The Effect of Selected Dental Materials Used in Conservative Dentistry, Endodontics, Surgery, and Orthodontics as Well as during the Periodontal Treatment on the Redox Balance in the Oral Cavity
Abstract
1. Introduction
2. Materials and Methods
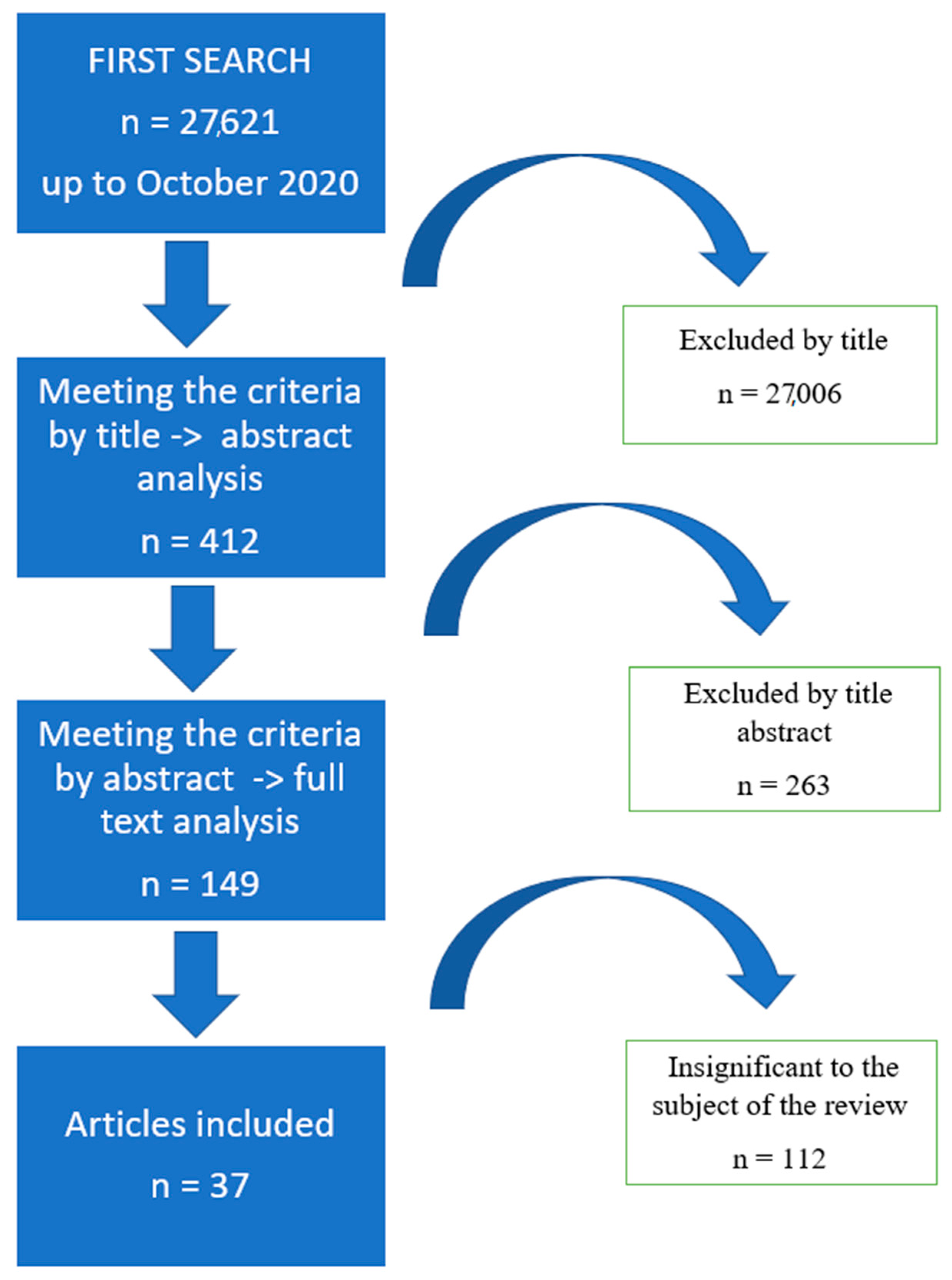
2.1. Search Strategy
2.2. Inclusion Criteria
- Works on redox disorders related to dental treatment, dental fillings, dental monomers, endodontic treatment, titanium implants, treatment of periodontal diseases, whitening.
- Results obtained from experiments participated by human subjects, as well as experimental works.
- Publications in English only.
- Clinical trials on a group of at least five individuals.
2.3. Exclusion Criteria
- Works written in languages other than English.
- Clinical trials on a group of fewer than five individuals.
- Meta-analyzes.
- Publications on the redox balance in the treatment of neurocranial diseases and cancer.
- Publications referring to prosthetic treatment and treatment of functional disorders of the masticatory organ.
- Case studies.
- Among the publications based on human material we excluded those that covered subjects with systemic diseases.
3. Data Extraction
4. Results
5. Amalgam
6. Glass-Ionomer Cement
7. Dental Resin Composites
8. Composite Resins
9. Endodontic Treatment
10. Orthodontic Braces
11. Fixations and Dental Implants
12. Periodontology
13. Whitening
14. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Maciejczyk, M.; Mikoluc, B.; Pietrucha, B.; Heropolitanska—Pliszka, E.; Pac, M.; Motkowski, R.; Car, H. Oxidative stress, mitochondrial abnormalities and antioxidant defense in Ataxia-telangiectasia, Bloom syndrome and Nijmegen breakage syndrome. Redox Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S. Oxidative Stress, Inflammation, and Disease; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 35–58. [Google Scholar]
- Sardaro, N.; della Vella, F.; Incalza, M.A.; Stasio, D.D.I.; Lucchese, A.; Contaldo, M.; Laudadio, C.; Petruzzi, M. Oxidative stress and oral mucosal diseases: An overview. In Vivo 2019, 33, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Gallorini, M.; Cataldi, A.; di Giacomo, V. HEMA-induced cytotoxicity: Oxidative stress, genotoxicity and apoptosis. Int. Endod. J. 2014, 47, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, A.; Maciejczyk, M.; Szulimowska, J.; Imierska, M.; Błachnio-Zabielska, A. High-fat diet affects ceramide content, disturbs mitochondrial redox balance, and induces apoptosis in the submandibular glands of mice. Biomolecules 2019, 9, 877. [Google Scholar] [CrossRef] [PubMed]
- Pawlukianiec, C.; Gryciuk, M.E.; Mil, K.M.; Żendzian-Piotrowska, M.; Zalewska, A.; Maciejczyk, M. A New Insight into Meloxicam: Assessment of Antioxidant and Anti-Glycating Activity in In Vitro Studies. Pharmaceuticals 2020, 13, 240. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar] [CrossRef] [PubMed]
- Reeg, S.; Grune, T. Protein Oxidation in Aging: Does It Play a Role in Aging Progression? Antioxid. Redox Signal. 2015, 23, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Maciejczyk, M.; Szulimowska, J.; Taranta-Janusz, K.; Wasilewska, A.; Zalewska, A. Salivary Gland Dysfunction, Protein Glycooxidation and Nitrosative Stress in Children with Chronic Kidney Disease. J. Clin. Med. 2020, 1285. [Google Scholar] [CrossRef] [PubMed]
- Branco, V.; Canário, J.; Holmgren, A.; Carvalho, C. Inhibition of the thioredoxin system in the brain and liver of zebra-seabreams exposed to waterborne methylmercury. Toxicol. Appl. Pharmacol. 2011, 251, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Naito, Y.; Yoshikawa, T.; Niki, E. Redox Biology Plasma lipid oxidation induced by peroxynitrite, hypochlorite, lipoxygenase and peroxyl radicals and its inhibition by antioxidants as assessed by diphenyl-1-pyrenylphosphine. Redox Biol. 2016, 8, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Skutnik-Radziszewska, A.; Maciejczyk, M.; Fejfer, K.; Krahel, J.; Flisiak, I.; Kołodziej, U.; Zalewska, A. Salivary Antioxidants and Oxidative Stress in Psoriatic Patients: Can Salivary Total Oxidant Status and Oxidative Status Index Be a Plaque Psoriasis Biomarker? Oxid. Med. Cell. Longev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Gerreth, P.; Maciejczyk, M.; Zalewska, A.; Gerreth, K.; Hojan, K. Comprehensive Evaluation of the Oral Health Status, Salivary Gland Function, and Oxidative Stress in the Saliva of Patients with Subacute Phase of Stroke: A Case-Control Study. J. Clin. Med. 2020, 2252. [Google Scholar] [CrossRef] [PubMed]
- Sawczuk, B.; Maciejczyk, M.; Sawczuk-Siemieniuk, M.; Posmyk, R.; Zalewska, A.; Car, H. Salivary gland function, antioxidant defence and oxidative damage in the saliva of patients with breast cancer: Does the BRCA1 mutation disturb the salivary redox profile? Cancers 2019, 11, 1501. [Google Scholar] [CrossRef] [PubMed]
- Waddington, R.J.; Moseley, R.; Embery, G. Reactive oxygen species: A potential role in the pathogenesis of periodontal diseases. Oral. Dis. 2000, 6, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Zińczuk, J.; Maciejczyk, M.; Zaręba, K.; Romaniuk, W.; Markowski, A.; Kędra, B.; Zalewska, A.; Pryczynicz, A.; Matowicka-Karna, J.; Guzińska-Ustymowicz, K. Antioxidant Barrier, Redox Status, and Oxidative Damage to Biomolecules in Patients with Colorectal Cancer. Can Malondialdehyde and Catalase Be Markers of Colorectal Cancer Advancement? Biomolecules 2019, 9, 637. [Google Scholar] [CrossRef] [PubMed]
- Maciejczyk, M.; Szulimowska, J.; Skutnik, A.; Taranta-Janusz, K.; Wasilewska, A.; Wiśniewska, N.; Zalewska, A. Salivary Biomarkers of Oxidative Stress in Children with Chronic Kidney Disease. J. Clin. Med. 2018, 7, 209. [Google Scholar] [CrossRef]
- Żukowski, P.; Maciejczyk, M.; Waszkiel, D. Sources of free radicals and oxidative stress in the oral cavity. Arch. Oral Biol. 2018, 92, 8–17. [Google Scholar] [CrossRef]
- Celik, N.; Binnetoglu, D.; Ozakar Ilday, N.; Hacimuftuoglu, A.; Seven, N. The cytotoxic and oxidative effects of restorative materials in cultured human gingival fibroblasts. Drug Chem. Toxicol. 2019, 31, 1–6. [Google Scholar] [CrossRef]
- Ramezani, G.H.; Moghadam, M.M.; Saghiri, M.A.; Garcia-Godoy, F.; Asatourian, A.; Aminsobhani, M.; Scarbecz, M.; Sheibani, N. Effect of dental restorative materials on total antioxidant capacity and calcium concentration of unstimulated saliva. J. Clin. Exp. Dent. 2017, 9, e71–e77. [Google Scholar] [CrossRef]
- Daokar, S.G.; Shahu, C.; Shikshan, M.; Mustafa, M. Assessment of Oxidative Stress Induced by Various Restorative Materials: An In Vivo Biochemical Study. J. Int. Oral Health 2016, 8, 1–6. [Google Scholar]
- Al-Saleh, I.; Al-Sedairi, A.; Elkhatib, R. Effect of mercury (Hg) dental amalgam fillings on renal and oxidative stress biomarkers in children. Sci. Total Environ. 2012, 431, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Cabaña-Muñoz, M.E.; Parmigiani-Izquierdo, J.M.; Bravo-González, L.A.; Kyung, H.M.; Merino, J.J. Increased Zn/glutathione levels and higher superoxide dismutase-1 activity as biomarkers of oxidative stress in women with long-term dental amalgam fillings: Correlation between mercury/aluminium levels (in hair) and antioxidant systems in plasma. PLoS ONE 2015, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yıldız, M.; Alp, H.H.; Gül, P.; Bakan, N.; Özcan, M. Lipid peroxidation and DNA oxidation caused by dental filling materials. J. Dent. Sci. 2017, 12, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Ma, S.; Wang, Y.; Li, J.; Shan, L.; Liu, Q.; Liu, Y.; Song, Q.; Yu, F.; Yu, H.; et al. N-Acetyl cysteine depletes reactive oxygen species and prevents dental monomer-induced intrinsic mitochondrial apoptosis in vitro in human dental pulp cells. PLoS ONE 2016, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.R.; Hakami-Tafreshi, R.; Tomasino-Perez, A.; Tayebi, L.; Lobner, D. Effects of dental composite resin monomers on dental pulp cells. Dent. Mater. J. 2019, 38, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Guo, M.K.; Kasten, F.H.; Chang, M.C.; Huang, G.F.; Wang, Y.L.; Wang, R.S.; Jeng, J.H. Stimulation of glutathione depletion, ROS production and cell cycle arrest of dental pulp cells and gingival epithelial cells by HEMA. Biomaterials 2005, 26, 745–753. [Google Scholar] [CrossRef]
