Abstract
Aeromonas veronii is one of the main pathogens causing various diseases in humans and animals. It is currently difficult to eradicate drug-resistant A. veronii due to the biofilm formation by conventional antibiotic treatments. In this study, a marine peptide-N6NH2 and its analogs were generated by introducing Orn or replacing with D-amino acids, Val and Pro; their enzymic stability and antibacterial/antibiofilm ability against multi-drug resistant (MDR) A. veronii ACCC61732 were detected in vitro and in vivo, respectively. The results showed that DN6NH2 more rapidly killed A. veronii ACCC61732 and had higher stability in trypsin, simulated gastric/intestinal fluid, proteinase K, and mouse serum than the parent peptide-N6NH2. DN6NH2 and other analogs significantly improved the ability of N6NH2 to penetrate the outer membrane of A. veronii ACCC61732. DN6NH2, N6PNH2 and V112N6NH2 protected mice from catheter-associated biofilm infection with MDR A. veronii ACCC61732, superior to N6NH2 and CIP. DN6NH2 had more potent efficacy at a dose of 5 μmol/kg (100% survival) in a mouse peritonitis model than other analogs (50–66.67%) and CIP (83.33%), and it inhibited the bacterial translocation, downregulated pro-inflammatory cytokines, upregulated the anti-inflammatory cytokine, and ameliorated multiple-organ injuries (including the liver, spleen, lung, and kidney). These data suggest that the analogs of N6NH2 may be a candidate for novel antimicrobial and antibiofilm agents against MDR A. veronii infections.
1. Introduction
Aeromonas veronii is an emerging aquatic pathogen that can cause diarrhea, wound infections, and hemorrhagic septicemia in humans and animals, including aquatic animals with 40–60% cumulative mortality [1,2]. In recent years, there has been an increasing number of cases of large-scale A. veronii outbreaks. It has been reported that A. veronii can infect freshwater fish [3,4], amphibians [5], and mammals [6], resulting in serious economic losses to the aquaculture industry and threatening food safety. In addition, people can infect through contacting with A. veronii-contaminated surfaces, such as contaminated livestock and poultry meat and seafood, resulting in gastroenteritis [7], wound infections [8], and other diseases in the elderly and children with low immunity [9]. A. veronii can form biofilms; aggregates of bacteria are embedded within a self-produced matrix of extracellular substances [10], which further complicates the treatment because biofilm-encased bacteria can be 10–1000-fold more tolerant to conventional antibiotics than their planktonic counterparts [11]. Approximately 96% of Aeromonas isolates from humans and water conditions are resistant to ampicillin, tetracycline, ciprofloxacin (CIP), chloramphenicol, tetracycline, cotrimoxazole, etc. [12,13]. Therefore, there is an urgent and growing need for the development of alternative antimicrobial agents with superior properties and less toxicity for the prevention and treatment of A. veronii infections.
Antimicrobial peptides (AMPs), also known as host defense peptides, are natural or synthetic peptides with antimicrobial activity against diverse pathogenic bacteria in the planktonic state and/or in biofilms [14]. Some cationic AMPs and their synthetic derivatives such as LL-37, PR-39, pexiganan, temporin A, and PXL01 have been proposed to be a promising alternative for the treatment of infections caused by antibiotic-resistant bacteria [15,16]. However, major obstacles to their success as therapeutics in clinical trials are their inherent susceptibility to proteolytic degradation, high toxicity to eukaryotic cells, or low stability under physiologically relevant conditions in vivo (e.g., in the presence of proteases (trypsin and chymotrypsin), salt, and serum) [17,18]. To overcome these problems, many researchers have tried to develop novel AMPs with improved stability and selective toxicity by several strategies, including the use of non-coded amino acids (D-amino acids, aminoisobutyric acid, etc.), chemical modification (N-acetylation, C-amidation, cyclization, etc.), and non-peptidic backbones of peptides [19,20]. Among them, the use of ornithine (Orn) as a charged moiety is preferable to the use of a non-coded amino acid, and it provides the stability of AMPs such as Api88, Api137, and Apidaecin against proteases [21,22,23,24]. Unfortunately, some modifications such as N-acetylation and D-enantiomer can improve the stability of AMPs towards proteases, accompanied by unfavorable effects on antibacterial activity or some toxicity towards eukaryotic cells [19].
In our previous studies, C-terminal amidated marine peptide-N6 (N6NH2) displayed potent intracellular and extracellular antibacterial activity against a variety of bacteria, especially Gram-negative bacteria including Escherichia and Salmonella strains [25]. However, N6NH2 is very sensitive to trypsin, which greatly limits its clinical application in the future. In this study, various modified peptides of N6NH2 were designed by using natural residues (Val and Pro), unnatural residue (Orn), and D-amino acids for the first time, aiming to improve its stability towards proteases and antibacterial activity, while keeping the structural features (β-sheet) known to be important for the antimicrobial activity of AMPs. The extracellular/intracellular antibacterial activity and anti-biofilm ability of N6NH2 and its analogs were tested against multidrug-resistant (MDR) A. veronii, as well as their stability, toxicity, resistance, and mechanisms of action. Additionally, the efficacy of all analogs was determined in a mouse model of catheter-associated biofilm infection and in a peritonitis mouse model of bacterial infection with MDR A. veronii.
2. Results
2.1. Design and Physicochemical Properties of N6NH2 and Its Analogs
To develop marine peptides with high stability, low toxicity, and high efficacy, four new analogs of N6NH2 were designed based on the following criteria: (a) keeping the structural features (β-sheet) that are crucial for effective antibacterial activity of N6NH2; (b) changing net charge and hydrophobicity; and (c) replacing residues with natural amino acids (Pro and Val) and non-natural ones (D-enantiomers and Orn). N6PNH2 and V112N6NH2 were generated by the substitution of Arg19 and Gly1,12 in the sequences of N6NH2 with Pro and Val, respectively. DN6NH2 and Guo-N6NH2 were produced by using D-enantiomers of all amino acids (in the exception of Gly) and Orn in N6NH2, respectively (Table 1).

Table 1.
Amino acid sequence and physicochemical properties of N6NH2 and its analogs.
The chemical structures of four analogs were similar to those of the parent N6NH2 (Figures S1 and S2), indicating the structural features (β-sheet) of N6NH2 were maintained after modification. The physicochemical properties (including pI, net charge, and hydrophobicity) of DN6NH2 (+5) are equal to those of N6NH2 (Table 1). The hydrophobicity of N6PNH2 and V112N6NH2 are higher than that of N6NH2. Both V112N6NH2 and N6NH2 have the same net charges (+5). Guo-N6NH2 (+6) has more positive charges than N6NH2 (+5) and N6PNH2 (+4), indicating their different antibacterial activity; more net charges of AMPs may facilitate the initial electrostatic attraction of peptides with negatively charged components on the bacterial membrane surface.
2.2. N6NH2 and Its Analogs Showed Potent Antimicrobial Activity
The minimum inhibitory concentration (MIC) values of N6NH2 against A. veronii, Escherichia coli, and Salmonella were 1.61–12.90, 0.81–1.61, and 0.81–3.23 μM, respectively, which were slightly lower than those of DN6NH2 (1.62–25.86, 1.62–3.23, and 0.81–6.46 μM) (Table 2). The activity of Guo-N6NH2 (3.04–12.16, 0.76–1.52, and 0.76–3.04 μM) was almost identical to that of N6NH2. However, N6PNH2 (6.62–26.49, 1.66–3.31, and 0.83–13.25 μΜ) and V112N6NH2 (6.25–12.51, 1.56–3.13, 0.78–6.25 μM) have higher MIC values, which indicated their lower antibacterial activity than their parent N6NH2. The MICs of DN6NH2 against Pseudomonas aeruginosa and Gram-positive bacteria such as Staphylococcus aureus and S. hyicus were 1.62–6.46 μM, lower than those of N6NH2 (6.46–25.8 µΜ), indicating that DN6NH2 possesses antibacterial activity than N6NH2. Additionally, all peptides did not display any activity against Candida albicans.

Table 2.
MIC values of N6NH2 and its analogs.
The bactericidal kinetics showed that 1× MIC of N6NH2 analogs could not completely kill A. veronii ACCC61732, while 2× MIC DN6NH2 could completely kill A. veronii ACCC61732 within 1 h, which is more rapid than N6NH2 (8 h), N6PNH2 (2 h), and Guo-N6NH2 (6 h) (Figure 1A); however, 2× MIC V112N6NH2 was unable completely kill A. veronii ACCC61732. Comparably, the bacteria treated with 2× MIC CIP showed a slow reduction and started to regrow after 2 h. The results indicate that DN6NH2 has the strongest bactericidal activity.
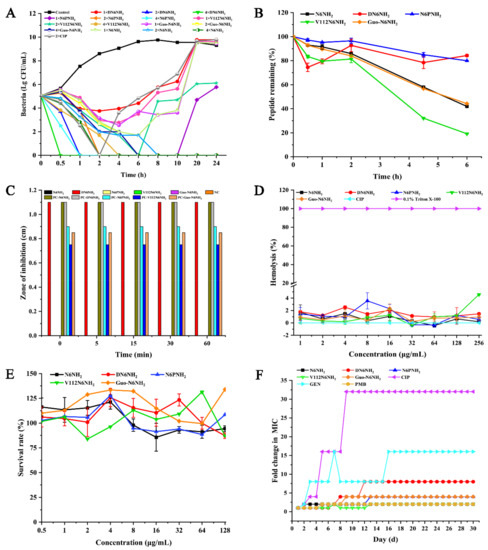
Figure 1.
Time-killing curves, toxicity, and stability of N6NH2 and its analogs: (A) time-killing curves of N6NH2 and its analogs (1×, 2×, or 4× MIC) against A. veronii ACCC61732. CIP (2× MIC) and PBS were used as the positive and negative control, respectively; (B) changes of peptide content of N6NH2 and its analogs in serum of mouse with time; (C) the stability of N6NH2 and its analogs in SIF (NC, negative control; PC, positive control); (D) hemolytic activity of N6NH2 and its analogs or CIP at different concentrations (1–256 μg/mL) against mouse erythrocytes; (E) cytotoxicity of peptides or CIP at different concentrations (0.5–128 μg/mL) against A. veronii ACCC61732 in RAW 264.7 cells; and (F) resistance of N6NH2 and its analogs.
2.3. N6NH2 and Its Analogs Showed Additive Effects with Antibiotics and Greater Post-Antibiotic Effect (PAE) Values
The efficacy of interactions between peptides and traditional antibiotics against A. veronii ACCC61732 is shown in Tables S1–S5. The fractional inhibitory concentration index (FICI) showed that the activity of N6NH2 combined with rifampicin, streptomycin sulfate, doxycycline hyclate, and kanamycin sulfate was 16–25-fold greater than that of N6NH2 alone. Furthermore, N6NH2 showed additive effects with rifampicin, streptomycin sulfate, doxycycline hyclate, and kanamycin sulfate (Table S1). Notably, antagonism between N6NH2 and antibiotics was not found in the combinations. Comparably, N6NH2 had synergic effect with polymyxin B (PMB). Other analogs showed an additive effect with some antibiotics including norfloxacin, streptomycin sulfate, rifampicin, and PMB (Tables S2–S5). Additionally, Guo-N6NH2 had a synergic effect with PMB (Table S5).
