Characterization of Inhibitory Effectiveness in Hyperpolarization-Activated Cation Currents by a Group of ent-Kaurane-Type Diterpenoids from Croton tonkinensis
Abstract
1. Introduction
2. Results
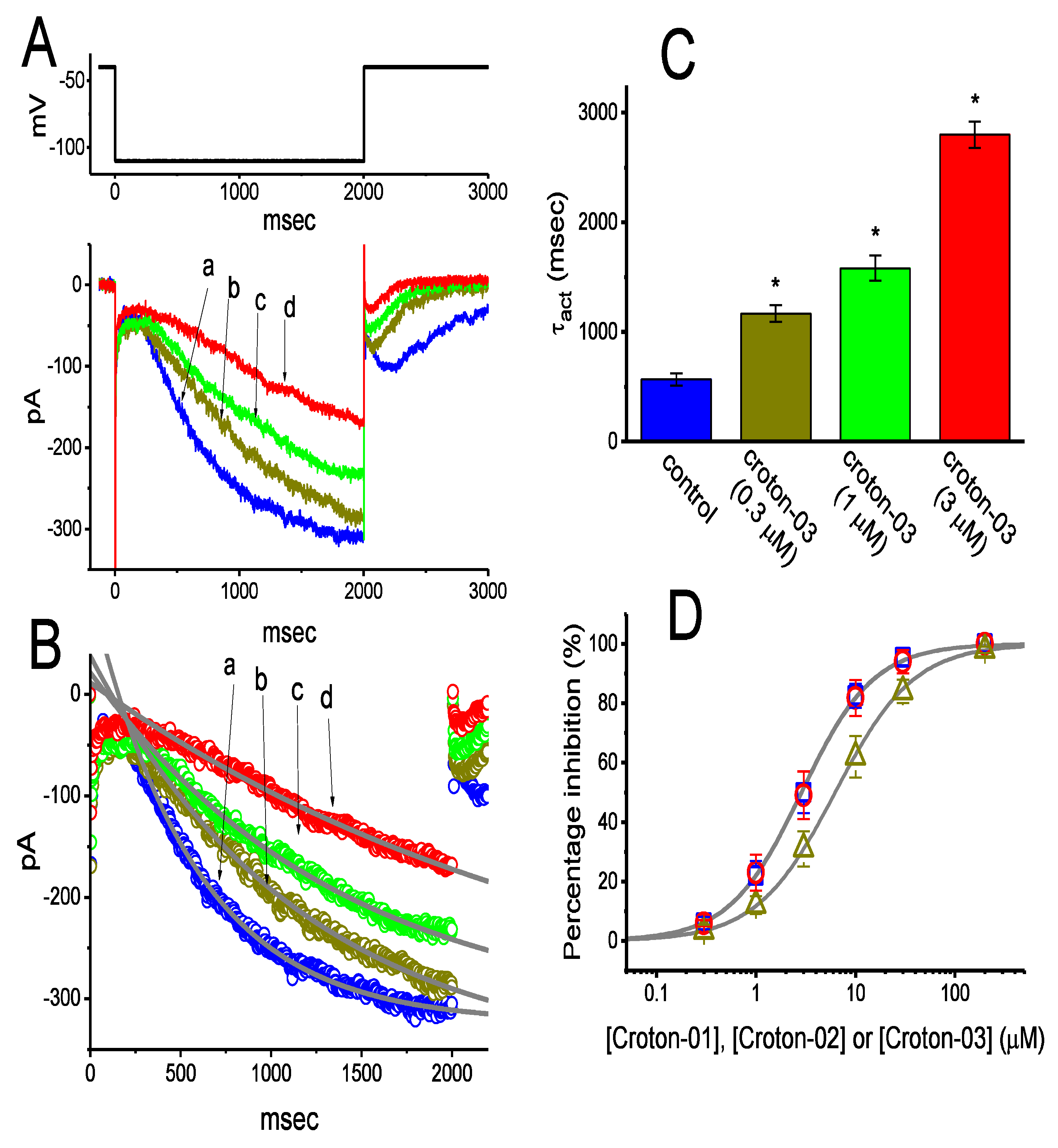
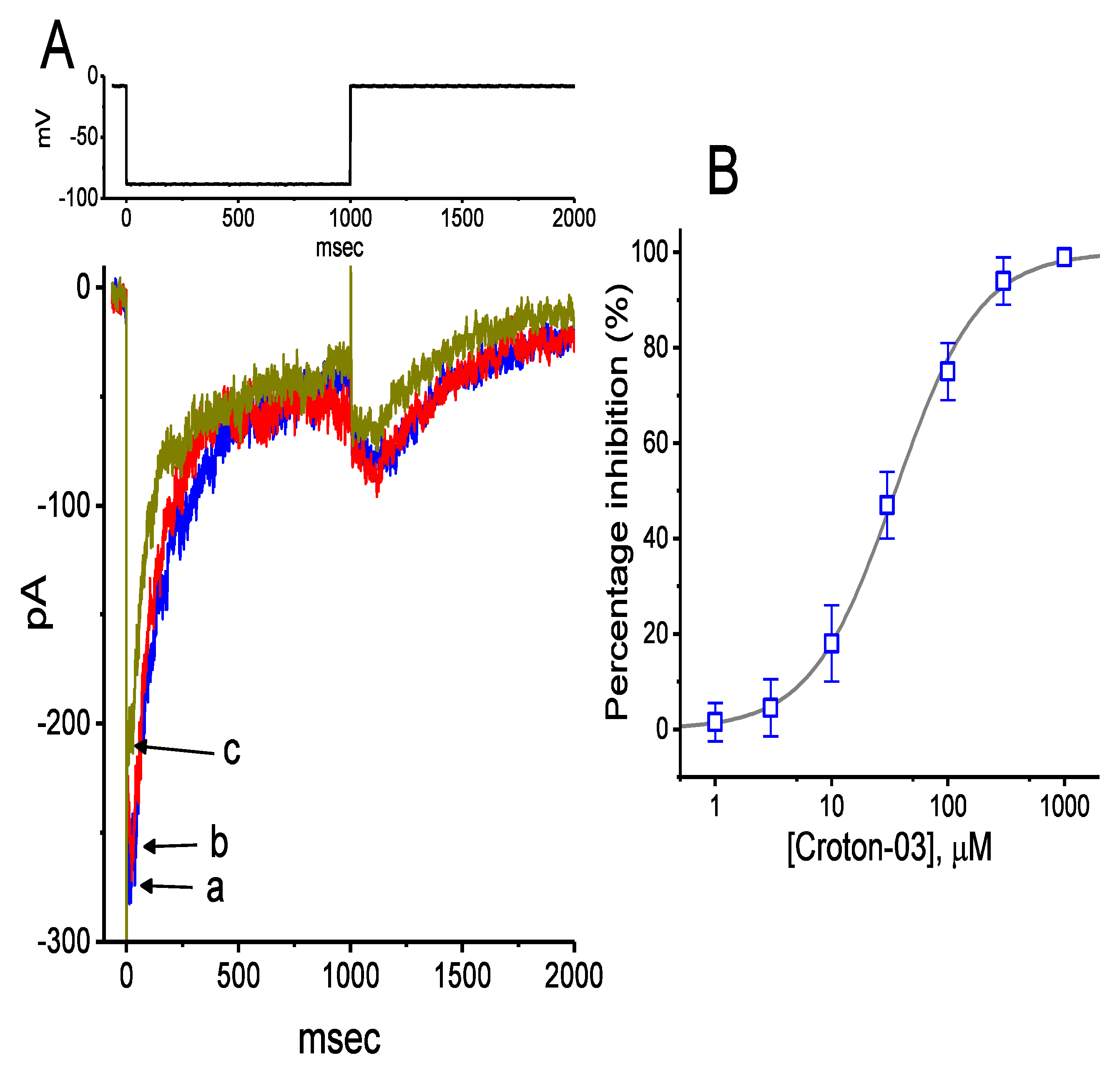
2.1. Effects of Croton-03 and Other ent-Kaurane Diterpenoids on Hyperpolarization-Activated Cation Current (Ih) Identified in Pituitary GH3 Cells
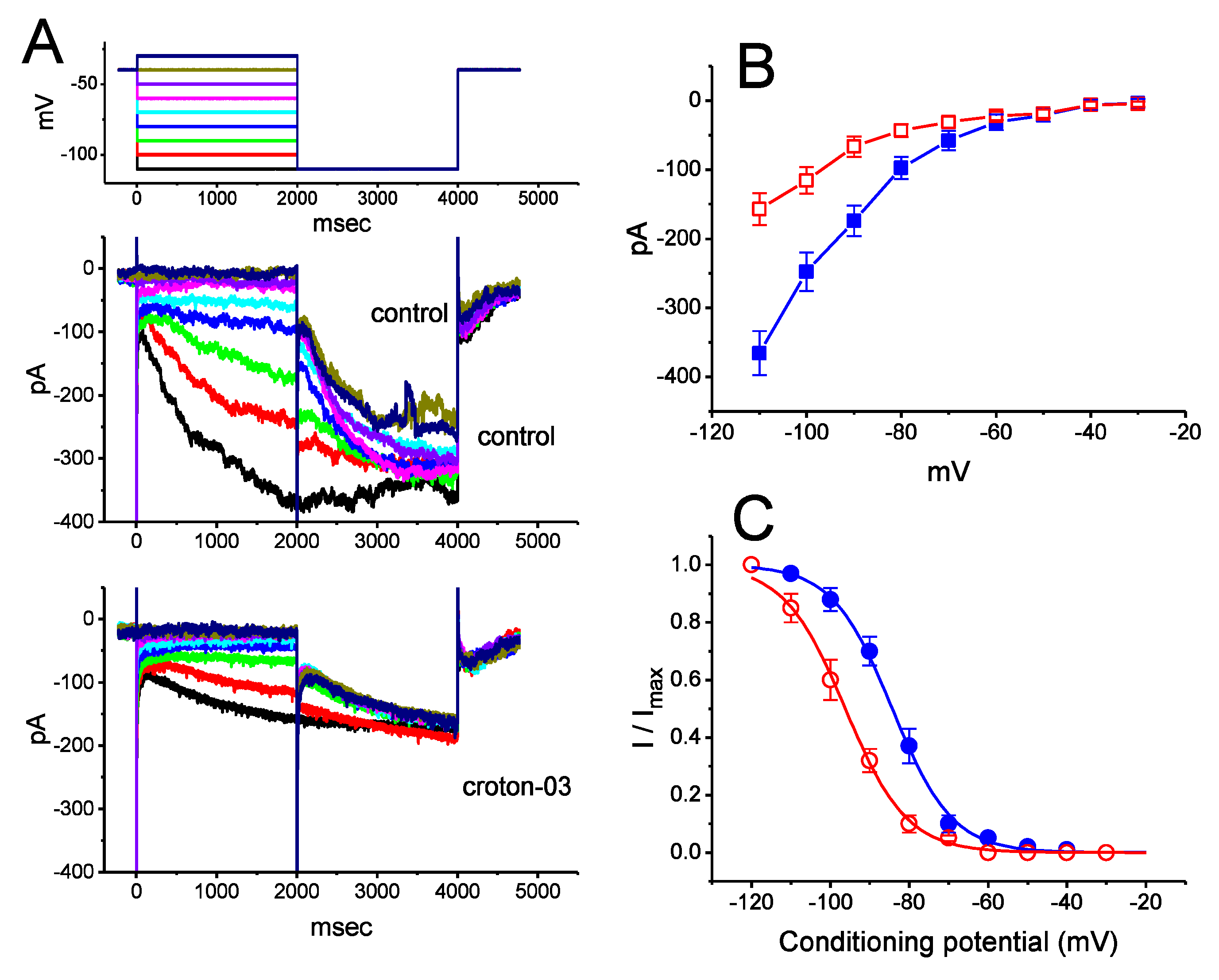
2.2. Effects of Croton-03 on I-V Relationship and Steady-State Activation Curve of Ih in GH3 Cells
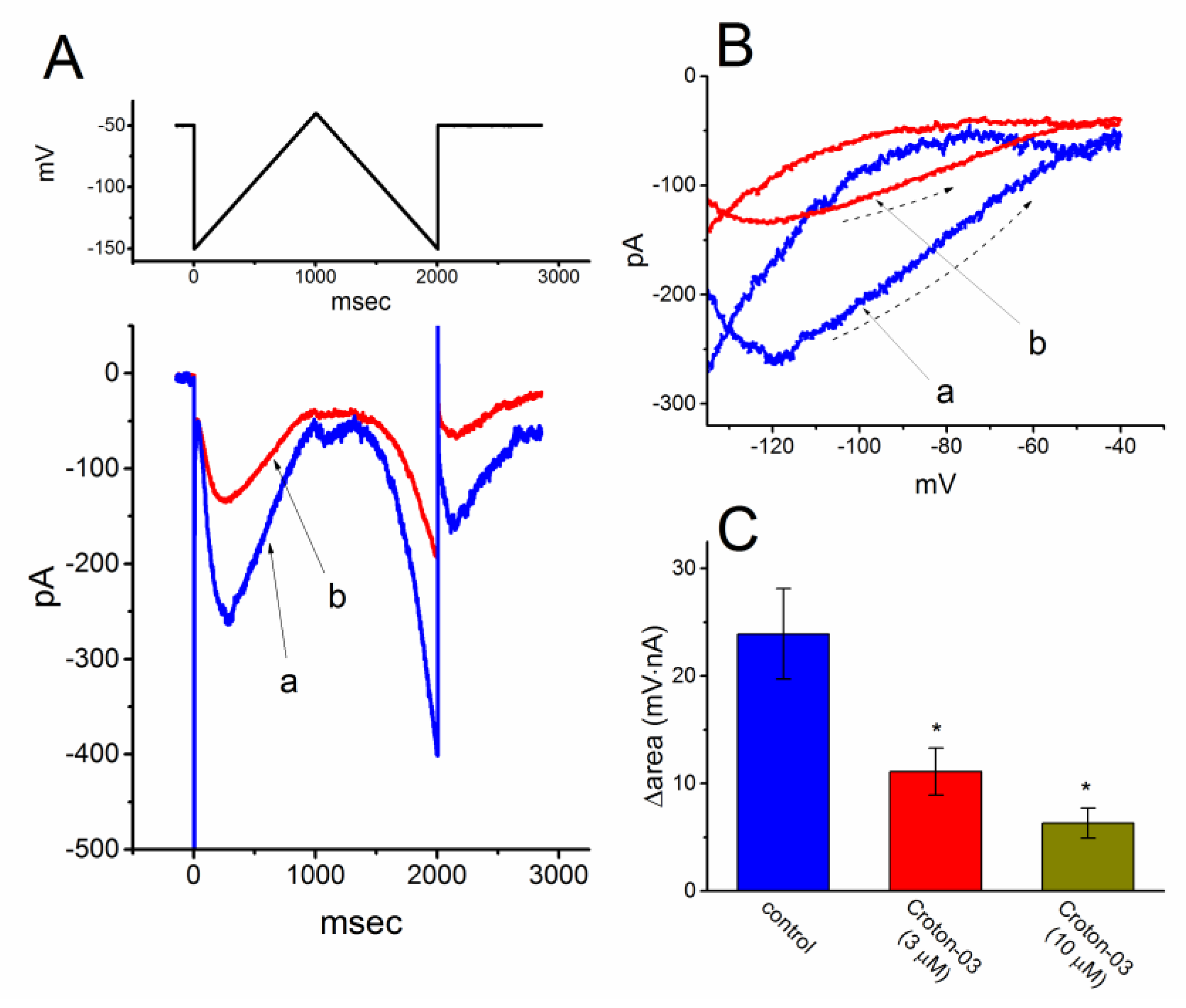
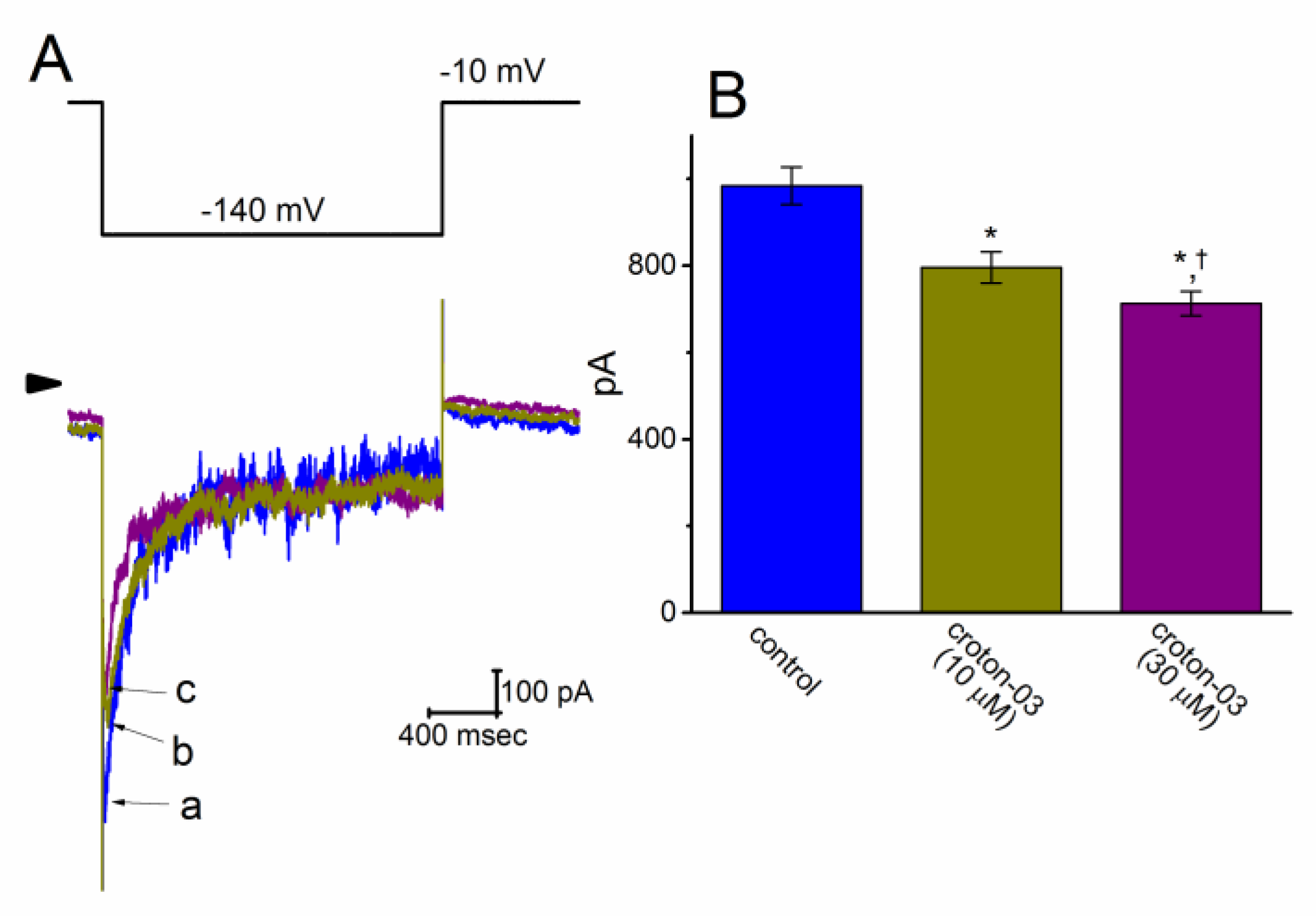
2.3. Effect of Croton-03 on the Voltage-Dependent Hysteresis of Ih Elicited in Response to Long-Lasting Triangular Ramp Pulse
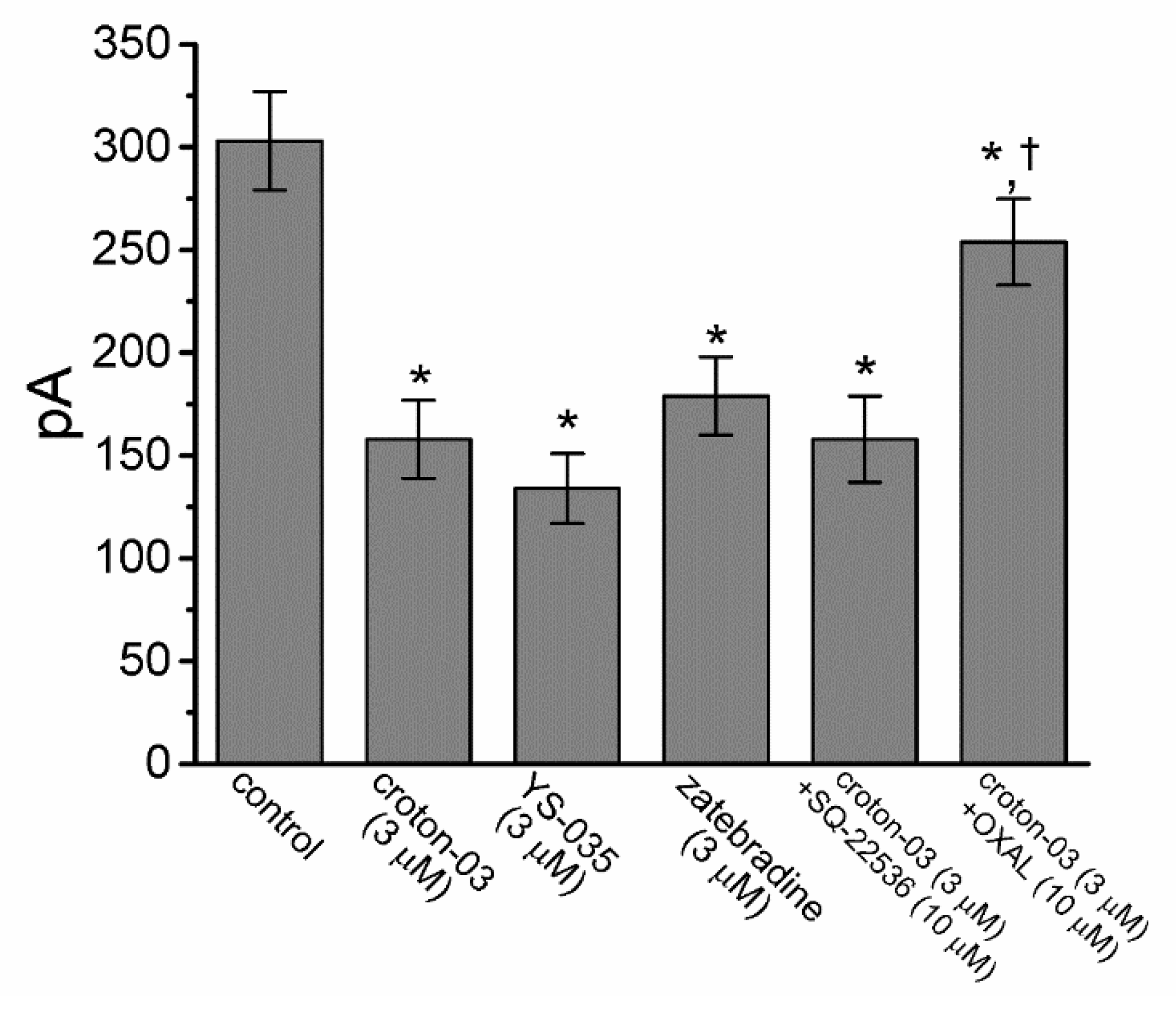
2.4. Effect of Croton-03, YS-035, Zatebradine, Croton-03 Plus SQ-22536, and Croton-03 Plus Oxaliplatin on Ih Amplitude
2.5. Effect of Croton-03 on erg-Mediated K+ Current (IK(erg)) in GH3 Cells
2.6. Effect of Croton-03 on Sag Potential Measured from GH3 Cells
2.7. Inhibitory Effect of Croton-03 on Ih in INS1 Insulin-Secreting Cells
2.8. Effect of Croton-03 on IK(erg) Identified in INS1 Cells
3. Discussion
4. Materials and Methods
4.1. Purification, Extraction, and Fractionation of Plant Materials
4.2. Drugs, Chemicals, and Solutions
4.3. Cell Preparations
4.4. Electrophysiological Measurements
4.5. Data Recordings
4.6. Data Analyses
4.7. Statistical Analyses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| croton-01 | ent-18-acetoxy-7α-hydroxykaur-16-en-15-one |
| croton-02 | ent-7α,14β-dihydroxykaur-16-en-15-one |
| croton-03 | ent-1β-acetoxy-7α,14β-dihydroxykaur-16-en-15-one |
| erg | ether-à-go-go-related gene |
| HCN channel | hyperpolarization-activated, cyclic nucleotide-gated channel |
