Novel 1,3,4-Oxadiazole Derivatives of Pyrrolo[3,4-d]pyridazinone Exert Antinociceptive Activity in the Tail-Flick and Formalin Test in Rodents and Reveal Reduced Gastrotoxicity
Abstract
1. Introduction
2. Results
2.1. Tail-Flick Test
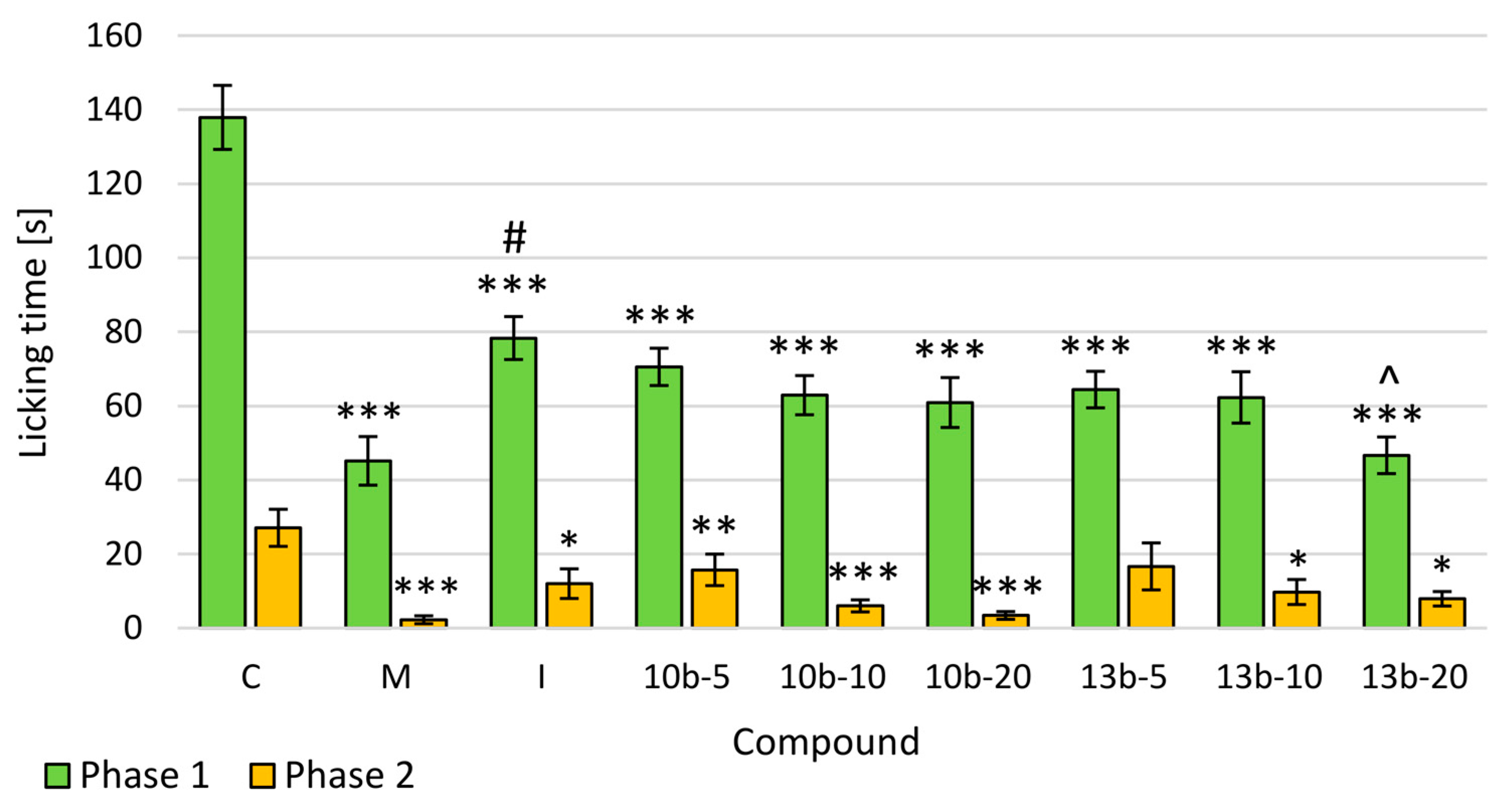
2.2. Formalin Test
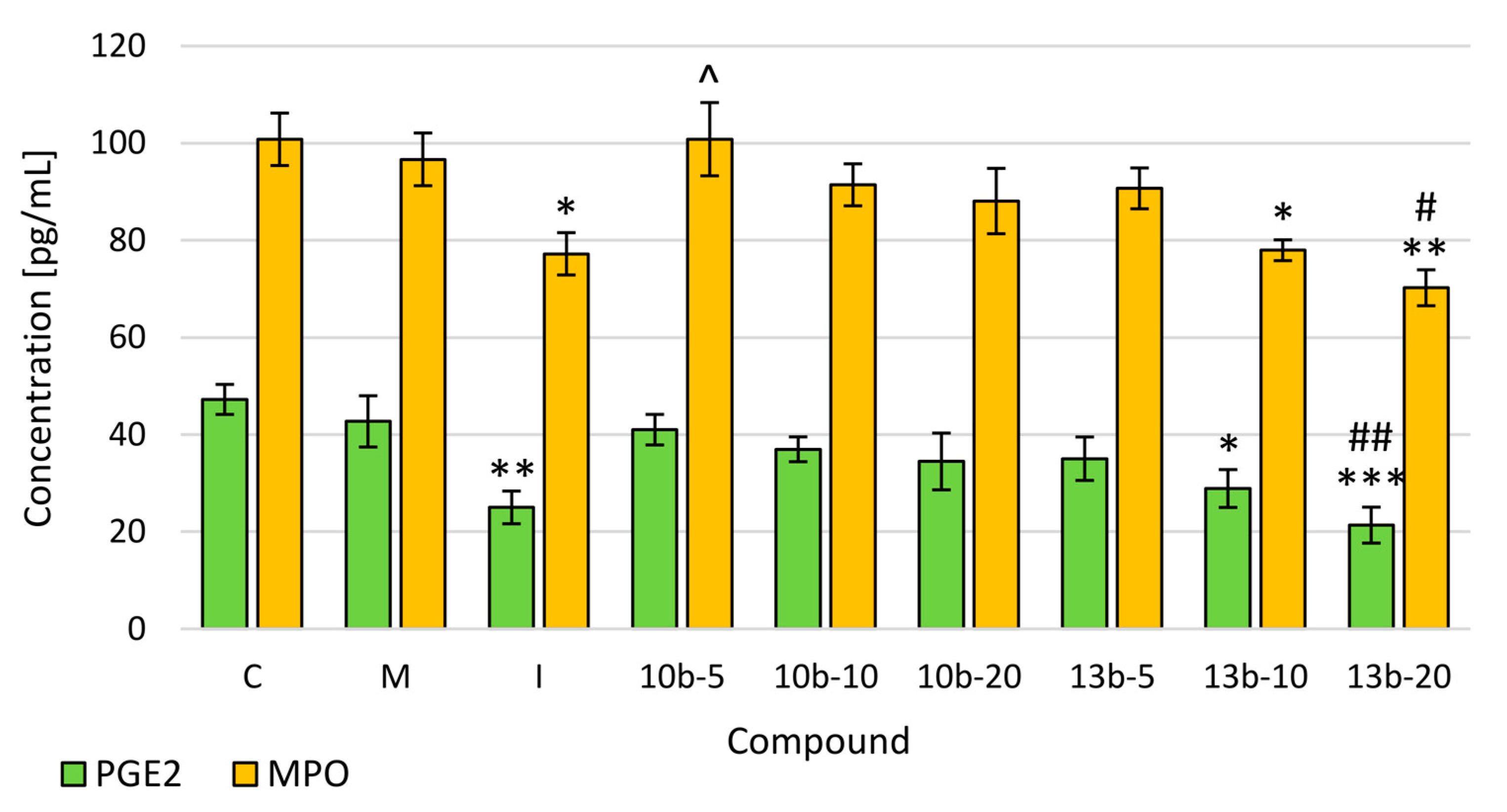
2.3. Evaluation of Prostaglandin E2 (PGE2) and Myeloperoxidase (MPO) Levels
2.4. Histopathological Assessment of Gastric Mucosa
2.5. Multi-Criteria Decision Analysis (MCDA)
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals
4.2. Animals
4.3. Ethics Statement
4.4. Drug Administration
4.5. Tail-Flick Test
4.6. Formalin Test
4.7. Evaluation of PGE2 and MPO Levels
4.8. Histopathological Assessment of Gastric Mucosa
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sneddon, L.U. Comparative physiology of nociception and pain. Physiology 2018, 33, 63–73. [Google Scholar] [CrossRef]
- Nagasako, E.M.; Oaklander, A.L.; Dworkin, R.H. Congenital insensitivity to pain: An update. Pain 2003, 101, 213–219. [Google Scholar] [CrossRef]
- Negus, S.S.; Vanderah, T.W.; Brandt, M.R.; Bilsky, E.J.; Becerra, L.; Borsook, D. Preclinical assessment of candidate analgesic drugs: Recent advances and future challenges. J. Pharmacol. Exp. Ther. 2006, 319, 507–514. [Google Scholar] [CrossRef]
- Malinka, W.; Redzicka, A.; Lozach, O. New derivatives of pyrrolo[3,4-d]pyridazinone and their anticancer effects. Farmaco 2004, 59, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Mogilski, S.; Kubacka, M.; Redzicka, A.; Kazek, G.; Dudek, M.; Malinka, W.; Filipek, B. Antinociceptive, anti-inflammatory and smooth muscle relaxant activities of the pyrrolo[3,4-d]pyridazinone derivatives: Possible mechanisms of action. Pharmacol. Biochem. Behav. 