Inhibition of the Wnt Signalling Pathway: An Avenue to Control Breast Cancer Aggressiveness
Abstract
1. Introduction
2. Wnt Signalling Components and Pathways
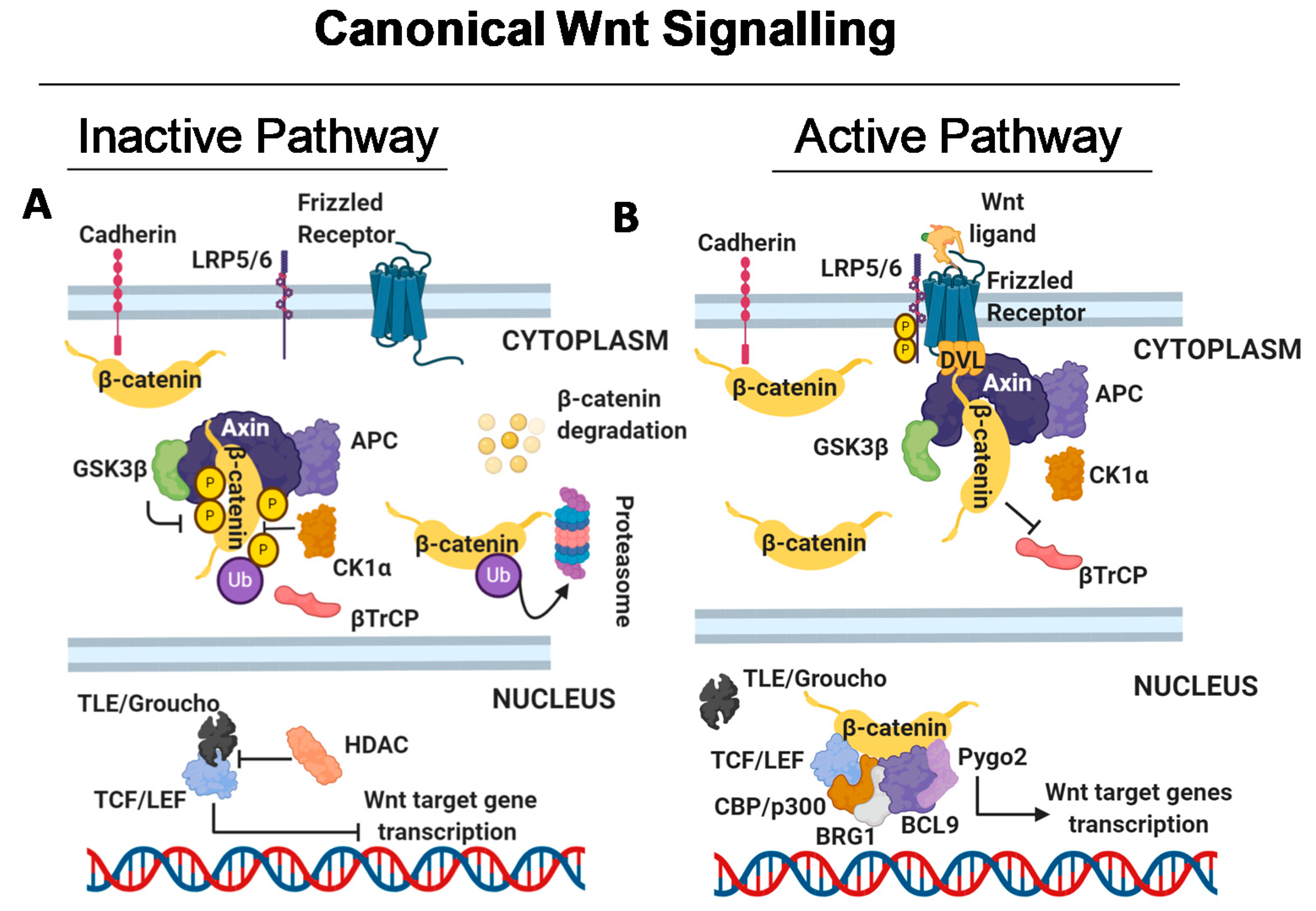
2.1. Canonical Wnt Signalling
2.1.1. Wnt Signalling Off
2.1.2. Wnt Signalling On
2.1.3. Canonical Wnt Signaling in CSCs, Therapy Resistance and Immunoevasion
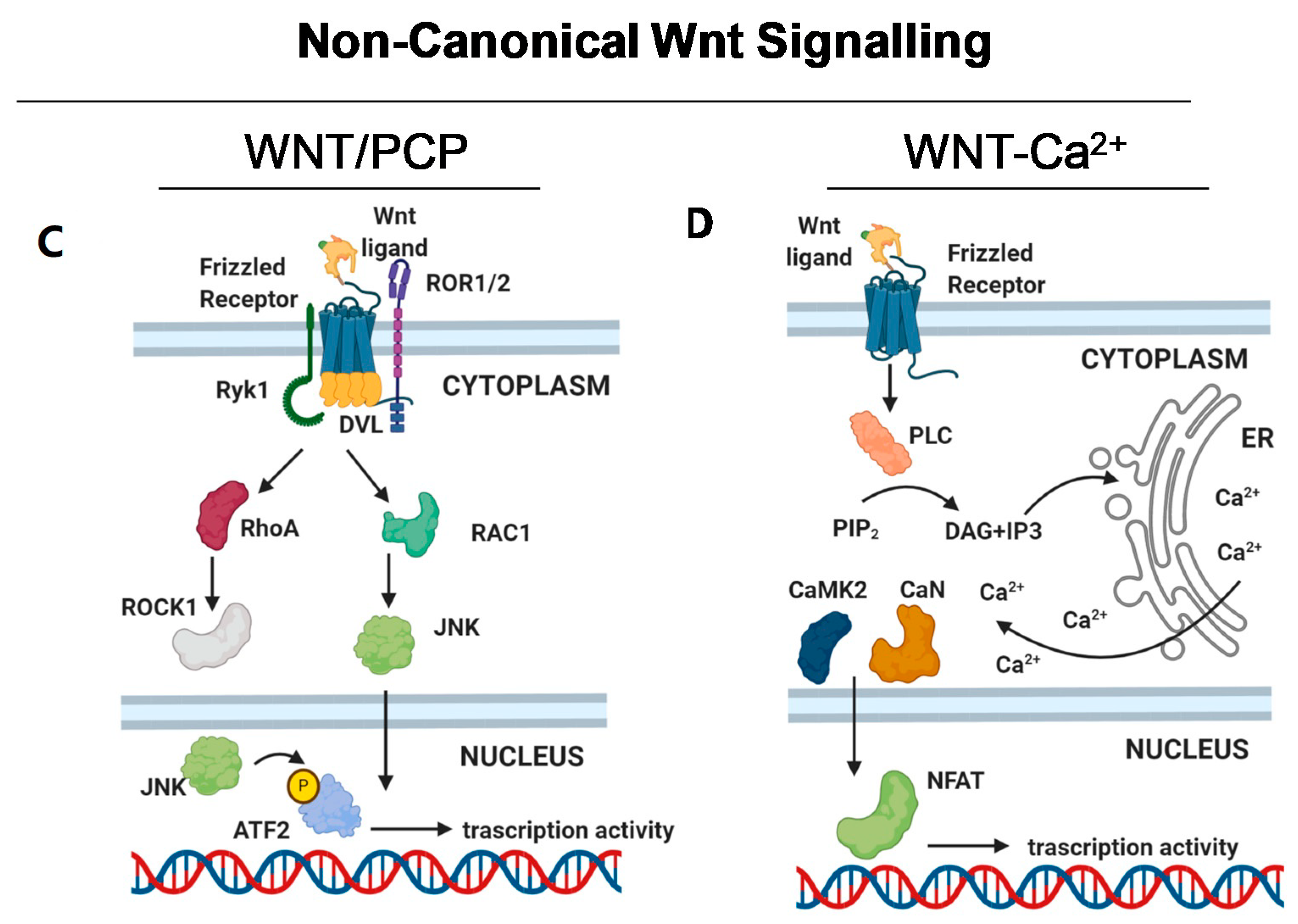
2.2. Non-Canonical Wnt Signalling
2.3. Imbalance of the Wnt Pathway in BC
2.3.1. Altered Expression of Destruction Complex Components
2.3.2. Altered Expression of FZD/LRP Receptor Complex
2.3.3. Altered Expression of β-Catenin
2.3.4. Altered Expression of Wnt Ligands
3. Wnt Signalling and Resistance to Anti-BC Therapy
3.1. Altered Wnt Signaling and Response to Therapy in the HER2+ BC Subtype
3.2. Altered Wnt Signaling and Response to Therapy in the Luminal BC Subtype
3.3. Altered Wnt Signaling and Response to Therapy in the TNBC BC Subtype
4. Anti-WNT Compounds in Clinical Trial
4.1. Porcupine Inhibitors
4.2. WNT5a Mimetics
4.3. FZD Receptors Inhibitors
4.4. Modulating Wnt Pathway in the Clinical Setting
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sorlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Sorlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef] [PubMed]
- Fragomeni, S.M.; Sciallis, A.; Jeruss, J.S. Molecular Subtypes and Local-Regional Control of Breast Cancer. Surg. Oncol. Clin. N. Am. 2018, 27, 95–120. [Google Scholar] [CrossRef] [PubMed]
- Aponte, P.M.; Caicedo, A. Stemness in Cancer: Stem Cells, Cancer Stem Cells, and Their Microenvironment. Stem. Cells Int. 2017, 2017, 5619472. [Google Scholar] [CrossRef]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2017, 36, 1461–1473. [Google Scholar] [CrossRef]
- Mohammed, M.K.; Shao, C.; Wang, J.; Wei, Q.; Wang, X.; Collier, Z.; Tang, S.; Liu, H.; Zhang, F.; Huang, J.; et al. Wnt/beta-catenin signaling plays an ever-expanding role in stem cell self-renewal, tumorigenesis and cancer chemoresistance. Genes Dis. 2016, 3, 11–40. [Google Scholar] [CrossRef]
- Castagnoli, L.; De Santis, F.; Volpari, T.; Vernieri, C.; Tagliabue, E.; Di Nicola, M.; Pupa, S.M. Cancer Stem Cells: Devil or Savior-Looking behind the Scenes of Immunotherapy Failure. Cells 2020, 9, 555. [Google Scholar] [CrossRef]
- Martin-Orozco, E.; Sanchez-Fernandez, A.; Ortiz-Parra, I.; yala-San, N.M. WNT signaling in tumors: The way to evade drugs and immunity. Front. Immunol. 2019, 10, 2854. [Google Scholar] [CrossRef]
- Hsu, J.M.; Xia, W.; Hsu, Y.H.; Chan, L.C.; Yu, W.H.; Cha, J.H.; Chen, C.T.; Liao, H.W.; Kuo, C.W.; Khoo, K.H.; et al. STT3-dependent PD-L1 accumulation on cancer stem cells promotes immune evasion. Nat. Commun. 2018, 9, 1908. [Google Scholar] [CrossRef] [PubMed]
