Abstract
Mechanical force is a key factor for the maintenance, adaptation, and function of tendons. Investigating the impact of mechanical loading in tenocytes and tendons might provide important information on in vivo tendon mechanobiology. Therefore, the study aimed at understanding if an in vitro loading set up of tenocytes leads to similar regulations of cell shape and gene expression, as loading of the Achilles tendon in an in vivo mouse model. In vivo: The left tibiae of mice (n = 12) were subject to axial cyclic compressive loading for 3 weeks, and the Achilles tendons were harvested. The right tibiae served as the internal non-loaded control. In vitro: tenocytes were isolated from mice Achilles tendons and were loaded for 4 h or 5 days (n = 6 per group) based on the in vivo protocol. Histology showed significant differences in the cell shape between in vivo and in vitro loading. On the molecular level, quantitative real-time PCR revealed significant differences in the gene expression of collagen type I and III and of the matrix metalloproteinases (MMP). Tendon-associated markers showed a similar expression profile. This study showed that the gene expression of tendon markers was similar, whereas significant changes in the expression of extracellular matrix (ECM) related genes were detected between in vivo and in vitro loading. This first pilot study is important for understanding to which extent in vitro stimulation set-ups of tenocytes can mimic in vivo characteristics.
1. Introduction
Tendons play a key role in the musculoskeletal system by transmitting forces from muscle to bone, allowing joint movement. The tissue is hypocellular and its extracellular matrix (ECM) is mainly composed of collagen type I as the predominant protein. For the maintenance of its structural integrity and function, tendons require mechanical loading. Mechanical forces include tension, hydrostatic pressure, fluid shear stress, and compression, and are transduced by cells to evoke a biochemical response. These responses affect processes such as proliferation, differentiation, tissue development, and skeletal maintenance [1]. Studies have shown that immobilization during embryonic development can lead to tendon degeneration, reduced size, or impaired formation of the tendon synovial sheath [2,3,4]. In mature tendons, mechanical loading prevents the degradation of the ECM and promotes tissue homeostasis [5]. However, depending on the strain rate, loading can have different effects on the tendon. Loading within a physiological range (4–8%) induces increased collagen production and has therapeutic effects in ex vivo tendon models [6,7,8]. Overloading (>9%) has severe effects on tendons, damaging the structural integrity, increasing cell apoptosis, and upregulating inflammatory pathways [9]. Similar effects were observed in underload (<4%) ex vivo tendon models, in which the ECM was degraded and matrix metalloproteinases (MMP) were upregulated [9]. In various in vitro cell culture studies, mechanical loading was applied to tenocytes, either biaxial or uniaxial in two-dimensional set ups [10]. Compared to ex vivo tendon models, physiological loading led to elevated collagen type I production, decorin expression, and tenogenic differentiation; however, did not activate inflammatory responses during bi-and uniaxial stretching [11,12,13,14,15]. Loading with a strain below 4% induced elevated levels of inflammatory factors, and ECM degrading enzymes, such as MMP3 [16]. High magnitude strains mimicking overuse conditions induced apoptosis, expression of various angiogenic factors, as well as inflammatory factors and MMP expression in both loading conditions [17,18,19]. For in vivo models, mechanical loading has been applied through treadmill running to induce certain conditions, such as Achilles tendinopathy [20,21]. Only a few studies exist that directly compare in vitro and in vivo mechanical loading [22,23].
