The Role of Arachidonic and Linoleic Acid Derivatives in Pathological Pregnancies and the Human Reproduction Process
Abstract
1. Introduction
Physiologic Mechanisms of Polyunsaturated Fatty Acids
2. Participation of AA Metabolites in Fertility
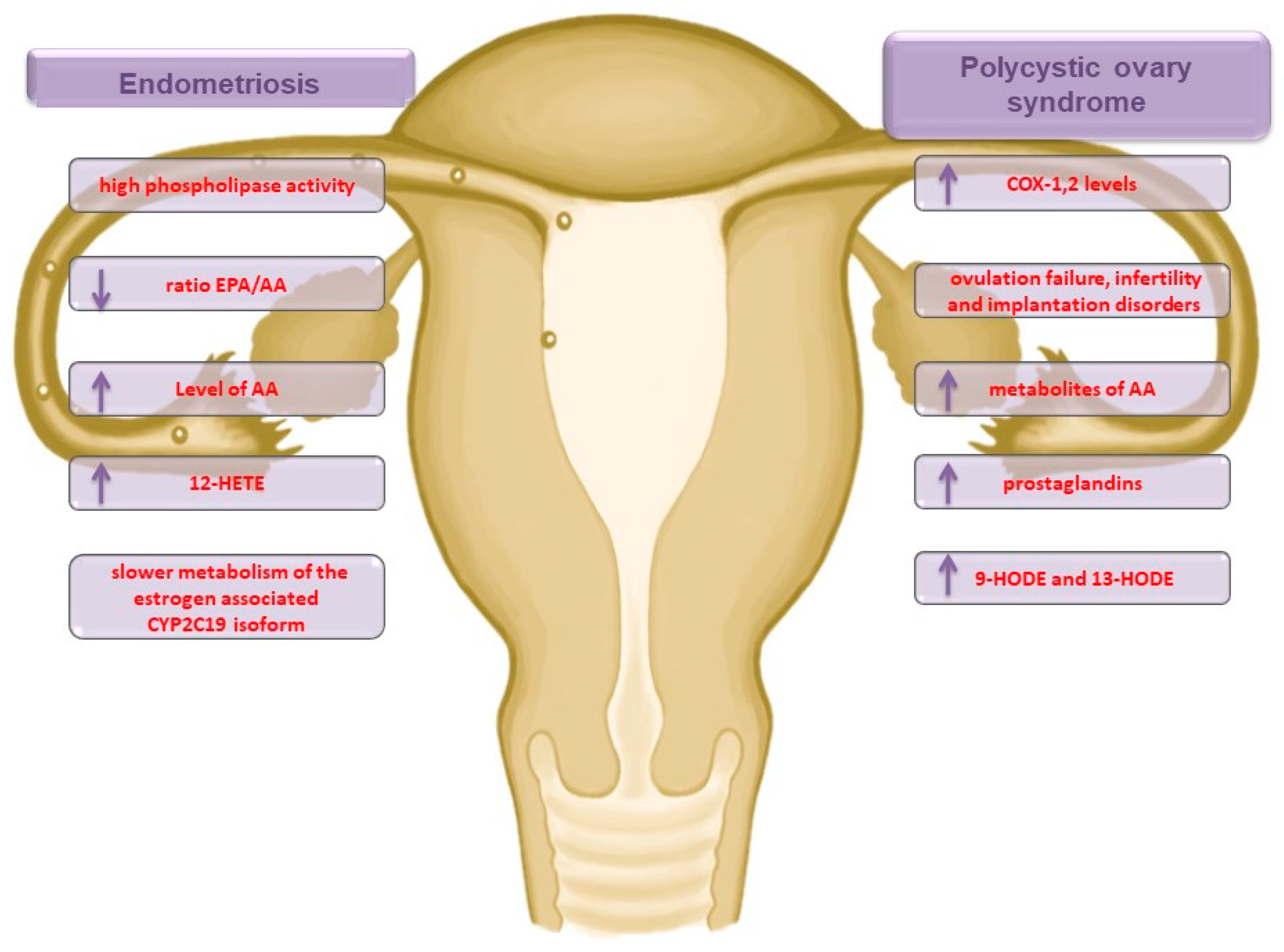
2.1. Endometriosis
2.2. Polycystic Ovary Syndrome
2.3. Long-Term Health Problems
2.4. Male Reproduction
3. Participation of AA Metabolites in Pregnancy
3.1. The Implantation and Fetal Growth
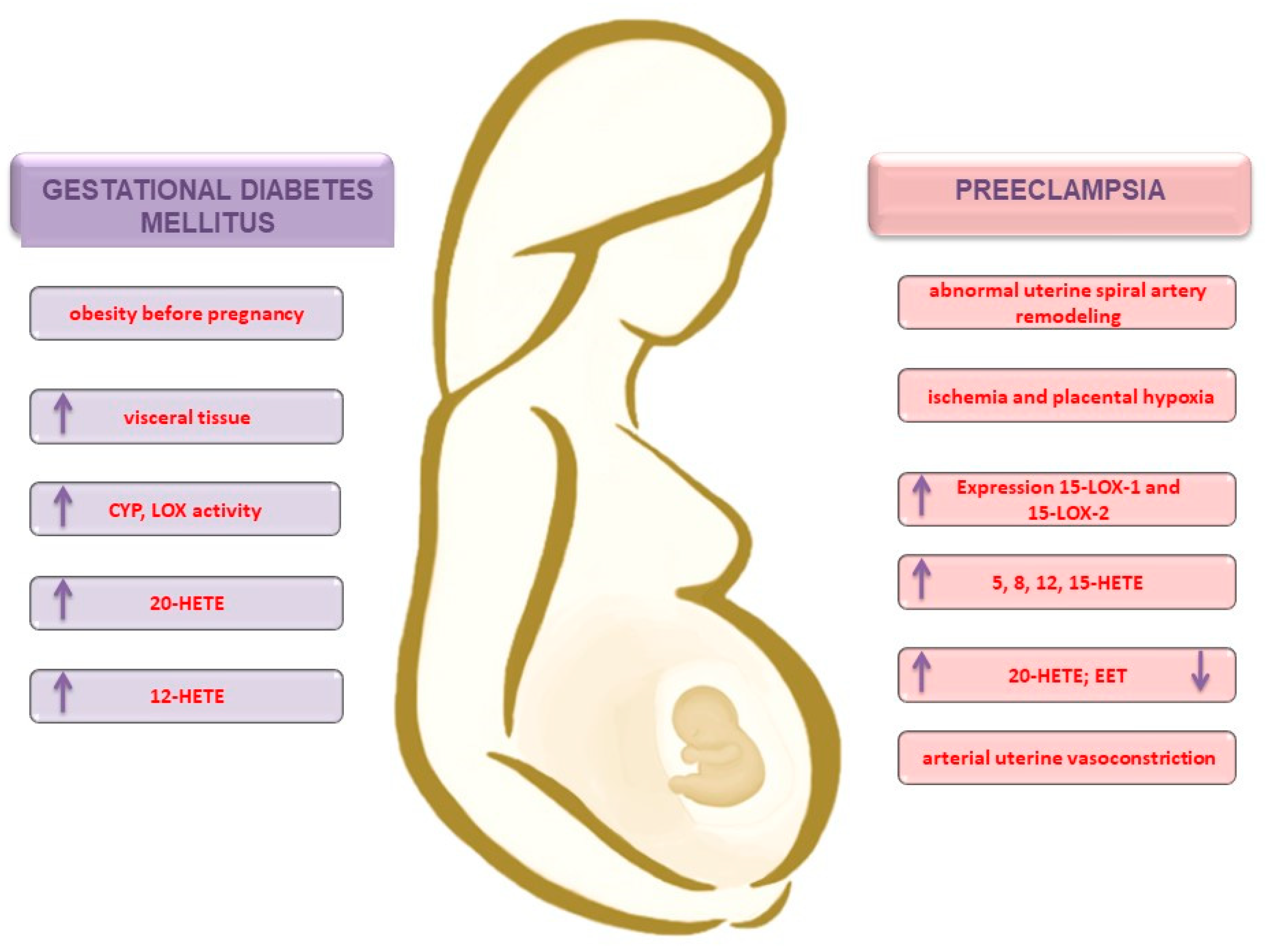
3.2. Obesity and the Development of Gestational Diabetes Mellitus
3.3. Preeclampsia
4. Summary
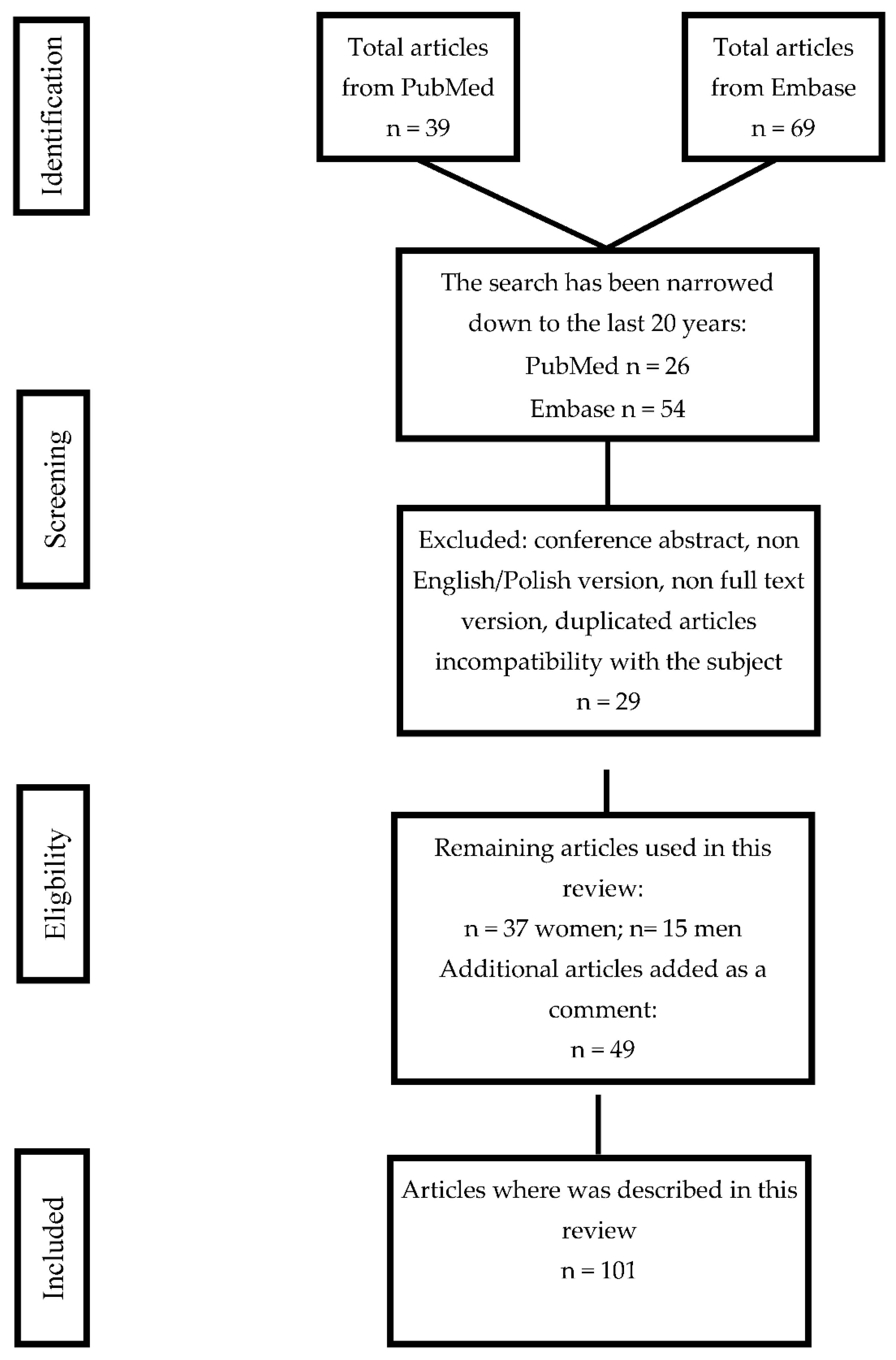
5. Data Search Algorithm
Author Contributions
Funding
Conflicts of Interest
References
- Jabbour, H.N.; Sales, K.J.; Catalano, R.D.; Norman, J.E. Inflammatory pathways in female reproductive health and disease. Reproduction 2009, 138, 903–919. [Google Scholar] [CrossRef]
- Kikut, J.; Komorniak, N.; Ziętek, M.; Palma, J.; Szczuko, M. Inflammation with the participation of arachidonic (AA) and linoleic acid (LA) derivatives (HETEs and HODEs) is necessary in the course of a normal reproductive cycle and pregnancy. J. Reprod. Immunol. 2020, 141, 103177. [Google Scholar] [CrossRef]
- Maybin, J.A.; Critchley, H.O.D.; Jabbour, H.N. Inflammatory pathways in endometrial disorders. Mol. Cell. Endocrinol. 2011, 335, 42–51. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef]
- Miles, E.A.; Calder, P.C. Can Early Omega-3 Fatty Acid Exposure Reduce Risk of Childhood Allergic Disease? Nutrients 2017, 9, 784. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I. REVIEW ARTICLE: The Immune System in Pregnancy: A Unique Complexity. Am. J. Reprod. Immunol. 2010, 63, 425–433. [Google Scholar] [CrossRef]
- Catalá, A. Five Decades with Polyunsaturated Fatty Acids: Chemical Synthesis, Enzymatic Formation, Lipid Peroxidation and Its Biological Effects. Available online: https://www.hindawi.com/journals/jl/2013/710290/ (accessed on 14 October 2020).
- Kroetz, D.L.; Xu, F. Regulation and inhibition of Arachidonic acid ω-Hydroxylases and 20-HETE formation. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 413–438. [Google Scholar] [CrossRef]
- Sonnweber, T.; Pizzini, A.; Nairz, M.; Weiss, G.; Tancevski, I. Arachidonic Acid Metabolites in Cardiovascular and Metabolic Diseases. Int. J. Mol. Sci. 2018, 19, 3285. [Google Scholar] [CrossRef]
- Hao, C.-M.; Breyer, M.D. Physiologic and pathophysiologic roles of lipid mediators in the kidney. Kidney Int. 2007, 71, 1105–1115. [Google Scholar] [CrossRef]
- Gouveia-Figueira, S.; Martens, D.S.; Nawrot, T.S.; Nording, M.L. Cord blood eicosanoid signatures and newborn gestational age. Prostaglandins Other Lipid Mediat. 2017, 133, 123–127. [Google Scholar] [CrossRef]
- Roman, R.J. P-450 Metabolites of Arachidonic Acid in the Control of Cardiovascular Function. Physiol. Rev. 2002, 82, 131–185. [Google Scholar] [CrossRef]
- Facchin, F.; Barbara, G.; Saita, E.; Mosconi, P.; Roberto, A.; Fedele, L.; Vercellini, P. Impact of endometriosis on quality of life and mental health: Pelvic pain makes the difference. J. Psychosom. Obs. Gynaecol. 2015, 36, 135–141. [Google Scholar] [CrossRef]
- Parazzini, F.; Esposito, G.; Tozzi, L.; Noli, S.; Bianchi, S. Epidemiology of endometriosis and its comorbidities. Eur. J. Obs. Gynecol. Reprod. Biol. 2017, 209, 3–7. [Google Scholar] [CrossRef]
