Peptide-Conjugated Phosphorodiamidate Morpholino Oligomers for In Situ Live-Cell Molecular Imaging of Dengue Virus Replication
Abstract
1. Introduction
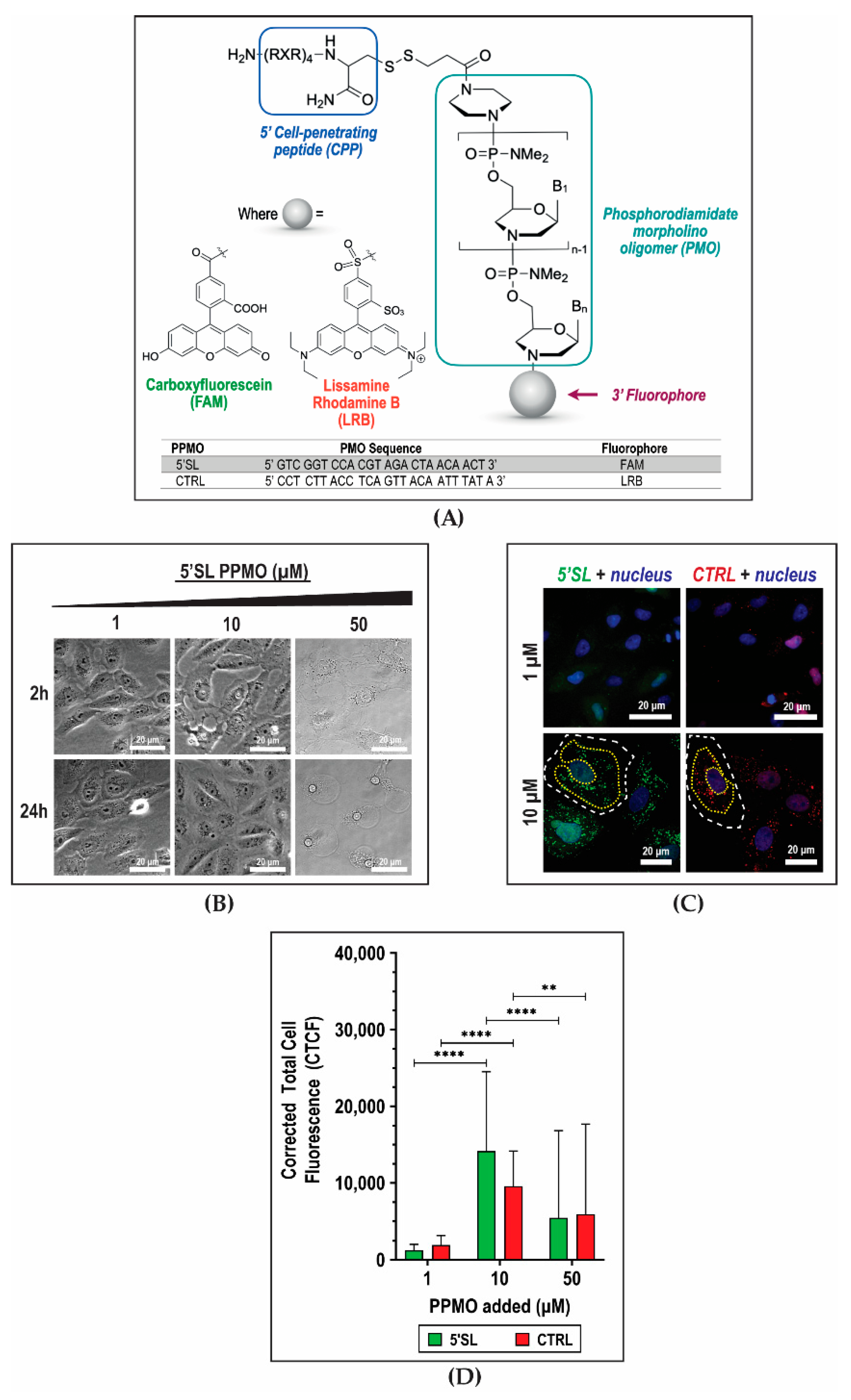
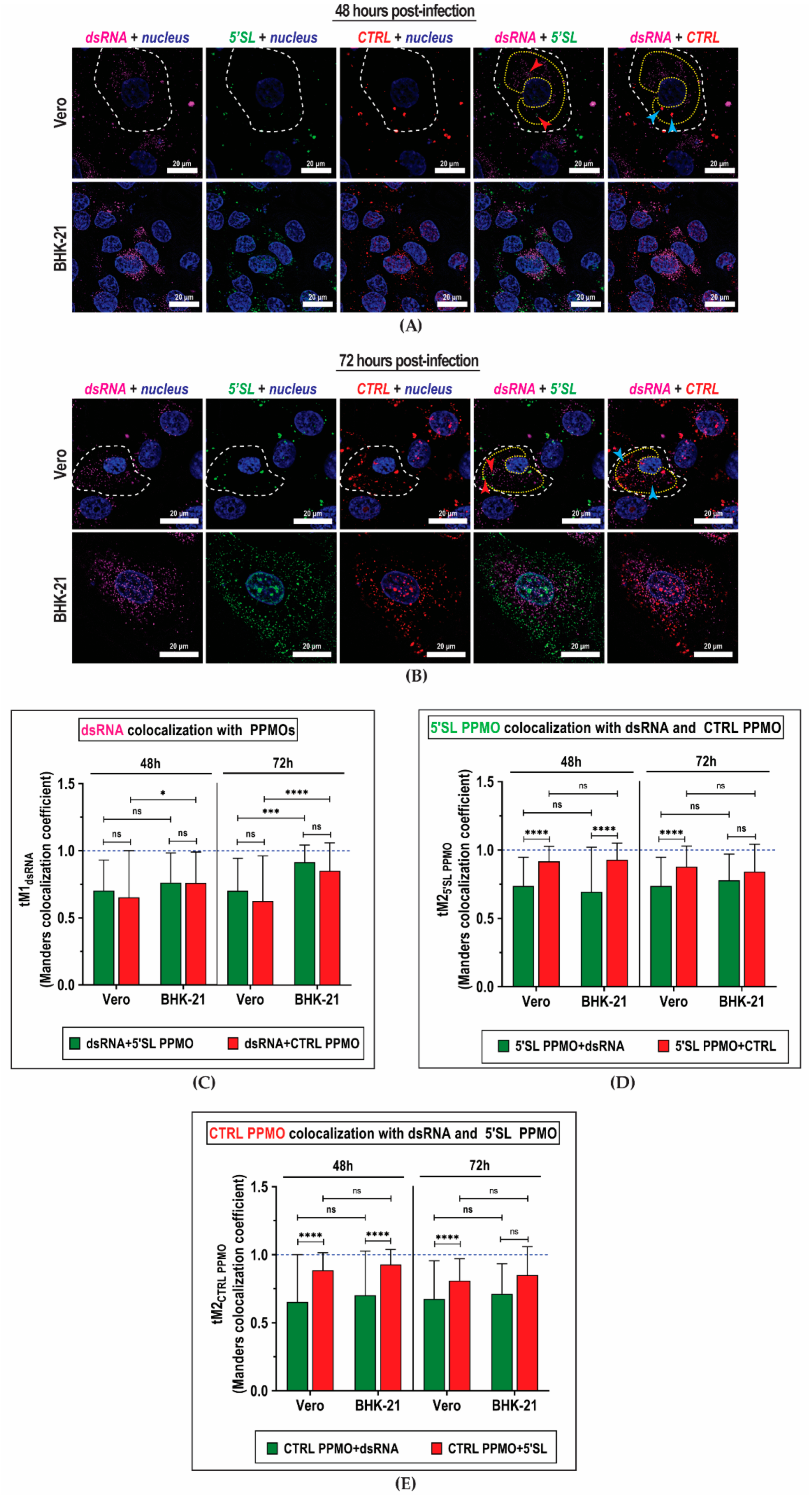
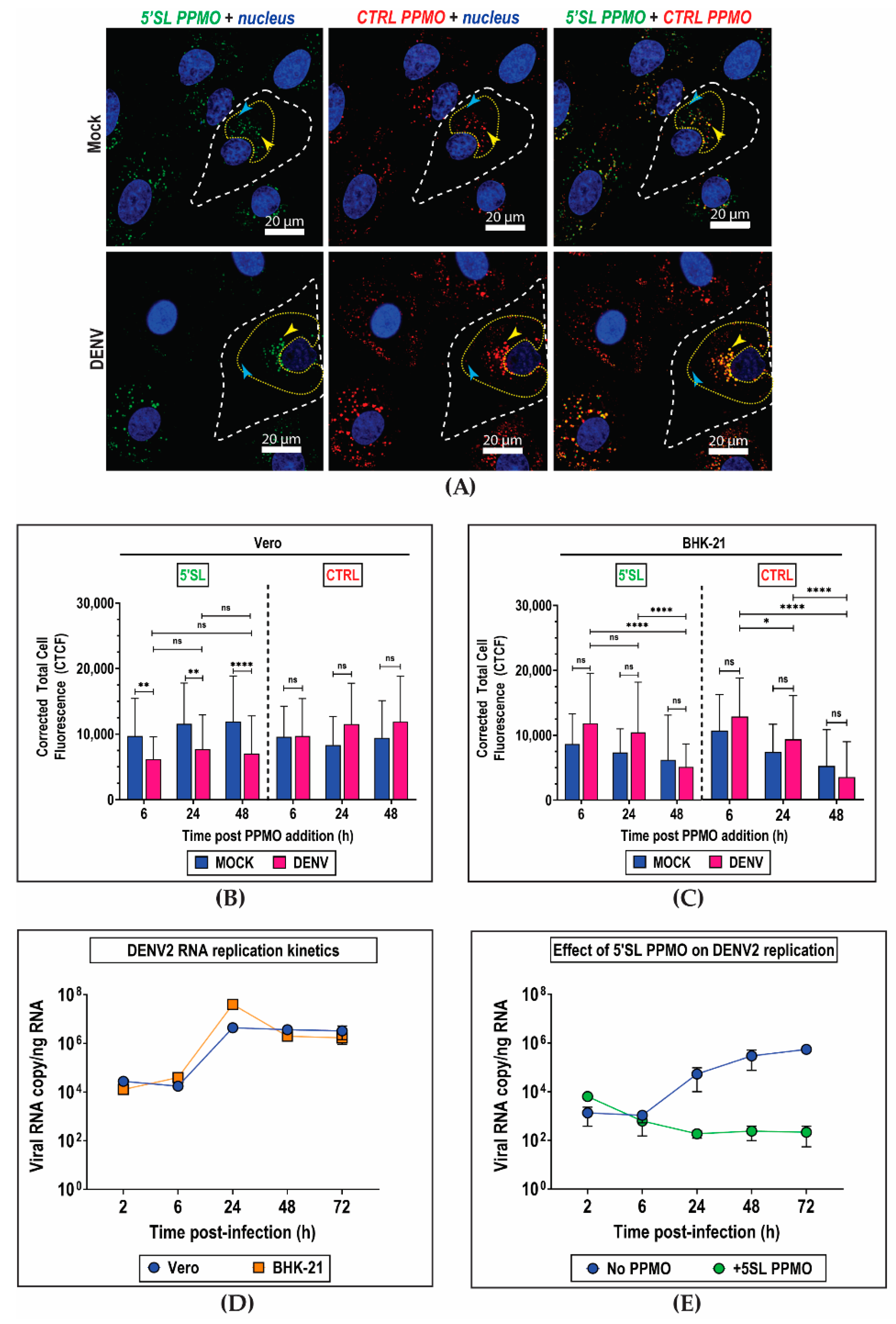
2. Results
3. Materials and Methods
3.1. Materials Used in This Study
3.2. Virus Infection and PPMO Incubation
3.3. Immunofluorescence (IF) Staining
3.4. Live-Cell Fluorescence Imaging
3.5. Viral Replication Assays
3.6. Image Analysis and Statistical Comparisons
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PMO | Phosphorodiamidate morpholino oligomers |
| PPMO | Peptide-conjugated PMO |
| DENV | Dengue virus |
| DENV2 | Dengue virus serotype-2 |
| MRI | Magnetic Resonance Imaging |
| PET | Positron Emission Tomography |
| SPECT | Single Photon Emission Computed Tomography |
| FDG | Fluorodeoxyglucose |
| ZIKV | Zika virus |
| CPP | Cell-penetrating peptide |
| CTCF | Corrected total cell fluorescence |
| ROI | Regions of interest |
| dsRNA | Double-stranded RNA |
References
- Gordon, O.; Ruiz-Bedoya, C.A.; Ordonez, A.A.; Tucker, E.W.; Jain, S.K. Molecular Imaging: A Novel Tool to Visualize Pathogenesis of Infections In Situ. mBio 2019, 10, e00317–e00319. [Google Scholar] [CrossRef]
- Jain, S.K. Introduction. In Imaging Infections: From Bench to Bedside; Jain, S.K., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–15. [Google Scholar]
- Signore, A.; Mather, S.J.; Piaggio, G.; Malviya, G.; Dierckx, R.A. Molecular imaging of inflammation/infection: Nuclear medicine and optical imaging agents and methods. Chem. Rev. 2010, 110, 3112–3145. [Google Scholar] [CrossRef]
- Douglas, A.; Lau, E.; Thursky, K.; Slavin, M. What, where and why: Exploring fluorodeoxyglucose-PET’s ability to localise and differentiate infection from cancer. Curr. Opin. Infect. Dis. 2017, 30, 552–564. [Google Scholar] [CrossRef]
- Chacko, A.M.; Watanabe, S.; Herr, K.J.; Kalimuddin, S.; Tham, J.Y.; Ong, J.; Reolo, M.; Serrano, R.M.; Cheung, Y.B.; Low, J.G.; et al. 18F-FDG as an inflammation biomarker for imaging dengue virus infection and treatment response. JCI Insight 2017, 2, e93474. [Google Scholar] [CrossRef]