The aim of this study was to understand if an in vitro loading set up of tenocytes leads to similar regulations of cell morphology and gene expression, as in axial compressive loading of the mouse tibia and Achilles tendon. We used comparable loading parameters in both models, and the in vivo model used in this study is a well-established model to investigate the bone formation in response of mechanical loading [24,25,26,27,28,29].
2. Results
To investigate the impact mechanical loading has on the Achilles tendon in vivo, and on mouse tenocytes in vitro, the loading protocols were chosen to be similar. The number of cycles and frequency in vitro was the same as the in vivo loading; however, the strain applied to the tendon was unknown, and therefore chosen to be 4%, as it is known that this strain is in a physiological range, and histological analysis of the loaded tendons did not show signs of degeneration. We investigated markers of tenocytes, tissue remodeling, as well as adipogenic, chondrogenic and osteogenic lineage, cytokine, and integrin expression.
2.1. Morphological Analysis
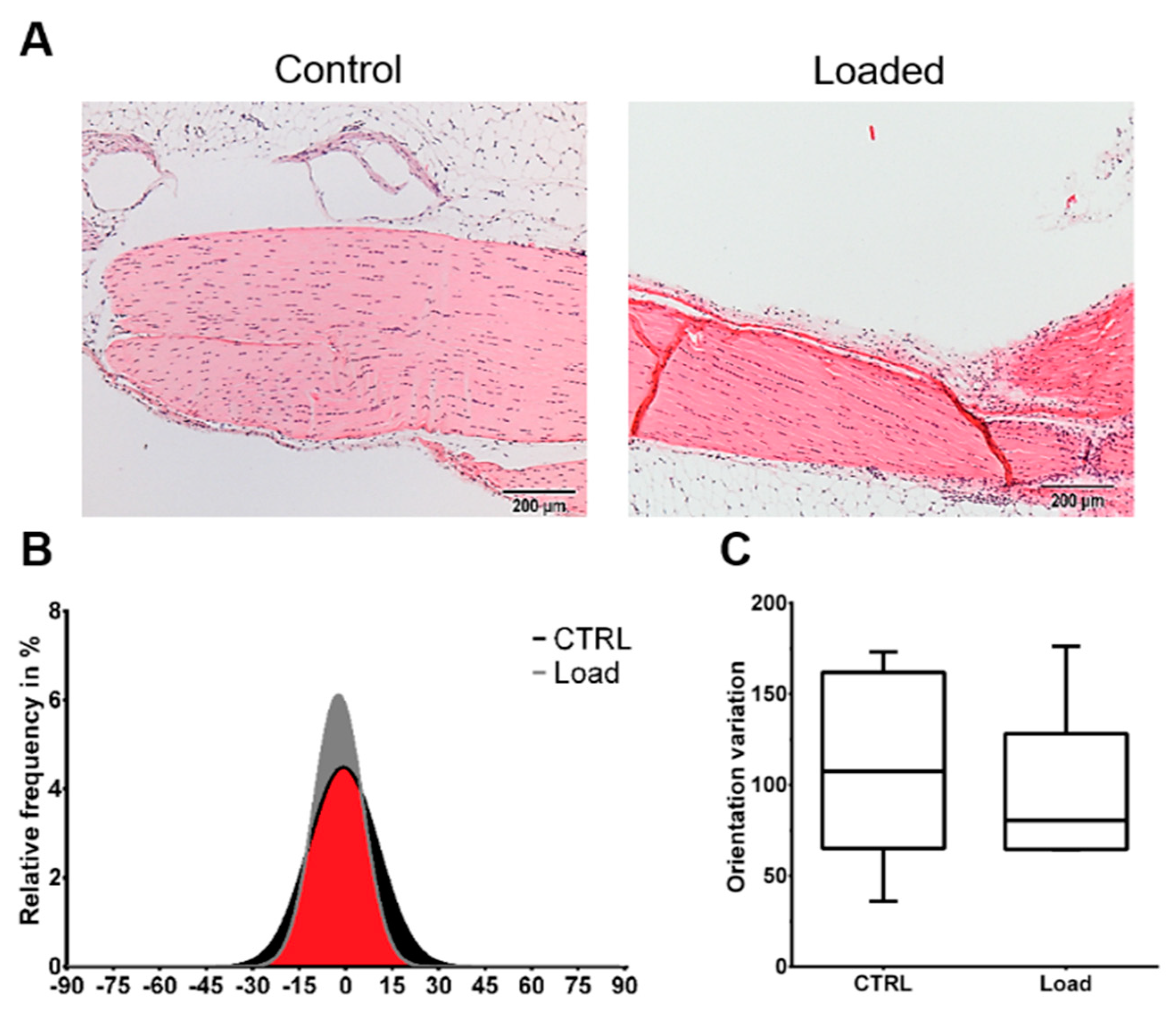
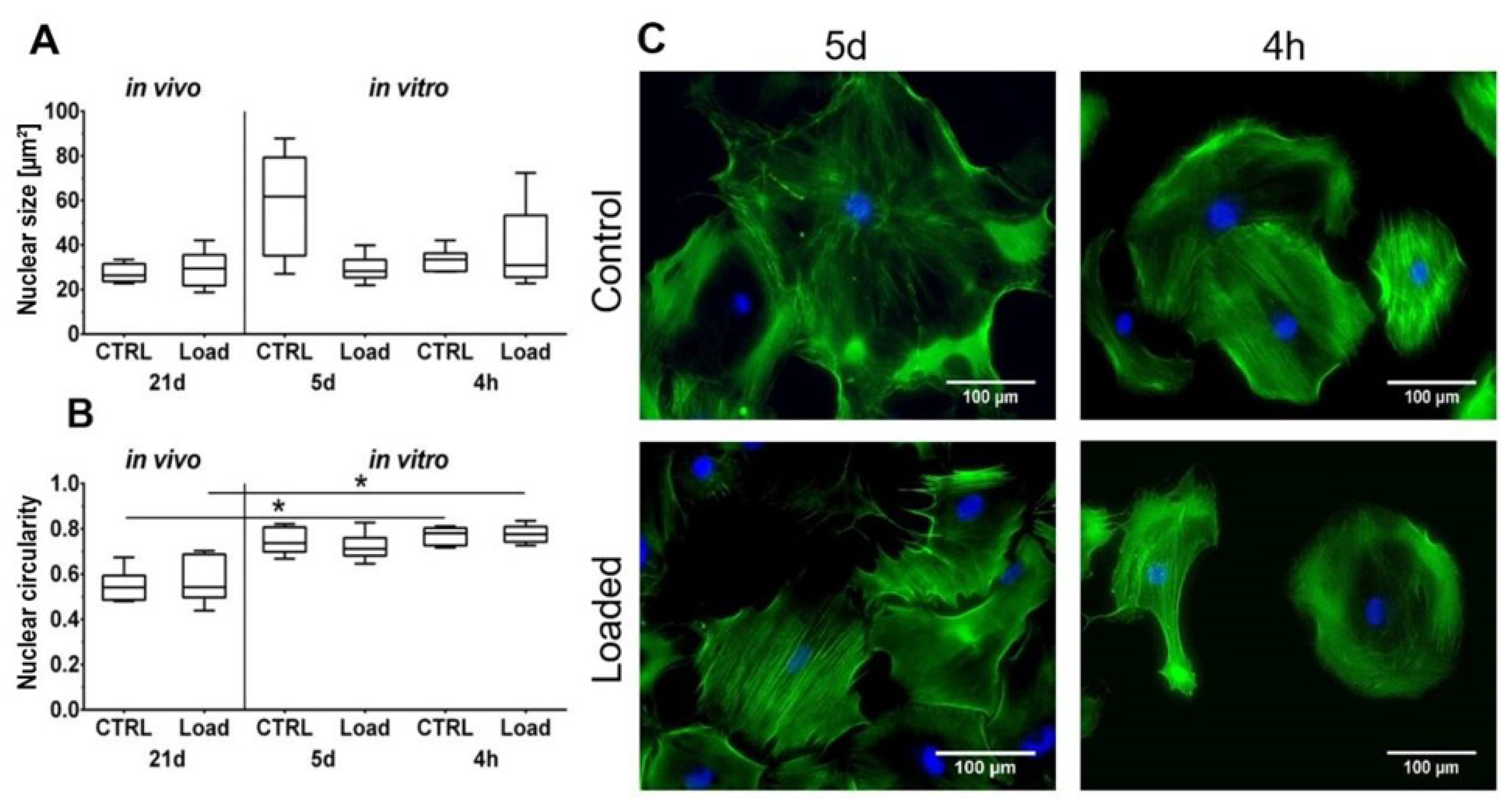
The Hematoxylin and Eosin staining showed the typical picture of a tendon with elongated tenocytes laying between the parallel-aligned collagen bundles. The overall structural integrity was not influenced by in vivo mechanical loading (Figure 1A). Analysis of the collagen fiber alignment demonstrated an overlapping area between the control and loaded tendon (Figure 1, red area). To investigate the organization of the fibers, the variation of the angle of the fibers was determined as the difference between the maximal and minimal angle. The variation was slightly smaller in the loaded Achilles tendon compared to the non-loaded control (Figure 1C). On the cellular level, the size of the nuclear was not significantly altered by loading in vivo or in vitro (Figure 2A). The nuclear size in the in vitro control group at day 5 was almost doubled compared to the other groups with a high variability, but the difference was not significant (p = 0.066). Analyzing the shape of the nuclei revealed that within the in vivo or the in vitro groups, the circularity was not influenced by mechanical stimulation. Comparing the in vitro groups to the in vivo groups, the nuclei were significantly rounder after 4 h in culture, independent of the mechanical stimulation. After 5 days of loading in vitro, no difference was seen compared to the in vivo results; however, compared to the 4-h group, a small shift to an elongated shape was observed (Figure 2B). Further, no differences regarding the amount or orientation of F-actin were found in the in vitro stimulated groups compared to the respective control (Figure 2C).
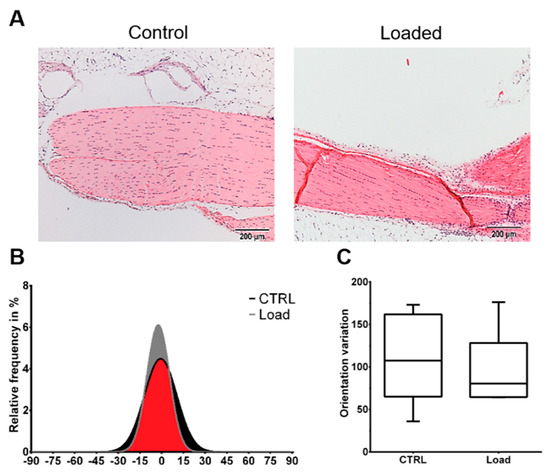
Figure 1.
Mechanical loading did not induce histological alterations in the in vivo loaded Achilles tendon. The loaded and non-loaded Achilles tendons were dissected and further processed for histological analysis. (A) Representative Hematoxylin and Eosin images of the loaded and non-loaded Achilles tendon. Scale bar: 200 µm. (B) Collagen fiber distribution. (C) Variation of fiber orientation (n = 6).
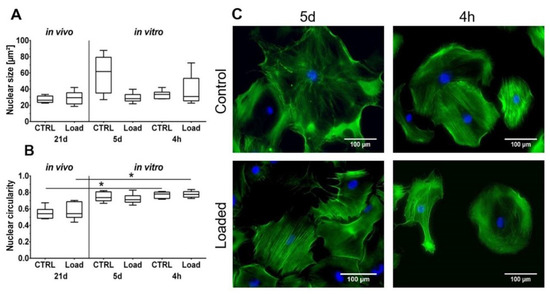
Figure 2.
Cell morphology is partially influenced by mechanical loading. (A) The nuclear size is not significantly affected. (B) Cell nuclei in vitro were significantly rounder compared to in vivo (* p < 0.05, n = 6). (C) Phalloidin/ 4′,6-Diamidin-2-phenylindol (DAPI) staining of loaded murine tenocytes in vitro. Scale bar: 100 µm.