The PAE results of peptides on A. veronii ACCC61732 are shown in Table 3. All peptides and CIP showed increased PAE values in a dose-dependent manner. The PAE values of DN6NH2 on A. veronii ACCC61732 was 3.36 h at 4× MIC, significantly greater than that of N6NH2 (1.17 h), N6PNH2 (0.68 h), V112N6NH2 (2.15 h), Guo-N6NH2 (2.07 h), and CIP (0.67 h). Meanwhile, the PAEs of V112N6NH2 (0.74–2.15 h) and Guo-N6NH2 (0.62–2.07 h) were higher than those of N6NH2 (0.69–1.17 h), with the exception of N6PNH2 (0.52–0.68 h).

Table 3.
PAE analysis of N6NH2 and its analogs on A. veronii ACCC61732.
2.4. DN6NH2 and N6PNH2 Had Higher Stability in Different Conditions
The temperature, salt, serum, enzyme, and pH stabilities of peptides were evaluated by the MIC assay (Table S6). After treatment at different temperatures (4–80 °C) for 1 h, pH values (2–10) for 3 h, and salt concentrations (50–500 mM) for 3 h, DN6NH2 retained its intrinsic antibacterial activity against MDR A. veronii ACCC61732, but its activity at 100 °C was slightly reduced. The activity of Guo-N6NH2, N6PNH2, and V112N6NH2 was reduced by 1–3-fold. Noticeably, Guo-N6NH2 had higher activity than N6PNH2 and V112N6NH2 at different salt conditions. In the enzyme stability, DN6NH2 retained the antibacterial activity against A. veronii ACCC61732 under pepsin, trypsin, and protease K conditions, but N6PNH2, V112N6NH2, and Guo-N6NH2 lost their activity in trypsin and protease K conditions. MS analysis of trypsin-treated N6NH2 and DN6NH2 showed that the structure of DN6NH2 is not affected by trypsin (Figures S3 and S4). Additionally, after incubation in the mouse serum for 2–4.5 h, 92.73–78.41% of DN6NH2 and 95.23–84.94% of N6PNH2 retained intact native patterns, higher than those of N6NH2 (85.89–57.98%), Guo-N6NH2 (84.03–56.75%), and V112N6NH2 (81.36–32.11%) (Figure 1B). After a 6 h-incubation in serum, the remaining rates of DN6NH2 and N6PNH2 were 84.25% and 79.94%, respectively, higher than those of N6NH2 (41.93%), Guo-N6NH2 (44.24%), and V112N6NH2 (19.12%). These results indicate that both DN6NH2 and N6PNH2 have higher stability in serum or enzyme conditions than N6NH2 and other analogs.
The susceptibility of peptides to simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) was detected by using the inhibition zone. The results showed that, after treatment in SGF for 60 min, DN6NH2 displayed higher antibacterial activity than N6NH2 and other analogs (Figure 1C). Meanwhile, DN6NH2 retained 100% activity against A. veronii ACCC61732, but N6NH2 and other analogs were degraded in less than 5 min in SIF (Figure S5), indicating higher stability of DN6NH2 than N6NH2 in SIF.
2.5. All Analogs Exhibited No or Low Toxicity and Resistance
The hemolysis activities of N6NH2, DN6NH2, N6PNH2, V112N6NH2, Guo-N6NH2, and CIP were 0.19%, 1.46%, 0.56%, 4.59%, 0.93%, and 0%, respectively, at a concentration of 256 μg/mL, indicating that, similar to N6NH2, all analogs have no or very low hemolysis to mouse erythrocytes (Figure 1D).
RAW 264.7 cells were used to evaluate the toxicity of peptides. The survival rate of DN6NH2 was over 100% at low concentrations; the cell survival rates of N6NH2, DN6NH2, N6PNH2, V112N6NH2, and Guo-N6NH2 were 94.39%, 87.26%, 108.44%, 87.18%, and 133.93%, respectively, at a concentration of 128 μg/mL, indicating that all peptides have no or very low toxic effects on animals (Figure 1E).
After 30 serial passages of A. veronii ACCC61732 in the presence of peptides, the MICs of N6NH2, DN6NH2, N6PNH2, V112N6NH2, and Guo-N6NH2 were increased by one-fold, seven-fold, three-fold, one-fold, and three-fold, respectively, indicating that DN6NH2, N6PNH2, and Guo-N6NH2 can induce very low bacterial resistances within 30 days (Figure 1F). In contrast, CIP and gentamicin (GEN) significantly induced bacterial resistances with the MIC increased by 15-fold and 31-fold, respectively, after 30 passages. The MIC of PMB was enhanced by one-fold, indicating that, similar to PMB, N6NH2 and its analogs have lower resistance than CIP and GEN, which may serve as novel antibacterial agents against MDR A. veronii ACCC61732.
2.6. Antibacterial Mechanism of N6NH2 and Its Analogs
2.6.1. N6NH2 and Its Analogs Permeabilized the Outer Cell Membranes and Disrupted the Membrane Potentials
The ability of peptides to permeabilize the bacterial outer membranes was measured by N-phenyl-1-naphthylamine (NPN) probe staining. As shown in Figure 2 and Figure S6A, all peptides induced a time- and concentration-dependent increase in fluorescence. The addition of 1× and 2× MIC of peptides to A. veronii ACCC61732 cells caused a slow increase in fluorescence intensity, indicating a weak outer membrane permeability of peptides. However, 4× MIC of peptides could permeabilize A. veronii ACCC61732 within 1 min and fluorescence was increased by one-fold, indicating that all peptides can instantly permeabilize the outer membrane of A. veronii cells. DN6NH2, V112N6NH2, Guo-N6NH2, and PMB have a higher ability to penetrate the outer membrane than N6NH2, while V112N6NH2 has the best ability to penetrate the outer membrane of A. veronii ACCC61732.
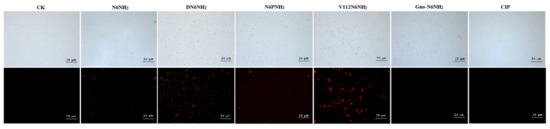
Figure 2.
Fluorescence inverted microscope analysis of N6NH2 and its analogs against A. veronii ACCC61732 membrane penetrating ability. The magnification of images is 20 μm.
In the absence of peptides, 100% of cells exhibited no propidium iodide (PI) staining, indicating the intact cell membranes (Figure 2 and Figure S8). After treatment with 4× MIC DN6NH2, the membrane permeabilizing ratio of bacteria increased to 41.93% (0.5 h) and 33.73% (2 h). After treatment with 4× MIC V112N6NH2 for 0.5–2 h, the penetration ratio increased up to 4.28% and 23.93%, respectively, higher than that of N6NH2 (0–1.12%) (Figure S8 and Table S7), indicating that both DN6NH2 and V112N6NH2 have stronger ability to destroy the integrity of the cell membrane of A. veronii ACCC61732 than their parent N6NH2. However, N6NH2, N6PNH2, Guo-N6NH2, and CIP have poor ability to destroy the cell membrane integrity of A. veronii ACCC61732.
After treatment of A. veronii ACCC61732 with peptides, the inner membrane potential changes are shown in Figure S6B–F. In 3,3′-dipropylthiadicarbocyanine iodide DiSC3(5) analysis, the fluorescence intensity of DiSC3(5) was significantly increased by the addition of peptides. Compared with CIP, N6NH2 and its analogs showed a relatively strong and concentration-dependent ability to depolarize cytoplasmic bacterial membranes.
2.6.2. Peptides Exposure Affects Adenosine Triphosphate (ATP) Release from Cells
ATP efflux measured by luminescent microbial cell viability assays, in contrast to CIP, indicates a rapid leakage of ATP following the addition of N6NH2 and its analogs to intermediate cultures of A. veronii ACCC61732. When incubated at 8×, 16×, and 32× MIC concentrations for 60 min, N6NH2 showed ATP release of 56.07–90.00%, DN6NH2 showed ATP release of 84.34–86.15%, N6PNH2 showed ATP release of 77.39–90.34%, V112N6NH2 showed ATP release of 88.14–90.34%, and V112N6NH2 showed ATP release of 88.14–90.00%. Guo-N6NH2 showed ATP release of 66.23–92.09%, higher than CIP (approximately 7.06–47.60%). DN6NH2 was also found to induce ATP leakage in bacteria even under low MIC conditions (Figure S6G). The results indicate that N6NH2 and its derivatives have a role in causing ATP leakage in bacteria.
2.6.3. All Analogs of N6NH2 Bound to Bacterial Genomic DNA and Changed DNA Structure
The interaction between DNA and peptides is shown in Figure S7A. The DNA-binding ability of all peptides was enhanced with the increased mass ratio of peptide-DNA. When the mass ratios were up to 10.0, DN6NH2 completely inhibited the migration of genomic DNA, more potently than its parent and other analogs. However, CIP could not inhibit the migration of genomic DNA. This result indicates that similar to N6NH2, all analogs can bind to genomic DNA.
The circular dichroism (CD) spectra were used to further confirm the DNA-binding affinity of peptides. As presented in Figure S7B, the CD spectra of A. veronii ACCC61732 genomic DNA showed a positive peak at 270 nm and a negative peak at 245 nm in the absence of peptides. After exposure to DN6NH2, the elliptic intensity of the positive band declined with the increased peptide concentration, suggesting that DN6NH2 may insert into base pairs of DNA and weakened stacking interaction. However, the peak positions of positive and negative peaks did not change and shift in the presence of other analogs of N6NH2, indicating that N6NH2, N6PNH2, V112N6NH2, and Guo-N6NH2 do not affect the double helix of genomic DNA.
2.7. All Analogs of N6NH2 Induced Morphological Changes in A. veronii
2.7.1. Scanning Electron Microscope (SEM) Observations
The untreated A. veronii ACCC61732 cells exhibited intact smooth surfaces, but the cells treated with peptides underwent considerable morphological changes (Figure 3). After treatment with DN6NH2, over 50% cells produced many filamentous substances and the collapsed cells were observed. After treatment with N6NH2, N6PNH2, and V112N6NH2, it appeared to numerous protrusions or blebs and filamentous substances outside of the cells. After treatment with V112N6NH2 and Guo-N6NH2, numerous protruding outer membrane vesicles (OMVs) were seen in the A. veronii ACCC61732 cells (Figure 3). After polymerase chain reaction (PCR) amplification with the primers of the outer membrane vesicle gene-vacJ (Table S8), the product (482 bp) was obtained in gel (Figure S9), indicating that the vacJ ABC (ATP-binding cassette) transport system may be involved in A. veronii ACCC61732 OMVs formation [26]. In contrast, the A. veronii ACCC61732 cells treated with CIP could not be divided normally and formed longer rod-shaped bacteria. These results suggest that there are different mechanisms of action between peptides and CIP.
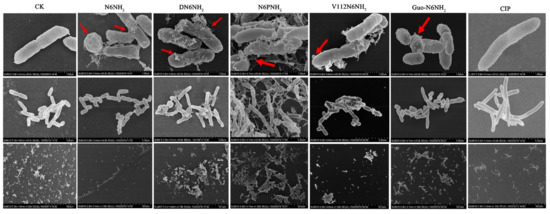
Figure 3.
SEM images of A. veronii ACCC61732 cells treated with N6NH2 and its analogs. Bacteria in mid-logarithmic growth were treated with peptides or antibiotic at 4× MIC or CIP for 2 h (n = 3 independent experiments). CIP was used as the positive control. Red arrows indicate typical disruptions, which were caused by peptides or CIP (filamentous substances, OMVs, protrusions, and leakage of contents).
