The Role of Macrophages in Oocyte Donation Pregnancy: A Systematic Review
Abstract
1. Introduction
1.1. Macrophages in Naturally Conceived Pregnancies
1.1.1. Macrophages in the Immune System
1.1.2. Macrophages in Endometrium before Pregnancy
1.1.3. Macrophages during Pregnancy (Decidual Macrophages)
2. Macrophages and OD Pregnancy (Systematic Review)
2.1. Methods
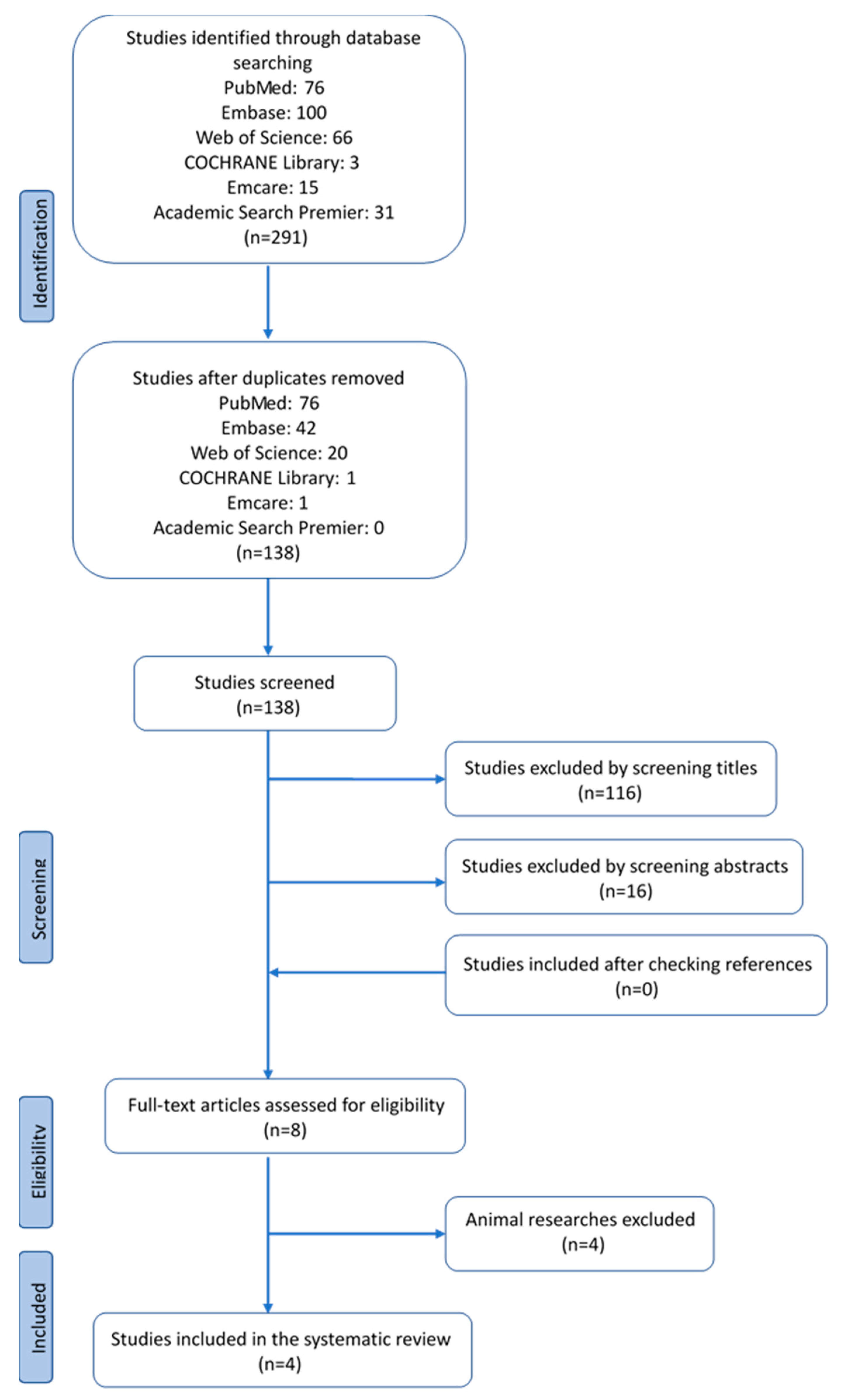
2.1.1. Search Strategy and Study Selection
2.1.2. Risk of Bias Assessment
2.2. Results
2.3. Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| OD | oocyte donation |
| EVTs | extra villous trophoblasts |
| NK | natural killer |
| HLA | human leukocyte antigen |
| IL | interleukin |
| Th2 NF-κB | T helper 2 nuclear factor kappa-light-chain-enhancer of activated B cells |
| MMPs | matrix metalloproteases |
| MIP-1B | macrophage inflammatory protein 1B |
| EMT | epithelial–mesenchymal transition |
| GM-CSF | granulocyte macrophage colony-stimulating factor |
| ILT CD | ig-like transcript cluster of differentiation |
| TNF-α | tumor necrosis factor alpha |
| M-CSF | macrophage colony-stimulating factor |
| TGF-β | transforming growth factor beta |
| NOS | Newcastle-Ottawa Scale |
| IVF | in vitro fertilization |
| NC | naturally conceived |
| SDF1α | stromal cell-derived factor-1alpha |
| VUE | villitis of unknown etiology |
| CK7 | cytokeratin 7 |
References
- Lutjen, P.; Trounson, A.; Leeton, J.; Findlay, J.; Wood, C.; Renou, P. The establishment and maintenance of pregnancy using in vitro fertilization and embryo donation in a patient with primary ovarian failure. Nature 1984, 307, 174–175. [Google Scholar] [CrossRef] [PubMed]
- Weissman, A.; Leong, M.; Sauer, M.V.; Shoham, Z. Characterizing the practice of oocyte donation: A web-based international survey. Reprod. Biomed. Online 2014, 28, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, V.A.; Gleicher, N. Fresh versus cryopreserved oocyte donation. Curr. Opin. Endocrinol. Diabetesand Obes. 2016, 23, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Jeve, Y.B.; Potdar, N.; Opoku, A.; Khare, M. Donor oocyte conception and pregnancy complications: A systematic review and meta-analysis. Bjog Int. J. Obstet. Gynaecol. 2016, 123, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, M.; Sunkara, S.K.; Antonisamy, B.; Kamath, M.S. Higher risk of preterm birth and low birth weight following oocyte donation: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 218, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Savasi, V.M.; Mandia, L.; Laoreti, A.; Cetin, I. Maternal and fetal outcomes in oocyte donation pregnancies. Hum. Reprod. Update 2016, 22, 620–633. [Google Scholar] [CrossRef]
- Storgaard, M.; Loft, A.; Bergh, C.; Wennerholm, U.B.; Soderstrom-Anttila, V.; Romundstad, L.B.; Aittomaki, K.; Oldereid, N.; Forman, J.; Pinborg, A. Obstetric and neonatal complications in pregnancies conceived after oocyte donation: A systematic review and meta-analysis. Bjog. Int. J. Obstet. Gynaecol. 2017, 124, 561–572. [Google Scholar] [CrossRef]