- Khanaki, K.; Nouri, M.; Ardekani, A.M.; Ghassemzadeh, A.; Shahnazi, V.; Sadeghi, M.R.; Darabi, M.; Mehdizadeh, A.; Dolatkhah, H.; Saremi, A.; et al. Evaluation of the Relationship between Endometriosis and Omega-3 and Omega-6 Polyunsaturated Fatty Acids. Iran. Biomed. J. 2012, 16, 38–43. [Google Scholar] [CrossRef]
- Li, J.; Guan, L.; Zhang, H.; Gao, Y.; Sun, J.; Gong, X.; Li, D.; Chen, P.; Liang, X.; Huang, M.; et al. Endometrium metabolomic profiling reveals potential biomarkers for diagnosis of endometriosis at minimal-mild stages. Reprod. Biol. Endocrinol. 2018, 16. [Google Scholar] [CrossRef]
- Hopeman, M.M.; Riley, J.K.; Frolova, A.I.; Jiang, H.; Jungheim, E.S. Serum Polyunsaturated Fatty Acids and Endometriosis. Reprod. Sci. 2015, 22, 1083–1087. [Google Scholar] [CrossRef]
- Lee, Y.H.; Cui, L.; Fang, J.; Chern, B.S.M.; Tan, H.H.; Chan, J.K.Y. Limited value of pro-inflammatory oxylipins and cytokines as circulating biomarkers in endometriosis—A targeted ‘omics study. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Buczynski, M.W.; Dumlao, D.S.; Dennis, E.A. Thematic Review Series: Proteomics. An integrated omics analysis of eicosanoid biology. J. Lipid Res. 2009, 50, 1015–1038. [Google Scholar] [CrossRef]
- Daly, A.K. Chapter Three—Polymorphic Variants of Cytochrome P450: Relevance to Cancer and Other Diseases. In Advances in Pharmacology; Hardwick, J.P., Ed.; Cytochrome P450 Function and Pharmacological Roles in Inflammation and Cancer; Academic Press: Cambridge, MA, USA, 2015; Volume 74, pp. 85–111. [Google Scholar]
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef]
- Patel, S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J. Steroid Biochem. Mol. Biol. 2018, 182, 27–36. [Google Scholar] [CrossRef]
- Sales, K.J.; Jabbour, H.N. Cyclooxygenase enzymes and prostaglandins in pathology of the endometrium. Reproduction 2003, 126, 559–567. [Google Scholar] [CrossRef]
- Huang, R.; Xue, X.; Li, S.; Wang, Y.; Sun, Y.; Liu, W.; Yin, H.; Tao, T. Alterations of polyunsaturated fatty acid metabolism in ovarian tissues of polycystic ovary syndrome rats. J. Cell Mol. Med. 2018, 22, 3388–3396. [Google Scholar] [CrossRef]
- Dong, F.; Deng, D.; Chen, H.; Cheng, W.; Li, Q.; Luo, R.; Ding, S. Serum metabolomics study of polycystic ovary syndrome based on UPLC-QTOF-MS coupled with a pattern recognition approach. Anal. Bioanal. Chem. 2015, 407, 4683–4695. [Google Scholar] [CrossRef]
- Vinaixa, M.; Rodriguez, M.A.; Samino, S.; Díaz, M.; Beltran, A.; Mallol, R.; Bladé, C.; Ibañez, L.; Correig, X.; Yanes, O. Metabolomics Reveals Reduction of Metabolic Oxidation in Women with Polycystic Ovary Syndrome after Pioglitazone-Flutamide-Metformin Polytherapy. PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Obinata, H.; Izumi, T. G2A as a receptor for oxidized free fatty acids. Prostaglandins Other Lipid Mediat. 2008, 89, 66–72. [Google Scholar] [CrossRef]
- Li, S.; Chu, Q.; Ma, J.; Sun, Y.; Tao, T.; Huang, R.; Liao, Y.; Yue, J.; Zheng, J.; Wang, L.; et al. Discovery of Novel Lipid Profiles in PCOS: Do Insulin and Androgen Oppositely Regulate Bioactive Lipid Production? J. Clin. Endocrinol. Metab. 2016, 102, 810–821. [Google Scholar] [CrossRef]
