The Pattern of AQP4 Expression in the Ageing Human Brain and in Cerebral Amyloid Angiopathy
Abstract
1. Introduction
2. Results
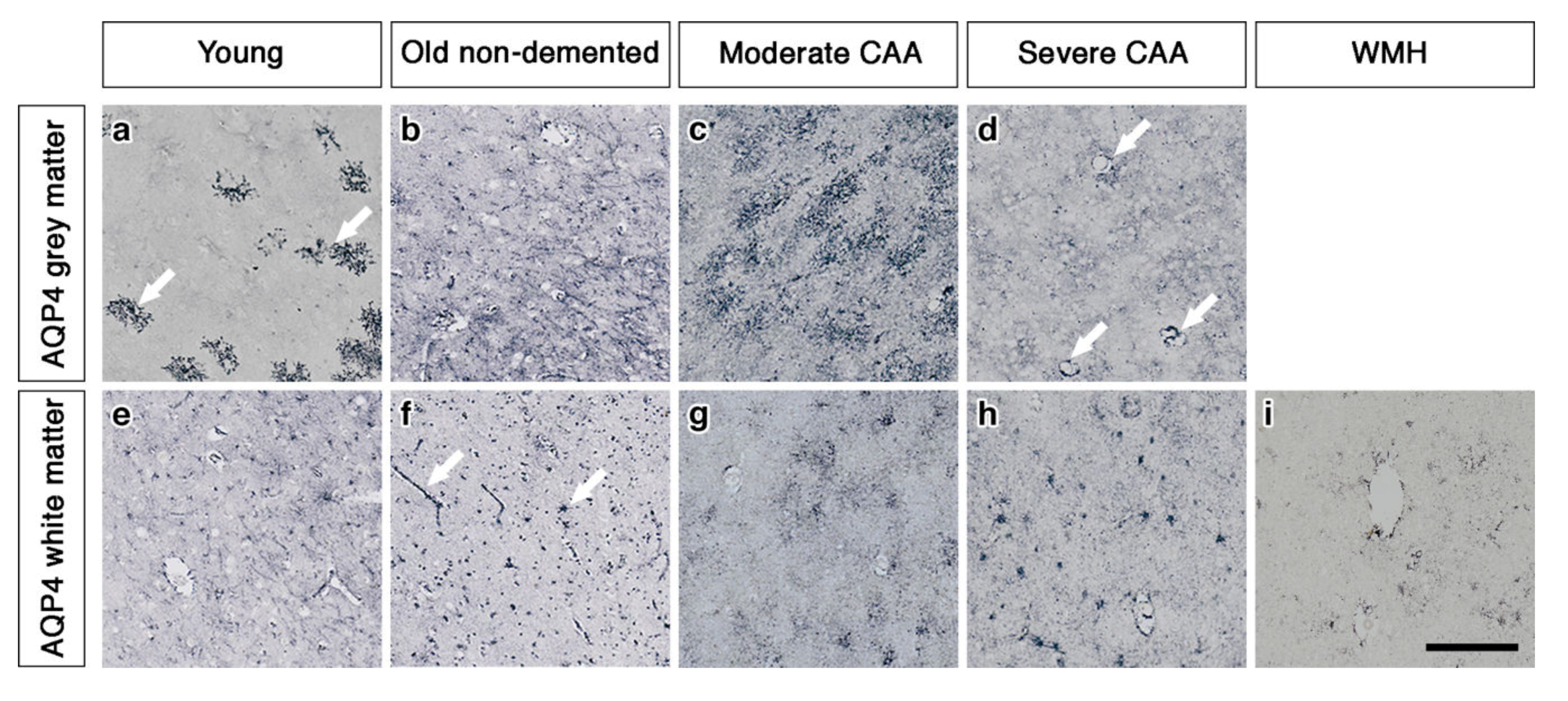
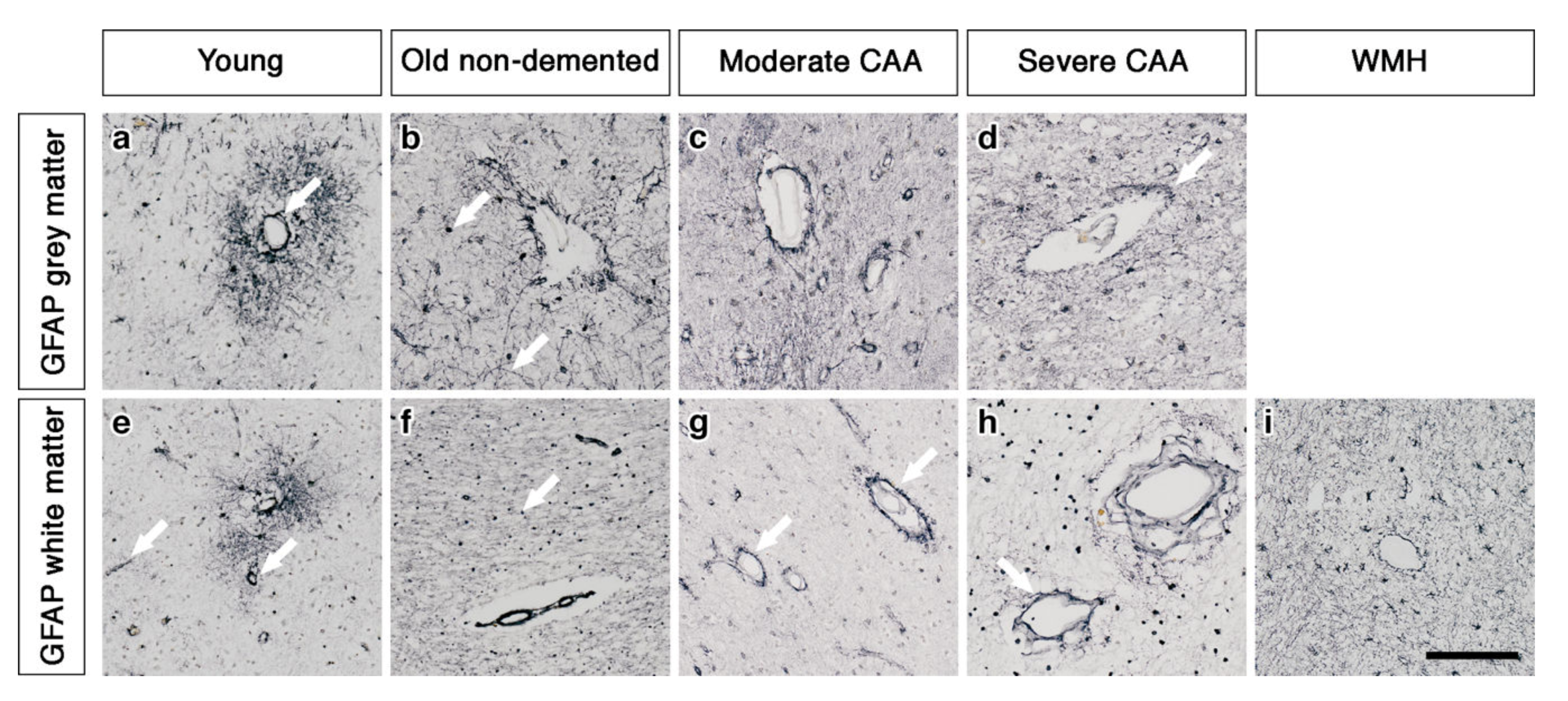
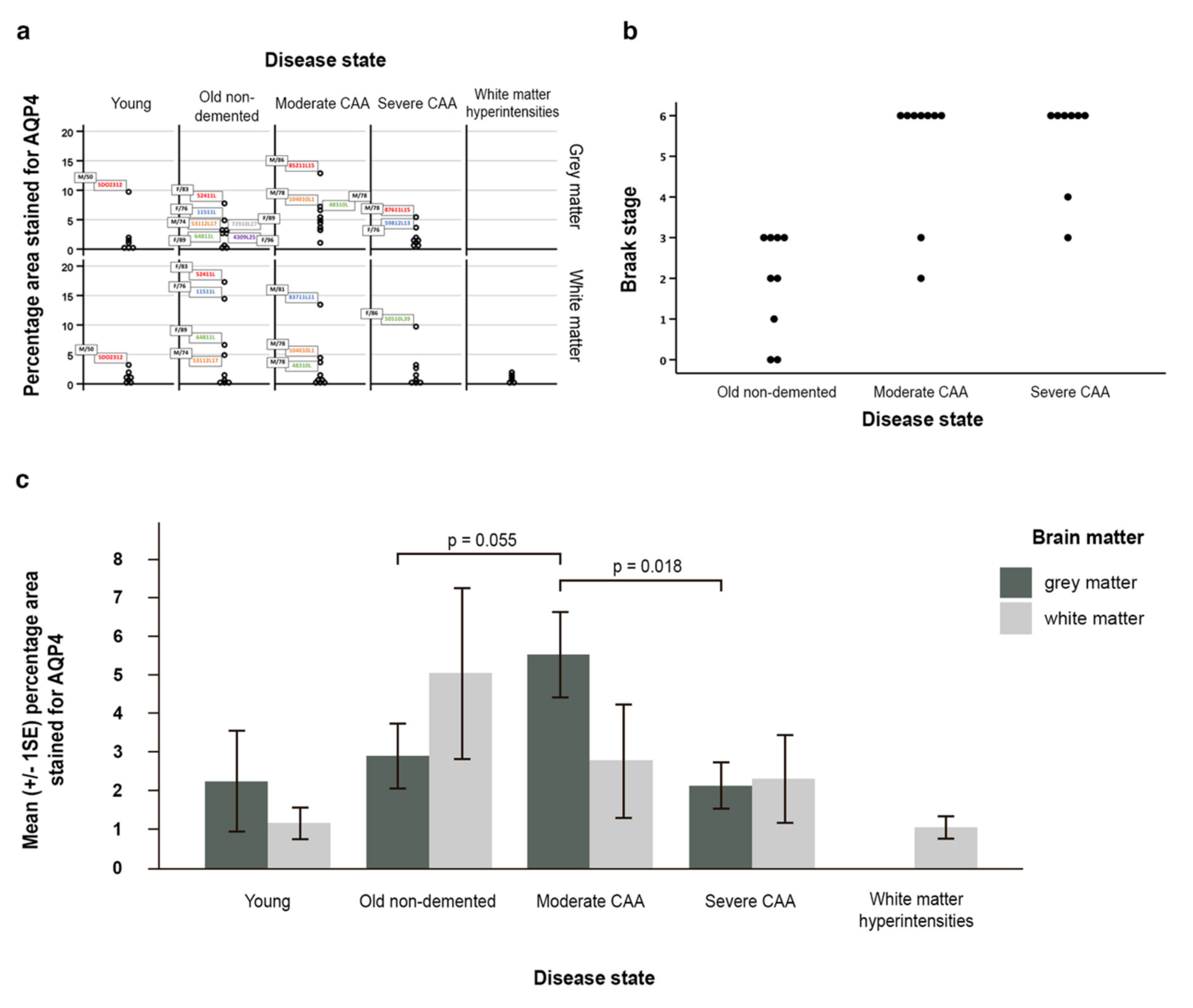
2.1. The Pattern of Immunostaining for AQP4 Is Different in the Grey Compared to White Matter and Changes with Age and with Severity of CAA
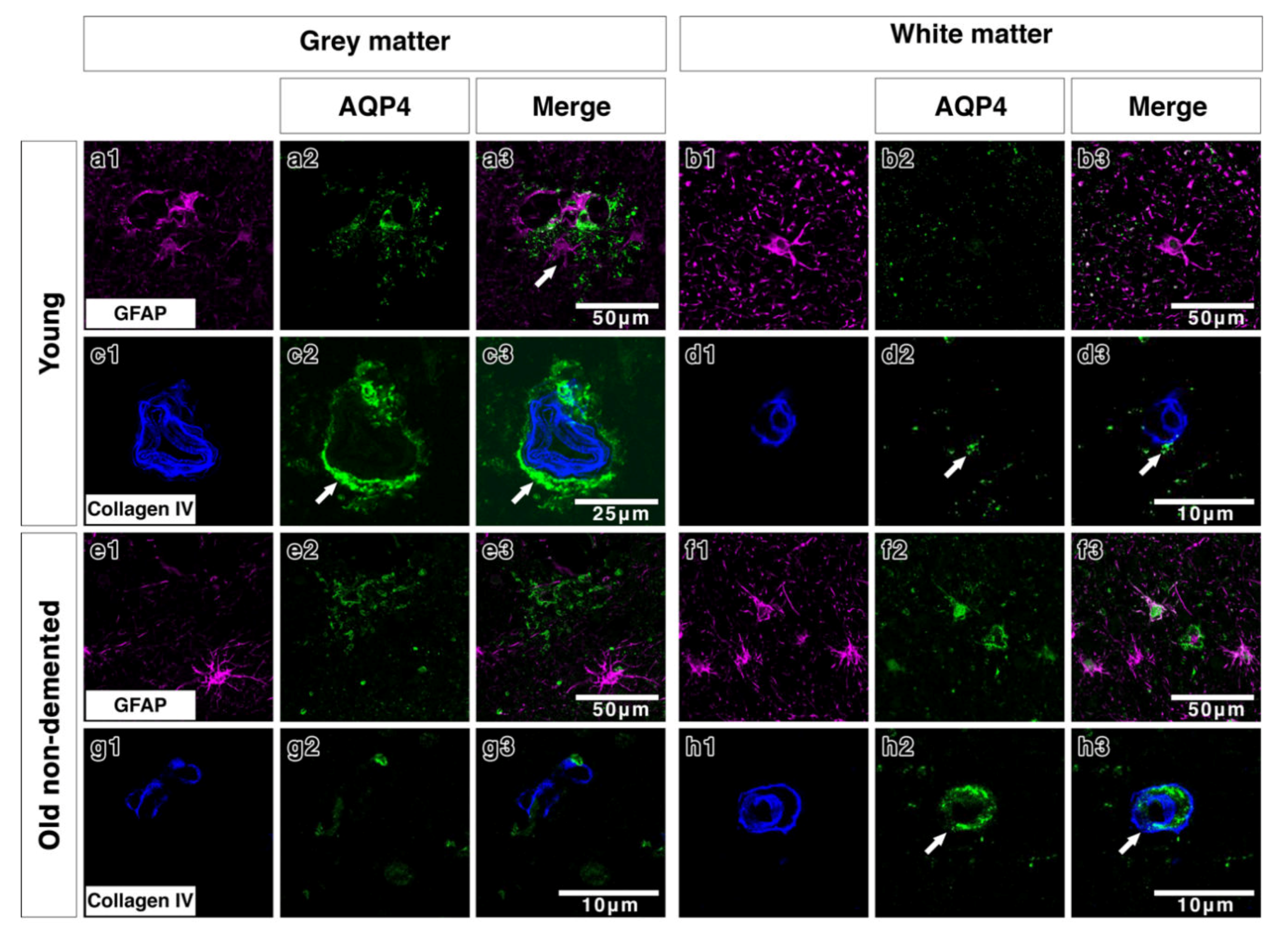
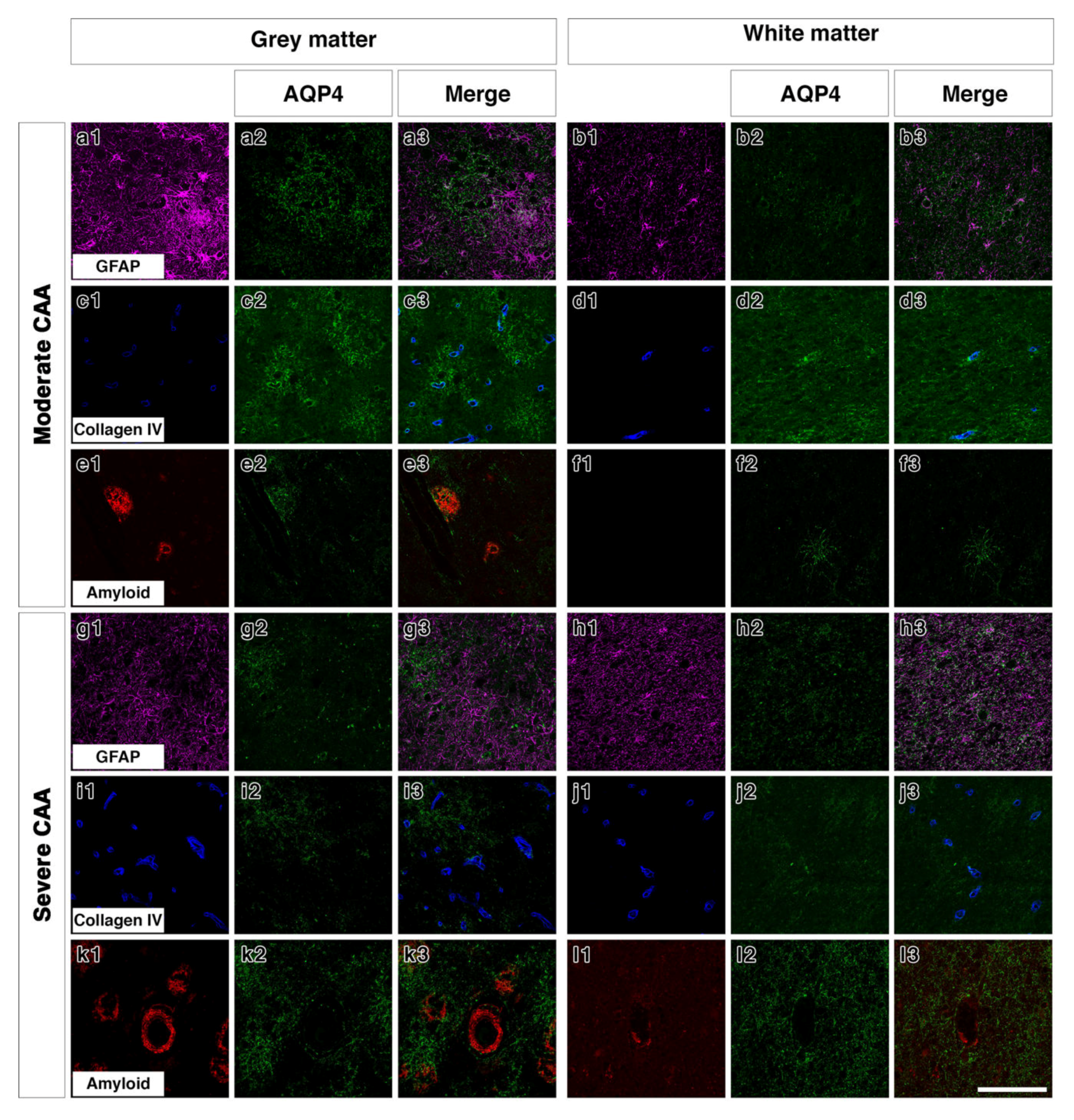
2.2. Double Immunostaining
2.3. AQP4 Expression Is Altered with Severity of CAA
3. Discussion
4. Materials and Methods
4.1. Experimental Subjects
4.2. Assessment of AQP4 Expression by Immunohistochemistry
4.3. Assessment of AQP4 and CAA Severity by Immunofluorescence
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AQP4 | Aquaporin 4 |
| CSF | CEREBROSPINAL fluid |
| CAA | Cerebral amyloid angiopathy |
| GFAP | Glial fibrillary acidic protein |
| WMH | White matter hyperintensities |
| AD | Alzheimer’s disease |
References
- Hawkes, C.A.; Carare, R.O.; Weller, R.O. Amyloid and tau in the brain in sporadic Alzheimer’s disease: Defining the chicken and the egg. Acta Neuropathol. 2014, 127, 617–618. [Google Scholar] [CrossRef] [PubMed]
- Weller, R.O.; Hawkes, C.A.; Kalaria, R.N.; Werring, D.J.; Carare, R.O. White matter changes in dementia: Role of impaired drainage of interstitial fluid. Brain Pathol. 2015, 25, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Weller, R.O.; Djuanda, E.; Yow, H.Y.; Carare, R.O. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 2009, 117, 1–14. [Google Scholar] [CrossRef]
- Carare, R.O.; Bernardes-Silva, M.; Newman, T.A.; Page, A.M.; Nicoll, J.A.; Perry, V.H.; Weller, R.O. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: Significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol. Appl. Neurobiol. 2008, 34, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.W.; Sharp, M.M.; Albargothy, N.J.; Fernandes, R.; Hawkes, C.A.; Verma, A.; Weller, R.O.; Carare, R.O. Vascular basement membranes as pathways for the passage of fluid into and out of the brain. Acta Neuropathol. 2016, 131, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Albargothy, N.J.; Johnston, D.A.; MacGregor-Sharp, M.; Weller, R.O.; Verma, A.; Hawkes, C.A.; Carare, R.O. Convective influx/glymphatic system: Tracers injected into the CSF enter and leave the brain along separate periarterial basement membrane pathways. Acta Neuropathol. 2018, 136, 139–152. [Google Scholar] [CrossRef]