- Dyall, J.; Johnson, R.F.; Chefer, S.; Leyson, C.; Thomasson, D.; Seidel, J.; Ragland, D.R.; Byrum, R.; Jett, C.; Cann, J.A.; et al. [18F]-Fluorodeoxyglucose Uptake in Lymphoid Tissue Serves as a Predictor of Disease Outcome in the Nonhuman Primate Model of Monkeypox Virus Infection. J. Virol. 2017, 91, e00897-17. [Google Scholar] [CrossRef] [PubMed]
- Dyall, J.; Johnson, R.F.; Chen, D.Y.; Huzella, L.; Ragland, D.R.; Mollura, D.J.; Byrum, R.; Reba, R.C.; Jennings, G.; Jahrling, P.B.; et al. Evaluation of monkeypox disease progression by molecular imaging. J. Infect. Dis. 2011, 204, 1902–1911. [Google Scholar] [CrossRef]
- Jonsson, C.B.; Camp, J.V.; Wu, A.; Zheng, H.; Kraenzle, J.L.; Biller, A.E.; Vanover, C.D.; Chu, Y.K.; Ng, C.K.; Proctor, M.; et al. Molecular imaging reveals a progressive pulmonary inflammation in lower airways in ferrets infected with 2009 H1N1 pandemic influenza virus. PLoS ONE 2012, 7, e40094. [Google Scholar] [CrossRef] [PubMed]
- Chefer, S.; Thomasson, D.; Seidel, J.; Reba, R.C.; Bohannon, J.K.; Lackemeyer, M.G.; Bartos, C.; Sayre, P.J.; Bollinger, L.; Hensley, L.E.; et al. Modeling [18F]-FDG lymphoid tissue kinetics to characterize nonhuman primate immune response to Middle East respiratory syndrome-coronavirus aerosol challenge. EJNMMI Res. 2015, 5, 65. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.R.; Jia, F. Antisense imaging: And miles to go before we sleep? J. Cell Biochem. 2003, 90, 464–472. [Google Scholar] [CrossRef]
- Fu, P.; Shen, B.; Zhao, C.; Tian, G. Molecular imaging of MDM2 messenger RNA with 99mTc-labeled antisense oligonucleotides in experimental human breast cancer xenografts. J. Nucl. Med. 2010, 51, 1805–1812. [Google Scholar] [CrossRef]
- Jia, F.; Figueroa, S.D.; Gallazzi, F.; Balaji, B.S.; Hannink, M.; Lever, S.Z.; Hoffman, T.J.; Lewis, M.R. Molecular imaging of bcl-2 expression in small lymphocytic lymphoma using 111In-labeled PNA-peptide conjugates. J. Nucl. Med. 2008, 49, 430–438. [Google Scholar] [CrossRef]