2015, 133, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Szczukowski, Ł.; Redzicka, A.; Wiatrak, B.; Krzyżak, E.; Marciniak, A.; Gębczak, K.; Gębarowski, T.; Świątek, P. Design, synthesis, biological evaluation and in silico studies of novel pyrrolo[3,4-d]pyridazinone derivatives with promising anti-inflammatory and antioxidant activity. Bioorg. Chem. 2020, 102, 104035. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, K.; Poojary, B.; Lobo, P.L.; Fernandes, J.; Kumari, N.S. Synthesis and biological evaluation of some 1,3,4-oxadiazole derivatives. Eur. J. Med. Chem. 2010, 45, 5225–5233. [Google Scholar] [CrossRef]
- Koksal, M.; Ozkan-Dagliyan, I.; Ozyazici, T.; Kadioglu, B.; Sipahi, H.; Bozkurt, A.; Bilge, S.S. Some Novel Mannich Bases of 5-(3,4-Dichlorophenyl)-1,3,4-oxadiazole-2(3H)-one and Their Anti-Inflammatory Activity. Arch. Pharm. (Weinheim) 2017, 350, e1700153. [Google Scholar] [CrossRef]
- Palkar, M.B.; Singhai, A.S.; Ronad, P.M.; Vishwanathswamy, A.H.M.; Boreddy, T.S.; Veerapur, V.P.; Shaikh, M.S.; Rane, R.A.; Karpoormath, R. Synthesis, pharmacological screening and in silico studies of new class of Diclofenac analogues as a promising anti-inflammatory agents. Bioorg. Med. Chem. 2014, 22, 2855–2866. [Google Scholar] [CrossRef]
- Wakulik, K.; Wiatrak, B.; Szczukowski, Ł.; Bodetko, D.; Szandruk-Bender, M.; Dobosz, A.; Świątek, P.; Gąsiorowski, K. Effect of Novel Pyrrolo[3,4-d]pyridazinone Derivatives on Lipopolysaccharide-Induced Neuroinflammation. Int. J. Mol. Sci. 2020, 21, 2575. [Google Scholar] [CrossRef]