- Keable, A.; Fenna, K.; Yuen, H.M.; Johnston, D.A.; Smyth, N.R.; Smith, C.; Al-Shahi Salman, R.; Samarasekera, N.; Nicoll, J.A.; Attems, J.; et al. Deposition of amyloid beta in the walls of human leptomeningeal arteries in relation to perivascular drainage pathways in cerebral amyloid angiopathy. Biochim. Biophys. Acta 2016, 1862, 1037–1046. [Google Scholar] [CrossRef]
- Hawkes, C.A.; Hartig, W.; Kacza, J.; Schliebs, R.; Weller, R.O.; Nicoll, J.A.; Carare, R.O. Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy. Acta Neuropathol. 2011, 121, 431–443. [Google Scholar] [CrossRef]
- Nielsen, S.; Arnulf Nagelhus, E.; Amiry-Moghaddam, M.; Bourque, C.; Agre, P.; Petter Ottersen, O. Specialized Membrane Domains for Water Transport in Glial Cells: High-Resolution Immunogold Cytochemistry of Aquaporin-4 in Rat Brain. J. Neurosci. 1997, 17, 171–180. [Google Scholar] [CrossRef]
- Amiry-Moghaddam, M.; Xue, R.; Haug, F.M.; Neely, J.D.; Bhardwaj, A.; Agre, P.; Adams, M.E.; Froehner, S.C.; Mori, S.; Ottersen, O.P. Alpha-syntrophin deletion removes the perivascular but not endothelial pool of aquaporin-4 at the blood-brain barrier and delays the development of brain edema in an experimental model of acute hyponatremia. FASEB J. 2004, 18, 542–544. [Google Scholar] [CrossRef]
- Nagelhus, E.A.; Ottersen, O.P. Physiological roles of aquaporin-4 in brain. Physiol. Rev. 2013, 93, 1543–1562. [Google Scholar] [CrossRef] [PubMed]
- Eidsvaag, V.A.; Enger, R.; Hansson, H.A.; Eide, P.K.; Nagelhus, E.A. Human and mouse cortical astrocytes differ in aquaporin-4 polarization toward microvessels. Glia 2017, 65, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.S.; Seldin, M.; Lee, D.J.; Seifert, G.; Steinhauser, C.; Binder, D.K. Laminar-specific and developmental expression of aquaporin-4 in the mouse hippocampus. Neuroscience 2011, 178, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Nagelhus, E.A.; Amiry-Moghaddam, M.; Agre, P.; Ottersen, O.P.; Nielsen, S. Ontogeny of water transport in rat brain: Postnatal expression of the aquaporin-4 water channel. Eur. J. Neurosci. 1999, 11, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Badaut, J.; Nehlig, A.; Verbavatz, J.; Stoeckel, M.; Freund-Mercier, M.J.; Lasbennes, F. Hypervascularization in the magnocellular nuclei of the rat hypothalamus: Relationship with the distribution of aquaporin-4 and markers of energy metabolism. J. Neuroendocr. 2000, 12, 960–969. [Google Scholar] [CrossRef]
