Prophylactic Therapy with Human Amniotic Fluid Stem Cells Improves Long-Term Cognitive Impairment in Rat Neonatal Sepsis Survivors
Abstract
1. Introduction
2. Results
2.1. Phenotypic Characterization of hAFSCs
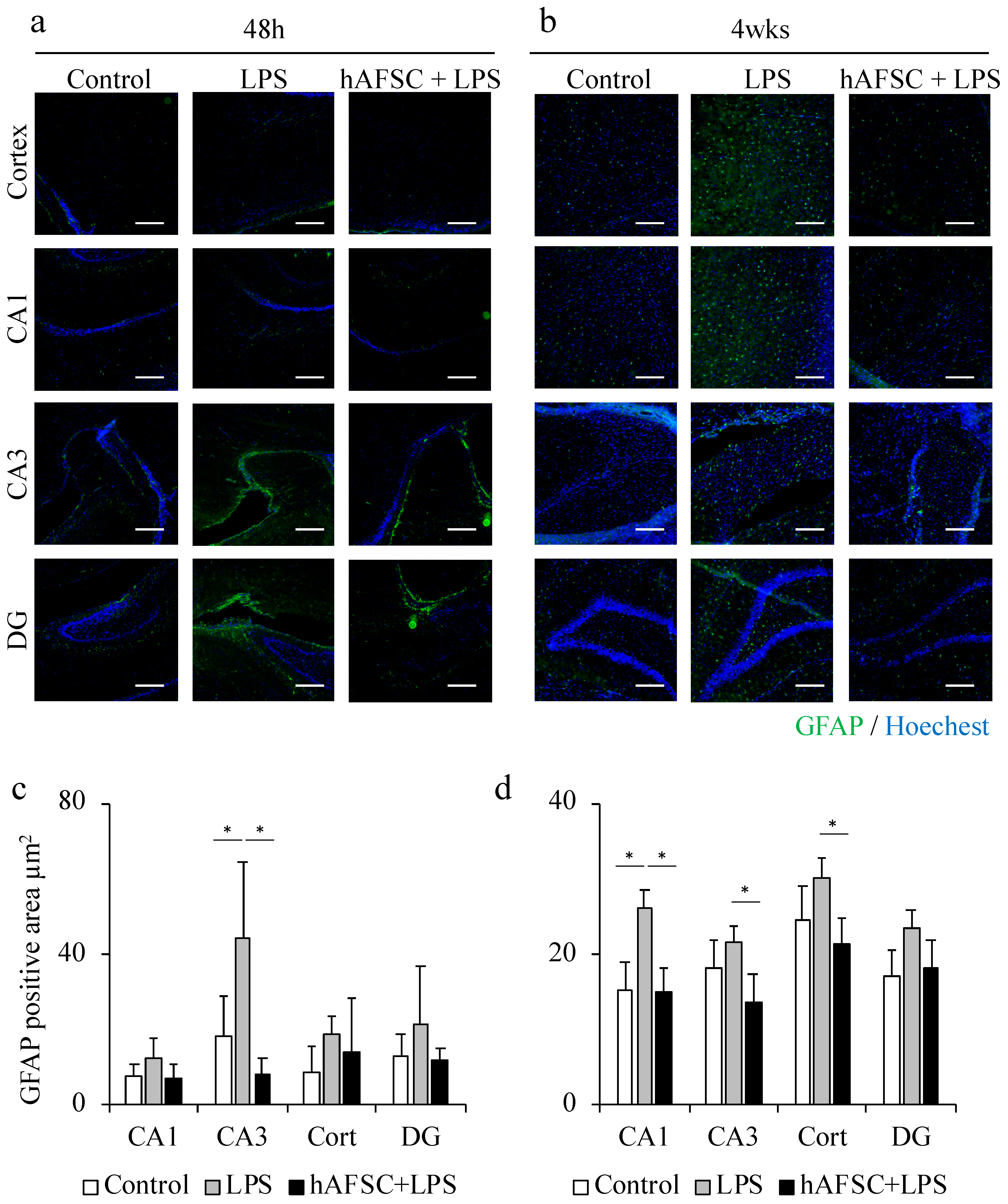
2.2. hAFSC Administration Reduced the Glial Fibrillary Acidic Protein (GFAP)-Positive Area after LPS Administration
2.3. hAFSC Administration Reduced Iba-1-Positive Area after LPS Administration
2.4. hAFSC Administration Did Not Affect Dysmyelination
2.5. hAFSC Administration Improved Spatial Awareness and Memory-Based Behavior
3. Discussion
4. Materials and Methods
4.1. Isolation, Culture, and Immunophenotypic Characterization of CD117+ Amniotic Fluid Cells
4.2. Animals
4.3. Immunohistochemical Analysis
4.4. RNA Extraction and Real-Time RT-PCR Analysis for MBP
4.5. Barnes Maze Testing
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DG | Dentate gyrus |
| GFAP | Glial fibrillary acidic protein |
| MSCs | Mesenchymal stem cells |
| PVL | Periventricular leukomalacia |
| hAFSCs | Human amniotic fluid stem cells |
| Iba-1 | Ionized calcium-binding adapter molecule 1 |
References
- Stoll, B.J.; Hansen, N.; Fanaroff, A.A.; Wright, L.L.; Carlo, W.A.; Ehrenkranz, R.A.; Lemons, J.A.; Donovan, E.F.; Stark, A.R.; Tyson, J.E.; et al. Late-onset sepsis in very low birth weight neonates: The experience of the NICHD Neonatal Research Network. Pediatrics 2002, 110 Pt 1, 285–291. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.I.; Sánchez, P.J.; Faix, R.G.; Poindexter, B.B.; Van Meurs, K.P.; Bizzarro, M.J.; Goldberg, R.N.; Frantz, I.D., III; Hale, E.C.; et al. Early onset neonatal sepsis: The burden of group B Streptococcal and E. coli disease continues. Pediatrics 2011, 127, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Wójkowska-Mach, J.; Chmielarczyk, A.; Strus, M.; Lauterbach, R.; Heczko, P. Neonate Bloodstream Infections in Organization for Economic Cooperation and Development Countries: An Update on Epidemiology and Prevention. J. Clin. Med. 2019, 8, 1750. [Google Scholar] [CrossRef] [PubMed]
- Haller, S.; Deindl, P.; Cassini, A.; Suetens, C.; Zingg, W.; Abu Sin, M.; Velasco, E.; Weiss, B.; Ducomble, T.; Sixtensson, M.; et al. Neurological sequelae of healthcare-associated sepsis in very-low-birthweight infants: Umbrella review and evidence-based outcome tree. Euro Surveill. 2016, 21, 30143. [Google Scholar] [CrossRef] [PubMed]
- Silveira, R.C.; Procianoy, R.S.; Dill, J.C.; da Costa, C.S. Periventricular leukomalacia in very low birth weight preterm neonates with high risk for neonatal sepsis. J. Pediatr. 2008, 84, 211–216. [Google Scholar] [CrossRef][Green Version]
- Hagberg, H.; Mallard, C. Effect of inflammation on central nervous system development and vulnerability. Curr. Opin. Neurol. 2005, 18, 117–123. [Google Scholar] [CrossRef]
