Urinary NMR Profiling in Pediatric Acute Kidney Injury—A Pilot Study
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Study Population
2.2. Nuclear Magnetic Resonance (NMR) Spectroscopy Analysis
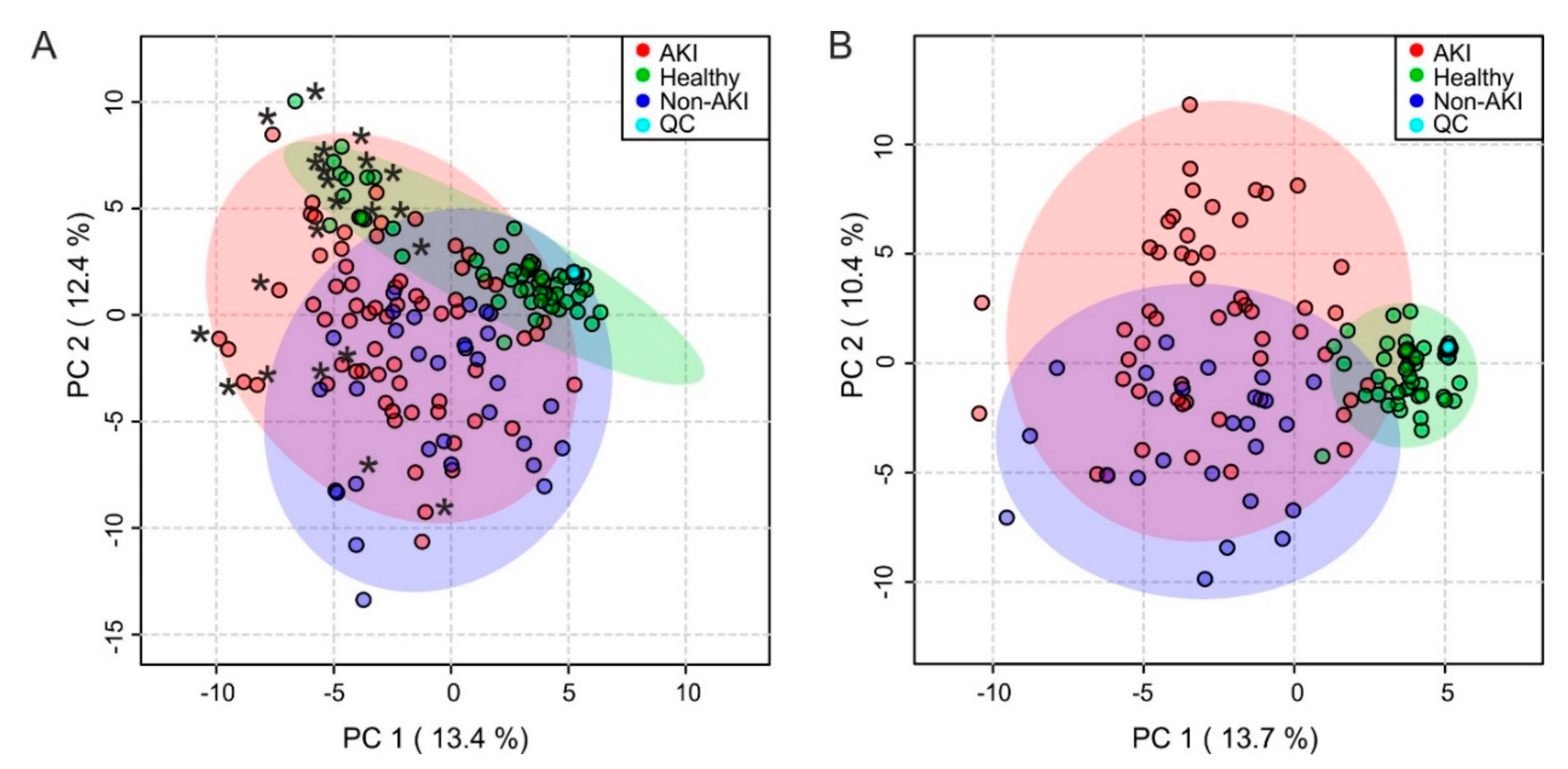
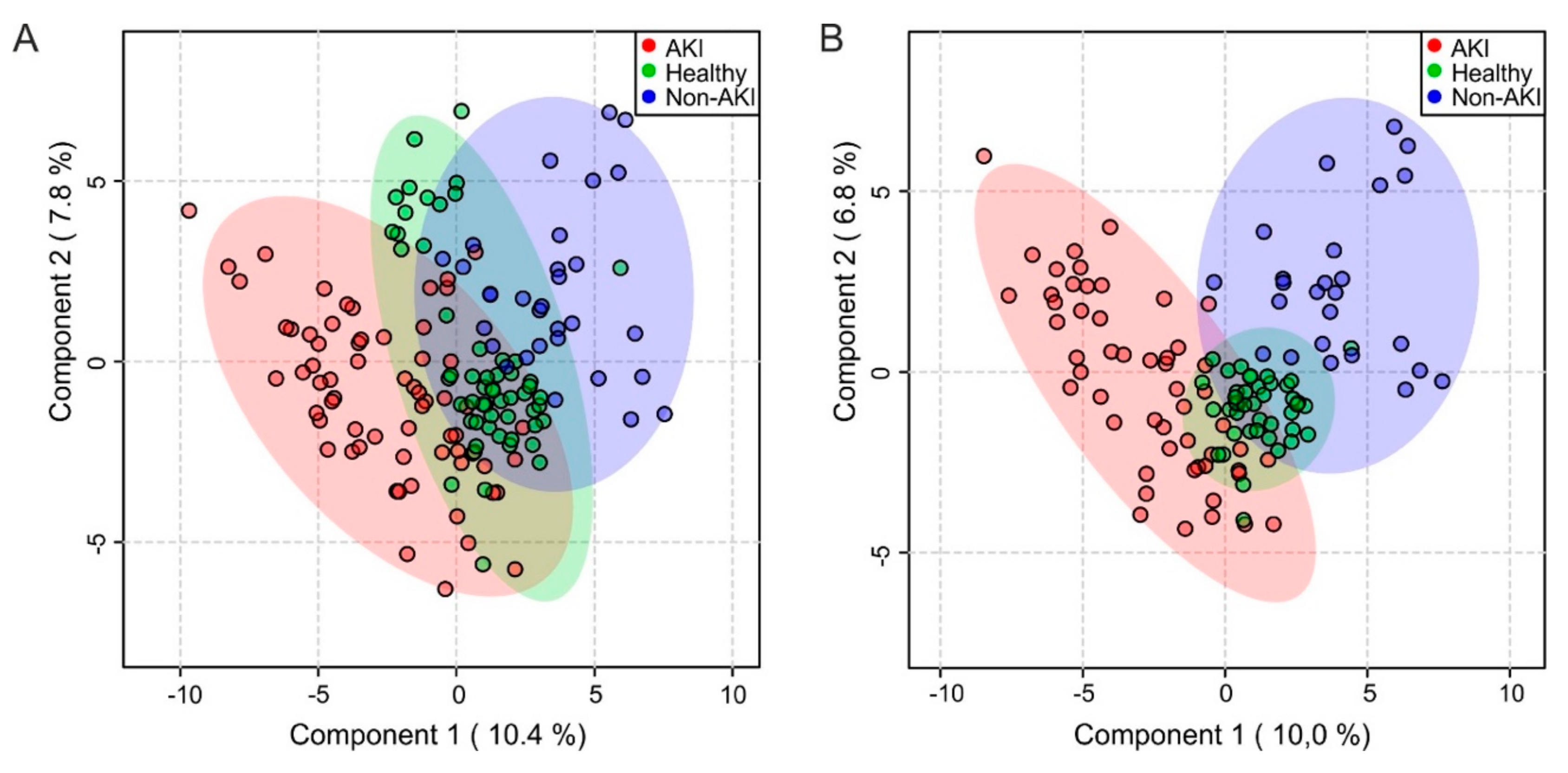
2.3. Multivariate Analysis
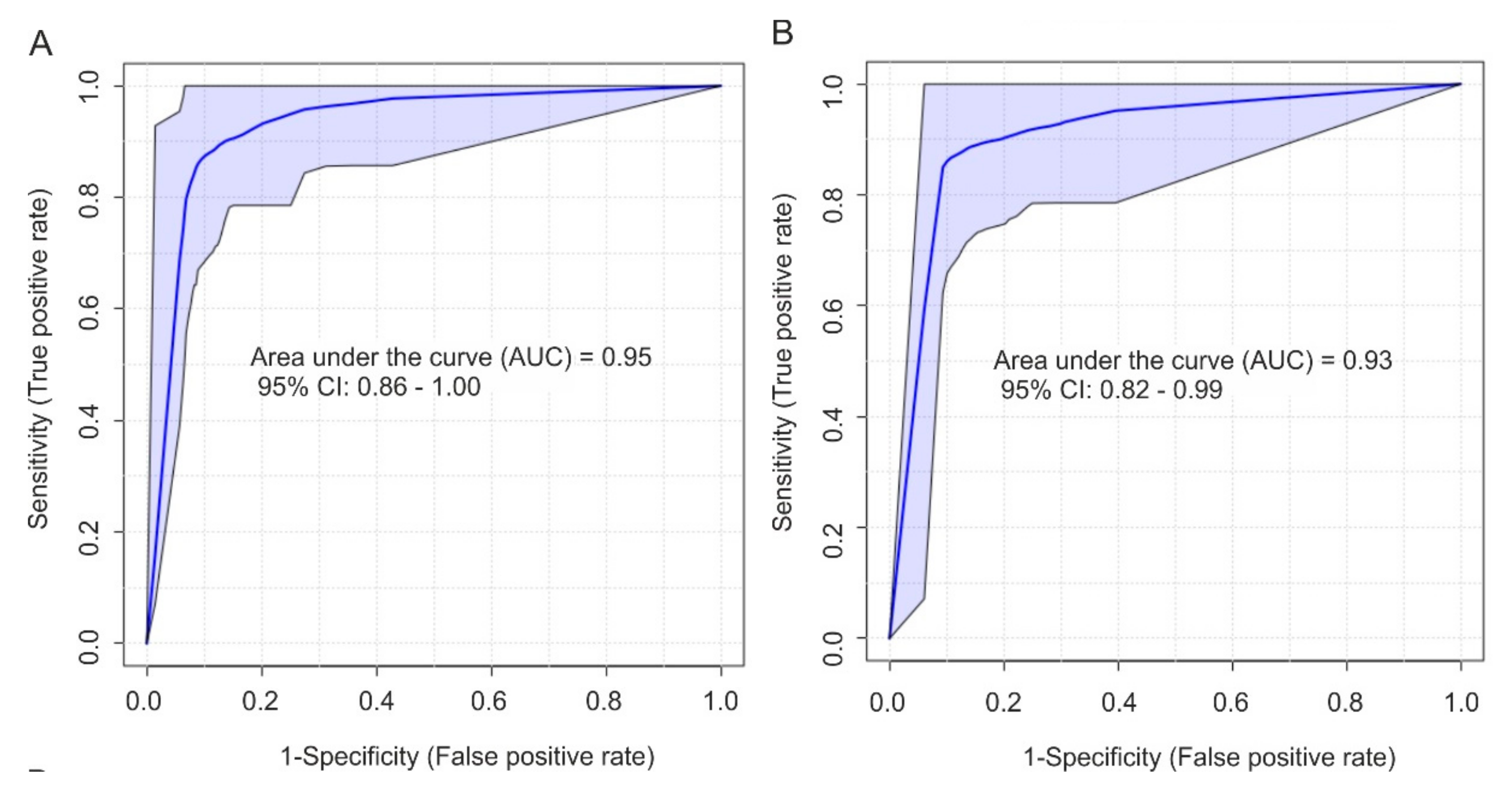
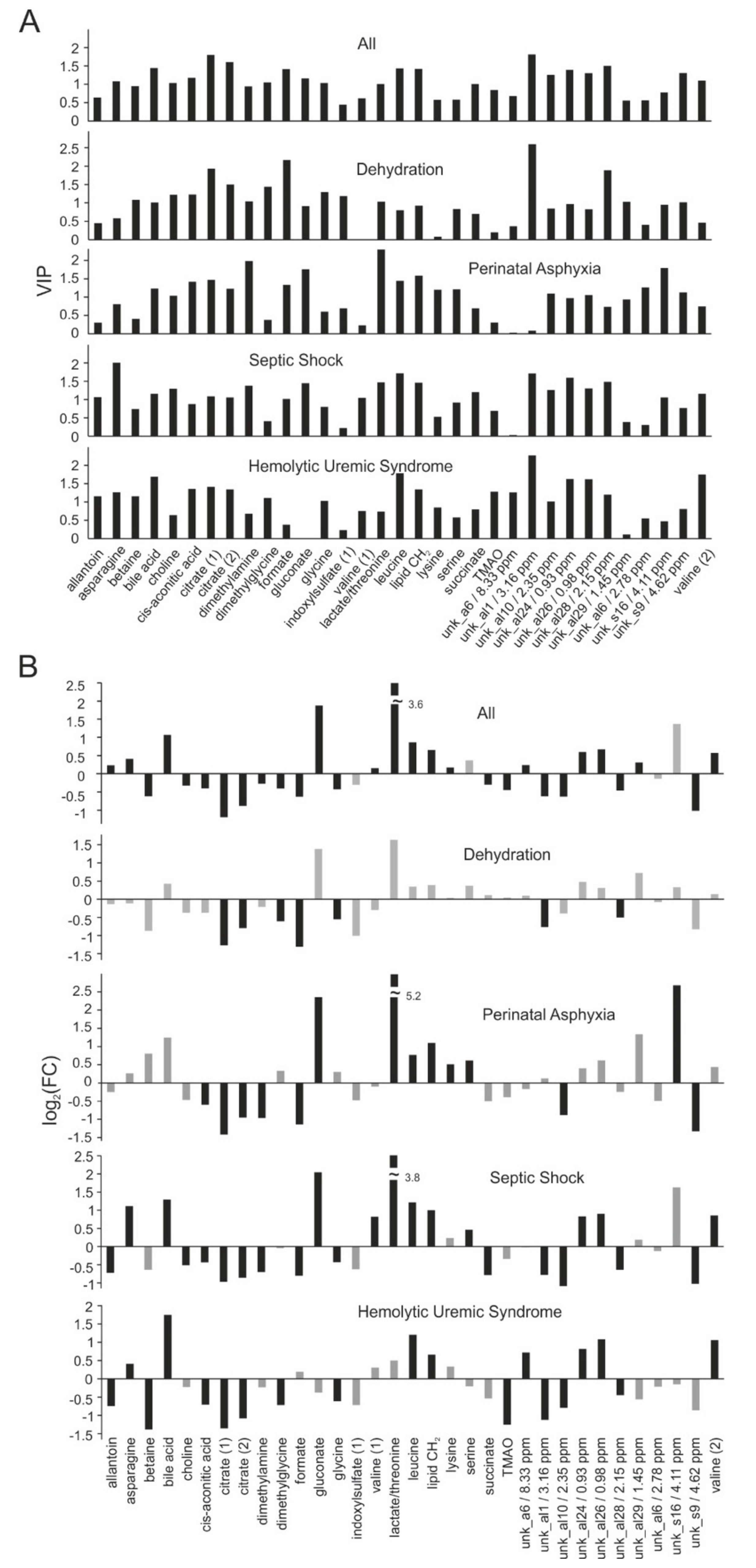
2.4. Biomarker Analysis
2.5. Differentiation of Acute Kidney Injury (AKI) Etiologies
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Study Design and Participants
4.3. Sample and Data Collection
4.4. 1H-NMR Spectroscopy of Urine Samples
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AKI | acute kidney injury |
| AUC | area under the curve |
| BMI | body mass index |
| eCCl | estimated creatinine clearance |
| KDIGO | Kidney Disease: Improving Global Outcomes |
| NICU | neonatal intensive care unit |
| NMR | nuclear magnetic resonance |
| PCA | principle component analysis |
| PICU | pediatric intensive care unit |
| PLS-DA | partial least squares discriminant analysis |
| QC | quality control |
| ROC | receiver operating characteristic |
| SDS | standard deviation score |
| VIP | variable importance in projection |
References
- Mammen, C.; Al Abbas, A.; Skippen, P.; Nadel, H.; Levine, D.; Collet, J.P.; Matsell, D.G. Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: A prospective cohort study. Am. J. Kidney Dis. 2012, 59, 523–530. [Google Scholar] [CrossRef]
- Menon, S.; Kirkendall, E.S.; Nguyen, H.; Goldstein, S.L. Acute kidney injury associated with high nephrotoxic medication exposure leads to chronic kidney disease after 6 months. J. Pediatr. 2014, 165, 522–527 e2. [Google Scholar] [CrossRef] [PubMed]
- Askenazi, D.J.; Feig, D.I.; Graham, N.M.; Hui-Stickle, S.; Goldstein, S.L. 3–5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int. 2006, 69, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Kaddourah, A.; Basu, R.K.; Bagshaw, S.M.; Goldstein, S.L. Investigators, A., Epidemiology of Acute Kidney Injury in Critically Ill Children and Young Adults. N. Engl. J. Med. 2017, 376, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Jetton, J.G.; Boohaker, L.J.; Sethi, S.K.; Wazir, S.; Rohatgi, S.; Soranno, D.E.; Chishti, A.S.; Woroniecki, R.; Mammen, C.; Swanson, J.R.; et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): A multicentre, multinational, observational cohort study. Lancet Child. Adolesc. Health 2017, 1, 184–194. [Google Scholar] [CrossRef]