- Castagnoli, L.; Cancila, V.; Romero-Cordoba, S.L.; Faraci, S.; Talarico, G.; Belmonte, B.; Iorio, M.V.; Volpari, T.; Chiodoni, C.; Hidalgo-Miranda, A.; et al. WNT signaling modulates PD-L1 expression in the stem cell compartment of triple-negative breast cancer. Oncogene 2019, 38, 4047–4060. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Deida, Y.; Alula, K.M.; Theiss, A.L. The influence of mitochondrial-directed regulation of Wnt signaling on tumorigenesis. Gastroenterol. Rep. 2020, 8, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Feng, Z.; He, M.L. Lipid metabolism alteration contributes to and maintains the properties of cancer stem cells. Theranostics 2020, 10, 7053–7069. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Sun, X.L.; Wang, Y.L.; Miao, Y.L. Agrin promotes the proliferation, invasion and migration of rectal cancer cells via the WNT signaling pathway to contribute to rectal cancer progression. J. Recept. Signal Transduct. Res. 2020, 1–8. [Google Scholar] [CrossRef]
- Duchartre, Y.; Kim, Y.M.; Kahn, M. The Wnt signaling pathway in cancer. Crit. Rev. Oncol. Hematol. 2016, 99, 141–149. [Google Scholar] [CrossRef]
- Niehrs, C. The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell Biol. 2012, 13, 767–779. [Google Scholar] [CrossRef]
- Koval, A.; Katanaev, V.L. Dramatic dysbalancing of the Wnt pathway in breast cancers. Sci. Rep. 2018, 8, 7329. [Google Scholar] [CrossRef]
- Hagen, T.; Di, D.E.; Culbert, A.A.; Reith, A.D. Expression and characterization of GSK-3 mutants and their effect on beta-catenin phosphorylation in intact cells. J. Biol. Chem. 2002, 277, 23330–23335. [Google Scholar] [CrossRef]
- Stamos, J.L.; Weis, W.I. The b-catenin destruction complex. Cold Spring Harb. Perspect. Biol. 2013, 5, a007898. [Google Scholar] [CrossRef]
- Metcalfe, C.; Mendoza-Topaz, C.; Mieszczanek, J.; Bienz, M. Stability elements in the LRP6 cytoplasmic tail confer efficient signalling upon DIX-dependent polymerization. J. Cell Sci. 2010, 123, 1588–1599. [Google Scholar] [CrossRef] [PubMed]
- Lien, W.H.; Fuchs, E. Wnt some lose some: Transcriptional governance of stem cells by Wnt/b-catenin signaling. Genes Dev. 2014, 28, 1517–1532. [Google Scholar] [CrossRef]
- Bienz, M.; Clevers, H. Linking colorectal cancer to Wnt signaling. Cell 2000, 103, 311–320. [Google Scholar] [CrossRef]
- Peifer, M.; Polakis, P. Wnt signaling in oncogenesis and embryogenesis-a look outside the nucleus. Science 2000, 287, 1606–1609. [Google Scholar] [CrossRef] [PubMed]
