X-ray Diffraction Studies on the Structural Origin of Dynamic Tension Recovery Following Ramp-Shaped Releases in High-Ca Rigor Muscle Fibers
Abstract
1. Introduction
2. Results
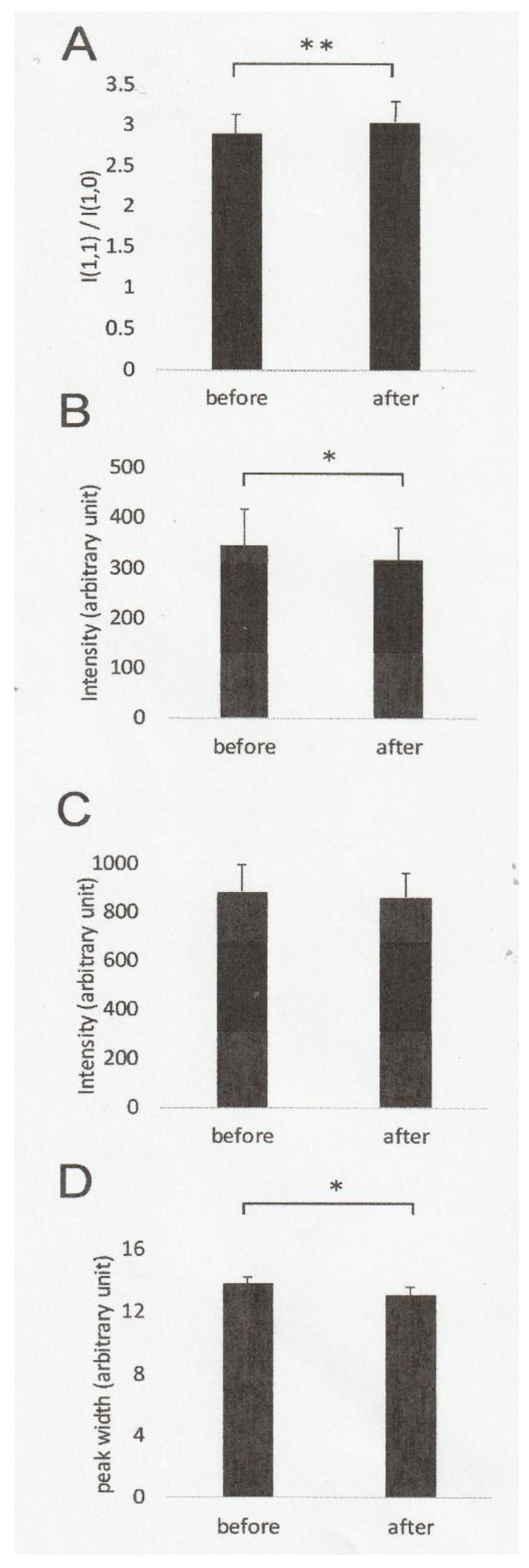
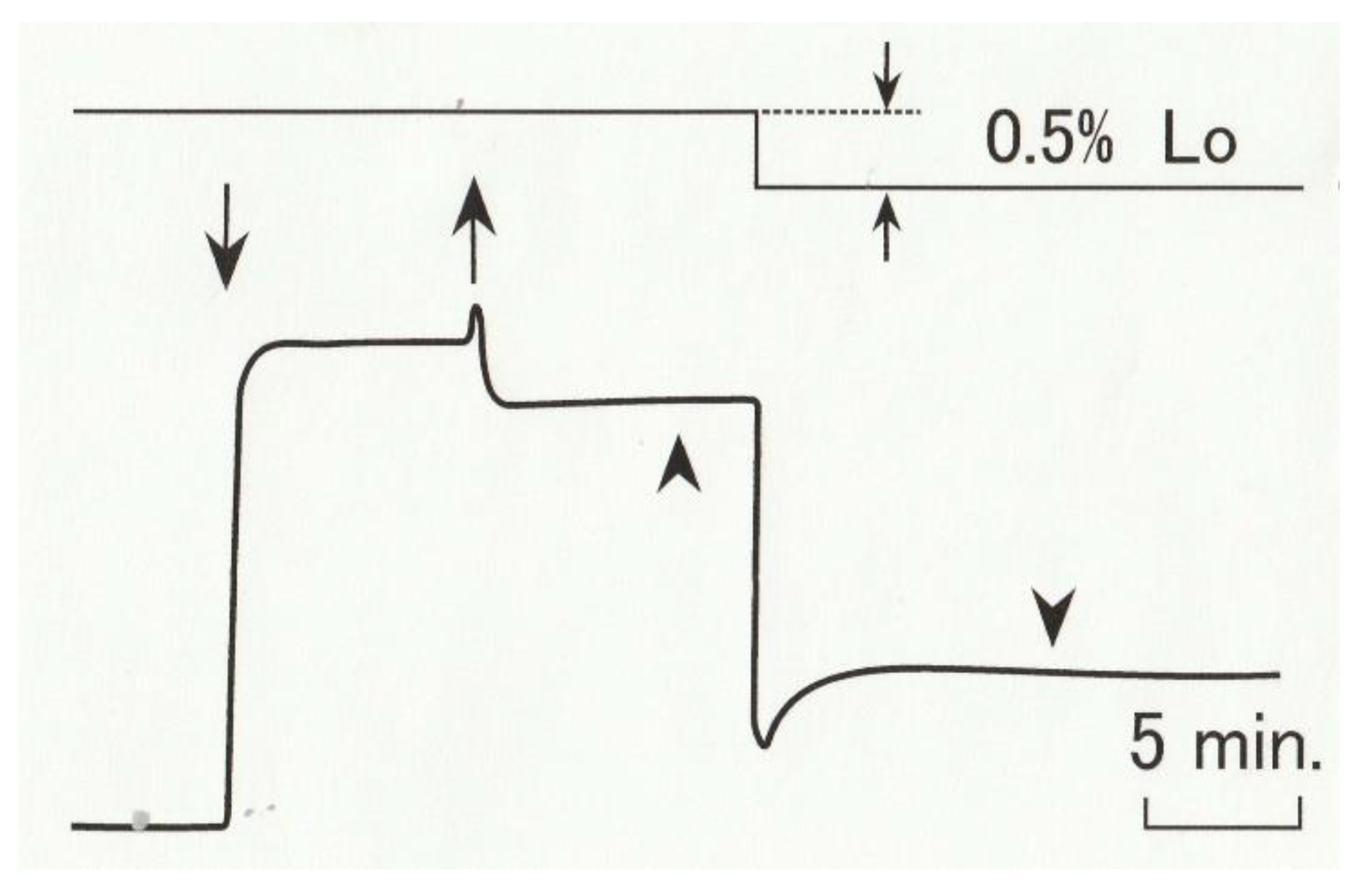
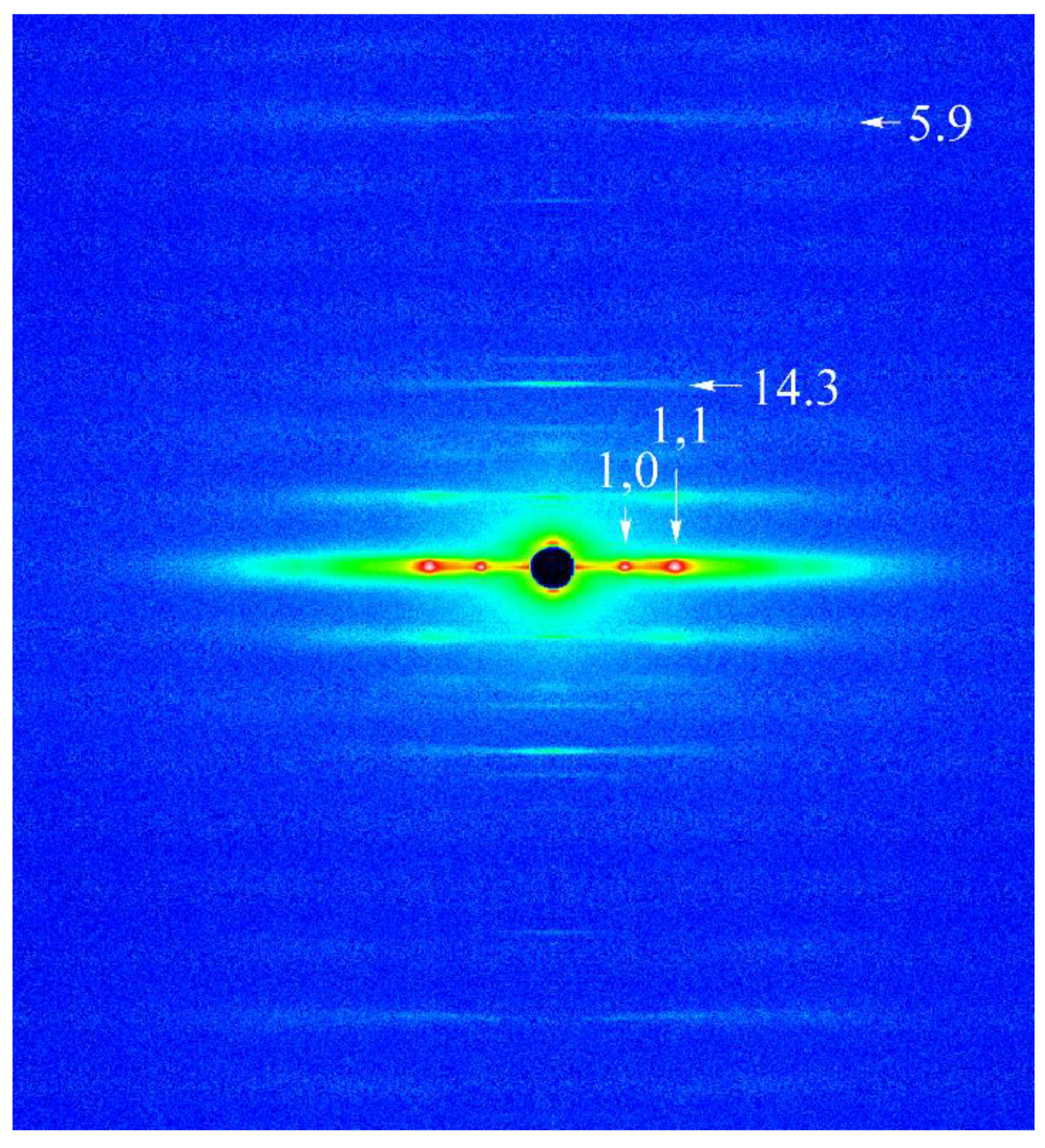
2.1. Effect of Ramp-Shaped Releases on the Equatorial Reflections of High-Ca Rigor Muscle Fibers
2.2. Effect of Ramp-shaped Releases on the Intensity Profile of 14.3 nm Meridional Reflection of High-Ca Rigor Muscle Fibers
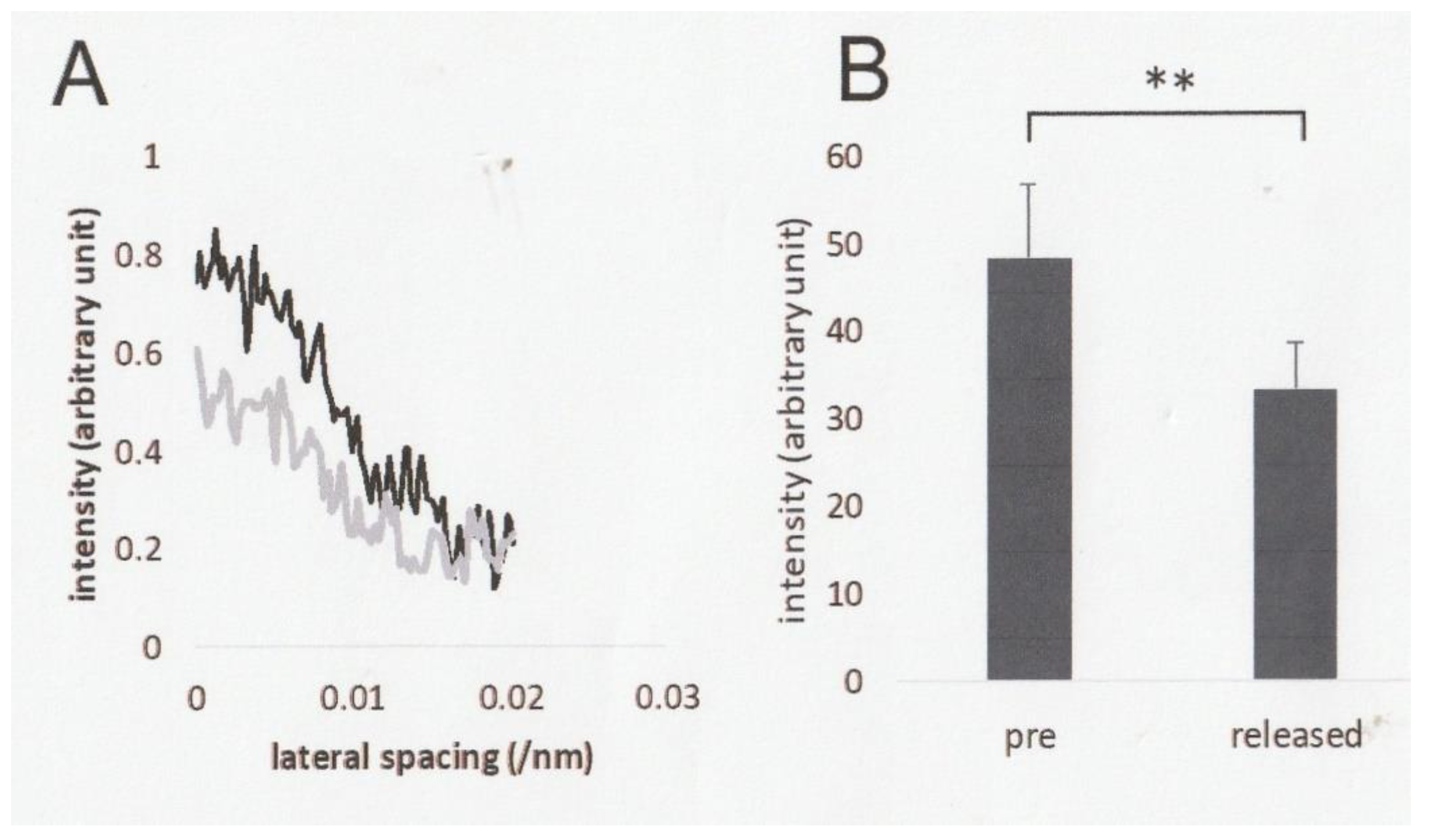
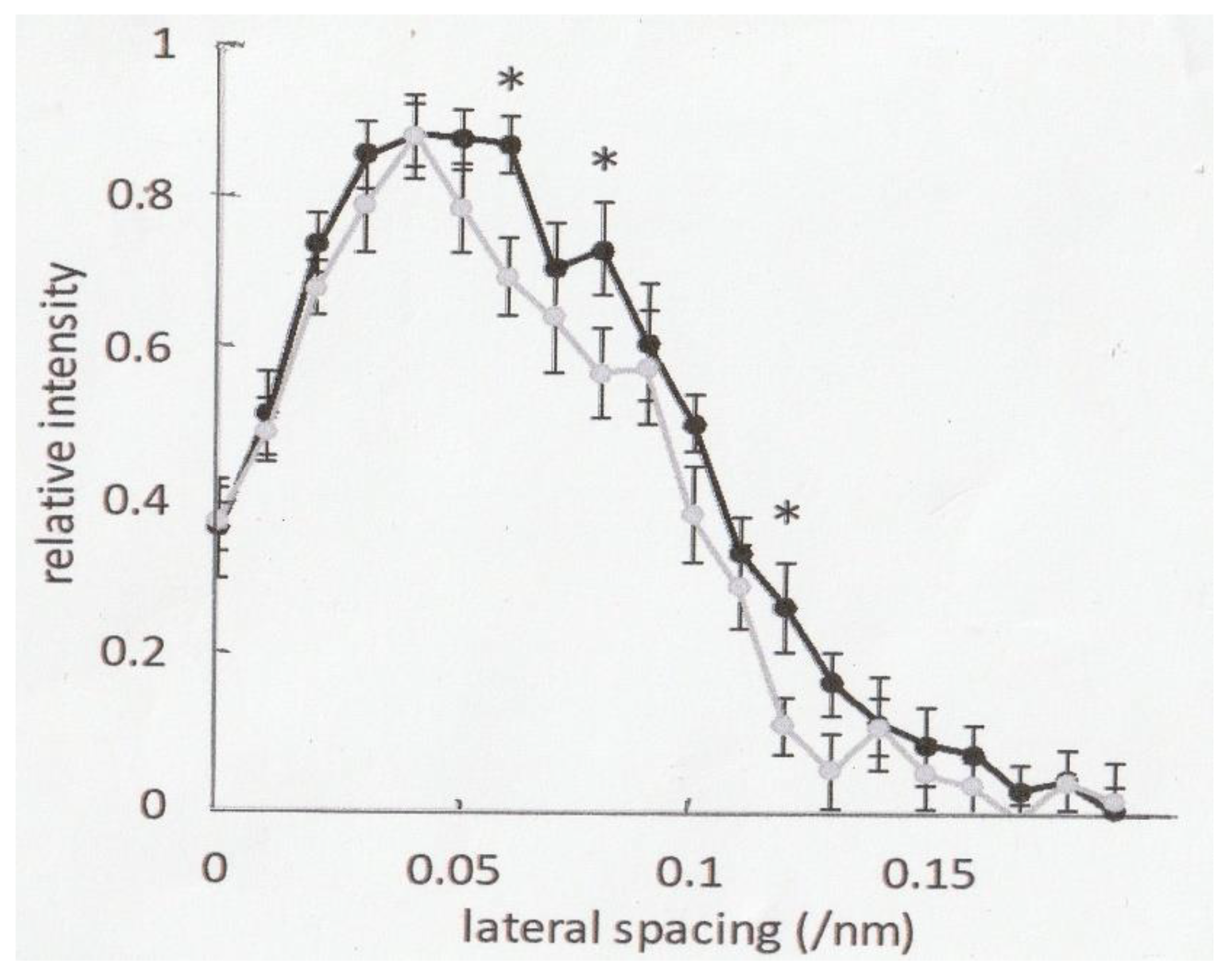
2.3. Effect of Ramp-Shaped Releases on the Intensity Profile of 5.9nm Actin-Based Layer line in high-Ca Rigor Muscle Fibers
3. Discussion
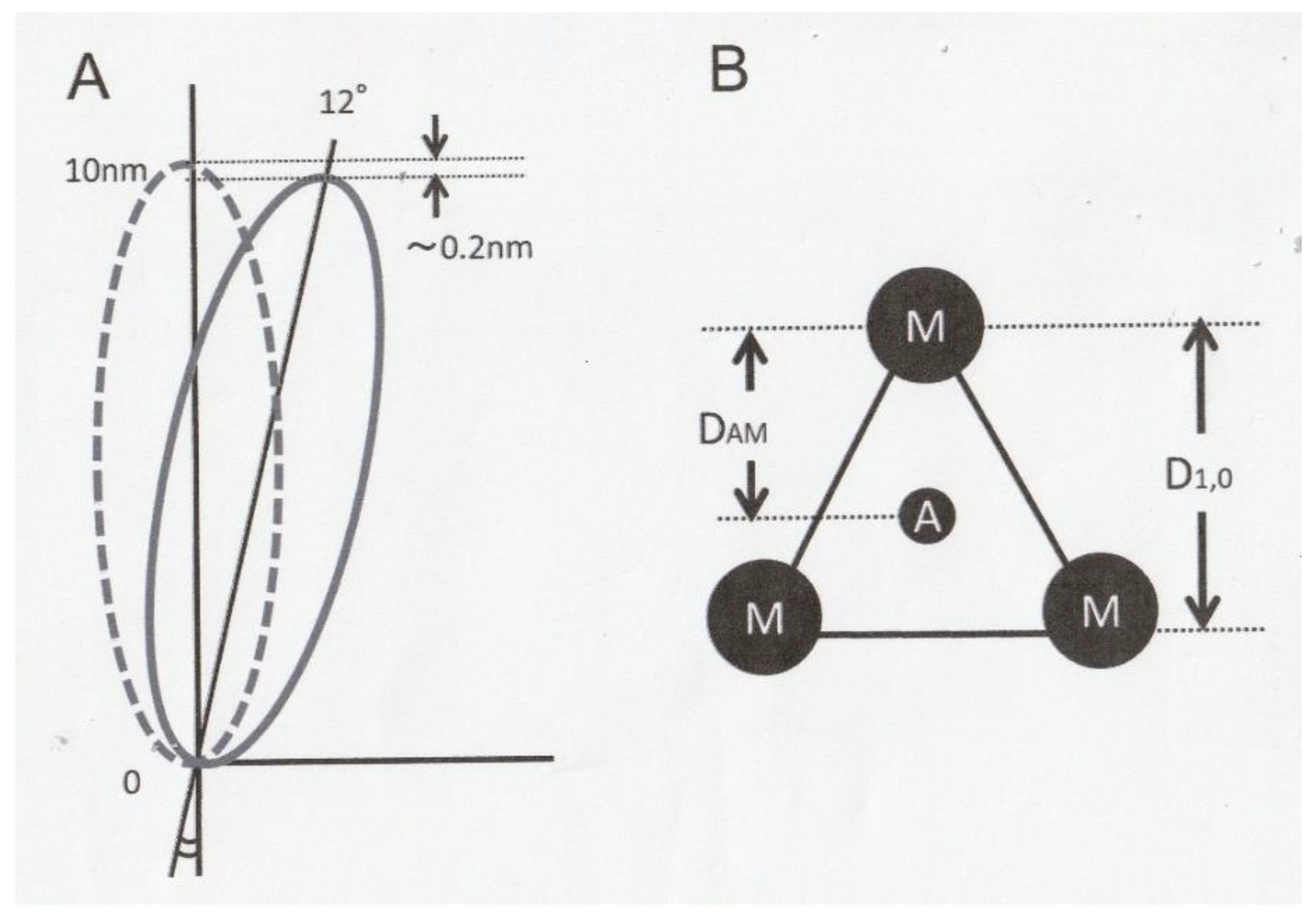
3.1. Tilting of Rigor Myosin Head CAD to Take up Ramp-Shaped Releases
3.2. Helical Rotation of Rigor Myosin Heads As a Possible Cause of the Tension Recovery of High-Ca Rigor Muscle Fibers After Ramp-Shaped Releases