- McGregor, T.L.; Jones, D.P.; Wang, L.; Danciu, I.; Bridges, B.C.; Fleming, G.M.; Shirey-Rice, J.; Chen, L.; Byrne, D.W.; Van Driest, S.L. Acute Kidney Injury Incidence in Noncritically Ill Hospitalized Children, Adolescents, and Young Adults: A Retrospective Observational Study. Am. J. Kidney Dis. 2016, 67, 384–390. [Google Scholar] [CrossRef]
- Schrezenmeier, E.V.; Barasch, J.; Budde, K.; Westhoff, T.; Schmidt-Ott, K.M. Biomarkers in acute kidney injury - pathophysiological basis and clinical performance. Acta Physiol. (Oxf) 2017, 219, 554–572. [Google Scholar] [CrossRef]
- Malhotra, R.; Siew, E.D. Biomarkers for the Early Detection and Prognosis of Acute Kidney Injury. Clin. J. Am. Soc. Nephrol. 2017, 12, 149–173. [Google Scholar] [CrossRef]
- Waldherr, S.; Fichtner, A.; Beedgen, B.; Bruckner, T.; Schaefer, F.; Tonshoff, B.; Poschl, J.; Westhoff, T.H.; Westhoff, J.H. Urinary acute kidney injury biomarkers in very low-birth-weight infants on indomethacin for patent ductus arteriosus. Pediatr. Res. 2019, 85, 678–686. [Google Scholar] [CrossRef]
- Greenberg, J.H.; Parikh, C.R. Biomarkers for Diagnosis and Prognosis of AKI in Children: One Size Does Not Fit All. Clin. J. Am. Soc Nephrol. 2017, 12, 1551–1557. [Google Scholar] [CrossRef]
- van Duijl, T.T.; Ruhaak, L.R.; de Fijter, J.W.; Cobbaert, C.M. Kidney Injury Biomarkers in an Academic Hospital Setting: Where Are We Now? Clin. Biochem. Rev. 2019, 40, 79–97. [Google Scholar] [PubMed]
- Cerda, J. A biomarker able to predict acute kidney injury before it occurs? Lancet 2019, 394, 448–450. [Google Scholar] [CrossRef]
- Weiss, R.H.; Kim, K. Metabolomics in the study of kidney diseases. Nat. Rev. Nephrol. 2011, 8, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Abbiss, H.; Maker, G.L.; Trengove, R.D. Metabolomics Approaches for the Diagnosis and Understanding of Kidney Diseases. Metabolites 2019, 9, 34. [Google Scholar] [CrossRef]
- Kalim, S.; Rhee, E.P. An overview of renal metabolomics. Kidney Int. 2017, 91, 61–69. [Google Scholar] [CrossRef]
- Izquierdo-Garcia, J.L.; Nin, N.; Cardinal-Fernandez, P.; Rojas, Y.; de Paula, M.; Granados, R.; Martinez-Caro, L.; Ruiz-Cabello, J.; Lorente, J.A. Identification of novel metabolomic biomarkers in an experimental model of septic acute kidney injury. Am. J. Physiol. Renal. Physiol. 2019, 316, F54–F62. [Google Scholar] [CrossRef] [PubMed]