- Steiner, E.; Enzmann, G.U.; Lin, S.; Ghavampour, S.; Hannocks, M.J.; Zuber, B.; Ruegg, M.A.; Sorokin, L.; Engelhardt, B. Loss of astrocyte polarization upon transient focal brain ischemia as a possible mechanism to counteract early edema formation. Glia 2012, 60, 1646–1659. [Google Scholar] [CrossRef]
- Chen, A.; Akinyemi, R.O.; Hase, Y.; Firbank, M.J.; Ndung’u, M.N.; Foster, V.; Craggs, L.J.; Washida, K.; Okamoto, Y.; Thomas, A.J.; et al. Frontal white matter hyperintensities, clasmatodendrosis and gliovascular abnormalities in ageing and post-stroke dementia. Brain 2016, 139, 242–258. [Google Scholar] [CrossRef]
- Warth, A.; Simon, P.; Capper, D.; Goeppert, B.; Tabatabai, G.; Herzog, H.; Dietz, K.; Stubenvoll, F.; Ajaaj, R.; Becker, R.; et al. Expression pattern of the water channel aquaporin-4 in human gliomas is associated with blood-brain barrier disturbance but not with patient survival. J. Neurosci. Res. 2007, 85, 1336–1346. [Google Scholar] [CrossRef]
- Saadoun, S.; Papadopoulos, M.C.; Davies, D.C.; Krishna, S.; Bell, B.A. Aquaporin-4 expression is increased in oedematous human brain tumours. J. Neurol. Neurosurg. Psychiatry 2002, 72, 262–265. [Google Scholar] [CrossRef]
- Ribeiro Mde, C.; Hirt, L.; Bogousslavsky, J.; Regli, L.; Badaut, J. Time course of aquaporin expression after transient focal cerebral ischemia in mice. J. Neurosci. Res. 2006, 83, 1231–1240. [Google Scholar] [CrossRef]
- Hirt, L.; Ternon, B.; Price, M.; Mastour, N.; Brunet, J.F.; Badaut, J. Protective role of early aquaporin 4 induction against postischemic edema formation. J. Cereb. Blood Flow Metab. J. Int. Soc. Cereb. Blood Flow Metab. 2009, 29, 423–433. [Google Scholar] [CrossRef]
- Sun, M.C.; Honey, C.R.; Berk, C.; Wong, N.L.; Tsui, J.K. Regulation of aquaporin-4 in a traumatic brain injury model in rats. J. Neurosurg. 2003, 98, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Badaut, J.; Fukuda, A.M.; Jullienne, A.; Petry, K.G. Aquaporin and brain diseases. Biochim. Biophys. Acta 2014, 1840, 1554–1565. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Derugin, N.; Manley, G.T.; Verkman, A.S. Reduced brain edema and infarct volume in aquaporin-4 deficient mice after transient focal cerebral ischemia. Neurosci. Lett. 2015, 584, 368–372. [Google Scholar] [CrossRef]