- Sherrington, C.S.; Laslett, E.E. Remarks on the dorsal spino-cerebellar tract. J. Physiol. 1903, 29, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Le Bars, D.; Gozariu, M.; Cadden, S. Animal Models of Nociception. Pharmacol. Rev. 2001, 53, 597–652. [Google Scholar] [PubMed]
- Mogil, J.S.; Davis, K.D.; Derbyshire, S.W. The necessity of animal models in pain research. Pain 2010, 151, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, K.J.; Gómez-Pinilla, F.; Crowe, M.J.; Ying, Z.; Basso, D.M. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain 2004, 127, 1403–1414. [Google Scholar] [CrossRef]
- Hunskaar, S.; Hole, K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 1987, 30, 103–114. [Google Scholar] [CrossRef]
- Tjølsen, A.; Berge, O.G.; Hunskaar, S.; Rosland, J.H.; Hole, K. The formalin test: An evaluation of the method. Pain 1992, 51, 5–17. [Google Scholar] [CrossRef]
- Yin, Z.Y.; Li, L.; Chu, S.S.; Sun, Q.; Ma, Z.L.; Gu, X.P. Antinociceptive effects of dehydrocorydaline in mouse models of inflammatory pain involve the opioid receptor and inflammatory cytokines. Sci. Rep. 2016, 6, 27129. [Google Scholar] [CrossRef]
- Dickerman, J.D. The Late Effects of Childhood Cancer Therapy. Pediatrics 2007, 119, 554–568. [Google Scholar] [CrossRef]
- Bastos, G.N.T.; Santos, A.R.S.; Ferreira, V.M.M.; Costa, A.M.R.; Bispo, C.I.; Silveira, A.J.A.; Do Nascimento, J.L.M. Antinociceptive effect of the aqueous extract obtained from roots of Physalis angulata L. on mice. J. Ethnopharmacol. 2006, 103, 241–245. [Google Scholar] [CrossRef]