- Chihanga, T.; Ma, Q.; Nicholson, J.D.; Ruby, H.N.; Edelmann, R.E.; Devarajan, P.; Kennedy, M.A. NMR spectroscopy and electron microscopy identification of metabolic and ultrastructural changes to the kidney following ischemia-reperfusion injury. Am. J. Physiol. Renal. Physiol. 2018, 314, F154–F166. [Google Scholar] [CrossRef]
- Jouret, F.; Leenders, J.; Poma, L.; Defraigne, J.O.; Krzesinski, J.M.; de Tullio, P. Nuclear Magnetic Resonance Metabolomic Profiling of Mouse Kidney, Urine and Serum Following Renal Ischemia/Reperfusion Injury. PLoS ONE 2016, 11, e0163021. [Google Scholar] [CrossRef]
- Archdekin, B.; Sharma, A.; Gibson, I.W.; Rush, D.; Wishart, D.S.; Blydt-Hansen, T.D. Non-invasive differentiation of non-rejection kidney injury from acute rejection in pediatric renal transplant recipients. Pediatr. Transplant. 2019, 23, e13364. [Google Scholar] [CrossRef]
- Mussap, M.; Antonucci, R.; Noto, A.; Fanos, V. The role of metabolomics in neonatal and pediatric laboratory medicine. Clin. Chim. Acta 2013, 426, 127–138. [Google Scholar] [CrossRef]
- Beger, R.D.; Holland, R.D.; Sun, J.; Schnackenberg, L.K.; Moore, P.C.; Dent, C.L.; Devarajan, P.; Portilla, D. Metabonomics of acute kidney injury in children after cardiac surgery. Pediatr. Nephrol. 2008, 23, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Bouatra, S.; Aziat, F.; Mandal, R.; Guo, A.C.; Wilson, M.R.; Knox, C.; Bjorndahl, T.C.; Krishnamurthy, R.; Saleem, F.; Liu, P.; et al. The human urine metabolome. PLoS ONE 2013, 8, e73076. [Google Scholar] [CrossRef] [PubMed]
- Westhoff, J.H.; Fichtner, A.; Waldherr, S.; Pagonas, N.; Seibert, F.S.; Babel, N.; Tonshoff, B.; Bauer, F.; Westhoff, T.H. Urinary biomarkers for the differentiation of prerenal and intrinsic pediatric acute kidney injury. Pediatr. Nephrol. 2016, 31, 2353–2363. [Google Scholar] [CrossRef] [PubMed]
- Fanos, V.; Fanni, C.; Ottonello, G.; Noto, A.; Dessi, A.; Mussap, M. Metabolomics in adult and pediatric nephrology. Molecules 2013, 18, 4844–4857. [Google Scholar] [CrossRef]
- Boudonck, K.J.; Mitchell, M.W.; Nemet, L.; Keresztes, L.; Nyska, A.; Shinar, D.; Rosenstock, M. Discovery of metabolomics biomarkers for early detection of nephrotoxicity. Toxicol. Pathol. 2009, 37, 280–292. [Google Scholar] [CrossRef]
- Won, A.J.; Kim, S.; Kim, Y.G.; Kim, K.B.; Choi, W.S.; Kacew, S.; Kim, K.S.; Jung, J.H.; Lee, B.M.; Kim, S.; et al. Discovery of urinary metabolomic biomarkers for early detection of acute kidney injury. Mol. Biosyst. 2016, 12, 133–144. [Google Scholar] [CrossRef]
