et al. Cis-regulatory changes at Flowering Locus T mediate natural variation in flowering responses of Arabidopsis thaliana. Genetics 2009, 183, 723–732. [Google Scholar] [CrossRef]
- Atif, M.J.; Amin, B.; Ghani, M.I.; Ali, M.; Cheng, Z. Variation in morphological and quality parameters in garlic (Allium sativum L.) bulb influenced by different photoperiod, temperature, sowing and harvesting time. Plants 2020, 9, 155. [Google Scholar] [CrossRef]
- Jin, Y.; Fei, M.; Rosenquist, S.; Jin, L.; Gohil, S.; Sandström, C.; Olsson, H.; Persson, C.; Höglund, A.S.; Fransson, G.; et al. A Dual-promoter gene orchestrates the sucrose-coordinated synthesis of starch and fructan in barley. Mol. Plant 2017, 10, 1556–1570. [Google Scholar] [CrossRef]
- Maeda, T.; Watanabe, A.; Wambrauw, D.Z.; Osanai, S.; Honda, K.; Oku, S.; Shimura, H.; Suzuki, T.; Yamasaki, A.; Okabe, Y.; et al. Analysis of varietal differences in the fructo-oligosaccharide accumulation profile among onion (Allium cepa L.) cultivars grown by spring-sown cultivation. Hortic. J. 2017, 86, 501–510. [Google Scholar] [CrossRef]
- Valluru, R. Fructan and hormone connections. Front. Plant Sci. 2015, 6, 180. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wei, H.; Bausewein, A.; Steininger, H.; Su, T.; Zhao, H.; Harms, K.; Greiner, S.; Rausch, T. Linking expression of fructan active enzymes, cell wall invertases and sucrose transporters with fructan profiles in growing taproot of chicory (Cichorium intybus): Impact of hormonal and environmental cues. Front. Plant Sci. 2016, 7, 1–15. [Google Scholar] [CrossRef][Green Version]
- Wei, H.; Bausewein, A.; Greiner, S.; Dauchot, N.; Harms, K.; Rausch, T. CiMYB17, a stress-induced chicory R2R3-MYB transcription factor, activates promoters of genes involved in fructan synthesis and degradation. New Phytol. 2017, 215, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Oku, S.; Ueno, K.; Tsuruta, Y.; Jitsuyama, Y.; Suzuki, T.; Onodera, S.; Maeda, T.; Shimura, H. Sugar accumulation and activities of enzymes involved in fructan dynamics from seedling to bulb formation in onion (Allium cepa L.). Sci. Hortic. 2019, 247, 147–155. [Google Scholar] [CrossRef]
- Tylewicz, S.; Petterle, A.; Marttila, S.; Miskolczi, P.; Azeez, A.; Singh, R.K.; Immanen, J.; Mähler, N.; Hvidsten, T.R.; Eklund, D.M.; et al. Photoperiodic control of seasonal growth is mediated by ABA acting on cell-cell communication. Science 2018, 360, 212–215. [Google Scholar] [CrossRef]
