Zinc Binds to RRM2 Peptide of TDP-43
Abstract
1. Introduction
2. Result and Discussion
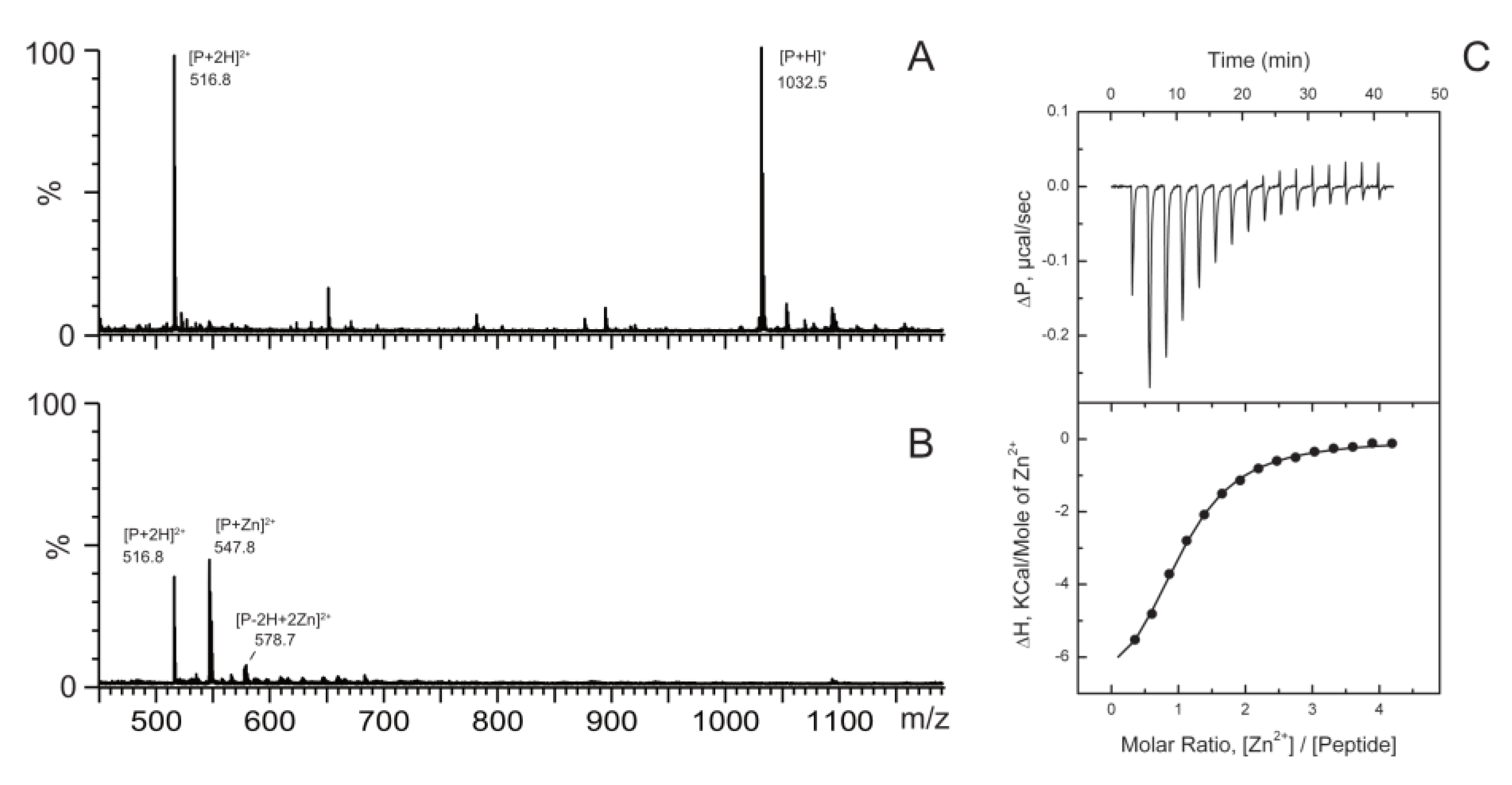
2.1. Zinc Binding to a Peptide from TDP-43 Is Enthalpy Driven
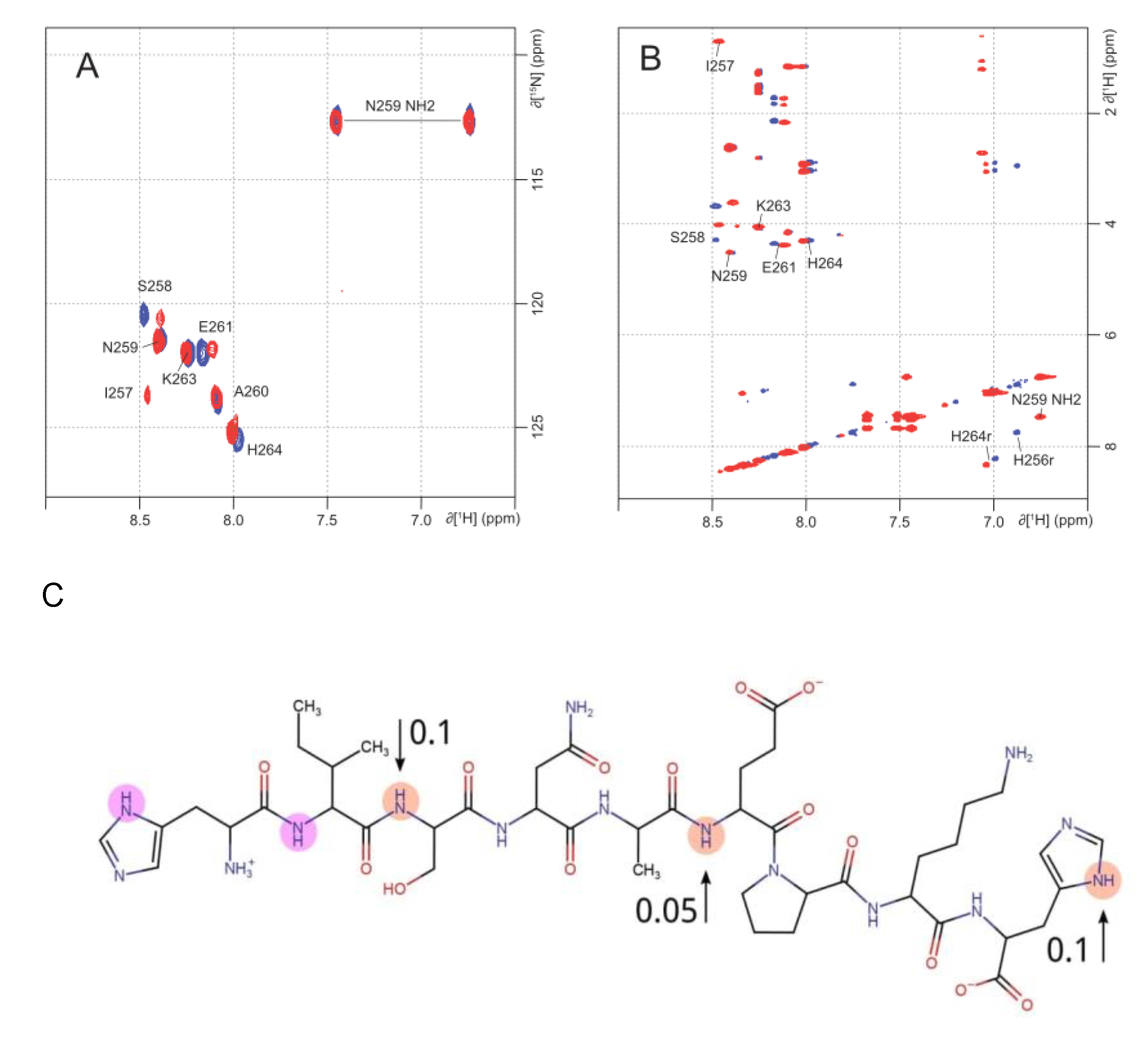
2.2. NMR Analysis of Zinc Coordination in ALR Peptide Points to Multiple Conformation Complex Supports
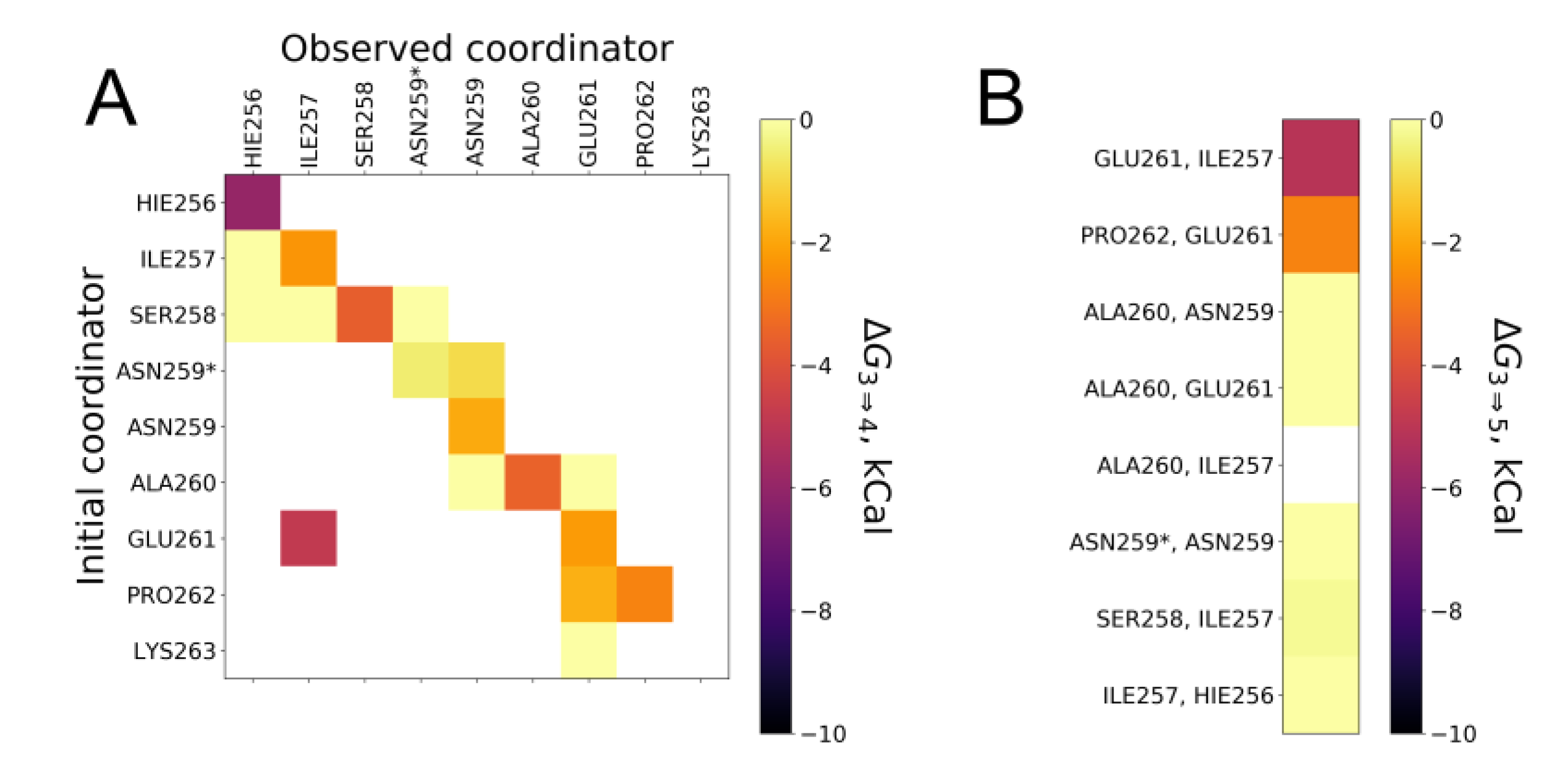
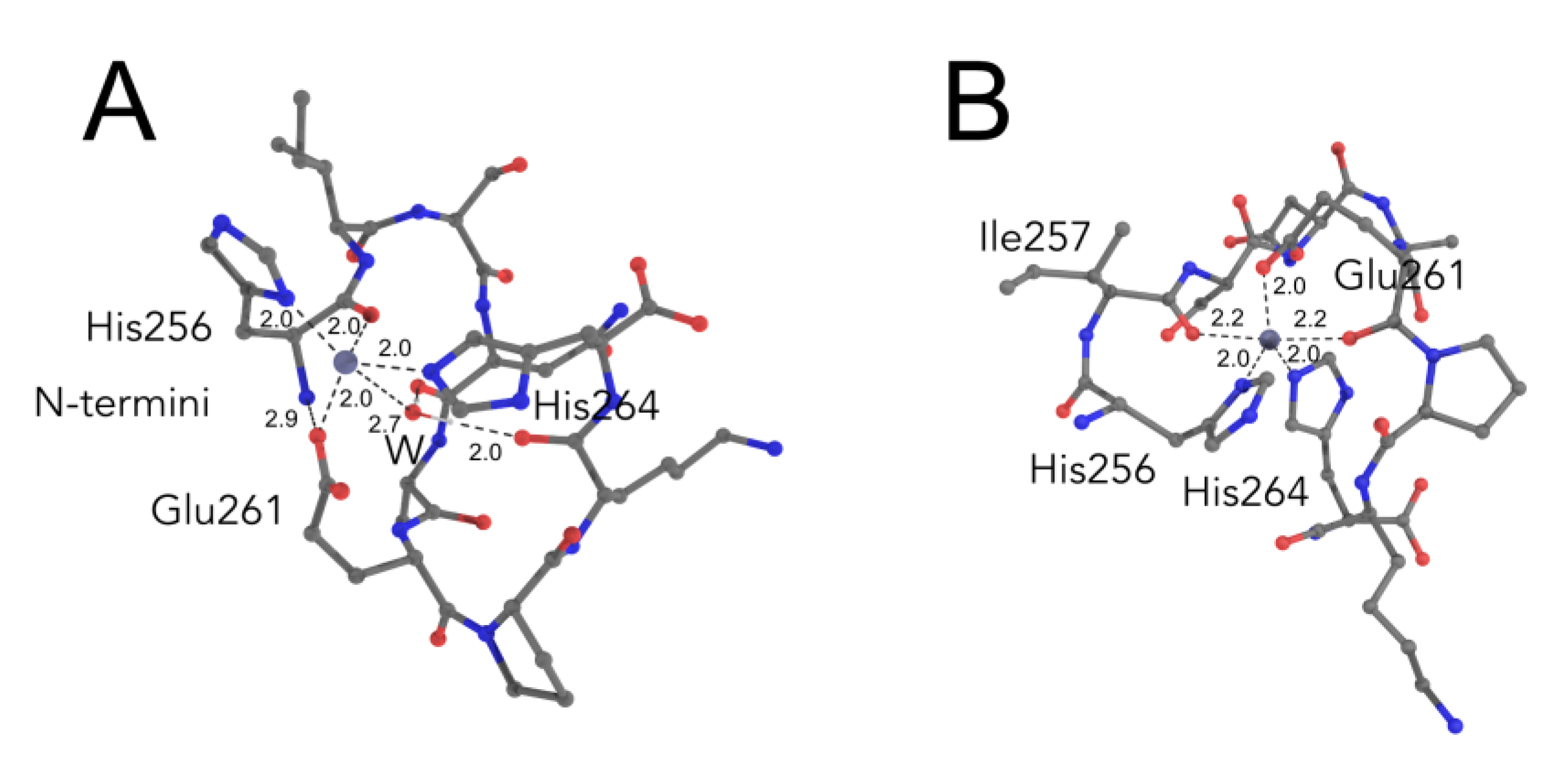
2.3. Comparative Simulations of All the Available Ways to Bind Zinc in ALR Peptide
2.4. Biological Relevance
3. Materials and Methods
3.1. Materials
3.2. Mass-Spectrometry
3.3. Isothermal Titration Calorimetry (ITC)
3.4. NMR Experiments
3.5. QM/MM Modeling
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TDP-43 | transactive response DNA binding protein 43 kDa |
| RRM | RNA recognition motif |
| ALS | amyotrophic lateral sclerosis |
| FTLD | frontotemporal lobar degeneration |
| AD | Alzheimer’s disease |
| NTD | N-terminal domain of TDP-43 |
| ITC | isothermal titration calorimetry |
| Aβ1–16 | amyloid-β metal-binding domain 1–16 |
References
- Cassandri, M.; Smirnov, A.; Novelli, F.; Pitolli, C.; Agostini, M.; Malewicz, M.; Melino, G.; Raschellà, G. Zinc-finger proteins in health and disease. Cell Death Discov. 2017, 3, 17071. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, P.O.; Kulikova, A.A.; Golovin, A.V.; Tkachev, Y.V.; Archakov, A.I.; Kozin, S.A.; Makarov, A.A. Minimal Zn(2+) binding site of amyloid-β. Biophys. J. 2010, 99, L84–L86. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, P.O.; Roman, A.Y.; Baksheeva, V.E.; Nazipova, A.A.; Shevelyova, M.P.; Vladimirov, V.I.; Buyanova, M.F.; Zinchenko, D.V.; Zamyatnin, A.A.J.; Devred, F.; et al. Functional Status of Neuronal Calcium Sensor-1 Is Modulated by Zinc Binding. Front. Mol. Neurosci. 