- Sieber, M.; Hoffmann, D.; Adler, M.; Vaidya, V.S.; Clement, M.; Bonventre, J.V.; Zidek, N.; Rached, E.; Amberg, A.; Callanan, J.J.; et al. Comparative analysis of novel noninvasive renal biomarkers and metabonomic changes in a rat model of gentamicin nephrotoxicity. Toxicol. Sci. 2009, 109, 336–349. [Google Scholar] [CrossRef]
- Martin-Lorenzo, M.; Gonzalez-Calero, L.; Ramos-Barron, A.; Sanchez-Nino, M.D.; Gomez-Alamillo, C.; Garcia-Segura, J.M.; Ortiz, A.; Arias, M.; Vivanco, F.; Alvarez-Llamas, G. Urine metabolomics insight into acute kidney injury point to oxidative stress disruptions in energy generation and H2S availability. J. Mol. Med. (Berl) 2017, 95, 1399–1409. [Google Scholar] [CrossRef]
- Mercier, K.; McRitchie, S.; Pathmasiri, W.; Novokhatny, A.; Koralkar, R.; Askenazi, D.; Brophy, P.D.; Sumner, S. Preterm neonatal urinary renal developmental and acute kidney injury metabolomic profiling: An exploratory study. Pediatr. Nephrol. 2017, 32, 151–161. [Google Scholar] [CrossRef]
- Elmariah, S.; Farrell, L.A.; Daher, M.; Shi, X.; Keyes, M.J.; Cain, C.H.; Pomerantsev, E.; Vlahakes, G.J.; Inglessis, I.; Passeri, J.J.; et al. Metabolite Profiles Predict Acute Kidney Injury and Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement. J. Am. Heart Assoc. 2016, 5, e002712. [Google Scholar] [CrossRef]
- Doskocz, M.; Marchewka, Z.; Jez, M.; Passowicz-Muszynska, E.; Dlugosz, A. Preliminary Study on J-Resolved NMR Method Usability for Toxic Kidney’s Injury Assessment. Adv. Clin. Exp. Med. 2015, 24, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Zacharias, H.U.; Hochrein, J.; Vogl, F.C.; Schley, G.; Mayer, F.; Jeleazcov, C.; Eckardt, K.U.; Willam, C.; Oefner, P.J.; Gronwald, W. Identification of Plasma Metabolites Prognostic of Acute Kidney Injury after Cardiac Surgery with Cardiopulmonary Bypass. J. Proteome Res. 2015, 14, 2897–2905. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Shannon, M.; Ando, Y.; Schnackenberg, L.K.; Khan, N.A.; Portilla, D.; Beger, R.D. Serum metabolomic profiles from patients with acute kidney injury: A pilot study. J. Chromatogr B Analyt Technol. Biomed. Life Sci. 2012, 893, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Portilla, D.; Schnackenberg, L.; Beger, R.D. Metabolomics as an extension of proteomic analysis: Study of acute kidney injury. Semin. Nephrol. 2007, 27, 609–620. [Google Scholar] [CrossRef]