- Papadopoulos, M.C.; Manley, G.T.; Krishna, S.; Verkman, A.S. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004, 18, 1291–1293. [Google Scholar] [CrossRef]
- Bloch, O.; Auguste, K.I.; Manley, G.T.; Verkman, A.S. Accelerated progression of kaolin-induced hydrocephalus in aquaporin-4-deficient mice. J. Cereb. Blood Flow Metab. 2006, 26, 1527–1537. [Google Scholar] [CrossRef]
- Stokum, J.A.; Mehta, R.I.; Ivanova, S.; Yu, E.; Gerzanich, V.; Simard, J.M. Heterogeneity of aquaporin-4 localization and expression after focal cerebral ischemia underlies differences in white versus grey matter swelling. Acta Neuropathol. Commun. 2015, 3, 61. [Google Scholar] [CrossRef]
- Fenske, A.; Samii, M.; Reulen, H.J.; Hey, O. Extracellular space and electrolyte distribution in cortex and white matter of dog brain in cold induced oedema. Acta Neurochir. 1973, 28, 81–94. [Google Scholar] [CrossRef]
- Aarabi, B.; Long, D.M. Dynamics of cerebral edema. The role of an intact vascular bed in the production and propagation of vasogenic brain edema. J. Neurosurg. 1979, 51, 779–784. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Valdes Hernandez, M.C.; Munoz-Maniega, S. What are White Matter Hyperintensities Made of? Relevance to Vascular Cognitive Impairment. J. Am. Heart Assoc. 2015, 4, 001140. [Google Scholar] [CrossRef]
- Banerjee, G.; Kim, H.J.; Fox, Z.; Jager, H.R.; Wilson, D.; Charidimou, A.; Na, H.K.; Na, D.L.; Seo, S.W.; Werring, D.J. MRI-visible perivascular space location is associated with Alzheimer’s disease independently of amyloid burden. Brain 2017, 140, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Wilcock, D.M.; Vitek, M.P.; Colton, C.A. Vascular amyloid alters astrocytic water and potassium channels in mouse models and humans with Alzheimer’s disease. Neuroscience 2009, 159, 1055–1069. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.; Barrachina, M.; Rodriguez, A.; Torrejon-Escribano, B.; Boada, M.; Hernandez, I.; Sanchez, M.; Ferrer, I. Aquaporin expression in the cerebral cortex is increased at early stages of Alzheimer disease. Brain Res. 2007, 1128, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, A.; Yamamoto, T.; Shimizu, K.; Ugawa, Y.; Nishizawa, M.; Takahashi, H.; Kakita, A. Characteristics of aquaporin expression surrounding senile plaques and cerebral amyloid angiopathy in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2012, 71, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, R.; Shi, C.; Mao, C.; Yang, Z.; Suo, Z.; Torp, R.; Xu, Y. AQP4 