- He, T.C.; Sparks, A.B.; Rago, C.; Hermeking, H.; Zawel, L.; Da Costa, L.T.; Morin, P.J.; Vogelstein, B.; Kinzler, K.W. Identification of c-MYC as a target of the APC pathway. Science 1998, 281, 1509–1512. [Google Scholar] [CrossRef] [PubMed]
- You, Z.; Saims, D.; Chen, S.; Zhang, Z.; Guttridge, D.C.; Guan, K.L.; MacDougald, O.A.; Brown, A.M.; Evan, G.; Kitajewski, J.; et al. Wnt signaling promotes oncogenic transformation by inhibiting c-Myc-induced apoptosis. J. Cell Biol. 2002, 157, 429–440. [Google Scholar] [CrossRef]
- Jang, G.B.; Kim, J.Y.; Cho, S.D.; Park, K.S.; Jung, J.Y.; Lee, H.Y.; Hong, I.S.; Nam, J.S. Blockade of Wnt/beta-catenin signaling suppresses breast cancer metastasis by inhibiting CSC-like phenotype. Sci. Rep. 2015, 5, 12465. [Google Scholar] [CrossRef]
- Bourguignon, L.Y.; Xia, W.; Wong, G. Hyaluronan-mediated CD44 interaction with p300 and SIRT1 regulates beta-catenin signaling and NFkappaB-specific transcription activity leading to MDR1 and Bcl-xL gene expression and chemoresistance in breast tumor cells. J. Biol. Chem. 2009, 284, 2657–2671. [Google Scholar] [CrossRef]
- Zhang, X.H.; Wang, Q.; Gerald, W.; Hudis, C.A.; Norton, L.; Smid, M.; Foekens, J.A.; Massagué, J. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell 2009, 16, 67–78. [Google Scholar] [CrossRef]
- Pate, K.T.; Stringari, C.; Sprowl-Tanio, S.; Wang, K.; TeSlaa, T.; Hoverter, N.P.; McQuade, M.M.; Garner, C.; Digman, M.A.; Teitell, M.A.; et al. Wnt signaling directs a metabolic program of glycolysis and angiogenesis in colon cancer. EMBO J. 2014, 33, 1454–1473. [Google Scholar] [CrossRef]
- Yang, L.; Perez, A.A.; Fujie, S.; Warden, C.; Li, J.; Wang, Y.; Yung, B.; Chen, Y.R.; Liu, X.; Zhang, H.; et al. Wnt modulates MCL1 to control cell survival in triple negative breast cancer. BMC Cancer 2014, 14, 124. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.L.; Chien, A.J.; Moon, R.T. Wnt/Fz signaling and the cytoskeleton: Potential roles in tumorigenesis. Cell Res. 2009, 19, 532–545. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Nishimura, O.; Misaki, K.; Nishita, M.; Minami, Y.; Yonemura, S.; Tarui, H.; Sasaki, H. Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Dev. Cell 2008, 15, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Chen, Y.G. Dishevelled: The hub of Wnt signaling. Cell. Signal. 2010, 22, 717–727. [Google Scholar] [CrossRef]
- Sheldahl, L.C.; Slusarski, D.C.; Pandur, P.; Miller, J.R.; Kuhl, M.; Moon, R.T. Dishevelled activates Ca2+ flux, PKC, and CamKII in vertebrate embryos. J. Cell Biol. 2003, 161, 769–777. [Google Scholar] [CrossRef]
