Transcriptome Analysis of Drought-Resistant and Drought-Sensitive Sorghum (Sorghum bicolor) Genotypes in Response to PEG-Induced Drought Stress
Abstract
1. Introduction
2. Results and Discussion
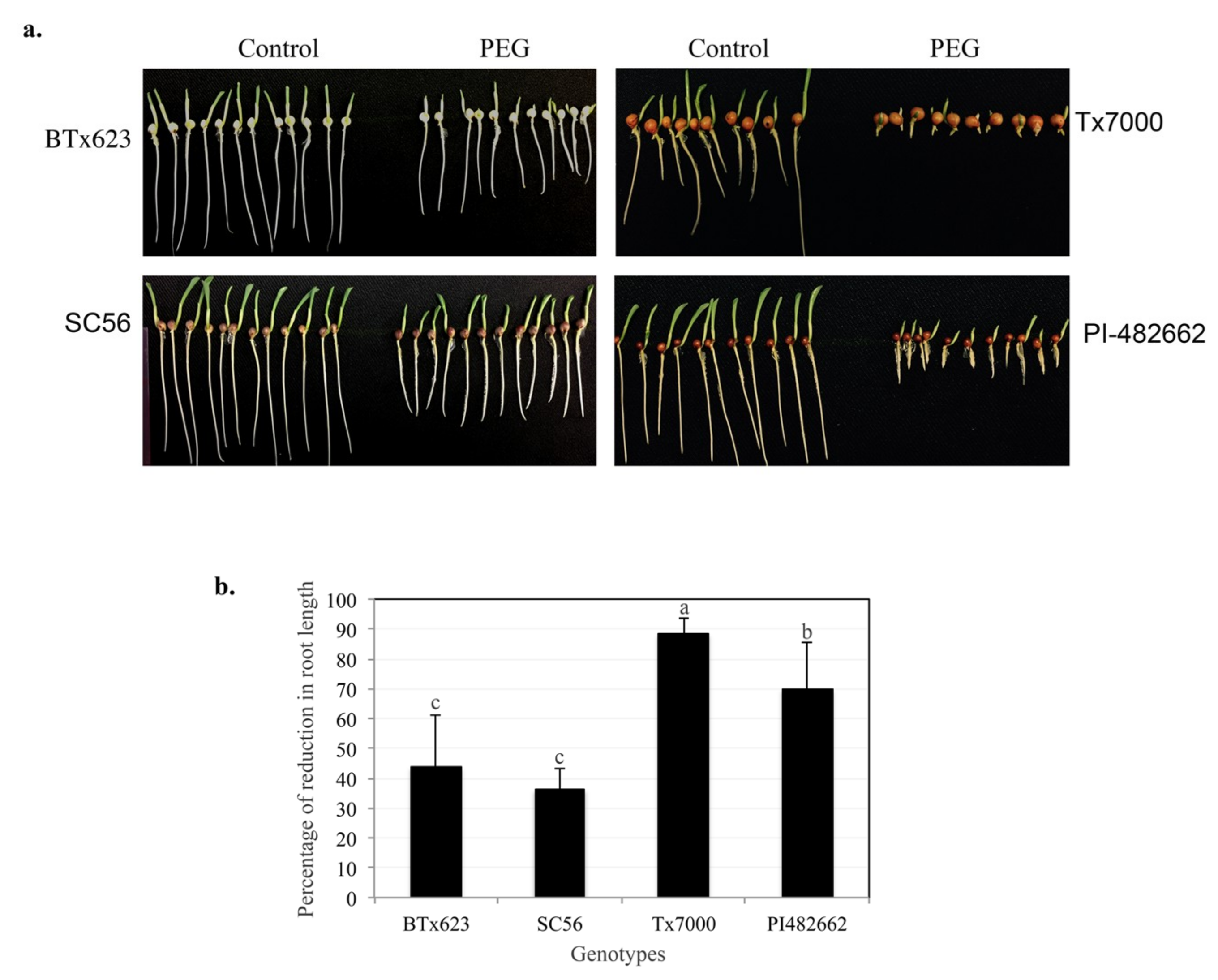
2.1. Selection of Drought Resistance Sorghum Genotypes at the Seedling Stage
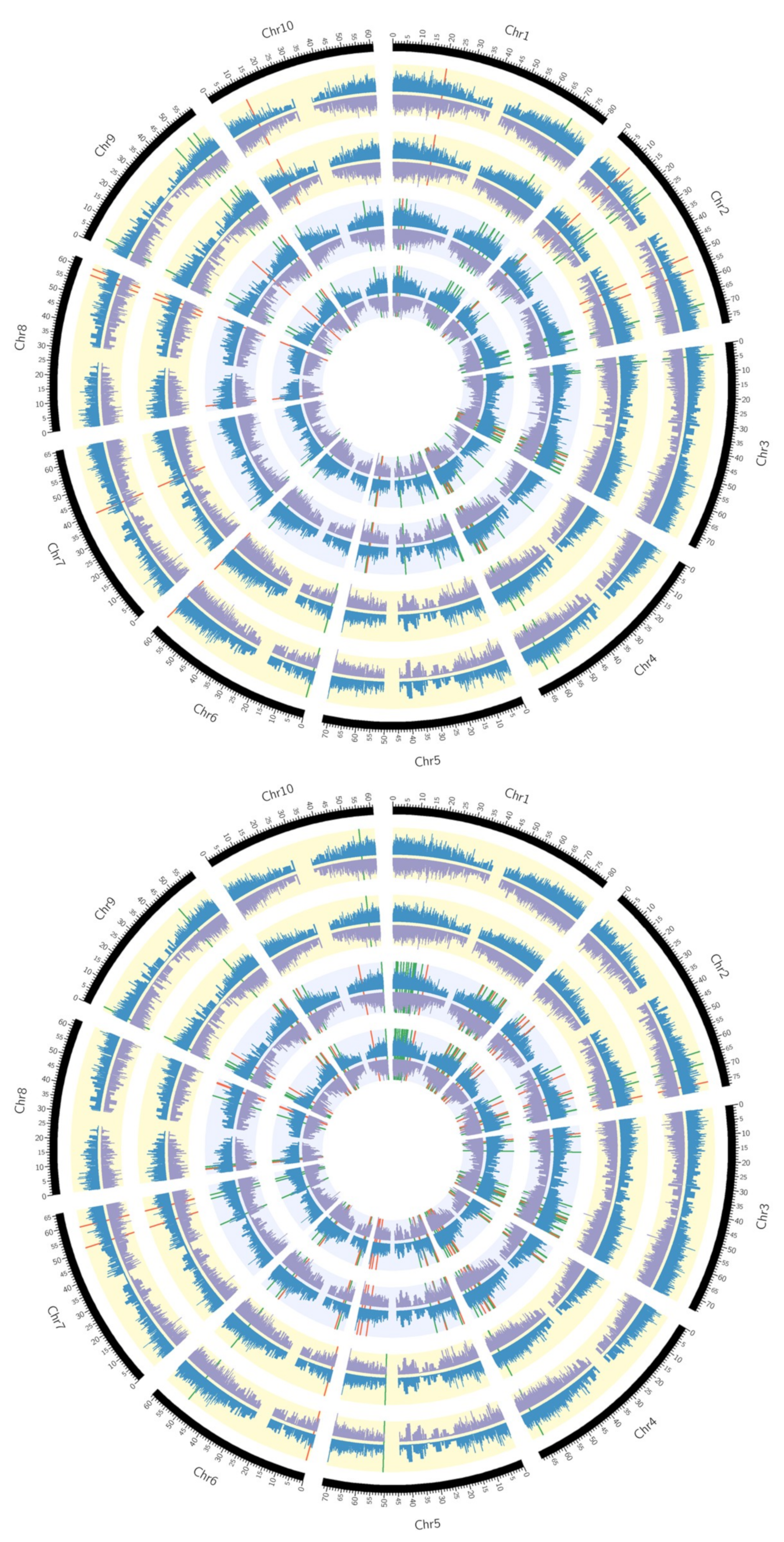
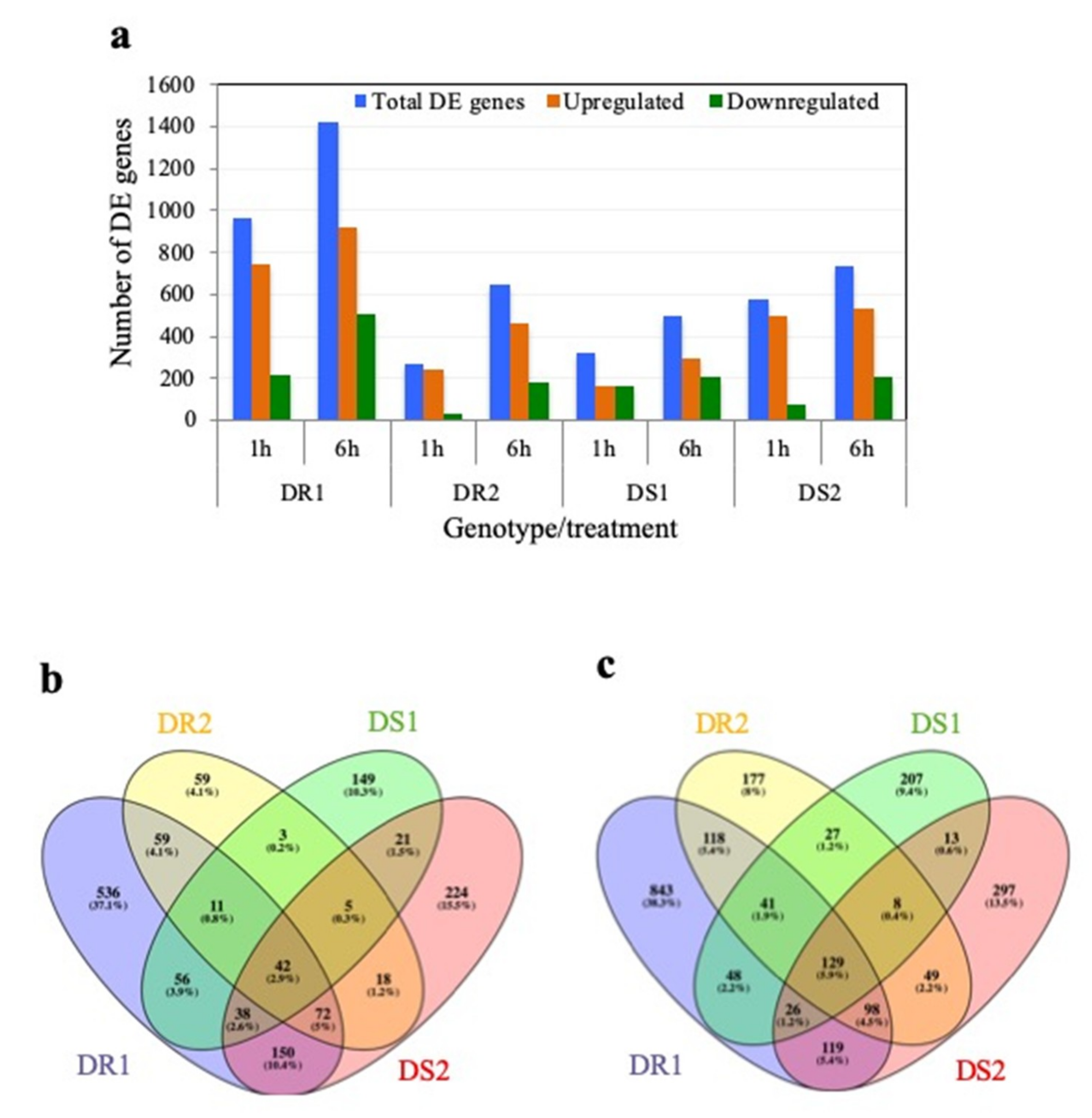
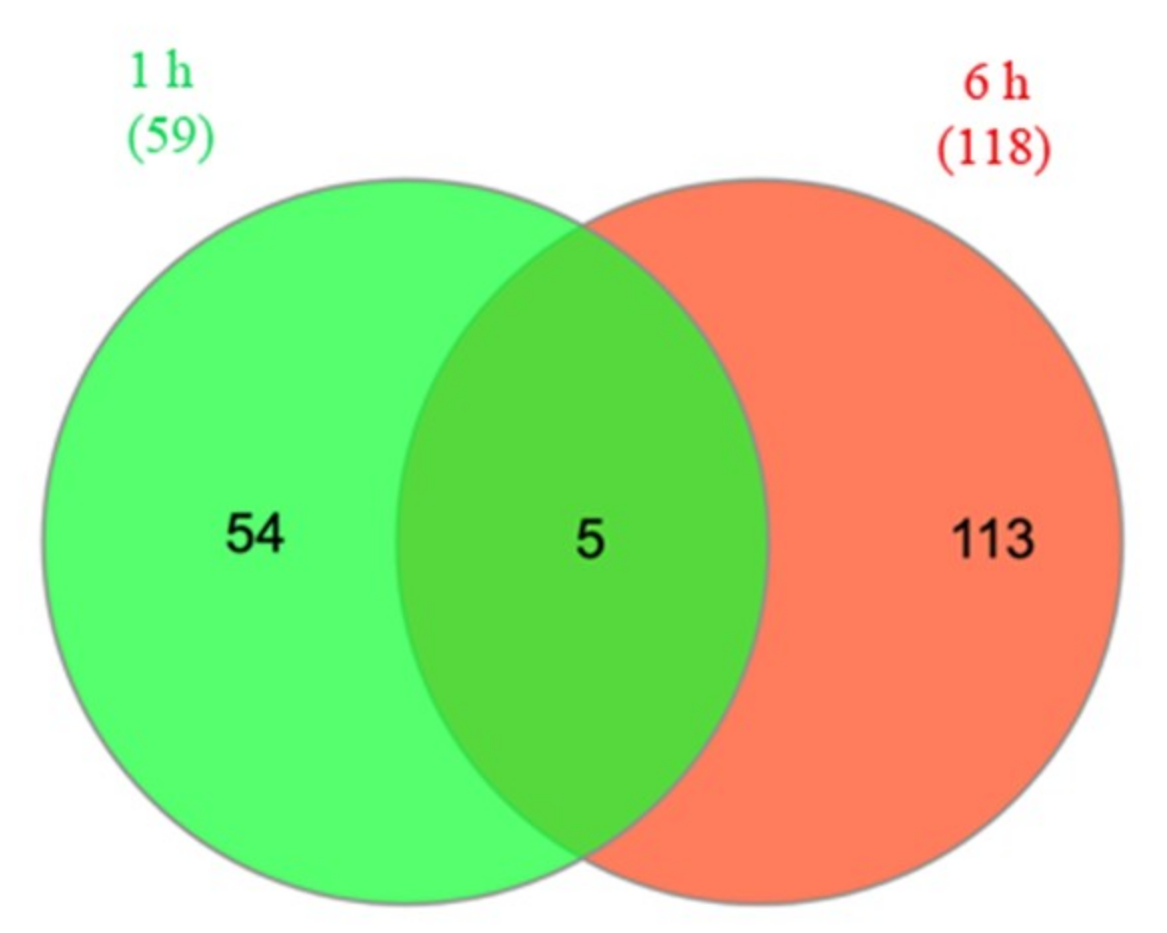
2.2. Transcriptome Analysis of Two Drought-Resistant and Two Drought-Sensitive Sorghum Genotypes in Response to PEG-Induced Drought
2.3. Differentially Expressed Genes Associated with Drought Resistance
2.3.1. Transcription Factors and Stress Tolerance
2.3.2. Damage Control/Repair and Detoxification
Cryo-Protectant
Detoxification-Related Genes
2.4. Osmoprotectants
Membrane Composition and Signaling Molecules
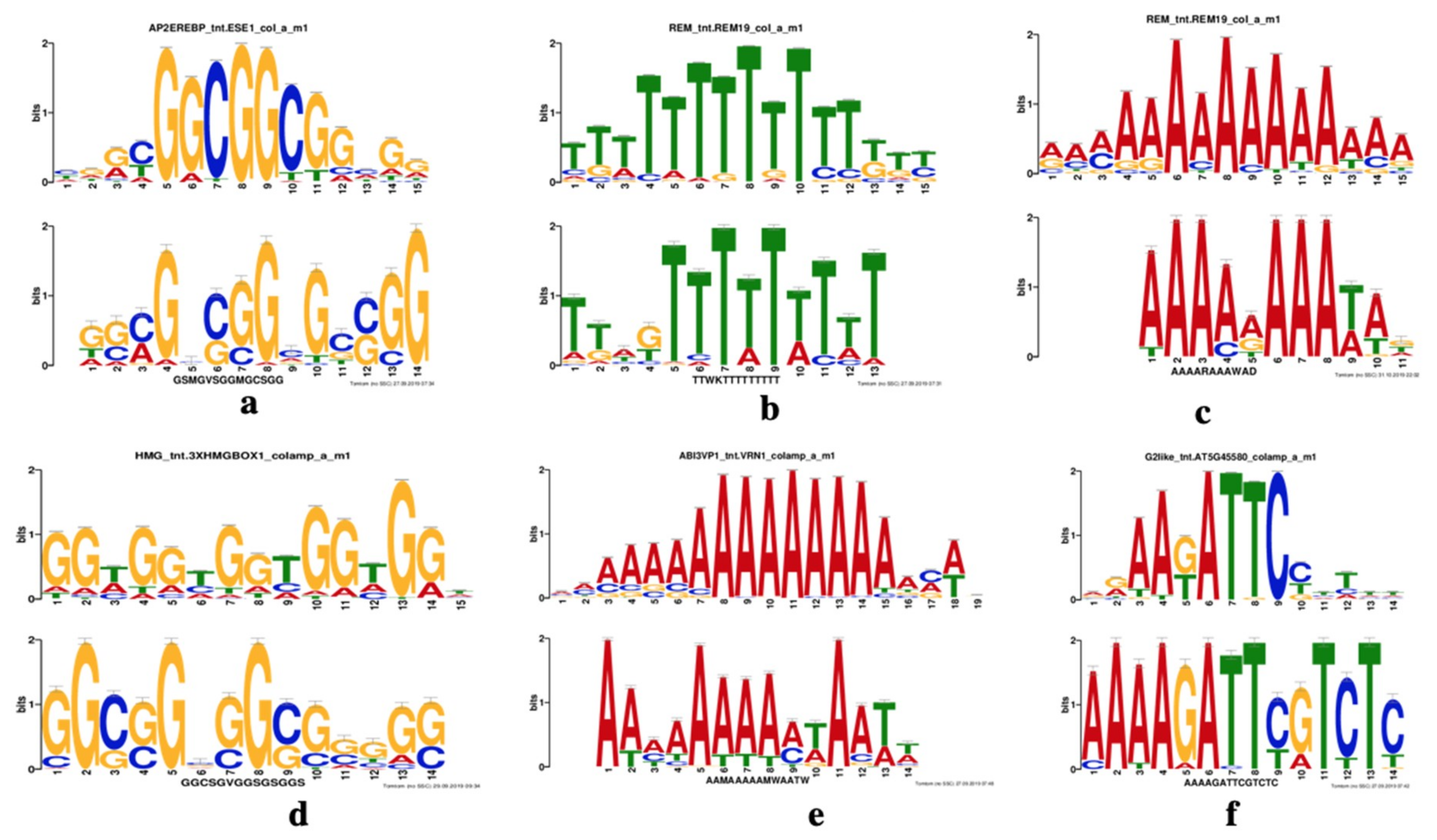
2.5. Motif Analysis in the Promoters of DE Genes
2.6. Conclusions
3. Materials and Methods
3.1. Plant Materials and Growth Condition
3.2. RNA Extraction and Library Preparation
3.3. Data Pre-Processing
3.4. Differential Gene Expression Analysis
3.5. Circos Plots
3.6. Motif Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DE | Differentially expressed |
References
- Ahuja, I.; de Vos, R.C.; Bones, A.M.; Hall, R.D. Plant molecular stress responses face climate change. Trends Plant Sci. 2010, 15, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Kogan, F.; Guo, W.; Yang, W.Z. Drought and food security prediction from NOAA new generation of operational satellites. Geomat. Nat. Hazards Risk 2019, 10, 651–666. [Google Scholar] [CrossRef]
- FAO. The Impact of Disasters and Crises on Agriculture and Food Security; FAO: Roma, Italy, 2018; pp. 1–168. [Google Scholar]
- Osmolovskaya, N.; Shumilina, J.; Kim, A.; Didio, A.; Grishina, T.; Bilova, T.; Keltsieva, O.A.; Zhukov, V.; Tikhonovich, I.; Tarakhovskaya, E.; et al. Methodology of Drought Stress Research: Experimental Setup and Physiological Characterization. Int. J. Mol. Sci. 2018, 19, 4089. [Google Scholar] [CrossRef] [PubMed]
- Thatcher, S.R.; Zhou, W.; Leonard, A.; Wang, B.B.; Beatty, M.; Zastrow-Hayes, G.; Zhao, X.; Baumgarten, A.; Li, B. Genome-wide analysis of alternative splicing in Zea mays: Landscape and genetic regulation. Plant Cell 2014, 26, 3472–3487. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, F.; Kuromori, T.; Sato, H.; Shinozaki, K. Regulatory Gene Networks in Drought Stress Responses and Resistance in Plants. Adv. Exp. Med. Biol. 2018, 1081, 189–214. [Google Scholar] [PubMed]
- Filichkin, S.A.; Hamilton, M.; Dharmawardhana, P.D.; Singh, S.K.; Sullivan, C.; Ben-Hur, A.; Reddy, A.S.N.; Jaiswal, P. Abiotic Stresses Modulate Landscape of Poplar Transcriptome via Alternative Splicing, Differential Intron Retention, and Isoform Ratio Switching. Front Plant Sci. 2018, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Provart, N.J.; Glazebrook, J.; Katagiri, F.; Chang, H.S.; Eulgem, T.; Mauch, F.; Luan, S.; Zou, G.; Whitham, S.A.; et al. Expression profile matrix of Arabidopsis transcription factor genes suggests their putative functions in response to environmental stresses. Plant Cell 2002, 14, 559–574. [Google Scholar] [CrossRef]
- Knight, H.; Knight, M.R. Abiotic stress signalling pathways: Specificity and cross-talk. Trends Plant Sci. 2001, 6, 262–267. [Google Scholar] [CrossRef]
- Liu, Q.; Kasuga, M.; Sakuma, Y.; Abe, H.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 1998, 10, 1391–1406. [Google Scholar] [CrossRef]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef]
- Sakuma, Y.; Maruyama, K.; Osakabe, Y.; Qin, F.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of an Arabidopsis transcription factor, DREB2A, involved in drought-responsive gene expression. Plant Cell 2006, 18, 1292–1309. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, M.M.; Muchow, R.C. A Critical Evaluation of Traits for Improving Crop Yields in Water-Limited Environments1. In Advances in Agronomy; Brady, N.C., Ed.; Academic Press: Cambridge, MA, USA, 1990; Volume 43, pp. 107–153. [Google Scholar]
- Tari, I.; Laskay, G.; Takács, Z.; Poór, P. Response of Sorghum to Abiotic Stresses: A Review. J. Agron. Crop Sci. 2013, 199, 264–274. [Google Scholar] [CrossRef]