- Van Bentem, K.; Bos, M.; van der Keur, C.; Brand-Schaaf, S.H.; Haasnoot, G.W.; Roelen, D.L.; Eikmans, M.; Heidt, S.; Claas, F.H.J.; Lashley, E.; et al. The development of preeclampsia in oocyte donation pregnancies is related to the number of fetal-maternal HLA class II mismatches. J. Reprod. Immunol. 2019, 137, 103074. [Google Scholar] [CrossRef]
- Bos, M.; Schoots, M.H.; Fernandez, B.O.; Mikus-Lelinska, M.; Lau, L.C.; Eikmans, M.; van Goor, H.; Gordijn, S.J.; Pasch, A.; Feelisch, M.; et al. Reactive Species Interactome Alterations in Oocyte Donation Pregnancies in the Absence and Presence of Pre-Eclampsia. Int. J. Mol. Sci. 2019, 20, 1150. [Google Scholar] [CrossRef]
- Negishi, Y.; Takahashi, H.; Kuwabara, Y.; Takeshita, T. Innate immune cells in reproduction. J. Obstet. Gynaecol. Res. 2018, 44, 2025–2036. [Google Scholar] [CrossRef]
- Trundley, A.; Moffett, A. Human uterine leukocytes and pregnancy. Tissue Antigens 2004, 63, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chazara, O.; Xiong, S.; Moffett, A. Maternal KIR and fetal HLA-C: A fine balance. J. Leukoc. Biol. 2011, 90, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Tilburgs, T.; Claas, F.H.; Scherjon, S.A. Elsevier Trophoblast Research Award Lecture: Unique properties of decidual T cells and their role in immune regulation during human pregnancy. Placenta 2010, 31, S82–S86. [Google Scholar] [CrossRef] [PubMed]
- Bulmer, J.N.; Williams, P.J.; Lash, G.E. Immune cells in the placental bed. Int. J. Dev. Biol. 2010, 54, 281–294. [Google Scholar] [CrossRef]
- Kabawat, S.E.; Mostoufi-Zadeh, M.; Driscoll, S.G.; Bhan, A.K. Implantation site in normal pregnancy. A study with monoclonal antibodies. Am. J. Pathol. 1985, 118, 76–84. [Google Scholar]
- Abrahams, V.M.; Kim, Y.M.; Straszewski, S.L.; Romero, R.; Mor, G. Macrophages and apoptotic cell clearance during pregnancy. Am. J. Reprod. Immunol. 2004, 51, 275–282. [Google Scholar] [CrossRef]
- Ning, F.; Liu, H.; Lash, G.E. The Role of Decidual Macrophages During Normal and Pathological Pregnancy. Am. J. Reprod. Immunol. 2016, 75, 298–309. [Google Scholar] [CrossRef]
- Svensson-Arvelund, J.; Ernerudh, J.; Buse, E.; Cline, J.M.; Haeger, J.D.; Dixon, D.; Markert, U.R.; Pfarrer, C.; De Vos, P.; Faas, M.M. The placenta in toxicology. Part II: Systemic and local immune adaptations in pregnancy. Toxicol. Pathol. 2014, 42, 327–338. [Google Scholar] [CrossRef]
- Mellembakken, J.R.; Aukrust, P.; Olafsen, M.K.; Ueland, T.; Hestdal, K.; Videm, V. Activation of leukocytes during the uteroplacental passage in preeclampsia. Hypertension 2002, 39, 155–160. [Google Scholar] [CrossRef]
- De Kleer, I.; Willems, F.; Lambrecht, B.; Goriely, S. Ontogeny of myeloid cells. Front. Immunol. 2014, 5, 423. [Google Scholar] [CrossRef]