- Comim, C.M.; Bussmann, R.M.; Simão, S.R.; Ventura, L.; Freiberger, V.; Patrício, J.J.; Palmas, D.; Mendonça, B.P.; Cassol, O.J., Jr.; Quevedo, J. Experimental Neonatal Sepsis Causes Long-Term Cognitive Impairment. Mol. Neurobiol. 2016, 53, 5928–5934. [Google Scholar] [CrossRef]
- Barichello, T.; Sayana, P.; Giridharan, V.V.; Arumanayagam, A.S.; Narendran, B.; Della Giustina, A.; Petronilho, F.; Quevedo, J.; Dal-Pizzol, F. Long-Term Cognitive Outcomes After Sepsis: A Translational Systematic Review. Mol. Neurobiol. 2019, 56, 186–251. [Google Scholar] [CrossRef]
- In’t Anker, P.S.; Scherjon, S.A.; Kleijburg-van der Keur, C.; Noort, W.A.; Claas, F.H.; Willemze, R.; Fibbe, W.E.; Kanhai, H.H. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 2003, 102, 1548–1549. [Google Scholar] [CrossRef]
- De Coppi, P.; Bartsch, G., Jr.; Siddiqui, M.M.; Xu, T.; Santos, C.C.; Perin, L.; Mostoslavsky, G.; Serre, A.C.; Snyder, E.Y.; Yoo, J.J.; et al. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 2007, 25, 100–106. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.; Kim, H.; Hwang, K.J.; Kwon, H.C.; Kim, S.K.; Cho, D.J.; Kang, S.G.; You, J. Human amniotic fluid-derived stem cells have characteristics of multipotent stem cells. Cell Prolif. 2007, 40, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Perin, L.; Sedrakyan, S.; Da Sacco, S.; De Filippo, R. Characterization of human amniotic fluid stem cells and their pluripotential capability. Methods Cell Biol. 2008, 86, 85–99. [Google Scholar] [PubMed]
- Moorefield, E.C.; McKee, E.E.; Solchaga, L.; Orlando, G.; Yoo, J.J.; Walker, S.; Furth, M.E.; Bishop, C.E. Cloned, CD117 selected human amniotic fluid stem cells are capable of modulating the immune response. PLoS ONE 2011, 6, e26535. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Ochiai, D.; Abe, Y.; Masuda, H.; Fukutake, M.; Ikenoue, S.; Kasuga, Y.; Shimoda, M.; Kanai, Y.; Tanaka, M. Prophylactic therapy with human amniotic fluid stem cells improved survival in a rat model of lipopolysaccharide-induced neonatal sepsis through immunomodulation via aggregates with peritoneal macrophages. Stem Cell Res. Ther. 2020, 11, 300. [Google Scholar] [CrossRef]
- Morioka, C.; Komaki, M.; Taki, A.; Honda, I.; Yokoyama, N.; Iwasaki, K.; Iseki, S.; Morio, T.; Morita, I. Neuroprotective effects of human umbilical cord-derived mesenchymal stem cells on periventricular leukomalacia-like brain injury in neonatal rats. Inflamm. Regen. 2017, 37, 1. [Google Scholar] [CrossRef]
- McLay, R.N.; Freeman, S.M.; Zadina, J.E. Chronic corticosterone impairs memory performance in the Barnes maze. Physiol. Behav. 1998, 63, 933–937. [Google Scholar] [CrossRef]