4. Materials and Methods
4.1. Preparation
4.2. Solutions
4.3. Recording of X-ray Diffraction Pattern from Rigor Fibers Before and After Ramp-Shaped Releases
4.4. General Procedure
4.5. Data Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Huxley, A.F.; Niedergerke, R. Interference microscopy of living muscle fibres. Nature 1954, 173, 971–972. [Google Scholar] [CrossRef] [PubMed]
- Huxley, H.E.; Hanson, J. Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature 1954, 173, 973–976. [Google Scholar] [CrossRef] [PubMed]
- Lymn, R.W.; Taylor, E.W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry 1971, 25, 4617–4624. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.A.; Moir, A.J.G.; Trayer, I.P.; Williams, R.J.P. Nuclear magnetic resonance studies of calcium-modulated proteins and actin-myosin interaction. In Molecular Mechanism in Muscle Contraction; Squire, J.M., Ed.; MacMillan Press: Haundmills, UK, 1990; pp. 171–209. [Google Scholar]
- Milligan, R.A.; Whittaker, M.; Safer, D. Molecular structure of F-actin and location of surface binding sites. Nature 1990, 348, 217–221. [Google Scholar] [CrossRef]
- Sutoh, K.; Tokunaga, M.; Wakabayashi, T. Electron microscopic mapping of myosin head with site-directed antibodies. J. Mol. Biol. 1989, 206, 357–363. [Google Scholar] [CrossRef]
- Sugi, H.; Chaen, S.; Kobayashi, T.; Abe, T.; Kimura, K.; Saeki, Y.; Ohnuki, Y.; Miyakawa, T.; Tanokura, M.; Sugiura, T. Definite differences between in vitro actin-myosin sliding and muscle contraction as revealed using antibodies to myosin head. PLoS ONE 2014, e93272. [Google Scholar] [CrossRef]
- Squire, J.M. The Structural Basis of Muscular Contraction; Plenum Press: New York, NY, USA; London, UK, 1981; p. 608. [Google Scholar]
- Sugi, H.; Yamaguchi, M.; Ohno, T.; Kobayashi, T.; Chaen, S.; Okuyama, H. Tension recovery following ramp-shaped release in high-Ca and Low-Ca rigor muscle fibers: Evidence for the dynamic state of AMADP myosin heads in the absence of ATP. PLoS ONE 2016. [Google Scholar] [CrossRef]