- Zgoda-Pols, J.R.; Chowdhury, S.; Wirth, M.; Milburn, M.V.; Alexander, D.C.; Alton, K.B. Metabolomics analysis reveals elevation of 3-indoxyl sulfate in plasma and brain during chemically-induced acute kidney injury in mice: Investigation of nicotinic acid receptor agonists. Toxicol. Appl. Pharmacol. 2011, 255, 48–56. [Google Scholar] [CrossRef]
- Hauet, T.; Baumert, H.; Gibelin, H.; Hameury, F.; Goujon, J.M.; Carretier, M.; Eugene, M. Noninvasive monitoring of citrate, acetate, lactate, and renal medullary osmolyte excretion in urine as biomarkers of exposure to ischemic reperfusion injury. Cryobiology 2000, 41, 280–291. [Google Scholar] [CrossRef]
- Xu, E.Y.; Perlina, A.; Vu, H.; Troth, S.P.; Brennan, R.J.; Aslamkhan, A.G.; Xu, Q. Integrated pathway analysis of rat urine metabolic profiles and kidney transcriptomic profiles to elucidate the systems toxicology of model nephrotoxicants. Chem. Res. Toxicol. 2008, 21, 1548–1561. [Google Scholar] [CrossRef]
- Portilla, D.; Li, S.; Nagothu, K.K.; Megyesi, J.; Kaissling, B.; Schnackenberg, L.; Safirstein, R.L.; Beger, R.D. Metabolomic study of cisplatin-induced nephrotoxicity. Kidney Int. 2006, 69, 2194–2204. [Google Scholar] [CrossRef]
- Shah, V.O.; Townsend, R.R.; Feldman, H.I.; Pappan, K.L.; Kensicki, E.; Vander Jagt, D.L. Plasma metabolomic profiles in different stages of CKD. Clin. J. Am. Soc. Nephrol. 2013, 8, 363–370. [Google Scholar] [CrossRef]
- Luck, M.; Bertho, G.; Bateson, M.; Karras, A.; Yartseva, A.; Thervet, E.; Damon, C.; Pallet, N. Rule-Mining for the Early Prediction of Chronic Kidney Disease Based on Metabolomics and Multi-Source Data. PLoS ONE 2016, 11, e0166905. [Google Scholar] [CrossRef]
- Sharma, K.; Karl, B.; Mathew, A.V.; Gangoiti, J.A.; Wassel, C.L.; Saito, R.; Pu, M.; Sharma, S.; You, Y.H.; Wang, L.; et al. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J. Am. Soc. Nephrol. 2013, 24, 1901–1912. [Google Scholar] [CrossRef]
- Gronwald, W.; Klein, M.S.; Zeltner, R.; Schulze, B.D.; Reinhold, S.W.; Deutschmann, M.; Immervoll, A.K.; Boger, C.A.; Banas, B.; Eckardt, K.U.; et al. Detection of autosomal dominant polycystic kidney disease by NMR spectroscopic fingerprinting of urine. Kidney Int. 2011, 79, 1244–1253. [Google Scholar] [CrossRef]
- Dantzler, W.H. Renal organic anion transport: A comparative and cellular perspective. Biochim Biophys Acta 2002, 1566, 169–181. [Google Scholar] [CrossRef][Green Version]
