Comparative Efficacy and Safety of Dupilumab and Benralizumab in Patients with Inadequately Controlled Asthma: A Systematic Review
Abstract
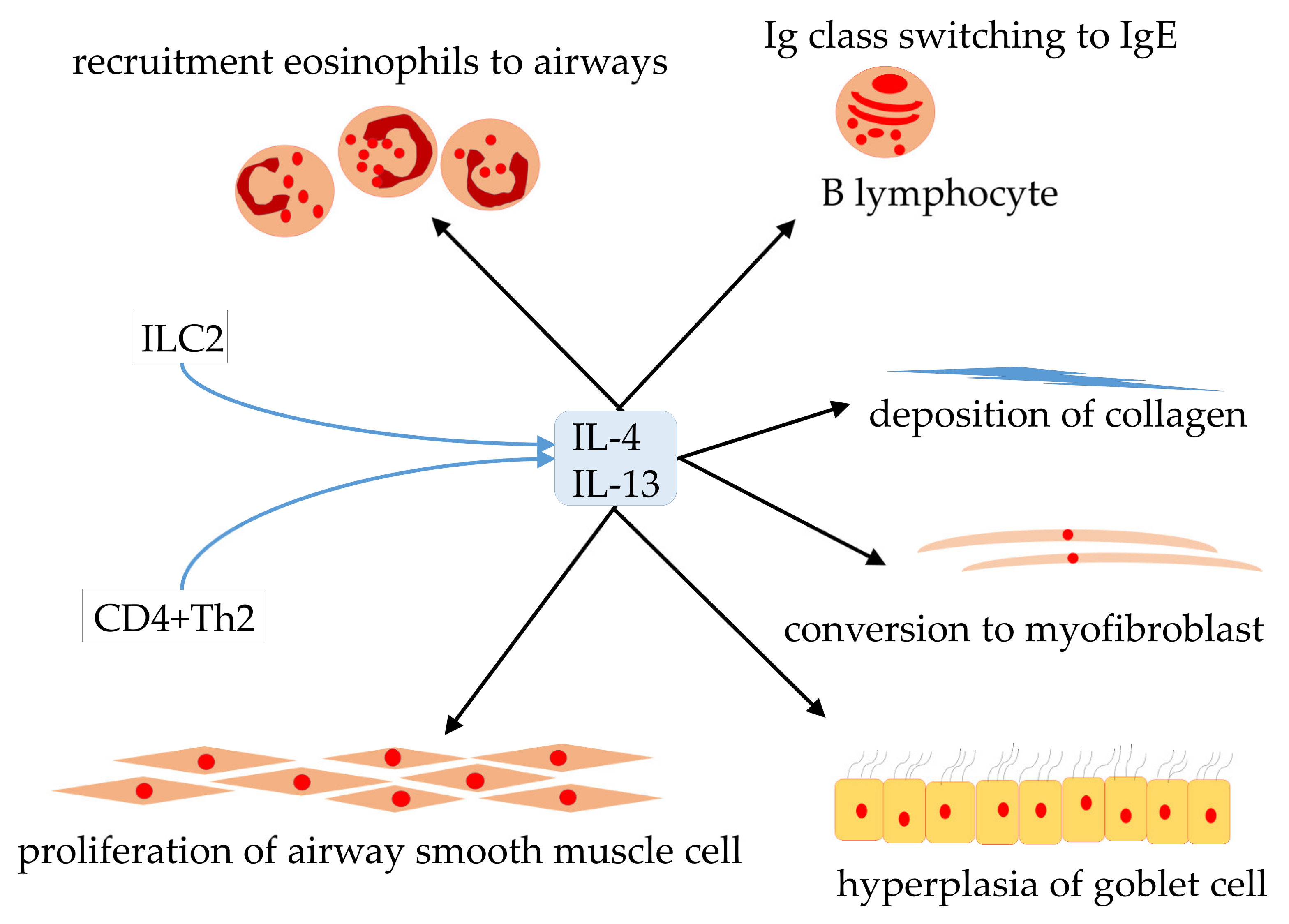
1. Introduction
2. Results
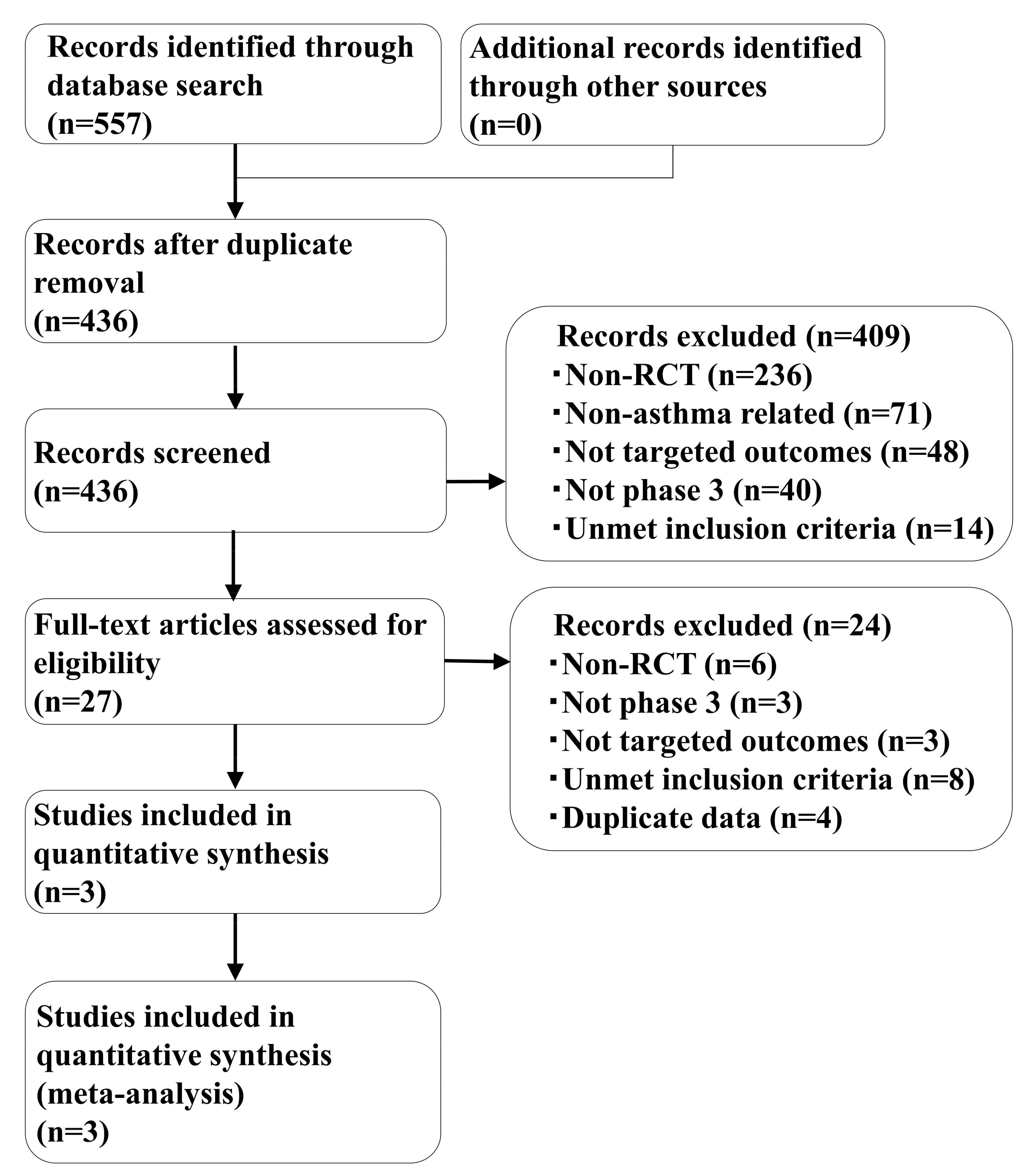
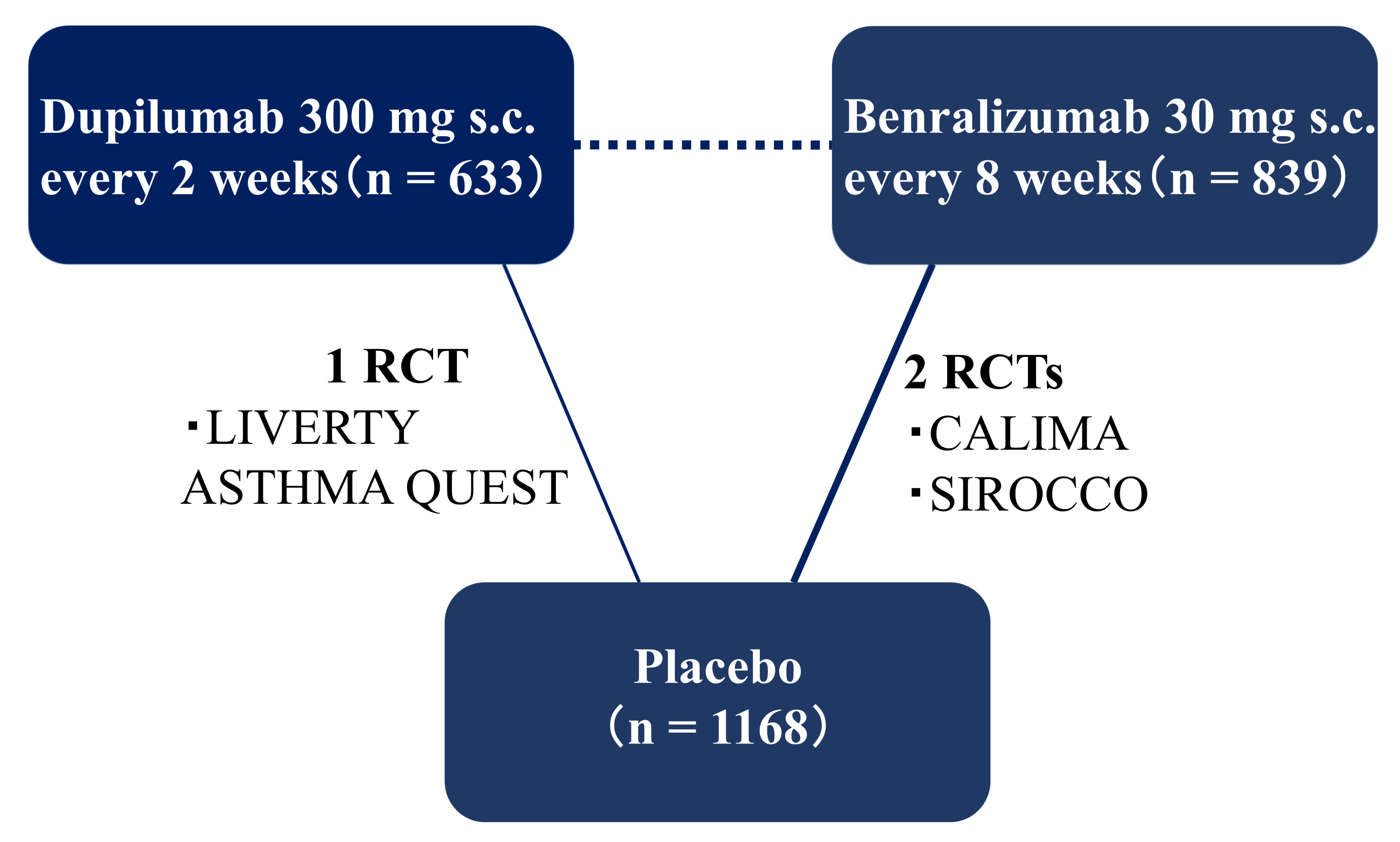
2.1. Systematic Review
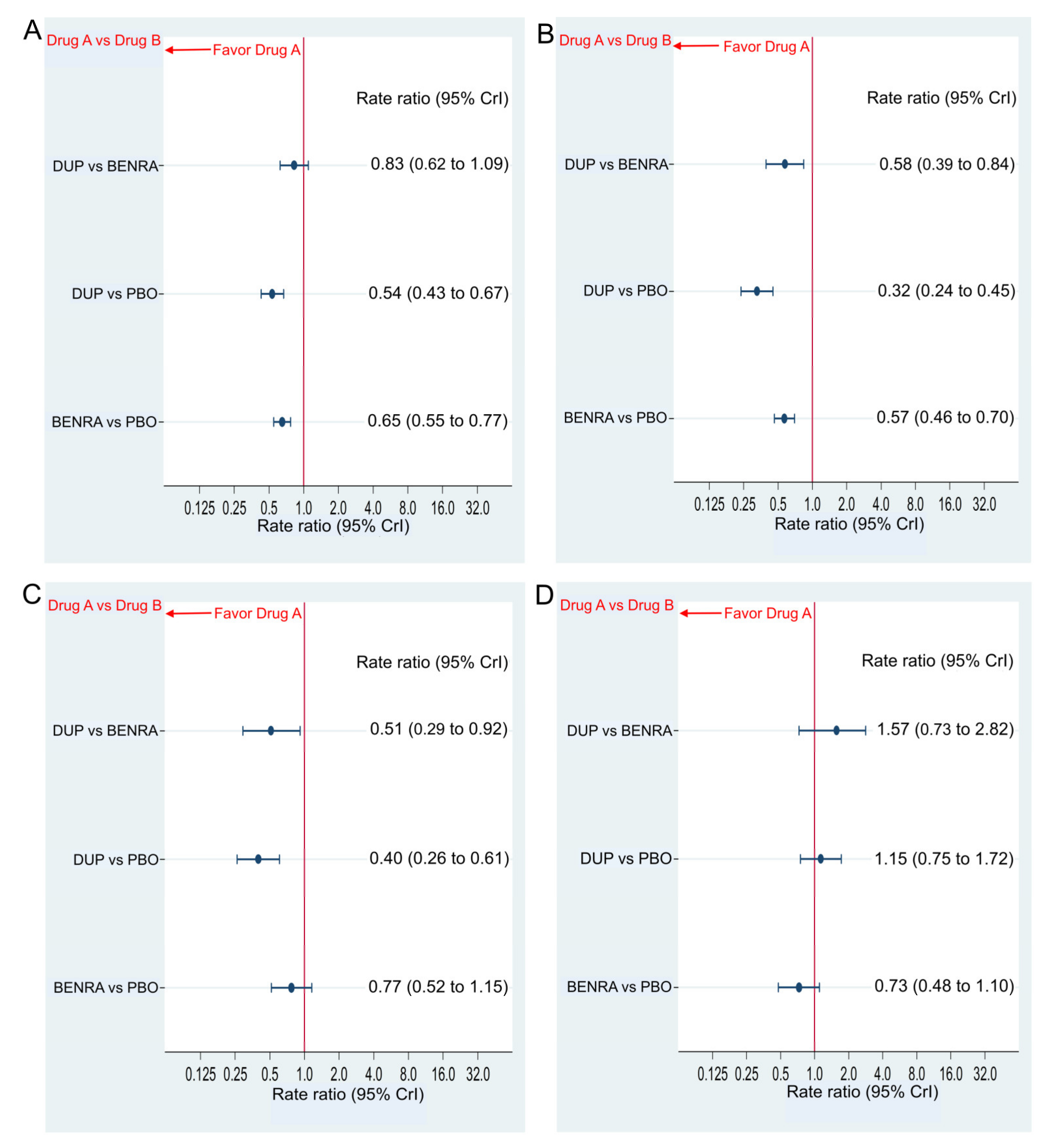
2.2. Primary Efficacy Endpoint
AER
2.3. Secondary Efficacy Endpoint
Changes in FEV1.0 and AQLQ Score from Baseline
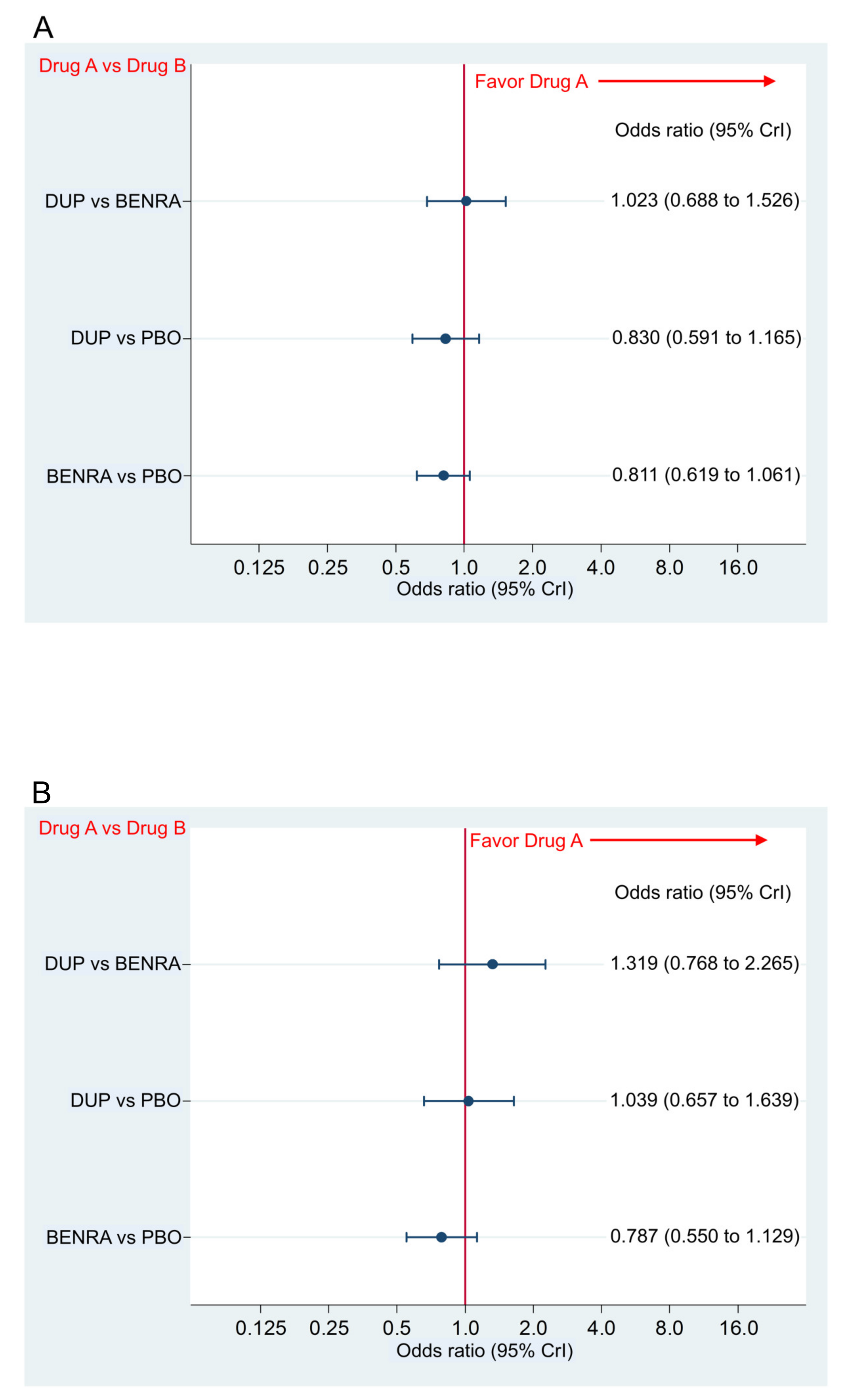
2.4. Primary and Secondary Safety Endpoint
Incidence of AAE and SAE
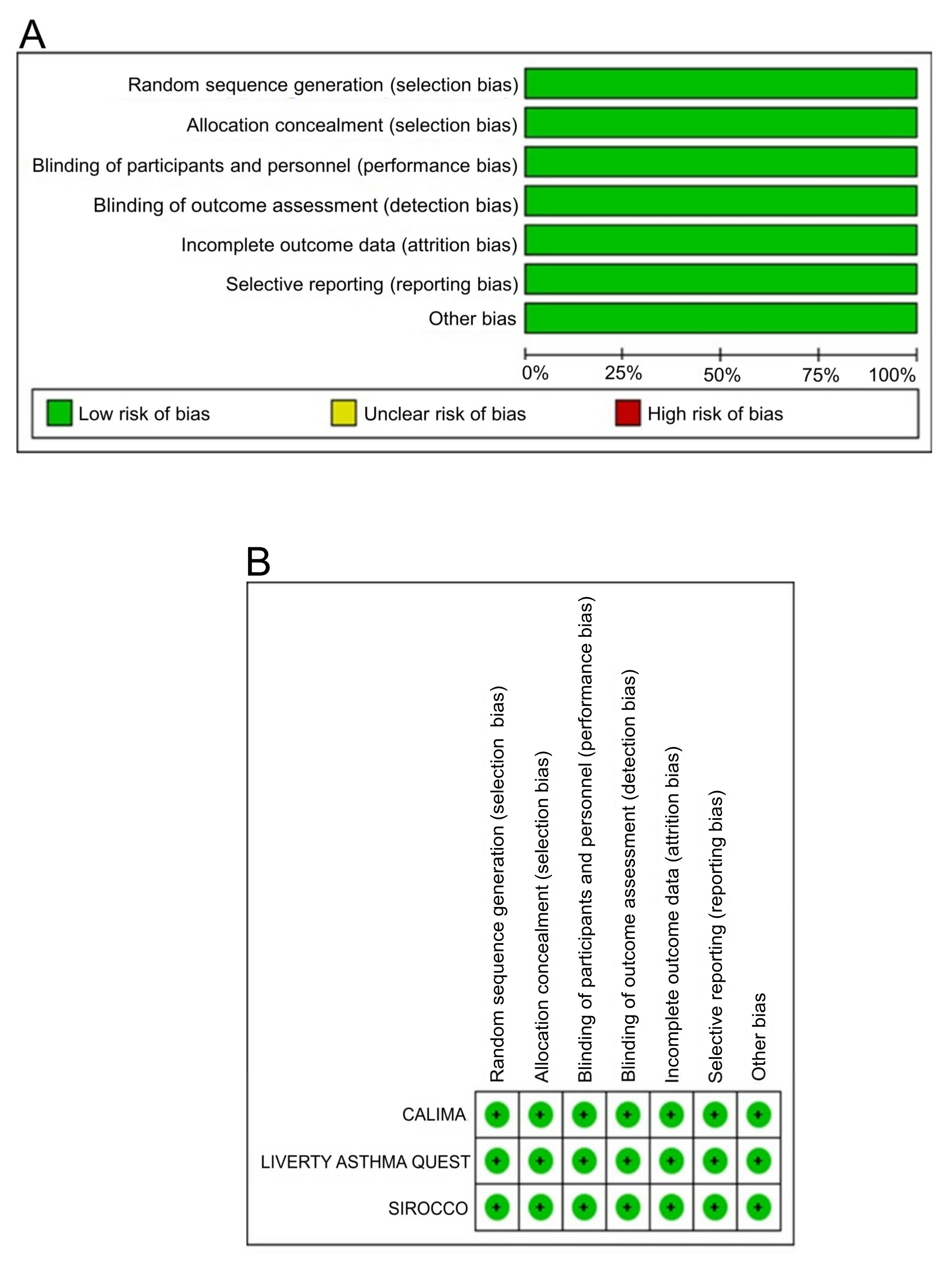
2.5. Bias Assessment
3. Discussion
4. Materials and Methods
4.1. Systematic Review
4.2. Quality Evaluation
4.3. Inclusion and Exclusion Criteria (Pre-Defined PICOS)
Patients
4.4. Interventions/Comparisons
4.5. Outcomes
4.6. Study Design
4.7. Statistical Analysis Method of Indirect Comparison
4.8. Ethical Aspects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAE | any adverse event |
| ACQ | asthma control questionnaire |
| AER | annualized exacerbation rate |
| AQLQ | asthma quality of life questionnaire |
| BCR | Brooks-Gelman-Rubin |
| BENRA | benralizumab |
| CrI | credible interval |
| DUP | dupilumab |
| FEV1.0 | forced expiratory volume at 1.0 s |
| GINA | Global Initiative for Asthma |
| ICS | inhaled corticosteroid |
| IL-4Rα | interleukin-4 receptor alfa |
| IL-5Rα | interleukin-5 receptor alfa |
| ISPOR | International Society for Pharmacoeconomics and Outcome Research |
| ITC | indirect treatment comparison |
| LABA | long acting beta-2 agonist |
| LTRA | leukotriene receptor antagonist |
| MD | mean difference |
| OR | odds ratio |
| PBO | placebo |
| PRISMA | Preferred Reporting Items for Systematic reviews |
| RCT | randomized controlled trial |
| RR | rate ratio |
| SAE | severe adverse event |
References
