Advances in HDL: Much More than Lipid Transporters
Abstract
1. Introduction
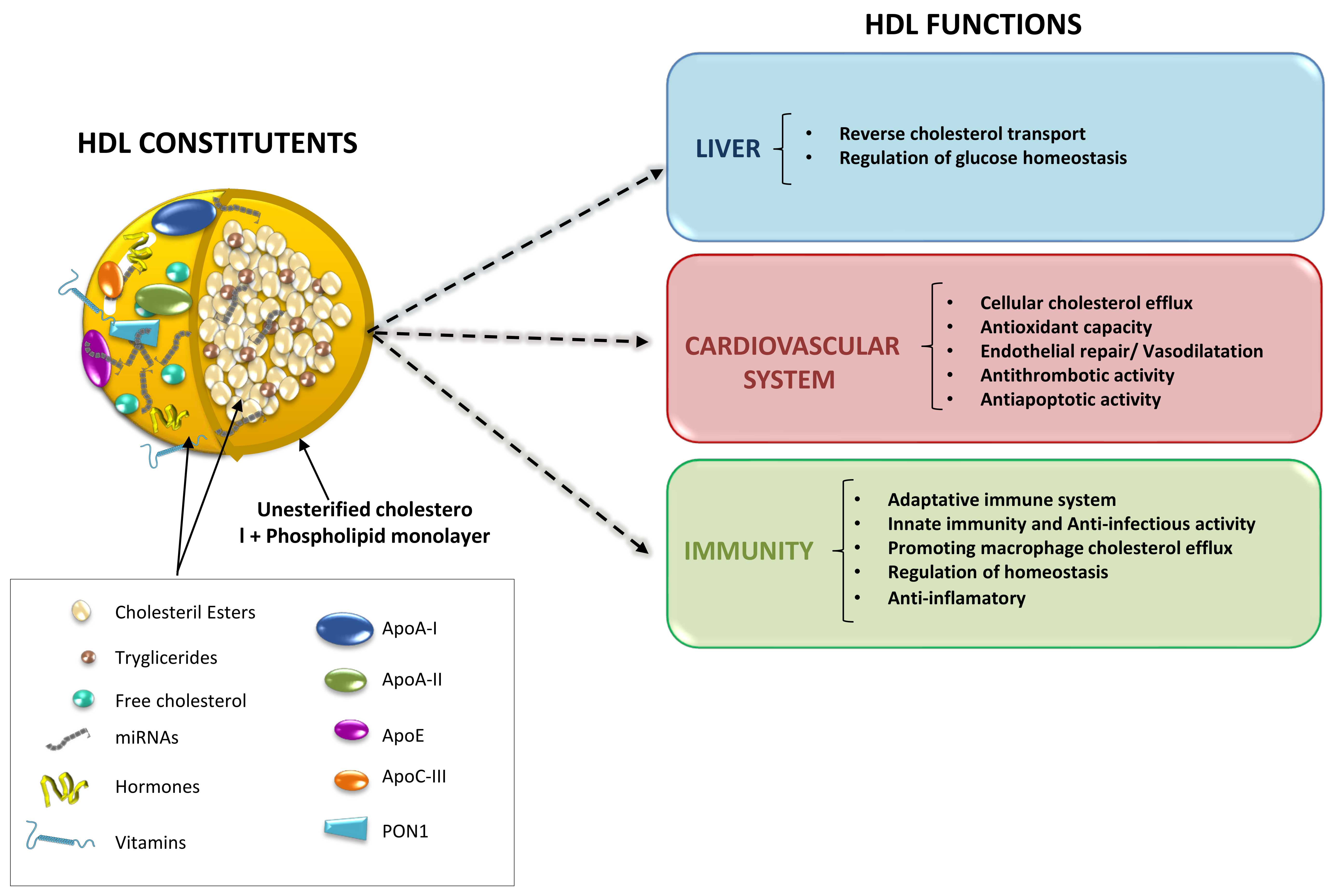
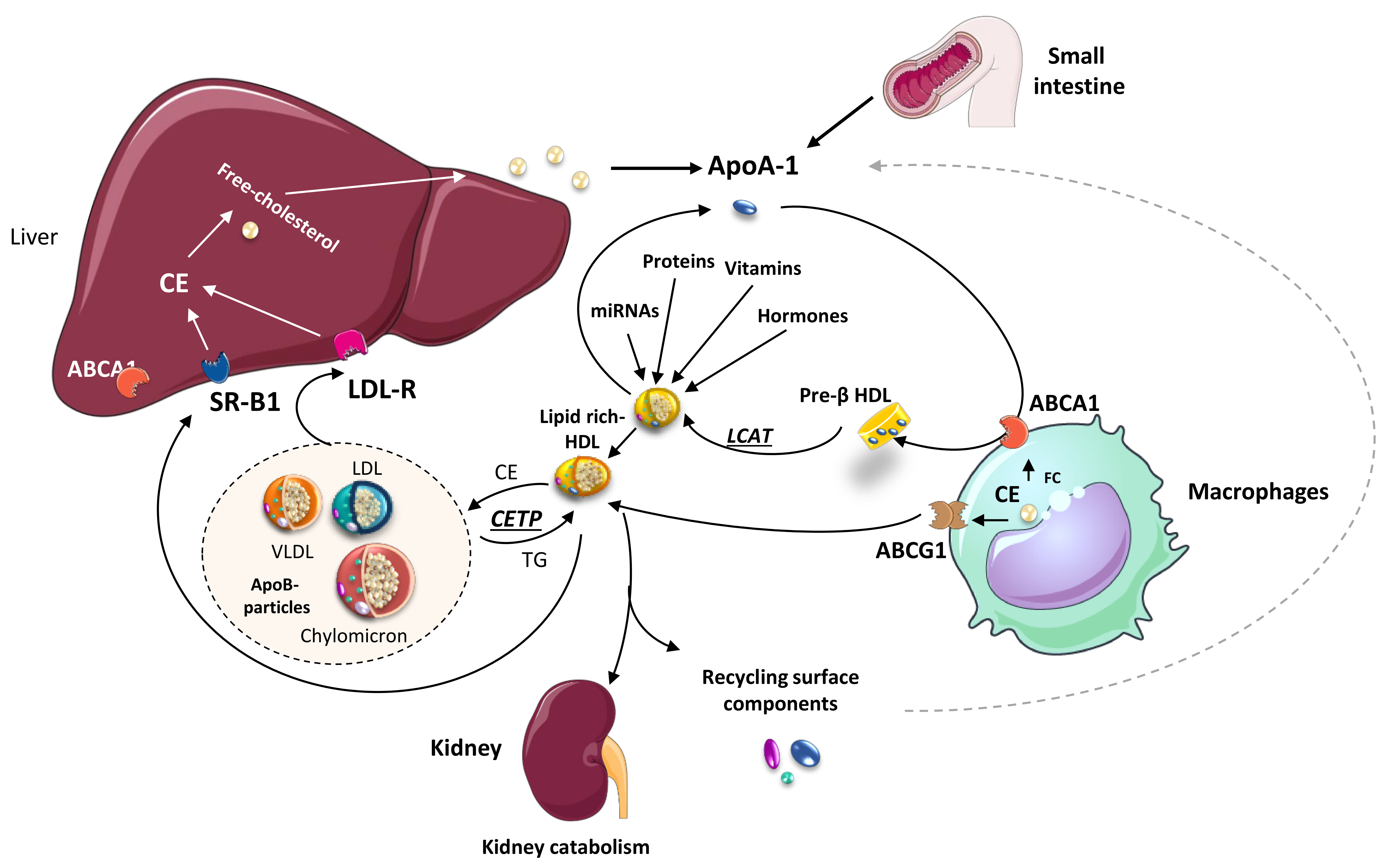
2. Molecules Carried by HDL Particles
3. Protective Effects of HDL Particles beyond Lipid Removal
3.1. Antioxidant and Anti-Inflammatory Effects
3.2. Protection against Ischemia/Reperfusion
3.3. Endothelial Progenitor Cell Recruitment
3.4. Antithrombotic Effects
3.5. Immunomodulatory Properties
4. Impact of Cardiovascular Risk Factor and Co-Morbidities on HDL Composition and Function
5. HDL-Based Approaches
5.1. HDL-Mimetics: Composition and Characteristics
| HDL-Mimetic | Composition | Mechanism of Action | Experimental Design | Added Capacity (Compared to Native HDLs) | Reference |
|---|---|---|---|---|---|
| ETC-216 | Human recombinant Apolipoprotein A-I Milano | ABCA1 | Stopped ClinicalTrials.gov Identifier: NCT02678923 | __ | [129] |
| MDCO-216 | Human recombinant Apolipoprotein A-I Milano | ABCA1 | Phase 2 clinical trial: ClinicalTrials.gov Identifier: NCT02678923 | Increases ABCA1-mediated cholesterol efflux and pre-beta 1 HDL. | [130] |
| CSL112 | Human plasma-derived apolipoprotein A-I (apoA-I) | ABCA1 | Phase 2a in stable atherothrombotic patients and Phase 2b for patients with acute MI: AEGIS-I trial ClinicalTrials.gov NCT02108262 | Increases cholesterol efflux capacity | [131] |
| rHDL-apoA-I | Human plasma-derived apolipoprotein A-I (apoA-I) | ABCA1 | Phase 2 on atherosclerosis ClinicalTrials.gov Identifier: NCT00225719 | Increases cholesterol efflux capacity | [132,133] |
| CER-001 | Lipoprotein complex mimicking discoidal pre-β HDL, consisting of recombinant human apoA-I | ABCA1 | Phase 2 studies: CHI-SQUARE and CARAT trials clinicaltrials.gov Identifier: NCT01201837 and NCT02484378 respectively | Can rapidly mobilise large amounts of cholesterol into the HDL fraction | [134,135] |
| Nanolipoprotein Particles (NLPs) | Phospholipid bilayer stabilized by an apolipoprotein scaffold protein | ABCA1 | Initial in vitro state | Enhanced particle stability | [114] |
| sHDL-T1317 | sHDL apoA-I peptide+A synthetic LXR agonist, T0901317 (T1317) | ABCA1 | Preclinical studies | Upregulates the expression of ATP-binding cassette transporters and increases cholesterol efflux in macrophages in vitro and in vivo. | [136] |
| ApoE-Based rHDL | rHDL particles containing ApoE3 | ABCA1 and LCAT | Phase 1 in China, preclinical studies in Europe | Enhances endosomal/lysosomal escape capacity | [137,138,139] |
| rHDL-DiR-BOA | Mimicking peptide phospholipid scaffold (HPPS) | SR-B1 | Initial in vitro state | Endosomal/lysosomal avoidance capacity which makes a highly biocompatible, exhibited long circulation half-life in serum nanocarrier | [140] |
| cp-rHDL | rHDL+cell penetrating peptides | __ | Initial in vitro state | Easily overcome the cellular plasma membrane | [141,142] |
| Receptor-Mediated rHDL Cellular Internalization | rHDL+cell receptor signalling structures | SR-B1 | Initial in vitro state | Enhances the accumulation of nanoparticles and increased uptaking | [143,144] |
| rHDL-siRNA | rHDL+siRNAs | SR-B1 | Initial in vitro state | Allows directed siRNA delivery | [142] |
| AT-DXS-LP-rHDL | Atorvastatin calcium (AT)-loaded dextran sulfate (DXS)-coated core-shell reconstituted high-density lipoprotein (rHDL) | SR-AI | Initial in vitro state | High-affinity SR-AI as well as depletion of intracellular cholesterol by apoA-I mediated cholesterol efflux | [145] |
| rHDL-AuNP | AuNPs+rHDL+ApoE | Receptor-mediated endocytosis in glioblastoma cells | Initial in vitro state | A platform for transport and delivery of hydrophobic gold nanoparticles | [146] |
| rHDL-rApoJ | phospholipids with recombinant human ApoJ (rApoJ) | Amyloid beta (Aβ) interaction | Preclinical studies | Maintains the ability to prevent the Aβ fibrillation and mediated higher cholesterol efflux from cultured macrophages | [147] |
| sHDL-EL | sHDL+Substrate for plasma endothelial lipase (EL) with useful specificity | Endothelial lipase | Initial in vitro state | Specificity for EL | [148] |
5.2. Potential Clinical Applicability of HDL Mimetics
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Havel, R.J.; Eder, H.A.; Bragdon, J.H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Investig. 1955, 34, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Heinecke, J.W. The HDL proteome: A marker—And perhaps mediator—Of coronary artery disease. J. Lipid Res. 2009, 50, S167–S171. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Vilahur, G. HDL particles—More complex than we thought. Thromb. Haemost. 2014, 112, 857. [Google Scholar] [PubMed]
- Parks, J.S.; Chung, S.; Shelness, G.S. Hepatic ABC transporters and triglyceride metabolism. Curr. Opin. Lipidol. 2012, 23, 196–200. [Google Scholar] [CrossRef]
- Rousset, X.; Vaisman, B.; Amar, M.; Sethi, A.A.; Remaley, A.T. Lecithin: Cholesterol acyltransferase—From biochemistry to role in cardiovascular disease. Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 163–171. [Google Scholar] [CrossRef]
- Hoekstra, M.; Van Berkel, T.-J.; Van Eck, M. Scavenger receptor BI: A multi-purpose player in cholesterol and steroid metabolism. World J. Gastroenterol. 2010, 16, 5916–5924. [Google Scholar]
- Lamarche, B.; Uffelman, K.D.; Steiner, G.; Barrett, P.H.; Lewis, G.F. Analysis of particle size and lipid composition as determinants of the metabolic clearance of human high density lipoproteins in a rabbit model. J. Lipid Res. 1998, 39, 1162–1172. [Google Scholar]