- Iwamoto, H.; Oiwa, K.; Suzuki, T.; Fujisawa, T. X-ray diffraction evidence for the lack of stereospecific protein in highly activated actomyosin complex. J. Mol. Biol. 2001, 305, 863–874. [Google Scholar] [CrossRef]
- Huxley, H.E.; Brown, W. The low angle X-ray diagram of vertebrate skeletal muscle and its behavior during contraction and rigor. J. Mol. Biol. 1967, 30, 384–434. [Google Scholar] [CrossRef]
- Haselgrove, J.C.; Huxley, H.E. X-ray edivence for radial cross-bridge movement and for the sliding filament model in actively contracting skeletal muscle. J. Mol. Biol. 1973, 77, 549–568. [Google Scholar] [CrossRef]
- Amemiya, Y.; Iwamoto, H.; Kobayashi, T.; Sugi, H.; Tanaka, H.; Wakabayashi, K. Time- resolved X-ray diffraction studies on the effect of slow length changes on tetanized frog skeletal muscle. J. Physiol. 1988, 407, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Sugi, H. Extensibility of the myofilaments in vertebrate skeletal muscle as revealed by stretching rigor muscle fibers. J. Gen. Physiol. 1983, 81, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Rayment, I.; Ripeniewski, W.R.; Schmidt-Base, K.; Smith, R.; Tomchick, D.R.; Benning, M.M.; Winkelmann, D.A.; Wesenberg, G.; Holden, H.M. Three-dimensional structure of myosin subfragment-1: A molecular motor. Science 1993, 261, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Jarosch, R. Breakthroughs and News: Muscle force arises by actin filament rotation and torque in the Z-filaments. Biochem. Biophys. Res. Commun. 2000, 276, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Jarosch, R. The alpha-helix, an overlooked molecular motor. Protoplasma 2005, 227, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Namba, K.; Fujii, T. Cardiac muscle thin filament structures reveal calcium regulatory mechanism. Nat. Commun. 2019, 11, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Brandt, P.W. Two rigor states in skinned crayfish single muscle fibers. J. Gen. Physiol. 1976, 68, 267–280. [Google Scholar] [CrossRef]
