Olive Leaf Polyphenols (OLPs) Stimulate GLUT4 Expression and Translocation in the Skeletal Muscle of Diabetic Rats
Abstract
1. Introduction
2. Results
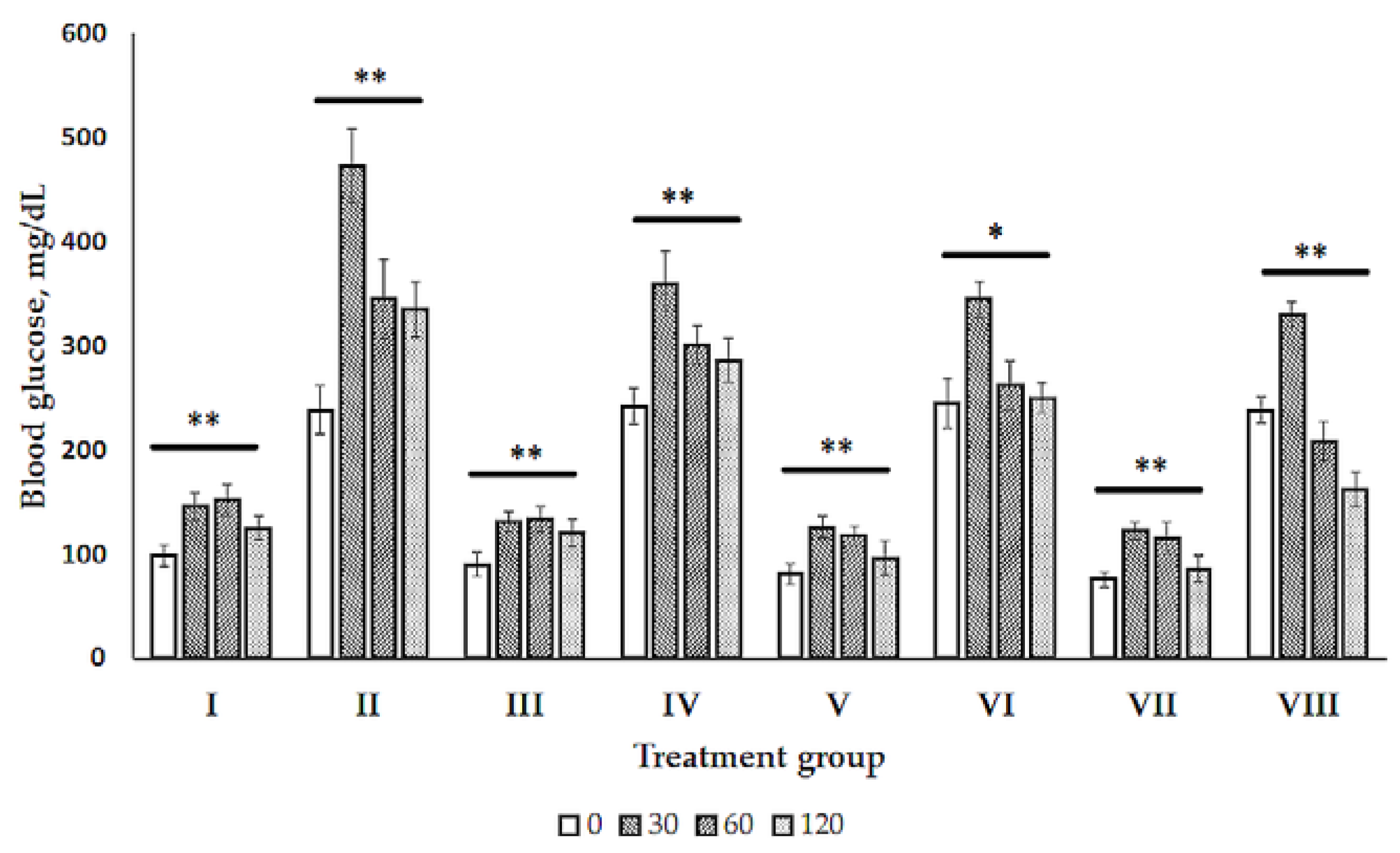
2.1. Glucose Tolerance Test (GTT)
2.2. Blood Biochemistry
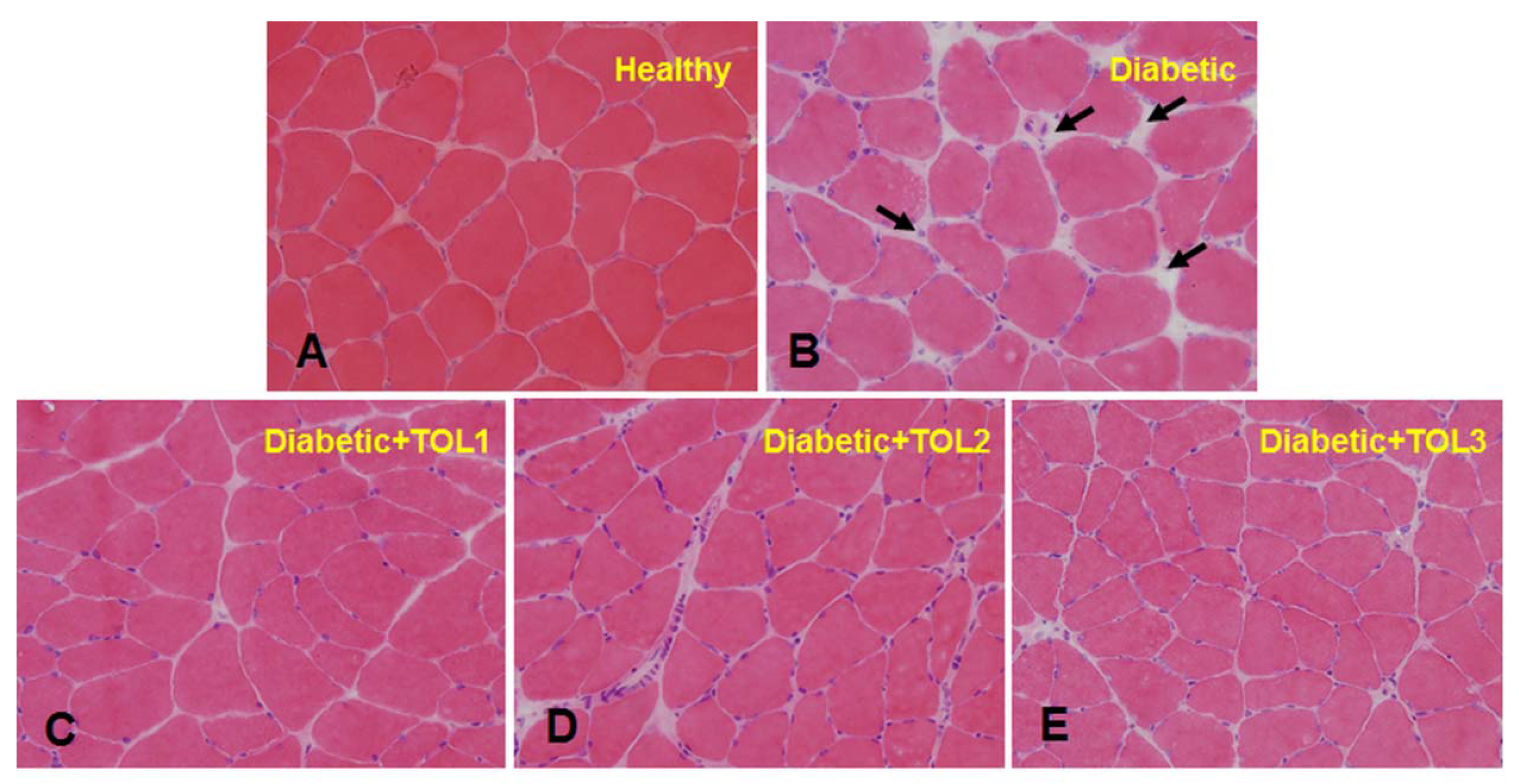
