Use of 3D Organoids as a Model to Study Idiopathic Form of Parkinson’s Disease
Abstract
1. Introduction
2. Results
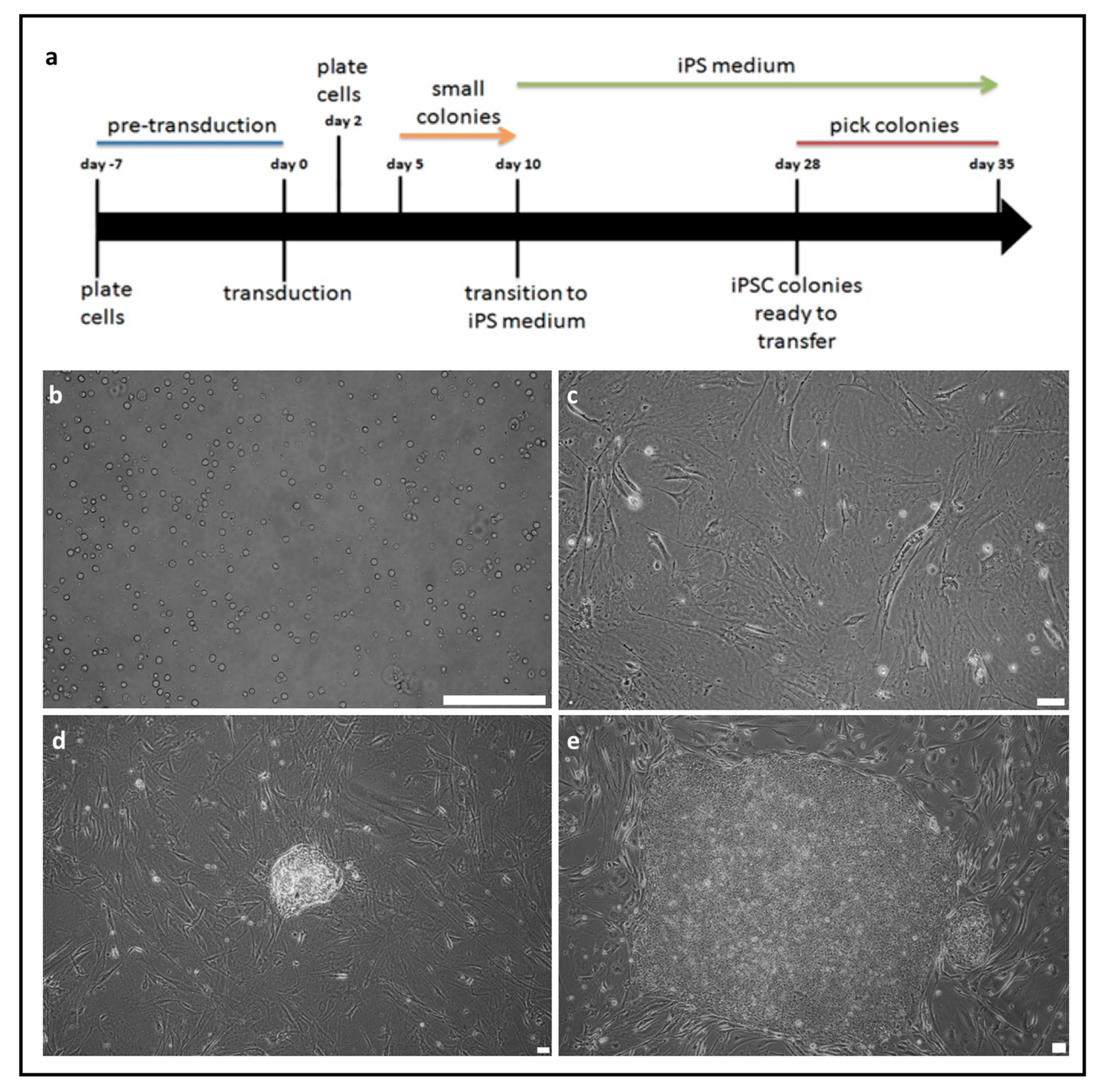
2.1. Generation of Induced Pluripotent Stem Cells from Peripheral Blood Mononuclear Cells
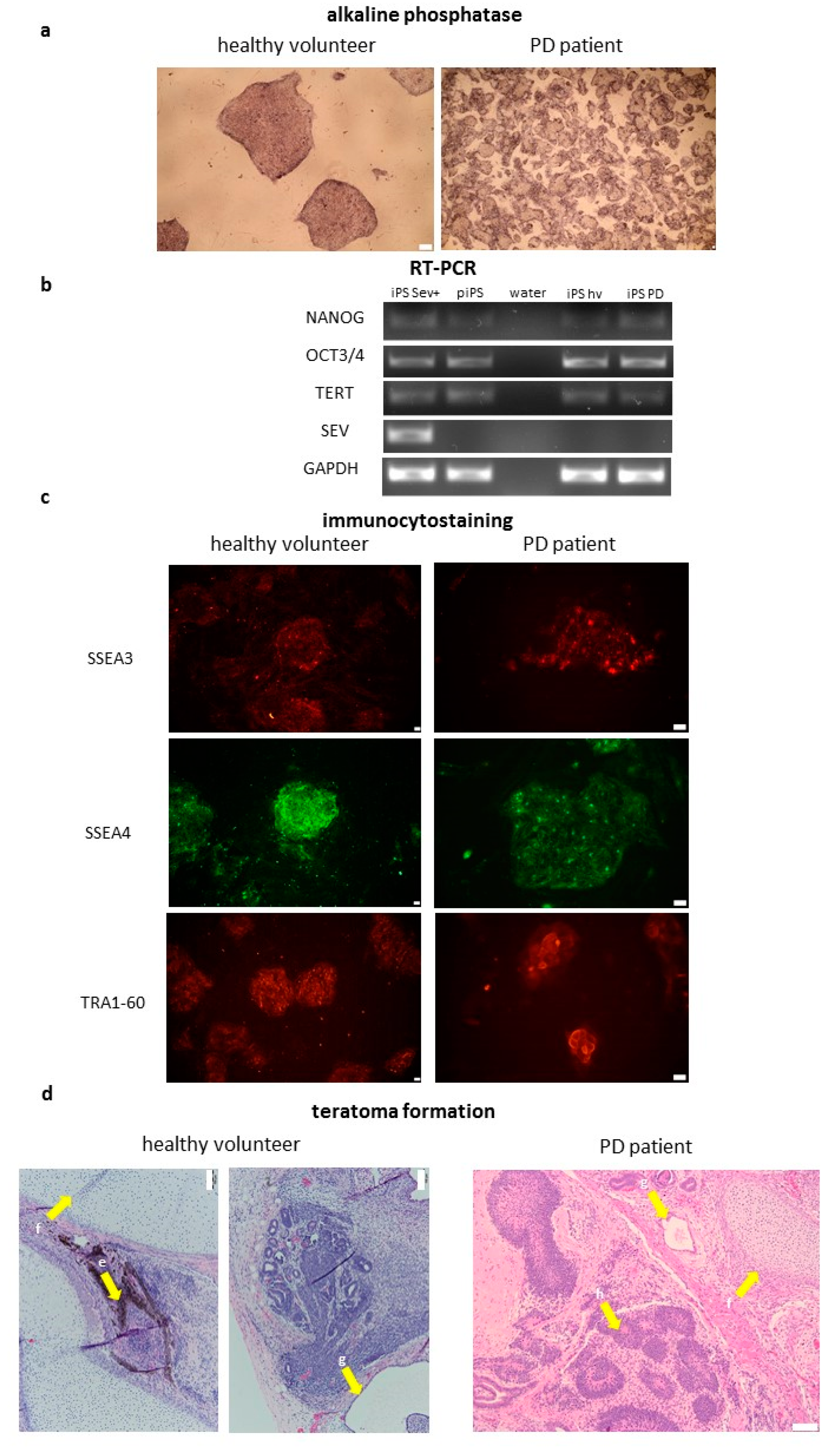
2.2. Characterization of Induced Pluripotent Stem Cells Generated from Healthy Volunteers and Parkinson’s Disease Patients
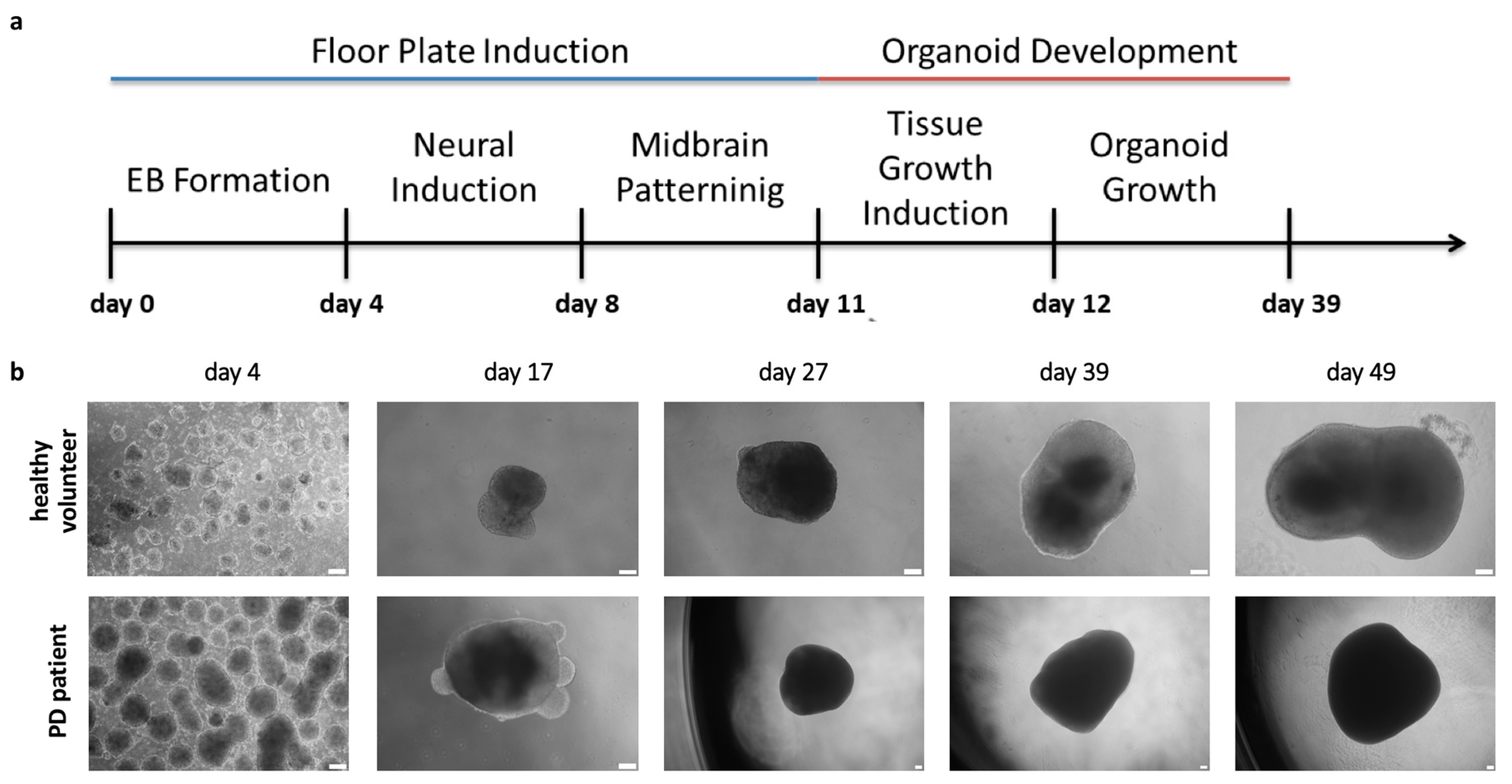
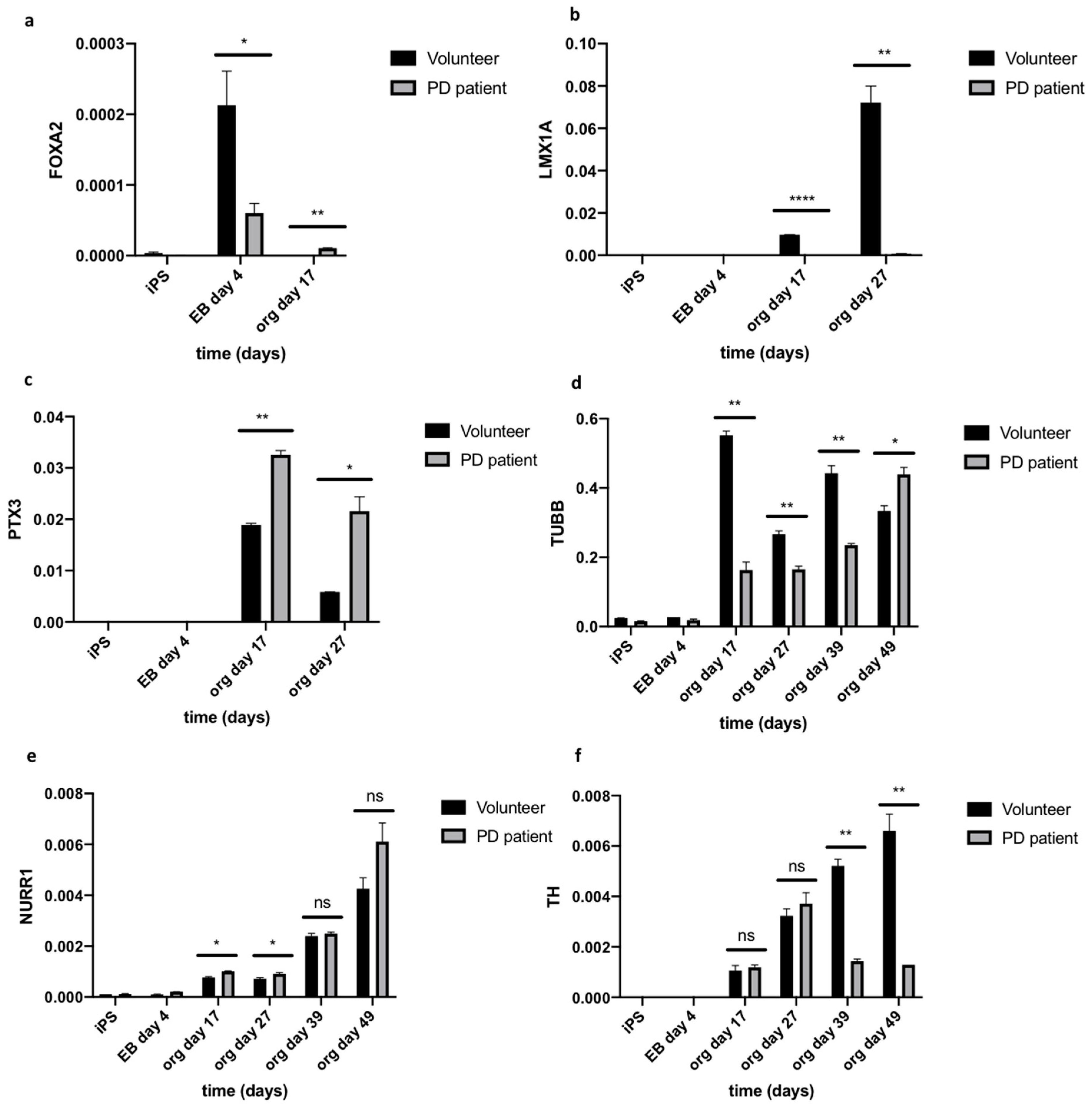
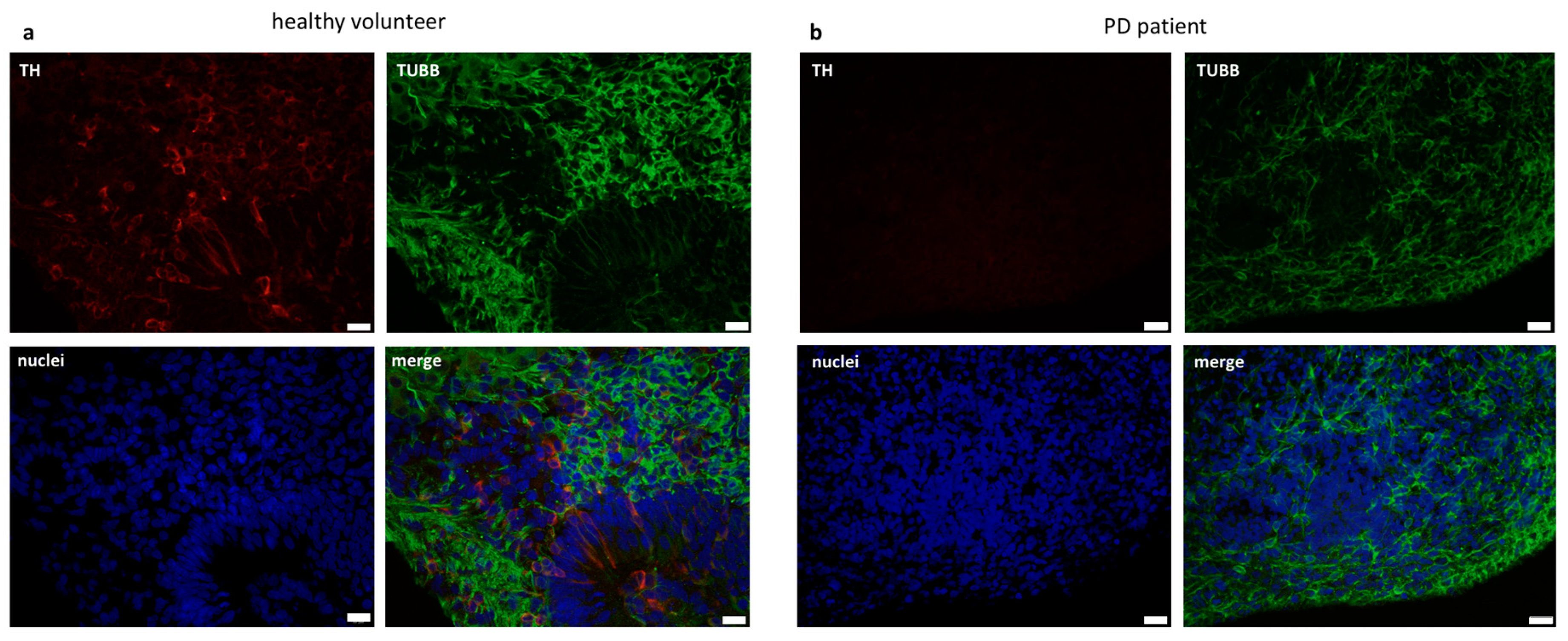
2.3. Organoids Formation from Induced Pluripotent Stem Cells from Healthy Volunteer and Parkinson’s Disease Patient
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Generation of iPSCs from Peripheral Blood Mononuclear Cells
4.3. Reverse Transcription-Polymerase Chain Reaction
4.4. Polymerase Chain Reaction Analysis
4.5. Quantitative Reverse Transcription Real Time Polymerase Chain Reaction
4.6. Immunofluorescent Staining
4.7. Alkaline Phosphatase Staining
4.8. Teratoma Formation Assay
4.9. Embryoid Bodies Formation
4.10. Midbrain Organoids Formation
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PD | Parkinson’s Disease |
| iPSCs | Induced Pluripotent Stem Cells |
| PBMCs | Peripheral Blood Mononuclear Cells |
