A Novel Quantitative Approach to Staging and Assessing Recovery from Type 1 Diabetes Mellitus: The Type 1 Diabetes Mellitus Metabolic Recovery Index
Abstract
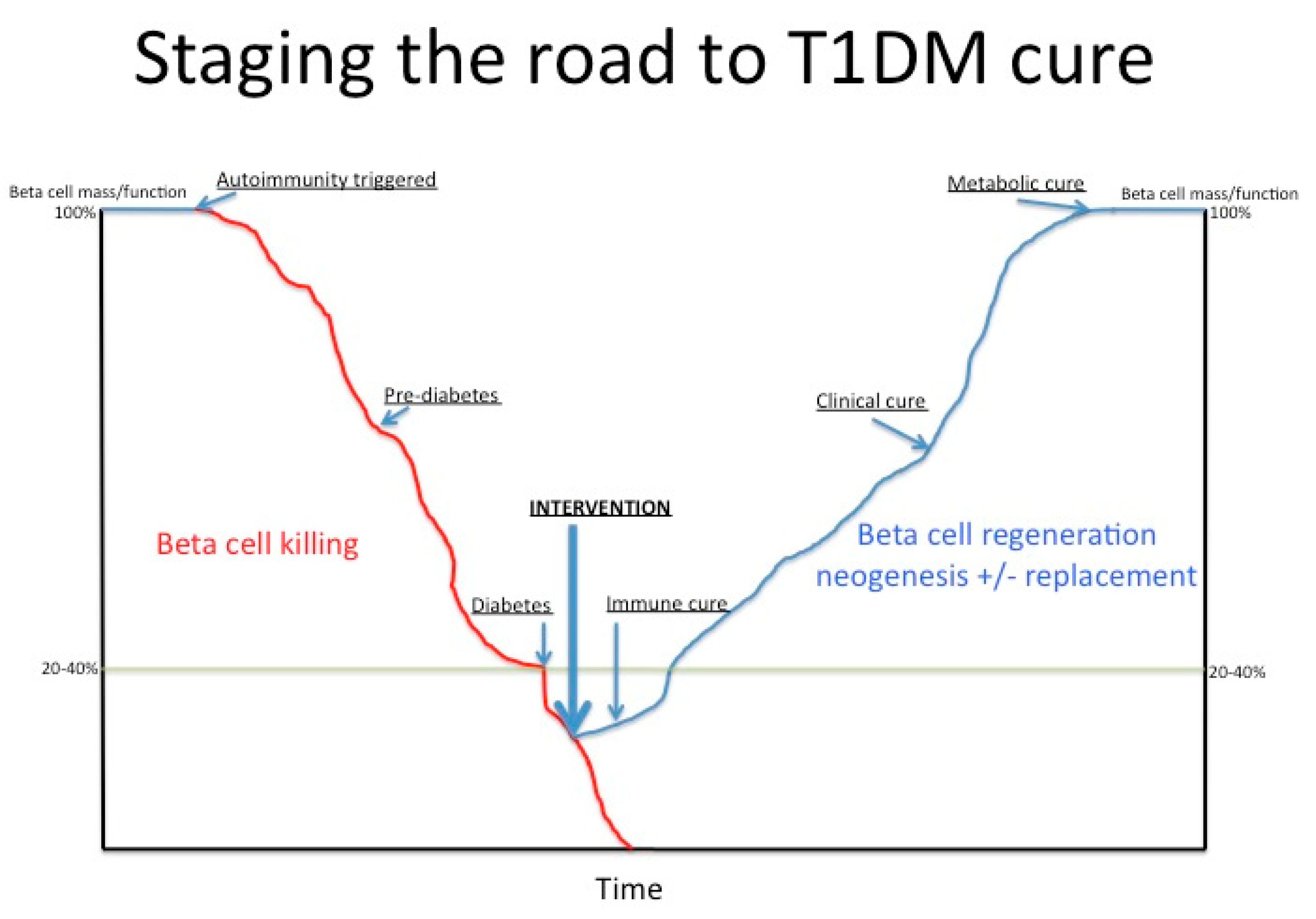
1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Banting, F.G.; Best, C.H.; Collip, J.B.; Campbell, W.R.; Fletcher, A.A. Pancreatic Extracts in the Treatment of Diabetes Mellitus. Can. Med. Assoc. J. 1922, 12, 141–146. [Google Scholar]
- Thabit, H.; Hovorka, R. Coming of age: The artificial pancreas for type 1 diabetes. Diabetologia 2016, 59, 1795–1805. [Google Scholar] [CrossRef] [PubMed]
- Home, P.D. Plasma insulin profiles after subcutaneous injection: How close can we get to physiology in people with diabetes? Diabetes Obes. Metab. 2015, 17, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- de Boer, I.H.; Rue, T.C.; Cleary, P.A.; Lachin, J.M.; Molitch, M.E.; Steffes, M.W.; Sun, W.; Zinman, B.; Brunzell, J.D.; White, N.H.; et al. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: An analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch. Intern. Med. 2011, 171, 412–420. [Google Scholar] [CrossRef] [PubMed]
- White, N.H.; Sun, W.; Cleary, P.A.; Tamborlane, W.V.; Danis, R.P.; Hainsworth, D.P.; Davis, M.D. Effect of prior intensive therapy in type 1 diabetes on 10-year progression of retinopathy in the DCCT/EDIC: Comparison of adults and adolescents. Diabetes 2010, 59, 1244–1253. [Google Scholar] [CrossRef]
- Albers, J.W.; Herman, W.H.; Pop-Busui, R.; Feldman, E.L.; Martin, C.L.; Cleary, P.A.; Waberski, B.H.; Lachin, J.M. Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Diabetes Care 2010, 33, 1090–1096. [Google Scholar] [CrossRef]
- Eisenbarth, G.S. Autoimmune beta cell insufficiency. Triangle 1984, 23, 111–124. [Google Scholar]
- Walker, L.S.; von Herrath, M. CD4 T cell differentiation in Type 1 Diabetes. Clin. Exp. Immunol. 2016, 183, 16–29. [Google Scholar] [CrossRef]
- Bluestone, J.A.; Buckner, J.H.; Fitch, M.; Gitelman, S.E.; Gupta, S.; Hellerstein, M.K.; Herold, K.C.; Lares, A.; Lee, M.R.; Li, K.; et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci. Transl. Med. 2015, 7, 315ra189. [Google Scholar] [CrossRef]
- Calderon, B.; Carrero, J.A.; Unanue, E.R. The central role of antigen presentation in islets of Langerhans in autoimmune diabetes. Curr. Opin. Immunol. 2014, 26, 32–40. [Google Scholar] [CrossRef]