2018, 11, 459. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, P.O.; Devred, F.; Makarov, A.A. Thermodynamics of zinc binding to human S100A2. Mol. Biol. 2010, 44, 832–835. [Google Scholar] [CrossRef]
- Eide, D.J. Zinc transporters and the cellular trafficking of zinc. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2006, 1763, 711–722. [Google Scholar] [CrossRef]
- Pfaender, S.; Grabrucker, A.M. Characterization of biometal profiles in neurological disorders. Metallomics 2014, 6, 960–977. [Google Scholar] [CrossRef]
- Shelkovnikova, T.A.; Kulikova, A.A.; Tsvetkov, P.O.; Peters, O.; Bachurin, S.O.; Buchman, V.L.; Ninkina, N.N. Proteinopathies, neurodegenerative disorders with protein aggregation-based pathology. Mol. Biol. 2012, 46, 362–374. [Google Scholar] [CrossRef]
- Rezaei-Ghaleh, N.; Giller, K.; Becker, S.; Zweckstetter, M. Effect of zinc binding on β-amyloid structure and dynamics: Implications for Aβ aggregation. Biophys. J. 2011, 101, 1202–1211. [Google Scholar] [CrossRef]
- Miller, Y.; Ma, B.; Nussinov, R. Zinc ions promote Alzheimer Abeta aggregation via population shift of polymorphic states. Proc. Natl. Acad. Sci. USA 2010, 107, 9490–9495. [Google Scholar] [CrossRef]
- Rana, M.; Sharma, A.K. Cu and Zn interactions with Aβ peptides: Consequence of coordination on aggregation and formation of neurotoxic soluble Aβ oligomers. Metallomics 2019, 11, 64–84. [Google Scholar] [CrossRef]
- Lovell, M.A.; Robertson, J.D.; Teesdale, W.J.; Campbell, J.L.; Markesbery, W.R. Copper, iron and zinc in Alzheimer’s disease senile plaques. J. Neurol. Sci. 1998, 158, 47–52. [Google Scholar] [CrossRef]