2.7.2. Transmission Electron Microscope (TEM) and Confocal Laser Scanning Microscope (CLSM) Observations
Effects of peptides on A. veronii ACCC61732 cells were also visualized using TEM. The untreated A. veronii ACCC61732 cells displayed normal morphology, intact cell membranes, and homogeneous electron density in cytoplasm. After treatment with 4× MIC peptides or CIP for 2 h, heterogeneous electron density, disappearance of the outer and inner cell membranes, leakage of cellular contents, and ghosts were observed in A. veronii ACCC61732 cells, which is a typical feature of cell death. DN6NH2, N6PNH2, Guo-N6NH2, and CIP induced more abnormal cells than N6NH2 (Figure 4). However, noticeably, after exposure to V112N6NH2, many OMVs were observed on the surface of the bacterial cell membrane (Figure 4 and Figure S10), which may be related to the host immune regulation and the promotion of bacterial survival during envelope stress [27].
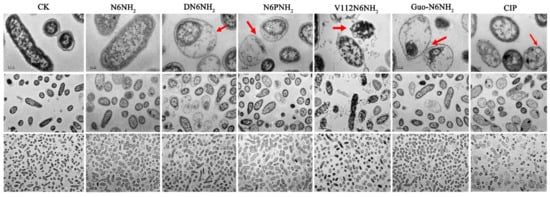
Figure 4.
TEM images of A. veronii ACCC61732 cells treated with N6NH2 and its analogs. Bacteria in mid-logarithmic growth were treated with peptides or antibiotic at 4× MIC or CIP for 2 h (n = 3 independent experiments). CIP was used as the positive control. Red arrows indicate typical disruptions, which were caused by peptides or CIP (filamentous substances, disappearance of membranes, leakage of contents, and ghosts). The scale of the top, middle and bottom images is 0.2, 1.0, and 2.0 μm, respectively.
2.8. N6NH2 and Its Analogs Eliminated MDR A. veronii Biofilms and Persisters
2.8.1. Inhibition of Biofilm Formation
To investigate the inhibitory effect of peptides on early biofilms, A. veronii ACCC61732 cells were exposed to different concentrations of peptides or CIP. After treatment with 16× MIC peptides, the biofilm of A. veronii ACCC61732 was inhibited by 57.59% (N6NH2), 71.33% (DN6NH2), 67.49% (N6PNH2), and 65.09% (Guo-N6NH2), respectively, indicating that DN6NH2 is more effective in preventing the formation of early biofilms. After treatment with 16× MIC CIP, the biofilm of A. veronii ACCC61732 was reduced by 70.75% (Figure 5A). Differently, however, A. veronii ACCC61732 treated with V112N6NH2 produced a large amount of precipitated substances, which may be related to the formation of OMVs. Therefore, it is difficult to evaluate the inhibitory effect of V112N6NH2 on early biofilms of A. veronii ACCC61732 by crystal violet staining. These data suggest that DN6NH2, N6PNH2, Guo-N6NH2, and CIP (with the exception of V112N6NH2) have a more potent ability to inhibit early biofilm formation than N6NH2.
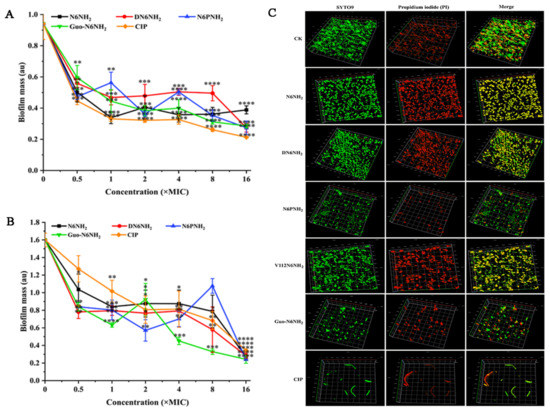
Figure 5.
Effects of N6NH2 and its analogs on biofilms of A. veronii ACCC61732: (A) effect of N6NH2 and its analogs on early biofilms of A. veronii ACCC61732; (B) effect of N6NH2 and its analogs on mature biofilms of A. veronii ACCC61732; (C) laser confocal observation of the effect of N6NH2 and its analogs on mature biofilm of A. veronii ACCC61732. The analyses were measured by one-way ANOVA, with Duncan’s multiple comparisons test. A p-value of <0.05 was considered significant. (*) Indicates the significance between control and treatment groups. * p < 0.05; ** p < 0.01, *** p < 0.001, **** p < 0.0001.
2.8.2. Eradication of Mature Biofilms
To determine the destructive potential of peptides to mature biofilms of MDR A. veronii ACCC61732, the bacteria were incubated for 24 h in advance. As shown in Figure 5B,C and Figure S11, the untreated bacterial cells formed thick biofilms on the surfaces of the glass plates; conversely, DN6NH2, N6PNH2, Guo-N6NH2, and CIP (in the exception of V112N6NH2) more potently inhibited A. veronii biofilm formation than N6NH2. Specifically, after treatment with 16× MIC peptides, A. veronii ACCC61732 biofilms were inhibited by 91.57% (N6NH2), 91.90% (DN6NH2), 97.16% (N6PNH2), and 97.04% (Guo-N6NH2). After treatment with 16× MIC CIP, the biofilm was inhibited by 89.87%. Similarly, the crystal violet staining method cannot be used to evaluate the effects of V112N6NH2 on mature biofilms of A. veronii ACCC61732. These data suggest that DN6NH2, N6PNH2, and Guo-N6NH2 have stronger destructive abilities on mature biofilms than N6NH2 and CIP.
2.8.3. Killing Persisters in Biofilm
MDR A. veronii significantly reduced after treatment with peptides; 8× MIC DN6NH2, N6PNH2, V112N6NH2, Guo-N6NH2, N6NH2 and CIP killed 64.22%, 57.99%, 46.21%, 46.26%, 65.49%, and 47.46% of A. veronii ACCC61732, respectively. Meanwhile, 66.79%, 67.44%, 52.04%, 54.31%, 75.36%, and 52.32% of bacteria cells were killed by 16× MIC DN6NH2, N6PNH2, V112N6NH2, Guo-N6NH2, N6NH2, and CIP, respectively (Figure 6A). This indicates that N6NH2, DN6NH2, N6PNH2, V112N6NH2, Guo-N6NH2, and CIP can kill bacteria in A. veronii biofilm in a concentration-dependent manner, which is consistent with the results of the biofilm inhibition.
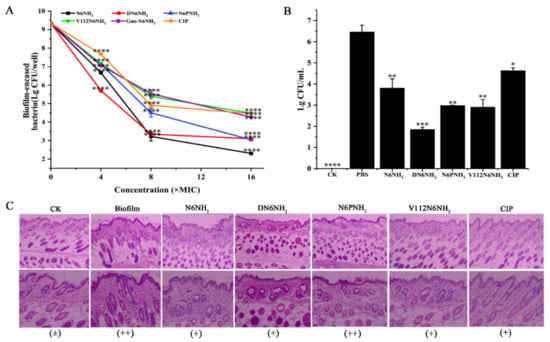
Figure 6.
Effects of N6NH2 and its analogs on biofilm in mice: (A) effects of N6NH2 and its analogs on retained bacteria in early biofilms; (B) effects of N6NH2 and its analogs on biofilm formation in mouse; (C) effects of N6NH2 and its analogs on skin injuries induced by A. veronii ACCC61732 biofilm. Catheters with A. veronii ACCC61732 biofilm were incubated in the skin of the backs of mice treated with N6NH2 and its analogs (5 μmol/kg). Skins were harvested and detected at 7 d post infection. (Top) Microscope magnification 100 times; (Bottom) microscope magnification 200 times. The analyses were measured by one-way ANOVA, with Duncan’s multiple comparisons test. A p-value of <0.05 was considered significant. (*) Indicates the significance between control and treatment groups. * p < 0.05; ** p < 0.01, *** p < 0.001, **** p < 0.0001.
2.9. N6NH2 and Its Analogs Protected Mice from Catheter-Associated Biofilm Infection with MDR A. veronii
2.9.1. Protection of Biofilm-Infected Mice
In a mouse model of catheter-associated biofilm infection, the mice were injected with 5 μmol/kg peptides or CIP at 24 h after catheter implantation with MDR A. veronii ACCC61732 biofilms. After treatment with 5 μmol/kg DN6NH2, N6PNH2, and V112N6NH2, the bacterial cells in the catheter were significantly decreased by 71.55%, 54.09%, and 55.29%, respectively, higher than those of N6NH2 (41.48%) and CIP (28.72%) (Figure 6B). Guo-N6NH2 was excluded due to its toxic effect on mice. This indicates that DN6NH2, N6PNH2, and V112N6NH2 exhibit more potent ability to inhibit the biofilm formation and alleviate abscesses in vivo than N6NH2 and CIP.
2.9.2. Protection of Tissues from A. veronii ACCC61732 Biofilm
To investigate whether peptides (excluding Guo-N6NH2) protect mice from A. veronii ACCC61732 biofilm-induced skin injury, the skin was examined at seven days after treatment with peptides. There was no abnormality in the mouse skin tissue in the blank control, indicating that the implantation of sterile catheters does not affect the mouse skin; however, in the negative control, infiltration of lymphocytes in the epidermis and obvious inflammation were observed in the local area, indicating that A. veronii ACCC61732 biofilms can severely damage the mouse skin tissues. In contrast, after treatment with 5 μmol/kg peptides (excluding Guo-N6NH2) or CIP, the swelling and injury of skin tissues were apparently alleviated and no obvious pathological changes were found in the mice at seven days, suggesting that N6NH2 and its analogs (excluding Guo-N6NH2) or CIP improve the A. veronii ACCC61732 biofilm-induced skin damage in mice (Figure 6C). The efficacy of DN6NH2 and V112N6NH2 is superior to N6PNH2 and CIP, but slightly inferior to N6NH2.
2.10. N6NH2 and Its Analogs Protected Mice from Bacterial Infection with MDR A. veronii
2.10.1. Protection of Mice
In the peritonitis model, the mice were injected with peptides (excluding Guo-N6NH2) (5 μmol/kg) or CIP (1 μmol/kg) at 0.5 h after infection with MDR A. veronii ACCC61732 (6 × 108 CFU/mL, 200 μL). The untreated mice began to die at 24 h after inoculation with A. veronii ACCC61732, and 50% died within 48 h. After treatment with 5 μmol/kg N6PNH2 and V112N6NH2, the survival rates of the mice were 50% and 66.67%, respectively. All mice survived when treated with 5 μmol/kg N6NH2 and DN6NH2. The survival rate of mice treated with 1 μmol/kg CIP was 83.33% (Figure 7A). The mice in the blank control survived throughout the experimental period, indicating that the therapeutic efficiency of N6NH2 and DN6NH2 in peritonitis mice is greater than that of N6PNH2, V112N6NH2, and CIP.