- Liu, M.; Wang, R.F.; Yan, P.; Zhang, C.L.; Cui, Y.G. Molecular imaging and pharmacokinetics of 99mTc-hTERT antisense oligonucleotide as a potential tumor imaging probe. J. Labelled Comp. Radiopharm. 2014, 57, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cheng, D.; Liu, G.; Dou, S.; Wang, Y.; Liu, X.; Liu, Y.; Rusckowski, M. Detection of Klebsiella. Pneumoniae Infection with an Antisense Oligomer Against its Ribosomal RNA. Mol. Imaging Biol. 2016, 18, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, L.; Liu, X.; Cheng, D.; Liu, G.; Liu, Y.; Dou, S.; Hnatowich, D.J.; Rusckowski, M. Detection of Aspergillus fumigatus pulmonary fungal infections in mice with 99mTc-labeled MORF oligomers targeting ribosomal RNA. Nucl. Med. Biol. 2013, 40, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, Y.; Cheng, D.; Liu, X.; Dou, S.; Liu, G.; Hnatowich, D.J.; Rusckowski, M. 99mTc-MORF oligomers specific for bacterial ribosomal RNA as potential specific infection imaging agents. Bioorg. Med. Chem. 2013, 21, 6523–6530. [Google Scholar] [CrossRef] [PubMed]
- Wickstrom, E. DNA and RNA derivatives to optimize distribution and delivery. Adv. Drug Deliv. Rev. 2015, 87, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Iversen, P.L.; Aird, K.M.; Wu, R.; Morse, M.M.; Devi, G.R. Cellular uptake of neutral phosphorodiamidate morpholino oligomers. Curr. Pharm. Biotechnol. 2009, 10, 579–588. [Google Scholar] [CrossRef]
- Ming, X.; Laing, B. Bioconjugates for targeted delivery of therapeutic oligonucleotides. Adv. Drug Deliv. Rev. 2015, 87, 81–89. [Google Scholar] [CrossRef]
- Komin, A.; Russell, L.M.; Hristova, K.A.; Searson, P.C. Peptide-based strategies for enhanced cell uptake, transcellular transport, and circulation: Mechanisms and challenges. Adv. Drug Deliv. Rev. 2017, 110–111, 52–64. [Google Scholar] [CrossRef]
- Bok, K.; Cavanaugh, V.J.; Matson, D.O.; Gonzalez-Molleda, L.; Chang, K.O.; Zintz, C.; Smith, A.W.; Iversen, P.; Green, K.Y.; Campbell, A.E. Inhibition of norovirus replication by morpholino oligomers targeting the 5′-end of the genome. Virology 2008, 380, 328–337. [Google Scholar] [CrossRef]