- Abelenda, J.A.; Bergonzi, S.; Oortwijn, M.; Sonnewald, S.; Du, M.; Visser, R.G.F.; Sonnewald, U.; Bachem, C.W.B. Source-sink regulation is mediated by interaction of an FT homolog with a SWEET protein in potato. Curr. Biol. 2019, 29, 1178–1186. [Google Scholar] [CrossRef]
- Barboza, K.; Salinas, M.C.; Acuña, C.V.; Bannoud, F.; Beretta, V.; García-Lampasona, S.; Burba, J.L.; Galmarini, C.R.; Cavagnaro, P.F. Assessment of genetic diversity and population structure in a garlic (Allium sativum L.) germplasm collection varying in bulb content of pyruvate, phenolics, and solids. Sci. Hortic. 2020, 261, 108900. [Google Scholar] [CrossRef]
- Ayub, S.; Hayat, R.; Zainab, Z.; Akhtar, W.; Mahmood, T. OsRGLP2 promoter derived GUS expression in transgenic tobacco in response to salicylic acid, H2O2, PEG, NaCl and auxins. Plant Gene 2019, 19, 100190. [Google Scholar] [CrossRef]
- Beinecke, F.A.; Grundmann, L.; Wiedmann, D.R.; Schmidt, F.J.; Caesar, A.S.; Zimmermann, M.; Lahme, M.; Twyman, R.M.; Prüfer, D.; Noll, G.A. The FT/FD-dependent initiation of flowering under long-day conditions in the day-neutral species Nicotiana tabacum originates from the facultative short-day ancestor Nicotiana tomentosiformis. Plant J. 2018, 96, 329–342. [Google Scholar] [CrossRef]
- Shukla, S.; Iquebal, M.A.; Jaiswal, S.; Angadi, U.B.; Fatma, S.; Kumar, N.; Jasrotia, R.S.; Fatima, Y.; Rai, A.; Kumar, D. The onion genomic resource: A genomics and bioinformatics driven resource for onion breeding. Plant Gene 2016, 8, 9–15. [Google Scholar] [CrossRef]
- Ragas, R.E.G.; Padron, F.K.J.R.; Ruedas, M.Y.A.D. Analysis of the morpho-anatomical traits of four major garlic (Allium sativum L.) cultivars in the Philippines. Appl. Ecol. Environ. Res. 2019, 17, 1143–1157. [Google Scholar] [CrossRef]
- Moraes, T.S.; Dornelas, M.C.; Martinelli, A.P. FT/TFL1: Calibrating plant architecture. Front. Plant Sci. 2019, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Ye, Y.; Song, C.; Chen, D.; Jiang, B.; Wang, Y. Cloning and functional identification of the AcLFY gene in Allium cepa. Biochem. Biophys. Res. Commun. 2016, 473, e1100–e1105. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xinping, X.; Qin, W. Cloning and functional analysis of flavanone 3-hydroxylase gene related to allelopathy in tillered onion. Allelopath. J. 2018, 45, 113. [Google Scholar] [CrossRef]