| I-V | current versus voltage |
| IC50 | the concentration required for 50% inhibition |
| Ih | hyperpolarization-activated cation current |
| IK(erg) | erg-mediated K+ current |
| act | activation time constant |
References
- Giang, P.M.; Son, P.T.; Lee, J.J.; Otsuka, H. Four ent-kaurane-type diterpenoids from Croton tonkinensis Gagnep. Chem. Pharm. Bull. 2004, 52, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.R.; Lira, G.H.; Oliveira Neto, R.M.; Graca, J.R.; Vasconcelos, P.R.; Nobre e Souza, M.A.; Magalhaes, P.J.; Rola, F.H.; Santos, A.A. 1.8 cineole decreases gastric compliance in anesthetized rats. Acta Cir. Bras. 2007, 22, 63–67. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Siqueira, R.J.; Magalhaes, P.J.; Leal-Cardoso, J.H.; Duarte, G.P.; Lahlou, S. Cardiovascular effects of the essential oil of Croton zehntneri leaves and its main constituents, anethole and estragole, in normotensive conscious rats. Life Sci. 2006, 78, 2365–2372. [Google Scholar] [CrossRef]
- de Siqueira, R.J.; Duarte, G.P.; Magalhaes, P.J.; Lahlou, S. Cardiovascular effects of the essential oil of Croton zehntneri leaves in DOCA-salt hypertensive, conscious rats. Nat. Prod. Commun. 2013, 8, 1167–1170. [Google Scholar] [CrossRef] [PubMed]
- Leal-Cardoso, J.H.; Lahlou, S.; Weinreich, D.; Caldas Magalhaes, P.J. The essential oil of Croton nepetaefolius selectively blocks histamine-augmented neuronal excitability in guinea-pig celiac ganglion. J. Pharm. Pharmacol. 2010, 62, 1045–1053. [Google Scholar] [CrossRef]
- Aguiar, L.A.; Porto, R.S.; Lahlou, S.; Ceccatto, V.M.; Barbosa, R.; Lemos, T.L.; dos Santos, H.S.; Coelho-de-Souza, A.N.; Magalhaes, P.J.; Zin, W.A.; et al. Antispasmodic effects of a new kaurene diterpene isolated from Croton argyrophylloides on rat airway smooth muscle. J. Pharm. Pharmacol. 2012, 64, 1155–1164. [Google Scholar] [CrossRef]
