Ion Channels in The Pathogenesis of Endometriosis: A Cutting-Edge Point of View
Abstract
1. Introduction
2. Materials and Methods
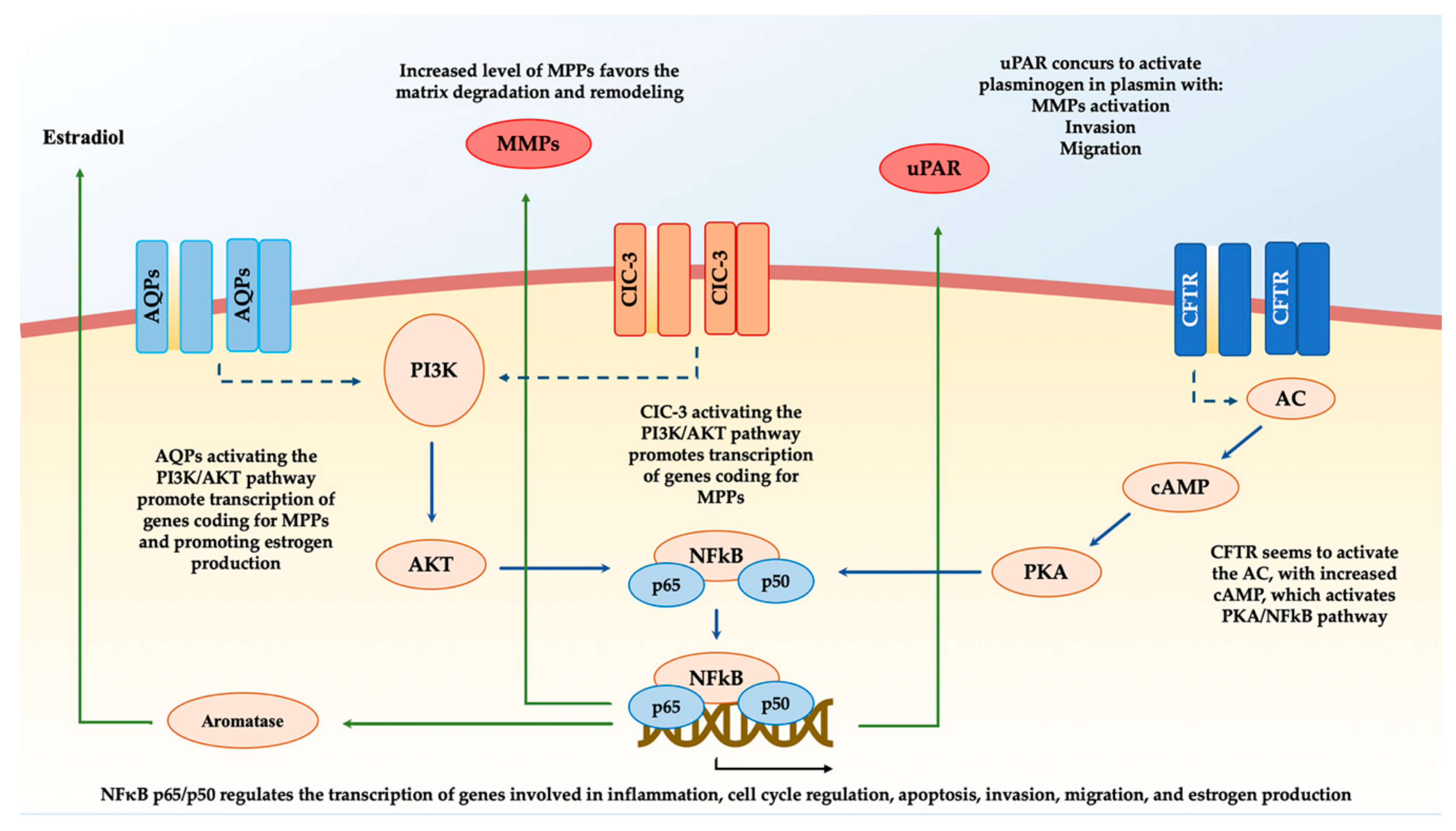
3. Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) and Endometriosis
4. Transient Receptor Potential (TRP) Channels and Endometriosis
5. Aquaporins (AQPs) and Endometriosis
6. Chloride Channel-3 (ClC-3) and Endometriosis
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DIE | Deep infiltrating endometriosis |
| Wnt | Wingless-related integration site gene cluster |
| Hox | Homeobox genes |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
| TRP | Transient receptor potential |
| AQP | Aquaporin |
| ClC | Chloride channel |
| NFκB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| uPAR | Urokinase plasminogen activator receptor |
| PI3K | Phosphatidylinositol-3-Kinase |
| AKT | Protein kinase B |
| ERK | Extracellular signal-regulated kinases |
| MAPK | Mitogen-activated protein kinases |
| MMP | Matrix metalloproteinase |
| MeSH | Medical subject headings |
| E2 | Estradiol |
| qPCR | Quantitative real-time polymerase chain reaction |
| ISK | Human endometrial Ishikawa cell |
| hESC | Human endometrial stromal cell |
| siRNA | small interfering RNA |
| ECM | Extracellular matrix |
| SNP | Single-nucleotide polymorphism |
| TNF-α | Tumor necrosis factor α |
References
- Laganà, A.S.; Garzon, S.; Götte, M.; Viganò, P.; Franchi, M.; Ghezzi, F.; Martin, D.C. The Pathogenesis of Endometriosis: Molecular and Cell Biology Insights. Int. J. Mol. Sci. 2019, 20, 5615. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.S.; Vitale, S.G.; Salmeri, F.M.; Triolo, O.; Ban Frangež, H.; Vrtačnik-Bokal, E.; Stojanovska, L.; Apostolopoulos, V.; Granese, R.; Sofo, V. Unus pro omnibus, omnes pro uno: A novel, evidence-based, unifying theory for the pathogenesis of endometriosis. Med. Hypotheses 2017, 103, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Peiris, A.N.; Chaljub, E.; Medlock, D. Endometriosis. JAMA 2018, 320, 2608. [Google Scholar] [CrossRef] [PubMed]
- Falcone, T.; Flyckt, R. Clinical Management of Endometriosis. Obstet. Gynecol. 2018, 131, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulos, N.V.; Alexandraki, K.I.; Gorry, A.; Coker, A. Association between chronic pelvic pain symptoms and the presence of endometriosis. Arch. Gynecol. Obstet. 2015, 293, 439–445. [Google Scholar] [CrossRef]
- Šalamun, V.; Verdenik, I.; Laganà, A.S.; Vrtačnik-Bokal, E. Should we consider integrated approach for endometriosis-associated infertility as gold standard management? Rationale and results from a large cohort analysis. Arch. Gynecol. Obstet. 2017, 297, 613–621. [Google Scholar] [CrossRef]
- Terzic, M.; Aimagambetova, G.; Garzon, S.; Bapayeva, G.; Ukybassova, T.; Terzic, S.; Norton, M.; Lagana, A.S. Ovulation induction in infertile women with endometriotic ovarian cysts: Current evidence and potential pitfalls. Minerva Med. 2019. [Google Scholar] [CrossRef]