- Burian, M.; Geisslinger, G. COX-dependent mechanisms involved in the antinociceptive action of NSAIDs at central and peripheral sites. Pharmacol. Ther. 2005, 107, 139–154. [Google Scholar] [CrossRef]
- Leuti, A.; Fazio, D.; Fava, M.; Piccoli, A.; Oddi, S.; Maccarrone, M. Bioactive lipids, inflammation and chronic diseases. Adv. Drug Deliv. Rev. 2020. in Press. [Google Scholar] [CrossRef] [PubMed]
- Veiga, A.P.C.; Duarte, I.D.G.; Ávila, M.N.; Da Motta, P.G.; Tatsuo, M.A.K.F.; Francischi, J.N. Prevention by celecoxib of secondary hyperalgesia induced by formalin in rats. Life Sci. 2004, 75, 2807–2817. [Google Scholar] [CrossRef] [PubMed]
- Daher, J.B.; Tonussi, C.R. A spinal mechanism for the peripheral anti-inflammatory action of indomethacin. Brain Res. 2003, 962, 207–212. [Google Scholar] [CrossRef]
- Kidd, B.L.; Urban, L.A. Mechanisms of inflammatory pain. Br. J. Anaesth. 2001, 87, 3–11. [Google Scholar] [CrossRef]
- Cunha, T.M.; Barsante, M.M.; Guerrero, A.T.; Verri, W.A.; Ferreira, S.H.; Coelho, F.M.; Bertini, R.; Di Giacinto, C.; Allegretti, M.; Cunha, F.Q.; et al. Treatment with DF 2162, a non-competitive allosteric inhibitor of CXCR1/2, diminishes neutrophil influx and inflammatory hypernociception in mice. Br. J. Pharmacol. 2008, 154, 460–470. [Google Scholar] [CrossRef]
- Guerrero, A.T.G.; Verri, W.A.; Cunha, T.M.; Silva, T.A.; Schivo, I.R.S.; Dal-Secco, D.; Canetti, C.; Rocha, F.A.C.; Parada, C.A.; Cunha, F.Q.; et al. Involvement of LTB 4 in zymosan-induced joint nociception in mice: Participation of neutrophils and PGE 2. J. Leukoc. Biol. 2008, 83, 122–130. [Google Scholar] [CrossRef]