- Jin, Z.; Tamura, G.; Tsuchiya, T.; Sakata, K.; Kashiwaba, M.; Osakabe, M.; Motoyama, T. Adenomatous polyposis coli (APC) gene promoter hypermethylation in primary breast cancers. Br. J. Cancer 2001, 85, 69–73. [Google Scholar] [CrossRef]
- Van der Auwera, I.; van Laere, S.J.; Van den Bosch, S.M.; Van den Eynden, G.G.; Trinh, B.X.; Van Dam, P.A.; Colpaert, C.G.; van Engeland, M.; van Marck, E.A.; Vermeulen, P.B.; et al. Aberrant methylation of the Adenomatous Polyposis Coli (APC) gene promoter is associated with the inflammatory breast cancer phenotype. Br. J. Cancer 2008, 99, 1735–1742. [Google Scholar] [CrossRef]
- Isobe, T.; Hisamori, S.; Hogan, D.J.; Zabala, M.; Hendrickson, D.G.; Dalerba, P.; Cai, S.; Scheeren, F.; Kuo, A.H.; Sikandar, S.S.; et al. miR-142 regulates the tumorigenicity of human breast cancer stem cells through the canonical WNT signaling pathway. Elife 2014, 3, e01977. [Google Scholar] [CrossRef]
- He, Y.; Liu, Z.; Qiao, C.; Xu, M.; Yu, J.; Li, G. Expression and significance of Wnt signaling components and their target genes in breast carcinoma. Mol. Med. Rep. 2014, 9, 137–143. [Google Scholar] [CrossRef]
- Milovanovic, T.; Planutis, K.; Nguyen, A.; Marsh, J.L.; Lin, F.; Hope, C.; Holcombe, R.F. Expression of Wnt genes and frizzled 1 and 2 receptors in normal breast epithelium and infiltrating breast carcinoma. Int. J. Oncol. 2004, 25, 1337–1342. [Google Scholar] [CrossRef]
- Lazzaroni, F.; Del Giacco, L.; Biasci, D.; Turrini, M.; Prosperi, L.; Brusamolino, R.; Cairoli, R.; Beghini, A. Intronless WNT10B-short variant underlies new recurrent allele-specific rearrangement in acute myeloid leukaemia. Sci. Rep. 2016, 6, 37201. [Google Scholar] [CrossRef] [PubMed]
- Corda, G.; Sala, G.; Lattanzio, R.; Iezzi, M.; Sallese, M.; Fragassi, G.; Lamolinara, A.; Mirza, H.; Barcaroli, D.; Ermler, S.; et al. Functional and prognostic significance of the genomic amplification of frizzled 6 (FZD6) in breast cancer. J. Pathol. 2017, 241, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Zhang, Y.; He, Y.; Zhang, K.; Wan, G.; Huang, Y.; Zhou, Z.; Huang, G.; Wang, J. A novel recombinant human Frizzled-7 protein exhibits anti-tumor activity against triple negative breast cancer via abating Wnt/b-catenin pathway. Int. J. Biochem. Cell Biol. 2018, 103, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Prior, J.; Piwnica-Worms, D.; Bu, G. LRP6 overexpression defines a class of breast cancer subtype and is a target for therapy. Proc. Natl. Acad. Sci. USA 2010, 107, 5136–5141. [Google Scholar] [CrossRef]
- Wang, H.; He, L.; Ma, F.; Regan, M.M.; Balk, S.P.; Richardson, A.L.; Yuan, X. SOX9 regulates low density lipoprotein receptor-related protein 6 (LRP6) and T-cell factor 4 (TCF4) expression and Wnt/b-catenin activation in breast cancer. J. Biol. Chem. 2013, 288, 6478–6487. [Google Scholar] [CrossRef]