- Karniski, L.P.; Aronson, P.S. Chloride/formate exchange with formic acid recycling: A mechanism of active chl oride transport across epithelial membranes. Proc. Natl. Acad Sci. USA 1985, 82, 6362–6365. [Google Scholar] [CrossRef]
- Scalabre, A.; Jobard, E.; Demede, D.; Gaillard, S.; Pontoizeau, C.; Mouriquand, P.; Elena-Herrmann, B.; Mure, P.Y. Evolution of Newborns’ Urinary Metabolomic Profiles According to Age and Growth. J. Proteome Res. 2017, 16, 3732–3740. [Google Scholar] [CrossRef]
- Gu, H.; Pan, Z.; Xi, B.; Hainline, B.E.; Shanaiah, N.; Asiago, V.; Gowda, G.A.; Raftery, D. 1H NMR metabolomics study of age profiling in children. NMR Biomed. 2009, 22, 826–833. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Yeh, K.W.; Lin, G.; Chiang, M.H.; Yang, S.C.; Chao, W.J.; Yao, T.C.; Tsai, M.H.; Hua, M.C.; Liao, S.L.; et al. Metabolomics Reveals Dynamic Metabolic Changes Associated with Age in Early Childhood. PLoS ONE 2016, 11, e0149823. [Google Scholar] [CrossRef]
- Westhoff, J.H.; Seibert, F.S.; Waldherr, S.; Bauer, F.; Tonshoff, B.; Fichtner, A.; Westhoff, T.H. Urinary calprotectin, kidney injury molecule-1, and neutrophil gelatinase-associated lipocalin for the prediction of adverse outcome in pediatric acute kidney injury. Eur. J. Pediatr. 2017, 176, 745–755. [Google Scholar] [CrossRef]
- Westhoff, J.H.; Tonshoff, B.; Waldherr, S.; Poschl, J.; Teufel, U.; Westhoff, T.H.; Fichtner, A. Urinary Tissue Inhibitor of Metalloproteinase-2 (TIMP-2) * Insulin-Like Growth Factor-Binding Protein 7 (IGFBP7) Predicts Adverse Outcome in Pediatric Acute Kidney Injury. PLoS ONE 2015, 10, e0143628. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N.; Group, K.A.G.W. Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Crit. Care. 2013, 17, 204. [Google Scholar] [CrossRef]
- Selewski, D.T.; Charlton, J.R.; Jetton, J.G.; Guillet, R.; Mhanna, M.J.; Askenazi, D.J.; Kent, A.L. Neonatal Acute Kidney Injury. Pediatrics 2015, 136, e463–e473. [Google Scholar] [CrossRef]
- Schwartz, G.J.; Munoz, A.; Schneider, M.F.; Mak, R.H.; Kaskel, F.; Warady, B.A.; Furth, S.L. New equations to estimate GFR in children with CKD. J. Am. Soc. Nephrol. 2009, 20, 629–637. [Google Scholar] [CrossRef]
- Chong, J.; Xia, J. MetaboAnalystR: An R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 2018, 34, 4313–4314. [Google Scholar] [CrossRef]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef]
| Characteristic | AKI Patients (n = 65) | Non-AKI Patients (n = 31) | Healthy Controls (n = 53) | p-Value |
|---|---|---|---|---|