2.2. Gene Expression
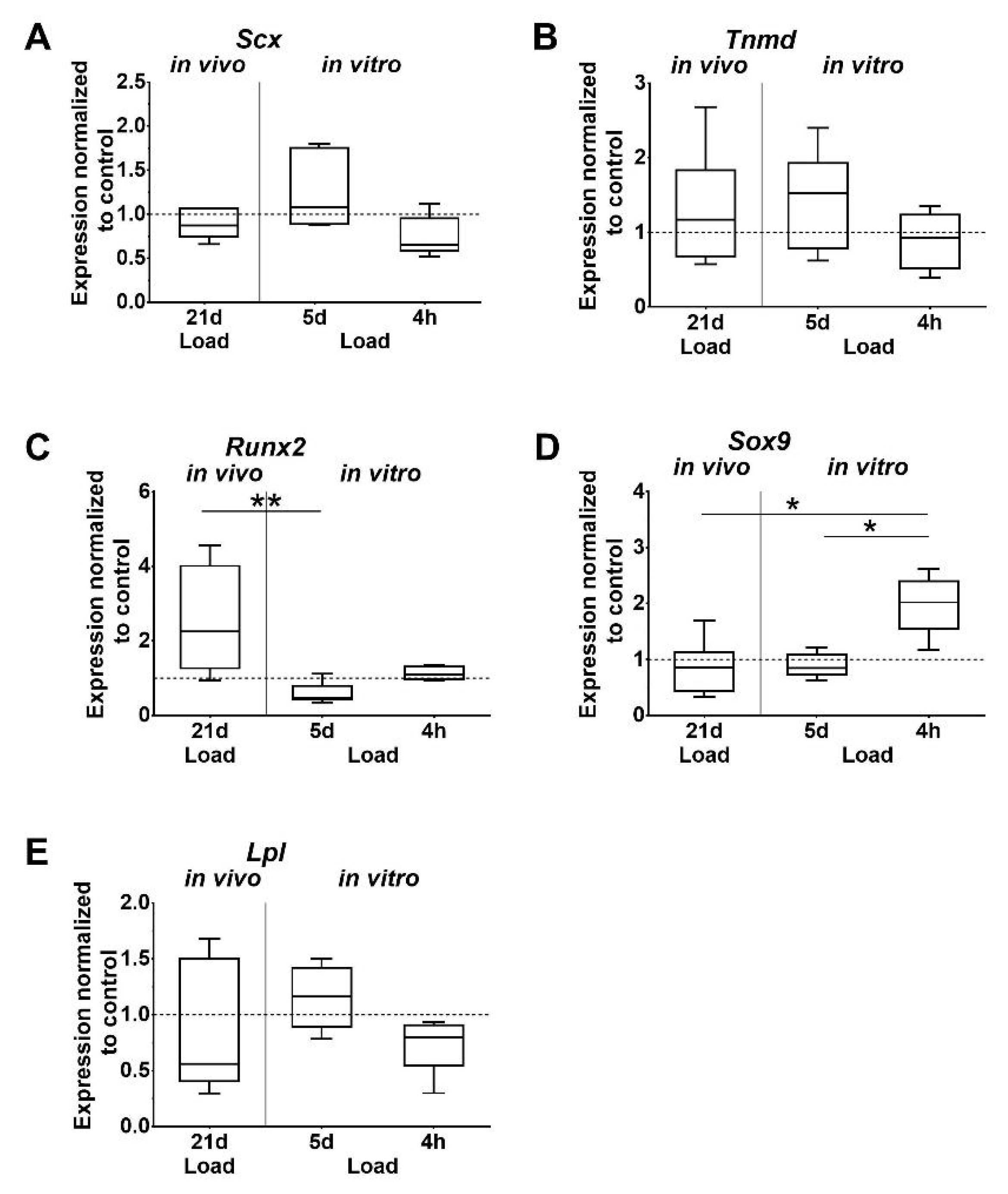
Analysis of gene expression showed that the tendon-associated markers Scleraxis (Scx) and Tenomodulin (Tnmd) were unaffected by mechanical loading in vivo. However, in vitro, the expression of Scx was lower after short-term stimulation compared to the 5 days of stimulation of tenocytes, but just missed significant difference (p = 0.052; Figure 3A,B). Changes of Tnmd expression were not detected. Expression of Runt related transcription factor 2 (Runx2), which is associated with osteogenic differentiation, was significantly higher after in vivo loading, compared to the in vitro stimulation of tenocytes for 5 days (Figure 3C). The chondrogenic marker sex determining region Y (SRY)-box 9 (Sox9) was significantly upregulated after 4 h in vitro compared to the other in vitro and in vivo loaded groups (Figure 3D). Expression of the adipogenic marker Lipoprotein lipase (Lpl) was not regulated by loading (Figure 3E).
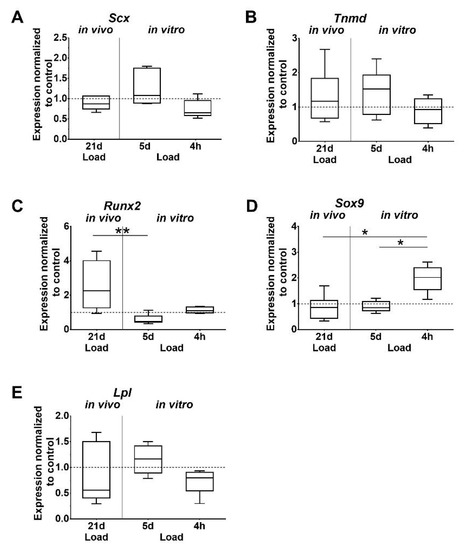
Figure 3.
Differential expression of tissue-specific markers. (A,B) Tenogenic, (C–E) osteogenic, chondrogenic, and adipogenic markers. Gene expression is given as fold change to non-loaded controls (horizontal line). Inter-group differences are marked with an asterisk (* p < 0.05, ** p < 0.01, n = 6).
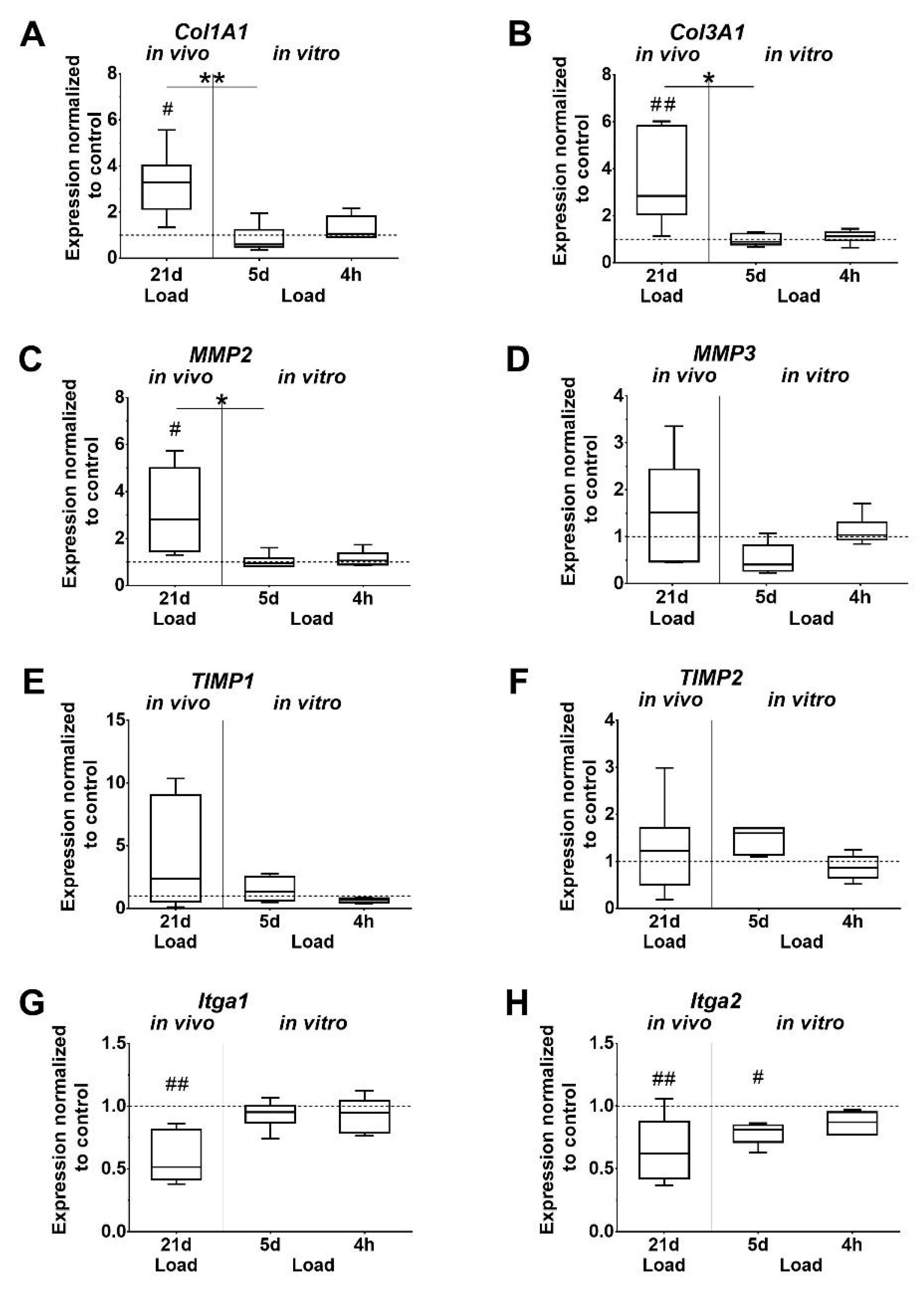
Expression levels of Col1A1 and Col3A1 were found to be significantly increased after in vivo loading, as well as compared to the 5-day in vitro loaded group. In vivo loading also increased Col3A1 expression compared to the 4-h in vitro loading group, but without reaching significant difference (p = 0.062). In vitro, the Col1A1 and Col3A1 expression was unaffected by short and long time loading of tenocytes (Figure 4A,B). A similar trend was observed for Matrix metalloproteinases 2 (MMP2), which was significantly upregulated in vivo compared to the unloaded control, the 5-day in vitro group, and by trend to the 4-h in vitro group (p = 0.052). In the in vitro groups, the expression of MMP2 and 3 was not regulated by loading. MMP3 expression showed a similar pattern compared to MMP2, but was not significantly affected (Figure 4C,D). Expression of Tissue inhibitor of metalloproteinase 1 and 2 (TIMP1; TIMP2) was unchanged by loading. The increase in the TIMP2 expression at 5 days missed significance compared to the unloaded in vitro control (p = 0.051) and the 4-h loading group (p = 0.060). Integrin α1 and α2 (Itga1, Itga2) expression was significantly downregulated in the in vivo loaded tendons compared to the unloaded controls. Furthermore, the expression of Itga2 was significantly lower in the 5-day in vitro stimulation group compared to the unloaded cells. In vitro loading for 4 h did not affect integrin expression (Figure 4G,H). Elastin (Eln), tumor necrosis factor alpha (TNF-α), and interleukin 1 beta (IL-1β) were expressed in mouse tendons, but only in very low amounts in isolated tenocytes, which could not be evaluated. In vivo, Eln expression was significantly increased whereas TNF-α expression was significantly decreased in loaded tendons compared to unloaded controls (p = 0.002, p = 0.048, respectively. The comparison of IL-1β expression in loaded versus unloaded tendons did not reveal significant differences.