- To, T.; Stanojevic, S.; Moores, G.; Gershon, A.S.; Bateman, E.D.; Cruz, A.A.; Boulet, L. Global asthma prevalence in adults: Findings from the cross-sectional world health survey. BMC Public Health 2012, 12, 204. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.B.; Abrams, E.M. Asthma guidelines: The Global Initiative for Asthma in relation to national guidelines. Curr. Opin. Allergy Clin. Immunol. 2017, 17, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Bateman, E.D.; Boushey, H.A.; Bousquet, J.; Busse, W.W.; Clark, T.J.; Pauwels, R.A.; Pedersen, S.E. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma Control study. Am. J. Respir. Crit. Care Med. 2004, 170, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Hermosa, J.L.; Sanchez, C.B.; Rubio, M.C.; Minguez, M.M.; Walther, J.L. Factors associated with the control of severe asthma. J. Asthma 2010, 47, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.P.; Ferguson, G.; Deniz, Y.; Reisner, C. Uncontrolled asthma: a review of the prevalence, disease burden and options for treatment. Respir. Med. 2006, 100, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Cytokine-directed therapies for asthma. J. Allergy Clin. Immunol. 2001, 108, S72–S76. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dunn, R.M.; Wechsler, M.E. Anti-interleukin therapy in asthma. Clin Pharmacol. Ther. 2015, 97, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, M.E. Getting control of uncontrolled asthma. Am. J. Med. 2014, 127, 1049–1059. [Google Scholar] [CrossRef]
- Chung, K.F. Targeting the interleukin pathway in the treatment of asthma. Lancet. 2015, 386, 1086–1096. [Google Scholar] [CrossRef]
- Fajt, M.L.; Wenzel, S.E. Asthma phenotypes and the use of biologic medications in asthma and allergic disease: the next steps toward personalized care. J. Allergy Clin. Immunol. 2015, 135, 299–310. [Google Scholar] [CrossRef]
- Olin, J.T.; Wechsler, M.E. Asthma: Pathogenesis and novel drugs for treatment. BMJ 2014, 349, g5517. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.D.; El-Gammal, A.I.; O’Byrne, P.M. Emerging monoclonal antibodies as targeted innovative therapeutic approaches to asthma. Clin Pharmacol. Ther. 2016, 99, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.A.; Bennett, B.L.; Graham, N.M.; Pirozzi, G.; Stahl, N.; Yancopoulos, G.D. Targeting key proximal drivers of type 2 inflammation in disease. Nat. Rev. Drug Discov. 2016, 15, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, T.; Skabytska, Y.; Kaesler, S.; Volz, T. Regulation of T cell immunity in atopic dermatitis by microbes: The Yin and Yang of cutaneous inflammation. Front Immunol. 2015, 6, 353. [Google Scholar] [CrossRef]