| ESCs | Embryonic Stem Cells |
| EBs | Embryoid Bodies |
| mDA | Midbrain Dopaminergic neurons |
| MEFs | Mouse Embryonic Fibroblasts |
| SeV | Sendai viral Vector |
References
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Egawa, N.; Kitaoka, S.; Tsukita, K.; Naitoh, M.; Takahashi, K.; Yamamoto, T.; Adachi, F.; Kondo, T.; Okita, K.; Asaka, I.; et al. Drug Screening for ALS Using Patient-Specific Induced Pluripotent Stem Cells. Sci. Transl. Med. 2012, 4, 104–145. [Google Scholar] [CrossRef]
- Romano, G.; Morales, F.; Marino, I.R.; Giordano, A. A Commentary on iPS Cells: Potential Applications in Autologous Transplantation, Study of Illnesses and Drug Screening. J. Cell. Physiol. 2014, 229, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Park, I.-H.; Arora, N.; Huo, H.; Maherali, N.; Ahfeldt, T.; Shimamura, A.; Lensch, M.W.; Cowan, C.; Hochedlinger, K.; Daley, G.Q. Disease-specific induced pluripotent stem (iPS) cells. Cell 2008, 134, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Vergara, X.; Sevilla, A.; D’Souza, S.L.; Ang, Y.-S.; Schaniel, C.; Lee, D.-F.; Yang, L.; Kaplan, A.D.; Adler, E.D.; Rozov, R.; et al. Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature 2010, 465, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Soldner, F.; Hockemeyer, D.; Beard, C.; Gao, Q.; Bell, G.W.; Cook, E.G.; Hargus, G.; Blak, A.; Cooper, O.; Mitalipova, M.; et al. Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell 2009, 136, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Ghatak, S.; Dolatabadi, N.; Trudler, D.; Zhang, X.; Wu, Y.; Mohata, M.; Ambasudhan, R.; Talantova, M.; Lipton, S.A. Mechanisms of hyperexcitability in Alzheimer’s disease hiPSC-derived neurons and cerebral organoids vs isogenic controls. eLife 2019, 8, e50333. [Google Scholar] [CrossRef]
- Mungenast, A.E.; Siegert, S.; Tsai, L.H. Modeling Alzheimer’s disease with human induced pluripotent stem (iPS) cells. Mol Cell Neurosci. 2016, 73, 13–31. [Google Scholar] [CrossRef]
- Tan, G.W.; Kondo, T.; Murakami, N.; Imamura, K.; Enami, T.; Tsukita, K.; Shibukawa, R.; Funayama, M.; Matsumoto, R.; Ikeda, A.; et al. Induced pluripotent stem cells derived from an autosomal dominant lateral temporal epilepsy (ADLTE) patient carrying S473L mutation in leucine-rich glioma inactivated 1 (LGI1). Stem Cell Res. 2017, 24, 12–15. [Google Scholar] [CrossRef]