- Ge, Q.; Pastey, M.; Kobasa, D.; Puthavathana, P.; Lupfer, C.; Bestwick, R.K.; Iversen, P.L.; Chen, J.; Stein, D.A. Inhibition of multiple subtypes of influenza A virus in cell cultures with morpholino oligomers. Antimicrob. Agents Chemother. 2006, 50, 3724–3733. [Google Scholar] [CrossRef] [PubMed]
- Lupfer, C.; Stein, D.A.; Mourich, D.V.; Tepper, S.E.; Iversen, P.L.; Pastey, M. Inhibition of influenza A H3N8 virus infections in mice by morpholino oligomers. Arch. Virol. 2008, 153, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.; Chen, H.; Chen, C.K.; Min, N.; Chu, J.J. Antiviral Phosphorodiamidate Morpholino Oligomers are Protective against Chikungunya Virus Infection on Cell-based and Murine Models. Sci. Rep. 2015, 5, 12727. [Google Scholar] [CrossRef] [PubMed]
- Iversen, P.L.; Warren, T.K.; Wells, J.B.; Garza, N.L.; Mourich, D.V.; Welch, L.S.; Panchal, R.G.; Bavari, S. Discovery and early development of AVI-7537 and AVI-7288 for the treatment of Ebola virus and Marburg virus infections. Viruses 2012, 4, 2806–2830. [Google Scholar] [CrossRef]
- Heald, A.E.; Charleston, J.S.; Iversen, P.L.; Warren, T.K.; Saoud, J.B.; Al-Ibrahim, M.; Wells, J.; Warfield, K.L.; Swenson, D.L.; Welch, L.S.; et al. AVI-7288 for Marburg Virus in Nonhuman Primates and Humans. N. Engl. J. Med. 2015, 373, 339–348. [Google Scholar] [CrossRef]
- Burrer, R.; Neuman, B.W.; Ting, J.P.; Stein, D.A.; Moulton, H.M.; Iversen, P.L.; Kuhn, P.; Buchmeier, M.J. Antiviral effects of antisense morpholino oligomers in murine coronavirus infection models. J. Virol. 2007, 81, 5637–5648. [Google Scholar] [CrossRef]
- Neuman, B.W.; Stein, D.A.; Kroeker, A.D.; Paulino, A.D.; Moulton, H.M.; Iversen, P.L.; Buchmeier, M.J. Antisense morpholino-oligomers directed against the 5′ end of the genome inhibit coronavirus proliferation and growth. J. Virol. 2004, 78, 5891–5899. [Google Scholar] [CrossRef]
- Popik, W.; Khatua, A.; Hildreth, J.E.K.; Lee, B.; Alcendor, D.J. Phosphorodiamidate morpholino targeting the 5′ untranslated region of the ZIKV RNA inhibits virus replication. Virology 2018, 519, 77–85. [Google Scholar] [CrossRef]