- Doebley, J.F.; Gaut, B.S.; Smith, B.D. The Molecular Genetics of Crop Domestication. Cell 2006, 127, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Legwaila, G.M.; Balole, T.V.; Karikari, S.K. Review of sweet sorghum: A potential cash and forage crop in Botswana. UNISWA J. Agric. 2003, 12, 5–14. [Google Scholar] [CrossRef]
- Berenji, J.; Dahlberg, J. Perspectives of Sorghum in Europe. J. Agron. Crop Sci. 2004, 190, 332–338. [Google Scholar] [CrossRef]
- Evans, J.; McCormick, R.F.; Morishige, D.; Olson, S.N.; Weers, B.; Hilley, J.; Klein, P.; Rooney, W.; Mullet, J. Extensive variation in the density and distribution of DNA polymorphism in sorghum genomes. PLoS ONE 2013, 8, e79192. [Google Scholar] [CrossRef]
- Rami, J.-F.; Dufour, P.; Trouche, G.; Fliedel, G.; Mestres, C.; Davrieux, F.; Blanchard, P.; Hamon, P. Quantitative trait loci for grain quality, productivity, morphological and agronomical traits in sorghum (Sorghum bicolor L. Moench). Theor. Appl. Genet. 1998, 97, 605–616. [Google Scholar] [CrossRef]
- Harris-Shultz, K.R.; Hayes, C.M.; Knoll, J.E. Mapping QTLs and Identification of Genes Associated with Drought Resistance in Sorghum. Methods Mol. Biol. 2019, 1931, 11–40. [Google Scholar]
- Ashraf, M. Inducing drought tolerance in plants: Recent advances. Biotechnol. Adv. 2010, 28, 169–183. [Google Scholar] [CrossRef]
- Kebede, H.; Subudhi, P.K.; Rosenow, D.T.; Nguyen, H.T. Quantitative trait loci influencing drought tolerance in grain sorghum (Sorghum bicolor L. Moench). Theor. Appl. Genet. 2001, 103, 266–276. [Google Scholar] [CrossRef]
- Phuong, N.; Afolayan, G.; Stutzel, H.; Uptmoor, R.; El-Soda, M. Unraveling the genetic complexity underlying sorghum response to water availability. PLoS ONE 2019, 14, e0215515. [Google Scholar]
- Paterson, A.H.; Bowers, J.E.; Bruggmann, R.; Dubchak, I.; Grimwood, J.; Gundlach, H.; Haberer, G.; Hellsten, U.; Mitros, T.; Poliakov, A.; et al. The Sorghum bicolor genome and the diversification of grasses. Nature 2009, 457, 551–556. [Google Scholar] [CrossRef]
- Mace, E.S.; Tai, S.; Gilding, E.K.; Li, Y.; Prentis, P.J.; Bian, L.; Campbell, B.C.; Hu, W.; Innes, D.J.; Han, X.; et al. Whole-genome sequencing reveals untapped genetic potential in Africa’s indigenous cereal crop sorghum. Nat. Commun. 2013, 4, 2320. [Google Scholar] [CrossRef]
- Johnson, S.M.; Lim, F.L.; Finkler, A.; Fromm, H.; Slabas, A.R.; Knight, M.R. Transcriptomic analysis of Sorghum bicolor responding to combined heat and drought stress. BMC Genom. 2014, 15, 456. [Google Scholar] [CrossRef]
- Dugas, D.V.; Monaco, M.K.; Olsen, A.; Klein, R.R.; Kumari, S.; Ware, D.; Klein, P.E. Functional annotation of the transcriptome of Sorghum bicolor in response to osmotic stress and abscisic acid. BMC Genom. 2011, 12, 514. [Google Scholar] [CrossRef]
- Fracasso, A.; Trindade, L.M.; Amaducci, S. Drought stress tolerance strategies revealed by RNA-Seq in two sorghum genotypes with contrasting WUE. BMC Plant Biol. 2016, 16, 115. [Google Scholar] [CrossRef]
- Agrawal, L.; Gupta, S.; Mishra, S.K.; Pandey, G.; Kumar, S.; Chauhan, P.S.; Chakrabarty, D.; Nautiyal, C.S. Elucidation of Complex Nature of PEG Induced Drought-Stress Response in Rice Root Using Comparative Proteomics Approach. Front. Plant Sci. 2016, 7, 1466. [Google Scholar] [CrossRef]
- Guo, J.; Ren, Y.; Tang, Z.; Shi, W.; Zhou, M. Characterization and expression profiling of the ICE-CBF-COR genes in wheat. PeerJ 2019, 7, e8190. [Google Scholar] [CrossRef]
- Zeng, W.; Peng, Y.; Zhao, X.; Wu, B.; Chen, F.; Ren, B.; Zhuang, Z.; Gao, Q.; Ding, Y. Comparative Proteomics Analysis of the Seedling Root Response of Drought-sensitive and Drought-tolerant Maize Varieties to Drought Stress. Int. J. Mol. Sci. 2019, 20, 2793. [Google Scholar] [CrossRef]
- Rabbani, M.A.; Maruyama, K.; Abe, H.; Khan, M.A.; Katsura, K.; Ito, Y.; Yoshiwara, K.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Monitoring expression profiles of rice genes under cold, drought, and high-salinity stresses and abscisic acid application using cDNA microarray and RNA gel-blot analyses. Plant Physiol. 2003, 133, 1755–1767. [Google Scholar] [CrossRef]
- Lagerwerff, J.V.; Ogata, G.; Eagle, H.E. Control of osmotic pressure of culture solutions with polyethylene glycol. Science 1961, 133, 1486–1487. [Google Scholar] [CrossRef] [PubMed]