- Lefeuvre, M.; Amjaad, W.; Goldberg, M.; Stanislawski, L. TEGDMA induces mitochondrial damage and oxidative stress in human gingival fibroblasts. Biomaterials 2005, 26, 5130–5137. [Google Scholar] [CrossRef]
- Diomede, F.; Marconi, G.D.; Guarnieri, S.; D’Attilio, M.; Cavalcanti, M.F.X.B.; Mariggiò, M.A.; Pizzicannella, J.; Trubiami, O. A Novel Role of Ascorbic Acid in Anti-Inflammatory Pathway and ROS Generation in HEMA Treated Dental Pulp Stem Cells. Materials 2020, 130. [Google Scholar] [CrossRef]
- Di Nisio, C.; Zara, S.; Cataldi, A.; di Giacomo, V. 2-Hydroxyethyl methacrylate inflammatory effects in human gingival fibroblasts. Int. Endod. J. 2013, 46, 466–476. [Google Scholar] [CrossRef]
- Perduns, R.; Volk, J.; Schertl, P.; Leyhausen, G.; Geurtsen, W. HEMA modulates the transcription of genes relatedto oxidative defense, inflammatory response andorganization of the ECM in human oral cells. Dent. Mater. 2019, 35, 501–510. [Google Scholar] [CrossRef]
- Jiao, Y.; Niu, T.; Liu, H.; Tay, F.R.; Chen, J. Protection against HEMA-Induced Mitochondrial Injury In Vitro by Nrf2 Activation. Oxid. Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Teti, G.; Orsini, G.; Salvatore, V.; Focaroli, S.; Mazzotti, M.C.; Ruggeri, A.; Mattioli-belmonte, M.; Falconi, M. HEMA but not TEGDMA induces autophagy in human gingival fibroblasts. Front. Physiol. 2015, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Krifka, S.; Seidenader, C.; Hiller, K.A.; Schmalz, G.; Schweikl, H. Oxidative stress and cytotoxicity generated by dental composites in human pulp cells. Clin. Oral Investig. 2012, 16, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Pauly, K.; Fritz, K.; Furey, A.; Lobner, D. Insulin-like Growth Factor 1 and Transforming Growth Factor-β Stimulate Cystine/Glutamate Exchange Activity in Dental Pulp Cells. J. Endod. 2011, 37, 943–947. [Google Scholar] [CrossRef][Green Version]
- Huang, F.; Li, Y.; Lee, S.; Chang, Y. Cytotoxicity of dentine bonding agents on human pulp cells is related to intracellular glutathione levels. Int. Endod. J. 2010, 43, 1091–1097. [Google Scholar] [CrossRef]
- Gul, P.; Akgul, N.; Hakan, H. Effects of composite restorations on oxidative stress in saliva: An in vivo study. J. Dent. Sci. 2015, 10, 394–400. [Google Scholar] [CrossRef]
- Blasiak, J.; Synowiec, E. Dental methacrylates may exert genotoxic effects via the oxidative induction of DNA double strand breaks and the inhibition of their repair. Mol. Biol. Rep. 2012, 39, 7487–7496. [Google Scholar] [CrossRef] [PubMed]
- Ferrúa, C.P.; Leal, F.B.; de Oliveira Gazal, M.; Ghisleni, G.C.; de Carvalho, R.V.; Demarco, F.F.; Ogliari, F.A.; Nedel, F. Iodonium salt incorporation in dental adhesives and its relation with degree of conversion, ultimate tensile strength, cell viability, and oxidative stress. Clin. Oral Investig. 2019, 23, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Spalj, S.; Mlacovic Zrinski, M.; Tudor Spalj, V.; Ivankovic Buljan, Z. In-vitro assessment of oxidative stress generated by orthodontic archwires. Am. J. Orthod. Dentofac. Orthop. 2012, 141, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Buljan, Z.I.; Ribaric, S.P.; Abram, M.; Ivankovic, A.; Spalj, S. In vitro oxidative stress induced by conventional and self-ligating brackets. Angle Orthod. 2012, 82, 340–345. [Google Scholar] [CrossRef]
- Portelli, M.; Militi, A.; Cervino, G.; Lauritano, F.; Sambataro, S.; Mainardi, A.; Nucera, R. Oxidative Stress Evaluation in Patients Treated with Orthodontic Self-ligating Multibracket Appliances: An in Vivo Case-Control Study. Open Dent. J. 2017, 11, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Atuǧ Özcan, S.S.; Ceylan, I.; Özcan, E.; Kurt, N.; Daǧsuyu, I.M.; Çanakçi, C.F. Evaluation of oxidative stress biomarkers in patients with fixed orthodontic appliances. Dis. Mark. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Buczko, P.; Knaś, M.; Grycz, M.; Szarmach, I.; Zalewska, A. Orthodontic treatment modifies the oxidant–antioxidant balance in saliva of clinically healthy subjects. Adv. Med. Sci. 2017, 62, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Gong, T.; Pow, E.H.N.; Botelho, M.G. Adhesive and oxidative response of stem cell and pre-osteoblasts on titanium and zirconia surfaces in vitro. J. Investig. Clin. Dent. 2019, 10, e12407. [Google Scholar] [CrossRef] [PubMed]
- Borys, J.; Maciejczyk, M.; Antonowicz, B.; Krętowski, A.; Sidun, J.; Domel, E.; Dąbrowski, J.; Ładny, J.; Morawska, K.; Zalewska, A. Glutathione Metabolism, Mitochondria Activity, and Nitrosative Stress in Patients Treated for Mandible Fractures. J. Clin. Med. 2019, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Pietropaoli, D.; Ortu, E.; Severino, M.; Ciarrocchi, I.; Gatto, R.; Monaco, A. Glycation and oxidative stress in the failure of dental implants: A case series. BMC Res. Notes 2013, 6, 1. [Google Scholar] [CrossRef]
- Borys, J.; Maciejczyk, M.; Krȩtowski, A.J.; Antonowicz, B.; Ratajczak-Wrona, W.; Jablonska, E.; Zaleski, P.; Waszkiel, D.; Ladny, J.R.; Zukowski, P.; et al. The redox balance in erythrocytes, plasma, and periosteum of patients with titanium fixation of the jaw. Front. Physiol. 2017, 8, 386. [Google Scholar] [CrossRef]
- Borys, J.; Maciejczyk, M.; Antonowicz, B.; Sidun, J.; Świderska, M.Z.A. Free Radical Production, Inflammation and Apoptosis in Patients Treated With Titanium Mandibular Fixations—An Observational Study. Front. Immunol. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Borys, J.; Maciejczyk, M.; Antonowicz, B.; Krętowski, A.; Waszkiel, D.; Bortnik, P.; Czarniecka-Bargłowska, K.; Kocisz, M.; Szulimowska, J.; Czajkowski, M.; et al. Exposure to Ti4Al4V titanium alloy leads to redox abnormalities, oxidative stress, and oxidative damage in patients treated for mandible fractures. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Pillusky, F.M.; Barcelos, R.C.S.; Vey, L.T.; Barin, L.M.; de Mello Palma, V.; Maciel, R.M.; Kantorski, K.Z.; Bürger, M.E.; Danesi, C.C. Antimicrobial photodynamic therapy with photosensitizer in ethanol improves oxidative status and gingival collagen in a short-term in periodontitis. Photodiagnosis Photodyn. Ther. 2017, 19, 119–127. [Google Scholar] [CrossRef]
- Boia, S.; Stratul, Ş.I.; Boariu, M.; Ursoniu, S.; Goţia, S.L.; Boia, E.R.; Borza, C. Evaluation of antioxidant capacity and clinical assessment of patients with chronic periodontitis treated with non-surgical periodontal therapy and adjunctive systemic antibiotherapy. Rom. J. Morphol. Embryol. 2018, 59, 1107–1113. [Google Scholar] [PubMed]
- Tamaki, N.; Tomofuji, T.; Ekuni, D.; Yamanaka, R.; Yamamoto, T. Short—Term Effects of Non-Surgical Periodontal Treatment on Plasma Level of Reactive Oxygen Metabolites in Patients With Chronic Periodontitis. J. Periodontol. 2009, 80, 901–906. [Google Scholar] [CrossRef]
- Guo, M.; Liu, L.; Zhang, J.; Liu, M. Role of Reactive Oxygen Species and Advanced Glycation End Products in the Malfunctioning of Dental Implants. West. Indian Med. J. 2015, 64, 419–423. [Google Scholar] [PubMed]