- Holden, K.L.; Stein, D.A.; Pierson, T.C.; Ahmed, A.A.; Clyde, K.; Iversen, P.L.; Harris, E. Inhibition of dengue virus translation and RNA synthesis by a morpholino oligomer targeted to the top of the terminal 3′ stem-loop structure. Virology 2006, 344, 439–452. [Google Scholar] [CrossRef]
- Kinney, R.M.; Huang, C.Y.; Rose, B.C.; Kroeker, A.D.; Dreher, T.W.; Iversen, P.L.; Stein, D.A. Inhibition of dengue virus serotypes 1 to 4 in vero cell cultures with morpholino oligomers. J. Virol. 2005, 79, 5116–5128. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.A.; Huang, C.Y.; Silengo, S.; Amantana, A.; Crumley, S.; Blouch, R.E.; Iversen, P.L.; Kinney, R.M. Treatment of AG129 mice with antisense morpholino oligomers increases survival time following challenge with dengue 2 virus. J. Antimicrob. Chemother. 2008, 62, 555–565. [Google Scholar] [CrossRef]
- Raviprakash, K.; Liu, K.; Matteucci, M.; Wagner, R.; Riffenburgh, R.; Carl, M. Inhibition of dengue virus by novel, modified antisense oligonucleotides. J. Virol. 1995, 69, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.A. Inhibition of RNA virus infections with peptide-conjugated morpholino oligomers. Curr. Pharm. Des. 2008, 14, 2619–2634. [Google Scholar] [CrossRef] [PubMed]
- Nan, Y.; Zhang, Y.J. Antisense Phosphorodiamidate Morpholino Oligomers as Novel Antiviral Compounds. Front. Microbiol. 2018, 9, 750. [Google Scholar] [CrossRef] [PubMed]
- Wilder-Smith, A.; Ooi, E.E.; Horstick, O.; Wills, B. Dengue. Lancet 2019, 393, 350–363. [Google Scholar] [CrossRef]
- Summerton, J.; Weller, D. Morpholino antisense oligomers: Design, preparation, and properties. Antisense Nucleic. Acid. Drug Dev. 1997, 7, 187–195. [Google Scholar] [CrossRef]
- Youngblood, D.S.; Hatlevig, S.A.; Hassinger, J.N.; Iversen, P.L.; Moulton, H.M. Stability of cell-penetrating peptide-morpholino oligomer conjugates in human serum and in cells. Bioconjug. Chem. 2007, 18, 50–60. [Google Scholar] [CrossRef]
- Barrows, N.J.; Campos, R.K.; Liao, K.C.; Prasanth, K.R.; Soto-Acosta, R.; Yeh, S.C.; Schott-Lerner, G.; Pompon, J.; Sessions, O.M.; Bradrick, S.S.; et al. Biochemistry and Molecular Biology of Flaviviruses. Chem. Rev. 2018, 118, 4448–4482. [Google Scholar] [CrossRef]