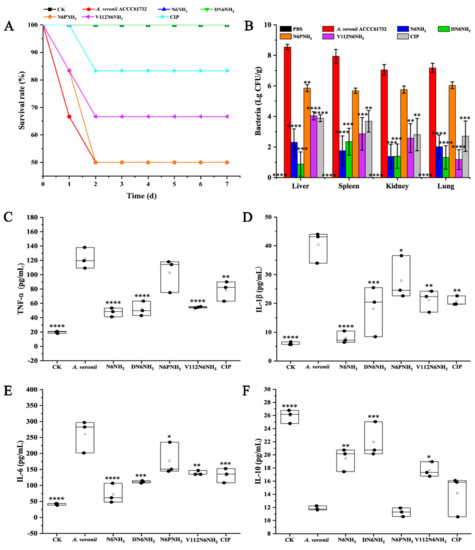
Figure 7.
Efficacy of N6NH2 and its analogs in a mouse peritonitis model infected with A. veronii ACCC61732. (A) Survival of mice. Mice were infected intraperitoneally with A. veroni ACCC61732 (6 × 108 CFU/mL, 200 μL) and treated with N6NH2, DN6NH2, N6PNH2, and V112N6NH2 (5 μmol/kg) or CIP (1 μmol/kg) after 0.5 h post infection. Survival was recorded for seven days; (B) the bacterial counts of mice in livers, spleens, kidneys, and lungs after treatment with peptides (5 μmol/kg) or CIP (1 μmol/kg). The untreated mice were used as the negative control. Data are expressed as mean ± standard error of mean (n = 6); (C–F) effects on sera cytokines. Mice were challenged with A. veronii ACCC61732 (6 × 108 CFU/mL, 200 μL) followed by injection with peptides (5 μmol/kg) or CIP (1 μmol/kg). Sera were collected and the levels of TNF-α (C), IL-1β (D), IL-6 (E), and IL-10 (F) were detected by using an ELISA kit 24 h after treatment. Each black circle represents data from a single mouse. The analyses were measured by one-way ANOVA, with Duncan’s multiple comparisons test. A p-value of <0.05 was considered significant. (*) Indicates the significance between control and treatment groups. * p < 0.05; ** p < 0.01, *** p < 0.001, **** p < 0.0001.
2.10.2. Inhibition of Bacterial Translocation
To test whether A. veronii ACCC61732 translocate from the peritoneal cavity to other deep organs, livers, spleens, kidneys, and lungs were harvested and homogenized at 24 h post-treatment with peptides (excluding Guo-N6NH2) or CIP; bacteria in tissue homogenates were counted. As shown in Figure 7B, bacterial counts (Lg CFU/g) in the livers, spleens, kidneys, and lungs of the untreated mice were 8.55, 7.94, 7.05, and 7.18, respectively. After treatment with 5 μmol/kg N6NH2, A. veronii ACCC61732 cells were significantly reduced in livers (72.94%), spleens (77.86%), kidneys (80.52%), and lungs (71.89%); 5 μmol/kg DN6NH2 reduced the bacterial burden in livers (89.65%), spleens (70.38%), kidneys (80.10%), and lungs (81.61%); after treatment with 5 μmol/kg N6PNH2, A. veronii ACCC61732 cells reduced in livers (31.54%), spleens (28.47%), kidneys (18.36%), and lungs (15.92%); and after treatment with 5 μmol/kg V112N6NH2, A. veronii ACCC61732 cells decreased in livers (52.71%), spleens (63.87%), kidneys (63.42%), and lungs (83.57%). Nevertheless, in the CIP-treated mice (1 μmol/kg), the bacterial counts were decreased in livers (54.55%), spleens (53.62%), kidneys (60.14%), and lung (62.27%), indicating that DN6NH2 (with the exception of spleens) has higher activity than N6NH2 and CIP. Both N6PNH2 and V112N6NH2 have intermediate therapeutic effects, with N6PNH2 being worse.
2.10.3. Regulation of Cytokines
To explore whether effects of peptides (excluding Guo-N6NH2) and CIP on the protection are associated with cytokines, the serum levels of pro-inflammatory (tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6)) and anti-inflammatory cytokine (interleukin-10 (IL-10)) were determined by the enzyme-linked immunosorbent assay (ELISA) kit. After 24 h treatment with N6NH2, the levels of TNF-α, IL-1β, IL-6, and IL-10 in mice were 40.65–55.67, 6.06–10.82, 47.22–108.59, and 17.02–20.87 pg/mL, respectively; after treatment with DN6NH2, the levels of TNF-α, IL-1β, IL-6, and IL-10 in mice were 40.02–66.20, 8.17–25.94, 104.48–115.43, and 15.94–25.46 pg/mL, respectively; after treatment with V112N6NH2, the levels of TNF-α, IL-1β, IL-6, and IL-10 in the mice were 52.05–59.01, 16.41–24.86, 133.08–150.00, and 16.48–19.60 pg/mL, respectively (Figure 7C–F). The levels of TNF-α, IL-1β, and IL-6 in the N6NH2- and DN6NH2-treated groups were remarkably lower than those of the untreated control (108.36–140.31, 29.63–44.87, and 200.48–304.48 pg/mL, respectively). The levels of IL-10 in N6NH2- and DN6NH2-treated mice were significantly greater than those of the untreated control (11.08–12.67 pg/mL) and CIP-treated groups (10.1–16.41 pg/mL). However, there was no discrepancy of IL-10 levels between the N6PNH2- or CIP-treated mice and untreated mice, indicating that N6PNH2 and CIP have poor ability to modulate immune factors. Similar to N6NH2 and CIP, DN6NH2 and V112N6NH2 can inhibit the production of proinflammatory cytokines and upregulate the anti-inflammatory cytokine level.
2.10.4. Alleviation of the Organ Injury
No pathological symptom was observed in the liver, spleen, kidney, and lung of the uninfected mice (Figure 8). In the untreated (24 h after infection) control, the liver tissue was severely damaged, including swollen and deformed liver cells, necrotic foci, necrotic debris, and eosinophilic bodies; many liver lobules were inflamed or degenerated. The veins in the red pulp area were dilated and congested, and the splenic sinus was dilated and congested. Scattered infiltration of inflammatory cells was found in the local renal interstitial tissue; renal tubular atrophy and degeneration could also be seen at local inflammatory sites. Lung tissue structure was blurred; most of the inflammatory cells around the blood vessels and the bronchi and bronchioles infiltrated, and fibroblasts proliferated heavily. In contrast, the livers and spleens were apparently less damaged in the DN6NH2- and N6NH2-treated mice, and no obvious pathological changes occurred in the lungs and kidneys. N6NH2 and DN6NH2 remarkably alleviated the injury of the liver and spleen. In the mice treated with V112N6NH2, livers, spleens, kidneys, and lungs were significantly less damaged, but it was slightly less effective than N6NH2 and DN6NH2. In the CIP-treated mice, there was a weak therapeutic effect on livers, spleens, kidneys, and lungs, but it was superior to V112N6NH2. N6PNH2 had the worst therapeutic effect with obvious inflammation in each tissue (Figure 8). The overall efficacy of peptides at five days was superior to that at 24 h (Figure S12). These results indicate that DN6NH2 and N6NH2 improve the tissue damage or injury in mice induced by A. veronii ACCC61732. In summary, the efficacy of peptides may be ranked from good to bad as follows: N6NH2 = DN6NH2 > V112N6NH2 > CIP > N6PNH2.
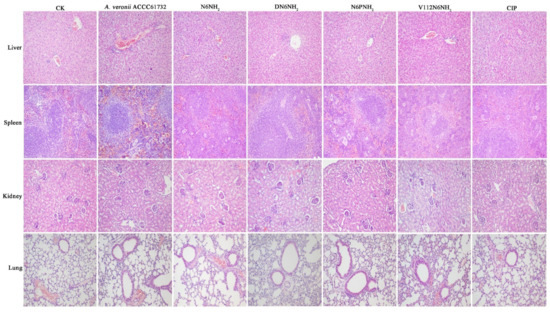
Figure 8.
Effects of N6NH2 and its analogs on organ injury in mice. Mice were infected intraperitoneally with A. veronii ACCC61732 (6 × 108 CFU/mL, 200 μL) and treated with N6NH2, DN6NH2, N6PNH2, and V112N6NH2 (5 μmol/kg) or CIP (1 μmol/kg). Livers, spleens, kidneys, and lungs were harvested from mice sacrificed at five days after infection. The magnification of images is 200 times.
3. Discussion
A. veronii is a widely distributed novel pathogen that can infect humans and animals. Due to overuse of broad-spectrum antibiotics in fish hatcheries, agriculture, and clinical settings, there has been an increase in antibiotic resistance of Aeromonas species [28], illustrating an emerging potential health concern [29]. Some marine AMPs such as N2, N6, A6, and G6 with low toxicity are highly effective against Gram-negative bacteria in vitro and in vivo and exhibit different antimicrobial mechanisms (including penetrating cell membrane and binding to genomic DNA), which open the door to the exploration of marine peptides [30,31]. In our previous study, the structure–activity relationship revealed that the analogs of N6 (including N2143, N2413, SN1, and SN3) without the structural features (β-sheet) lost the antimicrobial activity against Gram-negative bacteria (data not shown), indicating that the structural feature β-sheet is very important for the antimicrobial activity of marine peptides. Thus, in this study, to improve the stability and activity of peptide, four new modified analogs of marine peptide-N6NH2 in keeping the structural feature (β-sheet) were designed by changing charge and hydrophobicity based on natural or unnatural amino acids, namely Val, Pro, Orn, and D-ones, followed by the determination of antibacterial and anti-biofilm activity in vitro and in vivo for the first time.
Introduction of non-natural amino acids, especially Orn and D-amino acids, into the sequence of AMPs or substitution with some natural amino acids is an effective strategy to improve activity and prevent peptides from proteolytic degradation [19,23]. In our study, an addition of basic Orn at the N-terminus of N6NH2 did not improve the stability towards trypsin, serum, and SIF but slightly improved the activity against MDR A. veronii ACCC61732 compared to its parent N6NH2 (Figure 1B,C and Table 2), which is consistent with a previous report that found the activity of Api88 against E. coli and Klebsiella pneumoniae was enhanced by 1-fold and 127-fold, respectively, which may be attributed to the addition of Orn at its N-terminus [19]. The basic residue-Orn can increase positive net charges of peptides and most likely allow stronger electrostatic interactions with the negatively charged bacterial surface [19,32]. It has been reported that D-magainin, polybia-CP, and relevant derivatives may be of significant therapeutic potential due to their being highly resistant to proteolysis and nearly identical antibacterial activity [23,24]. Peptides containing D-amino acids might possess biological properties, which are similar to those of the respective natural L-enantiomer [24]. In our study, after replacement with D-residues, the antibacterial activity of DN6NH2 showed higher activity against some Gram-positive bacteria such as S. aureus and S. hyicus, indicating stronger broad-spectrum antibacterial activity than N6NH2 (Table 2). Compared with other analogs of N6NH2, DN6NH2 is more effective under physiological conditions (25% mouse serum, trypsin, and SIF) (Figure 1B,C, Figure S5, and Table S6). DN6NH2 remained intact even after 1 h of incubation in SIF, while all-L-enantiomer (N6NH2) was rapidly degraded (within 5 min) (Figure 1C). Similarly, the MIC value of DN6NH2 against A. veronii ACCC61732 was not changed (4 μg/mL) after 3 h incubation with trypsin, but N6NH2 lost its activity against A. veronii ACCC61732 (>128 μg/mL) (Table S6), which is consistent with magainin [24]. Moreover, PAE of DN6NH2 was greater than N6NH2 and other analogs (Table 3). DN6NH2 also enhanced the cell membrane penetrating ability and cytoplasmic membrane potential of A. veronii (Figure S6). More importantly, DN6NH2 and other analogs of N6NH2 induced lower resistance to A. veronii ACCC61732 (1–7-fold) than CIP (15-fold) and GEN (31-fold) after 30 passages (Figure 1F), which may be related to their rapid membrane permeabilizing mechanism at high concentrations of peptides (Figure 2 and Figure S6). For this reason, it is likely that more effective permeabilization of bacterial outer membrane by peptides than CIP may overcome intrinsic resistance pathways [33]. Furthermore, in the mouse peritonitis model, both N6NH2 and DN6NH2 were highly efficient in a mouse model of intraperitoneal infection, with 100% survival rates at doses of 5 μmol/kg, indicating its higher therapeutic effect than CIP and other analogs (Figure 7). The data further support our view that DN6NH2 can be a new potential antimicrobial candidate against A. veronii infections due to its very low toxicity and hemolysis (Figure 1D,E).