- Ratti, A.; Buratti, E. Physiological functions and pathobiology of TDP-43 and FUS/TLS proteins. J. Neurochem. 2016, 138 (Suppl. S1), 95–111. [Google Scholar] [CrossRef]
- McAlary, L.; Chew, Y.L.; Lum, J.S.; Geraghty, N.J.; Yerbury, J.J.; Cashman, N.R. Amyotrophic Lateral Sclerosis: Proteins, Proteostasis, Prions, and Promises. Front. Cell. Neurosci. 2020, 14, 581907. [Google Scholar] [CrossRef]
- Cohen, T.J.; Lee, V.M.Y.; Trojanowski, J.Q. TDP-43 functions and pathogenic mechanisms implicated in TDP-43 proteinopathies. Trends Mol. Med. 2011, 17, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Baralle, M.; Buratti, E.; Baralle, F.E. The role of TDP-43 in the pathogenesis of ALS and FTLD: Figure 1. Biochem. Soc. Trans. 2013, 41, 1536–1540. [Google Scholar] [CrossRef]
- Dewey, C.M.; Cenik, B.; Sephton, C.F.; Johnson, B.A.; Herz, J.; Yu, G. TDP-43 aggregation in neurodegeneration: Are stress granules the key? Brain Res. 2012, 1462, 16–25. [Google Scholar] [CrossRef]
- McAleese, K.E.; Walker, L.; Erskine, D.; Thomas, A.J.; McKeith, I.G.; Attems, J. TDP-43 pathology in Alzheimer’s disease, dementia with Lewy bodies and ageing. Brain Pathol. 2017, 27, 472–479. [Google Scholar] [CrossRef]
- Caragounis, A.; Price, K.A.; Soon, C.P.W.; Filiz, G.; Masters, C.L.; Li, Q.-X.; Crouch, P.J.; White, A.R. Zinc induces depletion and aggregation of endogenous TDP-43. Free Radic Biol. Med. 2010, 48, 1152–1161. [Google Scholar] [CrossRef]
- Garnier, C.; Devred, F.; Byrne, D.; Puppo, R.; Roman, A.Y.; Malesinski, S.; Golovin, A.V.; Lebrun, R.; Ninkina, N.N.; Tsvetkov, P.O. Zinc binding to RNA recognition motif of TDP-43 induces the formation of amyloid-like aggregates. Sci. Rep. 2017, 7, 6812. [Google Scholar] [CrossRef]