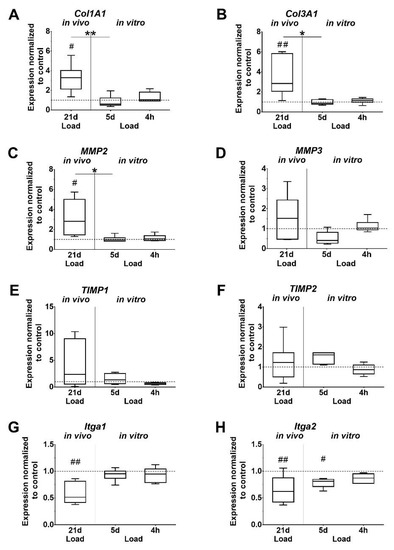
Figure 4.
Mechanical loading influences the expression of ECM components. (A,B) Collagens, (C,D) matrix metalloproteinases (MMPs), (E,F) Tissue inhibitor of metalloproteinase (TIMPs), (G,H) Integrins. Gene expression is given as fold change to non-loaded controls (horizontal line). Significant difference to non-loaded control is marked with a pound sign and inter-group differences with an asterisk (#, *: p < 0.05; ##, **: p < 0.01, n = 6).
3. Discussion
The ability of cells to sense mechanical loading is a fundamental process affecting tissue homeostasis, development, and repair. Physiologic forces are important to maintain tendon tissue homeostasis and adaptation, whereas unphysiological loading can lead to injuries, such as rupture or degeneration. The response of the tendon cells to adapt by anabolic or catabolic processes depends on the applied frequency, magnitude, duration, and direction. Therefore, deciphering the mechanobiology of tenocytes is a critical step to further understand the pathophysiology in a diseased tendon and the physiologic benefits of mechanical loading during tendon adaptation and healing [30,31]. In the present study, the impact of mechanical loading on the Achilles tendon of mice was assessed. In a second step, different regimens based on the in vivo protocol were applied to mouse tenocytes in vitro using equibiaxial loading.
Histological analysis of the Achilles tendons showed no alteration of the structural integrity in loaded tendons compared to the non-loaded control. The hierarchical structure with aligned collagen fibers was observed in both groups. The findings of the present study showed no signs of a degenerated tendon as the fibers were aligned and evidence of disorganization was not found [32,33,34]. Therefore, we conclude that axial compression of the tibia used in this study does not induce a degeneration process in the mouse Achilles tendon. Further morphological analysis revealed that mechanical in vivo loading did not influence the nuclear morphology in regard to size and shape, but the 2D in vitro culture had an effect on cell nuclei. The cell nuclei in the 4-h culture had a significantly rounder shape compared to the nuclei in vivo, independent of mechanical stimulation. After 5 days in vitro, a progression towards a more elongated morphology was observed, slightly more pronounced in the loaded group. This rounding of cell nuclei of tenocytes expanded in monolayer cultures was previously shown, and seems to be reverted by a 3D culturing of the cells [35]. The organization of the F-actin in the cells loaded in vitro was unchanged compared to the non-loaded control, with no major direction. Various studies have proven that different cell types are capable of sensing mechanical loading and align the cytoskeleton in the direction of uniaxial strain [36,37,38,39]. In the present study, the applied strain was equibiaxial, and theoretically comes from all directions. If the cell experiences strain from all directions, the cytoskeleton has no predominant direction to align with.
To investigate other possible effects of mechanical loading on the tendon and tenocytes, the expression of several genes responsible for cell differentiation and the ECM was analyzed. Scx has a well-defined role in the development of tendons during embryogenesis. Scx knockout mice exhibited a disordered tendon phenotype. In addition, Scx promotes the proliferation of tenocytes and the production of Col1 [40,41]. Another important factor involved in various processes, such as tenocyte proliferation, collagen organization, and fibril maturation is Tnmd [42]. In the present study, the expression of both factors was not affected in the loaded Achilles tendons in vivo. Studies showed that moderate as well as intense treadmill running is able to increase the expression of Scx and Tnmd in mice [23,43,44]. It is important to mention that they used a different loading regimen compared to the present study. Treadmill running is a well-established method to apply physiological and non-physiological strain to the Achilles tendon in rodents. In the present study, the histological analysis showed no degenerative signs and further indicated that the expression of tendon-associated markers was not influenced by the applied loading protocols. In vitro data showed, by trend, an increase of Scx expression from the 4-h stimulation to the 5-day stimulation. In contrast, Huisman et al. demonstrated that Scx expression is already upregulated after 4-h loading in human tenocytes [45]. This difference might be explained as Huisman et al. used a strain of 10% vs. 4% in the present study; in addition, the loading time differed.
Fat infiltration and ossification are pathological signs of tendon degeneration [46]. The differentiation into non-tenocytes is known to induce degenerative processes, which can later manifest as lipid depositions and calcified tissue. To test if mechanical loading of the tibia induces molecular processes that can lead to degeneration, the differentiation markers Lpl, Sox9, and Runx2 were examined for adipogenic, chondrogenic, and osteogenic differentiation, respectively. The expression of the differentiation markers was not significantly affected in the in vivo loaded Achilles tendons. However, the expression of Runx2 was significantly higher in vivo compared to the 5-day in vitro loading group, where loading resulted in a decreased expression. These results support our assumption that axial compression leads to no degeneration and alteration of the cells, and are in line with a previous study, where intensive treadmill running increased Runx2, Lpl and Sox9 expression in mouse Achilles tendons, while moderate running had no effect on the gene expression [23]. In the in vitro set-up, the differentiation markers were mostly unaffected from loading, except Sox9 in the 4-h group. Similar results were observed by Zhang et al. who determined no effect on gene expression at 4% strain in vitro; however, at 8% strain expression, levels were increased in tenocytes [23]. The expression of differentiation markers can be explained by the work of Bi et al. and Klatte-Schulz et al. They showed that human and mouse tendons harbor a cell population, called tendon stem/progenitor cells that exhibit stem cell characteristics, such as multipotency, self-renewal, and clonogenicity [47,48].
This study showed that the expression of Col1A1, Col3A1, and Elastin was significantly upregulated in the loaded Achilles tendon, which is consistent with the findings of other studies [23,49,50]. In the aforementioned studies and the present study, different methods of mechanical loading were applied to different animal species, but still, they all showed an adaptation of the Achilles tendon to mechanical loading by increasing the production of Col 1. The in vitro data showed an opposing trend wherein the expression of collagens was not affected by loading in both groups, and was significantly less expressed than after in vivo loading. The data suggest that the in vitro environment, such as loading regimen and culture conditions (e.g., coatings and media supplements) has to be adapted to further induce and mimic in vivo collagen expression. Kim et al. showed a similar trend of Col1 expression in rat tenocytes after loading for 12 h at a 4% strain rate [22]. Other studies, however, found an increase in collagen expression in their models [23,45]. A direct comparison of the results is difficult due to the different loading regimens used, but also indicates the variability of mechanobiology. Taken together, previous studies and the present study showed that the applied strain, frequency, and duration have a major impact and evoke different cell responses in tenocytes.
Comparing the results of the in vitro and in vivo effects to the study by Zhang et al., similar findings were observed. The effect of moderate treadmill running and tibial loading on gene expression were comparable. This was somewhat surprising considering that exercise has systemic effects, while tibial loading has only local effects on the bone and tendon. Our data indicate that a physiological stimulus evokes similar responses, independent of the type of loading. Mechanical loading of tenocytes in vitro revealed similar expression profiles at a strain rate of 4% in both studies, although frequency and loading time differed [23]. The comparability of in vitro loading studies is often limited because the loading protocols differ in strain rate, frequency, and time.