Association with Amyloid Deposition and Astrocyte Pathology in the Tg-ArcSwe Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, A.; Tsunoda, A.; Yamamoto, T.; Tada, M.; Kakita, A.; Ugawa, Y. Altered expression of glutamate transporter-1 and water channel protein aquaporin-4 in human temporal cortex with Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 2018, 44, 628–638. [Google Scholar] [CrossRef]
- Garwood, C.J.; Ratcliffe, L.E.; Simpson, J.E.; Heath, P.R.; Ince, P.G.; Wharton, S.B. Review: Astrocytes in Alzheimer’s disease and other age-associated dementias: A supporting player with a central role. Neuropathol. Appl. Neurobiol. 2017, 43, 281–298. [Google Scholar] [CrossRef]
- Ferrer, I. Diversity of astroglial responses across human neurodegenerative disorders and brain aging. Brain Pathol. 2017, 27, 645–674. [Google Scholar] [CrossRef]
- MacGregor Sharp, M.; Bulters, D.; Brandner, S.; Holton, J.; Verma, A.; Werring, D.J.; Carare, R.O. The fine anatomy of the perivascular compartment in the human brain: Relevance to dilated perivascular spaces in cerebral amyloid angiopathy. Neuropathol. Appl. Neurobiol. 2019, 45, 305–308. [Google Scholar] [CrossRef]
- Charidimou, A.; Meegahage, R.; Fox, Z.; Peeters, A.; Vandermeeren, Y.; Laloux, P.; Baron, J.C.; Jager, H.R.; Werring, D.J. Enlarged perivascular spaces as a marker of underlying arteriopathy in intracerebral haemorrhage: A multicentre MRI cohort study. J. Neurol. Neurosurg. Psychiatry 2013, 84, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Guillamon, M.; Martinez-Saez, E.; Delgado, P.; Domingues-Montanari, S.; Boada, C.; Penalba, A.; Boada, M.; Pagola, J.; Maisterra, O.; Rodriguez-Luna, D.; et al. MMP-2/MMP-9 plasma level and brain expression in cerebral amyloid angiopathy-associated hemorrhagic stroke. Brain Pathol. 2012, 22, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Zeppenfeld, D.M.; Simon, M.; Haswell, J.D.; D’Abreo, D.; Murchison, C.; Quinn, J.F.; Grafe, M.R.; Woltjer, R.L.; Kaye, J.; Iliff, J.J. Association of Perivascular Localization of Aquaporin-4 With Cognition and Alzheimer Disease in Aging Brains. JAMA Neurol. 2017, 74, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Moftakhar, P.; Lynch, M.D.; Pomakian, J.L.; Vinters, H.V. Aquaporin expression in the brains of patients with or without cerebral amyloid angiopathy. J. Neuropathol. Exp. Neurol. 