- Bussis, D.; Heineke, D. Acclimation of potato plants to polyethyleneglycol-induced water deficitII. Contents and subcellular distribution of organicsolutes. J. Exp. Bot. 1988, 49, 1361–1370. [Google Scholar] [CrossRef]
- Muscolo, A.; Sidari, M.; Anastaso, U.; Santonoceto, C.; Maggio, A. Effect of PEG-induced drought stress on seedgermination of four lentil genotypes. J. Plant Interact. 2014, 9, 354–363. [Google Scholar] [CrossRef]
- Abdel-Ghany, S.E.; Hamilton, M.; Jacobi, J.L.; Ngam, P.; Devitt, N.; Schilkey, F.; Ben-Hur, A.; Reddy, A.S. A survey of the sorghum transcriptome using single-molecule long reads. Nat. Commun. 2016, 7, 11706. [Google Scholar] [CrossRef]
- Chakrabarti, M.; de Lorenzo, L.; Abdel-Ghany, S.E.; Reddy, A.S.N.; Hunt, A.G. Wide-ranging transcriptome remodeling mediated by alternative polyadenylation in response to abiotic stresses in sorghum. Plant J. 2020. [Google Scholar] [CrossRef]
- Lata, C.; Prasad, M. Role of DREBs in regulation of abiotic stress responses in plants. J. Exp. Bot. 2011, 62, 4731–4748. [Google Scholar] [CrossRef]
- Bihani, P.; Char, B.; Bhargava, S. Transgenic expression of sorghum DREB2 in rice improves tolerance and yield under water limitation. J. Agric. Sci. 2011, 149, 95–101. [Google Scholar] [CrossRef]
- Buchanan, C.D.; Lim, S.; Salzman, R.A.; Kagiampakis, I.; Morishige, D.T.; Weers, B.D.; Klein, R.R.; Pratt, L.H.; Cordonnier-Pratt, M.M.; Klein, P.E.; et al. Sorghum bicolor’s transcriptome response to dehydration, high salinity and ABA. Plant Mol. Biol. 2005, 58, 699–720. [Google Scholar] [CrossRef]
- Rabara, R.C.; Tripathi, P.; Reese, R.N.; Rushton, D.L.; Alexander, D.; Timko, M.P.; Shen, Q.J.; Rushton, P.J. Tobacco drought stress responses reveal new targets for Solanaceae crop improvement. BMC Genom. 2015, 16, 484. [Google Scholar] [CrossRef]
- Kim, M.J.; Ruzicka, D.; Shin, R.; Schachtman, D.P. The Arabidopsis AP2/ERF Transcription Factor RAP2.11 Modulates Plant Response to Low-Potassium Conditions. Mol. Plant 2012, 5, 1042–1057. [Google Scholar] [CrossRef]
- Ma, H.-S.; Liang, D.; Shuai, P.; Xia, X.-L.; Yin, W.-L. The salt- and drought-inducible poplar GRAS protein SCL7 confers salt and drought tolerance in Arabidopsis thaliana. J. Exp. Bot. 2010, 61, 4011–4019. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Chen, S.; Li, T.; Ma, X.; Liang, X.; Ding, X.; Liu, H.; Luo, L. OsGRAS23, a rice GRAS transcription factor gene, is involved in drought stress response through regulating expression of stress-responsive genes. BMC Plant Biol. 2015, 15, 141. [Google Scholar] [CrossRef]
- Giri, J.; Vij, S.; Dansana, P.K.; Tyagi, A.K. Rice A20/AN1 zinc-finger containing stress-associated proteins (SAP1/11) and a receptor-like cytoplasmic kinase (OsRLCK253) interact via A20 zinc-finger and confer abiotic stress tolerance in transgenic Arabidopsis plants. New Phytol. 2011, 191, 721–732. [Google Scholar] [CrossRef]
- Lakra, N.; Nutan, K.K.; Das, P.; Anwar, K.; Singla-Pareek, S.L.; Pareek, A. A nuclear-localized histone-gene binding protein from rice (OsHBP1b) functions in salinity and drought stress tolerance by maintaining chlorophyll content and improving the antioxidant machinery. J. Plant Physiol. 2015, 176, 36–46. [Google Scholar] [CrossRef]
- Tunnacliffe, A.; Wise, M.J. The continuing conundrum of the LEA proteins. Naturwissenschaften 2007, 94, 791–812. [Google Scholar] [CrossRef]
- Hassan, N.M.; El-Bastawisy, Z.M.; El-Sayed, A.K.; Ebeed, H.T.; Nemat Alla, M.M. Roles of dehydrin genes in wheat tolerance to drought stress. J. Adv. Res. 2015, 6, 179–188. [Google Scholar] [CrossRef]
- Lopes-Caitar, V.S.; de Carvalho, M.C.; Darben, L.M.; Kuwahara, M.K.; Nepomuceno, A.L.; Dias, W.P.; Abdelnoor, R.V.; Marcelino-Guimarães, F.C. Genome-wide analysis of the Hsp 20 gene family in soybean: Comprehensive sequence, genomic organization and expression profile analysis under abiotic and biotic stresses. BMC Genom. 2013, 14, 577. [Google Scholar] [CrossRef]
- Seki, M.; Narusaka, M.; Ishida, J.; Nanjo, T.; Fujita, M.; Oono, Y.; Kamiya, A.; Nakajima, M.; Enju, A.; Sakurai, T.; et al. Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA microarray. Plant J. 2002, 31, 279–292. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, X.; Lu, C.; Zeng, X.; Li, Y.; Fu, D.; Wu, G. Non-specific lipid transfer proteins in plants: Presenting new advances and an integrated functional analysis. J. Exp. Bot. 2015, 66, 5663–5681. [Google Scholar] [CrossRef]