- Fischer, H.; Machen, T.E.; Widdicombe, J.H.; Carlson, T.J.; King, S.R.; Chow, J.W.; Illek, B. A novel extract SB-300 from the stem bark latex of Croton lechleri inhibits CFTR-mediated chloride secretion in human colonic epithelial cells. J. Ethnopharmacol. 2004, 93, 351–357. [Google Scholar] [CrossRef]
- Tradtrantip, L.; Namkung, W.; Verkman, A.S. Crofelemer, an antisecretory antidiarrheal proanthocyanidin oligomer extracted from Croton lechleri, targets two distinct intestinal chloride channels. Mol. Pharmacol. 2010, 77, 69–78. [Google Scholar] [CrossRef]
- Belardinelli, L.; Giles, W.R.; West, A. Ionic mechanisms of adenosine actions in pacemaker cells from rabbit heart. J. Physiol. 1988, 405, 615–633. [Google Scholar] [CrossRef]
- DiFrancesco, D. Serious workings of the funny current. Prog. Biophys. Mol. Biol. 2006, 90, 13–25. [Google Scholar] [CrossRef]
- Hao, X.M.; Xu, R.; Chen, A.Q.; Sun, F.J.; Wang, Y.; Liu, H.X.; Chen, H.; Xue, Y.; Chen, L. Endogenous HCN channels modulate the firing activity of globus pallidus neurons in Parkinsonian animals. Front. Aging Neurosci. 2019, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Chen, F.; Li, B.; Hu, Z. Neurophysiology of HCN channels: From cellular functions to multiple regulations. Prog. Neurobiol. 2014, 112, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Irisawa, H.; Brown, H.F.; Giles, W. Cardiac pacemaking in the sinoatrial node. Physiol. Rev. 1993, 73, 197–227. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Wang, Y.J.; Wu, P.Y.; Wu, S.N. Tramadol-induced block of hyperpolarization-activated cation current in rat pituitary lactotrophs. Naunyn. Schmiedebergs Arch. Pharmacol. 2009, 379, 127–135. [Google Scholar] [CrossRef]