- Donnez, J. Endometriosis: Enigmatic in the pathogenesis and controversial in its therapy. Fertil. Steril. 2012, 98, 509–510. [Google Scholar] [CrossRef]
- Bedaiwy, M.A.; Abdel-Aleem, M.A.; Miketa, A.; Falcone, T. Endometriosis: A critical appraisal of the advances and the controversies of a challenging health problem. Minerva Ginecol. 2009, 61, 285–298. [Google Scholar]
- Donnez, J. Introduction: From pathogenesis to therapy, deep endometriosis remains a source of controversy. Fertil. Steril. 2017, 108, 869–871. [Google Scholar] [CrossRef]
- Mele, D.; De Franciscis, P.; Cosenza, C.; Riemma, G.; D’eufemia, M.D.; Schettino, M.T.; Morlando, M.; Schiattarella, A. Surgical management of endometrioma for ovarian safety. Ital. J. Gynaecol. Obstet. 2019, 31, 49–55. [Google Scholar] [CrossRef]
- Siciliano, R.A.; Mazzeo, M.F.; Spada, V.; Facchiano, A.; D’acierno, A.; Stocchero, M.; De Franciscis, P.; Colacurci, N.; Sannolo, N.; Miraglia, N. Rapid peptidomic profiling of peritoneal fluid by MALDI-TOF mass spectrometry for the identification of biomarkers of endometriosis. Gynecol. Endocrinol. 2014, 30, 872–876. [Google Scholar] [CrossRef]
- Bozdag, G. Recurrence of Endometriosis: Risk Factors, Mechanisms and Biomarkers. Womens Health 2015, 11, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Sansone, A.; De Rosa, N.; Giampaolino, P.; Guida, M.; Laganà, A.S.; Di Carlo, C. Effects of etonogestrel implant on quality of life, sexual function, and pelvic pain in women suffering from endometriosis: Results from a multicenter, prospective, observational study. Arch. Gynecol. Obstet. 2018, 298, 731–736. [Google Scholar] [CrossRef]
- Márki, G.; Bokor, A.; Rigó, J.; Rigó, A. Physical pain and emotion regulation as the main predictive factors of health-related quality of life in women living with endometriosis. Hum. Reprod. 2017, 32, 1432–1438. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, V.L.; De Franciscis, P.; Barra, F.; Schiattarella, A.; Tropea, A.; Tesarik, J.; Shah, M.; Kahramanoglu, I.; Marques Cerentini, T.; Ponta, M.; et al. Sexuality in women with endometriosis: A critical narrative review. Minerva Med. 2019. [Google Scholar] [CrossRef]
- Soliman, A.M.; Coyne, K.S.; Zaiser, E.; Castelli-Haley, J.; Fuldeore, M.J. The burden of endometriosis symptoms on health-related quality of life in women in the United States: A cross-sectional study. J. Psychosom. Obstet. Gynaecol. 2017, 38, 238–248. [Google Scholar] [CrossRef]
- La Rosa, V.L.; De Franciscis, P.; Barra, F.; Schiattarella, A.; Török, P.; Shah, M.; Karaman, E.; Marques Cerentini, T.; Di Guardo, F.; Gullo, G.; et al. Quality of life in women with endometriosis: A narrative overview. Minerva Med. 2019. [Google Scholar] [CrossRef]
- Burney, R.O.; Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef]
- Rock, J.A.; Markham, S.M. Pathogenesis of endometriosis. Lancet 1992, 340, 1264–1267. [Google Scholar] [CrossRef]