- Abreu, J.R.; Martina, S.; Verrijn Stuart, A.A.; Fillie, Y.E.; Franken, K.L.; Drijfhout, J.W.; Roep, B.O. CD8 T cell autoreactivity to preproinsulin epitopes with very low human leucocyte antigen class I binding affinity. Clin. Exp. Immunol. 2012, 170, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Gitelman, S.E.; Gottlieb, P.A.; Rigby, M.R.; Felner, E.I.; Willi, S.M.; Fisher, L.K.; Moran, A.; Gottschalk, M.; Moore, W.V.; Pinckney, A.; et al. Antithymocyte globulin treatment for patients with recent-onset type 1 diabetes: 12-month results of a randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2013, 1, 306–316. [Google Scholar] [CrossRef]
- Keymeulen, B.; Vandemeulebroucke, E.; Ziegler, A.G.; Mathieu, C.; Kaufman, L.; Hale, G.; Gorus, F.; Goldman, M.; Walter, M.; Candon, S.; et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N. Engl. J. Med. 2005, 352, 2598–2608. [Google Scholar] [CrossRef] [PubMed]
- Orban, T.; Bundy, B.; Becker, D.J.; DiMeglio, L.A.; Gitelman, S.E.; Goland, R.; Gottlieb, P.A.; Greenbaum, C.J.; Marks, J.B.; Monzavi, R.; et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: A randomised, double-blind, placebo-controlled trial. Lancet 2011, 378, 412–419. [Google Scholar] [CrossRef]
- Pescovitz, M.D.; Greenbaum, C.J.; Krause-Steinrauf, H.; Becker, D.J.; Gitelman, S.E.; Goland, R.; Gottlieb, P.A.; Marks, J.B.; McGee, P.F.; Moran, A.M.; et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N. Engl. J. Med. 2009, 361, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Brooks-Worrell, B.; Gersuk, V.H.; Greenbaum, C.; Palmer, J.P. Intermolecular Antigen Spreading Occurs During the Preclinical Period of Human Type 1 Diabetes. J. Immunol. 2001, 166, 5265–5270. [Google Scholar] [CrossRef]
- Skyler, J.S.; Krischer, J.P.; Wolfsdorf, J.; Cowie, C.; Palmer, J.P.; Greenbaum, C.; Cuthbertson, D.; Rafkin-Mervis, L.E.; Chase, H.P.; Leschek, E. Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial—Type 1. Diabetes Care 2005, 28, 1068–1076. [Google Scholar]
- Beam, C.A.; MacCallum, C.; Herold, K.C.; Wherrett, D.K.; Palmer, J.; Ludvigsson, J. GAD vaccine reduces insulin loss in recently diagnosed type 1 diabetes: Findings from a Bayesian meta-analysis. Diabetologia 2016, 60, 43–49. [Google Scholar] [CrossRef]
- Orban, T.; Farkas, K.; Jalahej, H.; Kis, J.; Treszl, A.; Falk, B.; Reijonen, H.; Wolfsdorf, J.; Ricker, A.; Matthews, J.B.; et al. Autoantigen-specific regulatory T cells induced in patients with type 1 diabetes mellitus by insulin B-chain immunotherapy. J. Autoimmun. 2010, 34, 408–415. [Google Scholar] [CrossRef]
- Sosenko, J.M.; Skyler, J.S.; Beam, C.A.; Boulware, D.; Mahon, J.L.; Krischer, J.P.; Greenbaum, C.J.; Rafkin, L.E.; Matheson, D.; Herold, K.C.; et al. The development and utility of a novel scale that quantifies the glycemic progression toward type 1 diabetes over 6 months. Diabetes Care 2015, 38, 940–942. [Google Scholar] [CrossRef]