- McKinley, J.W.; Shi, Z.; Kawikova, I.; Hur, M.; Bamford, I.J.; Sudarsana Devi, S.P.; Vahedipour, A.; Darvas, M.; Bamford, N.S. Dopamine deficiency reduces striatal cholinergic interneuron function in models of Parkinson’s disease. Neuron 2019, 103, 1056–1072.e6. [Google Scholar] [CrossRef]
- Spinelli, V.; Sartiani, L.; Mugelli, A.; Romanelli, M.N.; Cerbai, E. Hyperpolarization-activated cyclic-nucleotide-gated channels: Pathophysiological, developmental, and pharmacological insights into their function in cellular excitability. Can. J. Physiol. Pharmacol. 2018, 96, 977–984. [Google Scholar] [CrossRef]
- Stojilkovic, S.S.; Tabak, J.; Bertram, R. Ion channels and signaling in the pituitary gland. Endocr. Rev. 2010, 31, 845–915. [Google Scholar] [CrossRef]
- Hsiao, H.T.; Liu, Y.C.; Liu, P.Y.; Wu, S.N. Concerted suppression of Ih and activation of IK(M) by ivabradine, an HCN-channel inhibitor, in pituitary cells and hippocampal neurons. Brain Res. Bull. 2019, 149, 11–20. [Google Scholar] [CrossRef]
- Romanelli, M.N.; Cerbai, E.; Dei, S.; Guandalini, L.; Martelli, C.; Martini, E.; Scapecchi, S.; Teodori, E.; Mugelli, A. Design, synthesis and preliminary biological evaluation of zatebradine analogues as potential blockers of the hyperpolarization-activated current. Bioorg. Med. Chem. 2005, 13, 1211–1220. [Google Scholar] [CrossRef]
- Mellon, D., Jr. Electrophysiological evidence for intrinsic pacemaker currents in crayfish parasol cells. PLoS ONE 2016, 11, e0146091. [Google Scholar] [CrossRef]