- Medina, J.; Ballesteros, M.L.; Salinas, J. Phylogenetic and functional analysis of Arabidopsis RCI2 genes. J. Exp. Bot. 2007, 58, 4333–4346. [Google Scholar] [CrossRef]
- Li, L.; Li, N.; Song, S.F.; Li, Y.X.; Xia, X.J.; Fu, X.Q.; Chen, G.H.; Deng, H.F. Cloning and characterization of the drought-resistance OsRCI2-5 gene in rice (Oryza sativa L.). Genet. Mol. Res. 2014, 13, 4022–4035. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Singla-Pareek, S.L.; Ray, M.; Reddy, M.K.; Sopory, S.K. Methylglyoxal levels in plants under salinity stress are dependent on glyoxalase I and glutathione. Biochem. Biophys. Res. Commun. 2005, 337, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Shen, B.; Jensen, R.G.; Bohnert, H.J. Increased resistance to oxidative stress in transgenic plants by targeting mannitol biosynthesis to chloroplasts. Plant Physiol. 1997, 113, 1177–1183. [Google Scholar] [CrossRef]
- Khan, M.S.; Ahmad, D.; Khan, M.A. Utilization of genes encoding osmoprotectants in transgenic plants for enhanced abiotic stress tolerance. Electron. J. Biotechnol. 2015, 18, 257–266. [Google Scholar] [CrossRef]
- Kinnersley, A.M.; Turano, F.J. Gamma Aminobutyric Acid (GABA) and Plant Responses to Stress. Crit. Rev. Plant Sci. 2000, 19, 479–509. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, P.; Yusuf, M.A.; Upadhyaya, C.P.; Roy, S.D.; Hohn, T.; Sarin, N.B. The Xerophyta viscosa aldose reductase (ALDRXV4) confers enhanced drought and salinity tolerance to transgenic tobacco plants by scavenging methylglyoxal and reducing the membrane damage. Mol. Biotechnol. 2013, 54, 292–303. [Google Scholar] [CrossRef]
- ElSayed, A.I.; Rafudeen, M.S.; Golldack, D. Physiological aspects of raffinose family oligosaccharides in plants: Protection against abiotic stress. Plant Biol. 2014, 16, 1–8. [Google Scholar] [CrossRef]
- Meijer, H.J.; Munnik, T. Phospholipid-based signaling in plants. Annu. Rev. Plant Biol. 2003, 54, 265–306. [Google Scholar] [CrossRef]
- Zhang, M.; Barg, R.; Yin, M.G.; Gueta-Dahan, Y.; Leikin-Frenkel, A.; Salts, Y.; Shabtai, S.; Ben-Hayyim, G. Modulated fatty acid desaturation via overexpression of two distinct omega-3 desaturases differentially alters tolerance to various abiotic stresses in transgenic tobacco cells and plants. Plant J. 2005, 44, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.M.; Perera, I.Y.; Heilmann, I.I.; Persson, S.; Boss, W.F. Inositol signaling and plant growth. Trends Plant Sci. 2000, 5, 357. [Google Scholar] [CrossRef]
- Perera, I.Y.; Hung, C.Y.; Moore, C.D.; Stevenson-Paulik, J.; Boss, W.F. Transgenic Arabidopsis plants expressing the type 1 inositol 5-phosphatase exhibit increased drought tolerance and altered abscisic acid signaling. Plant Cell 2008, 20, 2876–2893. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucl. Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Mogami, J.; Yamaguchi-Shinozaki, K. ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr. Opin. Plant Biol. 2014, 21, 133–139. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Tuinstra, M.R.; Grote, E.M.; Goldsbrough, P.B.; Ejeta, G. Genetic analysis of post-flowering drought tolerance and components of grain development in Sorghum bicolor (L.) Moench. Mol. Breed. 1997, 3, 439–448. [Google Scholar] [CrossRef]
- Van der Weele, C.M.; Spollen, W.G.; Sharp, R.E.; Baskin, T.I. Growth of Arabidopsis thaliana seedlings under water deficit studied by control of water potential in nutrient-agar media. J. Exp. Bot. 2000, 51, 1555–1562. [Google Scholar] [CrossRef]
- Tajima, R.; Kato, Y. A Quick Method to Estimate Root Length in Each Diameter Class Using Freeware ImageJ. Plant. Prod. Sci. 2013, 16, 9–11. [Google Scholar] [CrossRef]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef]
- Rogers, M.F.; Thomas, J.; Reddy, A.S.; Ben-Hur, A. SpliceGrapher: Detecting patterns of alternative splicing from RNA-Seq data in the context of gene models and EST data. Genome Biol. 2012, 13, R4. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, S.; Li, W. RSeQC: Quality control of RNA-seq experiments. Bioinformatics 2012, 28, 2184–2185. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [PubMed]