- Szczuko, M.; Zapałowska-Chwyć, M.; Maciejewska, D.; Drozd, A.; Starczewski, A.; Stachowska, E. Significant Improvement Selected Mediators of Inflammation in Phenotypes of Women with PCOS after Reduction and Low GI Diet. Mediat. Inflamm. 2017, 2017. [Google Scholar] [CrossRef]
- Zheng, H.; Tang, H.; Liu, M.; He, M.; Lai, P.; Dong, H.; Lin, J.; Jia, C.; Zhong, M.; Dai, Y.; et al. Inhibition of Endometrial Cancer by n-3 Polyunsaturated Fatty Acids in Preclinical Models. Cancer Prev. Res. 2014, 7, 824–834. [Google Scholar] [CrossRef]
- Ferrandina, G.; Legge, F.; Ranelletti, F.O.; Zannoni, G.F.; Maggiano, N.; Evangelisti, A.; Mancuso, S.; Scambia, G.; Lauriola, L. Cyclooxygenase-2 expression in endometrial carcinoma. Cancer 2002, 95, 801–807. [Google Scholar] [CrossRef]
- Cummings, M.; Massey, K.A.; Mappa, G.; Wilkinson, N.; Hutson, R.; Munot, S.; Saidi, S.; Nugent, D.; Broadhead, T.; Wright, A.I.; et al. Integrated eicosanoid lipidomics and gene expression reveal decreased prostaglandin catabolism and increased 5-lipoxygenase expression in aggressive subtypes of endometrial cancer. J. Pathol. 2019, 247, 21–34. [Google Scholar] [CrossRef]
- Macut, D.; Bjekić-Macut, J.; Rahelić, D.; Doknić, M. Insulin and the polycystic ovary syndrome. Diabetes Res. Clin. Pract. 2017, 130, 163–170. [Google Scholar] [CrossRef]
- Aitken, R.J. Sperm function tests and fertility. Int. J. Androl. 2006, 29, 69–75; discussion 105–108. [Google Scholar] [CrossRef]
- Andersen, J.M.; Rønning, P.O.; Herning, H.; Bekken, S.D.; Haugen, T.B.; Witczak, O. Fatty acid composition of spermatozoa is associated with BMI and with semen quality. Andrology 2016, 4, 857–865. [Google Scholar] [CrossRef]
- Ollero, M.; Gil-Guzman, E.; Lopez, M.C.; Sharma, R.K.; Agarwal, A.; Larson, K.; Evenson, D.; Thomas, A.J.; Alvarez, J.G. Characterization of subsets of human spermatozoa at different stages of maturation: Implications in the diagnosis and treatment of male infertility. Hum. Reprod 2001, 16, 1912–1921. [Google Scholar] [CrossRef]
- Hosseini, B.; Nourmohamadi, M.; Hajipour, S.; Taghizadeh, M.; Asemi, Z.; Keshavarz, S.A.; Jafarnejad, S. The Effect of Omega-3 Fatty Acids, EPA, and/or DHA on Male Infertility: A Systematic Review and Meta-analysis. J. Diet. Suppl. 2019, 16, 245–256. [Google Scholar] [CrossRef]
- Byrne, C.J.; Fair, S.; English, A.M.; Holden, S.A.; Dick, J.R.; Lonergan, P.; Kenny, D.A. Dietary polyunsaturated fatty acid supplementation of young post-pubertal dairy bulls alters the fatty acid composition of seminal plasma and spermatozoa but has no effect on semen volume or sperm quality. Theriogenology 2017, 90, 289–300. [Google Scholar] [CrossRef]
- Oborna, I.; Wojewodka, G.; De Sanctis, J.B.; Fingerova, H.; Svobodova, M.; Brezinova, J.; Hajduch, M.; Novotny, J.; Radova, L.; Radzioch, D. Increased lipid peroxidation and abnormal fatty acid profiles in seminal and blood plasma of normozoospermic males from infertile couples. Hum. Reprod. 2010, 25, 308–316. [Google Scholar] [CrossRef]
- Tavilani, H.; Doosti, M.; Abdi, K.; Vaisiraygani, A.; Joshaghani, H.R. Decreased polyunsaturated and increased saturated fatty acid concentration in spermatozoa from asthenozoospermic males as compared with normozoospermic males. Andrologia 2006, 38, 173–178. [Google Scholar] [CrossRef]