- Mackness, B.C.; Tran, M.T.; McClain, S.P.; Matthews, C.R.; Zitzewitz, J.A. Folding of the RNA recognition motif (RRM) domains of the amyotrophic lateral sclerosis (ALS)-linked protein TDP-43 reveals an intermediate state. J. Biol. Chem. 2014, 289, 8264–8276. [Google Scholar] [CrossRef]
- Kozin, S.A.; Mezentsev, Y.V.; Kulikova, A.A.; Indeykina, M.I.; Golovin, A.V.; Ivanov, A.S.; Tsvetkov, P.O.; Makarov, A.A. Zinc-induced dimerization of the amyloid-β metal-binding domain 1–16 is mediated by residues 11–14. Mol. Biosyst. 2011, 7, 1053–1055. [Google Scholar] [CrossRef] [PubMed]
- Kulikova, A.A.; Tsvetkov, P.O.; Indeykina, M.I.; Popov, I.A.; Zhokhov, S.S.; Golovin, A.V.; Polshakov, V.I.; Kozin, S.A.; Nudletr, E.; Makarov, A.A. Phosphorylation of Ser8 promotes zinc-induced dimerization of the amyloid-β metal-binding domain. Mol. Biosyst. 2014, 10, 2590–2596. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, P.O.; Popov, I.A.; Nikolaev, E.N.; Archakov, A.I.; Makarov, A.A.; Kozin, S.A. Isomerization of the Asp7 residue results in zinc-induced oligomerization of Alzheimer’s disease amyloid beta(1–16) peptide. ChemBioChem 2008, 9, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Atrián-Blasco, E.; Gonzalez, P.; Santoro, A.; Alies, B.; Faller, P.; Hureau, C. Cu and Zn coordination to amyloid peptides: From fascinating chemistry to debated pathological relevance. Coord. Chem. Rev. 2018, 371, 38–55. [Google Scholar] [CrossRef] [PubMed]