- Jie, S.; Cuicui, Y.; Xiaoxu, W.; Qiaoling, Y.; Dian, C.; Dongyuan, Z.; Yong, W. Molecular cloning and functional identification of photoperiod pathway transcription factor gene AcCOL7 in Allium cepa. Acta Hortic. Sin. 2018. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, B.; Ma, L.; Zhang, S.; Zhai, H.; Xu, X.; Hou, W.; Xia, Z.; Wu, C.; Sun, S.; et al. Functional diversification of Flowering Locus T homologs in soybean: GmFT1a and GmFT2a/5a have opposite roles in controlling flowering and maturation. New Phytol. 2018, 217, 1335–1345. [Google Scholar] [CrossRef]
| Highlights |
|---|
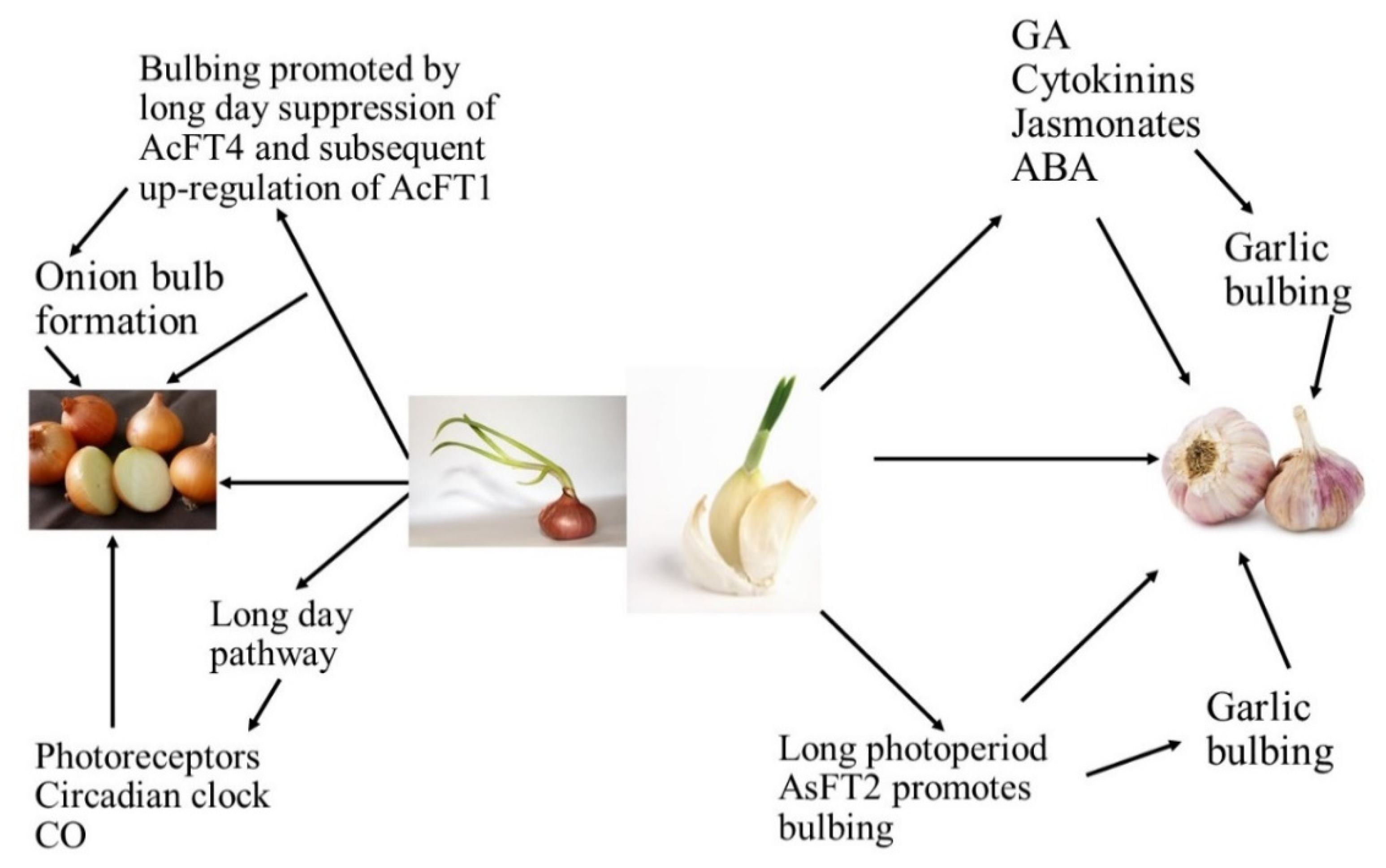
| Bulb enlargement and its subsequent development were influenced by photoperiod and bulbing was encouraged by long days [2,3]. |
| Bulbing is regulated by internal signals, which can be stimulated or inhibited by the environmental conditions. It has been widely reported that phytohormones regulate the plant growth and are considered to play an important role in the formation of bulbs [5,7]. |
| Long photoperiods are known to improve the levels of endogenous gibberellins, with consequent flower bud differentiation. Many studies have shown that gibberellic acid (GA) could partially or fully replace vernalization for some plants. Endogenous GA levels of long day or biennial plants during the process of floral induction increased. However, GA is considered to be an inhibitor of bulb formation. Exogenous GA inhibited the increase of the scape and bulb yield. It was likely that GA did not act directly on the inhibition of bulbing; instead, it enhanced the activity of a “bulbing inhibition substance” [5,7]. |
| Abscisic acid (ABA) generally plays an important role in plant defense against biotic or abiotic stresses. It was assumed that ABA acts similarly to GA in the early stage of plant bolting. Endogenous ABA levels of Welsh onion (Allium fistulosum L.) increased significantly during flower bud differentiation and decreased dramatically after the completion of flower bud differentiation [5,7] |
| Indoleacetic acid (IAA) showed the opposite effect, decreasing with increases in the flower bud differentiation rate but increasing significantly during the bolting process of Welsh onion. It is reasonable to assume that IAA inhibits flower bud differentiation but improves plant bolting [5,7]. |