- Safarinejad, M.R.; Hosseini, S.Y.; Dadkhah, F.; Asgari, M.A. Relationship of omega-3 and omega-6 fatty acids with semen characteristics, and anti-oxidant status of seminal plasma: A comparison between fertile and infertile men. Clin. Nutr. 2010, 29, 100–105. [Google Scholar] [CrossRef]
- Zhou, R.; Wu, J.; Liu, B.; Jiang, Y.; Chen, W.; Li, J.; He, Q.; He, Z. The roles and mechanisms of Leydig cells and myoid cells in regulating spermatogenesis. Cell. Mol. Life Sci. 2019, 76, 2681–2695. [Google Scholar] [CrossRef]
- Wang, X.J.; Dyson, M.T.; Jo, Y.; Eubank, D.W.; Stocco, D.M. Involvement of 5-lipoxygenase metabolites of arachidonic acid in cyclic AMP-stimulated steroidogenesis and steroidogenic acute regulatory protein gene expression. J. Steroid Biochem. Mol. Biol. 2003, 85, 159–166. [Google Scholar] [CrossRef]
- Wang, X.; Walsh, L.P.; Reinhart, A.J.; Stocco, D.M. The Role of Arachidonic Acid in Steroidogenesis and Steroidogenic Acute Regulatory (StAR) Gene and Protein Expression. J. Biol. Chem. 2000, 275, 20204–20209. [Google Scholar] [CrossRef]
- Oliw, E.H.; Fabiani, R.; Johansson, L.; Ronquist, G. Arachidonic acid 15-lipoxygenase and traces of E prostaglandins in purified human prostasomes. J. Reprod. Fertil. 1993, 99, 195–199. [Google Scholar] [CrossRef]
- Lax, Y.; Grossman, S.; Rubinstein, S.; Magid, N.; Breitbart, H. Role of lipoxygenase in the mechanism of acrosome reaction in mammalian spermatozoa. Biochim. Et Biophys. Acta (Bba) Lipids Lipid Metab. 1990, 1043, 12–18. [Google Scholar] [CrossRef]
- Konturek, S. Konturek Fizjologia Czlowieka. Podrecznik dla Studentów Medycyny, Wyd. II; Elsevier Urban & Partner: Amsterdam, The Netherlands, 2012; ISBN 978-83-7609-751-0. [Google Scholar]
- Rios, M.; Carreño, D.V.; Oses, C.; Barrera, N.; Kerr, B.; Villalón, M. Low physiological levels of prostaglandins E2 and F2α improve human sperm functions. Reprod. Fertil. Dev. 2016, 28, 434–439. [Google Scholar] [CrossRef]
- Perrotta, I.; Santoro, M.; Guido, C.; Avena, P.; Tripepi, S.; De Amicis, F.; Gervasi, M.C.; Aquila, S. Expression of cyclooxygenase-1 (COX-1) and COX-2 in human male gametes from normal patients, and those with varicocele and diabetes: A potential molecular marker for diagnosing male infertility disorders. J. Anat. 2012, 221, 209–220. [Google Scholar] [CrossRef]
- Hagan, S.; Khurana, N.; Chandra, S.; Abdel-Mageed, A.B.; Mondal, D.; Hellstrom, W.J.G.; Sikka, S.C. Differential expression of novel biomarkers (TLR-2, TLR-4, COX-2, and Nrf-2) of inflammation and oxidative stress in semen of leukocytospermia patients. Andrology 2015, 3, 848–855. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef]
- Chavan, A.R.; Griffith, O.W.; Wagner, G.P. The inflammation paradox in the evolution of mammalian pregnancy: Turning a foe into a friend. Curr. Opin. Genet. Dev. 2017, 47, 24–32. [Google Scholar] [CrossRef]
- McGregor, J.A.; Allen, K.G.; Harris, M.A.; Reece, M.; Wheeler, M.; French, J.I.; Morrison, J. The Omega-3 Story: Nutritional Prevention of Preterm Birth and Other Adverse Pregnancy Outcomes. Obstet. Gynecol. Surv. 2001, 56, S1. [Google Scholar] [CrossRef]
- Vrachnis, N.; Karavolos, S.; Iliodromiti, Z.; Sifakis, S.; Siristatidis, C.; Mastorakos, G.; Creatsas, G. Impact of Mediators Present in Amniotic Fluid on Preterm Labour. In Vivo 2012, 26, 799–812. [Google Scholar]