- Mousavi Jazi, M.; Sadeghi Pour Rodsari, H.R.; Mirmiran, F. Level of Oxidative Stress Markers in Peri-Implant Crevicular Fluid and Their Correlation with Clinical Parameters. J. Dent. 2015, 12, 340–346. [Google Scholar]
- Leewananthawet, A.; Arakawa, S.; Okano, T.; Daitoku Kinoshita, R.; Ashida, H.; Izumi, Y.; Suzuki, T. Ozone ultrafine bubble water induces the cellular signaling involved in oxidative stress responses in human periodontal ligament fibroblasts. Sci. Technol. Adv. Mater. 2019, 20, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Del Real García, J.F.; Saldaña-Velasco, F.R.; Sánchez-de la Rosa, S.V.; Ortiz-García, Y.M.; Morales-Velazquez, G.; Gómez-Meda, B.C.; Zúñiga-González, G.M.; Sánchez-Parada, M.G.; Zamora-Perez, A.L. In vivo evaluation of the genotoxicity and oxidative damage in individuals exposed to 10% hydrogen peroxide whitening strips. Clin. Oral Investig. 2019, 23, 3033–3046. [Google Scholar] [CrossRef] [PubMed]
- Farina, M.; Aschner, M.; Rocha, J.B.T. Oxidative stress in MeHg-induced neurotoxicity. Toxicol. Appl. Pharmacol. 2011, 256, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.L.; Posser, T.; Dunkley, P.R.; Dickson, P.W.; Mattos, J.J.; Martins, R.; Bainy, A.C.D.; Marques, M.R.; Dafre, A.L.; Farina, M. Methylmercury neurotoxicity is associated with inhibition of the antioxidant enzyme glutathione peroxidase Jeferson. Free Radic. Biol. Med. 2009, 47, 449–457. [Google Scholar] [CrossRef]
- Glaser, V.; Leipnitz, G.; Raniel, M.; Oliveira, J.; Wannmacher, D.; Fabro, A.; Bem, D.; Valgas, V. Oxidative stress-mediated inhibition of brain creatine kinase activity by methylmercury. NeuroToxicology 2010, 31, 454–460. [Google Scholar] [CrossRef]
- Glaser, V.; Maria, E.; Maria, Y.; Müller, R.; Feksa, L.; Milton, C.; Wannmacher, D.; Batista, J.; Rocha, T.; Fabro, A.; et al. Effects of inorganic selenium administration in methylmercury-induced neurotoxicity in mouse cerebral cortex. Int. J. Dev. Neurosci. 2010, 28, 631–637. [Google Scholar] [CrossRef]
- Rocha, J.B.; Freitas, A.J.; Marques, M.B.; Pereira, M.E.; Emanuelli, T.; Souza, D. Effects of methylmercury exposure during the second stage of rapid postnatal brain growth on negative geotaxis and on delta-aminolevulinate dehydratase of suckling rats. Braz J. Med. Biol Res. 1993, 10, 1077–1083. [Google Scholar]
- Barbosa, A.C.; Jardim, W.; Dórea, J.G.; Fosberg, B.; Souza, J. Hair Mercury Speciation as a Function of Gender, Age, and Body Mass Index in Inhabitants of the Negro River Basin, Amazon, Brazil. Arch. Environ. Contam. Toxicol. 2001, 40, 439–444. [Google Scholar] [PubMed]
- Dorea, J. Environmental contaminants as biomarkers of fish intake: A case for hair mercury concentrations. Eur. J. Clin. Nutr. 2011, 65, 419–420. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nogueira, C.W.; Rocha, J.B. Diphenyl Diselenide a Janus-Faced Molecule. J. Braz. Chem. Soc. 2010, 21, 2055–2071. [Google Scholar] [CrossRef]
- Araie, H.; Shiraiwa, Y. Selenium Utilization Strategy by Microalgae. Molecules 2009, 14, 4880–4891. [Google Scholar] [CrossRef]
- Hollenberg, S.M.; Cinel, I. Bench-to-bedside review: Nitric oxide in critical illness—Update 2008. Crit. Care 2009, 9. [Google Scholar] [CrossRef]
- Lobanov, A.V.; Hatfield, D.L.; Gladyshev, V.N. Eukaryotic selenoproteins and selenoproteomes. Biochim. Biophys. Acta Gen. Subj. 2012, 1790, 1424–1428. [Google Scholar] [CrossRef]
- Tsuzuki, Y.Y.T. Inhibitory actions of mercury compounds against glucose-6-phosphate dehydrogenase from yeast. J. Toxicol. Sci. 1979, 4, 105–113. [Google Scholar] [CrossRef]
- Stringari, J.; Nunes, A.K.C.; Franco, J.L.; Bohrer, D.; Solange, C.; Dafre, A.L.; Milatovic, D.; Souza, D.O.; Rocha, J.B.T.; Farina, M. Prenatal methylmercury exposure hampers glutathione antioxidant system ontogenesis and causes long-lasting oxidative stress in the mouse brain. Toxicol. Appl. Pharmacol. 2010, 227, 147–154. [Google Scholar] [CrossRef]
- Farina, M.; Campos, F.; Vendrell, I.; Berenguer, J.; Barzi, M. Probucol Increases Glutathione Peroxidase-1 Activity and Displays Long-Lasting Protection against Methylmercury Toxicity in Cerebellar Granule Cells. Toxicol. Sci. 2009, 112, 416–426. [Google Scholar] [CrossRef]
- Wagner, C.; Sudati, H.; Nogueira, W.; Rocha, J. In vivo and in vitro inhibition of mice thioredoxin reductase by methylmercury. Biometals 2010, 23, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, N.M.A.Y.K. Comparative study of activities in reactive oxygen species production/defense system in mitochondria of rat brain and liver, and their susceptibility to methylmercury toxicity. Arch. Toxicol 2007, 769–776. [Google Scholar]
- Wu, L.L.; Chiou, C.; Chang, P.; Wu, J.T. Urinary 8-OHdG: A marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin. Chim. Acta 2004, 339, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Mukherjee, S.; Ngo, L.; Christiani, D.C. Urinary 8-hydroxy-2′-deoxyguanosine as a biomarker of oxidative DNA damage in workers exposed to fine particulates. Environ. Health Perspect. 2004, 112, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Qu, L.; Li, B.; Xing, L.; Jia, G.; Wang, T.; Gao, Y.; Zhang, P.; Li, M.; Chen, W.; et al. Increased Oxidative DNA Damage, as Assessed by Urinary 8-Hydroxy-2′-Deoxyguanosine Concentrations, and Serum Redox Status in Persons Exposed to Mercury. Clin. Chem. 2005, 767, 759–767. [Google Scholar] [CrossRef]
- Ohno, T.; Sakamoto, M.; Kurosawa, T.; Dakeishi, M. Total mercury levels in hair, toenail, and urine among women free from occupational exposure and their relations to renal tubular function. Environ. Res. 2007, 103, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Selimović-Dragaš, M.; Huseinbegović, A.; Kobašlija, S.; Hatibović-Kofman, Š. A comparison of the in vitro cytotoxicity of conventional and resin modifi ed glass ionomer cements. Assoc. Basic Med. Sci. FBIH 2012, 12, 273–278. [Google Scholar] [CrossRef]
- Van Landuyt, K.L.; Nawrot, T.; Geebelen, B.; De Munck, J.; Snauwaert, J.; Yoshihara, K.; Scheers, H.; Godderis, L.; Hoet, P.; Van Meerbeek, B. How much do resin-based dental materials release? A meta-analytical approach. Dent. Mater. 2011, 27, 723–747. [Google Scholar] [CrossRef] [PubMed]
- Schweikl, H.; Spagnuolo, G.; Schmalz, G. Genetic and cellular toxicology of dental resin monomers. J. Dent. Res. 2006, 85, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Maciejczyk, M.; Heropolitanska-Pliszka, E.; Pietrucha, B.; Sawicka-Powierza, J.; Bernatowska, E.; Wolska-Kusnierz, B.; Pac, M.; Car, H.; Zalewska, A.; Mikoluc, B. Antioxidant defense, redox homeostasis, and oxidative damage in children with ataxia telangiectasia and nijmegen breakage syndrome. Front. Immunol. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Coelho, A.S.; Laranjo, M.; Gonçalves, A.C.; Paula, A.; Paulo, S.; Abrantes, A.M.; Caramelo, F.; Ferreira, M.M.; Silva, M.J.; Carrilho, E.; et al. Cytotoxic effects of a chlorhexidine mouthwash and of an enzymatic mouthwash on human gingival fibroblasts. Odontology 2020, 108, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Samuelsen, J.T.; Kopperud, H.M.; Holme, J.A.; Dragland, I.S.; Christensen, T.; Dahl, J.E. Role of thiol-complex formation in 2-hydroxyethyl- methacrylate-induced toxicity in vitro. J. Biomed. Mater. Res. 2010, 96, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Nocca, G.; De Palma, F.; Minucci, A.; De Sole, P.; Martorana, G.E.; Callà, C.; Morlacchi, C.; Gozzo, M.L.; Gambarini, G.; Chimenti, C.; et al. Alterations of energy metabolism and glutathione levels of HL-60 cells induced by methacrylates present in composite resins. J. Dent. 2007, 35, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Krifka, S.; Hiller, K.; Spagnuolo, G.; Jewett, A.; Schmalz, G.; Schweikl, H. The influence of glutathione on redox regulation by antioxidant proteins and apoptosis in macrophages exposed to 2-hydroxyethyl methacrylate (HEMA). Biomaterials 2012, 33, 5177–5186. [Google Scholar] [CrossRef] [PubMed]
