Mitochondrial E3 Ubiquitin Ligase Parkin: Relationships with Other Causal Proteins in Familial Parkinson’s Disease and Its Substrate-Involved Mouse Experimental Models
Abstract
1. Introduction
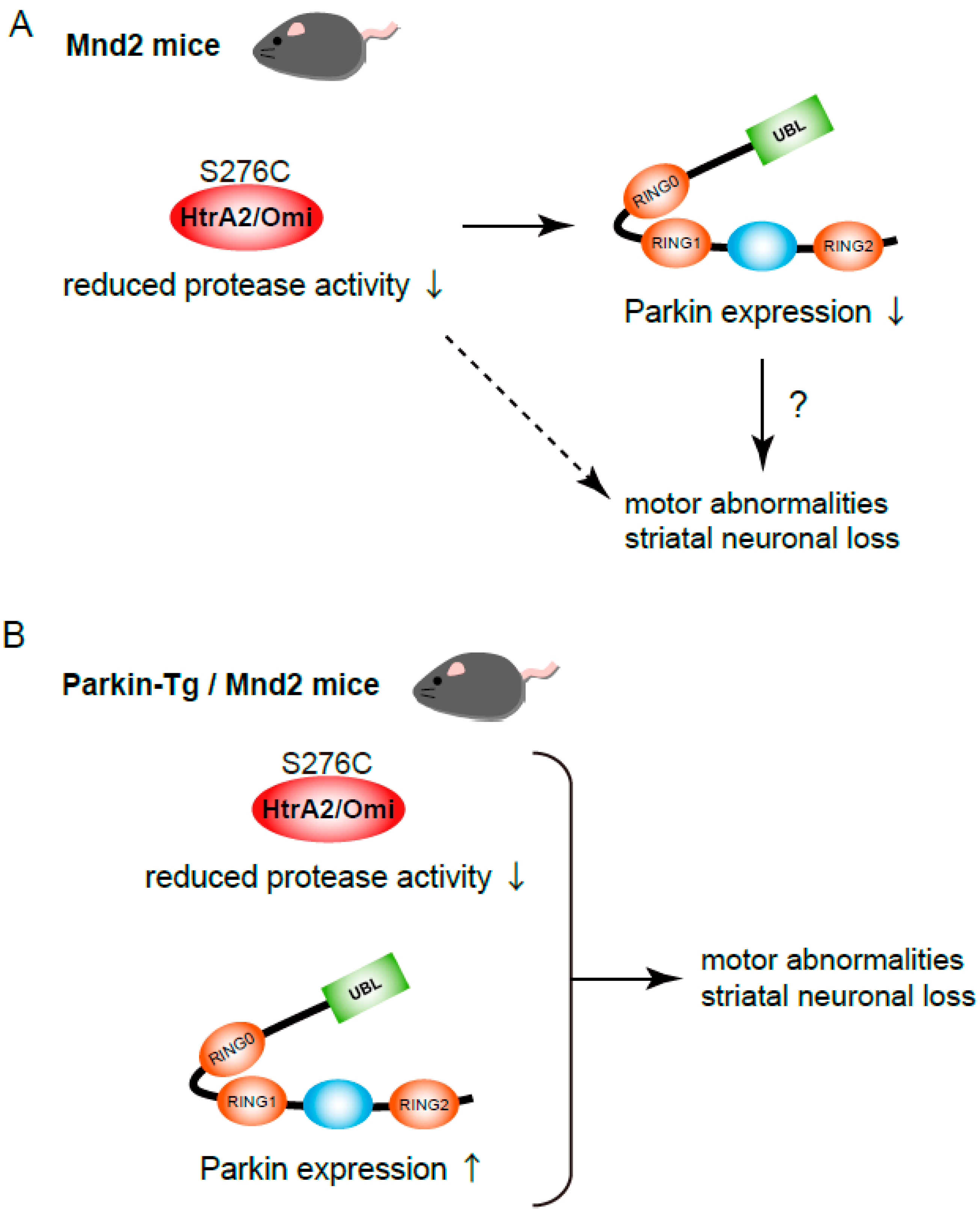
2. Parkin-Tg Introduction into mnd2 Mice; Neurodegeneration in HtrA2/Omi Mutant Mice Is Not Rescued by Parkin Transgene Expression
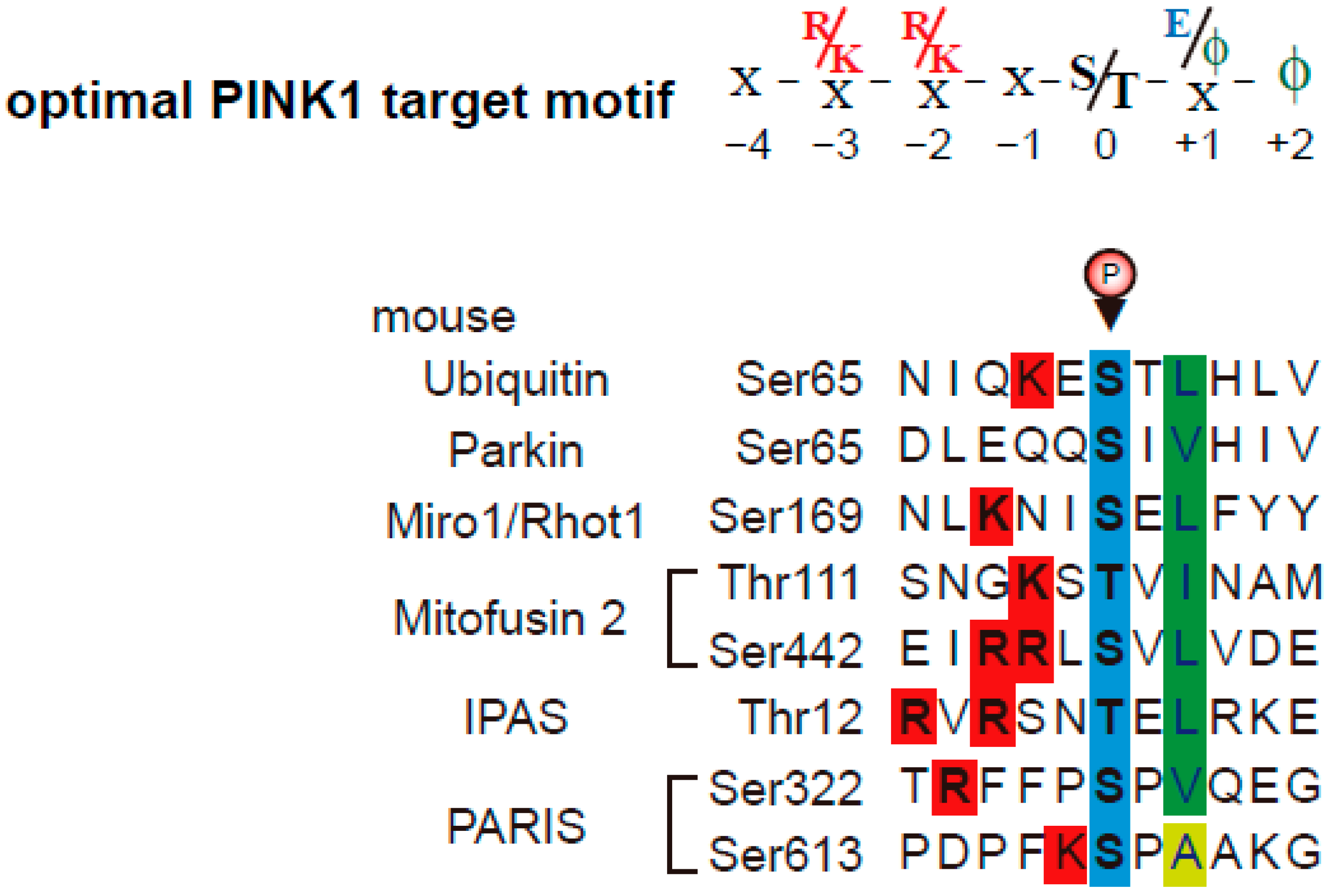
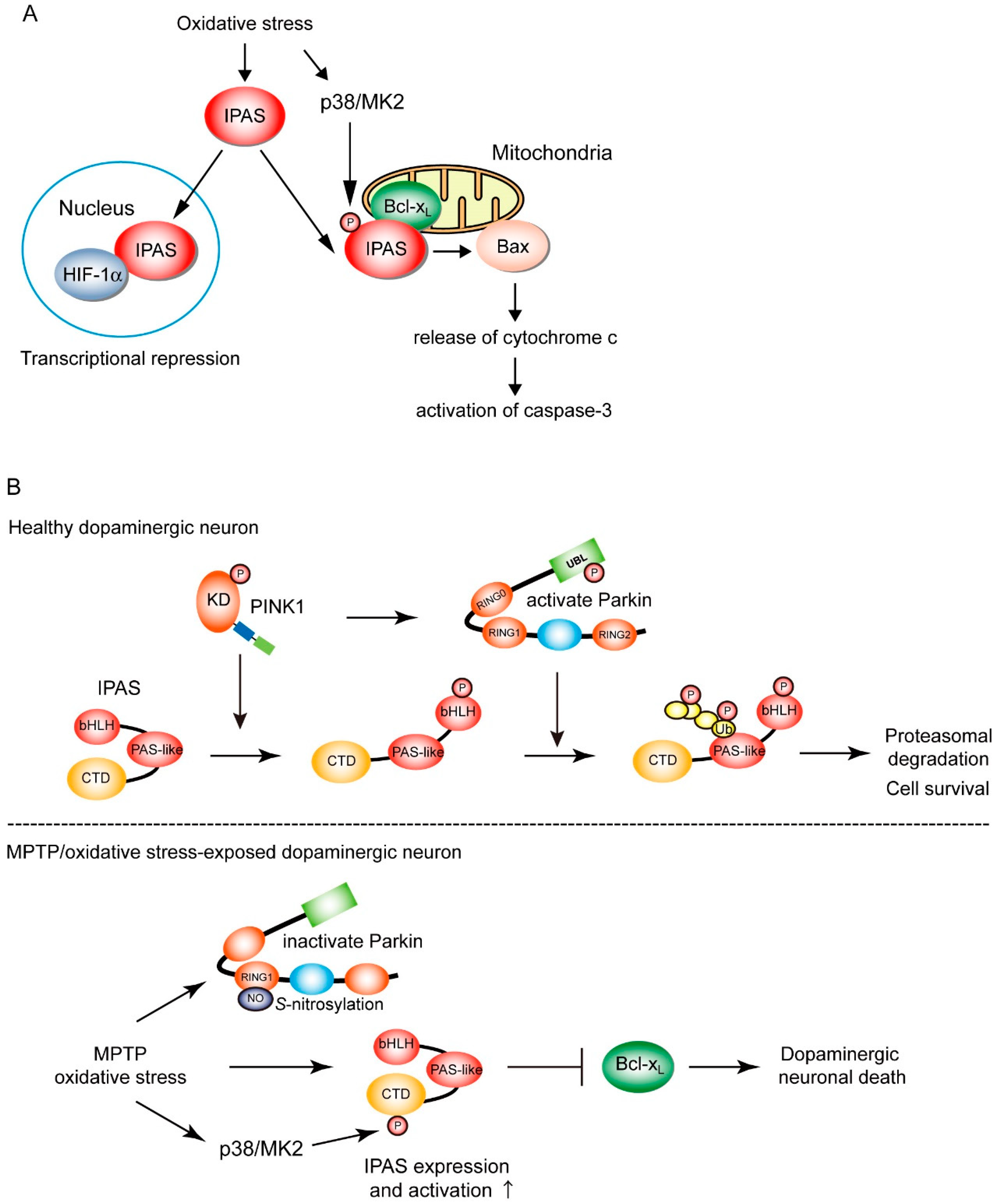
3. One of PINK1/Parkin-Target Proteins, IPAS; Its Molecular Mechanism of Pro-Apoptotic Activation, and Its Deficient Mouse Model
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Exner, N.; Lutz, A.K.; Haass, C.; Winklhofer, K.F. Mitochondrial dysfunction in Parkinson’s disease: Molecular mechanisms and pathophysiological consequences. EMBO J. 2012, 31, 3038–3062. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.E.; Paek, S.H. Mitochondrial dysfunction in Parkinson’s disease. Exp. Neurobiol. 2015, 24, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.; Westenberger, A. Genetics of Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a008888. [Google Scholar] [CrossRef] [PubMed]
- Langston, J.W.; Ballard, P.; Tetrud, J.W.; Irwin, I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 1983, 219, 979–980. [Google Scholar] [CrossRef]
- Singer, T.P.; Ramsay, R.R.; McKeown, K.; Trevor, A.; Castagnoli, N.E., Jr. Mechanism of the neurotoxicity of 1-methyl-4-phenylpyridinium (MPP+), the toxic bioactivation product of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Toxicology 1988, 49, 17–23. [Google Scholar] [CrossRef]
- Javitch, J.A.; D’Amato, R.J.; Strittmatter, S.M.; Snyder, S.H. Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine: Uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proc. Natl. Acad. Sci. USA 1985, 82, 2173–2177. [Google Scholar] [CrossRef]
- Berry, C.; La Vecchia, C.; Nicotera, P. Paraquat and Parkinson’s disease. Cell Death Differ. 2010, 17, 1115–1125. [Google Scholar] [CrossRef]
- Bove, J.; Perier, C. Neurotoxin-based models of Parkinson’s disease. Neuroscience 2012, 211, 51–76. [Google Scholar] [CrossRef]
- Jackson-Lewis, V.; Przedborski, S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat. Protoc. 2007, 2, 141–151. [Google Scholar] [CrossRef]
- Chiba, K.; Horii, H.; Kubota, E.; Ishizaki, T.; Kato, Y. Effects of N-methylmercaptoimidazole on the disposition of MPTP and its metabolites in mice. Eur. J. Pharmacol. 1990, 180, 59–67. [Google Scholar] [CrossRef]