- Barth, J.L.; Argraves, W.S. Cubilin and Megalin: Partners in Lipoprotein and Vitamin Metabolism. Trends Cardiovasc. Med. 2001, 11, 26–31. [Google Scholar] [CrossRef]
- Angeloni, E.; Paneni, F.; Landmesser, U.; Benedetto, U.; Melina, G.; Lüscher, T.F.; Volpe, M.; Sinatra, R.; Cosentino, F. Lack of protective role of HDL-C in patients with coronary artery disease undergoing elective coronary artery bypass grafting. Eur. Heart J. 2013, 34, 3557–3562. [Google Scholar] [CrossRef]
- Keene, D.; Price, C.; Shun-Shin, M.J.; Francis, D.P. Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: Meta-analysis of randomised controlled trials including 117,411 patients. BMJ 2014, 349, g4379. [Google Scholar] [CrossRef]
- Armitage, J.; Holmes, M.V.; Preiss, D. Cholesteryl Ester Transfer Protein Inhibition for Preventing Cardiovascular Events: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.; Tan, L.; Long, J.L.; Davidson, W.S. Proteomic diversity of high density lipoproteins: Our emerging understanding of its importance in lipid transport and beyond. J. Lipid Res. 2013, 54, 2575–2585. [Google Scholar] [CrossRef] [PubMed]
- Padró, T.; Cubedo, J.; Camino, S.; Béjar, M.T.; Ben-Aicha, S.; Mendieta, G.; Escolà-Gil, J.C.; Escate, R.; Gutiérrez, M.; Casani, L.; et al. Detrimental Effect of Hypercholesterolemia on High-Density Lipoprotein Particle Remodeling in Pigs. J. Am. Coll. Cardiol. 2017, 70, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Rader, D.J. Molecular regulation of HDL metabolism and function: Implications for novel therapies. J. Clin. Investig. 2006, 116, 3090–3100. [Google Scholar] [CrossRef]
- Okada, T.; Ohama, T.; Takafuji, K.; Kanno, K.; Matsuda, H.; Sairyo, M.; Zhu, Y.; Saga, A.; Kobayashi, T.; Masuda, D.; et al. Shotgun proteomic analysis reveals proteome alterations in HDL of patients with cholesteryl ester transfer protein deficiency. J. Clin. Lipidol. 2019, 13, 317–325. [Google Scholar] [CrossRef]
- Kontush, A.; Lhomme, M.; Chapman, M.J. Unraveling the complexities of the HDL lipidome. J. Lipid Res. 2013, 54, 2950–2963. [Google Scholar] [CrossRef]
- Vickers, K.C.; Remaley, A.T. HDL and cholesterol: Life after the divorce? J. Lipid Res. 2014, 55, 4–12. [Google Scholar] [CrossRef]
- Sulaiman, W.N.W.; Caslake, M.J.; Delles, C.; Karlsson, H.; Mulder, M.T.; Graham, D.; Freeman, D.J. Does high-density lipoprotein protect vascular function in healthy pregnancy? Clin. Sci. 2016, 130, 491–497. [Google Scholar] [CrossRef][Green Version]
- van Tienhoven-Wind, L.J.N.; Dullaart, R.P.F. Low-normal thyroid function and the pathogenesis of common cardio-metabolic disorders. Eur. J. Clin. Investig. 2015, 45, 494–503. [Google Scholar] [CrossRef]
- Harrison, E.H. Mechanisms of Transport and Delivery of Vitamin A and Carotenoids to the Retinal Pigment Epithelium. Mol. Nutr. Food Res. 2019, 63, 1801046. [Google Scholar] [CrossRef]
- Thomas, S.E.; Harrison, E.H. Mechanisms of selective delivery of xanthophylls to retinal pigment epithelial cells by human lipoproteins. J. Lipid Res. 2016, 57, 1865–1878. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.B.; Zhu, S.S.; Luo, F.; Li, W.Q.; Sun, X.R.; Wu, H.F. Effects of Astaxanthin on Reverse Cholesterol Transport and Atherosclerosis in Mice. BioMed Res. Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.H.; Navab, M.; Dwyer, K.M.; Hassan, K.; Sun, P.; Shircore, A.; Hama-Levy, S.; Hough, G.; Wang, X.; Drake, T.; et al. Oxygenated carotenoid lutein and progression of early atherosclerosis: The Los Angeles atherosclerosis study. Circulation 2001, 103, 2922–2927. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhang, J.; Xu, N.; Han, G.; Geng, Q.; Song, J.; Li, S.; Zhao, J.; Chen, H. Signature of circulating microRNAs as potential biomarkers in vulnerable coronary artery disease. PLoS ONE 2013, 8, e80738. [Google Scholar] [CrossRef]
- Wagner, J.; Riwanto, M.; Besler, C.; Knau, A.; Fichtlscherer, S.; Roxe, T.; Zeiher, A.M.; Landmesser, U.; Dimmeler, S. Characterization of Levels and Cellular Transfer of Circulating Lipoprotein-Bound MicroRNAs. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1392–1400. [Google Scholar] [CrossRef]
- Vickers, K.C.; Palmisano, B.T.; Shoucri, B.M.; Shamburek, R.D.; Remaley, A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 2011, 13, 423–433. [Google Scholar] [CrossRef]
- Vickers, K.C.; Landstreet, S.R.; Levin, M.G.; Shoucri, B.M.; Toth, C.L.; Taylor, R.C.; Palmisano, B.T.; Tabet, F.; Cui, H.L.; Rye, K.-A.; et al. MicroRNA-223 coordinates cholesterol homeostasis. Proc. Natl. Acad. Sci. USA 2014, 111, 14518–14523. [Google Scholar] [CrossRef]
- Ono, K. Functions of microRNA-33a/b and microRNA therapeutics. J. Cardiol. 2016, 67, 28–33. [Google Scholar] [CrossRef]
- Wilson, P.W.; Garrison, R.J.; Castelli, W.P.; Feinleib, M.; McNamara, P.M.; Kannel, W.B. Prevalence of coronary heart disease in the Framingham Offspring Study: Role of lipoprotein cholesterols. Am. J. Cardiol. 1980, 46, 649–654. [Google Scholar] [CrossRef]
- Hernáez, Á.; Castañer, O.; Elosua, R.; Pintó, X.; Estruch, R.; Salas-Salvadó, J.; Corella, D.; Arós, F.; Serra-Majem, L.; Fiol, M.; et al. Mediterranean Diet Improves High-Density Lipoprotein Function in High-Cardiovascular-Risk Individuals. Circulation 2017, 135, 633–643. [Google Scholar] [CrossRef]
- Tuteja, S.; Rader, D.J. High-Density Lipoproteins in the Prevention of Cardiovascular Disease: Changing the Paradigm. Clin. Pharmacol. Ther. 2014, 96, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Rye, K.-A.; Barter, P.J. Cardioprotective functions of HDLs. J. Lipid Res. 2014, 55, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Rader, D.J.; Hovingh, G.K. HDL and cardiovascular disease. Lancet 2014, 384, 618–625. [Google Scholar] [CrossRef]