- Schweikl, H.; Petzel, C.; Bolay, C.; Hiller, K.; Buchalla, W.; Krifka, S. 2-Hydroxyethyl methacrylate-induced apoptosis through the ATM- and p53-dependent intrinsic mitochondrial pathway. Biomaterials 2014, 35, 2890–2904. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, A.; Szarmach, I.; Żendzian-Piotrowska, M.; Maciejczyk, M. The effect of N-acetylcysteine on respiratory enzymes, ADP/ATP ratio, glutathione metabolism, and nitrosative stress in the salivary gland mitochondria of insulin resistant rats. Nutrients 2020, 458. [Google Scholar] [CrossRef]
- Mikulás, K.; Hermann, P.; Gera, I.; Komlódi, T. Triethylene glycol dimethacrylate impairs bioenergetic functions and induces oxidative stress in mitochondria via inhibiting respiratory Complex. Dent. Mater. 2018, 1–16. [Google Scholar] [CrossRef]
- Gomes, A.P.; Price, N.L.; Ling, A.J.Y.; Moslehi, J.J.; Montgomery, M.K.; Rajman, L.; Teodoro, S.; Wrann, C.D.; Hubbard, B.P.; Mercken, E.M.; et al. Declining NAD+ Induces a Pseudohypoxic State Disrupting Nuclear-Mitochondrial Communication during Aging. Cell 2013, 155. [Google Scholar] [CrossRef]
- Styllou, P.; Styllou, M.; Hickel, R.; Högg, C.; Reichl, F.X.; Scherthan, H. NAC ameliorates dental composite-induced DNA double-strand breaks and chromatin condensation. Dent. Mater. J. 2017, 36, 638–646. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Durante, W. Protective Role of Heme Oxygenase-1 against Inflammation in Atherosclerosis. Front. Biosci 2018, 16, 2372–2388. [Google Scholar] [CrossRef] [PubMed]
- Gozzelino, R.; Jeney, V.; Soares, M.P. Mechanisms of Cell Protection by Heme Oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 323–354. [Google Scholar] [CrossRef] [PubMed]
- Gallorini, M.; Petzel, C.; Bolay, C.; Hiller, K.; Cataldi, A.; Buchalla, W.; Krifka, S.; Schweikl, H. Biomaterials Activation of the Nrf2-regulated antioxidant cell response inhibits HEMA-induced oxidative stress and supports cell viability. Biomaterials 2015, 56, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lim, B.S.; Lee, Y.K.; Ahn, S.J.; Yang, H.C. Involvement of oxidative stress in mutagenicity and apoptosis caused by dental resin monomers in cell cultures. Dent. Mater. 2006, 22, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Krifka, S.; Spagnuolo, G.; Schmalz, G.; Schweikl, H. A review of adaptive mechanisms in cell responses towards oxidative stress caused by dental resin monomers. Biomaterials 2013, 34, 4555–4563. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.M.; Petermann, E. Replication fork dynamics and the DNA damage response. Biochem. J. 2012, 26, 13–26. [Google Scholar] [CrossRef]
- Ansteinsson, V.; Solhaug, A.; Samuelsen, J.T.; Holme, J.A.; Dahl, J.E. DNA-damage, cell-cycle arrest and apoptosis induced in BEAS-2B cells by 2-hydroxyethyl methacrylate (HEMA). Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2011, 723, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Ansteinsson, V. In Vitro Toxicity of Filler Particles and Methacrylates Used in Dental Composite Materials Cytokine Release and Cell Death. Ph.D. Thesis, The University of Bergen, Bergen, Norway, 2013. [Google Scholar]
- Popal, M.; Volk, J.; Leyhausen, G.; Geurtsen, W. Cytotoxic and genotoxic potential of the type I photoinitiators BAPO and TPO on human oral keratinocytes and V79 fibroblasts. Dent. Mater. 2018, 34, 1783–1796. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, F.; Yoshida, A.; Okada, E.; Okada, Y.; Maehata, Y.; Miyamoto, C.; Kishimoto, S.; Otsuka, T.; Nishimura, T.; Lee, M.C. Il Dental resin curing blue light induced oxidative stress with reactive oxygen species production. J. Photochem. Photobiol. B Biol. 2012, 114, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Oktay, E.A.; Tort, H.; Yıldız, O.; Ulusoy, K.G.; Topcu, F.T.; Ozer, C. Dental resin curing blue light induces vasoconstriction through release of hydrogen peroxide. J. Photochem. Photobiol. B Biol. 2018, 185, 41–45. [Google Scholar] [CrossRef]
- Botton, G.; Pires, C.W.; Cadoná, F.C.; Machado, A.K.; Azzolin, V.F.; Cruz, I.B.M.; Sagrillo, M.R.; Praetzel, J.R. Toxicity of irrigating solutions and pharmacological associations used in pulpectomy of primary teeth. Int. Endod. J. 2016, 49, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Saghiri, M.A.; Delvarani, A.; Mehrvarzfar, P.; Nikoo, M.; Lotfi, M.; Karamifar, K.; Asgar, K.; Dadvand, S. The impact of pH on cytotoxic effects of three root canal irrigants. Saudi Dent. J. 2011, 23, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.; Marins, R.; Sassone, L.M.; Fidel, S.R.; Ribeiro, D.A. In Vitro Genotoxicity and Cytotoxicity in Murine Fibroblasts Exposed to EDTA, NaOCl, MTAD and Citric Acid. Braz. Dent. J. 2012, 23, 527–533. [Google Scholar]
- Singh, H.; Khurana, H.; Singh, H.; Singh, M. Photodynamic therapy: Truly a marriage between a drug and a light. Muller J. Med. Sci. Res. 2014, 5, 48–55. [Google Scholar] [CrossRef]
- Pires, C.W.; Botton, G.; Cadoná, F.C.; Machado, A.K.; Azzolin, V.F.; da Cruz, I.B.M.; Sagrillo, M.R.; Praetzel, J.R. Induction of cytotoxicity, oxidative stress and genotoxicity by root filling pastes used in primary teeth. Int. Endod. J. 2016, 49, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Barbin, L.E.; Estrela, C.; Guedes, D.F.C.; Spanó, J.C.E.; Sousa-Neto, M.D.; Pécora, J.D. Detection of para-chloroaniline, reactive oxygen species, and 1-chloro-4-nitrobenzene in high concentrations of chlorhexidine and in a mixture of chlorhexidine and calcium hydroxide. J. Endod. 2013, 5, 664–668. [Google Scholar] [CrossRef]
- Victoria-Escandell, A.; Ibañez-Cabellos, J.S.; De Cutanda, S.B.; Berenguer-Pascual, E.; Beltrán-García, J.; García-López, E.; Pallardó, F.V.; García-Giménez, J.L.; Pallarés-Sabater, A.; Zarzosa-López, I.; et al. Cellular Responses in Human Dental Pulp Stem Cells Treated with Three Endodontic Materials. Stem. Cells Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.G.; Lee, Y.H.; Lee, N.H.; Bhattarai, G.; Lee, I.K.; Yun, B.S.; Yi, H.K. The Antioxidant Property of Pachymic Acid Improves Bone Disturbance against AH Plus–induced Inflammation in MC-3T3 E1 Cells. J. Endod. 2013, 39, 461–466. [Google Scholar] [CrossRef]
- Chang, S.-W.; Lee, S.-Y.; Ann, H.-J.; Kum, K.-Y.; Kim, E.-C. Effects of Calcium Silicate Endodontic Cements on Biocompatibility and Mineralization-inducing Potentials in Human Dental Pulp Cells. J. Endod. 2014, 40, 1194–1200. [Google Scholar] [CrossRef]
- Loison-robert, L.S.; Tassin, M.; Bonte, E. In vitro effects of two silicate-based materials, Biodentine and BioRoot RCS, on dental pulp stem cells in models of reactionary and reparative dentinogenesis. PLoS ONE 2018, 13, 1–19. [Google Scholar] [CrossRef]