- Lin, D.; Zuo, Z. Isoflurane induces hippocampal cell injury and cognitive impairments in adult rats. Neuropharmacology 2011, 61, 1354–1359. [Google Scholar] [CrossRef]
- Nobuta, H.; Ghiani, C.A.; Paez, P.M.; Spreuer, V.; Dong, H.; Korsak, R.A.; Manukyan, A.; Li, J.; Vinters, H.V.; Huang, E.J.; et al. STAT3-mediated astrogliosis protects myelin development in neonatal brain injury. Ann. Neurol. 2012, 72, 750–765. [Google Scholar] [CrossRef]
- Banker, B.Q.; Larroche, J.C. Periventricular leukomalacia of infancy. A form of neonatal anoxic encephalopathy. Arch. Neurol. 1962, 7, 386–410. [Google Scholar] [CrossRef]
- Bendel, O.; Bueters, T.; von Euler, M.; Ove Ogren, S.; Sandin, J.; von Euler, G. Reappearance of hippocampal CA1 neurons after ischemia is associated with recovery of learning and memory. J. Cereb. Blood Flow Metab. 2005, 25, 1586–1595. [Google Scholar] [CrossRef]
- Daval, J.L.; Pourié, G.; Grojean, S.; Lièvre, V.; Strazielle, C.; Blaise, S.; Vert, P. Neonatal hypoxia triggers transient apoptosis followed by neurogenesis in the rat CA1 hippocampus. Pediatr. Res. 2004, 55, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Schwalm, M.T.; Pasquali, M.; Miguel, S.P.; Dos Santos, J.P.; Vuolo, F.; Comim, C.M.; Petronilho, F.; Quevedo, J.; Gelain, D.P.; Moreira, J.C.; et al. Acute brain inflammation and oxidative damage are related to long-term cognitive deficits and markers of neurodegeneration in sepsis-survivor rats. Mol. Neurobiol. 2014, 49, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.L.; Herz, J.; Fernandes, A.; Rocha, J.; Sepodes, B.; Brito, M.A.; McGavern, D.B.; Brites, D. Systemic inflammation in early neonatal mice induces transient and lasting neurodegenerative effects. J. Neuroinflamm. 2015, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Favrais, G.; van de Looij, Y.; Fleiss, B.; Ramanantsoa, N.; Bonnin, P.; Stoltenburg-Didinger, G.; Lacaud, A.; Saliba, E.; Dammann, O.; Gallego, J.; et al. Systemic inflammation disrupts the developmental program of white matter. Ann. Neurol. 2011, 70, 550–565. [Google Scholar] [CrossRef] [PubMed]
- Allin, M.P.; Kontis, D.; Walshe, M.; Wyatt, J.; Barker, G.J.; Kanaan, R.A.; McGuire, P.; Rifkin, L.; Murray, R.M.; Nosarti, C. White matter and cognition in adults who were born preterm. PLoS ONE 2011, 6, e24525. [Google Scholar] [CrossRef]
- Forbes, T.A.; Goldstein, E.Z.; Dupree, J.L.; Jablonska, B.; Scafidi, J.; Adams, K.L.; Imamura, Y.; Hashimoto-Torii, K.; Gallo, V. Environmental enrichment ameliorates perinatal brain injury and promotes functional white matter recovery. Nat. Commun. 2020, 11, 964. [Google Scholar] [CrossRef]
- Counsell, S.J.; Edwards, A.D.; Chew, A.T.; Anjari, M.; Dyet, L.E.; Srinivasan, L.; Boardman, J.P.; Allsop, J.M.; Hajnal, J.V.; Rutherford, M.A.; et al. Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain 2008, 131 Pt 12, 3201–3208. [Google Scholar] [CrossRef]