- Hoeffel, G.; Ginhoux, F. Ontogeny of Tissue-Resident Macrophages. Front. Immunol. 2015, 6, 486. [Google Scholar] [CrossRef] [PubMed]
- Hoeffel, G.; Chen, J.; Lavin, Y.; Low, D.; Almeida, F.F.; See, P.; Beaudin, A.E.; Lum, J.; Low, I.; Forsberg, E.C.; et al. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 2015, 42, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Hoeffel, G.; Ginhoux, F. Fetal monocytes and the origins of tissue-resident macrophages. Cell. Immunol. 2018, 330, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.J.; Ruckerl, D.; Cook, P.C.; Jones, L.H.; Finkelman, F.D.; van Rooijen, N.; MacDonald, A.S.; Allen, J.E. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science (New Yorkn.Y.) 2011, 332, 1284–1288. [Google Scholar] [CrossRef]
- Gentek, R.; Molawi, K.; Sieweke, M.H. Tissue macrophage identity and self-renewal. Immunol. Rev. 2014, 262, 56–73. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Reviews. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Berbic, M.; Fraser, I.S. Immunology of normal and abnormal menstruation. Women’s Health 2013, 9, 387–395. [Google Scholar] [CrossRef]
- Salamonsen, L.A.; Zhang, J.; Brasted, M. Leukocyte networks and human endometrial remodelling. J. Reprod. Immunol. 2002, 57, 95–108. [Google Scholar] [CrossRef]
- Weigert, A.; von Knethen, A.; Fuhrmann, D.; Dehne, N.; Brune, B. Redox-signals and macrophage biology. Mol. Asp. Med. 2018, 63, 70–87. [Google Scholar] [CrossRef]
- DeLoia, J.A.; Stewart-Akers, A.M.; Brekosky, J.; Kubik, C.J. Effects of exogenous estrogen on uterine leukocyte recruitment. Fertil. Steril. 2002, 77, 548–554. [Google Scholar] [CrossRef]
- Bombail, V.; MacPherson, S.; Critchley, H.O.; Saunders, P.T. Estrogen receptor related beta is expressed in human endometrium throughout the normal menstrual cycle. Hum. Reprod. 2008, 23, 2782–2790. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.A.; Bulmer, J.N.; Murdoch, A.P. Endometrial leucocytes: Expression of steroid hormone receptors. J. Clin. Pathol. 1998, 51, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.; Masuzaki, H.; Fujishita, A.; Kitajima, M.; Sekine, I.; Matsuyama, T.; Ishimaru, T. Estrogen and progesterone receptor expression in macrophages and regulation of hepatocyte growth factor by ovarian steroids in women with endometriosis. Hum. Reprod. 2005, 20, 2004–2013. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, C.J. Mechanisms of normal and abnormal endometrial bleeding. Menopause 2011, 18, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Salamonsen, L.A. Inflammation, leukocytes and menstruation. Rev. Endocr. Metab. Disord. 2012, 13, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Thiruchelvam, U.; Dransfield, I.; Saunders, P.T.; Critchley, H.O. The importance of the macrophage within the human endometrium. J. Leukoc. Biol. 2013, 93, 217–225. [Google Scholar] [CrossRef]