2010, 69, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Duan, T.; Verkman, A.S. Aquaporin-4 reduces neuropathology in a mouse model of Alzheimer’s disease by remodeling peri-plaque astrocyte structure. Acta Neuropathol. Commun. 2019, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Tarasoff-Conway, J.M.; Carare, R.O.; Osorio, R.S.; Glodzik, L.; Butler, T.; Fieremans, E.; Axel, L.; Rusinek, H.; Nicholson, C.; Zlokovic, B.V.; et al. Clearance systems in the brain-implications for Alzheimer disease. Nat. Rev. Neurol. 2015, 11, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E.N.; Bacskai, B.J.; Arbel-Ornath, M.; Aldea, R.; Bedussi, B.; Morris, A.W.; Weller, R.O.; Carare, R.O. Lymphatic Clearance of the Brain: Perivascular, Paravascular and Significance for Neurodegenerative Diseases. Cell. Mol. Neurobiol. 2016, 36, 181–194. [Google Scholar] [CrossRef]
- Verkman, A.S.; Smith, A.J.; Phuan, P.W.; Tradtrantip, L.; Anderson, M.O. The aquaporin-4 water channel as a potential drug target in neurological disorders. Expert Opin. Targets 2017, 21, 1161–1170. [Google Scholar] [CrossRef]
- Braak, H.; Alafuzoff, I.; Arzberger, T.; Kretzschmar, H.; Del Tredici, K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006, 112, 389–404. [Google Scholar] [CrossRef]
- Thal, D.R.; Rub, U.; Orantes, M.; Braak, H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 2002, 58, 1791–1800. [Google Scholar] [CrossRef]
- Mirra, S.S.; Heyman, A.; McKeel, D.; Sumi, S.M.; Crain, B.J.; Brownlee, L.M.; Vogel, F.S.; Hughes, J.P.; van Belle, G.; Berg, L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 1991, 41, 479–486. [Google Scholar] [CrossRef]
- Montine, T.; Phelps, C.; Beach, T.; Bigio, E.; Cairns, N.; Dickson, D.; Duyckaerts, C.; Frosch, M.; Masliah, E.; Mirra, S.; et al. National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol. 2012, 123, 1–11. [Google Scholar] [CrossRef] [PubMed]
- McKeith, I.G.; Dickson, D.W.; Lowe, J.; Emre, M.; O’Brien, J.T.; Feldman, H.; Cummings, J.; Duda, J.E.; Lippa, C.; Perry, E.K.; et al. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Consortium. Neurology 2005, 65, 1863–1872. [Google Scholar] [CrossRef]