- Wynn, T.A. Type 2 cytokines: mechanisms and therapeutic strategies. Nat. Rev. Immunol. 2015, 15, 271–282. [Google Scholar] [CrossRef]
- Bai, B.; Yamamoto, K.; Sato, H.; Sugiura, H.; Tanaka, T. Complex regulation of S100A8 by IL-17, dexamethasone, IL-4 and IL-13 in HaCat cells (human keratinocyte cell line). J. Dermatol. Sci. 2007, 47, 259–262. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Nograles, K.E.; Krueger, J.G. Contrasting pathogenesis of atopic dermatitis and psoriasis—Part II: Immune cell subsets and therapeutic concepts. J. Allergy Clin. Immunol. 2011, 127, 1420–1432. [Google Scholar] [CrossRef]
- Noda, S.; Krueger, J.G.; Guttman-Yassky, E. The translational revolution and use of biologics in patients with inflammatory skin diseases. J. Allergy Clin Immunol. 2015, 135, 324–336. [Google Scholar] [CrossRef]
- Gittler, J.K.; Shemer, A.; Suarez-Farinas, M.; Fuentes-Duculan, J.; Gulewicz, K.J.; Wang, C.Q.; Mitsui, H.; Cardinale, I.; de Guzman Strong, C.; Krueger, J.G.; et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J. Allergy Clin. Immunol. 2012, 130, 1344–1354. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Dhingra, N.; Leung, D.Y. New era of biologic therapeutics in atopic dermatitis. Expert Opin. Biol. Ther. 2013, 13, 549–561. [Google Scholar] [CrossRef]
- Castro, M.; Corren, J.; Pavord, I.D.; Maspero, J.; Wenzel, S.; Rabe, K.F.; Busse, W.W.; Ford, L.; Sher, L.; FitzGerald, G.M.; et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N. Engl. J. Med. 2018, 378, 2486–2496. [Google Scholar] [CrossRef] [PubMed]
- Patterson, M.F.; Borish, L.; Kennedy, J.L. The past, present, and future of monoclonal antibodies to IL-5 and eosinophilic asthma: A review. J. Asthma Allergy 2015, 8, 125–134. [Google Scholar] [PubMed]