- Funayama, M.; Ohe, K.; Amo, T.; Furuya, N.; Yamaguchi, J.; Saiki, S.; Li, Y.; Ogaki, K.; Ando, M.; Yoshino, H.; et al. CHCHD2 mutations in autosomal dominant late-onset Parkinson’s disease: A genome-wide linkage and sequencing study. Lancet Neurol. 2015, 14, 274–282. [Google Scholar] [CrossRef]
- Jones, J.M.; Datta, P.; Srinivasula, S.M.; Ji, W.; Gupta, S.; Zhang, Z.; Davies, E.; Hajnoczky, G.; Saunders, T.L.; Van Keuren, M.L.; et al. Loss of Omi mitochondrial protease activity causes the neuromuscular disorder of mnd2 mutant mice. Nature 2003, 425, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, N. Phospho-ubiquitin: Upending the PINK-Parkin-ubiquitin cascade. J. Biochem. 2016, 159, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Truban, D.; Hou, X.; Caulfield, T.R.; Fiesel, F.C.; Springer, W. PINK1, parkin, and mitochondrial quality control: What can we learn about Parkinson’s disease pathobiology? J. Parkinson’s Dis. 2017, 7, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Meng, H.; Shiba-Fukushima, K.; Hattori, N. Twin CHCH proteins, CHCHD2, and CHCHD10: Key molecules of Parkinson’s disease, amyotrophic lateral sclerosis, and frontotemporal dementia. Int. J. Mol. Sci. 2019, 20, 908. [Google Scholar] [CrossRef]
- Meng, H.; Yamashita, C.; Shiba-Fukushima, K.; Inoshita, T.; Funayama, M.; Sato, S.; Hatta, T.; Natsume, T.; Umitsu, M.; Takagi, J.; et al. Loss of Parkinson’s disease-associated protein CHCHD2 affects mitochondrial crista structure and destabilizes cytochrome c. Nat. Commun. 2017, 8, 15500. [Google Scholar] [CrossRef]
- Sim, C.H.; Gabriel, K.; Mills, R.D.; Culvenor, J.G.; Cheng, H.C. Analysis of the regulatory and catalytic domains of PTEN-induced kinase-1 (PINK1). Hum. Mutat. 2012, 33, 1408–1422. [Google Scholar] [CrossRef]
- Matsuda, N.; Sato, S.; Shiba, K.; Okatsu, K.; Saisho, K.; Gautier, C.A.; Sou, Y.S.; Saiki, S.; Kawajiri, S.; Sato, F.; et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J. Cell Biol. 2010, 189, 211–221. [Google Scholar] [CrossRef]
- Deas, E.; Plun-Favreau, H.; Gandhi, S.; Desmond, H.; Kjaer, S.; Loh, S.H.; Renton, A.E.; Harvey, R.J.; Whitworth, A.J.; Martins, L.M.; et al. PINK1 cleavage at position A103 by the mitochondrial protease PARL. Hum. Mol. Genet. 2011, 20, 867–879. [Google Scholar] [CrossRef]
- Kondapalli, C.; Kazlauskaite, A.; Zhang, N.; Woodroof, H.I.; Campbell, D.G.; Gourlay, R.; Burchell, L.; Walden, H.; Macartney, T.J.; Deak, M.; et al. PINK1 is activated by mitochondrial membrane potential depolarization and stimulates Parkin E3 ligase activity by phosphorylating Serine 65. Open Biol. 2012, 2, 120080. [Google Scholar] [CrossRef]