- Vonsattel, J.P.; Myers, R.H.; Hedley-Whyte, E.T.; Ropper, A.H.; Bird, E.D.; Richardson, E.P., Jr. Cerebral amyloid angiopathy without and with cerebral hemorrhages: A comparative histological study. Ann. Neurol. 1991, 30, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Biffi, A.; Greenberg, S.M. Cerebral amyloid angiopathy: A systematic review. J. Clin. Neurol. 2011, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Love, S.; Chalmers, K.; Ince, P.; Esiri, M.; Attems, J.; Jellinger, K.; Yamada, M.; McCarron, M.; Minett, T.; Matthews, F.; et al. Development, appraisal, validation and implementation of a consensus protocol for the assessment of cerebral amyloid angiopathy in post-mortem brain tissue. Am. J. Neurodegener. Dis. 2014, 3, 19–32. [Google Scholar] [PubMed]
- Thal, D.R.; Ghebremedhin, E.; Rub, U.; Yamaguchi, H.; Del Tredici, K.; Braak, H. Two types of sporadic cerebral amyloid angiopathy. J. Neuropathol. Exp. Neurol. 2002, 61, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Attems, J.; Jellinger, K.; Thal, D.R.; Van Nostrand, W. Sporadic cerebral amyloid angiopathy. Neuropathol. Appl. Neurobiol. 2011, 37, 75–93. [Google Scholar] [CrossRef]
- Fernando, M.S.; O’Brien, J.T.; Perry, R.H.; English, P.; Forster, G.; McMeekin, W.; Slade, J.Y.; Golkhar, A.; Matthews, F.E.; Barber, R.; et al. Comparison of the pathology of cerebral white matter with post-mortem magnetic resonance imaging (MRI) in the elderly brain. Neuropathol. Appl. Neurobiol. 2004, 30, 385–395. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; de Zonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
| Source | Age | Sex | PM Delay /Hrs | Category | Braak Stage | Thal Phase |
|---|---|---|---|---|---|---|
| Edinburgh | 50 | M | 45 | Young | ||
| Edinburgh | 44 | M | 47 | Young | ||
| Edinburgh | 21 | M | 111 | Young | ||
| Edinburgh | 29 | M | 44 | Young | ||
| Edinburgh | 38 | M | 49 | Young | ||
| Edinburgh | 49 | M | 79 | Young | ||
| Edinburgh | 32 | M | 99 | Young | ||
| Newcastle | 96 | F | 114 | old non-demented | 2 | 3 |
| Newcastle | 95 | M | 21 | old non-demented | 3 | 3 |
| Newcastle | 83 | F | 24 | old non-demented | 1 | 1 |
| Newcastle | 74 | M | 70 | old non-demented | 0 | 0 |
| Newcastle | 77 | M | 46 | old non-demented | 3 | 0 |
| Newcastle | 89 | F | 98 | old non-demented | 3 | 2 |
| Newcastle | 70 | M | 72 | old non-demented | 0 | 1 |
| Newcastle | 81 | F | 31 | old non-demented | 3 | 5 |
| Newcastle | 76 | F | 41 | old non-demented | 2 | 3 |
| Newcastle | 99 | F | 71 | CAA/moderate CAA | 6 | n/a |
| Newcastle | 63 | M | 40 | CAA/moderate CAA | 6 | 5 |
| Newcastle | 78 | M | 37 | CAA/moderate CAA | 6 | 5 |
| Newcastle | 90 | F | 90 | CAA/moderate CAA | 6 | 5 |
| Newcastle | 87 | M | 22 | CAA/moderate CAA | 6 | 5 |