- Bleecker, E.R.; FitzGerald, J.M.; Chanez, P.; Papi, A.; Weinstein, S.F.; Barker, P.; Sproule, S.; Gilmartin, G.; Aurivillius, M.; Werkström, V.; et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): A randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016, 388, 2115–2127. [Google Scholar] [CrossRef]
- FitzGerald, J.M.; Bleecker, E.R.; Nair, P.; Korn, S.; Ohta, K.; Lommatzsch, M.; Ferguson, G.T.; Busse, W.W.; Barker, P.; Sproule, S.; et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016, 388, 2128–2141. [Google Scholar] [CrossRef]
- Chupp, G.L.; Bradford, E.S.; Albers, F.C.; Bratton, D.J.; Wang-Jairaj, J.; Nelsen, L.M.; Trevor, J.L.; Magnan, A.; Ten Brinke, A. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): A randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir. Med. 2017, 5, 390–400. [Google Scholar] [CrossRef]
- Goldman, M.; Hirsch, I.; Zangrilli, J.G.; Newbold, P.; Xu, X. The association between blood eosinophil count and benralizumab efficacy for patients with severe, uncontrolled asthma: Subanalyses of the Phase III SIROCCO and CALIMA studies. Curr. Med. Res. Opin. 2017, 33, 1605–1613. [Google Scholar] [CrossRef]
- Pelaia, C.; Vatrella, A.; Gallelli, L.; Terracciano, R.; Navalesi, P.; Maselli, R.; Pelaia, G. Dupilumab for the treatment of asthma. Expert Opin. Biol. Ther. 2017, 17, 1565–1572. [Google Scholar] [CrossRef]
- Saco, T.V.; Pepper, A.N.; Lockey, R.F. Benralizumab for the treatment of asthma. Expert Rev. Clin. Immunol. 2017, 13, 405–413. [Google Scholar] [CrossRef]
- Bucher, H.C.; Guyatt, G.H.; Griffith, L.E.; Walter, S.D. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J. Clin Epidemiol. 1997, 50, 683–691. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- O’Byrne, P.M.; Inman, M.D.; Parameswaran, K. The trials and tribulations of IL-5, eosinophils, and allergic asthma. J. Allergy Clin. Immunol. 2001, 108, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Moczygemba, M.; Huston, D.P. Biology of common beta receptor-signaling cytokines: IL-3, IL-5, and GM-CSF. J. Allergy Clin. Immunol. 2003, 112, 653–665. [Google Scholar] [PubMed]