- Manders, E.M.M.; Verbeek, F.J.; Aten, J.A. Measurement of co-localization of objects in dual-colour confocal images. J. Microsc. 1993, 169, 375–382. [Google Scholar] [CrossRef]
- Gagnon, K.T.; Corey, D.R. Guidelines for Experiments Using Antisense Oligonucleotides and Double-Stranded RNAs. Nucleic. Acid. Ther. 2019, 29, 116–122. [Google Scholar] [CrossRef]
- Dowdy, S.F. Overcoming cellular barriers for RNA therapeutics. Nat. Biotechnol. 2017, 35, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Erazo-Oliveras, A.; Muthukrishnan, N.; Baker, R.; Wang, T.Y.; Pellois, J.P. Improving the endosomal escape of cell-penetrating peptides and their cargos: Strategies and challenges. Pharmaceuticals 2012, 5, 1177–1209. [Google Scholar] [CrossRef]
- White, P.J.; Anastasopoulos, F.; Pouton, C.W.; Boyd, B.J. Overcoming biological barriers to in vivo efficacy of antisense oligonucleotides. Expert Rev. Mol. Med. 2009, 11, e10. [Google Scholar] [CrossRef]
- Bocan, T.M.; Panchal, R.G.; Bavari, S. Applications of in vivo imaging in the evaluation of the pathophysiology of viral and bacterial infections and in development of countermeasures to BSL3/4 pathogens. Mol. Imaging Biol. 2015, 17, 4–17. [Google Scholar] [CrossRef]
- Young, L.; Sung, J.; Stacey, G.; Masters, J.R. Detection of Mycoplasma in cell cultures. Nat. Protoc. 2010, 5, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Yauch, L.E.; Zellweger, R.M.; Kotturi, M.F.; Qutubuddin, A.; Sidney, J.; Peters, B.; Prestwood, T.R.; Sette, A.; Shresta, S. A protective role for dengue virus-specific CD8+ T cells. J. Immunol. 2009, 182, 4865–4873. [Google Scholar] [CrossRef] [PubMed]
- Abes, S.; Moulton, H.M.; Clair, P.; Prevot, P.; Youngblood, D.S.; Wu, R.P.; Iversen, P.L.; Lebleu, B. Vectorization of morpholino oligomers by the (R-Ahx-R)4 peptide allows efficient splicing correction in the absence of endosomolytic agents. J. Control. Release 2006, 116, 304–313. [Google Scholar] [CrossRef]