- Shiba-Fukushima, K.; Imai, Y.; Yoshida, S.; Ishihama, Y.; Kanao, T.; Sato, S.; Hattori, N. PINK1-mediated phosphorylation of the Parkin ubiquitin-like domain primes mitochondrial translocation of Parkin and regulates mitophagy. Sci. Rep. 2012, 2, 1002. [Google Scholar] [CrossRef] [PubMed]
- Koyano, F.; Okatsu, K.; Kosako, H.; Tamura, Y.; Go, E.; Kimura, M.; Kimura, Y.; Tsuchiya, H.; Yoshihara, H.; Hirokawa, T.; et al. Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature 2014, 510, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Shiba-Fukushima, K.; Arano, T.; Matsumoto, G.; Inoshita, T.; Yoshida, S.; Ishihama, Y.; Ryu, K.Y.; Nukina, N.; Hattori, N.; Imai, Y. Phosphorylation of mitochondrial polyubiquitin by PINK1 promotes Parkin mitochondrial tethering. PLoS Genet. 2014, 10, e1004861. [Google Scholar] [CrossRef] [PubMed]
- Bingol, B.; Tea, J.S.; Phu, L.; Reichelt, M.; Bakalarski, C.E.; Song, Q.; Foreman, O.; Kirkpatrick, D.S.; Sheng, M. The mitochondrial deubiquitinase USP30 opposes parkin-mediated mitophagy. Nature 2014, 510, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, T.; Haddad, D.; Wauters, F.; Van Humbeeck, C.; Mandemakers, W.; Koentjoro, B.; Sue, C.; Gevaert, K.; De Strooper, B.; Verstreken, P.; et al. The deubiquitinase USP15 antagonizes Parkin-mediated mitochondrial ubiquitination and mitophagy. Hum. Mol. Genet. 2014, 23, 5227–5242. [Google Scholar] [CrossRef]
- Malik, B.R.; Maddison, D.C.; Smith, G.A.; Peters, O.M. Autophagic and endo-lysosomal dysfunction in neurodegenerative disease. Mol. Brain 2019, 12, 100. [Google Scholar] [CrossRef]
- Ravenhill, B.J.; Boyle, K.B.; von Muhlinen, N.; Ellison, C.J.; Masson, G.R.; Otten, E.G.; Foeglein, A.; Williams, R.; Randow, F. The cargo receptor NDP52 initiates selective autophagy by recruiting the ULK complex to cytosol-invading bacteria. Mol. Cell 2019, 74, 320–329. [Google Scholar] [CrossRef]
- Yamano, K.; Wang, C.; Sarraf, S.A.; Munch, C.; Kikuchi, R.; Noda, N.N.; Hizukuri, Y.; Kanemaki, M.T.; Harper, W.; Tanaka, K.; et al. Endosomal Rab cycles regulate Parkin-mediated mitophagy. eLife 2018, 7, e31326. [Google Scholar] [CrossRef]
- Iwashita, H.; Torii, S.; Nagahora, N.; Ishiyama, M.; Shioji, K.; Sasamoto, K.; Shimizu, S.; Okuma, K. Live cell imaging of mitochondrial autophagy with a novel fluorescent small molecule. ACS Chem. Biol. 2017, 12, 2546–2551. [Google Scholar] [CrossRef]
- Plun-Favreau, H.; Klupsch, K.; Moisoi, N.; Gandhi, S.; Kjaer, S.; Frith, D.; Harvey, K.; Deas, E.; Harvey, R.J.; McDonald, N.; et al. The mitochondrial protease HtrA2 is regulated by Parkinson’s disease-associated kinase PINK1. Nat. Cell Biol. 2007, 9, 1243–1252. [Google Scholar] [CrossRef]