- Kolbeck, R.; Kozhich, A.; Koike, M.; Peng, L.; Andersson, C.K.; Damschroder, M.M.; Reed, J.L.; Woods, R.; Dall’acqua, W.W.; Stephens, G.L.; et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J. Allergy Clin. Immunol. 2010, 125, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, M.E.; Fulkerson, P.C.; Bochner, B.S.; Gauvreau, G.M.; Gleich, G.J.; Henkel, T.; Kolbeck, R.; Mathur, S.K.; Ortega, H.; Petel, J.; et al. Novel targeted therapies for eosinophilic disorders. J. Allergy Clin. Immunol. 2012, 130, 563–571. [Google Scholar] [CrossRef]
- Romeo, M.J.; Agrawal, R.; Pomes, A.; Woodfolk, J.A. A molecular perspective on TH2-promoting cytokine receptors in patients with allergic disease. J. Allergy Clin. Immunol. 2014, 133, 952–960. [Google Scholar] [CrossRef]
- Levine, S.J.; Wenzel, S.E. Narrative review: The role of Th2 immune pathway modulation in the treatment of severe asthma and its phenotypes. Ann Intern Med. 2010, 152, 232–237. [Google Scholar] [CrossRef]
- O’Byrne, P.M. Cytokines or their antagonists for the treatment of asthma. Chest 2006, 130, 244–250. [Google Scholar] [CrossRef]
- Izuhara, K.; Arima, K.; Yasunaga, S. IL-4 and IL-13: Their pathological roles in allergic diseases and their potential in developing new therapies. Curr. Drug Targets Inflamm. Allergy 2002, 1, 263–269. [Google Scholar] [CrossRef]
- May, R.D.; Fung, M. Strategies targeting the IL-4/IL-13 axes in disease. Cytokine 2015, 75, 89–116. [Google Scholar] [CrossRef]
- Mueller, T.D.; Zhang, J.L.; Sebald, W.; Duschl, A. Structure, binding, and antagonists in the IL-4/IL-13 receptor system. Biochim. Biophys. Acta 2002, 1592, 237–250. [Google Scholar] [CrossRef]
- Oh, C.K.; Geba, G.P.; Molfino, N. Investigational therapeutics targeting the IL-4/IL-13/STAT-6 pathway for the treatment of asthma. Eur. Respir. Rev. 2010, 19, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Steinke, J.W. Current prospective of anti-IL-4, -IL-9, and -IL-13 therapies in allergic disease. Recent Pat. Inflamm. Allergy Drug Discov. 2010, 4, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Gour, N.; Wills-Karp, M. IL-4 and IL-13 signaling in allergic airway disease. Cytokine 2015, 75, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Walsh, G.M. Biologics targeting IL-5, IL-4 or IL-13 for the treatment of asthma—An update. Expert Rev. Clin Immunol. 2017, 13, 143–149. [Google Scholar] [CrossRef]