- Vetvicka, V.; Laganà, A.S.; Salmeri, F.M.; Triolo, O.; Palmara, V.I.; Vitale, S.G.; Sofo, V.; Králíčková, M. Regulation of apoptotic pathways during endometriosis: From the molecular basis to the future perspectives. Arch. Gynecol. Obstet. 2016, 294, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Simonelli, A.; Guadagni, R.; De Franciscis, P.; Colacurci, N.; Pieri, M.; Basilicata, P.; Pedata, P.; Lamberti, M.; Sannolo, N.; Miraglia, N. Environmental and occupational exposure to bisphenol A and endometriosis: Urinary and peritoneal fluid concentration levels. Int. Arch. Occup. Environ. Health 2017, 90, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Maniglio, P.; Ricciardi, E.; Laganà, A.S.; Triolo, O.; Caserta, D. Epigenetic modifications of primordial reproductive tract: A common etiologic pathway for Mayer-Rokitansky-Kuster-Hauser Syndrome and endometriosis? Med. Hypotheses 2016, 90, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.S.; Salmeri, F.M.; Ban Frangež, H.; Ghezzi, F.; Vrtačnik-Bokal, E.; Granese, R. Evaluation of M1 and M2 macrophages in ovarian endometriomas from women affected by endometriosis at different stages of the disease. Gynecol. Endocrinol. 2019, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.C.; Chen, H.; Chan, H.C. Ion channels in the endometrium: Regulation of endometrial receptivity and embryo implantation. Hum. Reprod. Update 2014, 20, 517–529. [Google Scholar] [CrossRef]
- Goldstein, S.A.N. Ion channels: Structural basis for function and disease. Semin. Perinatol. 1996, 20, 520–530. [Google Scholar] [CrossRef][Green Version]
- Bagal, S.K.; Brown, A.D.; Cox, P.J.; Omoto, K.; Owen, R.M.; Pryde, D.C.; Sidders, B.; Skerratt, S.E.; Stevens, E.B.; Storer, R.I.; et al. Ion Channels as Therapeutic Targets: A Drug Discovery Perspective. J. Med. Chem. 2012, 56, 593–624. [Google Scholar] [CrossRef]
- Li, J.; Liang, X.; Chen, Z. Improving the embryo implantation via novel molecular targets. Curr. Drug Targets 2013, 14, 864–871. [Google Scholar] [CrossRef]
- Mathie, A. Ion channels as novel therapeutic targets in the treatment of pain. J. Pharm. Pharmacol. 2010, 62, 1089–1095. [Google Scholar] [CrossRef]
- Ashcroft, F.M. From molecule to malady. Nature 2006, 440, 440–447. [Google Scholar] [CrossRef]
- Davidson, L.M.; Coward, K. Molecular mechanisms of membrane interaction at implantation. Birth Defects Res. C Embryo Today Rev. 2016, 108, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tan, Y.-J.; Qu, F.; Sheng, J.-Z.; Huang, H.-F. Functions of water channels in male and female reproductive systems. Mol. Aspects Med. 2012, 33, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, S.; Shen, Q.; Wu, J.; Zhu, X. The expression and regulation of aquaporins in placenta and fetal membranes. Front. Biosci. 2012, 17, 2371–2382. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leanza, L.; Managò, A.; Zoratti, M.; Gulbins, E.; Szabo, I. Pharmacological targeting of ion channels for cancer therapy: In vivo evidences. Biochim. Biophys. Acta-Mol. Cell Res. 2016, 1863, 1385–1397. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Peng, S.; Zheng, Y.; Yang, X.; Zhang, H.; Tan, Q.; Liang, X.; Gao, H.; Li, Y.; Huang, Y.; et al. Estradiol activates chloride channels via estrogen receptor-α in the cell membranes of osteoblasts. Am. J. Physiol. Cell Physiol. 2017, 313, C162–C172. [Google Scholar] [CrossRef]