- Gnainsky, Y.; Granot, I.; Aldo, P.B.; Barash, A.; Or, Y.; Schechtman, E.; Mor, G.; Dekel, N. Local injury of the endometrium induces an inflammatory response that promotes successful implantation. Fertil. Steril. 2010, 94, 2030–2036. [Google Scholar] [CrossRef]
- Berbic, M.; Schulke, L.; Markham, R.; Tokushige, N.; Russell, P.; Fraser, I.S. Macrophage expression in endometrium of women with and without endometriosis. Hum. Reprod. 2009, 24, 325–332. [Google Scholar] [CrossRef]
- Braun, D.P.; Ding, J.; Shen, J.; Rana, N.; Fernandez, B.B.; Dmowski, W.P. Relationship between apoptosis and the number of macrophages in eutopic endometrium from women with and without endometriosis. Fertil. Steril. 2002, 78, 830–835. [Google Scholar] [CrossRef]
- Takebayashi, A.; Kimura, F.; Kishi, Y.; Ishida, M.; Takahashi, A.; Yamanaka, A.; Wu, D.; Zheng, L.; Takahashi, K.; Suginami, H.; et al. Subpopulations of macrophages within eutopic endometrium of endometriosis patients. Am. J. Reprod. Immunol. 2015, 73, 221–231. [Google Scholar] [CrossRef]
- Nie, M.F.; Xie, Q.; Wu, Y.H.; He, H.; Zou, L.J.; She, X.L.; Wu, X.Q. Serum and Ectopic Endometrium from Women with Endometriosis Modulate Macrophage M1/M2 Polarization via the Smad2/Smad3 Pathway. J. Immunol. Res. 2018, 2018, 6285813. [Google Scholar] [CrossRef] [PubMed]
- Santanam, N.; Murphy, A.A.; Parthasarathy, S. Macrophages, oxidation, and endometriosis. Ann. New York Acad. Sci. 2002, 955, 183–198; discussion 119–200, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Lagana, A.S.; Salmeri, F.M.; Ban Frangez, H.; Ghezzi, F.; Vrtacnik-Bokal, E.; Granese, R. Evaluation of M1 and M2 macrophages in ovarian endometriomas from women affected by endometriosis at different stages of the disease. Gynecol. Endocrinol. 2019, 1–4. [Google Scholar] [CrossRef]
- An, M.; Li, D.; Yuan, M.; Li, Q.; Zhang, L.; Wang, G. Interaction of macrophages and endometrial cells induces epithelial-mesenchymal transition-like processes in adenomyosis. Biol. Reprod. 2017, 96, 46–57. [Google Scholar] [CrossRef] [PubMed][Green Version]
- An, M.; Li, D.; Yuan, M.; Li, Q.; Zhang, L.; Wang, G. Different macrophages equally induce EMT in endometria of adenomyosis and normal. Reproduction 2017, 154, 79–92. [Google Scholar] [CrossRef]
- Faas, M.M.; de Vos, P. Uterine NK cells and macrophages in pregnancy. Placenta 2017, 56, 44–52. [Google Scholar] [CrossRef]
- Svensson-Arvelund, J.; Ernerudh, J. The Role of Macrophages in Promoting and Maintaining Homeostasis at the Fetal-Maternal Interface. Am. J. Reprod. Immunol. 2015, 74, 100–109. [Google Scholar] [CrossRef]
- Tang, M.X.; Hu, X.H.; Liu, Z.Z.; Kwak-Kim, J.; Liao, A.H. What are the roles of macrophages and monocytes in human pregnancy? J. Reprod. Immunol. 2015, 112, 73–80. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. New York Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef]