- Verri, W.A.; Cunha, T.M.; Magro, D.A.; Guerrero, A.T.G.; Vieira, S.M.; Carregaro, V.; Souza, G.R.; Henriques, M.D.G.M.O.; Ferreira, S.H. Targeting endothelin ETA and ETB receptors inhibits antigen-induced neutrophil migration and mechanical hypernociception in mice. Naunyn. Schmiedebergs Arch. Pharmacol. 2009, 379, 271–279. [Google Scholar] [CrossRef]
- Shih, C.M.; Cheng, S.N.; Wong, C.S.; Kuo, Y.L.; Chou, T.C. Antiinflammatory and antihyperalgesic activity of C-phycocyanin. Anesth. Analg. 2009, 108, 1303–1310. [Google Scholar] [CrossRef]
- Prokopowicz, Z.; Marcinkiewicz, J.; Katz, D.R.; Chain, B.M. Neutrophil myeloperoxidase: Soldier and statesman. Arch. Immunol. Ther. Exp. (Warsz.) 2012, 60, 43–54. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Porreca, F.; Cuzzocrea, S.; Galen, K.; Lightfoot, R.; Masini, E.; Muscoli, C.; Mollace, V.; Ndengele, M.; Ischiropoulos, H.; et al. A newly identified role for superoxide in inflammatory pain. J. Pharmacol. Exp. Ther. 2004, 309, 869–878. [Google Scholar] [CrossRef]
- Rossato, M.F.; Velloso, N.A.; de Oliveira Ferreira, A.P.; de Mello, C.F.; Ferreira, J. Spinal Levels of NonProtein Thiols Are Related to Nociception in Mice. J. Pain 2010, 11, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Alabi, A.O.; Ajayi, A.M.; Omorogbe, O.; Umukoro, S. Anti-nociceptive and anti-inflammatory effects of an aqueous extract of blended leaves of Ocimum gratissimum and Psidium guajava. Clin. Phytosci. 2019, 5, 34. [Google Scholar] [CrossRef]
- Laine, L. Gastrointestinal Effects of NSAIDs and Coxibs. J. Pain Symptom Manag. 2003, 25, 32–40. [Google Scholar]