- Corry, D.B.; Kheradmand, F. Biology and therapeutic potential of the interleukin-4/interleukin-13 signaling pathway in asthma. Am. J. Respir. Med. 2002, 1, 185–193. [Google Scholar] [CrossRef]
- Dunican, E.M.; Fahy, J.V. The role of type 2 inflammation in the pathogenesis of asthma exacerbations. Ann. Am. Thorac. Soc. 2015, 12, S144–S149. [Google Scholar]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Version 5.1. 0; The Cochrane Collaboration: Wiley-Blackwell: Hoboken, NJ, USA, 2011. [Google Scholar]
- Dias, S.; Sutton, A.J.; Ades, A.; Welton, N.J. Evidence synthesis for decision making 2: A generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med. Decis. Making 2013, 33, 607–617. [Google Scholar] [CrossRef]
- Dias, S.; Sutton, A.J.; Welton, N.J.; Ades, A.E. Evidence synthesis for decision making 3: Heterogeneity--subgroups, meta-regression, bias, and bias-adjustment. Med. Decis. Making 2013, 33, 618–640. [Google Scholar] [CrossRef]
- Dias, S.; Welton, N.J.; Sutton, A.J.; Caldwell, D.M.; Lu, G.; Ades, A.E. Evidence synthesis for decision making 4: Inconsistency in networks of evidence based on randomized controlled trials. Med. Decis. Making 2013, 33, 641–656. [Google Scholar] [CrossRef]
- Jansen, J.P.; Fleurence, R.; Devine, B.; Itzler, R.; Barrett, A.; Hawkins, N.; Lee, K.; Boersma, C.; Annemans, L.; Cappelleri, J.C. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: Part 1. Value Health 2011, 14, 417–428. [Google Scholar] [CrossRef]
- Hoaglin, D.C.; Hawkins, N.; Jansen, J.P.; Scott, D.A.; Itzler, R.; Cappelleri, J.C.; Boersma, C.; Thompson, D.; Larholt, K.M.; Diaz, M.; et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: Report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2. Value Health 2011, 14, 429–437. [Google Scholar] [CrossRef]