- Veeck, J.; Wild, P.J.; Fuchs, T.; Schuffler, P.J.; Hartmann, A.; Knuchel, R.; Dahl, E. Prognostic relevance of Wnt-inhibitory factor-1 (WIF1) and Dickkopf-3 (DKK3) promoter methylation in human breast cancer. BMC Cancer 2009, 9, 217–219. [Google Scholar] [CrossRef]
- Lorsy, E.; Topuz, A.S.; Geisler, C.; Stahl, S.; Garczyk, S.; von Stillfried, S.; Hoss, M.; Gluz, O.; Hartmann, A.; Knuchel, R.; et al. Loss of dickkopf 3 promotes the tumorigenesis of basal Breast Cancer. PLoS ONE 2016, 11, e0160077. [Google Scholar] [CrossRef]
- Xu, J.; Prosperi, J.R.; Choudhury, N.; Olopade, O.I.; Goss, K.H. beta-Catenin is required for the tumorigenic behavior of triple-negative breast cancer cells. PLoS ONE 2015, 10, e0117097. [Google Scholar]
- Wang, Z.; Zhang, H.; Hou, J.; Niu, J.; Ma, Z.; Zhao, H.; Liu, C. Clinical implications of β-catenin protein expression in breast cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 14989–14994. [Google Scholar]
- Zhang, D.; Fei, F.; Li, S.; Zhao, Y.; Yang, Z.; Qu, J.; Zhang, X.; Yin, Y.; Zhang, S. The role of b-catenin in the initiation and metastasis of TA2 mice spontaneous breast cancer. J. Cancer 2017, 8, 2114–2123. [Google Scholar] [CrossRef][Green Version]
- Bai, J.; Mei, P.; Zhang, C.; Chen, F.; Li, C.; Pan, Z.; Liu, H.; Zheng, J. BRG1 is a prognostic marker and potential therapeutic target in human breast cancer. PLoS ONE 2013, 8, e59772. [Google Scholar] [CrossRef] [PubMed]
- Elsarraj, H.S.; Hong, Y.; Valdez, K.E.; Michaels, W.; Hook, M.; Smith, W.P.; Chien, J.; Herschkowitz, J.I.; Troester, M.A.; Beck, M.; et al. Expression profiling of in vivo ductal carcinoma in situ progression models identified B cell lymphoma-9 as a molecular driver of breast cancer invasion. Breast Cancer Res. 2015, 17, 128. [Google Scholar] [CrossRef] [PubMed]
- Andrews, P.G.; Lake, B.B.; Popadiuk, C.; Kao, K.R. Requirement of Pygopus 2 in breast cancer. Int. J. Oncol. 2007, 30, 357–363. [Google Scholar] [PubMed]
- Brown, A.M. Wnt signaling in breast cancer: Have we come full circle? Breast Cancer Res. 2001, 3, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Nusse, R.; Varmus, H.E. Wnt genes. Cell 1992, 69, 1073–1087. [Google Scholar] [CrossRef]
- Ayyanan, A.; Civenni, G.; Ciarloni, L.; Morel, C.; Mueller, N.; Lefort, K.; Mandinova, A.; Raffoul, W.; Fiche, M.; Dotto, G.P.; et al. Increased Wnt signaling triggers oncogenic conversion of human breast epithelial cells by a Notch-dependent mechanism. Proc. Natl. Acad. Sci. USA 2006, 103, 3799–3804. [Google Scholar] [CrossRef]