| Zeatin riboside (ZR) also showed an enhancing effect on plant bolting. Cytokinin (CTK) was a bulbing initiator but had no visible effect on bulb enlargement, while IAA and ethylene improved bulb formation. However, few studies have investigated the role of abscisic acid (ABA) on garlic bolting or bulbing [5,7]. |
| Jasmonic acid (JA) and related compounds are widely distributed among higher plants and play important roles in the regulation of plant development. It was found that jasmonates were potent inducers of vegetative storage protein gene expression and proteinase inhibitors of defense proteins. It is generally believed that the bulbing process is regulated by the balance between the “bulbing hormones” and GA. By considering that bulbing was involved in the disruption of microtubules, jasmonic acid (JA) and methyl jasmonate (MeJA) were candidate bulbing hormones because of their microtubule-disrupting activities and wide distribution in higher plants [5,7]. |
| Salicylic acid (SA) played an important role in garlic bulb formation and MeJA likely enhanced the endogenous SA content of garlic plant, thus improving bulbing [5,7]. |
| Cultivars grown at diverse latitudes required a least day length for bulbing, and cultivars are classified on this into short-day (SD), intermediate, and long-day (LD) categories. The short-day cultivars procedure bulbs at low latitudes whenever the day length is close to 12 h, whereas intermediate ones grow bulbs at mid latitudes whenever the day length lies between 12 and 16 h, and long-day cultivars initiate bulbing at high latitudes whenever the day length is close to or above 16 h [6,7,8,9,10,11]. |
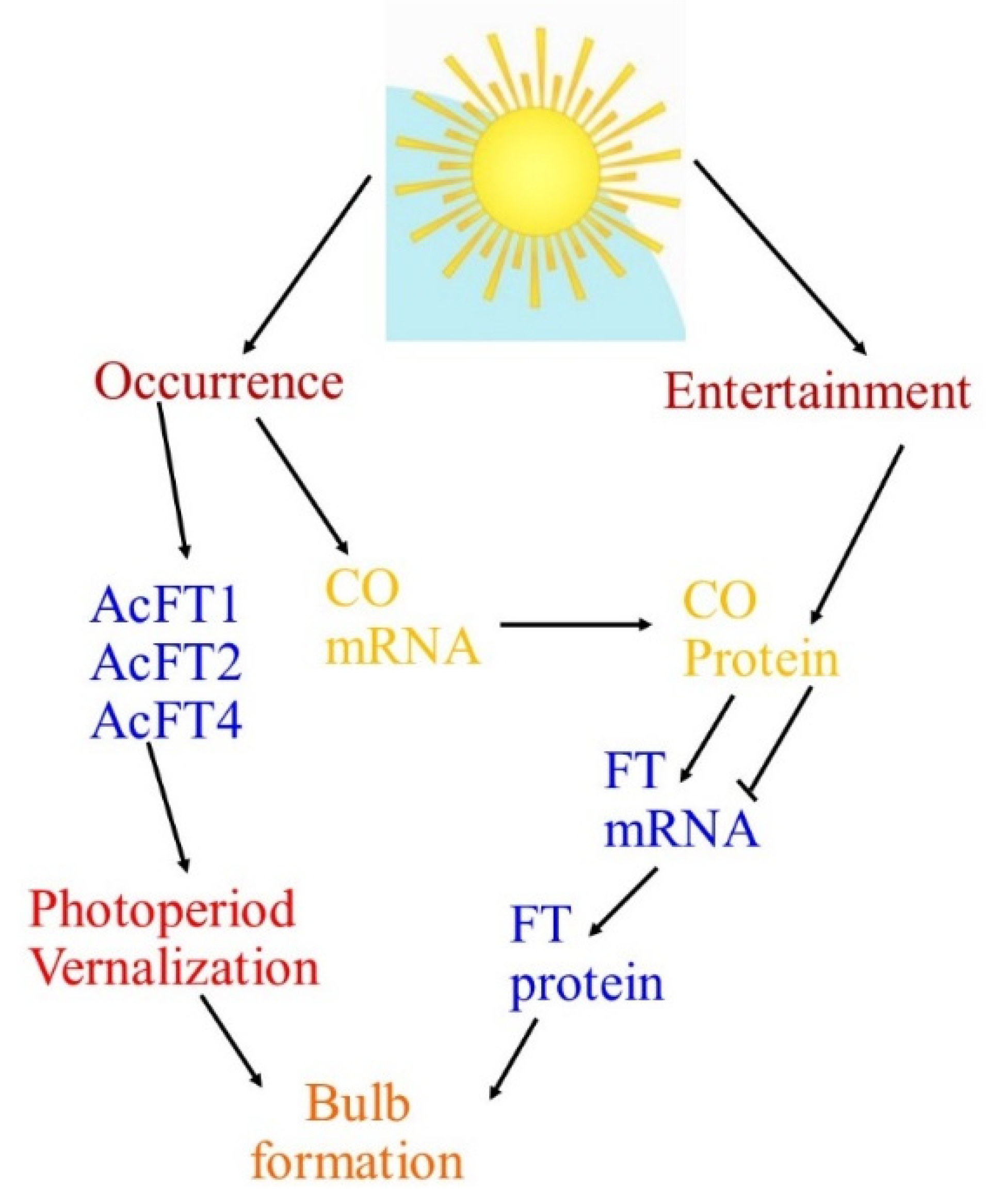
| Numerous key genes are intricate in circadian regulation, where the clock derives the rhythmic expression of key genes FLAVIN-BINDING, KELCH REPEAT, F-BOX (FKF1), GIGANTEA (GI), and CONSTANS (CO). FKF1 and GI promote CO expression and this CO positively controls FLOWERING LOCUS T (FT). Then, the FT protein is translocated to the apical meristem through the phloem and forms a FT/FD (FLOWERING LOCUS D) complex. This compound triggers the APETALA 1 (AP1) and suppressor of overexpression of CONSTANS 1 (SOC1) genes, which