- Groveman, B.R.; Foliaki, S.T.; Orru, C.D.; Zanusso, G.; Carroll, J.A.; Race, B.; Haigh, C.L. Sporadic Creutzfeldt-Jakob disease prion infection of human cerebral organoids. Acta Neuropathol Commun. 2019, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Giro, G.; Arias-Fuenzalida, J.; Jarazo, J.; Zeuschner, D.; Ali, M.; Possemis, N.; Bolognin, S.; Halder, R.; Jager, C.; Kuper, W.F.E.; et al. Synapse alterations precede neuronal damage and storage pathology in a human cerebral organoid model of CLN3-juvenile neuronal ceroid lipofuscinosis. Acta Neuropathol. Commun. 2019, 7, 222. [Google Scholar] [CrossRef] [PubMed]
- Chambers, S.M.; Fasano, C.A.; Papapetrou, E.P.; Tomishima, M.; Sadelain, M.; Studer, L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009, 27, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wilson, G.F.; Soerens, A.G.; Koonce, C.H.; Yu, J.; Palecek, S.P.; Thomson, J.A.; Kamp, T.J. Functional Cardiomyocytes Derived From Human Induced Pluripotent Stem Cells. Circ. Res. 2009, 104, e30–e41. [Google Scholar] [CrossRef]
- Jo, J.; Xiao, Y.; Sun, A.X.; Cukuroglu, E.; Tran, H.D.; Göke, J.; Tan, Z.Y.; Saw, T.Y.; Tan, C.P.; Lokman, H.; et al. Midbrain-like Organoids from Human Pluripotent Stem Cells Contain Functional Dopaminergic and Neuromelanin-Producing Neurons. Cell Stem Cell 2016, 19, 248–257. [Google Scholar] [CrossRef]