- Pridgeon, J.W.; Olzmann, J.A.; Chin, L.S.; Li, L. PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1. PLoS Biol. 2007, 5, e172. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Huang, Y.; Shao, Y.; May, J.; Prou, D.; Perier, C.; Dauer, W.; Schon, E.A.; Przedborski, S. The kinase domain of mitochondrial PINK1 faces the cytoplasm. Proc. Natl. Acad. Sci. USA 2008, 105, 12022–12027. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Winter, D.; Ashrafi, G.; Schlehe, J.; Wong, Y.L.; Selkoe, D.; Rice, S.; Steen, J.; LaVoie, M.J.; Schwarz, T.L. PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell 2011, 147, 893–906. [Google Scholar] [CrossRef]
- Okatsu, K.; Oka, T.; Iguchi, M.; Imamura, K.; Kosako, H.; Tani, N.; Kimura, M.; Go, E.; Koyano, F.; Funayama, M.; et al. PINK1 autophosphorylation upon membrane potential dissipation is essential for Parkin recruitment to damaged mitochondria. Nat. Commun. 2012, 3, 1016. [Google Scholar] [CrossRef]
- Chen, Y.; Dorn, G.W., II. PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science 2013, 340, 471–475. [Google Scholar] [CrossRef]
- Torii, S.; Kasai, S.; Suzuki, A.; Todoroki, Y.; Yokozawa, K.; Yasumoto, K.; Seike, N.; Kiyonari, H.; Mukumoto, Y.; Kakita, A.; et al. Involvement of inhibitory PAS domain protein in neuronal cell death in Parkinson’s disease. Cell Death Discov. 2015, 1, 15015. [Google Scholar] [CrossRef]
- Lee, Y.; Stevens, D.A.; Kang, S.U.; Jiang, H.; Lee, Y.I.; Ko, H.S.; Scarffe, L.A.; Umanah, G.E.; Kang, H.; Ham, S.; et al. PINK1 primes parkin-mediated ubiquitination of PARIS in dopaminergic neuronal survival. Cell Rep. 2017, 18, 918–932. [Google Scholar] [CrossRef]
- Tsai, P.I.; Lin, C.H.; Hsieh, C.H.; Papakyrikos, A.M.; Kim, M.J.; Napolioni, V.; Schoor, C.; Couthouis, J.; Wu, R.M.; Wszolek, Z.K.; et al. PINK1 phosphorylates MIC60/Mitofilin to control structural plasticity of mitochondrial crista junctions. Mol. Cell 2019, 69, 744–756. [Google Scholar] [CrossRef]
- Kinoshita, E.; Kinoshita-Kikuta, E.; Takiyama, K.; Koike, T. Phosphate-binding tag, a new tool to visualize phosphorylated proteins. Mol. Cell. Proteom. 2006, 5, 749–757. [Google Scholar] [CrossRef]
- Yoshida, T.; Mizuta, T.; Shimizu, S. Neurodegeneration in mnd2 mutant mice is not prevented by parkin transgene. Biochem. Biophys. Res. Commun. 2010, 402, 676–679. [Google Scholar] [CrossRef]