| Age (years) | 3.0 (0.6 to 13.5) | 1.0 (0.4 to 7.4) | 6.0 (1.0 to 10.0) | 0.21 |
| Male Female | 30 (46.2%) 35 (53.8%) | 15 (48.4%) 16 (51.6%) | 26 (49.1%) 27 (50.9%) | 0.57 |
| Neonates | 12 (18.5%) | 6 (19.4%) | 11 (20.8%) | 0.95 |
| BMI SDS | −0.5 (−1.2 to 0.5) | −0.8 (−1.9 to 0.6) | 0.25 | |
| AKI etiology | ||||
| Dehydration | 15 (23.0%) | |||
| Hemolytic uremic syndrome | 13 (20.0%) | |||
| Septic shock | 12 (18.5%) | |||
| Perinatal asphyxia | 7 (10.8%) | |||
| Hemodynamic | 4 (6.2%) | |||
| Interstitial nephritis | 4 (6.2%) | |||
| Other | 10 (15.4%) | |||
| Serum creatinine on study enrollment (mg/dL) | 1.60 (0.9 to 3.5) | 0.3 (0.2 to 0.5) | <0.001 | |
| eCCl on study enrollment (mL/min per 1.73 m2) | 19.2 (11.0 to 42.0) | 123.0 (80.9 to 170) | <0.001 | |
| KDIGO staging of AKI | ||||
| Stage 1 | 5 (7.7%) | |||
| Stage 2 | 9 (13.8%) | |||
| Stage 3 | 51 (78.5%) | |||
| Proteinuria (g/L) | 0.6 (0.2 to 1.5) | 0.1 (0.0 to 0.1) | <0.001 | |
| Urinary protein-to-creatinine ratio (mg/g) | 239 (48.6 to 955) | 34.6 (24.6 to 49.3) | <0.001 | |
| Urinary leukocytes (per μL) | 22.0 (4.5 to 93.0) | 5.0 (0.0 to 26.5) | <0.01 | |
| Urinary erythrocytes (per μL) | 15.0 (2.0 to 136.0) | 5.0 (2.0 to 37.3) | <0.05 | |
| Squamous epithelium (per μL) | 0.0 (0.0 to 1.5) | 0.0 (0.0 to 0.0) | 0.074 | |
| C-reactive protein (mg/L) | 30.9 (7.5 to 98.8) | 2.4 (0.0 to 19.0) | 0.001 | |
| Renal replacement therapy | 27 (41.5%) | 0 (0%) | 0 (0%) | <0.001 |
| 3-month mortality | 11 (16.9%) | 0 (0%) | 0 (0%) | <0.05 |
| Group | PLS-DA Parameters | |||
|---|---|---|---|---|
| No. of Components | Q2 | R2 | Permutation *: Empirical p-Value | |
| AKI (total) | 3 | 0.63 | 0.78 | < 0.001 |
| AKI (neonates excluded in AKI and CTL) | 4 | 0.73 | 0.89 | < 0.001 |
| Dehydration | 3 | 0.44 | 0.80 | < 0.001 |
| Perinatal asphyxia | 2 | 0.26 | 0.61 | 0.159 |
| Septic shock | 4 | 0.73 | 0.95 | < 0.001 |
| Hemolytic uremic syndrome | 4 | 0.69 | 0.90 | < 0.001 |
| Metabolite Importance Number | Metabolite | VIP Score | AUC * | 95% CI ** |
|---|---|---|---|---|
| 1 | Citrate1 *** | 2.73 | 0.94 | 0.88–0.98 |
| 2 | Bile acid | 2.52 | 0.91 | 0.84–0.97 |
| 3 | Citrate2 *** | 2.50 | 0.92 | 0.85–0.96 |
| 4 | Unk_al1 | 2.49 | 0.90 | 0.83–0.96 |
| 5 | unk_al28 | 2.19 | 0.84 | 0.75–0.91 |
| 6 | Leucine | 2.13 | 0.83 | 0.73–0.90 |
| 7 | unk_al24 | 2.07 | 0.83 | 0.74–0.92 |
| 8 | unk_al26 | 2.07 | 0.82 | 0.73–0.91 |
| 9 | Valine | 1.89 | 0.79 | 0.70–0.89 |