- Robertson, S.A.; Mayrhofer, G.; Seamark, R.F. Uterine epithelial cells synthesize granulocyte-macrophage colony-stimulating factor and interleukin-6 in pregnant and nonpregnant mice. Biol. Reprod. 1992, 46, 1069–1079. [Google Scholar] [CrossRef]
- Jaiswal, M.K.; Mallers, T.M.; Larsen, B.; Kwak-Kim, J.; Chaouat, G.; Gilman-Sachs, A.; Beaman, K.D. V-ATPase upregulation during early pregnancy: A possible link to establishment of an inflammatory response during preimplantation period of pregnancy. Reproduction (Camb. Engl.) 2012, 143, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Chen, L.; Guo, L.; Ou, X.; Xie, D.; Quan, S. Relationship between macrophages in mouse uteri and angiogenesis in endometrium during the peri-implantation period. Theriogenology 2014, 82, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Wakeland, A.K.; Parast, M.M. Trophoblast lineage specification, differentiation and their regulation by oxygen tension. J. Endocrinol. 2018, 236, R43–R56. [Google Scholar] [CrossRef] [PubMed]
- Faas, M.M.; De Vos, P. Innate immune cells in the placental bed in healthy pregnancy and preeclampsia. Placenta 2018, 69, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Diao, L.; Huang, C.; Li, Y.; Zeng, Y.; Kwak-Kim, J.Y.H. The role of decidual immune cells on human pregnancy. J. Reprod. Immunol. 2017, 124, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Ishitani, A.; Sageshima, N.; Lee, N.; Dorofeeva, N.; Hatake, K.; Marquardt, H.; Geraghty, D.E. Protein expression and peptide binding suggest unique and interacting functional roles for HLA-E, F, and G in maternal-placental immune recognition. J. Immunol. 2003, 171, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Shakhawat, A.; Shaikly, V.; Elzatma, E.; Mavrakos, E.; Jabeen, A.; Fernandez, N. Interaction between HLA-G and monocyte/macrophages in human pregnancy. J. Reprod. Immunol. 2010, 85, 40–46. [Google Scholar] [CrossRef]
- Zarif, J.C.; Hernandez, J.R.; Verdone, J.E.; Campbell, S.P.; Drake, C.G.; Pienta, K.J. A phased strategy to differentiate human CD14+monocytes into classically and alternatively activated macrophages and dendritic cells. BioTechniques 2016, 61, 33–41. [Google Scholar] [CrossRef]
- Alves, A.M.; Diel, L.F.; Lamers, M.L. Macrophages and prognosis of oral squamous cell carcinoma: A systematic review. J. Oral Pathol. Med. 2018, 47, 460–467. [Google Scholar] [CrossRef]
- Xu, W.; Schlagwein, N.; Roos, A.; van den Berg, T.K.; Daha, M.R.; van Kooten, C. Human peritoneal macrophages show functional characteristics of M-CSF-driven anti-inflammatory type 2 macrophages. Eur. J. Immunol. 2007, 37, 1594–1599. [Google Scholar] [CrossRef]