- Trombetta, D.; Mondello, M.R.; Cimino, F.; Cristani, M.; Pergolizzi, S.; Saija, A. Toxic effect of nickel in an in vitro model of human oral epithelium. Toxicol. Lett. 2005, 159, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Gholinejad, Z.; Ansari, M.H.K.; Rasmi, Y. Titanium dioxide nanoparticles induce endothelial cell apoptosis via cell membrane oxidative damage and p38, PI3K/Akt, NF-κB signaling pathways modulation. J. Trace Elem. Med. Biol. 2019, 54, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.C.; Pazin, M.; Franco-Bernardes, M.F.; Martins, C.; Barcelos, R.M.; Pereira, C.M.; Rodrigues, J.L.; Barbosa, F., Jr.; Dorta, D.J. A Perspective of Mitochondrial Dysfunction in Rats Treated with Silver and Titanium Nanoparticles (AgNPs and TiNPs). J. Trace Elem. Med. Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Xu, Y.; Zhang, L.; Ye, D.; Feng, X.; Fu, T. Enhancement of Apoptosis by Titanium Alloy Internal Fixations during Microwave Treatments for Fractures: An Animal Study. PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, A.S.; Macaulay, W.; Stefanovic-racic, M.; Rubash, H.E. Nitric oxide release by macrophages in response to particulate wear debris. J. Biomed. Maters Res. 1998, 3, 497–503. [Google Scholar] [CrossRef]
- Mouthuy, P.; Snelling, S.J.B.; Dakin, S.G.; Milković, L.; Gašparović, Č.; Carr, A.J.; Žarković, N. Biocompatibility of implantable materials: An oxidative stress viewpoint. Biomaterials 2016. [Google Scholar] [CrossRef]
- Cabaña-Muñoz, M.E.; Parmigiani-Izquierdo, J.M.; Alonso, F.C.; Merino, J. Increased Systemic Malondialdehyde Levels and Decreased Mo/Co, Mo/Hg2+, Co/Fe2+ Ratios in Patients with Long-Term Dental Titanium Implants and Amalgams. J. Clin. Med. 2019, 8, 86. [Google Scholar] [CrossRef]
- Sánchez-Siles, M.; Lucas-Azorin, J.; Salazar-Sánchez, N.; Carbonell-Meseguer, L.; Camacho-Alonso, F. Salivary Concentration of Oxidative Stress Biomarkers in a Group of Patients with Peri-Implantitis: A Transversal Study. Clin. Implant. Dent. 2015, 18, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Toczewska, J.; Maciejczyk, M.; Konopka, T.; Zalewska, A. Total Oxidant and Antioxidant Capacity of Gingival Crevicular Fluid and Saliva in Patients with Periodontitis: Review and Clinical Study. Antioxidants 2020, 9, 450. [Google Scholar] [CrossRef] [PubMed]
- Toczewska, J.; Konopka, T.; Zalewska, A. Nitrosative Stress Biomarkers in the Non-Stimulated and Stimulated Saliva, as well as Gingival Crevicular Fluid of Patients with Periodontitis: Review and Clinical Study. Antioxidants 2020, 9, 259. [Google Scholar] [CrossRef]
- Yeung, S.Y.; Huang, C.S.; Chan, C.P.; Lin, C.P.; Lin, H.N.; Lee, P.H.; Jia, H.W.; Huang, S.K.; Jeng, J.H.; Chang, M.C. Antioxidant and pro-oxidant properties of chlorhexidine and its interaction with calcium hydroxide solutions. Int. Endod. J. 2007, 40, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Iwata, S.; Iizuka, J.; Takahashi, S.; Wada-takahashi, S.; Miyamoto, C.; Maehata, Y.; Ogura, Y.; Lee, M.; Yo, F. Blue Light from Dental Resin Curing Unit Causes Light-Induced Vasocon- striction in Isolated Rat Aorta. OHDM 2014, 13, 1147–1151. [Google Scholar]
- Jha, N.; Ryu, J.J.; Choi, E.H.; Kaushik, N.K. Generation and Role of Reactive Oxygen and Nitrogen Species Induced by Plasma, Lasers, Chemical Agents, and Other Systems in Dentistry. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.T.; Li, L.F.; Du, R.; Jiang, L.; Zhu, Y.Q. Hydrogen peroxide induces apoptosis in human dental pulp cells via caspase-9 dependent pathway. J. Endod. 2013, 39, 1151–1155. [Google Scholar] [CrossRef]
- Marto, C.M.; Laranjo, M.; Paula, A.; Coelho, A.S.; Abrantes, A.M.; Casalta-Lopes, J.; Gonçalves, A.C.; Sarmento-Ribeiro, A.B.; Ferreira, M.M.; Cabrita, A.; et al. Cytotoxic effects of zoom® whitening product in human fibroblasts. Materials 2020, 13, 1491. [Google Scholar] [CrossRef]
- Zalewska, A.; Ziembicka, D.; Zendzian-Piotrowska, M.; MacIejczyk, M. The impact of high-fat diet on mitochondrial function, free radical production, and nitrosative stress in the salivary glands of wistar rats. Oxid. Med. Cell. Longev. 2019. [Google Scholar] [CrossRef]
- Matczuk, J.; Zendzian-Piotrowska, M.; Maciejczyk, M.; Kurek, K. Salivary lipids: A review. Adv. Clin. Exp. Med. 2017. [Google Scholar] [CrossRef]
- Lima, A.F.; Lessa, F.C.; Hebling, J.; de Souza Costa, C.A.; Marchie, G.M. Protective Effect of Sodium Ascorbate on MDPC-23 Odontoblast-Like Cells Exposed to a Bleaching Agent. Eur. J. Dent. 2010, 4, 238–244. [Google Scholar]
- Vargas, S.; Soares, D.G.; Paula, A.; Ribeiro, D.; Hebling, J.; Alberto, C.; Costa, D.S. Protective Effect of Alpha-Tocopherol Isomer from Vitamin E against the H2O2 Induced Toxicity on Dental Pulp Cells. Biomed. Res. Int. 2014, 2014, 1–5. [Google Scholar] [CrossRef]
| Experimental Model | Endpoints | References |
|---|---|---|
| Amalgam | ||
| Human gingival fibroblast cells (HGFCs) exposed to microhybrid resin-based composite, compomer resin, glass-ionomer cement and amalgam alloy for 7 and 21 days | The levels of total oxidant status (TOS) in the study groups (i.e., samples with the following materials: microhybrid resin-based composite, compomer resin, glass-ionomer cement and amalgam alloy; shaped as a 2-mm-thick disk with a diameter of 10 mm; exposed to light with the wavelength of 430–480 nm and intensity of 1200 mW/cm2) were significantly higher in freshly prepared samples compared to the control. After 7 and 21 days, TOS level in the amalgam sample was considerably lower than at the beginning of the study. The highest level of total antioxidant capacity (TAC) was observed after 7 days in the filling with glass-ionomer cement (which prevented TOS increase). In all studied groups, TAC level after 7 days was different than at the initial stage of the study. | [19] |
| Unstimulated saliva of 48 generally healthy children aged 6–10 (24 males, 24 females) with two class II dental composite or amalgam restorations and the control (caries-free) group | The saliva of patients with composite fillings had significantly higher TAC compared to patients with amalgam fillings as well as caries-free subjects. However, TAC in patients with amalgam restorations was also significantly higher compared to the caries-free control. Patients with composite fillings also demonstrated decreased salivary levels of Ca2+ ions. | [20] |
| Saliva from 60 generally healthy subjects aged 15–40 with class I restorations of: amalgam (20 participants), composite (20 subjects) and glass-ionomer (20 patients), collected before the filling as well as 24 h and 7 and 14 days after the filling | Malondialdehyde (MDA) level in the saliva of patients with an amalgam filling was found to be higher than in patients with a composite or glass-ionomer filling. Significant differences were also observed between MDA concentrations on day 7 and 14, and after 24 h and 7 days in patients with composite fillings. There were no differences in MDA levels before treatment and 7 days after, or before and 14 days after the treatment. In the case of glass-ionomer, a significant difference was found only between 24 h and 7 days after the treatment. | [21] |
| Urine collected from 106 generally healthy children aged 5–15.5 years with amalgam fillings | It was shown that in children with amalgam filling, there was a reduced excretion of 8-hydroxy-2-deoxyguanosine (8-OHdG) in the urine. It was also shown that the level of NAG in the urine of children with amalgam fillings was significantly higher compared to children without such fillings and was positively correlated with the level of MDA in the urine. There was no correlation between the concentration of 8-OHdG and malondialdehyde (MDA) in the urine of amalgam-filled children. The mercury (Hg) level was also significantly higher in children with amalgam fillings compared to children without amalgam fillings; however, no relationship was found between the Hg level and the number of fillings. | [22] |