- Chabbert-Buffeta, N. Neuroendocrine effects of progesterone. Steroids 2000, 65, 613–620. [Google Scholar] [CrossRef]
- Vega-Vela, N.E.; Osorio, D.; Avila-Rodriguez, M.; Gonzalez, J.; García-Segura, L.M.; Echeverria, V.; Barreto, G.E. L-Type Calcium Channels Modulation by Estradiol. Mol. Neurobiol. 2017, 54, 4996–5007. [Google Scholar] [CrossRef]
- Arnadóttir, J.; Chalfie, M. Eukaryotic mechanosensitive channels. Annu. Rev. Biophys. 2010, 39, 111–137. [Google Scholar] [CrossRef]
- Cuddapah, V.A.; Sontheimer, H. Ion channels and transporters [corrected] in cancer. 2. Ion channels and the control of cancer cell migration. Am. J. Physiol. Cell Physiol. 2011, 301, C541–C549. [Google Scholar] [CrossRef]
- Bohonyi, N.; Pohóczky, K.; Szalontai, B.; Perkecz, A.; Kovács, K.; Kajtár, B.; Orbán, L.; Varga, T.; Szegedi, S.; Bódis, J.; et al. Local upregulation of transient receptor potential ankyrin 1 and transient receptor potential vanilloid 1 ion channels in rectosigmoid deep infiltrating endometriosis. Mol. Pain 2017, 13. [Google Scholar] [CrossRef]
- Greaves, E.; Grieve, K.; Horne, A.W.; Saunders, P.T.K. Elevated peritoneal expression and estrogen regulation of nociceptive ion channels in endometriosis. J. Clin. Endocrinol. Metab. 2014, 99, E1738–E1743. [Google Scholar] [CrossRef]
- Jiang, X.-X.; Wu, R.-J.; Xu, K.-H.; Zhou, C.-Y.; Guo, X.-Y.; Sun, Y.-L.; Lin, J. Immunohistochemical detection of aquaporin expression in eutopic and ectopic endometria from women with endometriomas. Fertil. Steril. 2010, 94, 1229–1234. [Google Scholar] [CrossRef]
- Zheng, X.-Y.; Chen, G.-A.; Wang, H.-Y. Expression of cystic fibrosis transmembrane conductance regulator in human endometrium. Hum. Reprod. 2004, 19, 2933–2941. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Park, J.H.; Yoon, J.-K.; Yoon, J.S.; Kim, J.S.; Lee, J.H.; Yun, B.H.; Park, J.H.; Seo, S.K.; Cho, S.; et al. Potential roles of aquaporin 9 in the pathogenesis of endometriosis. MHR Basic Sci. Reprod. Med. 2019, 25, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Jin, A.; Zhang, J.; Wang, C.; Tsang, L.L.; Cai, Z.; Zhou, X.; Chen, H.; Chan, H.C. Upregulation of CFTR in patients with endometriosis and its involvement in NFκB-uPAR dependent cell migration. Oncotarget 2017, 8, 66951–66959. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.X.; Fei, X.W.; Zhao, L.; Ye, X.L.; Xin, L.B.; Qu, Y.; Xu, K.H.; Wu, R.J.; Lin, J. Aquaporin 5 Plays a Role in Estrogen-Induced Ectopic Implantation of Endometrial Stromal Cells in Endometriosis. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Persoons, E.; Hennes, A.; De Clercq, K.; Van Bree, R.; Vriens, G.; Dorien, F.O.; Peterse, D.; Vanhie, A.; Meuleman, C.; Voets, T.; et al. Functional Expression of TRP Ion Channels in Endometrial Stromal Cells of Endometriosis Patients. Int. J. Mol. Sci. 2018, 19, 2467. [Google Scholar] [CrossRef]