| Genotype | Total Reads (×106) | Mapped Reads (×106) | Uniquely Mapped Reads (×106) | Reads Mapped to Multiple Locatios (×106) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 h | 6 h | 1 h | 6 h | 1 h | 6 h | 1 h | 6 h | |||||||||
| Cont | PEG | Cont | PEG | Cont | PEG | Cont | PEG | Cont | PEG | Cont | PEG | Cont | PEG | Cont | PEG | |
| BTx623 (DR1) | 127.0 | 125.6 | 130.3 | 130.9 | 108.7 85.6% | 114.4 91.1% | 117.0 89.8% | 115.3 88.1% | 103.8 95.5% | 107.2 93.7% | 111.6 95.4% | 108.5 94.1% | 4.9 4.5% | 7.2 6.3% | 5.4 4.6% | 5.4 5.9% |
| SC56 (DR2) | 129.3 | 140.7 | 210.5 | 174.7 | 117.1 90.5% | 126.1 89.6% | 180.2 85.6% | 149.7 85.7% | 103.1 88.1% | 114.4 90.8% | 172.1 95.5% | 136.8 91.3% | 13.9 11.9% | 11.6 9.2% | 8.2 4.5% | 13.0 8.7% |
| Tx-7000 (DS1) | 131.6 | 123.4 | 126.3 | 137.1 | 117.3 89.1% | 109.1 88.4% | 111.1 87.9% | 119.7 87.3% | 108.6 92.6% | 104.4 95.7% | 106.3 97.7% | 112.2 93.8% | 8.7 7.4% | 4.7 4.3% | 4.7 4.3% | 7.4 6.2% |
| PI482662 (DS2) | 115.4 | 109.8 | 122.8 | 110.9 | 100.5 87.0% | 94.9 86.4% | 107.3 87.4% | 94.1 84.8% | 95.8 95.3% | 90.7 95.5% | 98.9 92.2% | 88.3 93.8% | 4.7 4.7% | 4.3 4.5% | 8.4 7.8% | 5.8 6.2% |
| Gene ID | Annotation | Functional Category | Log2FC | |
|---|---|---|---|---|
| DR1 | DR2 | |||
| SOBIC.001G319700 | Glutathione S-transferase, putative | Antioxidant/detoxification | 9.39 | 3.34 |
| SOBIC.005G112400 | Cysteine proteinase EP-B 1 precursor | Protein degradation | 8.01 | 5.76 |
| SOBIC.001G114800 | NA | NC | 7.67 | 3.19 |
| SOBIC.005G169300 | WIP5 - Wound-induced protein precursor | Stress (biotic) | 7.03 | 7.17 |
| SOBIC.002G063800 | Transferase family protein, putative | Stress (biotic) | 6.84 | 2.65 |
| SOBIC.003G425900 | Expressed protein | NC | 6.43 | 2.51 |
| SOBIC.003G063100 | NA | NC | 5.91 | 6.76 |
| SOBIC.009G159500 | PSF1 - Putative GINS complex subunit | NC | 5.87 | 4.58 |
| SOBIC.010G041900 | Dehydrin, putative, expressed | NC | 5.79 | 2.93 |
| SOBIC.007G029800 | LTPL24 - Protease inhibitor/seed storage/LTP protein precursor, expressed | NC | 5.33 | 9.45 |
| SOBIC.003G313800 | Cytokinin dehydrogenase precursor | Hormone metabolism | 5.13 | 2.15 |
| SOBIC.006G190100 | Naringenin, 2-oxoglutarate 3-dioxygenase | 2nd metabolism | 5.05 | 2.96 |
| SOBIC.001G058800 | Cysteine proteinase EP-B 1 precursor, putative | Protein degradation | 5.02 | 7.40 |
| SOBIC.002G251000 | Expressed protein | NC | 5.00 | 2.57 |
| SOBIC.004G309000 | Alpha-amylase precursor | Carbohydrate Digestion | 4.83 | 3.83 |
| SOBIC.002G324000 | Isocitrate lyase, putative | Glycolysis | 4.82 | 4.36 |
| SOBIC.010G166800 | Amino acid transporter | Amino acid transport | 4.63 | 2.77 |
| SOBIC.004G249950 | NA | NC | 4.56 | 2.13 |
| SOBIC.003G400400 | Cysteine proteinase inhibitor precursor protein | Protein degradation | 4.55 | 2.48 |
| SOBIC.003G395000 | Cysteine proteinase EP-B 1 precursor, putative | Protein degradation | 4.48 | 5.03 |
| SOBIC.001G344300 | NA | NC | 4.40 | 4.60 |
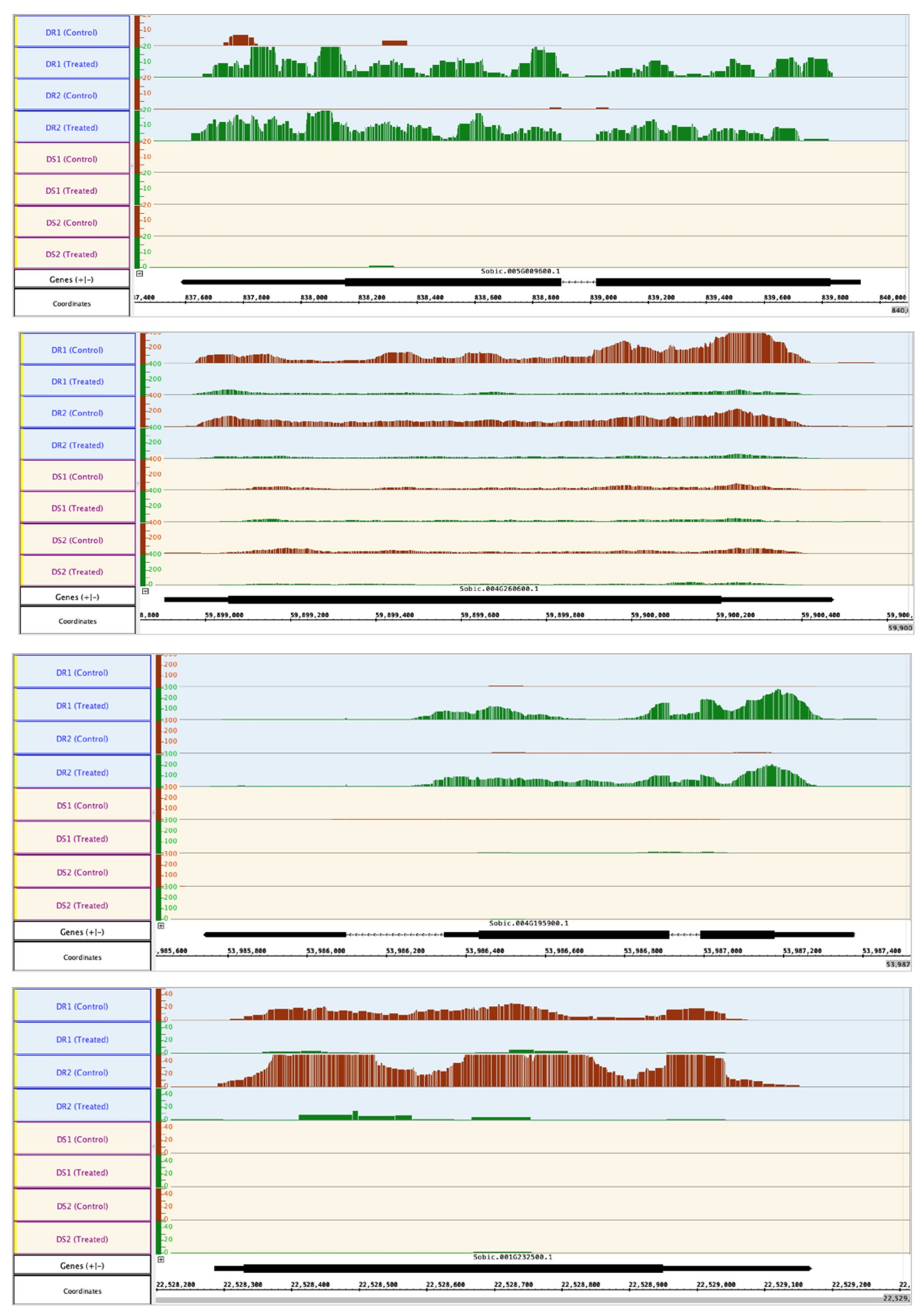
| SOBIC.005G009600 | Pectinesterase | NC | 4.01 | 7.11 |
| SOBIC.001G485200 | AP2 domain containing protein | Transcription factor | 4.01 | 2.31 |
| SOBIC.004G133600 | Nodulin mtn3 family protein, putative | NC | 3.84 | 4.46 |
| SOBIC.003G079200 | Transposon protein, putative, unclassified | NC | 3.53 | 2.43 |
| SOBIC.002G260100 | NA | NC | 3.39 | 2.21 |
| SOBIC.005G099000 | Glycosyl hydrolase, putative | Stress (biotic) | 3.34 | 2.11 |
| SOBIC.002G269032 | Dehydration-responsive element (DREB1B-like) proteinputaexpressed | Transcription factor | 3.33 | 2.40 |
| SOBIC.003G282901 | DUF584 domain containing protein | NC | 3.05 | 2.90 |
| SOBIC.001G401200 | Pathogenesis-related Bet v I family protein | NC | 2.97 | 2.43 |
| SOBIC.004G273300 | Ubiquitin family protein, putative | Protein degradation | 2.90 | 2.00 |
| SOBIC.004G315200 | Expressed protein | NC | 2.83 | 2.21 |
| SOBIC.004G147900 | Cupin domain containing protein | Stress (abioticv) | 2.82 | 2.26 |
| SOBIC.002G269100 | Similar to DREB1a-like protein | Transcription factor | 2.78 | 3.41 |
| SOBIC.001G443800 | Glutamate decarboxylase, putative | Amino acid metabolism | 2.78 | 2.01 |
| SOBIC.003G430400 | Oscml31 - Calmodulin-related calcium sensor proteinexpressed | NC | 2.76 | 2.00 |