- García Rodríguez, L.A.; Barreales Tolosa, L. Risk of Upper Gastrointestinal Complications Among Users of Traditional NSAIDs and COXIBs in the General Population. Gastroenterology 2007, 132, 498–506. [Google Scholar] [CrossRef]
- Malinka, W.; Redzicka, A.; Jastrzębska-Więsek, M.; Filipek, B.; Dybała, M.; Karczmarzyk, Z.; Urbańczyk-Lipkowska, Z.; Kalicki, P. Derivatives of pyrrolo[3,4-d]pyridazinone, a new class of analgesic agents. Eur. J. Med. Chem. 2011, 46, 4992–4999. [Google Scholar] [CrossRef]
- El-Sayed, N.A.; Nour, M.S.; Salem, M.A.; Arafa, R.K. New oxadiazoles with selective-COX-2 and EGFR dual inhibitory activity: Design, synthesis, cytotoxicity evaluation and in silico studies. Eur. J. Med. Chem. 2019, 183, 111693. [Google Scholar] [CrossRef]
- Avci, A.; Taşci, H.; Kandemir, Ü.; Can, Ö.D.; Gökhan-Kelekçi, N.; Tozkoparan, B. Synthesis, characterization, and in vivo pharmacological evaluation of novel mannich bases derived from 1,2,4-triazole containing a naproxen moiety. Bioorg. Chem. 2020, 100, 103892. [Google Scholar] [CrossRef]
- Bala, S.; Kamboj, S.; Saini, V.; Prasad, D.N. Anti-Inflammatory, Analgesic Evaluation and Molecular Docking Studies of N-Phenyl Anthranilic Acid-Based 1,3,4-Oxadiazole Analogues. J. Chem. 2013, 2013, 412053. [Google Scholar] [CrossRef]
- Świątek, P.; Strzelecka, M.; Urniaz, R.; Gębczak, K.; Gębarowski, T.; Gąsiorowski, K.; Malinka, W. Synthesis, COX-1/2 inhibition activities and molecular docking study of isothiazolopyridine derivatives. Bioorg. Med. Chem. 2017, 25, 316–326. [Google Scholar] [CrossRef]