- Gillen, A.E.; Harris, A. Transcriptional regulation of CFTR gene expression. Front. Biosci. 2012, 4, 587–592. [Google Scholar] [CrossRef]
- Vetter, A.J.; Karamyshev, A.L.; Patrick, A.E.; Hudson, H.; Thomas, P.J. N-Alpha-Acetyltransferases and Regulation of CFTR Expression. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Sweezey, N.B.; Gauthier, C.; Gagnon, S.; Ferretti, E.; Kopelman, H. Progesterone and estradiol inhibit CFTR-mediated ion transport by pancreatic epithelial cells. Am. J. Physiol. Liver Physiol. 1996, 271, G747–G754. [Google Scholar] [CrossRef]
- Mularoni, A.; Adessi, G.L.; Arbez-Gindre, F.; Agnani, G.; Nicollier, M. Competitive RT-PCR to quantify CFTR mRNA in human endometrium. Clin. Chem. 1996, 42, 1765–1769. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, Q.; Huang, W.; Xiao, L.; Shen, L.; Xu, W. NF κB expression increases and CFTR and MUC1 expression decreases in the endometrium of infertile patients with hydrosalpinx: A comparative study. Reprod. Biol. Endocrinol. 2012, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Z.; Ajonuma, L.C.; Tsang, L.L.; Lam, S.Y.; Rowlands, D.K.; Ho, L.S.; Zhou, C.X.; Chung, Y.W.; Chan, H.C. Differential expression and localization of CFTR and ENaC in mouse endometrium during pre-implantation. Cell Biol. Int. 2004, 28, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Knorre, A.; Wagner, M.; Schaefer, H.-E.; Colledge, W.H.; Pahl, H.L. DeltaF508-CFTR causes constitutive NF-kappaB activation through an ER-overload response in cystic fibrosis lungs. Biol. Chem. 2002, 383, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Chen, H.; Fok, K.L.; Tsang, L.L.; Yu, M.K.; Zhang, X.H.; Chen, J.; Jiang, X.; Chung, Y.W.; Ma, A.C.H.; et al. CFTR mediates bicarbonate-dependent activation of miR-125b in preimplantation embryo development. Cell Res. 2012, 22, 1453–1466. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, A. Transient Receptor Potential (TRP) Channels in Health and Disease. Cells 2019, 8, 413. [Google Scholar] [CrossRef]
- Voets, T.; Vriens, J.; Vennekens, R. Targeting TRP Channels—Valuable Alternatives to Combat Pain, Lower Urinary Tract Disorders, and Type 2 Diabetes? Trends Pharmacol. Sci. 2019, 40, 669–683. [Google Scholar] [CrossRef]
- Lamas, J.A.; Rueda-Ruzafa, L.; Herrera-Pérez, S. Ion Channels and Thermosensitivity: TRP, TREK, or Both? Int. J. Mol. Sci. 2019, 20, 2371. [Google Scholar] [CrossRef]
- Takayama, Y.; Derouiche, S.; Maruyama, K.; Tominaga, M. Emerging Perspectives on Pain Management by Modulation of TRP Channels and ANO1. Int. J. Mol. Sci. 2019, 20, 3411. [Google Scholar] [CrossRef]
- De Clercq, K.; Held, K.; Van Bree, R.; Meuleman, C.; Peeraer, K.; Tomassetti, C.; Voets, T.; D’Hooghe, T.; Vriens, J. Functional expression of transient receptor potential channels in human endometrial stromal cells during the luteal phase of the menstrual cycle. Hum. Reprod. 2015, 30, 1421–1436. [Google Scholar] [CrossRef]