- Feingold, K.R.; Grunfeld, C. Introduction to Lipids and Lipoproteins; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Allard-Ratick, M.P.; Kindya, B.R.; Khambhati, J.; Engels, M.C.; Sandesara, P.B.; Rosenson, R.S.; Sperling, L.S. HDL: Fact, fiction, or function? HDL cholesterol and cardiovascular risk. Eur. J. Prev. Cardiol. 2019. [Google Scholar] [CrossRef]
- Kontush, A.; Chapman, M.J. Antiatherogenic function of HDL particle subpopulations: Focus on antioxidative activities. Curr. Opin. Lipidol. 2010, 21, 312–318. [Google Scholar] [CrossRef]
- Barter, P.J.; Nicholls, S.; Rye, K.-A.; Anantharamaiah, G.M.; Navab, M.; Fogelman, A.M. Antiinflammatory Properties of HDL. Circ. Res. 2004, 95, 764–772. [Google Scholar] [CrossRef]
- Vilahur, G. High-density lipoprotein benefits beyond the cardiovascular system: A potential key role for modulating acquired immunity through cholesterol efflux. Cardiovasc. Res. 2017, 113, e51–e53. [Google Scholar] [CrossRef][Green Version]
- Riwanto, M.; Landmesser, U. High density lipoproteins and endothelial functions: Mechanistic insights and alterations in cardiovascular disease. J. Lipid Res. 2013, 54, 3227–3243. [Google Scholar] [CrossRef]
- Besler, C.; Heinrich, K.; Rohrer, L.; Doerries, C.; Riwanto, M.; Shih, D.M.; Chroni, A.; Yonekawa, K.; Stein, S.; Schaefer, N.; et al. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J. Clin. Investig. 2011, 121, 2693–2708. [Google Scholar] [CrossRef]
- Kontush, A.; Chapman, M.J. Functionally defective high-density lipoprotein: A new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol. Rev. 2006, 58, 342–374. [Google Scholar] [CrossRef]
- Rohatgi, A.; Khera, A.; Berry, J.D.; Givens, E.G.; Ayers, C.R.; Wedin, K.E.; Neeland, I.J.; Yuhanna, I.S.; Rader, D.R.; De Lemos, J.A.; et al. HDL cholesterol efflux capacity and incident cardiovascular events. N. Engl. J. Med. 2014, 371, 2383–2393. [Google Scholar] [CrossRef] [PubMed]
- Riwanto, M.; Rohrer, L.; Roschitzki, B.; Besler, C.; Mocharla, P.; Mueller, M.; Perisa, D.; Heinrich, K.; Altwegg, L.; von Eckardstein, A.; et al. Altered Activation of Endothelial Anti- and Proapoptotic Pathways by High-Density Lipoprotein from Patients with Coronary Artery Disease: Role of High-Density Lipoprotein-Proteome Remodeling. Circulation 2013, 127, 891–904. [Google Scholar] [CrossRef] [PubMed]
- Charakida, M.; Besler, C.; Batuca, J.R.; Sangle, S.; Marques, S.; Sousa, M.; Wang, G.; Tousoulis, D.; Delgado Alves, J.; Loukogeorgakis, S.P.; et al. Vascular abnormalities, paraoxonase activity, and dysfunctional HDL in primary antiphospholipid syndrome. JAMA 2009, 302, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Afonso, C.B.; Spickett, C.M. Lipoproteins as targets and markers of lipoxidation. Redox Biol. 2018, 23, 101066. [Google Scholar] [CrossRef]
- Sorrentino, S.A.; Besler, C.; Rohrer, L.; Meyer, M.; Heinrich, K.; Bahlmann, F.H.; Mueller, M.; Horváth, T.; Doerries, C.; Heinemann, M.; et al. Endothelial-vasoprotective effects of high-density lipoprotein are impaired in patients with type 2 diabetes mellitus but are improved after extended-release niacin therapy. Circulation 2010, 121, 110–122. [Google Scholar] [CrossRef]
- Cimmino, G.; Ibanez, B.; Vilahur, G.; Speidl, W.S.; Fuster, V.; Badimon, L.; Badimon, J.J. Up-regulation of reverse cholesterol transport key players and rescue from global inflammation by ApoA-IMilano. J. Cell. Mol. Med. 2009, 13, 3226–3235. [Google Scholar] [CrossRef]
- Mackness, M.; Mackness, B. Human paraoxonase-1 (PON1): Gene structure and expression, promiscuous activities and multiple physiological roles. Gene 2015, 567, 12–21. [Google Scholar] [CrossRef]
- Mineo, C.; Deguchi, H.; Griffin, J.H.; Shaul, P.W. Endothelial and Antithrombotic Actions of HDL. Circ. Res. 2006, 98, 1352–1364. [Google Scholar] [CrossRef]
- Tabet, F.; Vickers, K.C.; Cuesta Torres, L.F.; Wiese, C.B.; Shoucri, B.M.; Lambert, G.; Catherinet, C.; Prado-Lourenco, L.; Levin, M.G.; Thacker, S.; et al. HDL-transferred microRNA-223 regulates ICAM-1 expression in endothelial cells. Nat. Commun. 2014, 5, 3292. [Google Scholar] [CrossRef]
- Vilahur, G.; Juan-Babot, O.; Peña, E.; Oñate, B.; Casaní, L.; Badimon, L. Molecular and cellular mechanisms involved in cardiac remodeling after acute myocardial infarction. J. Mol. Cell. Cardiol. 2011, 50, 522–533. [Google Scholar] [CrossRef]
- Carden, D.L.; Granger, D.N. Pathophysiology of ischaemia-reperfusion injury. J. Pathol. 2000, 190, 255–266. [Google Scholar] [CrossRef]
- Theilmeier, G.; Schmidt, C.; Herrmann, J.; Keul, P.; Schäfers, M.; Herrgott, I.; Mersmann, J.; Larmann, J.; Hermann, S.; Stypmann, J.; et al. High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation 2006, 114, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Gomaraschi, M.; Calabresi, L.; Franceschini, G. Protective Effects of HDL Against Ischemia/Reperfusion Injury. Front. Pharmacol. 2016, 7, 2. [Google Scholar] [CrossRef] [PubMed][Green Version]
- James, R.W.; Frias, M.A. High density lipoproteins and ischemia reperfusion injury: The therapeutic potential of HDL to modulate cell survival pathways. Adv. Exp. Med. Biol. 2014, 824, 19–26. [Google Scholar] [PubMed]
- Vilahur, G.; Gutiérrez, M.; Casaní, L.; Cubedo, J.; Capdevila, A.; Pons-Llado, G.; Carreras, F.; Hidalgo, A.; Badimon, L. Hypercholesterolemia Abolishes High-Density Lipoprotein–Related Cardioprotective Effects in the Setting of Myocardial Infarction. J. Am. Coll. Cardiol. 2015, 66, 2469–2470. [Google Scholar] [CrossRef]