- Moreno-Fierros, L.; Garcia-Hernandez, A.L.; Ilhuicatzi-Alvarado, D.; Rivera-Santiago, L.; Torres-Martinez, M.; Rubio-Infante, N.; Legorreta-Herrera, M. Cry1Ac protoxin from Bacillus thuringiensis promotes macrophage activation by upregulating CD80 and CD86 and by inducing IL-6, MCP-1 and TNF-alpha cytokines. Int. Immunopharmacol. 2013, 17, 1051–1066. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; He, M.; Wang, Y.; Liao, A.H. Modulators of the Balance between M1 and M2 Macrophages during Pregnancy. Front. Immunol. 2017, 8, 120. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.R.; Je, S.; Seok, S.H. Metabolic features of macrophages in inflammatory diseases and cancer. Cancer Lett. 2018, 413, 46–58. [Google Scholar] [CrossRef]
- Houser, B.L. Decidual macrophages and their roles at the maternal-fetal interface. Yale J. Biol. Med. 2012, 85, 105–118. [Google Scholar]
- Erlebacher, A. Immunology of the maternal-fetal interface. Annu. Rev. Immunol. 2013, 31, 387–411. [Google Scholar] [CrossRef]
- Wang, H.; He, M.; Hou, Y.; Chen, S.; Zhang, X.; Zhang, M.; Ji, X. Role of decidual CD14(+) macrophages in the homeostasis of maternal-fetal interface and the differentiation capacity of the cells during pregnancy and parturition. Placenta 2016, 38, 76–83. [Google Scholar] [CrossRef]
- Prins, J.R.; Faas, M.M.; Melgert, B.N.; Huitema, S.; Timmer, A.; Hylkema, M.N.; Erwich, J.J. Altered expression of immune-associated genes in first-trimester human decidua of pregnancies later complicated with hypertension or foetal growth restriction. Placenta 2012, 33, 453–455. [Google Scholar] [CrossRef]
- Faas, M.M.; Spaans, F.; De Vos, P. Monocytes and macrophages in pregnancy and pre-eclampsia. Front. Immunol. 2014, 5, 298. [Google Scholar] [CrossRef]
- Busireddy, K.R.; Miller, J.A.; Ellison, K.; Ren, V.; Qayyum, R.; Panda, M. Efficacy of Interventions to Reduce Resident Physician Burnout: A Systematic Review. J. Grad Med. Educ 2017, 9, 294–301. [Google Scholar] [CrossRef]
- Schonkeren, D.; Swings, G.; Roberts, D.; Claas, F.; de Heer, E.; Scherjon, S. Pregnancy close to the edge: An immunosuppressive infiltrate in the chorionic plate of placentas from uncomplicated egg cell donation. Plos ONE 2012, 7, e32347. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, Y.; Nakashima, A.; Yoshino, O.; Shima, T.; Shiozaki, A.; Adachi, T.; Nakabayashi, M.; Okai, T.; Kushima, M.; Saito, S. Impairment of the accumulation of decidual T cells, NK cells, and monocytes, and the poor vascular remodeling of spiral arteries, were observed in oocyte donation cases, regardless of the presence or absence of preeclampsia. J. Reprod. Immunol. 2016, 114, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Gundogan, F.; Bianchi, D.W.; Scherjon, S.A.; Roberts, D.J. Placental pathology in egg donor pregnancies. Fertil. Steril. 2010, 93, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Varea, A.; Pellicer, B.; Serra, V.; Hervas-Marin, D.; Martinez-Romero, A.; Bellver, J.; Perales-Marin, A.; Pellicer, A. The Maternal Cytokine and Chemokine Profile of Naturally Conceived Gestations Is Mainly Preserved during In Vitro Fertilization and Egg Donation Pregnancies. J. Immunol. Res. 2015, 2015, 128616. [Google Scholar] [CrossRef]