- Faccio, L.; Fusco, C.; Chen, A.; Martinotti, S.; Bonventre, J.V.; Zervos, A.S. Characterization of a novel human serine protease that has extensive homology to bacterial heat shock endoprotease HtrA and is regulated by kidney ischemia. J. Biol. Chem. 2000, 275, 2581–2588. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.C.; Camprubi, M.D.; Dunn, L.; Wu, H.C.; Ip, N.Y.; Kruger, R.; Martins, L.M.; Wood, N.W.; Plun-Favreau, H. Phosphorylation of HtrA2 by cyclin-dependent kinase-5 is important for mitochondrial function. Cell Death Differ. 2012, 19, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Imai, Y.; Nakayama, H.; Takahashi, K.; Takio, K.; Takahashi, R. A serine protease, HtrA2, is released from the mitochondria and interacts with XIAP, inducing cell death. Mol. Cell 2001, 8, 613–621. [Google Scholar] [CrossRef]
- Martins, L.M.; Iaccarino, I.; Tenev, T.; Gschmeissner, S.; Totty, N.F.; Lemoine, N.R.; Savopoulos, J.; Gray, C.W.; Creasy, C.L.; Dingwall, C.; et al. The serine protease Omi/HtrA2 regulates apoptosis by binding XIAP through a reaper-like motif. J. Biol. Chem. 2002, 277, 439–444. [Google Scholar] [CrossRef]
- Jones, J.M.; Albin, R.L.; Feldman, E.L.; Simin, K.; Schuster, T.G.; Dunnick, W.A.; Collins, J.T.; Chrisp, C.E.; Taylor, B.A.; Meisler, M.H. mnd2: A new mouse model of inherited motor neuron disease. Genomics 1993, 16, 669–677. [Google Scholar] [CrossRef]
- Rathke-Hartlieb, S.; Schlomann, U.; Heimann, P.; Meisler, M.H.; Jockusch, H.; Bartsch, J.W. Progressive loss of striatal neurons causes motor dysfunction in MND2 mutant mice and is not prevented by Bcl-2. Exp. Neurol. 2002, 175, 87–97. [Google Scholar] [CrossRef]
- Ideguchi, K.; Shimizu, S.; Okumura, M.; Tsujimoto, Y. Cyclophilin D-dependent mitochondrial permeability transition is not involved in neurodegeneration in mnd2 mutant mice. Biochem. Biophys. Res. Commun. 2010, 393, 264–267. [Google Scholar] [CrossRef]
- Vercammen, L.; Van der Perren, A.; Vaudano, E.; Gijsbers, R.; Debyser, Z.; Van den Haute, C.; Baekelandt, V. Parkin protects against neurotoxicity in the 6-hydroxydopamine rat model for Parkinson’s disease. Mol. Ther. 2006, 14, 716–723. [Google Scholar] [CrossRef]
- Petrucelli, L.; O’Farrell, C.; Lockhart, P.J.; Baptista, M.; Kehoe, K.; Vink, L.; Choi, P.; Wolozin, B.; Farrer, M.; Hardy, J.; et al. Parkin protects against the toxicity associated with mutant alpha-synuclein: Proteasome dysfunction selectively affects catecholaminergic neurons. Neuron 2002, 36, 1007–1019. [Google Scholar] [CrossRef]
- Manfredsson, F.P.; Burger, C.; Sullivan, L.F.; Muzyczka, N.; Lewin, A.S.; Mandel, R.J. rAAV-mediated nigral human parkin over-expression partially ameliorates motor deficits via enhanced dopamine neurotransmission in a rat model of Parkinson’s disease. Exp. Neurol. 2007, 207, 289–301. [Google Scholar] [CrossRef]