- Bossy-Wetzel, E.; Talantova, M.V.; Lee, W.D.; Schölzke, M.N.; Harrop, A.; Mathews, E.; Götz, T.; Han, J.; Ellisman, M.H.; Perkins, G.A.; et al. Crosstalk between nitric oxide and zinc pathways to neuronal cell death involving mitochondrial dysfunction and p38-activated K+ channels. Neuron 2004, 41, 351–365. [Google Scholar] [CrossRef]
- Danielsson, J.; Pierattelli, R.; Banci, L.; Gräslund, A. High-resolution NMR studies of the zinc-binding site of the Alzheimer’s amyloid beta-peptide. FEBS J. 2007, 274, 46–59. [Google Scholar] [CrossRef]
- Laitaoja, M.; Valjakka, J.; Jänis, J. Zinc coordination spheres in protein structures. Inorg. Chem. 2013, 52, 10983–10991. [Google Scholar] [CrossRef]
- Cornell, W.D.; Cieplak, P.; Bayly, C.I.; Gould, I.R.; Merz, K.M.; Ferguson, D.M.; Spellmeyer, D.C.; Fox, T.; Caldwell, J.W.; Kollman, P.A. A Second Generation Force Field for the Simulation of Proteins, Nucleic Acids, and Organic Molecules. J. Am. Chem. Soc. 1995, 117, 5179–5197. [Google Scholar] [CrossRef]
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.L.; Dror, R.O.; Shaw, D.E. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins 2010, 78, 1950–1958. [Google Scholar] [CrossRef]
- Tribello, G.A.; Bonomi, M.; Branduardi, D.; Camilloni, C.; Bussi, G. PLUMED 2, New feathers for an old bird. Comput. Phys. Commun. 2014, 185, 604–613. [Google Scholar] [CrossRef]
- Tiwary, P.; Parrinello, M. A time-independent free energy estimator for metadynamics. J. Phys. Chem. B 2015, 119, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Lukavsky, P.J.; Daujotyte, D.; Tollervey, J.R.; Ule, J.; Stuani, C.; Buratti, E.; Baralle, F.E.; Damberger, F.F.; Allain, F.H. Molecular basis of UG-rich RNA recognition by the human splicing factor