Biofilm is considered a biological barrier, formed by bacteria that continuously divide and multiply on biological materials through substances such as extracellular polysaccharide complexes; it is a major virulence factor contributing to the chronicity of infections and one of the most important reasons for the failure of current anti-infective therapies [34,35]. In recent years, there are increasing reports about A. veronii-infected aquatic animals and humans, but few studies focus on A. veronii biofilms [35,36]. In this study, N6NH2 and its analogs could effectively inhibit the early and mature biofilm formation of A. veronii ACCC61732 and killed persisters in biofilms in vitro and in a mouse model of catheter-associated biofilm infection (Figure 5 and Figure 6). Noticeably, DN6NH2, N6PNH2, and Guo-N6NH2 exhibited more potent ability to eradicate biofilms and kill persisters in a concentration-dependent manner than N6NH2 and CIP, indicating these analogs are potential therapeutic agents.
An interesting characteristic was the formation of OMVs at the outer membrane of A. veronii after treatment with V112N6NH2 (Figure S10), which was similar to the previous reports that sub-MIC Api peptides induced OMVs at the cell membrane of E. coli and P. aeruginosa [32]. Particularly, for membrane permeabilizing peptides, Gram-negative bacteria may form OMVs to remove membrane-interacting drug molecules, even if the membrane is not the final target of these peptides [32]. Additionally, OMVs may be related to the overexpressed or misfolded proteins in the periplasmic space, which can trigger similar bacterial cell vesicle formations, including the outer membrane and periplasmic components, and thus help bacteria remove toxic compounds from cell surfaces [32]. Overall, 4× MIC V112N6NH2 could more rapidly permeabilize the outer membrane of A. veronii within 1 min than other analogs of N6NH2 (Figure S6A), and it interacted with intracellular genomic DNA (Figure S7).
In conclusion, N6NH2 and its analogs exhibited potent antibacterial activity against A. veronii, with low toxicity and no or low resistance. These analogs displayed different antimicrobial mechanisms, including the permeabilization of cell membranes, interaction with genomic DNA, and formation of OMVs on the cell surfaces. The A. veronii biofilms and persisters were eradicated or killed by DN6NH2, N6PNH2, and V112N6NH2 in vitro and in mice, which is superior to N6NH2 and CIP. DN6NH2 displayed higher stability to trypsin and serum and higher efficacy at a dose of 5 μmol/kg (100% survival) in a mouse peritonitis model than other peptides and CIP (83.33%); it also inhibited bacterial translocation, downregulated cytokines, and alleviated multiple-organ injuries. This provides a new guideline to design novel antibacterial and antibiofilm marine peptides in clinical applications.
4. Materials and Methods
4.1. Reagents, Cell Lines, and Model Animals
Mueller–Hinton Broth (MHB), Mueller–Hinton Agar (MHA), Tryptic Soy Broth (TSB), and Tryptic Soy Agar (TSA) were obtained from AoBoX (Shanghai, China); PI was purchased from Sigma (Shanghai, China). The whole bacterial genome extraction kit was purchased from Tiangen Biotechnology Co., Ltd. (Beijing, China,); all antibiotics were purchased from China Veterinary Drug Supervision Institute (Beijing, China). RAW 264.7 macrophages were obtained from Peking Union Medical College (Beijing, China). Furthermore, six-week old specific-pathogen-free (SPF) female ICR mice (approximately 20 g/mouse) were purchased from the Beijing Vital River Laboratory Animal Technology Co. Ltd (Beijing, China). All tests for the evaluation of microbial and animal cells were carried out in Class II biological safety cabinets. Other chemical reagents were of analytical grade. All peptides were synthesized by Mimotopes (Wuxi, China) and their purity was greater than 90%.
4.2. Physiochemical Properties of N6NH2 and Its Analogs
The major structural parameters of peptides, including MW, pI, and net charge were calculated by ProtParam (https://web.expasy.org/protparam/). Hydrophobicity was predicated by the HeliQuest analysis website (http://heliquest.ipmc.cnrs.fr/cgi-bin/ComputParamsV2.py).
4.3. Antibacterial Activities and Time-Killing Curves of N6NH2 and Its Analogs
The MIC values of peptides and CIP were determined by the microtiter broth dilution method [37]. Briefly, the tested strains including Gram-negative bacteria, Gram-positive bacteria, and fungi were cultured to the logarithmic growth phase, diluted to 105 CFU/mL, and added into 96-well plates (90 μL/well). Two-fold serial dilutions of peptides or antibiotics dissolved in PBS were added to each well (10 μL/well). CIP and PBS were used as the positive and negative control, respectively. The plates were incubated at 37 °C for 16–18 h until visible turbidity was observed in the negative control. The lowest concentration that can completely inhibit bacterial growth is the MIC value of the peptides or antibiotic against the tested strains. All experiments were repeated three times.
Time-killing curves were performed as previously described [38]. Briefly, A. veronii ACCC61732 was cultivated to mid-log phase at 37 °C (250 rpm), diluted to 1 × 105 CFU/mL, and incubated with peptides or antibiotic (1×, 2×, and 4× MIC). The sample of 100 μL was taken from each flask at 0, 0.5, 1, 2, 4, 6, 8, 10, 20, and 24 h, respectively, and serially diluted for colony counting. PBS and CIP were used as the negative and positive control, respectively.
4.4. Synergism and PAE of N6NH2 and Its Analogs against A. veronii
The checkerboard microtiter assay was used to determine the combinations of peptides with different antibiotics (including quinolone, rifamycin, peptide antibiotics, aminoglycoside, tetracycline, and chloramphenicol). Briefly, logarithmic A. veronii ACCC61732 (105 CFU/mL) was added into the 96-well plates; 25 μL peptides (from 1/32× to 4× MIC) were added into the 96-well plates, followed by antibiotics. The fractional inhibitory concentration (FIC) was used to calculate the combination effects using the following formula: FIC index (FICI) = MIC(peptide in combination)/MIC(peptide alone) + MIC(antibiotic in combination)/MIC(antibiotic alone). FICI ≤ 0.5 means synergistic, 0.5 < FICI ≤ 1 means additive action, 1 < FICI ≤ 4 means indifference, and FICI > 4 means antagonism [39].
After treatment with N6NH2, N6NH2 analogs, and CIP (1×, 2×, and 4× MIC) for 2 h, A. veronii ACCC61732 cells (1 × 108 CFU/mL) were diluted 1000 times by medium, transferred to new flasks, and incubated at 37 °C and 250 rpm. The samples were taken from flasks for counting each hour until bacterial cultures became turbid. The untreated bacteria severed as controls. The PAE was calculated using the following equation: PAE = T − C, where T is the time (h) required for the CFU in the test culture to increase by 10-fold from the count immediately after the drug removal and C is the corresponding time (h) for the control [40]. The experiment was performed in triplicate.
4.5. Stability of N6NH2 and Its Analogs
4.5.1. Temperature, pH, Salt, and Enzyme Sensitivity
The thermal stability of peptides was determined after treatment for 1 h at different temperatures (4, 20, 40, 60, 80, and 100 °C). The relative antibacterial activity of peptides against A. veronii ACCC61732 was determined by the MIC assay [41].
To evaluate pH stability, peptides were dissolved in glycine-HCl buffer (pH 2.0), sodium acetate buffer (pH 4.0), sodium phosphate buffer (pH 6.0), tris-HCl buffer (pH 8.0), or glycine-NaOH buffer (pH 10.0) and treated for 3 h. The MIC values of peptides against A. veronii ACCC61732 were measured as described above.
Similarly, salt stability of peptides was determined after a 3 h-incubation in different NaCl solutions (50, 100, 200, 300, 400, and 500 mM). Additionally, N6NH2 and N6NH2 analogs were mixed with pepsin (3000 U/mg, pH 2.0), trypsin (250 U/mg, pH 8.0), and proteinase K (40 mAU/mg, pH 7.0) solutions at a ratio of 10:1 (w/w) to evaluate the protease stability [42]. The activity of peptides against A. veronii ACCC61732 was determined as described above. The untreated peptides were used as the positive control and buffers alone were used as the negative control. All assays were conducted in triplicate.
4.5.2. Stability in Gastric/Intestinal Fluid and Serum
The stability of N6NH2 and its analogs in SGF and SIF was carried out as previously described [43]. Briefly, a final concentration of 200 μg/mL peptide was incubated in SGF and SIF at 37 °C. At different time intervals, an aliquot of 20 μL mixture was taken and the activity of peptides was tested against A. veronii ACCC61732 by the inhibition zone assay. A. veronii ACCC61732 (200 μg/mL) in PBS was used as the positive control; SGF and SIF were used as negative controls.
Peptides were dissolved in mouse serum at 37 °C to detect their serum stability and samples were removed at different time to determine their residual activity by reverse-phase high-performance liquid chromatography (RP-HPLC) [30]. All assays were conducted in triplicate.
4.6. Hemolysis, Cytotoxicity, and Resistance of N6NH2 and Its Analogs
4.6.1. Hemolysis
To determine the hemolysis of peptides, eyeball blood was collected from 6-week-old SPF ICR mice. The blood was centrifuged at 4 °C for 10 min (1500 rpm), washed three times with 0.9% physiological saline, and diluted to 8% suspension. The peptides were dissolved in 0.9% physiological saline (1–256 μg/mL). Red blood cell suspensions and peptide solutions (100 μL) were mixed in 96-well plates, centrifuged at 4 °C for 5 min (1500 rpm), and incubated at 37 °C for 1 h. The supernatant was added into a 96-well plate and the UV absorbance was measured at 540 nm using a microplate reader. Physiological saline and 0.1% Triton X-100 were used as 0% and 100% hemolytic controls, respectively. The hemolytic ratio was calculated as follows: Hemolysis (%) = [(Abspeptide − Abssaline)/(Abs0.1%Triton X-100 − Abssaline)] × 100% [38].
4.6.2. Cytotoxicity
To determine the cytotoxicity of peptides, RAW 264.7 cells (2.5 × 104 cells/well) were seeded into 96-well plates and incubated for 24 h. A serial concentration of peptides (0.5–128 μg/mL) were added into each well and incubated for another 24 h; supernatants were removed from the wells and washed with PBS twice. The cells were dyed with 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) (5 mg/mL) for 4 h, incubated with dimethyl sulfoxide (DMSO), and measured at 570 nm. PBS was used as controls. The cell survival rate was calculated by the equation: Survival rate (%) = (Abspeptide/AbsPBS) × 100 [25].