| Hair samples collected from 42 generally healthy women (mean age 44 years) with amalgam fillings applied at least 10 years earlier | An increased activity of SOD-1 and an increase in GSH concentration in the hair of women with amalgam fillings as compared to women without such fillings were observed. A positive correlation was also shown between the concentration of aluminum (Al) and the concentration of GSH, and between the level of mercury (Hg) and the activity of SOD-1. | [23] |
| Blood collected from 41 generally healthy patients (17–23 years old), which used amalgam (19) and dental resin composite (22) fillings | A significant increase in the level of malondialdehyde (MDA) was observed 24 h after placing amalgam and composite filling. There were no changes in the concentration of 8-OHdG in women 24 h after the placement of the amalgam filling. The 8-OHdG level increased 24 h after placing the dental resin composite filling. | [24] |
| Dental Resin Composites—monomers | ||
| Human dental pulp cells (hDPCs) exposed to dental monomers (1 mM HEMA, 5 mM MMA and 1 mM TEGDMA) without and in the presence of 10 mM NAC for 24, 48, 72 and 96 h | In response to 6 h of exposure to dental monomers: 2-hydroxyethyl methacrylate (HEMA), triethylene glycol dimethacrylate (TEGDMA) and methyl methacrylate (MMA), there was a significant increase in ROS production in hDPCs compared to the control group without dental monomers. The addition of N-acetyl cysteine (NAC) decreased ROS production in the monomer-treated group. The presence of monomers also GSH level, which was observed for NAC as well, but to a lesser extent. No significant differences in the content of GSSG (oxidized disulfide) were observed for HEMA and MMA monomers, and a slight GSSG decrease was noted for TEGDMA (triethylene glycol dimethacrylate). In the case of dental monomers, MDA level increased, and after adding NAC—MDA level dropped almost to its level observed in the control group. Moreover, SOD activity decreased in the presence of all dental monomers, which was not observed after the addition of NAC. After 24 h of cell exposure to monomers, CAT activity increased significantly, and decreased after the use of NAC. | [25] |
| Human dental pulp cells isolated form third molars, exposed to dental monomers (bisphenol-A-glycidyl Methacrylate, Bis-GMA; urethane dimethacrylate, UDMA; and triethylene glycol dimethacrylate, TEGDMA) at concentrations of 10, 30, 100, 300 µm for 48 h | The level of free radicals was measured after 48 h of monomer action by means of 2′,7′-dichlorodihydrofluorescein diacetate (DCF) fluorescent dye, and it was observed that Bis-GMA and UDMA, at high concentrations (30, 100), induced a significant increase in oxidative stress, while the TEGDMA monomer did not trigger OS at any concentration. All monomers reduced the level of GSH. | [26] |
| Smulow-Glickman (S-G) human gingival epithelial cells and pulp fibroblasts (HPF) exposed to HEMA at the concentrations of 0.01–10 mm for 24 h | Higher HEMA concentrations (1, 2.5, 5, 10) caused a significant increase in the level of intracellular ROS in cells exposed to the monomer. | [27] |
| Gingival fibroblasts obtained during the extraction of premolars for orthodontic reasons, exposed to TEGDMA at a concentration of 0.6 mM and 1 mM for 15 min to 6 h | 15-min exposure to TEGDMA significantly reduced the concentration of intracellular GSH compared to cells not exposed to this monomer. It was also demonstrated that TEGDMA-induced time-dependent increase of thiobarbituric acid reactive substances (TBARS), which indicates increased lipid peroxidation. | [28] |
| Human dental pulp stem cells (isolated from third molars) exposed to monomer HEMA (at a concentration of 2 mM) and AC (at 50 µg·mL−1) for 24 h | 2-hydroxyethyl methacrylate (HEMA) increased the level of reactive oxygen species (ROS), pro-inflammatory mediators such as nuclear factor-κB (NF-kB) and inflammatory cytokines such as interleukin. In the presence of vitamin C, these changes were less noticeable. This indicates a protective effect of vitamin C on the dental pulp cells. | [29] |
| Human gingival fibroblasts (HGFs) treated with a relatively low level of 2-hydroxyethyl methacrylate (HEMA) for 0, 24 and 96 h | After 24 and 96 h of HGF exposure to the HEMA monomer (3 mmol·L−1), it was observed that ROS levels increased 8 and 11 times compared to the control not exposed to the monomer. | [30] |
| Primary human gingival fibroblasts (HGFs) and immortalized oral keratinocyte cell line OKF6/TERT2 treated with 2-hydroxyethyl methacrylate (HEMA) at a concentration of 0.5–10 mM | Significantly induced transcription of genes related to defense against oxidative stress was demonstrated for: nuclear factor erythroid 2-related factor 2 (Nrf2), heme oxygenase (HO-1), quinone dehydrogenase 1 (NQO1), superoxide dismutase 1 (SOD1) in both cell types exposed to the HEMA monomer. The transcription of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and interleukin-6 (IL-6) was repressed in both cell types, while the transcription of tumor necrosis factor α (TNF-α) and interleukin-8 (IL-8) was repressed only in OKF6/TERT-2 cells. | [31] |
| Primary human dental pulp cells (hDPCs) obtained from healthy patients aged 18–25, during the extraction of healthy third molars, exposed to 1 mM 2-hydroxyethyl methacrylate (HEMA) for 18 and 12 h | It was demonstrated that the expression of NFE2L2 (nuclear factor, erythroid 2 like 2) and HMOX1 (heme oxygenase (decycling) 1) genes encoding the proteins: Nrf2 (nuclear factor erythroid 2-related factor 2) and HO-1 (heme oxygenase 1) in the HEMA-exposed group increased compared to the group not exposed to HEMA. | [32] |
| Human gingival fibroblasts (HGFs) exposed to 2-hydroxyethyl methacrylate (HEMA) and triethylene glycol dimethacrylate (TEGDMA) at a concentration of 3 mM for 24, 48 and 72 h | It was demonstrated that exposure to HEMA caused autophagy and apoptosis in each of the analyzed periods of time. No signs of autophagia were observed in TEGDMA-exposed cells | [33] |
| Dental Resin Composites—Cross-linked samples | ||
| Human dental pulp cells exposed to methacrylate-based dental resin composite, including triethylene glycol dimethacrylate and composites free of 2-hydroxyethyl methacrylate and silorane-based composite (5 mm in diameter and 2 mm high) cured with light (780 mW/cm2) for 40 s in the presence of dental polymers (reduction of free radical polymerization) and absence of polyester film | Flow cytometry showed increased ROS production in cells exposed to dental resin composite materials. A positive correlation was observed between ROS production and cell survival in groups not covered with polyester film. TEGDMA increases ROS production. | [34] |
| Human dental pulp cells (isolated from third molars) exposed to dental material dental resin composite s for 48 h with IGF-1 i TGF-b | Insulin-like growth factor (IGF-1) and transforming growth factor beta (TGF-β) increased cystine capture, resulting in elevated levels of cellular glutathione in a group of cells exposed to dental resin composite (Flow Line, 9.5 +/− 0.4 mg and Durafill VS, 10.0 +/− 0.4 mg). This provided increased protection against OS effect triggered by dental resin composite. | [35] |
| Human pulp cells obtained from impacted third molars, exposed to cured bonding agents (Clearfil SE Bond, CB; Prime & Bond 2.1, PB; and Single Bond, SB) at a concentration of 10 µL for 2 days | Dentine bonding agents decrease the level of GSH, which might be the reason for the cytotoxicity of resins. Cytotoxicity decreased when N-acetyl-L-cysteine (NAC) was added to the sample. | [36] |