- Bödding, M. TRP proteins and cancer. Cell. Signal. 2007, 19, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Fels, B.; Bulk, E.; Pethő, Z.; Schwab, A. The Role of TRP Channels in the Metastatic Cascade. Pharmaceuticals 2018, 11, 48. [Google Scholar] [CrossRef]
- Smani, T.; Gómez, L.J.; Regodon, S.; Woodard, G.E.; Siegfried, G.; Khatib, A.-M.; Rosado, J.A. TRP Channels in Angiogenesis and Other Endothelial Functions. Front. Physiol. 2018, 9, 1731. [Google Scholar] [CrossRef] [PubMed]
- Hiroi, H.; Momoeda, M.; Watanabe, T.; Ito, M.; Ikeda, K.; Tsutsumi, R.; Hosokawa, Y.; Koizumi, M.; Zenri, F.; Muramatsu, M.; et al. Expression and regulation of transient receptor potential cation channel, subfamily M, member 2 (TRPM2) in human endometrium. Mol. Cell. Endocrinol. 2013, 365, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Kawarabayashi, Y.; Hai, L.; Honda, A.; Horiuchi, S.; Tsujioka, H.; Ichikawa, J.; Inoue, R. Critical role of TRPC1-mediated Ca2+ entry in decidualization of human endometrial stromal cells. Mol. Endocrinol. 2012, 26, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Takata, K.; Matsuzaki, T.; Tajika, Y. Aquaporins: Water channel proteins of the cell membrane. Prog. Histochem. Cytochem. 2004, 39, 1–83. [Google Scholar] [CrossRef]
- King, L.S.; Agre, P. Pathophysiology of the Aquaporin Water Channels. Annu. Rev. Physiol. 1996, 58, 619–648. [Google Scholar] [CrossRef]
- Mobasheri, A.; Wray, S.; Marples, D. Distribution of AQP2 and AQP3 water channels in human tissue microarrays. J. Mol. Histol. 2005, 36, 1–14. [Google Scholar] [CrossRef]
- Meli, R.; Pirozzi, C.; Pelagalli, A. New Perspectives on the Potential Role of Aquaporins (AQPs) in the Physiology of Inflammation. Front. Physiol. 2018, 9, 101. [Google Scholar] [CrossRef]
- He, R.-H.; Sheng, J.-Z.; Luo, Q.; Jin, F.; Wang, B.; Qian, Y.-L.; Zhou, C.-Y.; Sheng, X.; Huang, H.-F. Aquaporin-2 expression in human endometrium correlates with serum ovarian steroid hormones. Life Sci. 2006, 79, 423–429. [Google Scholar] [CrossRef]
- Bostanci Durmus, A.; Dincer Cengiz, S.; Yılmaz, H.; Candar, T.; Gursoy, A.Y.; Sinem Caglar, G. The levels of matrix metalloproteinase-9 and neutrophil gelatinase-associated lipocalin in different stages of endometriosis. J. Obstet. Gynaecol. 2019, 39, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Szymanowski, K.; Mikołajczyk, M.; Wirstlein, P.; Dera-Szymanowska, A. Matrix metalloproteinase-2 (MMP-2), MMP-9, tissue inhibitor of matrix metalloproteinases (TIMP-1) and transforming growth factor-β2 (TGF-β2) expression in eutopic endometrium of women with peritoneal endometriosis. Ann. Agric. Environ. Med. 2016, 23, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiong, W.; Xiong, Y.; Liu, H.; Li, N.; Du, Y.; Liu, Y. Intracellular Wnt/Beta-Catenin Signaling Underlying 17beta-Estradiol-Induced Matrix Metalloproteinase 9 Expression in Human Endometriosis1. Biol. Reprod. 