TDP-43. Nat. Struct. Mol. Biol. 2013, 20, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Fichou, Y.; Al-Hilaly, Y.K.; Devred, F.; Smet-Nocca, C.; Tsvetkov, P.O.; Verelst, J.; Winderickx, J.; Geukens, N.; Vanmechelen, E.; Perrotin, A.; et al. The elusive tau molecular structures: Can we translate the recent breakthroughs into new targets for intervention? Acta Neuropathol. Commun. 2019, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Gillespie, J.R.; Millett, I.S.; Khodyakova, A.V.; Vasilenko, R.N.; Vasiliev, A.M.; Rodionov, I.L.; Kozlovskaya, G.D.; Dolgikh, D.A.; Fink, A.L.; et al. Zn2+-Mediated Structure Formation and Compaction of the “Natively Unfolded” Human Prothymosin α. Biochem. Biophys. Res. Commun. 2000, 267, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Boys, B.L.; Brickenden, A.; Konermann, L.; Choy, W.-Y. Effects of zinc binding on the structure and dynamics of the intrinsically disordered protein prothymosin alpha: Evidence for metalation as an entropic switch. Biochemistry 2007, 46, 13120–13130. [Google Scholar] [CrossRef]
- Wang, J.; Anastasia, A.; Bains, H.; Giza, J.I.; Clossey, D.G.; Deng, J.; Neubert, T.A.; Rice, W.J.; Lee, F.S.; Hempstead, B.L.; et al. Zinc induced structural changes in the intrinsically disordered BDNF Met prodomain confer synaptic elimination. Metallomics 2020, 12, 1208–1219. [Google Scholar] [CrossRef]
- Wang, X.; Schwartz, J.C.; Cech, T.R. Nucleic acid-binding specificity of human FUS protein. Nucleic Acids Res. 2015, 43, 7535–7543. [Google Scholar] [CrossRef]
- Huang, J.; Ringuet, M.; Whitten, A.E.; Caria, S.; Lim, Y.W.; Badhan, R.; Anggono, V.; Lee, M. Structural basis of the zinc-induced cytoplasmic aggregation of the RNA-binding protein SFPQ. Nucleic Acids Res. 2020, 48, 3356–3365. [Google Scholar] [CrossRef]