Matrix metalloproteases and tissue inhibitors of metalloproteases play an important role in the remodeling process. The balance between MMPs and TIMPs is critical for tendon integrity. MMP2 is a gelatinase, mainly degrading small collagen fragments and gelatin. The stromelysin MMP3 primarily degrades proteoglycans and glycoproteins. The activity of MMPs is regulated by TIMPs. MMP2 expression was significantly upregulated in the loaded Achilles tendon compared to non-loaded control. Other studies also reported changes in the expression of collagens, MMPs and TIMPs in response to physical activity [20,49,51,52,53,54,55]. The overall upregulation of MMPs and collagen expression indicates changes in the collagen composition, potentially leading to matrix turnover. In vitro, mechanical stimulation with 4% strain had no significant impact on the regulation of MMP and TIMP expression. In contrast, Huisman et al. showed that MMP2 and TIMP2 expression increased in human tenocytes subjected to 10% strain at a frequency of 10 Hz [55]. To confirm the direct regulatory effects, protein and activity levels of TIMPs and MMPs have to be determined. Another important MMP that plays a major role in collagen degradation is MMP1. It is known to be involved during human tendon healing and matrix remodeling [53,56,57]. In this study, MMP1 expression could not be detected neither in the tendons of the mice nor in the tenocytes of the mice. We tested several primer pairs, as well as murine control cells and tissues, but MMP1 expression was not detected in any of the experiments. However, it was shown that murine MMP1 is involved in various types of cancer, sepsis, and arthritis in mice [58,59,60,61]. This led us to the conclusion that MMP1 has regulatory effects in different diseases in mice, but, in contrast to humans, does not play a major role in tendon homeostasis.
Cytokines, such as interleukins, TNF-α, and interferon gamma are known to be key players in tendon disorders. They are released by the tendon stroma or immunoregulatory cells in response to mechanical stress or tissue injury, alter the cellular phenotype, and induce changes in matrix production [62]. Our in vitro data demonstrated the absence of endogenous expression of IL-1β and TNF-α in both groups. Studies show that tenocytes are able to express pro-inflammatory cytokines by themselves in response to cytokines or in co-culture with macrophages [63,64]. In vitro mechanical loading that induces an inflammatory response comparable to over-load can be used to investigate tenocyte behavior and their mechanism to cope with inflammation.
Integrins are cell surface receptors known to connect the ECM to the actin cytoskeleton, and transmit mechanical stimuli into the cell to evoke different cell responses. The collagen binding Itga1 and Itga2 were both down regulated due to in vivo loading, while only Itga2 was down regulated after 5 days in vitro loading. A study by Popov et al. found similar results after applying 5% stretch to human tendon progenitor stem cells for one day [65]. Another study by Jones et al. suggests that various integrins are involved in the interaction of the cell with ECM during load [66]. These results confirm that further research needs to be conducted to elucidate the role of integrins during mechanotransduction.
Taken together, the results of this study show that the applied in vivo and the in vitro loading protocols resulted only in a partially comparable reaction of the cells. The used in vitro loading protocol was designed to mimic the in vivo loading. The observed cellular reaction, however, was different between in vivo and in vitro, as well as the two in vitro protocols. On the cellular level, we observed that the nuclear size was comparable between in vivo and in vitro loading; however, a more elongated nuclei shape was seen in vivo, which was lost in tenocytes during 2D in vitro culture. On the molecular level, we observed partial similarity in the expression of tendon-associated and lineage-specific markers; however, for the ECM compartment, we determined a difference in gene expression. This can be explained by the differences in the protocols, by the different behavior of cells in a tissue or cells in a 2D culture, the normal activity of the mice between the stimulation periods, or the combination of all. Designing in vitro studies that mirror the in vivo situation is a challenging task, but this pilot study opens the discussion on how to design appropriate in vitro studies. In conclusion, either of the in vitro loading protocols could mimic some aspects of in vivo loading. For future studies, in vitro loading protocols might be extended to 21 days to have a more direct comparison to the in vivo loading protocol. Additionally, it should be considered to embed the cells in a 3D environment to better mimic the physical environment of tendons in vivo.
4. Limitations
In the present study, two different kinds of mechanical loading were applied. In the in vivo stimulation, axial compression of the tibia was applied, and it is assumed that it resulted in a mechanical stimulation of the Achilles tendon, and resembled a load and relieve situation through uniaxial tension. On the other hand, equibiaxial tension was applied to the cells in vitro. In addition, it has to be considered that mechanical stimulation was applied in a 3D environment in vivo, whereas, in vitro, the cells were cultured and stimulated in a 2D environment. In the 2D in vitro environment, the cells were no longer embedded in their ECM, and this might have affected the mechanoresponsiveness of the cells. Further studies should try to mimic the in vivo situation by using a 3D set-up by embedding the isolated cells in a collagen matrix. Additionally, the comparison with other studies is often limited due to different loading parameters, cell source, and culture conditions.
A second limitation of the study was that we were unable to quantify the load experienced by the tendon. In vivo strain gauging confirmed that 2000 µε was engendered at the tibial mid-shaft of young BALB/c mice (unpublished data). During in vivo loading, the muscle is thought to be inactive as a result of the anesthesia, but the Achilles tendon is likely undergoing loading as it is compressed against the foot platen. Further measurements need to be performed to determine the force that is transmitted to the tendon during in vivo tibial loading. Additionally, we were unable to determine if the effects we observed in the tendon at the molecular level were due to direct mechanical stimulation of the tendon or if they were a result of bone-tendon crosstalk. It is likely that molecular changes occuring in the bone influence tenocytes, since it is known that bone through osteocytes functions as a secretory endocrine organ [67].
In the study, two different inbred mouse strains were used: BALB/c for the in vivo study and C57BL/6J for the in vitro study. Holguin et al. compared the outcome of two tibial axial compression regimens in BALB/c and C57BL/6J mice. They found that the loading effects on cortical bone formation were similar in both mouse strains, and concluded that the adaption of the tibia to loading was more influenced by the loading regimen than the mouse strain [27]. Although the study was focused on bone, based on these findings, we conclude that the response of the tenocytes might be similar in the BALB/c and C57BL/6J mouse strains. In accordance with the Replace, Reduce, Refine (3R) rules, tenocytes were isolated from a different mouse strain than the in vivo mouse strain, due to the availability of the animals from an independent experiment, and to avoid killing animals just for cell isolation.
5. Materials and Methods
5.1. In Vivo Mechanical Stimulation of Achilles Tendon
Mechanical loading was applied (Testbench ElectroForce LM1, Bose, Framingham, MA, USA) to 10-week old female BALB/c mice (n = 12). The left tibia was subject to controlled axial compression (216 cycles/day, f = 4Hz, -10N load, 5 days/week, 3 weeks, εmax = 2000με at tibial mid-shaft determined by strain gauging). The right tibia served as the non-loaded control. During mechanical stimulation, mice were anesthetized with 2.5% isoflurane. At day 21, mice were sacrificed through cervical dislocation, and the Achilles tendons, with adjacent bone and muscle, were dissected for histological (n = 6) and gene expression analysis (n = 6). All animal experiments were performed according to the procedures and policies approved by the legal research animal welfare representative (LAGeSo Berlin, G0027/15, approved 14 April 2015).
5.2. In Vitro Mechanical Stimulation of Mouse Tenocytes
Achilles tendons from female C57BL/6J mice (26 weeks) were harvested and the whole tendon was used to isolate tenocytes by enzymatic digestion with Collagenase II (3mg/mL, Biochrom AG, Berlin, Germany) for 2 h at 37 °C. Cells were grown in growth medium (Dulbecco’s Modified Eagle Medium (DMEM), with 10% fetal calf serum (FCS), and 1% Penicillin/Streptomycin (P/S), all Biochrom AG, Berlin, Germany) and passaged at a confluency of 90%. At passage 3, cells were trypsinized and seeded with a concentration of 3 × 104 cells/well onto Collagen type I coated plates (Flexcell International Corporation, Burlington, USA). Prior to mechanical loading, cells were starved overnight for cell cycle synchronization in growth medium supplemented with 1% FCS. Mechanical stimulation was performed with the FX-4000TM Tension system (Flexcell International Corporation, Burlington, USA) with two different loading protocols: 1) 216 cycles at 4 Hz, 4% elongation per day, 5 days; 2) 216 cycles at 4 Hz, 4% elongation, 30 min rest insertions, 5 repetitions, 4 h. Non-loaded controls were cultured in the same plates, but did not receive mechanical loading. RNA isolation and staining of the cytoskeleton was performed after the last cycle.
5.3. Histological Evaluation
Tendon samples were washed with 70% ethanol and fixed in a 4% paraformaldehyde solution for 24 h. Samples were decalcified for 2 weeks in ethylenediaminetetraacetic acid (EDTA) and automatically dehydrated; afterwards, tendons were paraffin embedded. For histological analysis, 4 µm thin tissue sections were cut. A Hematoxylin and Eosin (H&E) staining was performed to get an overall impression of the tendon structure. Images were taken with an AxioCam MRc5 (Zeiss, Jena, Germany) and the associated software AxioVision Rel 4.8 (Zeiss, Jena, Germany). The cell nuclei from the H&E staining were automatically analyzed in regard to their size and shape using the image analysis software ImageJ (ImageJ 1.48 v, Wayne Rasband, National Institute of Health, Bethesda, MD, USA). The Sirius Red staining was used to stain the collagen fibers and visualized under a polarization microscope. In order to visualize all birefringent collagen fibers, images were taken at six different angles (0°, 15°, 30°, 45°, 60°, 75°) and subsequently, an overlay was created with the image analysis software Fiji (Fiji 1.2 v Johannes Schindelin, Max Planck Institute of Molecular Cell Biology and Genetics, Dresden, Germany). The orientation and anisotropy of the fibers was analyzed with the FibrilTool plugin for ImageJ. The score of anisotropy was defined as the following: 0 for no order (isotropic regions) and 1 for high order (anisotropic region), such as parallel fibrils [68].