4.6.3. Resistance
The development of bacterial resistance of peptides was performed by the MIC assays. The mid-log phase of A. veronii ACCC61732 (1 × 105 CFU/mL) (90 μL/well) was added to a 10 μL fresh medium, containing 64×, 32×, 16×, 8×, 4×, 2×, 1×, 0.5×, 0.25×, 0.125×, and 0.0625× MIC of peptides. GEN, CIP, and PMB were used as controls. After 16–18 h incubation at 37 °C, cells from the second highest concentration, which shows visible growth, were used to inoculate the subsequent culture. The MIC values were measured as described above. The serial passaging was repeated for 30 days [30].
4.7. Mechanism of N6NH2 and Its Analogs
4.7.1. Effects on the Cell Membrane and Membrane Potential
The outer membrane permeabilization abilities of peptides were determined using the fluorescent NPN assay. Mid-log phase A. veronii ACCC61732 was collected by centrifugation, washed twice, and then suspended in N-2-hydroxyethylpiperazine-n-2-ethane sulfonic acid (HEPES) buffer (pH 7.4) to an OD600nm = 0.4. Cell suspensions and NPN solutions (10 μM) were added into the 96-well black plates, followed by the addition of peptide solutions (1×, 2×, and 4× MIC). Fluorescence intensity was recorded until no further increase with a microplate reader (excitation/emission, 328/438 nm). The cells treated with PMB and CIP were used as positive controls; A. veronii ACCC61732 treated with PBS was used as the negative control; the untreated A. veronii ACCC61732 cells were used as the blank control [44].
To analyze effects of peptides on the inner membrane, A. veronii ACCC61732 cells were cultured to mid-log phase at 37 °C in MHB medium, washed thrice with PBS, and resuspended in the PBS buffer to 1 × 108 CFU/mL. The cells were then incubated with 1×, 2×, and 4× MIC peptides or CIP at 37 °C for 0.5 and 2 h, respectively. After fixation with PI, the samples were detected by a fluorescent inverted microscope and FACS Calibur Flow Cytometer (BD, San Jose, CA, USA), respectively. Cell Quest Pro software (BD, San Jose, CA, USA) was used for the data analysis [22,45].
The DISC3(5)assay [46] was employed to investigate cytoplasmic membrane depolarization. Cultured A. veronii ACCC61732 cells were washed in 5 mM HEPES buffer (pH 7.2) containing 20 mM glucose and resuspended in buffer (5 mM HEPES buffer, 20 mM glucose, and 100 mM KCl, pH 7.2) to an OD600 of 0.1. The uptake and self-quench of DISC3(5) dye into bacterial cells was monitored for 13 min. Peptide solutions were added and the fluorescence intensity (λexc = 620 nm, λem = 670 nm) was measured using a spectro fluorophotometer. PBS and 2% Triton X-100 were used as the blank and positive control, respectively.
4.7.2. Measure of ATP Release
A. veronii ACCC61732 cells (1 × 105 CFU/mL mid-log phase in MHB) were exposed to various concentrationds (1/2×, 2×, 4×, 8×, 16×, and 32× MIC) of N6NH2 (MIC 4 μg/mL), DN6NH2 (MIC 4 μg/mL), N6PNH2 (MIC 16 μg/mL), V112N6NH2 (MIC 16 μg/mL), Guo-N6NH2 (MIC 8 μg/mL), and CIP (MIC 0.125 μg/mL) for 60 min. ATP was measured using the BacTiter-GloTM Microbial Cell Viability Assay (Promega) following the manufacturer’s instructions. The assay was performed for three biological replicates at 37 °C, with luminescence recorded using a Tecan Infinite M1000 Pro plate reader. The fold reduction of ATP was calculated as: Fold reduction ATP = 1 − (Ltreat − Lmedia/Lcontrol − Lmedia), where Ltreat is the luminescence of treated cells, Lmedia is the luminescence of MHB without cells, and Lcontrol is the luminescence of untreated cells. Resultant graphs show mean (n = 3) and SEM for each data point, prepared in Prism 7 [46].
4.7.3. Effects on Bacterial Genomic DNA
The gel retardation assay was used to determine the ability of peptides to bind to bacterial genomic DNA. The genomic DNA was extracted from the A. veronii ACCC61732 strain by using a bacterial DNA Kit (TIANGEN Biotech Co., Ltd., Beijing, China). N6NH2 and its analogs were dissolved in DNA-binding buffer. The samples containing peptides and genomic DNA (0.8 µg) in 20 µL DNA binding buffer were incubated at 37 °C for 10 min. The peptide/DNA ratios ranged from 0.0 to 10.0 (w/w). After electrophoresis, genomic DNA was analyzed using a Geliance 200 imaging system (PerkinElmer, Waltham, MA, USA) [37,44].
CD spectra were performed to examine the secondary structure of genomic DNA from A. veronii ACCC61732 after incubation with peptides. N6NH2 and its analogs were incubated with genomic DNA at mass ratios of 2.5 and 10.0 for 10 min at room temperature. The mixtures were loaded into a cuvette of 1.0-mm path length and scanned from 190 to 330 nm at 25 °C using a Pistar π-180 CD spectrometer (Applied Photophysics Ltd., Surry, UK) [37].
4.7.4. Effects of N6NH2 and Its Analogs on Bacterial Morphology
MDR A. veronii ACCC61732 cells were cultured in MHB at 37 °C (250 rpm) until the mid-log phase of growth, harvested by centrifugation, and diluted with 0.01 M PBS (pH 7.4) to 0.5–5 × 108 CFU/mL. Bacterial cells were incubated with 4× MIC peptides or CIP at 37 °C for 2 h. For SEM, the samples were harvested (5000× g, 5 min) and fixed with 2.5% glutaraldehyde at 4 °C overnight. The cells were dehydrated for 10 min in a graded ethanol series (50%, 70%, 90%, and 100%) and transferred to a mixture of 100% ethanol, tertiary butanol, and absolute tertiary butanol (v:v = 1:1) for 15 min. Finally, the specimens were dehydrated in a critical point dryer with liquid CO2, coated with gold–palladium, and observed using a QUANTA200 (FEI, Philips, Amsterdam, Netherlands) [47].
For TEM, after treatment with peptides and a series of ethanol solutions (50%, 70%, 90%, and 100%) for 8 min, the samples were transferred to a mixture (v:v = 1:1) of 100% ethanol, acetone, and absolute acetone for 15 min. Subsequently, the specimens were transferred to 1:1 mixture of absolute acetone and resin for 30 min and then to absolute epoxy resin overnight. Finally, the specimens were stained with uranyl acetate and lead citrate and observed using a JEM1400 (JEDL, Tokyo, Japan) [40].
4.8. Effects of N6NH2 and Its Analogs on Biofilms and Persisters of MDR A. veronii
4.8.1. Early and Mature Biofilms
Mid-log phase A. veronii ACCC61732 cells (1 × 108 CFU/mL, 180 µL) were added into the 96-well plates. Peptides (0.5–16× MIC, 20 μL) were added into the plates and incubated for 24 h. Effects of peptides on biofilms formation was evaluated by the crystal violet staining as previously described [48,49]. The untreated bacteria were used as the negative control (A) and fresh TSB medium was used as the blank control (A0). The inhibition effect of peptides on early biofilms was determined by the following equation: Biofilms (%) = [(Apeptides − A0)/(A − A0)] × 100.
A. veronii ACCC61732 cells (1 × 108 CFU/mL) were cultured in TSB medium in the 96-well plates at 37 °C for 24 h. A series of concentrations of peptides (0.5–16× MIC) were added into the plates and cultured for another 24 h to form mature biofilms. The plates were dyed by crystal violet and effects of peptides or CIP on mature biofilms was determined as described above.
The logarithmic phase of A. veronii ACCC61732 (1 × 108 CFU/mL) was added into the orifice plate; each orifice plate was placed in a sterile guide piece to biofilm attached. Then, 16× MIC of peptides or CIP were added and incubated for 24 h; the guide piece was taken out, flushed with PBS buffer for three times to remove planktonic bacteria, and fixed by 2.5% glutaraldehyde overnight at 4 °C. The cells were then dehydrated for 10 min in a graded ethanol series (50%, 70%, 90%, and 100%) and transferred to a mixture (v:v = 1:1) of 100% ethanol, tertiary butanol, and absolute tertiary butanol for 15 min. Finally, the specimens were dehydrated in a critical point dryer with liquid CO2, coated with gold–palladium and observed using a QUANTA200 (FEI, Philips, Amsterdam, Netherlands) [50].
A. veronii ACCC61732 cells (1 × 108 CFU/mL) were seeded in a confocal dish and incubated for 24 h; 16× MIC peptides were added into the dish and continued to incubate for another 24 h. The untreated bacteria were used as a control. Planktonic bacteria were gently rinsed twice, and the biofilms were dyed with SYTO9 and PI (LIVE/DEAD Bac Light Bacterial Viability Kit) for 15 min. After washing with PBS, the biofilms were observed by Zeiss LSM880 confocal microscope (CLSM) (Zeiss, Oberkochen, Germany) [48,51].
4.8.2. Persisters in Biofilms
The mid-log phase A. veronii ACCC61732 cells (1 × 108 CFU/mL) were inoculated into in the 96-well plates and cultured for 24 h at 37 °C. The plates were washed twice with PBS. Subsequently, the final concentration of 4–16× MIC peptides or CIP were added into the plates and incubated for 2 h at 37 °C. PBS was used as the negative control. The plates were treated with ultrasound for 5 min and the viable bacteria were counted on the TSA plates [48,51].
4.9. Efficacy of N6NH2 and Its Analogs in a Mouse Model of Catheter-Associated Biofilm Infection
4.9.1. Effects on Catheter-Associated Biofilms
The mouse experiment was performed according to the Animal Care and Use Committee of the Feed Research Institute of Chinese Academy of Agricultural Sciences (CAAS) and approved by the Laboratory Animal Ethical Committee and its Inspection of the Feed Research Institute of CAAS (AEC-CAAS-20090609).
A. veronii ACCC61732 biofilms were developed on a mouse intravenous catheter 0.6 × 0.3 mm (outer diameter × inner diameter). Briefly, the catheter was cut into 1 cm segments; each piece was sterilized with 70% ethanol and then dried by air. Bacterial biofilms were developed on the catheter by placing individual segments into tubes containing 1.0 mL of a cell suspension (1 × 108 CFU/mL) in TSB. After incubation for 24 h at 37 °C, colonized catheters were recovered aseptically and rinsed with PBS to remove unbound bacteria.
ICR female mice (20 ± 5 g) were anesthetized with isoflurane; the flanks were shaved and the skin was cleansed with alcohol. An 8–10-mm skin incision was made and dissected to create a subcutaneous tunnel with the 1cm segment of carrying A. veronii ACCC61732 biofilms catheter implanted at a distance of at least 2 cm from the incision. The incision was then covered with intact skin and closed with surgical suture line and the skin was disinfected. After 24 h of infection as an in vivo biological model, six mice in each group were injected with 5 μmol/kg peptides or CIP on the mouse back. The untreated mice were used as the negative control and the mice with sterile catheter were used as the blank control. The mice were anesthetized and sacrificed after seven days. The catheters in the back of the mice were collected and thoroughly shaken in sterile PBS. Effects of peptides or CIP on A. veronii ACCC61732 in mice was evaluated by the colony counting method [52].