- Nadeau-Vallée, M.; Obari, D.; Palacios, J.; Brien, M.-È.; Duval, C.; Chemtob, S.; Girard, S. Sterile inflammation and pregnancy complications: A review. Reproduction 2016, 152, R277–R292. [Google Scholar] [CrossRef]
- Murthi, P.; Vaillancourt, C. Placental serotonin systems in pregnancy metabolic complications associated with maternal obesity and gestational diabetes mellitus. Biochim. Et Biophys. Acta (Bba) Mol. Basis Dis. 2019, 165391. [Google Scholar] [CrossRef]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef]
- Johns, E.C.; Denison, F.C.; Norman, J.E.; Reynolds, R.M. Gestational Diabetes Mellitus: Mechanisms, Treatment, and Complications. Trends Endocrinol. Metab. 2018, 29, 743–754. [Google Scholar] [CrossRef]
- Pickens, C.A.; Sordillo, L.M.; Comstock, S.S.; Harris, W.S.; Hortos, K.; Kovan, B.; Fenton, J.I. Plasma phospholipids, non-esterified plasma polyunsaturated fatty acids and oxylipids are associated with BMI. Prostaglandins Leukot. Essent. Fat. Acids 2015, 95, 31–40. [Google Scholar] [CrossRef]
- Cinelli, G.; Fabrizi, M.; Ravà, L.; Ciofi degli Atti, M.; Vernocchi, P.; Vallone, C.; Pietrantoni, E.; Lanciotti, R.; Signore, F.; Manco, M. Influence of Maternal Obesity and Gestational Weight Gain on Maternal and Foetal Lipid Profile. Nutrients 2016, 8, 368. [Google Scholar] [CrossRef]
- Peterson, S.J.; Vanella, L.; Gotlinger, K.; Jiang, H.; Singh, S.P.; Sodhi, K.; Maher, E.; O’Hanlon, K.; Shapiro, J.I.; Abraham, N.G. Oxidized HDL is a potent inducer of adipogenesis and causes activation of the Ang-II and 20-HETE systems in human obese females. Prostaglandins Other Lipid Mediat. 2016, 123, 68–77. [Google Scholar] [CrossRef]
- Tsai, I.-J.; Croft, K.D.; Mori, T.A.; Falck, J.R.; Beilin, L.J.; Puddey, I.B.; Barden, A.E. 20-HETE and F2-isoprostanes in the metabolic syndrome: The effect of weight reduction. Free Radic. Biol. Med. 2009, 46, 263–270. [Google Scholar] [CrossRef]
- Issan, Y.; Hochhauser, E.; Guo, A.; Gotlinger, K.H.; Kornowski, R.; Leshem-Lev, D.; Lev, E.; Porat, E.; Snir, E.; Thompson, C.I.; et al. Elevated level of pro-inflammatory eicosanoids and EPC dysfunction in diabetic patients with cardiac ischemia. Prostaglandins Other Lipid Mediat 2013, 100–101, 15–21. [Google Scholar] [CrossRef]
- Gilani, A.; Pandey, V.; Garcia, V.; Agostinucci, K.; Singh, S.P.; Schragenheim, J.; Bellner, L.; Falck, J.R.; Paudyal, M.P.; Capdevila, J.H.; et al. High-fat diet-induced obesity and insulin resistance in CYP4a14−/− mice is mediated by 20-HETE. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R934–R944. [Google Scholar] [CrossRef]
- Facchinetti, F.; Appetecchia, M.; Aragona, C.; Bevilacqua, A.; Bezerra Espinola, M.S.; Bizzarri, M.; D’Anna, R.; Dewailly, D.; Diamanti-Kandarakis, E.; Hernández Marín, I.; et al. Experts’ opinion on inositols in treating polycystic ovary syndrome and non-insulin dependent diabetes mellitus: A further help for human reproduction and beyond. Expert Opin. Drug Metab. Toxicol. 2020, 16, 255–274. [Google Scholar] [CrossRef]
- Laganà, A.S.; Garzon, S.; Casarin, J.; Franchi, M.; Ghezzi, F. Inositol in Polycystic Ovary Syndrome: Restoring Fertility through a Pathophysiology-Based Approach. Trends Endocrinol. Metab. 2018, 29, 768–780. [Google Scholar] [CrossRef]