- Sosenko, J.M.; Skyler, J.S.; DiMeglio, L.A.; Beam, C.A.; Krischer, J.P.; Greenbaum, C.J.; Boulware, D.; Rafkin, L.E.; Matheson, D.; Herold, K.C.; et al. A new approach for diagnosing type 1 diabetes in autoantibody-positive individuals based on prediction and natural history. Diabetes Care 2015, 38, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Krischer, J.P. The use of intermediate endpoints in the design of type 1 diabetes prevention trials. Diabetologia 2013, 56, 1919–1924. [Google Scholar] [CrossRef] [PubMed]
- Wherrett, D.K.; Bundy, B.; Becker, D.J.; DiMeglio, L.A.; Gitelman, S.E.; Goland, R.; Gottlieb, P.A.; Greenbaum, C.J.; Herold, K.C.; Marks, J.B.; et al. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: A randomised double-blind trial. Lancet 2011, 378, 319–327. [Google Scholar] [CrossRef]
| Rituximab [15] (Age: 8–40 Years; Duration 1 Year) | Abatacept [14] (Age: 6–45 Years; Duration 2 Years) | GAD Vaccine [23] (Age: 3–45 Years; Duration 1 Year) | ||
|---|---|---|---|---|
| HbA1c (%) | Placebo | 7.0 | 7.9 | 7.1 |
| Treated | 6.76 | 7.2 | 7.1 | |
| Insulin dose (U/kg/day) | Placebo | 0.48 | 0.65 | 0.56 |
| Treated | 0.39 | 0.61 | 0.63 | |
| Stimulated C-peptide AUC (nmol/L) | Placebo | 0.47 | 0.238 | 0.413 |
| Treated | 0.56 | 0.378 | 0.412 | |
| DMMRI | 6.22 | 6.74 | 4.69 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orban, T.; Orban, N.T.; Jalahej, H.; Daubeney, P.E.F. A Novel Quantitative Approach to Staging and Assessing Recovery from Type 1 Diabetes Mellitus: The Type 1 Diabetes Mellitus Metabolic Recovery Index. Int. J. Mol. Sci. 2020, 21, 992. https://doi.org/10.3390/ijms21030992
Orban T, Orban NT, Jalahej H, Daubeney PEF. A Novel Quantitative Approach to Staging and Assessing Recovery from Type 1 Diabetes Mellitus: The Type 1 Diabetes Mellitus Metabolic Recovery Index. International Journal of Molecular Sciences. 2020; 21(3):992. https://doi.org/10.3390/ijms21030992
Chicago/Turabian StyleOrban, Tihamer, Nara T. Orban, Heyam Jalahej, and Piers E. F. Daubeney. 2020. "A Novel Quantitative Approach to Staging and Assessing Recovery from Type 1 Diabetes Mellitus: The Type 1 Diabetes Mellitus Metabolic Recovery Index" International Journal of Molecular Sciences 21, no. 3: 992. https://doi.org/10.3390/ijms21030992
APA StyleOrban, T., Orban, N. T., Jalahej, H., & Daubeney, P. E. F. (2020). A Novel Quantitative Approach to Staging and Assessing Recovery from Type 1 Diabetes Mellitus: The Type 1 Diabetes Mellitus Metabolic Recovery Index. International Journal of Molecular Sciences, 21(3), 992. https://doi.org/10.3390/ijms21030992