- Tigerholm, J.; Poulsen, A.H.; Andersen, O.K.; Morch, C.D. From perception threshold to ion channels-a computational study. Biophys. J. 2019, 117, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.C.; Silva, M.F.; Maciel, M.A.; Pinto, A.C.; Nunes, D.S.; Lima, R.M.; Bastos, J.K.; Sarti, S.J. Investigation of anti-inflammatory and antinociceptive activities of trans-dehydrocrotonin, a 19-nor-clerodane diterpene from Croton cajucara. Part 1. Planta Med. 1996, 62, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, K.W.; Felipe, J.L.; Malange, K.F.; do Prado, P.R.; de Oliveira Figueiredo, P.; Garcez, F.R.; de Cassia Freitas, K.; Garcez, W.S.; Toffoli-Kadri, M.C. Anti-inflammatory and antinociceptive activities of Croton urucurana Baillon bark. J. Ethnopharmacol. 2016, 183, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Simasko, S.M.; Sankaranarayanan, S. Characterization of a hyperpolarization-activated cation current in rat pituitary cells. Am. J. Physiol. 1997, 272, E405–E414. [Google Scholar] [CrossRef]
- Barthel, L.; Reetz, O.; Strauss, U. Use dependent attenuation of rat HCN1-mediated Ih in Intact HEK293 Cells. Cell Physiol. Biochem. 2016, 38, 2079–2093. [Google Scholar] [CrossRef]
- Fürst, O.; D’Avanzo, N. Isoform dependent regulation of human HCN channels by cholesterol. Sci. Rep. 2015, 5, 14270. [Google Scholar] [CrossRef]
- Männikkö, R.; Pandey, S.; Larsson, H.P.; Elinder, F. Hysteresis in the voltage dependence of HCN channels: Conversion between two modes affects pacemaker properties. J. Gen. Physiol. 2005, 125, 305–326. [Google Scholar] [CrossRef]
- Cabral, P.H.; de Morais Campos, R.; Fonteles, M.C.; Santos, C.F.; Leal Cardoso, J.H.; do Nascimento, N.R. Effects of the essential oil of Croton zehntneri and its major components, anethole and estragole, on the rat corpora cavernosa. Life Sci. 2014, 112, 74–81. [Google Scholar] [CrossRef]
- Berger, F.; Borchard, U.; Hafner, D.; Kammer, T.; Weis, T. Inhibition of potassium outward currents and pacemaker current in sheep cardiac Purkinje fibres by the verapamil derivative YS 035. Naunyn. Schmiedebergs Arch. Pharmacol. 1991, 344, 653–661. [Google Scholar] [CrossRef]