- Shoji, E.; Woltjen, K.; Sakurai, H. Directed Myogenic Differentiation of Human Induced Pluripotent Stem Cells. Methods Mol. Biol. 2016, 1353, 89–99. [Google Scholar]
- Kelava, I.; Lancaster, M.A. Stem Cell Models of Human Brain Development. Cell Stem Cell 2016, 18, 736–748. [Google Scholar] [CrossRef]
- Smits, L.M.; Reinhardt, L.; Reinhardt, P.; Glatza, M.; Monzel, A.S.; Stanslowsky, N.; Rosato-Siri, M.D.; Zanon, A.; Antony, P.M.; Bellmann, J.; et al. Modeling Parkinson’s disease in midbrain-like organoids. NPJ Park. Dis. 2019, 5, 5. [Google Scholar] [CrossRef]
- Sułkowski, M.; Konieczny, P.; Chlebanowska, P.; Majka, M. Introduction of exogenous HSV-TK suicide gene increases safety of keratinocyte-derived induced pluripotent stem cells by providing genetic “emergency exit” switch. Int. J. Mol. Sci. 2018, 19, 197. [Google Scholar] [CrossRef]
- Dong, J.; Li, S.; Mo, J.L.; Cai, H.B.; Le, W.D. Nurr1-Based Therapies for Parkinson’s Disease. CNS Neurosci. Ther. 2016, 22, 351–359. [Google Scholar] [CrossRef]
- Kriks, S.; Shim, J.-W.; Piao, J.; Ganat, Y.M.; Wakeman, D.R.; Xie, Z.; Carrillo-Reid, L.; Auyeung, G.; Antonacci, C.; Buch, A.; et al. Floor plate-derived dopamine neurons from hESCs efficiently engraft in animal models of PD HHS Public Access. Nature 2012, 480, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Nolbrant, S.; Heuer, A.; Parmar, M.; Kirkeby, A. Generation of high-purity human ventral midbrain dopaminergic progenitors for in vitro maturation and intracerebral transplantation. Nat. Protoc. 2017, 12, 1962–1979. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sheng, C.; Liu, Z.; Jia, W.; Wang, B.; Li, M.; Fu, L.; Ren, Z.; An, J.; Sang, L.; et al. Lmx1a enhances the effect of iNSCs in a PD model. Stem Cell Res. 2015, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Doucet-Beaupré, H.; Gilbert, C.; Profes, M.S.; Chabrat, A.; Pacelli, C.; Giguère, N.; Rioux, V.; Charest, J.; Deng, Q.; Laguna, A.; et al. Lmx1a and Lmx1b regulate mitochondrial functions and survival of adult midbrain dopaminergic neurons. Proc. Natl. Acad. Sci. USA 2016, 113, E4387–E4396. [Google Scholar] [CrossRef]
- Bergman, O.; Håkansson, A.; Westberg, L.; Belin, A.C.; Sydow, O.; Olson, L.; Holmberg, B.; Fratiglioni, L.; Bäckman, L.; Eriksson, E.; et al. Do polymorphisms in transcription factors LMX1A and LMX1B influence the risk for Parkinson’s disease? J. Neural Transm. 2009, 116, 333–338. [Google Scholar] [CrossRef]
- Arenas, E. Foxa2: The Rise and Fall of Dopamine Neurons. Cell Stem Cell 2008, 2, 110–112. [Google Scholar] [CrossRef]