| Saliva collected from 52 patients (32 women and 20 men) who had been treated with Filtek Z250 dental resin composite fillings, before the filling and 1 h, 1 day, 7 and 30 days after the filling | Patients with dental resin composite fillings demonstrated a significantly increased MDA level compared to subjects without fillings, but there were no statistical differences between the studied time periods. There was also a significant decrease in SOD activity 7 days after the filling compared to the controls. No significant differences were noted in SOD values between day 7 and 30 in patients with dental resin composite fillings. | [37] |
| Composite resins | ||
| Human gingival fibroblasts (HGFs) exposed to composite resin (consisting of 45% 2-hydroxyethyl methacrylate—HEMA and 55% bisphenol A-glycidyl dimethacrylate—Bis-GMA) at concentrations of up to 0.25 mM | It was demonstrated that the expression of 8-hydroxyguanine in DNA– hydrolase I, the main enzyme for repairing 8-oxoG damage in composite resin-exposed cells, was elevated compared to cells not exposed to monomers. | [38] |
| Mouse fibroblast cells (NIH/3T3) exposed to camphorquinone (CQ), CQ and diphenyleneiodonium hexafluorophosphate (DPI), CQ and ethyl 4-dimethylamino benzoate (EDAB), and CQ, EDAB and DPI, with EDAB in high and low concentration, for 10 and 20 s | Increased activity of SOD was observed after 10 s of polymerization vs 20 s in NIH/3T3. | [39] |
| Orthodontic braces | ||
| L929 mouse fibroblast cell line exposed to six types of orthodontic archiwires (stainless steel, nickel-titanium, copper-nickel-titanium, rhodium-coated nickel-titanium, cobalt-chromium Blue Elgiloy, titanium-molybdenum) in 1-cm-long pieces (1 mL saliva per 0.2 g of the wire) | It was demonstrated that a standard nickel-titanium orthodontic archiwire generates the strongest oxidative stress, while stainless steel and titanium-molybdenum wire triggers the lowest OS in a mouse fibroblast cell culture. | [40] |
| L929 mouse fibroblast cell line exposed to three conventional (stainless steel, monocrystalline sapphire ceramics, polyurethane) and four self-ligating brackets (stainless steel body with a nickel-titanium clip, aluminum oxide ceramics with a cobalt-chromium clip, aluminum oxide ceramics with a nickel-cobalt clip coated with rhodium, polycarbonate-stainless steel brackets) made of different materials | The assessment of 8-hydroxy-29-deoxyguanosine (8-OHdG) in DNA of L929 murine fibroblast cell line demonstrated that the lowest OS is triggered by a conventional sapphire ceramic bracket. Full metal conventional and self-ligating brackets and conventional polyurethane brackets showed higher OS compared to cells not exposed to these brackets. The highest OS is caused by full metal and polyurethane brackets. | [41] |
| Saliva of 23 patients aged 12–16 enrolled in the study (12 female, 11 male subjects), treated with multibracket self-ligating vestibular orthodontic appliances | During the first 10 weeks of treatment with multibracket self-ligating vestibular orthodontic appliances, no statistically significant changes in the salivary antioxidant test (SAT) were observed. | [42] |
| Unstimulated saliva and gingival fluid of 50 generally healthy patients (27 females and 23 males) aged 13–20, treated with permanent brackets, collected before the treatment as well as in the 1st and 6th month of the treatment | There was no increase in oxidative damage (8-OHdG, MDA) in the saliva and gingival fluid of patients treated with permanent brackets compared to pre-treatment results. | [43] |
| Unstimulated (UWS) and stimulated (SWS) saliva of 37 generally healthy subjects treated with permanent orthodontic brackets, collected immediately after the fitting of the brackets as well as 1 week and 24 weeks after the fitting | There was a significant increase in thiobarbituric acid reactive substance (TBARS) in UWS and SWS one week after braces were fitted. The measured values returned to their initial state 24 weeks after the beginning of the treatment. There were no significant differences between the levels of SOD1, CAT, UA and Px activity in UWS 1 week and 24 weeks after the start of treatment. SOD1 activity was found to be significantly lower in SWS, and Px activity was considerably higher 1 week after the placement of the brackets compared to the values before the treatment and 24 weeks after its commencement. The total antioxidant status (TAS) in UWS and SWS was also found to be considerably lower 24 weeks after the start of the treatment compared to the values before the treatment as well as 1 week after its start. The highest oxidative stress index (OSI) values were observed 1 week after the treatment. 24 weeks after the treatment these values were identical to pre-treatment results. | [44] |
| Fixations and dental implants | ||
| Human dental pulp stem cells (DPSC) and murine pre- osteoblast (MC3T3-E1) cells exposed to zirconium and titanium oxide for 24 h | Intracellular oxidation of 5-(and -6)- chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate and acetyl ester (CM-H2DCFDA), a ROS indicator dye, demonstrated relatively higher average ROS levels in both types of cells exposed to zirconium compared to titanium. | [45] |
| Periosteum of 30 patients (8 women and 22 men) with bilateral fractures of the mandible, treated with Ti6Al4V titanium alloy | The periosteum of patients treated with titanium implants showed significantly higher concentrations of the biomarkers of nitrosative (S-nitrosothiols, peroxynitrite, nitrotyrosine) and oxidative stress (malondialdehyde, protein carbonyls, dityrosine, kynurenine and N-formylkynurenine) compared to the control without titanium fixations. Osteosynthesis patients also demonstrated increased antioxidant protection expressed in elevated levels of reduced glutathione (↑GSH) and glutathione reductase (↑GR). The periosteum of patients with titanium fixations revealed a considerable decrease in the activity of mitochondrial complex I (−77.8%) and CS (citrate synthase) (−166.7%) compared to the control. There were no statistically significant differences in the activity of complex II and cytochrome C oxidase (COX) between patients after osteosynthesis as compared to healthy controls. In the periosteum of osteosynthesis patients, the production of hydrogen peroxide as well as the rate of ROS production were also significantly increased. Titanium implants caused oxidative/nitrosative stress and mitochondrial dysfunction. Moreover, a positive correlation between ROS production rate and GSH concentration was observed, which may suggest increased antioxidant defense in patients after osteosynthesis. | [46] |
| Whole saliva of patients aged 43–57 with peri-implantitis and five titanium implants (collected from five patients) that were rejected up to 6 months after their implantation (3 from the mandible, 2 from the maxilla); oxidative stress parameters | In the course of peri-implantitis, a significant increase was observed in AGE compared to the control. In the saliva of peri-implantitis patients the level of OS was higher than in healthy individuals. | [47] |
| Periosteum of 32 patients operated on due to class III dentofacial deformities (21 women and 11 men aged 20–30), who had had titanium implants inserted and then removed 12–30 months after the implantation | Decreased activity of superoxide dismutase-1 (SOD1) (↓37%) and tryptophan level (↓34%) as well as significantly higher content of advanced oxidation protein products (AOPP) (↑25%), total oxidant status (TOS) (↑80%) and oxidative stress index (OSI) (↑101%) were observed in the maxillary periosteum of osteotomized patients compared to the controls. The mandibular periosteum demonstrated a significant decrease in SOD-1 activity (↓55%), total oxidant status (TAC) (↓58.6%), advanced glycation end products (AGE) (↓60%) and N-formylkynurenine (↓34%), and considerably increased content of AOPP (↑38%), malondialdehyde (MDA) (↑29%), 4-hydroxynonenal (4-HNE) (↑114%), TOS (↑99%) and OSI (↑381%) compared to the controls. Further weakening of the redox economy and increased ROS production were demonstrated in the mandibular periosteum compared to the maxillary periosteum. | [48] |