- Wang, X.; Wang, F. Vascular protection by high-density lipoprotein-associated sphingosine-1-phosphate. J. Geriatr. Cardiol. 2017, 14, 696–702. [Google Scholar]
- Swendeman, S.L.; Xiong, Y.; Cantalupo, A.; Yuan, H.; Burg, N.; Hisano, Y.; Cartier, A.; Liu, C.H.; Engelbrecht, E.; Blaho, V.; et al. An engineered S1P chaperone attenuates hypertension and ischemic injury. Sci. Signal. 2017, 10, eaal2722. [Google Scholar] [CrossRef]
- Santos-Gallego, C.G.; Vahl, T.P.; Goliasch, G.; Picatoste, B.; Arias, T.; Ishikawa, K.; Njerve, I.U.; Sanz, J.; Narula, J.; Sengupta, P.P.; et al. Sphingosine-1-Phosphate Receptor Agonist Fingolimod Increases Myocardial Salvage and Decreases Adverse Postinfarction Left Ventricular Remodeling in a Porcine Model of Ischemia/Reperfusion. Circulation 2016, 133, 954–966. [Google Scholar] [CrossRef]
- Michell, D.L.; Vickers, K.C. Lipoprotein carriers of microRNAs. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2016, 1861, 2069–2074. [Google Scholar] [CrossRef]
- Rogg, E.M.; Abplanalp, W.T.; Bischof, C.; John, D.; Schulz, M.H.; Krishnan, J.; Fischer, A.; Poluzzi, C.; Schaefer, L.; Bonauer, A.; et al. Analysis of Cell Type-Specific Effects of MicroRNA-92a Provides Novel Insights into Target Regulation and Mechanism of Action. Circulation 2018, 138, 2545–2558. [Google Scholar] [CrossRef]
- Niculescu, L.S.; Simionescu, N.; Sanda, G.M.; Carnuta, M.G.; Stancu, C.S.; Popescu, A.C.; Popescu, M.R.; Vlad, A.; Dimulescu, D.R.; Simionescu, M.; et al. MiR-486 and miR-92a Identified in Circulating HDL Discriminate between Stable and Vulnerable Coronary Artery Disease Patients. PLoS ONE 2015, 10, e0140958. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.H.; Wang, X.; Zhang, Y.; Hui, J. Exosomes of bone-marrow stromal cells inhibit cardiomyocyte apoptosis under ischemic and hypoxic conditions via miR-486-5p targeting the PTEN/PI3K/AKT signaling pathway. Thromb. Res. 2019, 177, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Rink, J.S.; Sun, W.; Misener, S.; Wang, J.-J.; Zhang, Z.J.; Kibbe, M.R.; Dravid, V.P.; Venkatraman, S.; Thaxton, C.S. Nitric Oxide-Delivering High-Density Lipoprotein-like Nanoparticles as a Biomimetic Nanotherapy for Vascular Diseases. ACS Appl. Mater. Interfaces 2018, 10, 6904–6916. [Google Scholar] [CrossRef] [PubMed]
- Besler, C.; Heinrich, K.; Riwanto, M.; Lüscher, T.F.; Landmesser, U. High-density lipoprotein-mediated anti-atherosclerotic and endothelial-protective effects: A potential novel therapeutic target in cardiovascular disease. Curr. Pharm. Des. 2010, 16, 1480–1493. [Google Scholar] [CrossRef] [PubMed]
- Tso, C.; Martinic, G.; Fan, W.-H.; Rogers, C.; Rye, K.-A.; Barter, P.J. High-density lipoproteins enhance progenitor-mediated endothelium repair in mice. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1144–1149. [Google Scholar] [CrossRef]
- Noor, R.; Shuaib, U.; Wang, C.X.; Todd, K.; Ghani, U.; Schwindt, B.; Shuaib, A. High-density lipoprotein cholesterol regulates endothelial progenitor cells by increasing eNOS and preventing apoptosis. Atherosclerosis 2007, 192, 92–99. [Google Scholar] [CrossRef]
- Vanags, L.Z.; Tan, J.T.M.; Galougahi, K.K.; Schaefer, A.; Wise, S.G.; Murphy, A.; Ali, Z.A.; Bursill, C.A. Apolipoprotein A-I Reduces In-Stent Restenosis and Platelet Activation and Alters Neointimal Cellular Phenotype. JACC Basic Transl. Sci. 2018, 3, 200–209. [Google Scholar] [CrossRef]
- Van Der Vorst, E.P.C.; Vanags, L.Z.; Dunn, L.L.; Prosser, H.C.; Rye, K.A.; Bursill, C.A. High-density lipoproteins suppress chemokine expression and proliferation in human vascular smooth muscle cells. FASEB J. 2013, 27, 1413–1425. [Google Scholar] [CrossRef]
- Vanags, L.Z.; Wong, N.K.P.; Nicholls, S.J.; Bursill, C.A. High-Density Lipoproteins and Apolipoprotein A-I Improve Stent Biocompatibility. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1691–1701. [Google Scholar] [CrossRef]
- Yuhanna, I.S.; Zhu, Y.; Cox, B.E.; Hahner, L.D.; Osborne-Lawrence, S.; Lu, P.; Marcel, Y.L.; Anderson, R.G.; Mendelsohn, M.E.; Hobbs, H.H.; et al. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat. Med. 2001, 7, 853–857. [Google Scholar] [CrossRef]
- Wu, J.; He, Z.; Gao, X.; Wu, F.; Ding, R.; Ren, Y.; Jiang, Q.; Fan, M.; Liang, C.; Wu, Z. Oxidized high-density lipoprotein impairs endothelial progenitor cells’ function by activation of CD36-MAPK-TSP-1 pathways. Antioxid. Redox Signal. 2015, 22, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Holy, E.W.; Besler, C.; Reiner, M.F.; Camici, G.G.; Manz, J.; Beer, J.H.; Lüscher, T.F.; Landmesser, U.; Tanner, F.C. High-density lipoprotein from patients with coronary heart disease loses anti-thrombotic effects on endothelial cells: Impact on arterial thrombus formation. Thromb. Haemost. 2014, 112, 1024–1035. [Google Scholar] [CrossRef] [PubMed]
- van der Stoep, M.; Korporaal, S.J.A.; Van Eck, M. High-density lipoprotein as a modulator of platelet and coagulation responses. Cardiovasc. Res. 2014, 103, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Szabó, M.Z.; Szodoray, P.; Kiss, E. Dyslipidemia in systemic lupus erythematosus. Immunol. Res. 2017, 65, 543–550. [Google Scholar] [CrossRef] [PubMed]
- van der Valk, F.M.; Bernelot Moens, S.J.; Verweij, S.L.; Strang, A.C.; Nederveen, A.J.; Verberne, H.J.; Nurmohamed, M.T.; Baeten, D.L.; Stroes, E.S.G. Increased arterial wall inflammation in patients with ankylosing spondylitis is reduced by statin therapy. Ann. Rheum. Dis. 2016, 75, 1848–1851. [Google Scholar] [CrossRef] [PubMed]