- Lieb, D.C.; Brotman, J.J.; Hatcher, M.A.; Aye, M.S.; Cole, B.K.; Haynes, B.A.; Wohlgemuth, S.D.; Fontana, M.A.; Beydoun, H.; Nadler, J.L.; et al. Adipose Tissue 12/15 Lipoxygenase Pathway in Human Obesity and Diabetes. J. Clin. Endocrinol. Metab. 2014, 99, E1713–E1720. [Google Scholar] [CrossRef]
- Song, Y.-S.; Lee, D.H.; Yu, J.-H.; Oh, D.-K.; Hong, J.T.; Yoon, D.-Y. Promotion of adipogenesis by 15-(S)-hydroxyeicosatetraenoic acid. Prostaglandins Other Lipid Mediat. 2016, 123, 1–8. [Google Scholar] [CrossRef]
- Shokry, E.; Marchioro, L.; Uhl, O.; Bermúdez, M.G.; García-Santos, J.A.; Segura, M.T.; Campoy, C.; Koletzko, B. Impact of maternal BMI and gestational diabetes mellitus on maternal and cord blood metabolome: Results from the PREOBE cohort study. Acta Diabetol. 2019, 56, 421–430. [Google Scholar] [CrossRef]
- Pintaudi, B.; Fresa, R.; Dalfrà, M.; Dodesini, A.R.; Vitacolonna, E.; Tumminia, A.; Sciacca, L.; Lencioni, C.; Marcone, T.; Lucisano, G.; et al. The risk stratification of adverse neonatal outcomes in women with gestational diabetes (STRONG) study. Acta Diabetol. 2018, 55, 1261–1273. [Google Scholar] [CrossRef]
- O’Neill, K.; Alexander, J.; Azuma, R.; Xiao, R.; Snyder, N.W.; Mesaros, C.A.; Blair, I.A.; Pinney, S.E. Gestational Diabetes Alters the Metabolomic Profile in 2nd Trimester Amniotic Fluid in a Sex-Specific Manner. Int. J. Mol. Sci. 2018, 19, 2696. [Google Scholar] [CrossRef]
- Kuhn, D.C.; Botti, J.J.; Cherouny, P.H.; Demers, L.M. Eicosanoid production and transfer in the placenta of the diabetic pregnancy. Prostaglandins 1990, 40, 205–215. [Google Scholar] [CrossRef]
- Ferchaud-Roucher, V.; Barner, K.; Jansson, T.; Powell, T.L. Maternal obesity results in decreased syncytiotrophoblast synthesis of palmitoleic acid, a fatty acid with anti-inflammatory and insulin-sensitizing properties. FASEB J. 2019, 33, 6643–6654. [Google Scholar] [CrossRef]
- Umeno, A.; Shichiri, M.; Ishida, N.; Hashimoto, Y.; Abe, K.; Kataoka, M.; Yoshino, K.; Hagihara, Y.; Aki, N.; Funaki, M.; et al. Singlet Oxygen Induced Products of Linoleates, 10- and 12-(Z,E)-Hydroxyoctadecadienoic Acids (HODE), Can Be Potential Biomarkers for Early Detection of Type 2 Diabetes. PLoS ONE 2013, 8, e63542. [Google Scholar] [CrossRef]
- Laffer, C.L.; Laniado-Schwartzman, M.; Nasjletti, A.; Elijovich, F. 20-HETE and Circulating Insulin in Essential Hypertension with Obesity. Hypertension 2004, 43, 388–392. [Google Scholar] [CrossRef]
- Chen, X.; Stein, T.P.; Steer, R.A.; Scholl, T.O. Individual free fatty acids have unique associations with inflammatory biomarkers, insulin resistance and insulin secretion in healthy and gestational diabetic pregnant women. BMJ Open Diabetes Res. Care 2019, 7. [Google Scholar] [CrossRef]
- Thomas, B.; Ghebremeskel, K.; Lowy, C.; Min, Y.; Crawford, M.A. Plasma AA and DHA levels are not compromised in newly diagnosed gestational diabetic women. Eur. J. Clin. Nutr. 2004, 58, 1492–1497. [Google Scholar] [CrossRef][Green Version]
- Walsh, S.W. Eicosanoids in preeclampsia. Prostaglandins Leukot Essent. Fat. Acids 2004, 70, 223–232. [Google Scholar] [CrossRef]
- Gyselaers, W. Preeclampsia Is a Syndrome with a Cascade of Pathophysiologic Events. J. Clin. Med. 2020, 9, 2245. [Google Scholar] [CrossRef]