- Schoenberg, M.; Eisenberg, E. Muscle cross-bridge kinetics in rigor and in the presence of ATP analogues. Biophys. J. 1985, 48, 863–871. [Google Scholar] [CrossRef]
- Linari, M.; Dobbie, I.; Vanzi, F.; Trorok, K.; Irving, M.; Piazzesi, G.; Lombardi, V. Comparison between tension transients during isometric contraction and in rigor in isolated fibers from frog skeletal muscle. Biophys. J. 1995, 68, 218s. [Google Scholar]
- Chaen, S.; Shimada, M.; Sugi, H. Evidence for cooperative interaction of myosin heads with thin filament in the force generation of vertebrate skeletal muscle fibers. J. Biol. Chem. 1986, 261, 13632–13636. [Google Scholar]
- Nyitrai, M.; Geeves, M.A. Adenosine diphosphate and strain sensitivity in myosin motors. Philos. Trans. R. Soc. B 2004, 359, 1867–1877. [Google Scholar]
- Albet-Torres, N.; Blowmink, M.J.; Barman, T.; Candau, R.; Flolander, K.; Geeves, M.A.; Golker, K.; Herrmann, C.; Lionne, C.; Piperio, C.; et al. Drug effect unveils inter-head cooperativity and strain-dependent ADP release in fast skeletal actomyosin. J. Biol. Chem. 2009, 284, 22926–22937. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Kimura, M.; Li, Z.; Ohno, T.; Takemori, J.; Hoh, J.F.Y.; Yagi, N. X-ray diffraction analysis of the effects of myosin regulatory light chain phosphorylation and butandione monoxime on skinned skeletal muscle fibers. Am. J. Physiol. Cell Physiol. 2016, 310, C692–C700. [Google Scholar] [CrossRef]
- Podolsky, R.J.; Onge, R.; Yu, L.; Lymn, R.W. X-ray diffraction of actively shortening muscle. Proc. Natl. Acad. Sci. USA 1976, 73, 813–817. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugi, H.; Yamaguchi, M.; Ohno, T.; Okuyama, H.; Yagi, N. X-ray Diffraction Studies on the Structural Origin of Dynamic Tension Recovery Following Ramp-Shaped Releases in High-Ca Rigor Muscle Fibers. Int. J. Mol. Sci. 2020, 21, 1244. https://doi.org/10.3390/ijms21041244
Sugi H, Yamaguchi M, Ohno T, Okuyama H, Yagi N. X-ray Diffraction Studies on the Structural Origin of Dynamic Tension Recovery Following Ramp-Shaped Releases in High-Ca Rigor Muscle Fibers. International Journal of Molecular Sciences. 2020; 21(4):1244. https://doi.org/10.3390/ijms21041244
Chicago/Turabian StyleSugi, Haruo, Maki Yamaguchi, Tetsuo Ohno, Hiroshi Okuyama, and Naoto Yagi. 2020. "X-ray Diffraction Studies on the Structural Origin of Dynamic Tension Recovery Following Ramp-Shaped Releases in High-Ca Rigor Muscle Fibers" International Journal of Molecular Sciences 21, no. 4: 1244. https://doi.org/10.3390/ijms21041244
APA StyleSugi, H., Yamaguchi, M., Ohno, T., Okuyama, H., & Yagi, N. (2020). X-ray Diffraction Studies on the Structural Origin of Dynamic Tension Recovery Following Ramp-Shaped Releases in High-Ca Rigor Muscle Fibers. International Journal of Molecular Sciences, 21(4), 1244. https://doi.org/10.3390/ijms21041244