4.9.2. Effects on Skin Ulcer in the Mouse Back
Catheters with A. veronii ACCC61732 biofilm were implanted in the skin of the mouse backs and treated with 5 μmol/kg peptides or CIP after infection 24 h. The mouse back skin tissues were taken at seven days, washed with PBS, and placed in 4% paraformaldehyde for 24 h at 4 °C. After washing with PBS, the tissues were dehydrated by different concentrations of ethanol (75%, 85%, 90%, and 95%) and then were immersed in xylene for paraffin embedding. After sectioning, the samples were stained with hematoxylin and observed under the OLYMPUS BX43 microscope [53]. The mice challenged with A. veronii ACCC61732 biofilm and sterile catheter served as the negative and blank control.
4.10. Efficacy of N6NH2 and Its Analogs in a Mouse Peritonitis Model
4.10.1. Survival of Mice
To establish a mouse peritonitis model, the six-week-old female ICR mice (approximately 20 g/mouse) were intraperitoneally injected with the MDR A. veronii ACCC61732 (6 × 108 CFU/mL, 0.2 mL) strain. Therapeutic groups with six mice were treated with peptides (5 μmol/kg of body weight, 0.2 mL) and CIP (1 μmol/kg of body weight, 0.2 mL) at 0.5 h post-infection by intraperitoneal injection [31]. The mice injected with only bacteria or saline served as the negative and blank control. The mouse survival was recorded daily for seven days.
4.10.2. Effects on Bacterial Translocation and Cytokines
The mice (15 mice/group) were challenged with A. veronii ACCC61732 (6 × 108 CFU/mL, 0.2 mL) by intraperitoneal injection and treated with a single dose of peptides (5 μmol/kg) or CIP (1 μmol/kg). Sera and organs (livers, kidneys, spleens, and lungs) were collected from the mice at 24 h post-treatment. The organs were homogenized in sterile PBS to evaluate bacterial translocation by colony counting. The levels of cytokines (TNF-α, IL-1β, IL-6, and IL-10) in sera were detected using the ELISA kits. The uninfected and untreated mice served as the blank control; the infected mice treated with CIP and PBS were used as the positive and negative controls, respectively.
4.10.3. Effects on the Injury of Multiple Organs
The mice were intraperitoneally injected with peptides and CIP at 0.5 h post-infection of A. veronii ACCC61732 as described above. Livers, kidneys, spleens, and lungs were removed from mice at 24 h and five days post treatment to evaluate the organ injury. After washing with PBS, the organs were fixed in 4% paraform-aldehyde for 24 h at 4 °C, dehydrated by a graded series of ethanol (75‒95%), and infiltrated with xylene. The organs were then embedded in paraffin wax, sectioned, and stained with hematoxylin and eosin. Finally, the tissue samples were fixed and observed by the light microscope. The CIP-treated mice served as the positive control and the PBS group served as the negative control.
4.11. Statistical Analysis
All data are presented as means ± standard error of mean. Statistical analyses between treatments or groups were determined using one-way analysis of variance (ANOVA) models in SAS 9.2 (SAS Institute Inc., Cary, NC, USA), followed by Dunnett’s multiple comparisons test. A p-value of <0.05 was considered statistically significant.
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/21/24/9637/s1.
Author Contributions
Conceptualization, T.L. and X.W.; methodology, T.L., X.W. and Z.W.; investigation, H.H. and N.Y.; writing—original draft preparation, T.L. and X.W.; writing—review and editing, T.L., X.W., Z.W. and Y.H.; visualization, Y.H. and N.Y.; supervision, R.M., D.T. and J.W.; and project administration, X.W. and J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 31772640, 31572444, and 31572445), and its Key Project of Alternatives to Antibiotic for Feed Usages from Chinese Academy of Agricultural Sciences, China (Grant No. CAAS-ZDXT2018008).
Acknowledgments
We acknowledge Chunli Li and Tong Zhao from the Core Facility at the Institute of Microbiology at the Chinese Academy of Sciences (CAS) for their technical support with SEM, TEM, and flow cytometer analysis and Dan Zhang from the Core Facility at the Center of Biomedical Analysis at Tsinghua University for her CLSM analysis.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CIP | ciprofloxacin |
| AMP | antimicrobial peptide(s) |
| Orn | ornithine |
| MDR | multidrug resistant |
| MIC | minimum inhibitory concentration |
| MW | molecular weight |
| MS | mass spectroscopy |
| GRAVY | grand average of hydropathicity |
| AI | aliphatic index |
| BI | boman index |
| pI | isoelectric point |
| FICI | fractional inhibitory concentration index |
| PAE | postantibiotic effect |
| MTT | methylthiazolyldiphenyl-tetrazolium |
| SGF | simulated gastric fluid |
| SIF | simulated intestinal fluid |
| GEN | gentamicin |
| NPN | n-phenyl-1-naphthylamine |
| PI | propidium iodide |
| DISC3(5)) | 3,3′-dipropylthiadicarbocyanine iodide |
| ATP | adenosine triphosphate |
| CD | circular dichroism |
| SEM | scanning electron microscope |
| OMVs | outer membrane vesicles |
| PCR | polymerase chain reaction |
| TEM | transmission electron microscope |
| CLSM | confocal laser scanning microscope |
| TNF | tumor necrosis factor |
| IL | interleukin |
| ELISA | enzyme-linked immunosorbent assay |
| HEPES | n-2-hydroxyethylpiperazine-n-2-ethane sulfonic acid |
References
- Hoai, T.D.; Trang, T.T.; Van Tuyen, N.; Giang, N.T.H.; Van Van, K. Aeromonas veronii caused disease and mortality in channel catfish in Vietnam. Aquaculture 2019, 513, 734425. [Google Scholar] [CrossRef]
- Huang, H.; Zhou, P.; Chen, P.; Xia, L.; Hu, S.; Yi, G.; Lu, J.; Yang, S.; Xie, J.; Peng, J.; et al. Alteration of the gut microbiome and immune factors of grass carp infected with Aeromonas veronii and screening of an antagonistic bacterial strain (Streptomyces flavotricini). Microb. Pathog. 2020, 143, 104092. [Google Scholar] [CrossRef]
- Li, T.; Raza, S.H.A.; Yang, B.; Sun, Y.; Wang, G.-Q.; Sun, W.; Qian, A.; Wang, C.; Shan, X.; Shan, X. Aeromonas veronii Infection in Commercial Freshwater Fish: A Potential Threat to Public Health. Animal 2020, 10, 608. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Serrano, C.J.; Santos, J.A.; Garcia-Lopez, M.L.; Otero, A. Virulence markers in Aeromonas hydrophila and Aeromonas veronii biovar sobria isolates from freshwater fish and from a diarrhoea case. J. Appl. Microbiol. 2002, 93, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Pearson, M.D.; Hirono, I.; Aoki, T.; Miranda, R.; Inglis, V. Virulence properties of motile aeromonads isolated from farmed frogs Rana tigerina and R. rugulosa. Dis. Aquat. Org. 2000, 40, 185–193. [Google Scholar] [CrossRef]
- Bhowmick, U.D.; Bhattacharjee, S. Bacteriological, Clinical and Virulence Aspects of Aeromonas-associated Diseases in Humans. Pol. J. Microbiol. 2018, 67, 137–149. [Google Scholar] [CrossRef]
- Pund, R.P.; Theegarten, D. The importance of aeromonads as a human pathogen. Bundesgesundheitsblatt Gesundh. Gesundh. 2008, 51, 569–576. [Google Scholar] [CrossRef]
- Hadi, N.; Mahmoodi, Z.; Emami, A.; Malekzadegan, Y.; Valadbeygi, T. Isolation and Molecular Identification of Aeromonas Wound Infection in Iranian Burn Patients. Infect. Disord. Drug Targets 2019, 19, 269–273. [Google Scholar] [CrossRef]
- Wu, C.-J.; Ko, W.-C.; Lee, N.-Y.; Su, S.-L.; Li, C.-W.; Li, M.-C.; Chen, Y.-W.; Su, Y.-C.; Shu, C.-Y.; Lin, Y.-T.; et al. Aeromonas Isolates from Fish and Patients in Tainan City, Taiwan: Genotypic and Phenotypic Characteristics. Appl. Environ. Microbiol. 2019, 85, e01360-19. [Google Scholar] [CrossRef]
- Lazado, C.C.; Zilberg, D. Pathogenic characteristics of Aeromonas veronii isolated from the liver of a diseased guppy (Poecilia reticulata). Lett. Appl. Microbiol. 2018, 67, 476–483. [Google Scholar] [CrossRef]
- Olsen, I. Biofilm-specific antibiotic tolerance and resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Skwor, T.A.; Shinko, J.; Augustyniak, A.; Gee, C.; Andraso, G. Aeromonas hydrophila and Aeromonas veronii Predominate among Potentially Pathogenic Ciprofloxacin- and Tetracycline-Resistant Aeromonas Isolates from Lake Erie. Appl. Environ. Microbiol. 2014, 80, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Vila, J.R.J.; Gallardo, F.; Vargas, M.; Soler, L.; Figueras, M.J.; Gascon, J. Aeromonas spp. and traveler’s diarrhea: Clinical features and antimicrobial resistance. Emerg. Infect. Dis. 2003, 9, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Haney, E.F.; Hancock, R.E. Peptide design for antimicrobial and immunomodulatory applications. Biopolymers 2013, 100, 572–583. [Google Scholar] [CrossRef]
- Thapa, R.K.; Diep, D.B.; Tønnesen, H.H. Topical antimicrobial peptide formulations for wound healing: Current developments and future prospects. Acta Biomater. 2020, 103, 52–67. [Google Scholar] [CrossRef]
- Jaśkiewicz, M.; Neubauer, D.; Kazor, K.; Bartoszewska, S.; Kamysz, W. Antimicrobial Activity of Selected Antimicrobial Peptides against Planktonic Culture and Biofilm of Acinetobacter baumannii. Probiotics Antimicrob. Proteins 2019, 11, 317–324. [Google Scholar] [CrossRef]
- Sieprawska-Lupa, M.; Mydel, P.; Krawczyk, K.; Wójcik, K.; Puklo, M.; Lupa, B.; Suder, P.; Silberring, J.; Reed, M.; Pohl, J.; et al. Degradation of Human Antimicrobial Peptide LL-37 by Staphylococcus aureus-Derived Proteinases. Antimicrob. Agents Chemother. 2004, 48, 4673–4679. [Google Scholar] [CrossRef]
- Kim, H.; Jang, J.H.; Kim, S.C.; Cho, J.H. De novo generation of short antimicrobial peptides with enhanced stability and cell specificity. J. Antimicrob. Chemother. 2014, 69, 121–132. [Google Scholar] [CrossRef]