| 10 | unk_al10 | 1.86 | 0.80 | 0.70–0.89 |
| 11 | cis-Aconitic acid | 1.86 | 0.79 | 0.69–0.88 |
| 12 | Formate | 1.80 | 0.84 | 0.75–0.91 |
| 13 | LipidCH2 | 1.65 | 0.82 | 0.72–0.90 |
| 14 | Glycine | 1.50 | 0.71 | 0.61–0.81 |
| 15 | Asparagine | 1.48 | 0.76 | 0.65–0.85 |
| (A) logit(p) = log(p/(1 − P)) = −0.667 − 2.723 citrate + 2.538 leucine − 3.42 valine + 3.164 bile acid | |||||
| Estimate | Std. Error | z Value | Pr(>|z|) | Odds | |
| (Intercept) | −0.667 | 0.571 | −1.167 | 0.243 | - |
| citrate (2.69 ppm) | −2.723 | 0.83 | −3.281 | 0.001 | 0.07 |
| leucine | 2.538 | 1.089 | 2.33 | 0.02 | 12.65 |
| valine | −3.42 | 1.374 | −2.489 | 0.013 | 0.03 |
| bile acid | 3.164 | 1.096 | 2.887 | 0.004 | 23.67 |
| (B) logit(P) = log(P/(1 − P)) = 0.034 − 3.193 citrate + 4.859 leucine − 6.191 valine + 4.207 bile acid - 2.694 unk_al1 − 2.489 unk_al28 | |||||
| Estimate | Std. Error | z value | Pr(>|z|) | Odds | |
| (Intercept) | 0.034 | 0.998 | 0.034 | 0.973 | - |
| citrate (2.69 ppm) | −3.193 | 1.682 | −1.898 | 0.058 | 0.04 |
| leucine | 4.859 | 2.182 | 2.227 | 0.026 | 128.88 |
| valine | −6.191 | 2.617 | −2.366 | 0.018 | 0 |
| bile acid | 4.207 | 1.774 | 2.372 | 0.018 | 67.18 |
| unk_al1/3.16 ppm | 2.694 | 1.414 | −1.906 | 0.057 | 0.07 |
| unk_al28/2.15 ppm | −2.489 | 1.093 | −2.278 | 0.023 | 0.08 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhle-Goll, C.; Eisenmann, P.; Luy, B.; Kölker, S.; Tönshoff, B.; Fichtner, A.; Westhoff, J.H. Urinary NMR Profiling in Pediatric Acute Kidney Injury—A Pilot Study. Int. J. Mol. Sci. 2020, 21, 1187. https://doi.org/10.3390/ijms21041187
Muhle-Goll C, Eisenmann P, Luy B, Kölker S, Tönshoff B, Fichtner A, Westhoff JH. Urinary NMR Profiling in Pediatric Acute Kidney Injury—A Pilot Study. International Journal of Molecular Sciences. 2020; 21(4):1187. https://doi.org/10.3390/ijms21041187
Chicago/Turabian StyleMuhle-Goll, Claudia, Philipp Eisenmann, Burkhard Luy, Stefan Kölker, Burkhard Tönshoff, Alexander Fichtner, and Jens H. Westhoff. 2020. "Urinary NMR Profiling in Pediatric Acute Kidney Injury—A Pilot Study" International Journal of Molecular Sciences 21, no. 4: 1187. https://doi.org/10.3390/ijms21041187
APA StyleMuhle-Goll, C., Eisenmann, P., Luy, B., Kölker, S., Tönshoff, B., Fichtner, A., & Westhoff, J. H. (2020). Urinary NMR Profiling in Pediatric Acute Kidney Injury—A Pilot Study. International Journal of Molecular Sciences, 21(4), 1187. https://doi.org/10.3390/ijms21041187