| SOBIC.002G139400 | Expressed protein | Hormone metabolism | 2.76 | 2.49 |
| SOBIC.002G416600 | Peroxidase precursor | Antioxidant/detoxification | 2.72 | 2.10 |
| SOBIC.002G415600 | AP2 domain containing protein | Transcription factor | 2.67 | 2.28 |
| SOBIC.009G217600 | STE_MEKK_ste11_MAP3K.19 - STE kinases incluhomologs to sterile 7, sterile 11 and sterile 20 from yeast, expressed | Posttransilation modificati. | 2.66 | 2.12 |
| SOBIC.001G398500 | Long cell-linked locus protein | NC | 2.61 | 2.70 |
| SOBIC.005G016600 | DUF567 domain containing protein | NC | 2.58 | 2.30 |
| SOBIC.009G071800 | 6-phosphofructokinase | Glycolysis | 2.55 | 2.52 |
| SOBIC.009G043700 | Glutathione S-transferase | Antioxidant/detoxification | 2.49 | 2.16 |
| SOBIC.002G250900 | Expressed protein | NC | 2.35 | 2.95 |
| SOBIC.003G361100 | Hs1, putative | NC | 2.33 | 2.18 |
| SOBIC.003G264500 | Glutathione S-transferase, putative | Antioxidant/detoxification | 2.25 | 2.19 |
| SOBIC.001G319600 | Glutathione S-transferase | Antioxidant/detoxification | 2.22 | 2.38 |
| SOBIC.005G163800 | Laccase precursor protein | 2nd metabolism | −2.01 | −2.12 |
| SOBIC.004G299600 | Transposon protein, putative | NC | −2.07 | −2.16 |
| SOBIC.009G250200 | Transmembrane amino acid transporter proteinputatexpressed | Amino acid transport | −2.37 | −2.07 |
| SOBIC.008G036800 | Chalcone synthase | 2nd metabolism | −2.38 | −2.21 |
| SOBIC.001G082700 | TKL_IRAK_crrlk1l-1.1 - The crrlk1l-1 subfamily hahomology to the crrlk1l homolog, expressed | Signaling | −2.39 | −2.34 |
| SOBIC.003G348300 | Expressed protein | NC | −2.47 | −2.12 |
| SOBIC.004G260600 | Fatty acid desaturase | Lipid metabolism | −2.47 | −2.45 |
| SOBIC.009G096300 | ATOFP17/OFP17 | NC | −2.48 | −2.67 |
| SOBIC.003G278100 | Expressed protein | NC | −3.19 | −2.87 |
| SOBIC.002G071600 | AP2 domain containing protein | Transcription factor | −3.55 | −2.20 |
| SOBIC.008G177400 | Strictosidine synthase | 2nd metabolism | −4.20 | −4.51 |
| Gene ID | Annotation | Functional Category | Log2FC | |
|---|---|---|---|---|
| DR1 | DR2 | |||
| SOBIC.009G025500 | Osrci2-5 -low temperature and salt responsive protein | NC | 9.63 | 6.75 |
| SOBIC.003G295600 | DUF1264 domain containing protein | NC | 9.36 | 7.53 |
| SOBIC.001G311200 | NA | NC | 9.00 | 6.78 |
| SOBIC.001G381700 | NA | NC | 8.95 | 6.77 |
| SOBIC.002G151200 | LEA 4, seed maturation protein | Development | 8.68 | 7.02 |
| SOBIC.003G372600 | NA | NC | 8.62 | 4.47 |
| SOBIC.004G114600 | NA | NC | 8.03 | 6.70 |
| SOBIC.001G090900 | LEA, late embryogenesis abundant protein D-34 | Development | 7.72 | 6.19 |
| SOBIC.004G195900 | LEA, late embryogenesis abundant protein D-34 | Development | 7.40 | 7.73 |
| SOBIC.001G177600 | Osfbx103 - F-box domain containing protein | NC | 7.32 | 7.44 |
| SOBIC.010G016500 | Dehydration-responsive element-binding protein | Transcription factor | 7.24 | 4.10 |
| SOBIC.001G425900 | Edm2 | Transcription factor | 7.15 | 7.94 |
| SOBIC.001G091000 | LEA, late embryogenesis abundant protein D-34 | Development | 6.70 | 4.52 |
| SOBIC.001G208500 | Aquaporin protein, putative, expressed | Transport/major proteins | 5.91 | 5.50 |
| SOBIC.007G156700 | AP2 domain containing protein, expressed | Transcription factor | 5.82 | 6.33 |
| SOBIC.009G037100 | Oxidoreductase | Misc. (oxidoreductase) | 5.57 | 4.81 |
| SOBIC.003G198100 | NA | NC | 5.47 | 3.17 |
| SOBIC.004G092800 | Hsp20/alpha crystallin family protein | NC | 5.45 | 2.76 |
| SOBIC.004G196000 | Late embryogenesis abundant protein D-34 | Development | 5.45 | 3.18 |
| SOBIC.008G040600 | Bzip transcription factor domain containing protein | Transcription factor | 5.38 | 4.05 |
| SOBIC.004G185000 | STRUBBELIG-RECEPTOR FAMILY 7 precursor | Protein/post-translation modification | 5.31 | 3.73 |
| SOBIC.003G241400 | Seed maturation protein PM41 | NC | 5.13 | 3.08 |
| SOBIC.002G391100 | Peroxiredoxin | Redox/oxidation/reduction | 4.91 | 4.72 |
| SOBIC.001G149300 | Expressed protein | NC | 4.71 | 2.82 |
| SOBIC.001G497700 | Late embryogenesis abundant protein D-34 | Development | 4.61 | 3.94 |
| SOBIC.007G138000 | Zinc finger A20 and AN1 domain-containing stress-associated protein | Transcription factor | 4.59 | 3.65 |
| SOBIC.006G210600 | NA | NC | 4.56 | 2.43 |
| SOBIC.001G342500 | PDI | NC | 4.33 | 3.80 |