- Dini, L.; Del Lungo, M.; Resta, F.; Melchiorre, M.; Spinelli, V.; Di Cesare Mannelli, L.; Ghelardini, C.; Laurino, A.; Sartiani, L.; Coppini, R.; et al. Selective blockade of HCN1/HCN2 channels as a potential pharmacological strategy against pain. Front. Pharmacol. 2018, 9, 1252. [Google Scholar] [CrossRef]
- Resta, F.; Micheli, L.; Laurino, A.; Spinelli, V.; Mello, T.; Sartiani, L.; Di Cesare Mannelli, L.; Cerbai, E.; Ghelardini, C.; Romanelli, M.N.; et al. Selective HCN1 block as a strategy to control oxaliplatin-induced neuropathy. Neuropharmacology 2018, 131, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.N.; Yang, W.H.; Yeh, C.C.; Huang, H.C. The inhibition by di(2-ethylhexyl)-phthalate of erg-mediated K+ current in pituitary tumor (GH3) cells. Arch. Toxicol. 2012, 86, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Albertson, A.J.; Bohannon, A.S.; Hablitz, J.J. HCN channel modulation of synaptic integration in GABAergic interneurons in malformed rat neocortex. Front. Cell Neurosci. 2017, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Datunashvili, M.; Chaudhary, R.; Zobeiri, M.; Luttjohann, A.; Mergia, E.; Baumann, A.; Balfanz, S.; Budde, B.; van Luijtelaar, G.; Pape, H.C.; et al. Modulation of hyperpolarization-activated inward current and thalamic activity modes by different cyclic nucleotides. Front. Cell Neurosci. 2018, 12, 369. [Google Scholar] [CrossRef]
- Kuo, P.C.; Yang, C.J.; Lee, Y.C.; Chen, P.C.; Liu, Y.C.; Wu, S.N. The comprehensive electrophysiological study of curcuminoids on delayed-rectifier K+ currents in insulin-secreting cells. Eur. J. Pharmacol. 2018, 819, 233–241. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Qu, J.; Hardy, A.; Zhang, N.; Diao, J.; Strijbos, P.J.; Tsushima, R.; Robinson, R.B.; Gaisano, H.Y.; et al. Functional characterization of hyperpolarization-activated cyclic nucleotide-gated channels in rat pancreatic beta cells. J. Endocrinol. 2009, 203, 45–53. [Google Scholar] [CrossRef][Green Version]
- Skelin, M.; Rupnik, M.; Cencic, A. Pancreatic beta cell lines and their applications in diabetes mellitus research. ALTEX 2010, 27, 105–113. [Google Scholar] [CrossRef]
- Lainez, S.; Tsantoulas, C.; Biel, M.; McNaughton, P.A. HCN3 ion channels: Roles in sensory neuronal excitability and pain. J. Physiol. 2019, 597, 4661–4675. [Google Scholar] [CrossRef]
- Zhao, W.; Liang, P.; Liu, J.; Li, H.; Liao, D.; Chen, X.; Li, Q.; Zhou, C. Capsazepine prolongation of the duration of lidocaine block of sensory transmission in mice may be mediated by modulation of HCN channel currents. PeerJ 2019, 7, e7111. [Google Scholar] [CrossRef]
- Djouhri, L.; Smith, T.; Ahmeda, A.; Alotaibi, M.; Weng, X. Hyperpolarization-activated cyclic nucleotide-gated channels contribute to spontaneous activity in L4 C-fiber nociceptors, but not Aβ-non-nociceptors, after axotomy of L5-spinal nerve in the rat in vivo. Pain 2018, 159, 1392–1402. [Google Scholar] [CrossRef]
- Kuo, P.C.; Shen, Y.C.; Yang, M.L.; Wang, S.H.; Thang, T.D.; Dung, N.X.; Chiang, P.C.; Lee, K.H.; Lee, E.J.; Wu, T.S. Crotonkinins A and B and related diterpenoids from Croton tonkinensis as anti-inflammatory and antitumor agents. J. Nat. Prod. 2007, 70, 1906–1909. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, H.T.; Lee, Y.C.; Liu, Y.C.; Kuo, P.C.; Wu, S.N. Differential suppression of delayed-rectifier and inwardly rectifier K+ currents by a group of ent-kaurane-type diterpenoids from Croton tonkinensis, in microglial cells. Eur. J. Pharmacol. 2019, 856, 172414. [Google Scholar] [CrossRef]