5.4. Phalloidin/DAPI Staining
Mechanically stimulated mouse tenocytes were fixed in 4% PFA, washed, and permeabilized three times with Phosphate Buffered Saline (PBS) + 0.025% Triton. Then, the cells were incubated with a Phalloidin probe (Invitrogen, Karlsruhe, Germany) diluted 1:200 in PBS, for 1h at room temperature (RT). The cells were washed three times with PBS + 0.025% Triton and one time with Aqua Dest (Ampuwa, Bad Homburg, Germany). The nuclei of the cells were stained with DAPI (Molecular probes, Eugene, OR, USA) diluted 1:1500 in Aqua Dest for 15 min at RT. Subsequently, the cells were washed three times with Aqua Dest. The flexible membrane was cut out and placed on top of a slide. Lastly, the membrane was mounted with Fluoromount (Southern Biotech, Birmingham, AL, USA). Cells were visualized with a fluorescence microscope (Leica DMRB, Leica, Nussloch, Germany) and excited at 358 nm or 488 nm. Morphology of the nuclei was analyzed as described above.
5.5. Gene Expression Analysis
Total RNA was isolated from the dissected Achilles tendon with a precooled mortar system to homogenize the tissue. Afterwards, the powder was incubated in the lysis buffer provided by the RNA isolation kit (Qiagen, Hilden, Germany). Cells from the in vitro experiment were directly lysed in the plates through the addition of the lysis buffer. Phase separation was achieved through the addition of chloroform. After centrifugation, the aqueous phase containing the RNA was extracted and further purified with the miRNeasy Mini Kit according to the manufacturer’s protocol (Qiagen, Hilden, Germany). The purity and quantity was analyzed with the Nanodrop ND1000 system (Peqlab, Erlangen, Germany). A total amount of 100 ng RNA was transcribed into cDNA with the qScript cDNA Mix (Quanta Bioscience, Beverly, MA, USA). Quantitative real-time PCR was performed with the SYBR Green Mastermix (Quanta Bioscience) using the LightCycler 480 System (Roche, Mannheim, Germany). Primers were designed with the Primer 3 software (Table 1), except the primer pair for Scx (Qiagen, Hilden, Germany), and tested for their specificity and efficiency. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as the housekeeping gene, which has proven to be unaffected by the experimental set up. The normalized gene expression was calculated using an efficiency corrected equation.

Table 1.
List of qRT-Primers.
Normalized gene expression is given as a fold change to the non-loaded control.
5.6. Statistics
Statistical analysis was performed with GraphPad version 6 (GraphPad software, La Jolla California USA). The results are presented in box plots as median with 25 and 75 percentiles. The Kruskal–Wallis test and Dunn’s multiple comparison test were used to determine statistical differences between the 3 in vivo and in vitro loading groups, as well as the 3 loading groups compared to the unloaded control. For Eln, TNF-α, and IL-1β expression, the Mann-Whitney U test was performed to investigate significant differences between loaded and unloaded tendons. A p-value <0.05 was considered as statistical significant.
6. Conclusions
The findings of the present study provide first insights into changes of the mouse Achilles tendon after axial compression of the tibia in vivo. Histological analysis revealed only slight changes in the structural integrity; however, on a molecular level, significant differences were observed in the expression of collagens and MMPs, indicating changes in the ECM. This set up allows further investigation of mechanical loading without inducing degeneration to provide knowledge on the mechanobiology of tendons. The results of the in vitro stimulation mimicking the in vivo loading regimen and showed that partial aspects, such as nuclear size and the expression of tendon-associated markers, were similar. However, the differences of gene expression in the 2 in vitro loading protocols support the importance of strain, frequency, duration, and culturing condition. This pilot study is important for the development of an in vitro stimulation set up of tenocytes that mimics in vivo characteristics.
Author Contributions
B.W. and B.M.W. conceptualized the study. V.F., S.M., F.K.-S., and B.W. designed the experiments. A.S. (Anne Seliger), M.R., and B.M.W. provided the in vivo model and performed the animal work. V.F., A.S. (Aysha Schmock), F.K.-S., and S.M. performed the experiments and analyzed the data. V.F. and B.W. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This study was supported by institutional funding from the Charité-Universitätsmedizin Berlin. B.M.W. is supported by the FRQS Programme de bourses de chercheur. We acknowledge support by the German Research Foundation and the Open Access Publication Fund of the Thueringer Universitaets- und Landesbibliothek Jena Project-Nr. 433052568.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Col1A1 | Collagen type 1α1 |
| Col3A1 | Collagen type 3α1 |
| ECM | Extracellular matrix |
| Eln | Elastin |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| IL-1β | Interleukin-1beta |
| Itga | Integrin alpha |
| Lpl | Lipoprotein lipase |
| MNE | Mean normalized expression |
| MMP | Matrix metalloproteinase |
| qRT-PCR | Quantitative real-time PCR |
| Runx2 | Runt related transcription factor 2 |
| Scx | Scleraxis |
| Sox9 | SRY (sex determining region Y)-box 9 |
| TIMP | Tissue inhibitor of metalloproteinase |
| TNF-α | Tumor necrosis factor alpha |
| Tnmd | Tenomodulin |
References
- Sarasa-Renedo, A.; Chiquet, M. Mechanical signals regulating extracellular matrix gene expression in fibroblasts. Scand. J. Med. Sci. Sports 2005, 15, 223–230. [Google Scholar] [CrossRef]
- Beckham, C.; Dimond, R.; Greenlee, T.K. The role of movement in the development of a digital flexor tendon. Am. J. Anat. 1977, 150, 443–459. [Google Scholar] [CrossRef]
- Germiller, J.A.; Lerner, A.L.; Pacifico, R.J.; Loder, R.T.; Hensinger, R.N. Muscle and tendon size relationships in a paralyzed chick embryo model of clubfoot. J. Pediatr. Orthop. 1998, 18, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Mikic, B.; Johnson, T.L.; Chhabra, A.B.; Schalet, B.J.; Wong, M.; Hunziker, E.B. Differential effects of embryonic immobilization on the development of fibrocartilaginous skeletal elements. J. Rehabil. Res. Dev. 2000, 37, 127–133. [Google Scholar] [PubMed]
- Flynn, B.P.; Bhole, A.P.; Saeidi, N.; Liles, M.; Dimarzio, C.A.; Ruberti, J.W. Mechanical strain stabilizes reconstituted collagen fibrils against enzymatic degradation by mammalian collagenase matrix metalloproteinase 8 (MMP-8). PLoS ONE 2010, 5, 12337. [Google Scholar] [CrossRef] [PubMed]
- Legerlotz, K.; Jones, G.C.; Screen, H.R.C.; Riley, G.P. Cyclic loading of tendon fascicles using a novel fatigue loading system increases interleukin-6 expression by tenocytes. Scand. J. Med. Sci. Sports 2013, 23, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lin, Z.; Ni, M.; Thien, C.; Day, R.E.; Gardiner, B.; Rubenson, J.; Kirk, T.B.; Smith, D.W.; Wang, A.; et al. Cyclic mechanical stimulation rescues achilles tendon from degeneration in a bioreactor system. J. Orthop. Res. 2015, 33, 1888–1896. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lin, Z.; Day, R.E.; Gardiner, B.; Landao-Bassonga, E.; Rubenson, J.; Kirk, T.B.; Smith, D.W.; Lloyd, D.G.; Hardisty, G.; et al. Programmable mechanical stimulation influences tendon homeostasis in a bioreactor system. Biotechnol. Bioeng. 2013, 110, 1495–1507. [Google Scholar] [CrossRef]