2016, 94. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.-W.; Wen, Y.; Chun, S.-H.; Nezhat, C.; Woo, B.-H.; Lake Polan, M. Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-3 mRNA expression in ectopic and eutopic endometrium in women with endometriosis: A rationale for endometriotic invasiveness. Fertil. Steril. 2001, 75, 152–159. [Google Scholar] [CrossRef]
- Collette, T.; Maheux, R.; Mailloux, J.; Akoum, A. Increased expression of matrix metalloproteinase-9 in the eutopic endometrial tissue of women with endometriosis. Hum. Reprod. 2006, 21, 3059–3067. [Google Scholar] [CrossRef]
- Xin, L.; Hou, Q.; Xiong, Q.I.; Ding, X. Association between matrix metalloproteinase-2 and matrix metalloproteinase-9 polymorphisms and endometriosis: A systematic review and meta-analysis. Biomed. Rep. 2015, 3, 559–565. [Google Scholar] [CrossRef]
- Weigel, M.T.; Krämer, J.; Schem, C.; Wenners, A.; Alkatout, I.; Jonat, W.; Maass, N.; Mundhenke, C. Differential expression of MMP-2, MMP-9 and PCNA in endometriosis and endometrial carcinoma. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 160, 74–78. [Google Scholar] [CrossRef]
- Hara-Chikuma, M.; Yang, B.; Sonawane, N.D.; Sasaki, S.; Uchida, S.; Verkman, A.S. ClC-3 Chloride Channels Facilitate Endosomal Acidification and Chloride Accumulation. J. Biol. Chem. 2004, 280, 1241–1247. [Google Scholar] [CrossRef]
- Li, M.; Wu, D.B.; Wang, J. Effects of volume-activated chloride channels on the invasion and migration of human endometrial cancer cells. Eur. J. Gynaecol. Oncol. 2013, 34, 60–64. [Google Scholar]
- Mao, J.; Chen, L.; Xu, B.; Wang, L.; Wang, W.; Li, M.; Zheng, M.; Li, H.; Guo, J.; Li, W.; et al. Volume-activated chloride channels contribute to cell-cycle-dependent regulation of HeLa cell migration. Biochem. Pharmacol. 2009, 77, 159–168. [Google Scholar] [CrossRef]
- Ransom, C.B.; O’Neal, J.T.; Sontheimer, H. Volume-activated chloride currents contribute to the resting conductance and invasive migration of human glioma cells. J. Neurosci. 2001, 21, 7674–7683. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Chen, L.; Xu, B.; Wang, L.; Li, H.; Guo, J.; Li, W.; Nie, S.; Jacob, T.J.C.; Wang, L. Suppression of ClC-3 channel expression reduces migration of nasopharyngeal carcinoma cells. Biochem. Pharmacol. 2008, 75, 1706–1716. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Jin, X.; Min, L.; Li, Q.; Deng, L.; Wu, H.; Lin, G.; Chen, L.; Zhang, H.; Li, C.; et al. Chloride channel-3 promotes tumor metastasis by regulating membrane ruffling and is associated with poor survival. Oncotarget 2015, 6, 2434–2450. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Yuan, J.; Wang, L.; Zhang, H.; Jin, X.; Zhu, J.; Li, H.; Xu, B.; Chen, L. Tamoxifen inhibits migration of estrogen receptor-negative hepatocellular carcinoma cells by blocking the swelling-activated chloride current. J. Cell. Physiol. 