- Klein, R.L.; Dayton, R.D.; Henderson, K.M.; Petrucelli, L. Parkin is protective for substantia nigra dopamine neurons in a tau gene transfer neurodegeneration model. Neurosci. Lett. 2006, 401, 130–135. [Google Scholar] [CrossRef]
- Hattori, N.; Mizuno, Y. Twenty years since the discovery of the parkin gene. J. Neural Transm. (Vienna) 2017, 124, 1037–1054. [Google Scholar] [CrossRef]
- Imai, Y.; Soda, M.; Inoue, H.; Hattori, N.; Mizuno, Y.; Takahashi, R. An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell 2001, 105, 891–902. [Google Scholar] [CrossRef]
- Sul, J.W.; Park, M.Y.; Shin, J.; Kim, Y.R.; Yoo, S.E.; Kong, Y.Y.; Kwon, K.S.; Lee, Y.H.; Kim, E. Accumulation of the parkin substrate, FAF1, plays a key role in the dopaminergic neurodegeneration. Hum. Mol. Genet. 2013, 22, 1558–1573. [Google Scholar] [CrossRef]
- Gao, F.; Chen, D.; Si, J.; Hu, Q.; Qin, Z.; Fang, M.; Wang, G. The mitochondrial protein BNIP3L is the substrate of PARK2 and mediates mitophagy in PINK1/PARK2 pathway. Hum. Mol. Genet. 2015, 24, 2528–2538. [Google Scholar] [CrossRef]
- Koyano, F.; Yamano, K.; Kosako, H.; Kimura, Y.; Kimura, M.; Fujiki, Y.; Tanaka, K.; Matsuda, N. Parkin-mediated ubiquitylation redistributes MITOL/March5 from mitochondria to peroxisomes. EMBO Rep. 2019, 20, e47728. [Google Scholar] [CrossRef]
- Yao, D.; Gu, Z.; Nakamura, T.; Shi, Z.Q.; Ma, Y.; Gaston, B.; Palmer, L.A.; Rockenstein, E.M.; Zhang, Z.; Masliah, E.; et al. Nitrosative stress linked to sporadic Parkinson’s disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc. Natl. Acad. Sci. USA 2004, 101, 10810–10814. [Google Scholar] [CrossRef]
- Imai, Y.; Inoue, H.; Kataoka, A.; Hua-Qin, W.; Masuda, M.; Ikeda, T.; Tsukita, K.; Soda, M.; Kodama, T.; Fuwa, T.; et al. Pael receptor is involved in dopamine metabolism in the nigrostriatal system. Neurosci. Res. 2007, 59, 413–425. [Google Scholar] [CrossRef]
- Makino, Y.; Cao, R.; Svensson, K.; Bertilsson, G.; Asman, M.; Tanaka, H.; Cao, Y.; Berkenstam, A.; Poellinger, L. Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature 2001, 414, 550–554. [Google Scholar] [CrossRef]
- Hirota, K.; Semenza, G.L. Regulation of hypoxia-inducible factor 1 by prolyl and asparaginyl hydroxylases. Biochem. Biophys. Res. Commun. 2005, 338, 610–616. [Google Scholar] [CrossRef]