- Watanabe, S.; Tan, N.W.W.; Chan, K.W.K.; Vasudevan, S.G. Dengue Virus and Zika Virus Serological Cross-reactivity and Their Impact on Pathogenesis in Mice. J. Infect. Dis. 2019, 219, 223–233. [Google Scholar] [CrossRef]
- Hammond, L. Measuring cell fluorescence using ImageJ. Available online: https://theolb.readthedocs.io/en/latest/imaging/measuring-cell-fluorescence-using-imagej.html (accessed on 1 May 2020).
- Dunn, K.W.; Kamocka, M.M.; McDonald, J.H. A practical guide to evaluating colocalization in biological microscopy. Am. J. Physiol. Cell Physiol. 2011, 300, C723–C742. [Google Scholar] [CrossRef]
- Eliceiri, K.T.P.; Jug, F.; Carpenter, A.; Berthold, M.; Swedlow, J.; Rasband, W.; Rueden, C.; Dietz, C.; Northan, B.; Hiner, M.; et al. Colocalization Analysis with ImageJ. Available online: https://imagej.net/Colocalization_Analysis (accessed on 1 May 2020).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Victorio, C.B.L.; Novera, W.; Tham, J.Y.; Watanabe, S.; Vasudevan, S.G.; Chacko, A.-M. Peptide-Conjugated Phosphorodiamidate Morpholino Oligomers for In Situ Live-Cell Molecular Imaging of Dengue Virus Replication. Int. J. Mol. Sci. 2020, 21, 9260. https://doi.org/10.3390/ijms21239260
Victorio CBL, Novera W, Tham JY, Watanabe S, Vasudevan SG, Chacko A-M. Peptide-Conjugated Phosphorodiamidate Morpholino Oligomers for In Situ Live-Cell Molecular Imaging of Dengue Virus Replication. International Journal of Molecular Sciences. 2020; 21(23):9260. https://doi.org/10.3390/ijms21239260
Chicago/Turabian StyleVictorio, Carla Bianca Luena, Wisna Novera, Jing Yang Tham, Satoru Watanabe, Subhash G. Vasudevan, and Ann-Marie Chacko. 2020. "Peptide-Conjugated Phosphorodiamidate Morpholino Oligomers for In Situ Live-Cell Molecular Imaging of Dengue Virus Replication" International Journal of Molecular Sciences 21, no. 23: 9260. https://doi.org/10.3390/ijms21239260
APA StyleVictorio, C. B. L., Novera, W., Tham, J. Y., Watanabe, S., Vasudevan, S. G., & Chacko, A.-M. (2020). Peptide-Conjugated Phosphorodiamidate Morpholino Oligomers for In Situ Live-Cell Molecular Imaging of Dengue Virus Replication. International Journal of Molecular Sciences, 21(23), 9260. https://doi.org/10.3390/ijms21239260