- Gawel, K.; Gibula, E.; Marszalek-Grabska, M.; Filarowska, J.; Kotlinska, J.H. Assessment of spatial learning and memory in the Barnes maze task in rodents-methodological consideration. Naunyn. Schmiedebergs. Arch. Pharmacol. 2019, 392, 1–18. [Google Scholar] [CrossRef]
- Rosenfeld, C.S.; Ferguson, S.A. Barnes maze testing strategies with small and large rodent models. J. Vis. Exp. 2014, e51194. [Google Scholar] [CrossRef]
- Barnes, C.A. Memory deficits associated with senescence: A neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol. 1979, 93, 74–104. [Google Scholar] [CrossRef]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Crippa, I.A.; Subirà, C.; Vincent, J.L.; Fernandez, R.F.; Hernandez, S.C.; Cavicchi, F.Z.; Creteur, J.; Taccone, F.S. Impaired cerebral autoregulation is associated with brain dysfunction in patients with sepsis. Crit. Care 2018, 22, 327. [Google Scholar] [CrossRef] [PubMed]
- Volpe, J.J.; Kinney, H.C.; Jensen, F.E.; Rosenberg, P.A. Reprint of “The developing oligodendrocyte: Key cellular target in brain injury in the premature infant”. Int. J. Dev. Neurosci. 2011, 29, 565–582. [Google Scholar] [CrossRef] [PubMed]
- Bertling, F.; Bendix, I.; Drommelschmidt, K.; Wisniewski, H.G.; Felderhoff-Mueser, U.; Keller, M.; Prager, S. Tumor necrosis factor-inducible gene 6 protein: A novel neuroprotective factor against inflammation-induced developmental brain injury. Exp. Neurol. 2016, 279, 283–289. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abe, Y.; Ochiai, D.; Sato, Y.; Kanzaki, S.; Ikenoue, S.; Kasuga, Y.; Tanaka, M. Prophylactic Therapy with Human Amniotic Fluid Stem Cells Improves Long-Term Cognitive Impairment in Rat Neonatal Sepsis Survivors. Int. J. Mol. Sci. 2020, 21, 9590. https://doi.org/10.3390/ijms21249590
Abe Y, Ochiai D, Sato Y, Kanzaki S, Ikenoue S, Kasuga Y, Tanaka M. Prophylactic Therapy with Human Amniotic Fluid Stem Cells Improves Long-Term Cognitive Impairment in Rat Neonatal Sepsis Survivors. International Journal of Molecular Sciences. 2020; 21(24):9590. https://doi.org/10.3390/ijms21249590
Chicago/Turabian StyleAbe, Yushi, Daigo Ochiai, Yu Sato, Seiji Kanzaki, Satoru Ikenoue, Yoshifumi Kasuga, and Mamoru Tanaka. 2020. "Prophylactic Therapy with Human Amniotic Fluid Stem Cells Improves Long-Term Cognitive Impairment in Rat Neonatal Sepsis Survivors" International Journal of Molecular Sciences 21, no. 24: 9590. https://doi.org/10.3390/ijms21249590
APA StyleAbe, Y., Ochiai, D., Sato, Y., Kanzaki, S., Ikenoue, S., Kasuga, Y., & Tanaka, M. (2020). Prophylactic Therapy with Human Amniotic Fluid Stem Cells Improves Long-Term Cognitive Impairment in Rat Neonatal Sepsis Survivors. International Journal of Molecular Sciences, 21(24), 9590. https://doi.org/10.3390/ijms21249590