- Makino, Y.; Uenishi, R.; Okamoto, K.; Isoe, T.; Hosono, O.; Tanaka, H.; Kanopka, A.; Poellinger, L.; Haneda, M.; Morimoto, C. Transcriptional up-regulation of inhibitory PAS domain protein gene expression by hypoxia-inducible factor 1 (HIF-1): A negative feedback regulatory circuit in HIF-1-mediated signaling in hypoxic cells. J. Biol. Chem. 2007, 282, 14073–14082. [Google Scholar] [CrossRef] [PubMed]
- Torii, S.; Kobayashi, K.; Takahashi, M.; Katahira, K.; Goryo, K.; Matsushita, N.; Yasumoto, K.; Fujii-Kuriyama, Y.; Sogawa, K. Magnesium deficiency causes loss of response to intermittent hypoxia in paraganglion cells. J. Biol. Chem. 2009, 284, 19077–19089. [Google Scholar] [CrossRef] [PubMed]
- Goryo, K.; Torii, S.; Yasumoto, K.; Sogawa, K. Tumour necrosis factor-alpha suppresses the hypoxic response by NF-kappaB-dependent induction of inhibitory PAS domain protein in PC12 cells. J. Biochem. 2011, 150, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Torii, S.; Goto, Y.; Ishizawa, T.; Hoshi, H.; Goryo, K.; Yasumoto, K.; Fukumura, H.; Sogawa, K. Pro-apoptotic activity of inhibitory PAS domain protein (IPAS), a negative regulator of HIF-1, through binding to pro-survival Bcl-2 family proteins. Cell Death Differ. 2011, 18, 1711–1725. [Google Scholar] [CrossRef][Green Version]
- Torii, S.; Sakaki, K.; Otomo, M.; Saka, K.; Yasumoto, K.; Sogawa, K. Nucleocytoplasmic shuttling of IPAS by its unique nuclear import and export signals unshared with other HIF-3alpha splice variants. J. Biochem. 2013, 154, 561–567. [Google Scholar] [CrossRef]
- Ravenna, L.; Salvatori, L.; Russo, M.A. HIF3alpha: The little we know. FEBS J. 2016, 283, 993–1003. [Google Scholar] [CrossRef]
- Yao, Q.; Zhang, P.; Lu, L.; Liu, Y.; Li, Y.; Duan, C. Nuclear localization of Hif-3alpha requires two redundant NLS motifs in its unique C-terminal region. FEBS Lett. 2018, 592, 2769–2775. [Google Scholar] [CrossRef]
- Kasai, S.; Kajimoto, S.; Ito, Y.; Saito, T.; Yasumoto, K.; Tokunaga, M.; Sakata-Sogawa, K.; Fukumura, H.; Sogawa, K. Conformational changes in inhibitory PAS domain protein associated with binding of HIF-1alpha and Bcl-xL in living cells. J. Biochem. 2017, 161, 291–296. [Google Scholar]
- Kasai, S.; Richardson, M.J.E.; Torii, S.; Yasumoto, K.; Shima, H.; Igarashi, K.; Itoh, K.; Sogawa, K.; Murayama, K. Increase in proapoptotic activity of inhibitory PAS domain protein via phosphorylation by MK2. FEBS J. 2017, 284, 4115–4127. [Google Scholar] [CrossRef]
- Yamamoto, H.; Higa-Nakamine, S.; Noguchi, N.; Maeda, N.; Kondo, Y.; Toku, S.; Kukita, I.; Sugahara, K. Desensitization by different strategies of epidermal growth factor receptor and ErbB4. J. Pharmacol. Sci. 2014, 124, 287–293. [Google Scholar] [CrossRef]