- Jansen, J.P.; Crawford, B.; Bergman, G.; Stam, W. Bayesian meta-analysis of multiple treatment comparisons: An introduction to mixed treatment comparisons. Value Health 2008, 11, 956–964. [Google Scholar] [CrossRef]
- Lumley, T. Network meta-analysis for indirect treatment comparisons. Stat. Med. 2002, 21, 2313–2324. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.A.; Elders, A.; Shyangdan, D.; Royle, P.; Waugh, N. The relative clinical effectiveness of ranibizumab and bevacizumab in diabetic macular oedema: An indirect comparison in a systematic review. BMJ 2012, 345, e5182. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thompson Coon, J.S.; Liu, Z.; Hoyle, M.; Rogers, G.; Green, C.; Moxham, T.; Welch, K.; Stein, K. Sunitinib and bevacizumab for first-line treatment of metastatic renal cell carcinoma: A systematic review and indirect comparison of clinical effectiveness. Br. J. Cancer 2009, 101, 238–343. [Google Scholar] [CrossRef]
- Medic, G.; Lindner, L.; van der Weijden, M.; Karabis, A. Efficacy and Safety of Aclidinium/Formoterol versus Tiotropium in COPD: Results of an Indirect Treatment Comparison. Adv. Ther. 2016, 33, 379–399. [Google Scholar] [CrossRef]
- Cure, S.; Diels, J.; Gavart, S.; Bianic, F.; Jones, E. Efficacy of telaprevir and boceprevir in treatment-naive and treatment-experienced genotype 1 chronic hepatitis C patients: An indirect comparison using Bayesian network meta-analysis. Curr. Med. Res. Opin. 2012, 28, 1841–1856. [Google Scholar] [CrossRef]
- Betts, K.A.; Griffith, J.; Friedman, A.; Zhou, Z.Y.; Signorovitch, J.E.; Ganguli, A. An indirect comparison and cost per responder analysis of adalimumab, methotrexate and apremilast in the treatment of methotrexate-naive patients with psoriatic arthritis. Curr. Med. Res. Opin. 2016, 32, 721–729. [Google Scholar] [CrossRef]
- Freemantle, N.; Ginsberg, D.A.; McCool, R.; Fleetwood, K.; Arber, M.; Khalaf, K.; Loveman, C.; Ni, Q.; Glanville, J. Comparative assessment of onabotulinumtoxinA and mirabegron for overactive bladder: An indirect treatment comparison. BMJ Open 2016, 6, e009122. [Google Scholar] [CrossRef]