- Wu, Y.; Ginther, C.; Kim, J.; Mosher, N.; Chung, S.; Slamon, D.; Vadgama, J.V. Expression of Wnt3 activates Wnt/b-catenin pathway and promotes EMT-like phenotype in trastuzumab-resistant HER2-overexpressing breast cancer cells. Mol. Cancer Res. 2012, 10, 1597–1606. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kadoya, T.; Amioka, A.; Hanaki, H.; Sasada, S.; Masumoto, N.; Yamamoto, H.; Arihiro, K.; Kikuchi, A.; Okada, M. Wnt5a-induced cell migration is associated with the aggressiveness of estrogen receptor-positive breast cancer. Oncotarget 2018, 9, 20979–20992. [Google Scholar] [CrossRef]
- Leris, A.C.; Roberts, T.R.; Jiang, W.G.; Newbold, R.F.; Mokbel, K. WNT5A expression in human breast cancer. Anticancer Res. 2005, 25, 731–734. [Google Scholar]
- Jiang, S.; Zhang, M.; Zhang, Y.; Zhou, W.; Zhu, T.; Ruan, Q.; Chen, H.; Fang, J.; Zhou, F.; Sun, J.; et al. WNT5B governs the phenotype of basal-like breast cancer by activating WNT signaling. Cell Commun. Signal. 2019, 17, 109. [Google Scholar] [CrossRef]
- Chen, J.; Liu, T.Y.; Peng, H.T.; Wu, Y.Q.; Zhang, L.L.; Lin, X.H.; Lai, Y.H. Up-regulation of Wnt7b rather than Wnt1, Wnt7a, and Wnt9a indicates poor prognosis in breast cancer. Int. J. Clin. Exp. Pathol. 2018, 11, 4552–4561. [Google Scholar]
- Yi, K.; Min, K.W.; Wi, Y.C.; Kim, Y.; Shin, S.J.; Chung, M.S.; Jang, K.; Paik, S.S. Wnt7a deficiency could predict worse disease-free and overall survival in estrogen receptor-positive Breast Cancer. J. Breast Cancer 2017, 20, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Avgustinova, A.; Iravani, M.; Robertson, D.; Fearns, A.; Gao, Q.; Klingbeil, P.; Hanby, A.M.; Speirs, V.; Sahai, E.; Calvo, F.; et al. Tumour cell-derived Wnt7a recruits and activates fibroblasts to promote tumour aggressiveness. Nat. Commun. 2016, 7, 10305. [Google Scholar] [CrossRef] [PubMed]
- Benhaj, K.; Akcali, K.C.; Ozturk, M. Redundant expression of canonical Wnt ligands in human breast cancer cell lines. Oncol. Rep. 2006, 15, 701–707. [Google Scholar] [CrossRef] [PubMed]
- El Ayachi, I.; Fatima, I.; Wend, P.; va-Ornelas, J.A.; Runke, S.; Kuenzinger, W.L.; Silva, J.; Silva, W.; Gray, J.K.; Lehr, S.; et al. The WNT10B Network Is Associated with Survival and Metastases in Chemoresistant Triple-Negative Breast Cancer. Cancer Res. 2019, 79, 982–993. [Google Scholar] [CrossRef]
- Wu, Y.; Sarkissyan, M.; Vadgama, J.V. Epithelial-Mesenchymal Transition and Breast Cancer. J. Clin. Med. 2016, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Jin, S.; Cho, H.; Won, H.Y.; An, H.W.; Jeong, G.Y.; Park, Y.U.; Kim, H.Y.; Park, M.K.; Son, T.; et al. CDK12 drives breast tumor initiation and trastuzumab resistance via WNT and IRS1-ErbB-PI3K signaling. EMBO Rep. 2019, 20, e48058. [Google Scholar] [CrossRef]