| Newcastle | 81 | M | 43 | CAA/moderate CAA | 2 | 0 |
| Newcastle | 86 | M | 44 | CAA/moderate CAA | 3 | 4 |
| Newcastle | 62 | M | 28 | CAA/moderate CAA | 6 | 5 |
| Newcastle | 78 | M | 17 | CAA/moderate CAA | 6 | 5 |
| Newcastle | 73 | F | 47 | CAA/severe CAA | 4 | 3 |
| Newcastle | 79 | M | 13 | CAA/severe CAA | 3 | 4 |
| Newcastle | 86 | F | 51 | CAA/severe CAA | 6 | 5 |
| Newcastle | 86 | F | 47 | CAA/severe CAA | 6 | 5 |
| Newcastle | 73 | M | 7 | CAA/severe CAA | 6 | 5 |
| Newcastle | 76 | F | 37 | CAA/severe CAA | 6 | 5 |
| Newcastle | 78 | M | 18 | CAA/severe CAA | 6 | 5 |
| Newcastle | 77 | M | 78 | CAA/severe CAA | 6 | 5 |
| Shefffield | 84 | F | 36 | White matter hyperintensity | ||
| Shefffield | 78 | F | 47 | White matter hyperintensity | ||
| Shefffield | 91 | F | 35 | White matter hyperintensity | ||
| Shefffield | 91 | F | 36 | White matter hyperintensity | ||
| Shefffield | 89 | M | 46 | White matter hyperintensity | ||
| Shefffield | 87 | F | 17 | White matter hyperintensity |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owasil, R.; O’Neill, R.; Keable, A.; Nimmo, J.; MacGregor Sharp, M.; Kelly, L.; Saito, S.; Simpson, J.E.; Weller, R.O.; Smith, C.; et al. The Pattern of AQP4 Expression in the Ageing Human Brain and in Cerebral Amyloid Angiopathy. Int. J. Mol. Sci. 2020, 21, 1225. https://doi.org/10.3390/ijms21041225
Owasil R, O’Neill R, Keable A, Nimmo J, MacGregor Sharp M, Kelly L, Saito S, Simpson JE, Weller RO, Smith C, et al. The Pattern of AQP4 Expression in the Ageing Human Brain and in Cerebral Amyloid Angiopathy. International Journal of Molecular Sciences. 2020; 21(4):1225. https://doi.org/10.3390/ijms21041225
Chicago/Turabian StyleOwasil, Raisah, Ronan O’Neill, Abby Keable, Jacqui Nimmo, Matthew MacGregor Sharp, Louise Kelly, Satoshi Saito, Julie E. Simpson, Roy O. Weller, Colin Smith, and et al. 2020. "The Pattern of AQP4 Expression in the Ageing Human Brain and in Cerebral Amyloid Angiopathy" International Journal of Molecular Sciences 21, no. 4: 1225. https://doi.org/10.3390/ijms21041225
APA StyleOwasil, R., O’Neill, R., Keable, A., Nimmo, J., MacGregor Sharp, M., Kelly, L., Saito, S., Simpson, J. E., Weller, R. O., Smith, C., Attems, J., Wharton, S. B., Yuen, H. M., & Carare, R. O. (2020). The Pattern of AQP4 Expression in the Ageing Human Brain and in Cerebral Amyloid Angiopathy. International Journal of Molecular Sciences, 21(4), 1225. https://doi.org/10.3390/ijms21041225