- Lim, Y.W.; James, D.; Huang, J.; Lee, M. The Emerging Role of the RNA-Binding Protein SFPQ in Neuronal Function and Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 7151. [Google Scholar] [CrossRef]
- Peters, M.B.; Yang, Y.; Wang, B.; Füsti-Molnár, L.; Weaver, M.N.; Merz, K.M., Jr. Structural Survey of Zinc Containing Proteins and the Development of the Zinc AMBER Force Field (ZAFF). J. Chem. Theory Comput. 2010, 6, 2935–2947. [Google Scholar] [CrossRef]
- Gaus, M.; Cui, Q.; Elstner, M. DFTB3, Extension of the self-consistent-charge density-functional tight-binding method (SCC-DFTB). J. Chem. Theory Comput. 2012, 7, 931–948. [Google Scholar] [CrossRef] [PubMed]
- Gaus, M.; Goez, A.; Elstner, M. Parametrization and Benchmark of DFTB3 for Organic Molecules. J. Chem. Theory Comput. 2013, 9, 338–354. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Gaus, M.; Elstner, M.; Cui, Q. Parametrization of DFTB3/3OB for magnesium and zinc for chemical and biological applications. J. Phys. Chem. B 2015, 119, 1062–1082. [Google Scholar] [CrossRef] [PubMed]
- Kubař, T.; Welke, K.; Groenhof, G. New QM/MM implementation of the DFTB3 method in the gromacs package. J. Comput. Chem. 2015, 36, 1978–1989. [Google Scholar] [CrossRef] [PubMed]

| # | A.A. | Zn2+ | NH | HA | Hsc | Hsc | Hsc | Hsc | Hsc | Hsc |
|---|---|---|---|---|---|---|---|---|---|---|
| 256 | His | − | 3.95 | 2.94 | 6.87 | 7.74 | ||||
| + | ||||||||||
| 257 | Ile | − | ||||||||
| + | 8.46 | 3.99 | 1.63 | 0.67 | ||||||
| 258 | Ser | − | 8.48 | 4.25 | 3.64 | |||||