- Wang, H. Lapatinib for the treatment of breast cancer in the People’s Republic of China. Onco Targets Ther. 2014, 7, 1367–1373. [Google Scholar] [CrossRef][Green Version]
- Won, H.S.; Lee, K.M.; Oh, J.E.; Nam, E.M.; Lee, K.E. Inhibition of b-Catenin to overcome endocrine resistance in Tamoxifen-resistant Breast Cancer Cell Line. PLoS ONE 2016, 11, e0155983. [Google Scholar] [CrossRef]
- Loh, Y.N.; Hedditch, E.L.; Baker, L.A.; Jary, E.; Ward, R.L.; Ford, C.E. The Wnt signalling pathway is upregulated in an in vitro model of acquired tamoxifen resistant breast cancer. BMC Cancer 2013, 13, 174. [Google Scholar] [CrossRef]
- Piva, M.; Domenici, G.; Iriondo, O.; Rábano, E.; Simoes, B.M.; Comaills, V.; Barredo, I.; López-Ruiz, J.A.; Zabalza, I.; Kypta, R.; et al. Sox2 promotes tamoxifen resistance in breast cancer cells. EMBO Mol. Med. 2014, 6, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xu, C.; Xiao, G.; Meng, J.; Wang, J.; Tang, S.C.; Qin, S.; Du, N.; Li, G.; Ren, H.; et al. Breast cancer stem-like cells are sensitized to tamoxifen induction of self-renewal inhibition with enforced Let-7c dependent on Wnt blocking. Int. J. Mol. Med. 2018, 41, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Huang, Y.; Lou, C.; He, Y.; Zhang, Y.; Zhang, Q. FSTL1 enhances chemoresistance and maintains stemness in breast cancer cells via integrin b3/Wnt signaling under miR-137 regulation. Cancer Biol. Ther. 2019, 20, 328–337. [Google Scholar] [CrossRef]
- Al-Dhfyan, A.; Alhoshani, A.; Korashy, H.M. Aryl hydrocarbon receptor/cytochrome P450Â1A1 pathway mediates breast cancer stem cells expansion through PTEN inhibition and b-Catenin and Akt activation. Mol. Cancer 2017, 16, 671–675. [Google Scholar]
- Lathia, J.D.; Liu, H. Overview of Cancer Stem Cells and Stemness for Community Oncologists. Target. Oncol. 2017, 12, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pan, S.; Hsieh, M.H.; Ng, N.; Sun, F.; Wang, T.; Kasibhatla, S.; Schuller, A.G.; Li, A.G.; Cheng, D.; et al. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc. Natl. Acad. Sci. USA 2013, 110, 20224–20229. [Google Scholar] [CrossRef]
- Safholm, A.; Tuomela, J.; Rosenkvist, J.; Dejmek, J.; Harkonen, P.; Andersson, T. The Wnt-5a-derived hexapeptide Foxy-5 inhibits breast cancer metastasis in vivo by targeting cell motility. Clin. Cancer Res. 2008, 14, 6556–6563. [Google Scholar] [CrossRef]
- Gurney, A.; Axelrod, F.; Bond, C.J.; Cain, J.; Chartier, C.; Donigan, L.; Fischer, M.; Chaudhari, A.; Ji, M.; Kapoun, A.M.; et al. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 11717–11722. [Google Scholar] [CrossRef]