- Connelly, M.A.; Shalaurova, I.; Otvos Raleigh, J.D. High-density lipoprotein and inflammation in cardiovascular disease. Transl. Res. 2016, 173, 7–18. [Google Scholar] [CrossRef]
- Hu, J.; Xi, D.; Zhao, J.; Luo, T.; Liu, J.; Lu, H.; Li, M.; Xiong, H.; Guo, Z. High-density Lipoprotein and Inflammation and Its Significance to Atherosclerosis. Am. J. Med. Sci. 2016, 352, 408–415. [Google Scholar] [CrossRef]
- Larbi, A.; Fortin, C.; Dupuis, G.; Berrougui, H.; Khalil, A.; Fulop, T. Immunomodulatory role of high-density lipoproteins: Impact on immunosenescence. Age 2014, 36, 9712. [Google Scholar] [CrossRef]
- Catapano, A.L.; Pirillo, A.; Bonacina, F.; Norata, G.D. HDL in innate and adaptive immunity. Cardiovasc. Res. 2014, 103, 372–383. [Google Scholar] [CrossRef]
- Yu, B.; Wang, S.; Peng, D.; Zhao, S. HDL and immunomodulation: An emerging role of HDL against atherosclerosis. Immunol. Cell Biol. 2010, 88, 285–290. [Google Scholar] [CrossRef]
- Annema, W.; Willemsen, H.M.; de Boer, J.F.; Dikkers, A.; van der Giet, M.; Nieuwland, W.; Muller Kobold, A.C.; van Pelt, L.J.; Slart, R.H.J.A.; van der Horst, I.C.C.; et al. HDL function is impaired in acute myocardial infarction independent of plasma HDL cholesterol levels. J. Clin. Lipidol. 2016, 10, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Oberbach, A.; Adams, V.; Schlichting, N.; Heinrich, M.; Kullnick, Y.; Lehmann, S.; Lehmann, S.; Feder, S.; Correia, J.C.; Mohr, F.-W.; et al. Proteome profiles of HDL particles of patients with chronic heart failure are associated with immune response and also include bacteria proteins. Clin. Chim. Acta 2016, 453, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Munoz, G.; Couret, D.; Lapergue, B.; Bruckert, E.; Meseguer, E.; Amarenco, P.; Meilhac, O. Dysfunctional HDL in acute stroke. Atherosclerosis 2016, 253, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Zewinger, S.; Kleber, M.E.; Rohrer, L.; Lehmann, M.; Triem, S.; Jennings, R.T.; Petrakis, I.; Dressel, A.; Lepper, P.M.; Scharnagl, H.; et al. Symmetric dimethylarginine, high-density lipoproteins and cardiovascular disease. Eur. Heart J. 2017, 38, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Niesor, E.J.; Schwartz, G.G.; Perez, A.; Stauffer, A.; Durrwell, A.; Bucklar-Suchankova, G.; Benghozi, R.; Abt, M.; Kallend, D. Statin-induced decrease in ATP-binding cassette transporter A1 expression via microRNA33 induction may counteract cholesterol efflux to high-density lipoprotein. Cardiovasc. Drugs Ther. 2015, 29, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Versmissen, J.; Vongpromek, R.; Yahya, R.; van der Net, J.B.; van Vark-van der Zee, L.; Blommesteijn-Touw, J.; Wattimena, D.; Rietveld, T.; Pullinger, C.R.; Christoffersen, C.; et al. Familial hypercholesterolaemia: Cholesterol efflux and coronary disease. Eur. J. Clin. Investig. 2016, 46, 643–650. [Google Scholar] [CrossRef]
- Woudberg, N.J.; Goedecke, J.H.; Blackhurst, D.; Frias, M.; James, R.; Opie, L.H.; Lecour, S. Association between ethnicity and obesity with high-density lipoprotein (HDL) function and subclass distribution. Lipids Health Dis. 2016, 15, 92. [Google Scholar] [CrossRef]
- Davidson, W.S.; Shah, A.S. High-Density Lipoprotein Subspecies in Health and Human Disease: Focus on Type 2 Diabetes. Methodist Debakey Cardiovasc. J. 2019, 15, 55–61. [Google Scholar]
- Hermans, M.P.; Ahn, S.A.; Rousseau, M.F. Crossing family histories of diabetes and cardiovascular disease leads to unexpected outcomes in diabetic offspring. J. Diabetes 2019, 11, 301–308. [Google Scholar] [CrossRef]
- Ruiz-Ramie, J.J.; Barber, J.L.; Sarzynski, M.A. Effects of exercise on HDL functionality. Curr. Opin. Lipidol. 2019, 30, 16–23. [Google Scholar] [CrossRef]
- Pedret, A.; Fernández-Castillejo, S.; Valls, R.-M.; Catalán, Ú.; Rubió, L.; Romeu, M.; Macià, A.; López de las Hazas, M.C.; Farràs, M.; Giralt, M.; et al. Cardiovascular Benefits of Phenol-Enriched Virgin Olive Oils: New Insights from the Virgin Olive Oil and HDL Functionality (VOHF) Study. Mol. Nutr. Food Res. 2018, 62, 1800456. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.V.; Yu, J.; Guo, Y.; Byun, J.; Chen, Y.E.; Wang, L.; Liu, M.; Bard, R.L.; Morishita, M.; Huang, W.; et al. Effect of Ambient Fine Particulate Matter Air Pollution and Colder Outdoor Temperatures on High-Density Lipoprotein Function. Am. J. Cardiol. 2018, 122, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.V.; Cuchel, M.; de la Llera-Moya, M.; Rodrigues, A.; Burke, M.F.; Jafri, K.; French, B.C.; Phillips, J.A.; Mucksavage, M.L.; Wilensky, R.L.; et al. Cholesterol Efflux Capacity, High-Density Lipoprotein Function, and Atherosclerosis. N. Engl. J. Med. 2011, 364, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S.; Brewer, H.B.; Barter, P.J.; Björkegren, J.L.M.; Chapman, M.J.; Gaudet, D.; Kim, D.S.; Niesor, E.; Rye, K.-A.; Sacks, F.M.; et al. HDL and atherosclerotic cardiovascular disease: Genetic insights into complex biology. Nat. Rev. Cardiol. 2018, 15, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, E.J.; Tsunoda, F.; Diffenderfer, M.; Polisecki, E.; Thai, N.; Asztalos, B. The Measurement of Lipids, Lipoproteins, Apolipoproteins, Fatty Acids, and Sterols, and Next Generation Sequencing for the Diagnosis and Treatment of Lipid Disorders. In Endotext; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Kratzer, A.; Jakob, P. Catch miR if you can—Transcoronary gradients of HDL-bound microRNAs. Int. J. Cardiol. 2018, 253, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Tabet, F.; Cuesta Torres, L.F.; Ong, K.L.; Shrestha, S.; Choteau, S.A.; Barter, P.J.; Clifton, P.; Rye, K.-A. High-Density Lipoprotein-Associated miR-223 Is Altered after Diet-Induced Weight Loss in Overweight and Obese Males. PLoS ONE 2016, 11, e0151061. [Google Scholar] [CrossRef]