| SOBIC.003G268800 | STE_MEKK_ste11_MAP3K.5-STE kinases | Protein/post-translation modification | 4.30 | 2.20 |
| SOBIC.004G221900 | DUF584 domain containing protein | NC | 4.24 | 2.58 |
| SOBIC.008G050600 | Ethylene-responsive transcription factor ERF114 | Transcription factor | 4.14 | 2.58 |
| SOBIC.003G429401 | NA | NC | 4.04 | 2.51 |
| SOBIC.005G003200 | Asparagine synthetase | Amino acid metabolism | 3.98 | 2.33 |
| SOBIC.003G270200 | Dehydrin family protein | NC | 3.97 | 2.96 |
| SOBIC.003G156200 | Cytochrome P450 | Misc. (Cytochrome P450) | 3.94 | 5.12 |
| SOBIC.003G312300 | Homocysteine S-methyltransferase protein | Amino acid metabolism | 3.85 | 3.00 |
| SOBIC.009G230000 | Transcription factor HBP-1b | Transcription factor | 3.72 | 2.79 |
| SOBIC.002G117100 | Cyclin-dependent kinase G-1 | Protein/post-translation modification | 3.67 | 2.28 |
| SOBIC.003G442601 | Transcription initiation factor IIA gamma chain | Transcription factor | 3.63 | 2.21 |
| SOBIC.003G082500 | Hsp20/alpha crystallin family protein | Stress/abiotic | 3.62 | 6.29 |
| SOBIC.003G293500 | Heat shock protein 101 | Stress/abiotic | 3.57 | 2.46 |
| SOBIC.002G308900 | Ubiquitin fusion protein | Protein/degradation | 3.49 | -2.46 |
| SOBIC.005G016400 | DUF567 domain containing protein | NC | 3.46 | 2.49 |
| SOBIC.010G276100 | Transporter family protein | Transport/Sugar | 3.42 | 3.05 |
| SOBIC.001G391300 | Glycosyl transferase 8 domain containing protein | Carbohydrate metabolism | 3.37 | 2.18 |
| SOBIC.001G035000 | ZOS3-22-C2H2 zinc finger protein | NC | 3.33 | 2.58 |
| SOBIC.001G027800 | Osman05-Endo-Beta-Mannanase | Cell wall modification | 3.31 | 2.09 |
| SOBIC.001G114800 | NA | NC | 3.27 | 4.11 |
| SOBIC.009G054500 | Lipase | Lipid metabolism | 3.19 | 2.03 |
| SOBIC.001G044300 | Transcription factor | Transcription factor | 3.14 | 3.41 |
| SOBIC.006G025066 | Lysm domain containing protein | NC | 3.11 | 7.10 |
| SOBIC.004G286600 | Dehydrin | NC | 3.02 | 2.41 |
| SOBIC.006G119600 | GRAM and C2 domains containing protein | Protein/degradation | 2.98 | 4.04 |
| SOBIC.001G128900 | Cytochrome P450 | Misc. (Cytochrome P450) | 2.98 | 3.16 |
| SOBIC.003G369100 | AP2 domain containing protein | NC | 2.96 | 2.39 |
| SOBIC.010G045100 | Osftl2 FT-Like2 homologous to Flowering Locus T | Development | 2.93 | 6.35 |
| SOBIC.010G088500 | Phosphate-induced protein 1 | Signaling | 2.89 | 2.84 |
| SOBIC.005G085400 | DNA-directed RNA polymerase subunit beta | NC | 2.87 | 2.60 |
| SOBIC.001G147500 | Cytochrome P450 | Misc. (Cytochrome P450) | 2.83 | 2.03 |
| SOBIC.001G162700 | Endonuclease/exonuclease/phosphatase family | Chromatic/DNA structure | 2.80 | 2.23 |
| SOBIC.008G109600 | Oxidoreductase, 2OG-Fe oxygenase family protein | Secondary metabolism | 2.69 | 2.13 |
| SOBIC.001G068600 | App1 | NC | 2.68 | 2.60 |
| SOBIC.001G509300 | NA | NC | 2.66 | 2.24 |
| SOBIC.001G174100 | GRAS family transcription factor containing protein | Transcription factor | 2.60 | 2.24 |
| SOBIC.004G321800 | Saccharopine dehydrogenase | Amino acid metabolism | 2.58 | 2.09 |
| SOBIC.002G247900 | Hypothetical protein | NC | 2.50 | 3.27 |
| SOBIC.004G192800 | Mitochondrial prohibitin complex protein 1 | Stress/abiotic | 2.39 | 2.39 |
| SOBIC.009G080400 | Hydroquinone glucosyltransferase | Secondary metabolism | 2.38 | 2.33 |
| SOBIC.006G097800 | Anthocyanin 5-O-glucosyltransferase | Hormone metabolism | 2.37 | 2.01 |
| SOBIC.004G088551 | NA | NC | 2.37 | 2.11 |
| SOBIC.002G393100 | Dehydrogenase/reductase | Misc. (dehydrogenase/reductase) | 2.32 | 2.42 |
| SOBIC.007G108800 | NA | NC | 2.29 | 2.70 |
| SOBIC.002G089600 | DEF8 - Defensin and Defensin-like DEFL family | NC | 2.28 | 2.35 |
| SOBIC.001G170301 | GASR2 - Gibberellin-regulated GASA/GAST/Snakin | Hormone Metabolism | 2.24 | 2.14 |
| SOBIC.005G103600 | NA | NC | 2.17 | 2.12 |
| SOBIC.009G171400 | Oxidoreductase, aldo/keto reductase family protein | Carbohydrate metabolism | 2.12 | 2.28 |
| SOBIC.001G482800 | ZIM domain containing protein | NC | 2.07 | 2.16 |
| SOBIC.002G043400 | Erythrocyte binding protein | NC | 2.04 | 2.35 |
| SOBIC.009G080300 | Uncharacterized protein KIAA1310 | NC | −2.00 | −2.19 |
| SOBIC.006G028000 | Amidase | Misc. (nitrile lyase) | −2.05 | −2.72 |
| SOBIC.004G110200 | Zinc finger, C3HC4 type domain containing protein | Transcription factor | −2.12 | −2.56 |