- Mita, M.M.; Becerra, C.; Richards, D.A.; Mita, A.C.; Shagisultanova, E.; Osborne, C.R.; O’Shaughnessy, J.; Zhang, C.; Henner, R.; Kapoun, A.M.; et al. Phase 1b study of WNT inhibitor vantictumab (VAN, human monoclonal antibody) with paclitaxel (P) in patients (pts) with 1st- to 3rd-line metastatic HER2-negative breast cancer (BC). J. Clin. Oncol. 2020, 34, 2516. [Google Scholar] [CrossRef]
- Mukherjee, N.; Panda, C.K. Wnt/b-catenin signaling pathway as chemotherapeutic target in Breast Cancer: An update on pros and cons. Clin. Breast Cancer 2020, 20, 361–370. [Google Scholar] [CrossRef]
- Behari, J.; Zeng, G.; Otruba, W.; Thompson, M.D.; Muller, P.; Micsenyi, A.; Sekhon, S.S.; Leoni, L.; Monga, S.P. R-Etodolac decreases beta-catenin levels along with survival and proliferation of hepatoma cells. J. Hepatol. 2007, 46, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.J.; Pei, L.B.; Zhuang, J.P.; Ji, Y.; Xu, G.P.; Zhang, Z.P.; Li, N.; Yan, J.L. Celecoxib inhibits b-catenin-dependent survival of the human osteosarcoma MG-63 cell line. J. Int. Med. Res. 2010, 38, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Coombes, R.C.; Tovey, H.; Kilburn, L.; Mansi, J.; Palmieri, C.; Bartlett, J.; Hicks, J.; Makris, A.; Evans, A.; Loibl, S.; et al. A phase III multicentre double blind randomised trial of celecoxib versus placebo in primary breast cancer patients (REACT–Randomised EuropeAn celecoxib trial). In Proceedings of the 2017 San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 5–9 December 2017. Cancer Research 78 [4 Supplement], 2020. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castagnoli, L.; Tagliabue, E.; Pupa, S.M. Inhibition of the Wnt Signalling Pathway: An Avenue to Control Breast Cancer Aggressiveness. Int. J. Mol. Sci. 2020, 21, 9069. https://doi.org/10.3390/ijms21239069
Castagnoli L, Tagliabue E, Pupa SM. Inhibition of the Wnt Signalling Pathway: An Avenue to Control Breast Cancer Aggressiveness. International Journal of Molecular Sciences. 2020; 21(23):9069. https://doi.org/10.3390/ijms21239069
Chicago/Turabian StyleCastagnoli, Lorenzo, Elda Tagliabue, and Serenella M. Pupa. 2020. "Inhibition of the Wnt Signalling Pathway: An Avenue to Control Breast Cancer Aggressiveness" International Journal of Molecular Sciences 21, no. 23: 9069. https://doi.org/10.3390/ijms21239069
APA StyleCastagnoli, L., Tagliabue, E., & Pupa, S. M. (2020). Inhibition of the Wnt Signalling Pathway: An Avenue to Control Breast Cancer Aggressiveness. International Journal of Molecular Sciences, 21(23), 9069. https://doi.org/10.3390/ijms21239069