- Alijotas-Reig, J.; Esteve-Valverde, E.; Ferrer-Oliveras, R.; Llurba, E.; Gris, J.M. Tumor Necrosis Factor-Alpha and Pregnancy: Focus on Biologics. An Updated and Comprehensive Review. Clin. Rev. Allergy Immunol. 2017, 53, 40–53. [Google Scholar] [CrossRef]
- Kim, C.J.; Romero, R.; Chaemsaithong, P.; Chaiyasit, N.; Yoon, B.H.; Kim, Y.M. Acute chorioamnionitis and funisitis: Definition, pathologic features, and clinical significance. Am. J. Obstet. Gynecol. 2015, 213, S29–S52. [Google Scholar] [CrossRef]
- Hoffmann, U.; Banas, B.; Kruger, B.; Banas, M.; Bergler, T.; Boger, C.; Kammerl, M.; Obed, A.; Rummele, P.; Segerer, S.; et al. SDF-1 expression is elevated in chronic human renal allograft rejection. Clin. Transplant. 2006, 20, 712–718. [Google Scholar] [CrossRef]
- Lan, X.; Wang, G.; Xu, X.; Lu, S.; Li, X.; Zhang, B.; Shi, G.; Zhao, Y.; Du, C.; Wang, H. Stromal Cell-Derived Factor-1 Mediates Cardiac Allograft Tolerance Induced by Human Endometrial Regenerative Cell-Based Therapy. Stem Cells Transl. Med. 2017, 6, 1997–2008. [Google Scholar] [CrossRef]
- Wan, X.; Xia, W.; Gendoo, Y.; Chen, W.; Sun, W.; Sun, D.; Cao, C. Upregulation of stromal cell-derived factor 1 (SDF-1) is associated with macrophage infiltration in renal ischemia-reperfusion injury. Plos ONE 2014, 9, e114564. [Google Scholar] [CrossRef]
- Beider, K.; Bitner, H.; Leiba, M.; Gutwein, O.; Koren-Michowitz, M.; Ostrovsky, O.; Abraham, M.; Wald, H.; Galun, E.; Peled, A.; et al. Multiple myeloma cells recruit tumor-supportive macrophages through the CXCR4/CXCL12 axis and promote their polarization toward the M2 phenotype. Oncotarget 2014, 5, 11283–11296. [Google Scholar] [CrossRef]
- Perni, S.C.; Predanic, M.; Cho, J.E.; Baergen, R.N. Placental pathology and pregnancy outcomes in donor and non-donor oocyte in vitro fertilization pregnancies. J. Perinat. Med. 2005, 33, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.J.; Romero, R.; Chaemsaithong, P.; Kim, J.S. Chronic inflammation of the placenta: Definition, classification, pathogenesis, and clinical significance. Am. J. Obstet. Gynecol. 2015, 213, S53–S69. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Aoki, A.; Kusabiraki, T.; Cheng, S.B.; Sharma, S.; Saito, S. Autophagy regulation in preeclampsia: Pros and cons. J. Reprod. Immunol. 2017, 123, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W.; Sargent, I.L. Immunology of pre-eclampsia. Am. J. Reprod. Immunol. 2010, 63, 534–543. [Google Scholar] [CrossRef]
| First Author | Year | Journal | Study | Samples | Delivery | Material | Methods | Main Outcomes | |
|---|---|---|---|---|---|---|---|---|---|