- Wu, S.N.; Chern, J.H.; Shen, S.; Chen, H.H.; Hsu, Y.T.; Lee, C.C.; Chan, M.H.; Lai, M.C.; Shie, F.S. Stimulatory actions of a novel thiourea derivative on large-conductance, calcium-activated potassium channels. J. Cell Physiol. 2017, 232, 3409–3421. [Google Scholar] [CrossRef] [PubMed]
- Gadagkar, S.R.; Call, G.B. Computational tools for fitting the Hill equation to dose-response curves. J. Pharmacol. Toxicol. Methods 2015, 71, 68–76. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, P.-C.; Liu, Y.-C.; Lo, Y.-C.; Wu, S.-N. Characterization of Inhibitory Effectiveness in Hyperpolarization-Activated Cation Currents by a Group of ent-Kaurane-Type Diterpenoids from Croton tonkinensis. Int. J. Mol. Sci. 2020, 21, 1268. https://doi.org/10.3390/ijms21041268
Kuo P-C, Liu Y-C, Lo Y-C, Wu S-N. Characterization of Inhibitory Effectiveness in Hyperpolarization-Activated Cation Currents by a Group of ent-Kaurane-Type Diterpenoids from Croton tonkinensis. International Journal of Molecular Sciences. 2020; 21(4):1268. https://doi.org/10.3390/ijms21041268
Chicago/Turabian StyleKuo, Ping-Chung, Yen-Chin Liu, Yi-Ching Lo, and Sheng-Nan Wu. 2020. "Characterization of Inhibitory Effectiveness in Hyperpolarization-Activated Cation Currents by a Group of ent-Kaurane-Type Diterpenoids from Croton tonkinensis" International Journal of Molecular Sciences 21, no. 4: 1268. https://doi.org/10.3390/ijms21041268
APA StyleKuo, P.-C., Liu, Y.-C., Lo, Y.-C., & Wu, S.-N. (2020). Characterization of Inhibitory Effectiveness in Hyperpolarization-Activated Cation Currents by a Group of ent-Kaurane-Type Diterpenoids from Croton tonkinensis. International Journal of Molecular Sciences, 21(4), 1268. https://doi.org/10.3390/ijms21041268