- Lopez-Jaramillo, P.; Barajas, J.; Rueda-Quijano, S.M.; Lopez-Lopez, C.; Felix, C. Obesity and Preeclampsia: Common Pathophysiological Mechanisms. Front. Physiol. 2018, 9. [Google Scholar] [CrossRef]
- Plenty, N.L.; Faulkner, J.L.; Cotton, J.; Spencer, S.-K.; Wallace, K.; LaMarca, B.; Murphy, S.R. Arachidonic acid metabolites of CYP4A and CYP4F are altered in women with preeclampsia. Prostaglandins Other Lipid Mediat. 2018, 136, 15–22. [Google Scholar] [CrossRef]
- Parkington, H.C.; Coleman, H.A.; Tare, M. Prostacyclin and endothelium-dependent hyperpolarization. Pharmacol. Res. 2004, 49, 509–514. [Google Scholar] [CrossRef]
- Lu, S.; Zhu, C.; Long, A.; Tan, L.; Li, Q.; Zhu, Y. Effect of 20-hydroxyeicosatetraenoic acid on biological behavior of human villous trophoblasts and uterine vascular smooth muscle cells. Mol. Med. Rep. 2014, 9, 1889–1894. [Google Scholar] [CrossRef][Green Version]
- Ashton, S.V.; Whitley, G.St.J.; Dash, P.R.; Wareing, M.; Crocker, I.P.; Baker, P.N.; Cartwright, J.E. Uterine Spiral Artery Remodeling Involves Endothelial Apoptosis Induced by Extravillous Trophoblasts Through Fas/FasL Interactions. Arter. Thromb. Vasc. Biol. 2005, 25, 102–108. [Google Scholar] [CrossRef]
- Jiang, H.; McGiff, J.C.; Fava, C.; Amen, G.; Nesta, E.; Zanconato, G.; Quilley, J.; Minuz, P. Maternal and Fetal Epoxyeicosatrienoic Acids in Normotensive and Preeclamptic Pregnancies. Am. J. Hypertens. 2013, 26, 271–278. [Google Scholar] [CrossRef]
- Reyes-Hernández, C.G.; Ramiro-Cortijo, D.; Rodríguez-Rodríguez, P.; Giambelluca, S.; Simonato, M.; González, M.D.C.; López de Pablo, A.L.; López-Giménez, M.D.R.; Cogo, P.; Sáenz de Pipaón, M.; et al. Effects of Arachidonic and Docosohexahenoic Acid Supplementation during Gestation in Rats. Implication of Placental Oxidative Stress. Int. J. Mol. Sci. 2018, 19, 3863. [Google Scholar] [CrossRef]
- Herrera, E. Implications of dietary fatty acids during pregnancy on placental, fetal and postnatal development—A review. Placenta 2002, 23 (Suppl. A), S9–S19. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, D.; An, Y.; Sun, J.; Cai, L.; Zheng, J. Preeclampsia activates 15-lipoxygenase and its metabolite 15-hydroxyeicosatetraenoic acid enhances constriction in umbilical arteries. Prostaglandins Leukot Essent. Fat. Acids 2012, 86, 79–84. [Google Scholar] [CrossRef]
- Yuan, D.; Ran, Y.; Liu, Q.; Zhang, Y.; Li, H.; Li, P.; Zhu, D. Enhancement of the HIF-1α/15-LO/15-HETE Axis Promotes Hypoxia-Induced Endothelial Proliferation in Preeclamptic Pregnancy. PLoS ONE 2014, 43, e96510. [Google Scholar] [CrossRef]
- Llinás Maria, T.; Alexander Barbara, T.; Capparelli Maria, F.; Carroll Mairead, A.; Granger Joey, P. Cytochrome P-450 Inhibition Attenuates Hypertension Induced by Reductions in Uterine Perfusion Pressure in Pregnant Rats. Hypertension 2004, 43, 623–628. [Google Scholar] [CrossRef]
- Sarkis, A.; Roman, R. Role of Cytochrome P450 Metabolites of Arachidonic Acid in Hypertension. CDM 2004, 5, 245–256. [Google Scholar] [CrossRef]
- Williams, J.M.; Murphy, S.; Burke, M.; Roman, R.J. 20-HETE: A new target for the treatment of hypertension. J. Cardiovasc. Pharm. 2010, 56, 336–344. [Google Scholar] [CrossRef]