- Choteau, S.A.; Cuesta Torres, L.F.; Barraclough, J.Y.; Elder, A.M.M.; Martínez, G.J.; Chen Fan, W.Y.; Shrestha, S.; Ong, K.L.; Barter, P.J.; Celermajer, D.S.; et al. Transcoronary gradients of HDL-associated MicroRNAs in unstable coronary artery disease. Int. J. Cardiol. 2018, 253, 138–144. [Google Scholar] [CrossRef]
- Mangat, R.; Borthwick, F.; Haase, T.; Jacome, M.; Nelson, R.; Kontush, A.; Vine, D.F.; Proctor, S.D. Intestinal lymphatic HDL miR-223 and ApoA-I are reduced during insulin resistance and restored with niacin. FASEB J. 2018, 32, 1602–1612. [Google Scholar] [CrossRef]
- Li, K.; Wong, D.K.; Luk, F.S.; Kim, R.Y.; Raffai, R.L. Isolation of Plasma Lipoproteins as a Source of Extracellular RNA. In Extracellular RNA; Humana Press: New York, NY, USA, 2018; Volume 1740, pp. 139–153. [Google Scholar]
- Axmann, M.; Meier, S.M.; Karner, A.; Strobl, W.; Stangl, H.; Plochberger, B. Serum and Lipoprotein Particle miRNA Profile in Uremia Patients. Genes 2018, 9, 533. [Google Scholar] [CrossRef]
- Ben-Aicha, S.; Escate, R.; Casaní, L.; Padró, T.; Peña, E.; Arderiu, G.; Mendieta, G.; Badimón, L.; Vilahur, G. HDL remodelled in hypercholesterolemic blood induce epigenetically driven downregulation of endothelial HIF-1α expression in a preclinical animal model. Cardiovasc. Res. 2019. [Google Scholar] [CrossRef]
- Hermans, M.P.; Amoussou-Guenou, K.D.; Bouenizabila, E.; Sadikot, S.S.; Ahn, S.A.; Rousseau, M.F. Size, density and cholesterol load of HDL predict microangiopathy, coronary artery disease and β-cell function in men with T2DM. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Asztalos, B.F.; Horvath, K.V.; Mehan, M.; Yokota, Y.; Schaefer, E.J. Influence of HDL particles on cell-cholesterol efflux under various pathological conditions. J. Lipid Res. 2017, 58, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Martínez-López, D.; Camafeita, E.; Cedó, L.; Roldan-Montero, R.; Jorge, I.; García-Marqués, F.; Gómez-Serrano, M.; Bonzon-Kulichenko, E.; Blanco-Vaca, F.; Blanco-Colio, L.M.; et al. APOA1 oxidation is associated to dysfunctional high-density lipoproteins in human abdominal aortic aneurysm. EBioMedicine 2019, 43, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, N.; Zacharia, E.; Androulakis, E.; Briasoulis, A.; Charakida, M.; Tousoulis, D. HDL as a prognostic biomarker for coronary atherosclerosis: The role of inflammation. Expert Opin. Ther. Targets 2016, 20, 907–921. [Google Scholar] [CrossRef] [PubMed]
- Cubedo, J.; Padró, T.; García-Moll, X.; Pintó, X.; Cinca, J.; Badimon, L. Proteomic signature of Apolipoprotein J in the early phase of new-onset myocardial infarction. J. Proteome Res. 2011, 10, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Vilahur, G.; Cubedo, J.; Padró, T.; Casaní, L.; Mendieta, G.; González, A.; Badimon, L. Intake of cooked tomato sauce preserves coronary endothelial function and improves apolipoprotein A-I and apolipoprotein J protein profile in high-density lipoproteins. Transl. Res. 2015, 166, 44–56. [Google Scholar] [CrossRef]
- Padro, T.; Muñoz-García, N.; Vilahur, G.; Chagas, P.; Deyà, A.; Antonijoan, R.M.; Badimon, L. Moderate Beer Intake and Cardiovascular Health in Overweight Individuals. Nutrients 2018, 10, 1237. [Google Scholar] [CrossRef]
- Vilahur, G.; Casani, L.; Mendieta, G.; Lamuela-Raventos, R.M.; Estruch, R.; Badimon, L. Beer elicits vasculoprotective effects through Akt/eNOS activation. Eur. J. Clin. Investig. 2014, 44, 1177–1188. [Google Scholar] [CrossRef]
- Vilahur, G.; Casani, L.; Guerra, J.M.; Badimon, L. Intake of fermented beverages protect against acute myocardial injury: Target organ cardiac effects and vasculoprotective effects. Basic Res. Cardiol. 2012, 107, 291. [Google Scholar] [CrossRef]
- Liu, L.; Bortnick, A.E.; Nickel, M.; Dhanasekaran, P.; Subbaiah, P.V.; Lund-Katz, S.; Rothblat, G.H.; Phillips, M.C. Effects of Apolipoprotein A-I on ATP-binding Cassette Transporter A1-mediated Efflux of Macrophage Phospholipid and Cholesterol. J. Biol. Chem. 2003, 278, 42976–42984. [Google Scholar] [CrossRef]
- Gilmore, S.F.; Carpenter, T.S.; Ingólfsson, H.I.; Peters, S.K.G.; Henderson, P.T.; Blanchette, C.D.; Fischer, N.O. Lipid composition dictates serum stability of reconstituted high-density lipoproteins: Implications for in vivo applications. Nanoscale 2018, 10, 7420–7430. [Google Scholar] [CrossRef] [PubMed]
- Van Linthout, S.; Frias, M.; Singh, N.; De Geest, B. Therapeutic Potential of HDL in Cardioprotection and Tissue Repair. In Handbook of Experimental Pharmacology; Springer: Cham, Germany, 2015; Volume 224, pp. 527–565. [Google Scholar]
- Shen, W.-J.; Asthana, S.; Kraemer, F.B.; Azhar, S. Scavenger receptor B type 1: Expression, Molecular Regulation, and Cholesterol Transport Function. J. Lipid Res. 2018, 59, 1114–1131. [Google Scholar] [CrossRef] [PubMed]
- Bricarello, D.A.; Smilowitz, J.T.; Zivkovic, A.M.; German, J.B.; Parikh, A.N. Reconstituted Lipoprotein: A Versatile Class of Biologically-Inspired Nanostructures. ACS Nano 2011, 5, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Vedhachalam, C.; Duong, P.T.; Nickel, M.; Nguyen, D.; Dhanasekaran, P.; Saito, H.; Rothblat, G.H.; Lund-Katz, S.; Phillips, M.C. Mechanism of ATP-binding Cassette Transporter A1-mediated Cellular Lipid Efflux to Apolipoprotein A-I and Formation of High Density Lipoprotein Particles. J. Biol. Chem. 2007, 282, 25123–25130. [Google Scholar] [CrossRef] [PubMed]