| + | 8.38 | 3.57 | ||||||||
| 259 | Asn | − | 8.39 | 4.51 | 2.56 | 6.74 | 7.47 | |||
| + | 8.39 | 4.51 | 2.56 | 6.74 | 7.47 | |||||
| 260 | Ala | − | 8.1 | 4.1 | 1.13 | |||||
| + | 8.1 | 4.1 | 1.13 | |||||||
| 261 | Glu | − | 8.17 | 4.31 | 2.1 | 1.79 | 1.67 | |||
| + | 8.12 | 4.28 | 2.09 | 1.8 | 1.66 | |||||
| 263 | Lys | − | 8.23 | 4.02 | 2.77 | 1.55 | 1.49 | 1.25 | ||
| + | 8.23 | 4.02 | 2.77 | 1.55 | 1.49 | 1.25 | ||||
| 264 | His | − | 7.98 | 4.25 | 2.99 | 2.85 | 6.99 | 8.23 | ||
| + | 8.01 | 4.28 | 3.02 | 2.88 | 7.04 | 8.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golovin, A.V.; Devred, F.; Yatoui, D.; Roman, A.Y.; Zalevsky, A.O.; Puppo, R.; Lebrun, R.; Guerlesquin, F.; Tsvetkov, P.O. Zinc Binds to RRM2 Peptide of TDP-43. Int. J. Mol. Sci. 2020, 21, 9080. https://doi.org/10.3390/ijms21239080
Golovin AV, Devred F, Yatoui D, Roman AY, Zalevsky AO, Puppo R, Lebrun R, Guerlesquin F, Tsvetkov PO. Zinc Binds to RRM2 Peptide of TDP-43. International Journal of Molecular Sciences. 2020; 21(23):9080. https://doi.org/10.3390/ijms21239080
Chicago/Turabian StyleGolovin, Andrey V., Francois Devred, Dahbia Yatoui, Andrei Yu. Roman, Arthur O. Zalevsky, Remy Puppo, Regine Lebrun, Francoise Guerlesquin, and Philipp O. Tsvetkov. 2020. "Zinc Binds to RRM2 Peptide of TDP-43" International Journal of Molecular Sciences 21, no. 23: 9080. https://doi.org/10.3390/ijms21239080
APA StyleGolovin, A. V., Devred, F., Yatoui, D., Roman, A. Y., Zalevsky, A. O., Puppo, R., Lebrun, R., Guerlesquin, F., & Tsvetkov, P. O. (2020). Zinc Binds to RRM2 Peptide of TDP-43. International Journal of Molecular Sciences, 21(23), 9080. https://doi.org/10.3390/ijms21239080