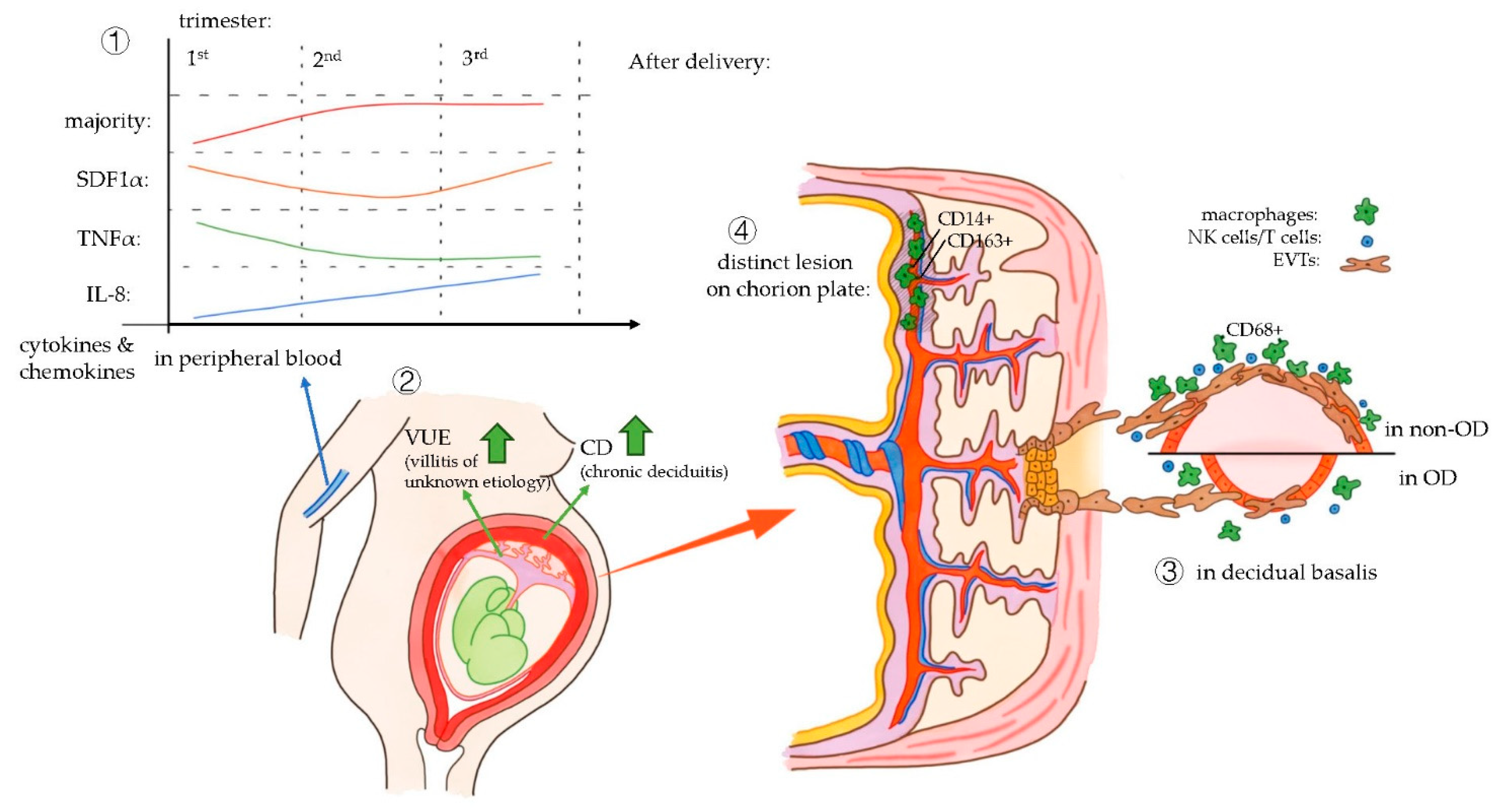
| Martinez-Varea et al. | 2015 | Journal of Immunology Research | Prospective longitudinal study | 25 OD 25 IVF 25 NC | Vaginal and c-section | Peripheral maternal blood at different time points during gestational age | Cytokine analysis (Luminex) | three study groups displayed similar cytokine and chemokine patterns throughout pregnancy | OD pregnancies showed lower SDF1α levels in the third trimester compared with NC and IVF pregnancies |
| Gundogan et al. | 2010 | Fertility and sterility | Retrospective case control | 20 OD 33 non donor IVF | Vaginal and c-section | Placenta macro and microscopy. Perinatal data | IHC Pathological investigation | Significant histological and immunohistochemical differences between the placentas of OD and nondonor IVF pregnancies | Representation of a host versus graft rejection-like phenomenon in OD |
| Nakabayashi et al. | 2016 | Journal of reproductive Immunology | Case control | 19 OD 22 NC 7 IVF | All c-sections | Decidua basalis form placental site uterus. Implantation site biopsy. Uterus after hysterectomy (n = 3). | IHC | Frequencies in normotensive OD pregnancies or preeclamptic cases in OD pregnancies were similar to those in preeclamptic cases in NC/IVF | Numbers of decidual CD3+ T cells, CD8+ T cells, CD4+ T cells, Foxp3+ T cells, CD56+ NK cells, and CD68+ macrophages were significantly decreased in the decidua basalis of OD patients compared with those in normal pregnant subjects impaired autophagy of EVTs was higher in normotensive OD pregnancy than in normotensive NC pregnancy |
| Schonkeren et al. | 2012 | PLOSone | Prospective cohort study | 26 OD placentas | Vaginal and c-section | Placenta macro and microscopy. Perinatal data | IHC FISH HLA typing KIR genotyping | Distinct lesion only in OD placentas, and with this lesion, the incidence of PE is low | Expression of CD14+ and CD163+ cells in the lesions |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, X.; Eikmans, M.; Hoorn, M.-L.v.d. The Role of Macrophages in Oocyte Donation Pregnancy: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 939. https://doi.org/10.3390/ijms21030939
Tian X, Eikmans M, Hoorn M-Lvd. The Role of Macrophages in Oocyte Donation Pregnancy: A Systematic Review. International Journal of Molecular Sciences. 2020; 21(3):939. https://doi.org/10.3390/ijms21030939
Chicago/Turabian StyleTian, Xuezi, Michael Eikmans, and Marie-Louise van der Hoorn. 2020. "The Role of Macrophages in Oocyte Donation Pregnancy: A Systematic Review" International Journal of Molecular Sciences 21, no. 3: 939. https://doi.org/10.3390/ijms21030939
APA StyleTian, X., Eikmans, M., & Hoorn, M.-L. v. d. (2020). The Role of Macrophages in Oocyte Donation Pregnancy: A Systematic Review. International Journal of Molecular Sciences, 21(3), 939. https://doi.org/10.3390/ijms21030939