- Auton, M.; Bassett, G.R.; Gillard, B.K.; Pownall, H.J. Free Cholesterol Determines Reassembled High-Density Lipoprotein Phospholipid Phase Structure and Stability. Biochemistry 2013, 52, 4324–4330. [Google Scholar] [CrossRef]
- Bassett, G.R.; Gillard, B.K.; Pownall, H.J. Cholesterol Determines and Limits rHDL Formation from Human Plasma Apolipoprotein A-II and Phospholipid Membranes. Biochemistry 2012, 51, 8627–8635. [Google Scholar] [CrossRef]
- Pownall, H.J.; Massey, J.B.; Kusserow, S.K.; Gotto, A.M. Kinetics of lipid-protein interactions: Effect of cholesterol on the association of human plasma high-density apolipoprotein A-I with L-.alpha.-dimyristoylphosphatidylcholine. Biochemistry 1979, 18, 574–579. [Google Scholar] [CrossRef]
- Gilman, T.; Kauffman, J.W.; Pownall, H.J. Raman spectroscopy of the thermal properties of reassembled high-density lipoprotein: Apolipoprotein A-I complexes of dimyristoylphosphatidylcholine. Biochemistry 1981, 20, 656–661. [Google Scholar] [CrossRef]
- Atkinson, D.; Smith, H.M.; Dickson, J.; Austin, J.P. Interaction of apoprotein from porcine high-density lipoprotein with dimyristoyl lecithin: 1. The structure of the complexes. Eur. J. Biochem. 1976, 64, 541–547. [Google Scholar] [CrossRef]
- Tall, A.R.; Small, D.M.; Deckelbaum, R.J.; Shipley, G.G. Structure and thermodynamic properties of high density lipoprotein recombinants. J. Biol. Chem. 1977, 252, 4701–4711. [Google Scholar]
- Jonas, A.; Krajnovich, D.J. Interaction of human and bovine A-1 apolipoproteins with L-alpha-dimyristoyl phosphadicylcholine and L-alpha-myristoyl lysophosphatidylcholine. J. Biol. Chem. 1977, 252, 2194–2199. [Google Scholar] [PubMed]
- Jonas, A. Reconstitution of high-density lipoproteins. Methods Enzymol. 1986, 128, 553–582. [Google Scholar] [PubMed]
- Schwendeman, A.; Sviridov, D.O.; Yuan, W.; Guo, Y.; Morin, E.E.; Yuan, Y.; Stonik, J.; Freeman, L.; Ossoli, A.; Thacker, S.; et al. The effect of phospholipid composition of reconstituted HDL on its cholesterol efflux and anti-inflammatory properties. J. Lipid Res. 2015, 56, 1727–1737. [Google Scholar] [CrossRef] [PubMed]
- Wolfrum, C.; Shi, S.; Jayaprakash, K.N.; Jayaraman, M.; Wang, G.; Pandey, R.K.; Rajeev, K.G.; Nakayama, T.; Charrise, K.; Ndungo, E.M.; et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat. Biotechnol. 2007, 25, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Kallend, D.G.; Reijers, J.A.A.; Bellibas, S.E.; Bobillier, A.; Kempen, H.; Burggraaf, J.; Moerland, M.; Wijngaard, P.L.J. A single infusion of MDCO-216 (ApoA-1 Milano/POPC) increases ABCA1-mediated cholesterol efflux and pre-beta 1 HDL in healthy volunteers and patients with stable coronary artery disease. Eur. Heart J. Cardiovasc. Pharmacother. 2016, 2, 23–29. [Google Scholar] [CrossRef]
- Reijers, J.A.A.; Kallend, D.G.; Malone, K.E.; Jukema, J.W.; Wijngaard, P.L.J.; Burggraaf, J.; Moerland, M. MDCO-216 Does Not Induce Adverse Immunostimulation, in Contrast to Its Predecessor ETC-216. Cardiovasc. Drugs Ther. 2017, 31, 381–389. [Google Scholar] [CrossRef]
- Capodanno, D.; Mehran, R.; Gibson, C.M.; Angiolillo, D.J. CSL112, a reconstituted, infusible, plasma-derived apolipoprotein A-I: Safety and tolerability profiles and implications for management in patients with myocardial infarction. Expert Opin. Investig. Drugs 2018, 27, 997–1005. [Google Scholar] [CrossRef]
- Theofilatos, D.; Fotakis, P.; Valanti, E.; Sanoudou, D.; Zannis, V.; Kardassis, D. HDL-apoA-I induces the expression of angiopoietin like 4 (ANGPTL4) in endothelial cells via a PI3K/AKT/FOXO1 signaling pathway. Metabolism 2018, 87, 36–47. [Google Scholar] [CrossRef]
- Tardif, J.-C.; Grégoire, J.; L’Allier, P.L.; Ibrahim, R.; Lespérance, J.; Heinonen, T.M.; Kouz, S.; Berry, C.; Basser, R.; Lavoie, M.-A.; et al. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: A randomized controlled trial. JAMA 2007, 297, 1675–1682. [Google Scholar] [CrossRef]
- Tardif, J.-C.; Ballantyne, C.M.; Barter, P.; Dasseux, J.-L.; Fayad, Z.A.; Guertin, M.-C.; Kastelein, J.J.P.; Keyserling, C.; Klepp, H.; Koenig, W.; et al. Effects of the high-density lipoprotein mimetic agent CER-001 on coronary atherosclerosis in patients with acute coronary syndromes: A randomized trial. Eur. Heart J. 2014, 35, 3277–3286. [Google Scholar] [CrossRef]
- Andrews, J.; Janssan, A.; Nguyen, T.; Pisaniello, A.D.; Scherer, D.J.; Kastelein, J.J.P.; Merkely, B.; Nissen, S.E.; Ray, K.; Schwartz, G.G.; et al. Effect of serial infusions of reconstituted high-density lipoprotein (CER-001) on coronary atherosclerosis: Rationale and design of the CARAT study. Cardiovasc. Diagn. Ther. 2017, 7, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yuan, W.; Yu, B.; Kuai, R.; Hu, W.; Morin, E.E.; Garcia-Barrio, M.T.; Zhang, J.; Moon, J.J.; Schwendeman, A.; et al. Synthetic High-Density Lipoprotein-Mediated Targeted Delivery of Liver X Receptors Agonist Promotes Atherosclerosis Regression. EBioMedicine 2018, 28, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-L.; Jiang, G.; Song, Q.-X.; Gu, X.; Hu, M.; Wang, X.-L.; Song, H.-H.; Chen, L.-P.; Lin, Y.-Y.; Jiang, D.; et al. Lipoprotein-biomimetic nanostructure enables efficient targeting delivery of siRNA to Ras-activated glioblastoma cells via macropinocytosis. Nat. Commun. 2017, 8, 15144. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Liu, J.; Wang, W.; Wang, M.; Zhao, F.; Sun, J.; Liu, J.; Zhao, D. Apolipoprotein E-containing high-density lipoprotein (HDL) modifies the impact of cholesterol-overloaded HDL on incident coronary heart disease risk: A community-based cohort study. J. Clin. Lipidol. 2018, 12, 89–98.e2. [Google Scholar] [CrossRef]