- Redzicka, A.; Szczukowski, Ł.; Kochel, A.; Wiatrak, B.; Gębczak, K.; Czyżnikowska, Ż. COX-1/COX-2 inhibition activities and molecular docking study of newly designed and synthesized pyrrolo[3,4-c]pyrrole Mannich bases. Bioorg. Med. Chem. 2019, 27, 3918–3928. [Google Scholar] [CrossRef]
- Florentino, I.F.; Silva, D.P.B.; Galdino, P.M.; Lino, R.C.; Martins, J.L.R.; Silva, D.M.; De Paula, J.R.; Tresvenzol, L.M.F.; Costa, E.A. Antinociceptive and anti-inflammatory effects of Memora nodosa and allantoin in mice. J. Ethnopharmacol. 2016, 186, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Rezaee-Asl, M.; Sabour, M.; Nikoui, V.; Ostadhadi, S.; Bakhtiarian, A. The Study of Analgesic Effects of Leonurus cardiaca L. in Mice by Formalin, Tail Flick and Hot Plate Tests. Int. Sch. Res. Not. 2014, 2014, 687697. [Google Scholar] [CrossRef] [PubMed]
- D’Amour, F.E.; Smith, D.L. A method for determining loss of pain sensation. J. Pharmacol. Exp. Ther. 1941, 72, 74–79. [Google Scholar]
- Szabo, S.; Trier, J.S.; Brown, A.; Schnoor, J.; Homan, H.D.; Bradford, J.C. A quantitative method for assessing the extent of experimental gastric erosions and ulcers. J. Pharmacol. Methods 1985, 13, 59–66. [Google Scholar] [CrossRef]


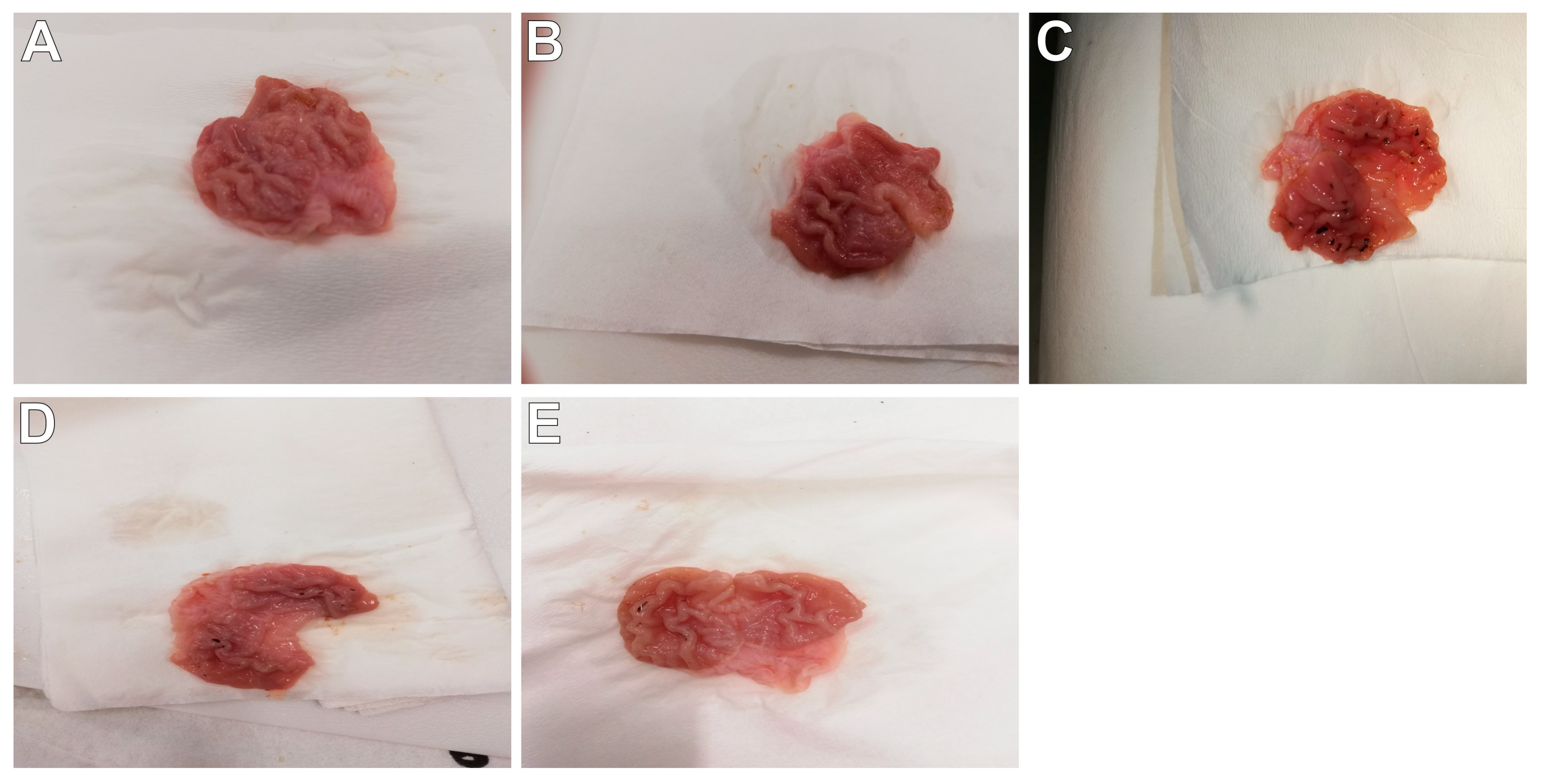
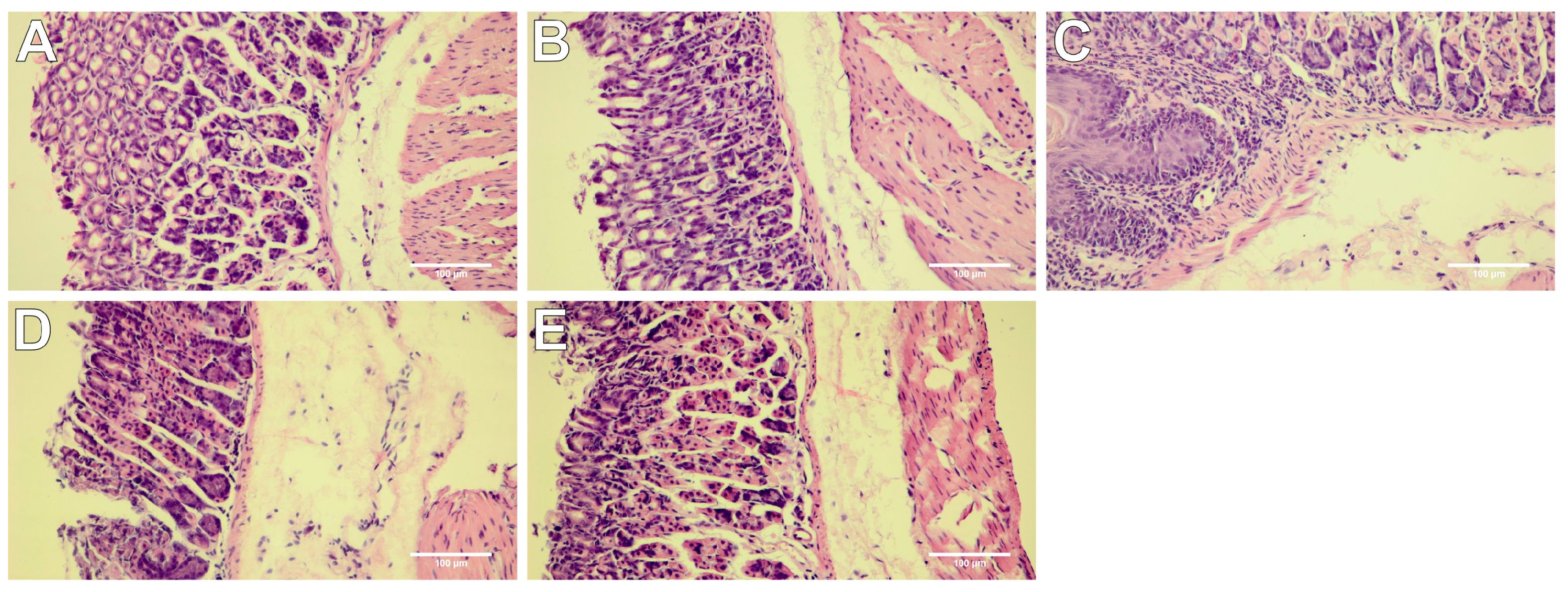


| Group | Macroscopic Lesions (0–3 Points) | Microscopic Lesions, H&E Staining (0–3 Points) |
|---|---|---|
| control | 0 | 0 |
| M | 0 | 0.43 ± 0.21 |
| I | 2.00 ± 0.31 ^^^ | 2.43 ± 0.30 ^^^ |
| 10b-5 | 0 | 0 |
| 10b-10 | 0 | 0.29 ± 0.18 |
| 10b-20 | 0.57 ± 0.20 *** | 0.71 ± 0.29 *** |
| 13b-5 | 0 | 0 |
| 13b-10 | 0 | 0 |