- Thorpe, C.T.; Chaudhry, S.; Lei, I.I.; Varone, A.; Riley, G.P.; Birch, H.L.; Clegg, P.D.; Screen, H.R.C. Tendon overload results in alterations in cell shape and increased markers of inflammation and matrix degradation. Scand. J. Med. Sci. Sports 2015, 25, 381–391. [Google Scholar] [CrossRef]
- Wang, T.; Chen, P.; Zheng, M.; Wang, A.; Lloyd, D.; Leys, T.; Zheng, Q.; Zheng, M.H. In vitro loading models for tendon mechanobiology. J. Orthop. Res. 2017, 36, 566–575. [Google Scholar] [CrossRef]
- Crockett, R.J.; Centrella, M.; McCarthy, T.L.; Grant Thomson, J. Effects of cyclic strain on rat tail tenocytes. Mol. Biol. Rep. 2010, 37, 2629–2634. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Im, H.-J.; Wang, J.H.-C. Repetitive mechanical stretching modulates IL-1beta induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene 2005, 363, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Shao, L.; Wang, Q.; Dong, Y. Repetitive mechanical stretching modulates transforming growth factor-β induced collagen synthesis and apoptosis in human patellar tendon fibroblasts. Biochem. Cell Biol. 2012, 90, 667–674. [Google Scholar] [CrossRef]
- Tian, Y.; Gawlak, G.; O’Donnell, J.J.; Mambetsariev, I.; Birukova, A.A. Modulation of Endothelial Inflammation by Low and High Magnitude Cyclic Stretch. PLoS ONE 2016, 11, e0153387. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, A.; Richardson, D.W. Regulation of the tenogenic gene expression in equine tenocyte-derived induced pluripotent stem cells by mechanical loading and Mohawk. Stem Cell Res. 2019, 39, e101489. [Google Scholar] [CrossRef] [PubMed]
- Tsuzaki, M.; Bynum, D.; Almekinders, L.; Yang, X.; Faber, J.; Banes, A.J. ATP modulates load-inducible IL-1beta, COX 2, and MMP-3 gene expression in human tendon cells. J. Cell. Biochem. 2003, 89, 556–562. [Google Scholar] [CrossRef]
- Mousavizadeh, R.; Khosravi, S.; Behzad, H.; McCormack, R.G.; Duronio, V.; Scott, A. Cyclic Strain Alters the Expression and Release of Angiogenic Factors by Human Tendon Cells. PLoS ONE 2014, 9, e97356. [Google Scholar] [CrossRef]
- Mousavizadeh, R.; Duronio, V.; McCormack, B.; Khosravi, S.; Scott, A. Mechanical loading modulates angiogenic factors in tendon cell. Br. J. Sports Med. 2013, 47. [Google Scholar] [CrossRef]
- Li, Z.; Yang, G.; Khan, M.; Stone, D.; Woo, S.L.-Y.; Wang, J.H.-C. Inflammatory Response of Human Tendon Fibroblasts to Cyclic Mechanical Stretching. Am. J. Sports Med. 2004, 32, 435–440. [Google Scholar] [CrossRef]
- Heinemeier, K.M.; Skovgaard, D.; Bayer, M.L.; Qvortrup, K.; Kjaer, A.; Kjaer, M.; Magnusson, S.P.; Kongsgaard, M. Uphill running improves rat Achilles tendon tissue mechanical properties and alters gene expression without inducing pathological changes. J. Appl. Physiol. 2012, 113, 827–836. [Google Scholar] [CrossRef]
- Thampatty, B.P.; Wang, J.H.-C. Mechanobiology of young and aging tendons: In vivo studies with treadmill running. J. Orthop. Res. 2018, 36, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Joo Kim, J.; Musson, D.S.; Matthews, B.G.; Cornish, J.; Anderson, I.A.; Shim, V.B. Applying Physiologically Relevant Strains to Tenocytes in an In Vitro Cell Device Induces In Vivo Like Behaviors. J. Biomech. Eng. 2016, 138, 121003–121012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.H.-C.; Lake, S.; Hill, A.; Glaser, D. The Effects of Mechanical Loading on Tendons—An In Vivo and In Vitro Model Study. PLoS ONE 2013, 8, e71740. [Google Scholar] [CrossRef] [PubMed]
- Birkhold, A.I.; Razi, H.; Duda, G.N.; Checa, S.; Willie, B.M. Tomography-Based Quantification of Regional Differences in Cortical Bone Surface Remodeling and Mechano-Response. Calcif. Tissue Int. 2017, 100, 255–270. [Google Scholar] [CrossRef]
- Birkhold, A.I.; Razi, H.; Duda, G.N.; Weinkamer, R.; Checa, S.; Willie, B.M. The Periosteal Bone Surface is Less Mechano-Responsive than the Endocortical. Sci. Rep. 2016, 6, 23480. [Google Scholar] [CrossRef]
- Checa, S.; Hesse, B.; Roschger, P.; Aido, M.; Duda, G.N.; Raum, K.; Willie, B.M. Skeletal maturation substantially affects elastic tissue properties in the endosteal and periosteal regions of loaded mice tibiae. Acta Biomater. 2015, 21, 154–164. [Google Scholar] [CrossRef]
- Holguin, N.; Brodt, M.D.; Sanchez, M.E.; Kotiya, A.A.; Silva, M.J. Adaptation of tibial structure and strength to axial compression depends on loading history in both C57BL/6 and BALB/c mice. Calcif. Tissue Int. 2013, 93, 211–221. [Google Scholar] [CrossRef]
- Razi, H.; Birkhold, A.I.; Weinkamer, R.; Duda, G.N.; Willie, B.M.; Checa, S. Aging Leads to a Dysregulation in Mechanically Driven Bone Formation and Resorption. J. Bone Miner. Res. 2015, 30, 1864–1873. [Google Scholar] [CrossRef]
- Main, R.P.; Shefelbine, S.J.; Meakin, L.B.; Silva, M.J.; van der Meulen, M.C.H.; Willie, B.M. Murine Axial Compression Tibial Loading Model to Study Bone Mechanobiology: Implementing the Model and Reporting Results. J. Orthop. Res. 2020, 38, 233–252. [Google Scholar] [CrossRef]
- Arnoczky, S.P.; Lavagnino, M.; Egerbacher, M. The mechanobiological aetiopathogenesis of tendinopathy: Is it the over-stimulation or the under-stimulation of tendon cells? Int. J. Exp. Pathol. 2007, 88, 217–226. [Google Scholar] [CrossRef]
- Lavagnino, M.; Wall, M.E.; Little, D.; Banes, A.J.; Guilak, F.; Arnoczky, S.P. Tendon mechanobiology: Current knowledge and future research opportunities. J. Orthop. Res. 2015, 33, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Maffulli, N.; Longo, U.G.; Maffulli, G.D.; Rabitti, C.; Khanna, A.; Denaro, V. Marked pathological changes proximal and distal to the site of rupture in acute Achilles tendon ruptures. Knee Surg. Sport. Traumatol. Arthrosc. 2011, 19, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Klatte-Schulz, F.; Minkwitz, S.; Schmock, A.; Bormann, N.; Kurtoglu, A.; Tsitsilonis, S.; Manegold, S.; Wildemann, B. Different Achilles Tendon Pathologies Show Distinct Histological and Molecular Characteristics. Int. J. Mol. Sci. 2018, 19, 404. [Google Scholar] [CrossRef] [PubMed]
- Pingel, J.; Lu, Y.; Starborg, T.; Fredberg, U.; Langberg, H.; Nedergaard, A.; Weis, M.; Eyre, D.; Kjaer, M.; Kadler, K.E. 3-D ultrastructure and collagen composition of healthy and overloaded human tendon: Evidence of tenocyte and matrix buckling. J. Anat. 2014, 224, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Stoll, C.; John, T.; Endres, M.; Rosen, C.; Kaps, C.; Kohl, B.; Sittinger, M.; Ertel, W.; Schulze-Tanzil, G. Extracellular matrix expression of human tenocytes in three-dimensional air-liquid and PLGA cultures compared with tendon tissue: Implications for tendon tissue engineering. J. Orthop. Res. 2010, 28, 1170–1177. [Google Scholar] [CrossRef]
- Gonzales, D.A.; Ferng, A.S.; Geffre, C.P.; Borg, J.L.; Miller, M.; Szivek, J.A. Mechanical loading of adipose derived stromal cells causes cell alignment. J. Biomed. Sci. Eng. 2011, 4, 357–361. [Google Scholar] [CrossRef]
- Grenier, G.; Rémy-Zolghadri, M.; Larouche, D.; Gauvin, R.; Baker, K.; Bergeron, F.; Dupuis, D.; Langelier, E.; Rancourt, D.; Auger, F.A.; et al. Tissue Reorganization in Response to Mechanical Load Increases Functionality. Tissue Eng. 2005, 11, 90–100. [Google Scholar] [CrossRef]
- Cardwell, R.D.; Kluge, J.A.; Thayer, P.S.; Guelcher, S.A.; Dahlgren, L.A.; Kaplan, D.L.; Goldstein, A.S. Static and Cyclic Mechanical Loading of Mesenchymal Stem Cells on Elastomeric, Electrospun Polyurethane Meshes. J. Biomech. Eng. 2015, 137, 071010. [Google Scholar] [CrossRef]