- Miwa, H.; Igarashi, A.; Teng, L.; Uda, A.; Deguchi, H.; Tango, T. Systematic review with network meta-analysis: Indirect comparison of the efficacy of vonoprazan and proton-pump inhibitors for maintenance treatment of gastroesophageal reflux disease. J. Gastroenterol. 2019, 54, 718–729. [Google Scholar] [CrossRef]
- Dias, S.; Welton, N.J.; Sutton, A.J.; Ades, A.E. NICE DSU Technical Support Document 2: A Generalised Linear Modelling Framework for Pairwise and Network Meta-Analysis of Randomised Controlled Trials; National Institute for Health and Clinical Excellence (NICE): London, UK, 2011. [Google Scholar]
- Gould, A.L. Using prior findings to augment active-controlled trials and trials with small placebo groups. Drug Inf. J. 1991, 25, 369–380. [Google Scholar] [CrossRef]
- Brooks, S.P.; Gelman, A. General methods for monitoring convergence of iterative simulations. J. Comput. Graph Stat. 1998, 7, 434–455. [Google Scholar]
- Brooks, S.P.; Roberts, G.O. Convergence assessment techniques for Markov chain Monte Carlo. Stat. Comput. 1998, 8, 319–335. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ando, K.; Tanaka, A.; Sagara, H. Comparative Efficacy and Safety of Dupilumab and Benralizumab in Patients with Inadequately Controlled Asthma: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 889. https://doi.org/10.3390/ijms21030889
Ando K, Tanaka A, Sagara H. Comparative Efficacy and Safety of Dupilumab and Benralizumab in Patients with Inadequately Controlled Asthma: A Systematic Review. International Journal of Molecular Sciences. 2020; 21(3):889. https://doi.org/10.3390/ijms21030889
Chicago/Turabian StyleAndo, Koichi, Akihiko Tanaka, and Hironori Sagara. 2020. "Comparative Efficacy and Safety of Dupilumab and Benralizumab in Patients with Inadequately Controlled Asthma: A Systematic Review" International Journal of Molecular Sciences 21, no. 3: 889. https://doi.org/10.3390/ijms21030889
APA StyleAndo, K., Tanaka, A., & Sagara, H. (2020). Comparative Efficacy and Safety of Dupilumab and Benralizumab in Patients with Inadequately Controlled Asthma: A Systematic Review. International Journal of Molecular Sciences, 21(3), 889. https://doi.org/10.3390/ijms21030889