| 13b-20 | 0.29 ± 0.18 *** | 0.57 ± 0.20 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szandruk-Bender, M.; Wiatrak, B.; Szczukowski, Ł.; Świątek, P.; Rutkowska, M.; Dzimira, S.; Merwid-Ląd, A.; Danielewski, M.; Szeląg, A. Novel 1,3,4-Oxadiazole Derivatives of Pyrrolo[3,4-d]pyridazinone Exert Antinociceptive Activity in the Tail-Flick and Formalin Test in Rodents and Reveal Reduced Gastrotoxicity. Int. J. Mol. Sci. 2020, 21, 9685. https://doi.org/10.3390/ijms21249685
Szandruk-Bender M, Wiatrak B, Szczukowski Ł, Świątek P, Rutkowska M, Dzimira S, Merwid-Ląd A, Danielewski M, Szeląg A. Novel 1,3,4-Oxadiazole Derivatives of Pyrrolo[3,4-d]pyridazinone Exert Antinociceptive Activity in the Tail-Flick and Formalin Test in Rodents and Reveal Reduced Gastrotoxicity. International Journal of Molecular Sciences. 2020; 21(24):9685. https://doi.org/10.3390/ijms21249685
Chicago/Turabian StyleSzandruk-Bender, Marta, Benita Wiatrak, Łukasz Szczukowski, Piotr Świątek, Maria Rutkowska, Stanisław Dzimira, Anna Merwid-Ląd, Maciej Danielewski, and Adam Szeląg. 2020. "Novel 1,3,4-Oxadiazole Derivatives of Pyrrolo[3,4-d]pyridazinone Exert Antinociceptive Activity in the Tail-Flick and Formalin Test in Rodents and Reveal Reduced Gastrotoxicity" International Journal of Molecular Sciences 21, no. 24: 9685. https://doi.org/10.3390/ijms21249685
APA StyleSzandruk-Bender, M., Wiatrak, B., Szczukowski, Ł., Świątek, P., Rutkowska, M., Dzimira, S., Merwid-Ląd, A., Danielewski, M., & Szeląg, A. (2020). Novel 1,3,4-Oxadiazole Derivatives of Pyrrolo[3,4-d]pyridazinone Exert Antinociceptive Activity in the Tail-Flick and Formalin Test in Rodents and Reveal Reduced Gastrotoxicity. International Journal of Molecular Sciences, 21(24), 9685. https://doi.org/10.3390/ijms21249685