- Faulkner, J.L.; Plenty, N.L.; Wallace, K.; Amaral, L.M.; Cunningham, M.W.; Murphy, S.; LaMarca, B. Selective inhibition of 20-hydroxyeicosatetraenoic acid lowers blood pressure in a rat model of preeclampsia. Prostaglandins Other Lipid Mediat. 2018, 134, 108–113. [Google Scholar] [CrossRef]
- Wang, M.-H.; Wang, J.; Chang, H.-H.; Zand, B.A.; Jiang, M.; Nasjletti, A.; Laniado-Schwartzman, M. Regulation of renal CYP4A expression and 20-HETE synthesis by nitric oxide in pregnant rats. Am. J. Physiol. Ren. Physiol. 2003, 285, F295–F302. [Google Scholar] [CrossRef]
- Johnson, R.D.; Polakoski, K.L.; Huang, X.; Sadovsky, Y.; Nelson, D.M. The release of 15-hydroxyeicosatetraenoic acid by human placental trophoblast is increased in preeclampsia. Am. J. Obstet. Gynecol. 1998, 178, 54–58. [Google Scholar] [CrossRef]
- Pearson, T.; Warren, A.Y.; Barrett, D.A.; Khan, R.N. Detection of EETs and HETE-generating cytochrome P-450 enzymes and the effects of their metabolites on myometrial and vascular function. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E647–E656. [Google Scholar] [CrossRef][Green Version]
- Soler, A.; Hunter, I.; Joseph, G.; Hutcheson, R.; Hutcheson, B.; Yang, J.; Zhang, F.F.; Joshi, S.R.; Bradford, C.; Gotlinger, K.H.; et al. Elevated 20-HETE in Metabolic Syndrome Regulates Arterial Stiffness and Systolic Hypertension via MMP12 Activation. J. Mol. Cell Cardiol. 2018, 117, 88–99. [Google Scholar] [CrossRef]
- Pearson, T.; Zhang, J.; Arya, P.; Warren, A.; Ortori, C.; Fakis, A.; Khan, R.; Barrett, D. Measurement of vasoactive metabolites (hydroxyeicosatetraenoic and epoxyeicosatrienoic acids) in uterine tissues of normal and compromised human pregnancy. J. Hypertens. 2010, 28, 2429–2437. [Google Scholar] [CrossRef]
- Long, A.; Ma, S.; Li, Q.; Lin, N.; Zhan, X.; Lu, S.; Zhu, Y.; Jiang, L.; Tan, L. Association between the maternal serum levels of 19 eicosanoids and pre-eclampsia. Int. J. Gynecol. Obstet. 2016, 133, 291–296. [Google Scholar] [CrossRef]
- Chatuphonprasert, W.; Jarukamjorn, K.; Ellinger, I. Physiology and Pathophysiology of Steroid Biosynthesis, Transport and Metabolism in the Human Placenta. Front. Pharm. 2018, 9. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczuko, M.; Kikut, J.; Komorniak, N.; Bilicki, J.; Celewicz, Z.; Ziętek, M. The Role of Arachidonic and Linoleic Acid Derivatives in Pathological Pregnancies and the Human Reproduction Process. Int. J. Mol. Sci. 2020, 21, 9628. https://doi.org/10.3390/ijms21249628
Szczuko M, Kikut J, Komorniak N, Bilicki J, Celewicz Z, Ziętek M. The Role of Arachidonic and Linoleic Acid Derivatives in Pathological Pregnancies and the Human Reproduction Process. International Journal of Molecular Sciences. 2020; 21(24):9628. https://doi.org/10.3390/ijms21249628
Chicago/Turabian StyleSzczuko, Małgorzata, Justyna Kikut, Natalia Komorniak, Jacek Bilicki, Zbigniew Celewicz, and Maciej Ziętek. 2020. "The Role of Arachidonic and Linoleic Acid Derivatives in Pathological Pregnancies and the Human Reproduction Process" International Journal of Molecular Sciences 21, no. 24: 9628. https://doi.org/10.3390/ijms21249628
APA StyleSzczuko, M., Kikut, J., Komorniak, N., Bilicki, J., Celewicz, Z., & Ziętek, M. (2020). The Role of Arachidonic and Linoleic Acid Derivatives in Pathological Pregnancies and the Human Reproduction Process. International Journal of Molecular Sciences, 21(24), 9628. https://doi.org/10.3390/ijms21249628