2013, 228, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Huang, Y.; Wu, J.; Deng, Z.; Wang, Y.; Lai, Z.; Wang, H.; Sun, X.; Zhu, Y.; Du, M.; et al. Overexpression of chloride channel-3 is associated with the increased migration and invasion ability of ectopic endometrial cells from patients with endometriosis. Hum. Reprod. 2016, 31, 986–998. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Du, H.; Taylor, H.S. Experimental murine endometriosis induces DNA methylation and altered gene expression in eutopic endometrium. Biol. Reprod. 2009, 80, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Volk, A.P.D.; Heise, C.K.; Hougen, J.L.; Artman, C.M.; Volk, K.A.; Wessels, D.; Soll, D.R.; Nauseef, W.M.; Lamb, F.S.; Moreland, J.G. ClC-3 and IClswell are required for normal neutrophil chemotaxis and shape change. J. Biol. Chem. 2008, 283, 34315–34326. [Google Scholar] [CrossRef]
- Yang, H.; Huang, L.-Y.; Zeng, D.-Y.; Huang, E.-W.; Liang, S.-J.; Tang, Y.-B.; Su, Y.-X.; Tao, J.; Shang, F.; Wu, Q.-Q.; et al. Decrease of intracellular chloride concentration promotes endothelial cell inflammation by activating nuclear factor-κB pathway. Hypertens 2012, 60, 1287–1293. [Google Scholar] [CrossRef]
| Ion Channel | Regulation | Main Pathway | Action | References |
|---|---|---|---|---|
| CFTR | Upregulation | NFκB-p-65-uPAR | Migration; proliferation | [45] |
| AQP5 | Upregulation | PI3K/AKT—MMP2, MMP9 | Implantation | [46] |
| AQP9 | Downregulation | ERK/p38 MAPK - MMP2, MMP9 | Migration; implantation | [44] |
| ClC-3 | Upregulation | MMP9 | Implantation; inflammation | [85] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riemma, G.; Laganà, A.S.; Schiattarella, A.; Garzon, S.; Cobellis, L.; Autiero, R.; Licciardi, F.; Della Corte, L.; La Verde, M.; De Franciscis, P. Ion Channels in The Pathogenesis of Endometriosis: A Cutting-Edge Point of View. Int. J. Mol. Sci. 2020, 21, 1114. https://doi.org/10.3390/ijms21031114
Riemma G, Laganà AS, Schiattarella A, Garzon S, Cobellis L, Autiero R, Licciardi F, Della Corte L, La Verde M, De Franciscis P. Ion Channels in The Pathogenesis of Endometriosis: A Cutting-Edge Point of View. International Journal of Molecular Sciences. 2020; 21(3):1114. https://doi.org/10.3390/ijms21031114
Chicago/Turabian StyleRiemma, Gaetano, Antonio Simone Laganà, Antonio Schiattarella, Simone Garzon, Luigi Cobellis, Raffaele Autiero, Federico Licciardi, Luigi Della Corte, Marco La Verde, and Pasquale De Franciscis. 2020. "Ion Channels in The Pathogenesis of Endometriosis: A Cutting-Edge Point of View" International Journal of Molecular Sciences 21, no. 3: 1114. https://doi.org/10.3390/ijms21031114
APA StyleRiemma, G., Laganà, A. S., Schiattarella, A., Garzon, S., Cobellis, L., Autiero, R., Licciardi, F., Della Corte, L., La Verde, M., & De Franciscis, P. (2020). Ion Channels in The Pathogenesis of Endometriosis: A Cutting-Edge Point of View. International Journal of Molecular Sciences, 21(3), 1114. https://doi.org/10.3390/ijms21031114