- Kypreos, K.E.; Zannis, V.I. Pathway of biogenesis of apolipoprotein E-containing HDL in vivo with the participation of ABCA1 and LCAT. Biochem. J. 2007, 403, 359–367. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, W.; Jin, H.; Lovell, J.F.; Yang, M.; Ding, L.; Chen, J.; Corbin, I.; Luo, Q.; Zheng, G. Biomimetic nanocarrier for direct cytosolic drug delivery. Angew. Chem. Int. Ed. Engl. 2009, 48, 9171–9175. [Google Scholar] [CrossRef]
- Gao, H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm. Sin. B 2016, 6, 268–286. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y.; Opoku-Damoah, Y.; Wang, C.; Shen, L.; Yin, L.; Zhou, J. Dual-functional bio-derived nanoparticulates for apoptotic antitumor therapy. Biomaterials 2015, 72, 90–103. [Google Scholar] [CrossRef]
- Rui, M.; Xin, Y.; Li, R.; Ge, Y.; Feng, C.; Xu, X. Targeted Biomimetic Nanoparticles for Synergistic Combination Chemotherapy of Paclitaxel and Doxorubicin. Mol. Pharm. 2017, 14, 107–123. [Google Scholar] [CrossRef]
- Brulhart-Meynet, M.-C.; Braunersreuther, V.; Brinck, J.; Montecucco, F.; Prost, J.-C.; Thomas, A.; Galan, K.; Pelli, G.; Pedretti, S.; Vuilleumier, N.; et al. Improving reconstituted HDL composition for efficient post-ischemic reduction of ischemia reperfusion injury. PLoS ONE 2015, 10, e0119664. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, C.; He, J.; Guo, Q.; Lu, J.; Yang, Y.; Zhang, W.; Liu, J. Multifunctional Dextran Sulfate-Coated Reconstituted High Density Lipoproteins Target Macrophages and Promote Beneficial Antiatherosclerotic Mechanisms. Bioconj. Chem. 2017, 28, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.T.; Shon, Y.-S.; Narayanaswami, V. Apolipoprotein E3-mediated cellular uptake of reconstituted high-density lipoprotein bearing core 3, 10, or 17 nm hydrophobic gold nanoparticles. Int. J. Nanomed. 2017, 12, 8495–8510. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-Retana, S.; Cano-Sarabia, M.; Marazuela, P.; Sánchez-Quesada, J.L.; Garcia-Leon, A.; Montañola, A.; Montaner, J.; Maspoch, D.; Hernández-Guillamon, M. Characterization of ApoJ-reconstituted high-density lipoprotein (rHDL) nanodisc for the potential treatment of cerebral β-amyloidosis. Sci. Rep. 2017, 7, 14637. [Google Scholar] [CrossRef] [PubMed]
- Papillon, J.P.N.; Pan, M.; Brousseau, M.E.; Gilchrist, M.A.; Lou, C.; Singh, A.K.; Stawicki, T.; Thompson, J.E. Synthetic phospholipids as specific substrates for plasma endothelial lipase. Bioorg. Med. Chem. Lett. 2016, 26, 3514–3517. [Google Scholar] [CrossRef] [PubMed]
- Ikenaga, M.; Higaki, Y.; Saku, K.; Uehara, Y. High-Density Lipoprotein Mimetics: A Therapeutic Tool for Atherosclerotic Diseases. J. Atheroscler. Thromb. 2016, 23, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Badimon, J.J.; Badimon, L.; Galvez, A.; Dische, R.; Fuster, V. High density lipoprotein plasma fractions inhibit aortic fatty streaks in cholesterol-fed rabbits. Lab. Investig. 1989, 60, 455–461. [Google Scholar]
- Karalis, I.; Jukema, J.W. HDL Mimetics Infusion and Regression of Atherosclerosis: Is It Still Considered a Valid Therapeutic Option? Curr. Cardiol. Rep. 2018, 20, 66. [Google Scholar] [CrossRef]
- Nissen, S.E.; Tsunoda, T.; Tuzcu, E.M.; Schoenhagen, P.; Cooper, C.J.; Yasin, M.; Eaton, G.M.; Lauer, M.A.; Sheldon, W.S.; Grines, C.L.; et al. Effect of Recombinant ApoA-I Milano on Coronary Atherosclerosis in Patients with Acute Coronary Syndromes: A Randomized Controlled Trial. J. Am. Med. Assoc. 2003, 290, 2292–2300. [Google Scholar] [CrossRef]
- Ibanez, B.; Vilahur, G.; Cimmino, G.; Speidl, W.S.; Pinero, A.; Choi, B.G.; Zafar, M.U.; Santos-Gallego, C.G.; Krause, B.; Badimon, L.; et al. Rapid Change in Plaque Size, Composition, and Molecular Footprint After Recombinant Apolipoprotein A-IMilano (ETC-216) Administration. Magnetic Resonance Imaging Study in an Experimental Model of Atherosclerosis. J. Am. Coll. Cardiol. 2008, 51, 1104–1109. [Google Scholar] [CrossRef]
- Aboumsallem, J.; Muthuramu, I.; Mishra, M.; Kempen, H.; De Geest, B. Effective Treatment of Diabetic Cardiomyopathy and Heart Failure with Reconstituted HDL (Milano) in Mice. Int. J. Mol. Sci. 2019, 20, 1273. [Google Scholar] [CrossRef]
- Mishra, M.; Muthuramu, I.; Aboumsallem, J.; Kempen, H.; De Geest, B. Reconstituted HDL (Milano) Treatment Efficaciously Reverses Heart Failure with Preserved Ejection Fraction in Mice. Int. J. Mol. Sci. 2018, 19, 3399. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben-Aicha, S.; Badimon, L.; Vilahur, G. Advances in HDL: Much More than Lipid Transporters. Int. J. Mol. Sci. 2020, 21, 732. https://doi.org/10.3390/ijms21030732
Ben-Aicha S, Badimon L, Vilahur G. Advances in HDL: Much More than Lipid Transporters. International Journal of Molecular Sciences. 2020; 21(3):732. https://doi.org/10.3390/ijms21030732
Chicago/Turabian StyleBen-Aicha, Soumaya, Lina Badimon, and Gemma Vilahur. 2020. "Advances in HDL: Much More than Lipid Transporters" International Journal of Molecular Sciences 21, no. 3: 732. https://doi.org/10.3390/ijms21030732
APA StyleBen-Aicha, S., Badimon, L., & Vilahur, G. (2020). Advances in HDL: Much More than Lipid Transporters. International Journal of Molecular Sciences, 21(3), 732. https://doi.org/10.3390/ijms21030732