- Oh, S.-M.; Chang, M.-Y.; Song, J.-J.; Rhee, Y.-H.; Joe, E.-H.; Lee, H.-S.; Yi, S.-H.; Lee, S.-H. Combined Nurr1 and Foxa2 roles in the therapy of Parkinson’s disease. EMBO Mol. Med. 2015, 7, 510–525. [Google Scholar] [CrossRef]
- Van den Munckhof, P.; Luk, K.C.; Ste-Marie, L.; Montgomery, J.; Blanchet, P.J.; Sadikot, A.F.; Drouin, J. Pitx3 is required for motor activity and for survival of a subset of midbrain dopaminergic neurons. Development 2003, 130, 2535–2542. [Google Scholar] [CrossRef]
- Rajkovic, I.; Denes, A.; Allan, S.M.; Pinteaux, E. Emerging roles of the acute phase protein pentraxin-3 during central nervous system disorders. J. Neuroimmunol. 2016, 292, 27–33. [Google Scholar] [CrossRef]
- Lee, H.W.; Choi, J.; Suk, K. Increases of pentraxin 3 plasma levels in patients with Parkinson’s disease. Mov. Disord. 2011, 26, 2364–2370. [Google Scholar] [CrossRef] [PubMed]
- Zetterstrom, R.H.; Solomin, L.; Jansson, L.; Hoffer, B.J.; Olson, L.; Perlmann, T. Dopamine Neuron Agenesis in Nurr1-Deficient Mice. Science 1997, 276, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Decressac, M.; Volakakis, N.; Björklund, A.; Perlmann, T. NURR1 in Parkinson disease - From pathogenesis to therapeutic potential. Nat. Rev. Neurol. 