- Nam, H.Y.; Pingguan-Murphy, B.; Abbas, A.A.; Merican, A.M.; Kamarul, T. Uniaxial Cyclic Tensile Stretching at 8% Strain Exclusively Promotes Tenogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stromal Cells. Stem Cells Int. 2019, 2019, 9723025. [Google Scholar] [CrossRef]
- Scott, A.; Sampaio, A.; Abraham, T.; Duronio, C.; Underhill, T.M. Scleraxis expression is coordinately regulated in a murine model of patellar tendon injury. J. Orthop. Res. 2011, 29, 289–296. [Google Scholar] [CrossRef]
- Schweitzer, R.; Chyung, J.H.; Murtaugh, L.C.; Brent, A.E.; Rosen, V.; Olson, E.N.; Lassar, A.; Tabin, C.J. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 2001, 128, 3855–3866. [Google Scholar] [PubMed]
- Docheva, D.; Hunziker, E.B.; Fässler, R.; Brandau, O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol. Cell. Biol. 2005, 25, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Mendias, C.L.; Gumucio, J.P.; Bakhurin, K.I.; Lynch, E.B.; Brooks, S. V Physiological loading of tendons induces scleraxis expression in epitenon fibroblasts. J. Orthop. Res. 2012, 30, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Sakabe, T.; Sunaga, A.; Sakai, K.; Rivera, A.L.; Keene, D.R.; Sasaki, T.; Stavnezer, E.; Iannotti, J.; Schweitzer, R.; et al. Conversion of mechanical force into TGF-β-mediated biochemical signals. Curr. Biol. 2011, 21, 933–941. [Google Scholar] [CrossRef]
- Huisman, E.; Lu, A.; McCormack, R.G.; Scott, A. Enhanced collagen type I synthesis by human tenocytes subjected to periodic in vitro mechanical stimulation. BMC Musculoskelet. Disord. 2014, 15, 386. [Google Scholar] [CrossRef]
- Klatte-Schulz, F.; Gerhardt, C.; Scheibel, M.; Wildemann, B.; Pauly, S. Relationship between muscle fatty infiltration and the biological characteristics and stimulation potential of tenocytes from rotator cuff tears. J. Orthop. Res. 2014, 32, 129–137. [Google Scholar] [CrossRef]
- Bi, Y.; Ehirchiou, D.; Kilts, T.M.; Inkson, C.A.; Embree, M.C.; Sonoyama, W.; Li, L.; Leet, A.I.; Seo, B.-M.; Zhang, L.; et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 2007, 13, 1219–1227. [Google Scholar] [CrossRef]
- Klatte-Schulz, F.; Pauly, S.; Scheibel, M.; Greiner, S.; Gerhardt, C.; Schmidmaier, G.; Wildemann, B. Influence of age on the cell biological characteristics and the stimulation potential of male human tenocyte-like cells. Eur. Cell. Mater. 2012, 24, 74–89. [Google Scholar] [CrossRef]
- Heinemeier, K.M.; Olesen, J.L.; Haddad, F.; Langberg, H.; Kjaer, M.; Baldwin, K.M.; Schjerling, P. Expression of collagen and related growth factors in rat tendon and skeletal muscle in response to specific contraction types. J. Physiol. 2007, 582, 1303–1316. [Google Scholar] [CrossRef]
- Archambault, J.M.; Hart, D.A.; Herzog, W. Response of rabbit Achilles tendon to chronic repetitive loading. Connect. Tissue Res. 2001, 42, 13–23. [Google Scholar] [CrossRef]
- Legerlotz, K.; Schjerling, P.; Langberg, H.; Brüggemann, G.-P.; Niehoff, A. The effect of running, strength, and vibration strength training on the mechanical, morphological, and biochemical properties of the Achilles tendon in rats. J. Appl. Physiol. 2007, 102, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Lo, I.K.Y.; Marchuk, L.L.; Hollinshead, R.; Hart, D.A.; Frank, C.B. Matrix Metalloproteinase and Tissue Inhibitor of Matrix Metalloproteinase mRNA Levels are Specifically Altered in Torn Rotator Cuff Tendons. Am. J. Sports Med. 2004, 32, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Riley, G.P.; Curry, V.; DeGroot, J.; van El, B.; Verzijl, N.; Hazleman, B.L.; Bank, R.A. Matrix metalloproteinase activities and their relationship with collagen remodelling in tendon pathology. Matrix Biol. 2002, 21, 185–195. [Google Scholar] [CrossRef]
- Sun, H.B.; Andarawis-Puri, N.; Li, Y.; Fung, D.T.; Lee, J.Y.; Wang, V.M.; Basta-Pljakic, J.; Leong, D.J.; Sereysky, J.B.; Ros, S.J.; et al. Cycle-dependent matrix remodeling gene expression response in fatigue-loaded rat patellar tendons. J. Orthop. Res. 2010, 28, 1380–1386. [Google Scholar] [CrossRef]
- Huisman, E.; Lu, A.; Jamil, S.; Mousavizadeh, R.; McCormack, R.; Roberts, C.; Scott, A. Influence of repetitive mechanical loading on MMP2 activity in tendon fibroblasts. J. Orthop. Res. 2016, 34, 1991–2000. [Google Scholar] [CrossRef]
- Del Buono, A.; Oliva, F.; Longo, U.G.; Rodeo, S.A.; Orchard, J.; Denaro, V.; Maffulli, N. Metalloproteases and rotator cuff disease. J. Shoulder Elb. Surg. 2012, 21, 200–208. [Google Scholar] [CrossRef]
- Minkwitz, S.; Schmock, A.; Kurtoglu, A.; Tsitsilonis, S.; Manegold, S.; Wildemann, B.; Klatte-Schulz, F. Time-dependent alterations of MMPs, TIMPs and tendon structure in human achilles tendons after acute rupture. Int. J. Mol. Sci. 2017, 18, 2199. [Google Scholar] [CrossRef]
- Silva, S.M.; Jerônimo, M.S.; Silva-Pereira, I.; Bocca, A.L.; Sousa, J.B. Expression of metalloproteinases and interleukins on anastomoses in septic rats. J. Surg. Res. 2013, 183, 777–782. [Google Scholar] [CrossRef]
- Fanjul-Fernández, M.; Folgueras, A.R.; Fueyos, A.; Balbín, M.; Suárez, M.F.; Fernández-García, M.S.; Shapiro, S.D.; Freije, J.M.P.; López-Otín, C. Matrix metalloproteinase Mmp-1a is dispensable for normal growth and fertility in mice and promotes lung cancer progression by modulating inflammatory responses. J. Biol. Chem. 2013, 288, 14647–14656. [Google Scholar] [CrossRef]
- Foley, C.J.; Luo, C.; O’Callaghan, K.; Hinds, P.W.; Covic, L.; Kuliopulos, A. Matrix metalloprotease-1a promotes tumorigenesis and metastasis. J. Biol. Chem. 2012, 287, 24330–24338. [Google Scholar] [CrossRef]
- Tressel, S.L.; Kaneider, N.C.; Kasuda, S.; Foley, C.; Koukos, G.; Austin, K.; Agarwal, A.; Covic, L.; Opal, S.M.; Kuliopulos, A. A matrix metalloprotease-PAR1 system regulates vascular integrity, systemic inflammation and death in sepsis. EMBO Mol. Med. 2011, 3, 370–384. [Google Scholar] [CrossRef]
- Morita, W.; Dakin, S.G.; Snelling, S.J.B.; Carr, A.J. Cytokines in tendon disease: A systematic review. Bone Jt. Res. 2017, 6, 656–664. [Google Scholar] [CrossRef]
- Stolk, M.; Klatte-Schulz, F.; Schmock, A.; Minkwitz, S.; Wildemann, B.; Seifert, M. New insights into tenocyte-immune cell interplay in an in vitro model of inflammation. Sci. Rep. 2017, 7, 9801. [Google Scholar] [CrossRef]
- John, T.; Lodka, D.; Kohl, B.; Ertel, W.; Jammrath, J.; Conrad, C.; Stoll, C.; Busch, C.; Schulze-Tanzil, G. Effect of pro-inflammatory and immunoregulatory cytokines on human tenocytes. J. Orthop. Res. 2010, 28, 1071–1077. [Google Scholar] [CrossRef]
- Popov, C.; Burggraf, M.; Kreja, L.; Ignatius, A.; Schieker, M.; Docheva, D. Mechanical stimulation of human tendon stem/progenitor cells results in upregulation of matrix proteins, integrins and MMPs, and activation of p38 and ERK1/2 kinases. BMC Mol. Biol. 2015, 16, 6. [Google Scholar] [CrossRef]
- Jones, E.; Legerlotz, K.; Riley, G. Mechanical regulation of integrins in human tenocytes in collagen and fibrin matrices. Orthop. Proc. 2014, 96-B, 161. [Google Scholar]
- Bonewald, L. Use it or lose it to age: A review of bone and muscle communication. Bone 2019, 120, 212–218. [Google Scholar] [CrossRef]
- Boudaoud, A.; Burian, A.; Borowska-Wykręt, D.; Uyttewaal, M.; Wrzalik, R.; Kwiatkowska, D.; Hamant, O. FibrilTool, an ImageJ plug-in to quantify fibrillar structures in raw microscopy images. Nat. Protoc. 2014, 9, 457–463. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).