- Jia, F.; Wang, J.; Peng, J.; Zhao, P.; Kong, Z.; Wang, K.; Yan, W.; Wang, R. D-amino acid substitution enhances the stability of antimicrobial peptide polybia-CP. Acta Biochim. Biophys. Sin. 2017, 49, 916–925. [Google Scholar] [CrossRef]
- Bessalle, R.; Kapitkovsky, A.; Gorea, A.; Shalit, I.; Fridkin, M. All-D-magainin: Chirality, antimicrobial activity and proteolytic resistance. FEBS Lett. 1990, 274, 151–155. [Google Scholar] [CrossRef]
- Czihal, P.; Knappe, D.; Fritsche, S.; Zahn, M.; Berthold, N.; Piantavigna, S.; Müller, U.; Van Dorpe, S.; Herth, N.; Binas, A.; et al. Api88 Is a Novel Antibacterial Designer Peptide To Treat Systemic Infections with Multidrug-Resistant Gram-Negative Pathogens. ACS Chem. Biol. 2012, 7, 1281–1291. [Google Scholar] [CrossRef]
- Bluhm, M.E.; Knappe, D.; Hoffmann, R. Structure-activity relationship study using peptide arrays to optimize Api137 for an increased antimicrobial activity against Pseudomonas aeruginosa. Eur. J. Med. Chem. 2015, 103, 574–582. [Google Scholar] [CrossRef]
- López-Pérez, P.M.; Grimsey, E.; Bourne, L.; Mikut, R.; Hilpert, K. Screening and Optimizing Antimicrobial Peptides by Using SPOT-Synthesis. Front. Chem. 2017, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Knappe, D.; Wende, E.; Ostorhazi, E.; Hoffmann, R. In vivo Efficacy and Pharmacokinetics of Optimized Apidaecin Analogs. Front. Chem. 2017, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Teng, D.; Mao, R.; Wang, X.; Hao, Y.; Wang, X.; Wang, J. Improved Antibacterial Activity of the Marine Peptide N6 against Intracellular Salmonella Typhimurium by Conjugating with the Cell-Penetrating Peptide Tat11 via a Cleavable Linker. J. Med. Chem. 2018, 61, 7991–8000. [Google Scholar] [CrossRef]
- Roier, S.; Zingl, F.G.; Cakar, F.; Durakovic, S.; Kohl, P.; Eichmann, T.O.; Klug, L.; Gadermaier, B.; Weinzerl, K.; Prassl, R.; et al. A novel mechanism for the biogenesis of outer membrane vesicles in Gram-negative bacteria. Nat. Commun. 2016, 7, 10515. [Google Scholar] [CrossRef]
- Avila-Calderon, E.D.; Otero-Olarra, J.E.; Flores-Romo, L.; Peralta, H.; Aguilera-Arreola, M.G.; Morales-Garcia, M.R.; Calderon-Amador, J.; Medina-Chavez, O.; Donis-Maturano, L.; Ruiz-Palma, M.D.S.; et al. The outer membrane vesicles of Aeromonas hydrophila ATCC((R)) 7966(TM): A proteomic analysis and effect on host cells. Front. Microbiol. 2018, 9, 2765. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.C.; Yu, K.W.; Liu, C.Y.; Huang, C.T.; Leu, H.S.; Chuang, Y.C. Increasing antibiotic resistance in clinical isolates of aeromonas strains in Taiwan. Antimicrob. Agents Chemother. 1996, 40, 1260–1262. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; De Silva, B.; Dahanayake, P.; Heo, G. Characterization of virulence properties and multi-drug resistance profiles in motile Aeromonas spp. isolated from zebrafish (Danio rerio). Lett. Appl. Microbiol. 2018, 67, 598–605. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Mao, R.; Hao, Y.; Yang, N.; Wang, X.; Li, Z.; Wang, X.; Wang, J.; Teng, D. Development of chimeric peptides to facilitate the neutralisation of lipopolysaccharides during bactericidal targeting of multidrug-resistant Escherichia coli. Commun. Biol. 2020, 3, 1–15. [Google Scholar] [CrossRef]
- Yang, N.; Liu, X.; Teng, D.; Li, Z.; Wang, X.; Mao, R.; Wang, X.; Hao, Y.; Wang, J. Antibacterial and detoxifying activity of NZ17074 analogues with multi-layers of selective antimicrobial actions against Escherichia coli and Salmonella enteritidis. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Bluhm, M.E.C.; Schneider, V.A.F.; Schäfer, I.; Piantavigna, S.; Goldbach, T.; Knappe, D.; Seibel, P.; Martin, L.L.; Veldhuizen, E.J.A.; Hoffmann, R. N-Terminal Ile-Orn- and Trp-Orn-Motif Repeats Enhance Membrane Interaction and Increase the Antimicrobial Activity of Apidaecins against Pseudomonas aeruginosa. Front. Cell Dev. Biol. 2016, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- Wimley, W.C. Describing the Mechanism of Antimicrobial Peptide Action with the Interfacial Activity Model. ACS Chem. Biol. 2010, 5, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-F.; Kang, Y.-H.; Zhang, D.-X.; Chen, L.; Bi, J.-F.; Zhang, H.-P.; Zhang, L.; Qian, A.; Shan, X. Immunogenicity of extracellular products from an inactivated vaccine against Aeromonas veronii TH0426 in koi, Cyprinus carpio. Fish Shellfish. Immunol. 2018, 81, 176–181. [Google Scholar] [CrossRef]
- Roilides, E.; Simitsopoulou, M.; Katragkou, A.; Walsh, T.J. How Biofilms Evade Host Defenses. Microbiol. Spectr. 2015, 3, 287–300. [Google Scholar] [CrossRef]
- Sanchez, C.J., Jr.; Mende, K.; Beckius, M.L.; Akers, K.S.; Romano, D.R.; Wenke, J.C.; Murray, C.K. Biofilm formation by clinical isolates and the implications in chronic infections. BMC Infect. Dis. 2013, 13, 47. [Google Scholar] [CrossRef]
- Zhao, F.; Yang, N.; Wang, X.; Mao, R.; Hao, Y.; Li, Z.; Wang, X.; Teng, D.; Fan, H.; Wang, J. In vitro/vivo Mechanism of Action of MP1102 with Low/Nonresistance against Streptococcus suis Type 2 Strain CVCC 3928. Front. Cell. Infect. Microbiol. 2019, 9, 48. [Google Scholar] [CrossRef]
- Liu, H.; Yang, N.; Mao, R.; Teng, D.; Hao, Y.; Wang, X.; Wang, J. A new high-yielding antimicrobial peptide NZX and its antibacterial activity against Staphylococcus hyicus in vitro/vivo. Appl. Microbiol. Biotechnol. 2020, 104, 1555–1568. [Google Scholar] [CrossRef]
- White, R.L.B.D.S.; Manduru, M.; Bosso, J.A. Comparison of three different in vitro methods of detecting synergy: Time-kill, checkerboard, and E test. Antimicrob. Agents Chemother. 1996, 40, 1914–1918. [Google Scholar] [CrossRef]
- Giguère, S.; Lee, E.A.; Guldbech, K.M.; Berghaus, L.J. In vitro synergy, pharmacodynamics, and postantibiotic effect of 11 antimicrobial agents against Rhodococcus equi. Vet. Microbiol. 2012, 160, 207–213. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, W.; Dong, M.; Hang, B.; Sun, Y.; Wang, L.; Wang, Y.; Hu, J.; Zhang, W. HJH-1, a Broad-Spectrum Antimicrobial Activity and Low Cytotoxicity Antimicrobial Peptide. Molecules 2018, 23, 2026. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Lai, Z.; Zhu, Y.; Shao, C.; Akhtar, M.U.; Li, W.; Zheng, X.; Shan, A. Multiple Strategy Optimization of Specifically Targeted Antimicrobial Peptide Based on Structure–Activity Relationships to Enhance Bactericidal Efficiency. ACS Biomater. Sci. Eng. 2019, 6, 398–414. [Google Scholar] [CrossRef]
- Li, B.; Yang, N.; Shan, Y.; Wang, X.; Hao, Y.; Mao, R.; Teng, D.; Fan, H.; Wang, J. Therapeutic potential of a designed CSalphabeta peptide ID13 in Staphylococcus aureus-induced endometritis of mice. Appl. Microbiol. Biotechnol. 2020, 104, 6693–6705. [Google Scholar] [CrossRef] [PubMed]
- Teng, D.; Wang, X.; Xi, D.; Mao, R.; Zhang, Y.; Guan, Q.; Zhang, J.; Wang, J. A dual mechanism involved in membrane and nucleic acid disruption of AvBD103b, a new avian defensin from the king penguin, against Salmonella enteritidis CVCC3377. Appl. Microbiol. Biotechnol. 2014, 98, 8313–8325. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ji, Y.; Zhu, Y.; Wu, X.; Mei, L.; Zhang, H.; Deng, J.; Wang, S. Antibacterial effect of chitosan and its derivative on Enterococcus faecalis associated with endodontic infection. Exp. Ther. Med. 2020, 19, 3805–3813. [Google Scholar] [CrossRef] [PubMed]
- Elliott, A.G.; Huang, J.X.; Neve, S.; Zuegg, J.; Edwards, I.A.; Cain, A.K.; Boinett, C.J.; Barquist, L.; Lundberg, C.V.; Steen, J.; et al. An amphipathic peptide with antibiotic activity against multidrug-resistant Gram-negative bacteria. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef]
- Hao, Y.; Yang, N.; Wang, X.; Teng, D.; Mao, R.; Wang, X.; Li, Z.; Wang, J. Killing of Staphylococcus aureus and Salmonella enteritidis and neutralization of lipopolysaccharide by 17-residue bovine lactoferricins: Improved activity of Trp/Ala-containing molecules. Sci. Rep. 2017, 7, srep44278. [Google Scholar] [CrossRef]
- Yang, N.; Teng, D.; Mao, R.; Hao, Y.; Wang, X.; Wang, Z.; Wang, X.; Wang, J. A recombinant fungal defensin-like peptide-P2 combats multidrug-resistant Staphylococcus aureus and biofilms. Appl. Microbiol. Biotechnol. 2019, 103, 5193–5213. [Google Scholar] [CrossRef]
- Mohamed, M.F.; Brezden, A.; Mohammad, H.; Chmielewski, J.; Seleem, M.N. A short D-enantiomeric antimicrobial peptide with potent immunomodulatory and antibiofilm activity against multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Sci. Rep. 2017, 7, 6953. [Google Scholar] [CrossRef]
- Asahi, Y.; Miura, J.; Tsuda, T.; Kuwabata, S.; Tsunashima, K.; Noiri, Y.; Sakata, T.; Ebisu, S.; Hayashi, M. Simple observation of Streptococcus mutans biofilm by scanning electron microscopy using ionic liquids. AMB Express 2015, 5. [Google Scholar] [CrossRef]
- Shan, Y.; Yang, N.; Teng, D.; Wang, X.; Mao, R.; Hao, Y.; Ma, X.; Fan, H.; Wang, J. Recombinant of the Staphylococcal Bacteriophage Lysin CHAPk and Its Elimination against Streptococcus agalactiae Biofilms. Microorganisms 2020, 8, 216. [Google Scholar] [CrossRef] [PubMed]
- Kadurugamuwa, J.L.; Sin, L.; Albert, E.; Yu, J.; Francis, K.; DeBoer, M.; Rubin, M.; Bellinger-Kawahara, C.; Parr, T.R., Jr.; Contag, P.R. Direct continuous method for monitoring biofilm infection in a mouse model. Infect. Immun. 2003, 71, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Mao, R.; Teng, D.; Hao, Y.; Chen, H.; Wang, X.; Wang, X.; Yang, N.; Wang, J. Antibacterial and immunomodulatory activities of insect defensins-DLP2 and DLP4 against multidrug-resistant Staphylococcus aureus. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).