| SOBIC.002G115600 | Membrane protein | NC | −2.16 | −2.29 |
| SOBIC.001G232200 | Glycine-rich cell wall structural protein 2 precursor | Cell wall | −2.21 | −6.49 |
| SOBIC.005G163800 | Laccase precursor protein | Secondary metabolism | −2.23 | −3.17 |
| SOBIC.002G286400 | Uncharacterized protein PA4923 | Amino acid metabolism | −2.31 | −2.10 |
| SOBIC.002G063700 | NB-ARC domain containing protein | Stress/biotic | −2.35 | −3.04 |
| SOBIC.008G158400 | LTPL116 - Protease inhibitor/seed storage protein | Misc. (seed storage protein) | −2.40 | −5.38 |
| SOBIC.002G163200 | NA | NC | −2.45 | −3.11 |
| SOBIC.008G158600 | LTPL116 - Protease inhibitor/seed storage protein | Misc. (seed storage protein) | −2.47 | −2.64 |
| SOBIC.008G162700 | Fatty acid hydroxylase | Lipid metabolism | −2.51 | −2.00 |
| SOBIC.005G195400 | Helix-loop-helix DNA-binding domain containing-P | Transcription factor | −2.66 | −2.47 |
| SOBIC.002G286566 | Carrier | NC | −2.67 | −2.30 |
| SOBIC.008G158501 | LTPL116 - Protease inhibitor/seed storage protein | Misc. (seed storage protein) | −2.72 | −3.93 |
| SOBIC.005G086600 | O-methyltransferase | Secondary metabolism | −2.75 | −3.16 |
| SOBIC.004G180700 | NA | Misc. (oxidase/copper) | −2.77 | −2.52 |
| SOBIC.001G317800 | Glutathione S-transferase | Misc. (Oxidoreductase) | −2.91 | −2.94 |
| SOBIC.008G158266 | NA | NC | −3.03 | −6.59 |
| SOBIC.005G002700 | Fatty acid desaturase, putative | Lipid metabolism | −3.11 | −2.11 |
| SOBIC.008G036800 | Chalcone synthase | Secondary metabolism | −3.15 | −2.35 |
| SOBIC.003G278100 | Expressed protein | NC | −3.20 | −2.45 |
| SOBIC.001G232500 | Glycine-rich cell wall structural protein 2 precursor | Cell wall | −3.23 | −3.79 |
| SOBIC.004G260600 | Fatty acid desaturase | Lipid metabolism | −3.26 | −3.19 |
| SOBIC.010G156500 | NA | NC | −3.29 | −3.99 |
| SOBIC.006G096100 | Oxidoreductase, aldo/keto reductase family protein | Secondary metabolism | −3.58 | −8.28 |
| SOBIC.004G228600 | NA | NC | −3.78 | −8.33 |
| SOBIC.005G215000 | Cytochrome P450 | Misc. (Cytochrome P450) | −3.80 | −2.69 |
| SOBIC.003G059900 | Expansin precursor | Cell wall modification | −3.82 | −2.42 |
| SOBIC.008G158900 | Chitinase 2 | NC | −3.88 | −2.63 |
| SOBIC.002G087200 | Osfbx231 - F-box domain containing protein | NC | −3.99 | −6.20 |
| SOBIC.001G502000 | Inorganic phosphate transporter | Transport/Phosphate | −4.05 | −2.21 |
| SOBIC.007G059200 | Transferase family protein | Secondary metabolism | −4.62 | 2.54 |
| SOBIC.009G158500 | NA | Signaling | −4.75 | −2.79 |
| SOBIC.010G044001 | NA | NC | −6.16 | −3.26 |
| SOBIC.008G158332 | LTPL116 - Protease inhibitor/seed storage protein | Misc. (seed storage protein) | −6.38 | −5.31 |
| SOBIC.002G228800 | Expressed protein | NC | −6.63 | −7.25 |
| SOBIC.001G403350 | NA | NC | −6.91 | −2.68 |
| SOBIC.009G244600 | MYB family transcription factor | RNA/Transcription | −7.01 | −8.60 |
| SOBIC.003G437900 | Decarboxylase | Amino acid metabolism | −7.50 | −5.70 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Ghany, S.E.; Ullah, F.; Ben-Hur, A.; Reddy, A.S.N. Transcriptome Analysis of Drought-Resistant and Drought-Sensitive Sorghum (Sorghum bicolor) Genotypes in Response to PEG-Induced Drought Stress. Int. J. Mol. Sci. 2020, 21, 772. https://doi.org/10.3390/ijms21030772
Abdel-Ghany SE, Ullah F, Ben-Hur A, Reddy ASN. Transcriptome Analysis of Drought-Resistant and Drought-Sensitive Sorghum (Sorghum bicolor) Genotypes in Response to PEG-Induced Drought Stress. International Journal of Molecular Sciences. 2020; 21(3):772. https://doi.org/10.3390/ijms21030772
Chicago/Turabian StyleAbdel-Ghany, Salah E., Fahad Ullah, Asa Ben-Hur, and Anireddy S. N. Reddy. 2020. "Transcriptome Analysis of Drought-Resistant and Drought-Sensitive Sorghum (Sorghum bicolor) Genotypes in Response to PEG-Induced Drought Stress" International Journal of Molecular Sciences 21, no. 3: 772. https://doi.org/10.3390/ijms21030772
APA StyleAbdel-Ghany, S. E., Ullah, F., Ben-Hur, A., & Reddy, A. S. N. (2020). Transcriptome Analysis of Drought-Resistant and Drought-Sensitive Sorghum (Sorghum bicolor) Genotypes in Response to PEG-Induced Drought Stress. International Journal of Molecular Sciences, 21(3), 772. https://doi.org/10.3390/ijms21030772