triggers LEAFY (LFY) gene expression and causes flowering at the floral apical meristem in Arabidopsis. The expression of GI, FKF1, and ZTL homologs under short-day and long-day environments was observed using quantitative reverse transcription-PCR (qRT-PCR), where the results presented that key genes—namely GI, CO, and FT—controlling photoperiodic flowering in Arabidopsis are conserved in Alliums, and a role for these genes in the photoperiodic control of bulb instigation is anticipated [12,13]. |
| The FLOWERING LOCUS T gene (FT), which was first documented in Arabidopsis thaliana, has been discovered to be the main feature of the floral signal molecule florigen. FT plays a key role in the photoperiodic pathway for the initiation of flowering in the apical meristem with the help of other floral homeotic genes such as LFY. Moreover, FT is a target of CONSTANS (CO) and turns upstream of suppressor of CONSTANS overexpression (SOC1) and can act as a mobile flowering signal to encourage flowering by long-distance transport. For bulbing, as with flowering, photoperiod insight develops in the leaves, while the response is in the meristem. These indorse that a mobile signal with properties similar to FT might be involved [12,13]. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atif, M.J.; Ahanger, M.A.; Amin, B.; Ghani, M.I.; Ali, M.; Cheng, Z. Mechanism of Allium Crops Bulb Enlargement in Response to Photoperiod: A Review. Int. J. Mol. Sci. 2020, 21, 1325. https://doi.org/10.3390/ijms21041325
Atif MJ, Ahanger MA, Amin B, Ghani MI, Ali M, Cheng Z. Mechanism of Allium Crops Bulb Enlargement in Response to Photoperiod: A Review. International Journal of Molecular Sciences. 2020; 21(4):1325. https://doi.org/10.3390/ijms21041325
Chicago/Turabian StyleAtif, Muhammad Jawaad, Mohammad Abass Ahanger, Bakht Amin, Muhammad Imran Ghani, Muhammad Ali, and Zhihui Cheng. 2020. "Mechanism of Allium Crops Bulb Enlargement in Response to Photoperiod: A Review" International Journal of Molecular Sciences 21, no. 4: 1325. https://doi.org/10.3390/ijms21041325
APA StyleAtif, M. J., Ahanger, M. A., Amin, B., Ghani, M. I., Ali, M., & Cheng, Z. (2020). Mechanism of Allium Crops Bulb Enlargement in Response to Photoperiod: A Review. International Journal of Molecular Sciences, 21(4), 1325. https://doi.org/10.3390/ijms21041325