| Periosteum of 29 patients (aged 19–29) treated with titanium implants (due to a bilateral mandibular shaft fracture) that were removed 3–5 months after the procedure | The periosteum of patients after osteosynthesis showed significantly higher activity of NADPH and xanthine oxidase, and increased rate of free radical production compared to the control. The periosteum of patients after osteosynthesis also demonstrated a considerable increase in the levels of inflammation markers: interleukin 1 (IL-1), interleukin 6 (IL-6), tumor necrosis factor α (TNF-α), transforming growth factor β (TGF-β) and β-glucuronidase (GLU) as well as markers of apoptosis (Bax, Bax/Bcl-2), caspase-3 (CAS-3) and nitric oxide (NO) compared to the control. Titanium implants increased the production of proinflammatory cytokines and oxygen free radicals. A positive correlation between titanium content and CAS-3 activity was also demonstrated. | [49] |
| Periosteum, plasma, and erythrocytes collected from 31 generally healthy subjects aged 21–29 (11 women and 20 men) with bilateral mandibular fractures treated with titanium miniplates (Ti4Al4V) | Decreased CAT activity in the mandibular periosteum and its increase in erythrocytes of patients with mandibular fracture treated with titanium miniplates were demonstrated compared to the subjects not exposed to titanium implants. SOD activity and UA concentration were significantly higher in both plasma and periosteum of fracture patients compared to healthy individuals. No differences were found in GPx activity between the studied groups. There was an increase in TAC, FRAP, TOS, AGE, AOPP, 4-HNE and a decrease in OSI level in the maxillary periosteum of patients with fracture compared to healthy subjects. There were no significant differences in plasma TAC, TOS, OSI, FRAP AGE, AOPP, 4-HNE and 8-OHdG levels between patients with a fracture and healthy subjects. A positive correlation was observed between TAC concentration in the mandibular periosteum and plasma UA level in patients with a mandibular fracture. A positive correlation was also found between TOS concentration in the periosteum and CAT activity in erythrocytes, and between 8-OHdG level in the periosteum and GPx activity in erythrocytes. | [50] |
| Periodontology | ||
| Male adult Wistar rats (2 months of age) with periodontitis, subjected to antimicrobial photodynamic therapy (aPDT) | PDT was shown to increase ROS formation as well as boost the antioxidant response. | [51] |
| Whole saliva of patients aged 43–57 with peri-implantitis and 5 titanium implants (collected from five patients), which were rejected up to 6 months after their implantation (3 from the mandible, 2 from the maxilla) | In patients with peri-implantitis, the western blot technique revealed a significant increase in AGE compared to healthy controls. By means of TBARS assays, a higher level of OS was also observed in the saliva of peri-implantitis patients compared to healthy subjects. | [47] |
| Sixteen patients with chronic periodontitis (CP), undergoing non-surgical periodontal therapy alone as well as non-surgical therapy accompanied by antibiotic therapy of Amoxicillin + Metronidazole, 500 mg each, 3 times daily, for 7 days | It was demonstrated that after 3 months OS levels decreased from very high to average during antibiotic therapy, as shown by reduced derivatives of reactive oxygen metabolites (d-ROMs) (from 491.83 ± 134.85 U CARR to 375.58 ± 126.06 U CARR) and reduced glutathione (GSH) (from 48.73 ± 33.89 μmol/L to 46.46 ± 21.59 μmol/L) in plasma. | [52] |
| Nineteen patients with chronic periodontitis (average age: 46.8 years) examined before the therapy (scaling and root planing) as well as 1 and 2 months after the therapy. | Non-surgical treatment of periodontitis reduced plasma ROM levels compared to pre-treatment levels. | [53] |
| Tissues and saliva of 10 patients with peri-implantitis and 10 with chronic periodontitis, aged 40–60 | In both the saliva and tissues of patients with peri-implantitis and chronic periodontitis, AGE levels more than doubled compared to healthy individuals. A strong positive correlation was also observed between ROS and AGE in the examined patients. | [54] |
| Peri-implant crevicular fluid (PICF) collected from 31 patients | The concentration of MDA, SOD and TAC in peri-implant crevicular fluid did not differ from that in healthy subjects. However, there was a positive correlation between periodontal pocket depth (PPD) around the implant and MDA and TAC levels. | [55] |
| Whitening | ||
| Human primary periodontal ligament fibroblasts (hPDLFs) and Ca9-22 human gingival epithelial cells treated with stable aqueous ozone ultrafine bubble water (OUFBW; ozone concentration: 2.5 ppm) or UV-inactivated OUFBW | OUFBW (30 min of incubation) stimulated ROS production in both cell lines, thus activating the MAPK pathway. OUFBW triggered the activation of c-Fos, a major component of the transcription factor activator protein 1 (AP-1), and also nuclear factor erythroid 2 (NF-E2)-related factor 2 (Nrf2), which demonstrated high sensitivity to oxidative stress. | [56] |
| One-hundred thirteen patients (60 people using Crest® 3D Whitestrips® premium plus, 10% hydrogen peroxide, 53 subjects in the control group). Oral epithelial cells and saliva samples were collected at the beginning of the study and 30 days later from the control group, and immediately before whitening as well as 15 and 30 days after the completion of the whitening procedure | After the whitening procedure, an elevated level of 8-OHdG in saliva and a positive correlation between oxidative stress produced by hydrogen peroxide and micronuclei were found. | [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zieniewska, I.; Maciejczyk, M.; Zalewska, A. The Effect of Selected Dental Materials Used in Conservative Dentistry, Endodontics, Surgery, and Orthodontics as Well as during the Periodontal Treatment on the Redox Balance in the Oral Cavity. Int. J. Mol. Sci. 2020, 21, 9684. https://doi.org/10.3390/ijms21249684
Zieniewska I, Maciejczyk M, Zalewska A. The Effect of Selected Dental Materials Used in Conservative Dentistry, Endodontics, Surgery, and Orthodontics as Well as during the Periodontal Treatment on the Redox Balance in the Oral Cavity. International Journal of Molecular Sciences. 2020; 21(24):9684. https://doi.org/10.3390/ijms21249684
Chicago/Turabian StyleZieniewska, Izabela, Mateusz Maciejczyk, and Anna Zalewska. 2020. "The Effect of Selected Dental Materials Used in Conservative Dentistry, Endodontics, Surgery, and Orthodontics as Well as during the Periodontal Treatment on the Redox Balance in the Oral Cavity" International Journal of Molecular Sciences 21, no. 24: 9684. https://doi.org/10.3390/ijms21249684
APA StyleZieniewska, I., Maciejczyk, M., & Zalewska, A. (2020). The Effect of Selected Dental Materials Used in Conservative Dentistry, Endodontics, Surgery, and Orthodontics as Well as during the Periodontal Treatment on the Redox Balance in the Oral Cavity. International Journal of Molecular Sciences, 21(24), 9684. https://doi.org/10.3390/ijms21249684