2013, 9, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H.V. Etiology and Pathogenesis of Parkinson Disease. Neurol. Clin. 2009, 27, 583–603. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chlebanowska, P.; Tejchman, A.; Sułkowski, M.; Skrzypek, K.; Majka, M. Use of 3D Organoids as a Model to Study Idiopathic Form of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 694. https://doi.org/10.3390/ijms21030694
Chlebanowska P, Tejchman A, Sułkowski M, Skrzypek K, Majka M. Use of 3D Organoids as a Model to Study Idiopathic Form of Parkinson’s Disease. International Journal of Molecular Sciences. 2020; 21(3):694. https://doi.org/10.3390/ijms21030694
Chicago/Turabian StyleChlebanowska, Paula, Anna Tejchman, Maciej Sułkowski, Klaudia Skrzypek, and Marcin Majka. 2020. "Use of 3D Organoids as a Model to Study Idiopathic Form of Parkinson’s Disease" International Journal of Molecular Sciences 21, no. 3: 694. https://doi.org/10.3390/ijms21030694
APA StyleChlebanowska, P., Tejchman, A., Sułkowski, M., Skrzypek, K., & Majka, M. (2020). Use of 3D Organoids as a Model to Study Idiopathic Form of Parkinson’s Disease. International Journal of Molecular Sciences, 21(3), 694. https://doi.org/10.3390/ijms21030694