- Kasai, S.; Torii, S.; Kakita, A.; Sogawa, K. Inhibitory PAS domain protein is a substrate of PINK1 and Parkin and mediates cell death in a Parkinson’s disease model. Cell Death Dis. 2015, 6, e1886. [Google Scholar] [CrossRef]
- Thomas, T.; Timmer, M.; Cesnulevicius, K.; Hitti, E.; Kotlyarov, A.; Gaestel, M. MAPKAP kinase 2-deficiency prevents neurons from cell death by reducing neuroinflammation—Relevance in a mouse model of Parkinson’s disease. J. Neurochem. 2008, 105, 2039–2052. [Google Scholar] [CrossRef]
- Karunakaran, S.; Saeed, U.; Mishra, M.; Valli, R.K.; Joshi, S.D.; Meka, D.P.; Seth, P.; Ravindranath, V. Selective activation of p38 mitogen-activated protein kinase in dopaminergic neurons of substantia nigra leads to nuclear translocation of p53 in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice. J. Neurosci. 2008, 28, 12500–12509. [Google Scholar] [CrossRef]
- Ikeda, A.; Nishioka, K.; Meng, H.; Takanashi, M.; Inoshita, T.; Shiba-Fukushima, K.; Li, Y.; Yoshino, H.; Mori, A.; Okuzumi, A.; et al. Mutations in CHCHD2 cause alpha-synuclein aggregation. Hum. Mol. Genet. 2019, 28, 3895–3911. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torii, S.; Kasai, S.; Yoshida, T.; Yasumoto, K.-i.; Shimizu, S. Mitochondrial E3 Ubiquitin Ligase Parkin: Relationships with Other Causal Proteins in Familial Parkinson’s Disease and Its Substrate-Involved Mouse Experimental Models. Int. J. Mol. Sci. 2020, 21, 1202. https://doi.org/10.3390/ijms21041202
Torii S, Kasai S, Yoshida T, Yasumoto K-i, Shimizu S. Mitochondrial E3 Ubiquitin Ligase Parkin: Relationships with Other Causal Proteins in Familial Parkinson’s Disease and Its Substrate-Involved Mouse Experimental Models. International Journal of Molecular Sciences. 2020; 21(4):1202. https://doi.org/10.3390/ijms21041202
Chicago/Turabian StyleTorii, Satoru, Shuya Kasai, Tatsushi Yoshida, Ken-ichi Yasumoto, and Shigeomi Shimizu. 2020. "Mitochondrial E3 Ubiquitin Ligase Parkin: Relationships with Other Causal Proteins in Familial Parkinson’s Disease and Its Substrate-Involved Mouse Experimental Models" International Journal of Molecular Sciences 21, no. 4: 1202. https://doi.org/10.3390/ijms21041202
APA StyleTorii, S., Kasai, S., Yoshida, T., Yasumoto, K.-i., & Shimizu, S. (2020). Mitochondrial E3 Ubiquitin Ligase Parkin: Relationships with Other Causal Proteins in Familial Parkinson’s Disease and Its Substrate-Involved Mouse Experimental Models. International Journal of Molecular Sciences, 21(4), 1202. https://doi.org/10.3390/ijms21041202