2.3. Morphological Analysis
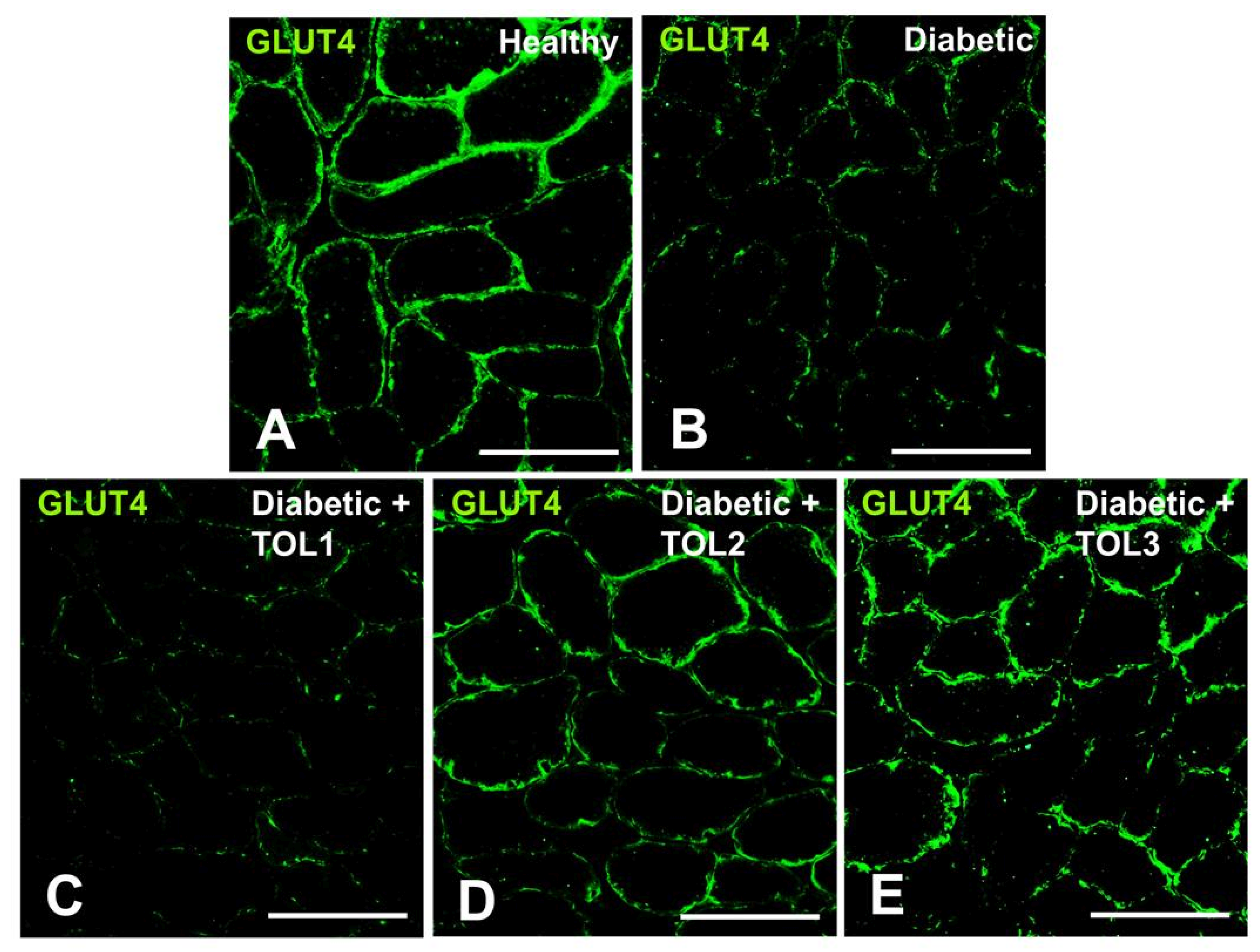
2.4. GLUT4 Expression on Muscle Fiber Membrane
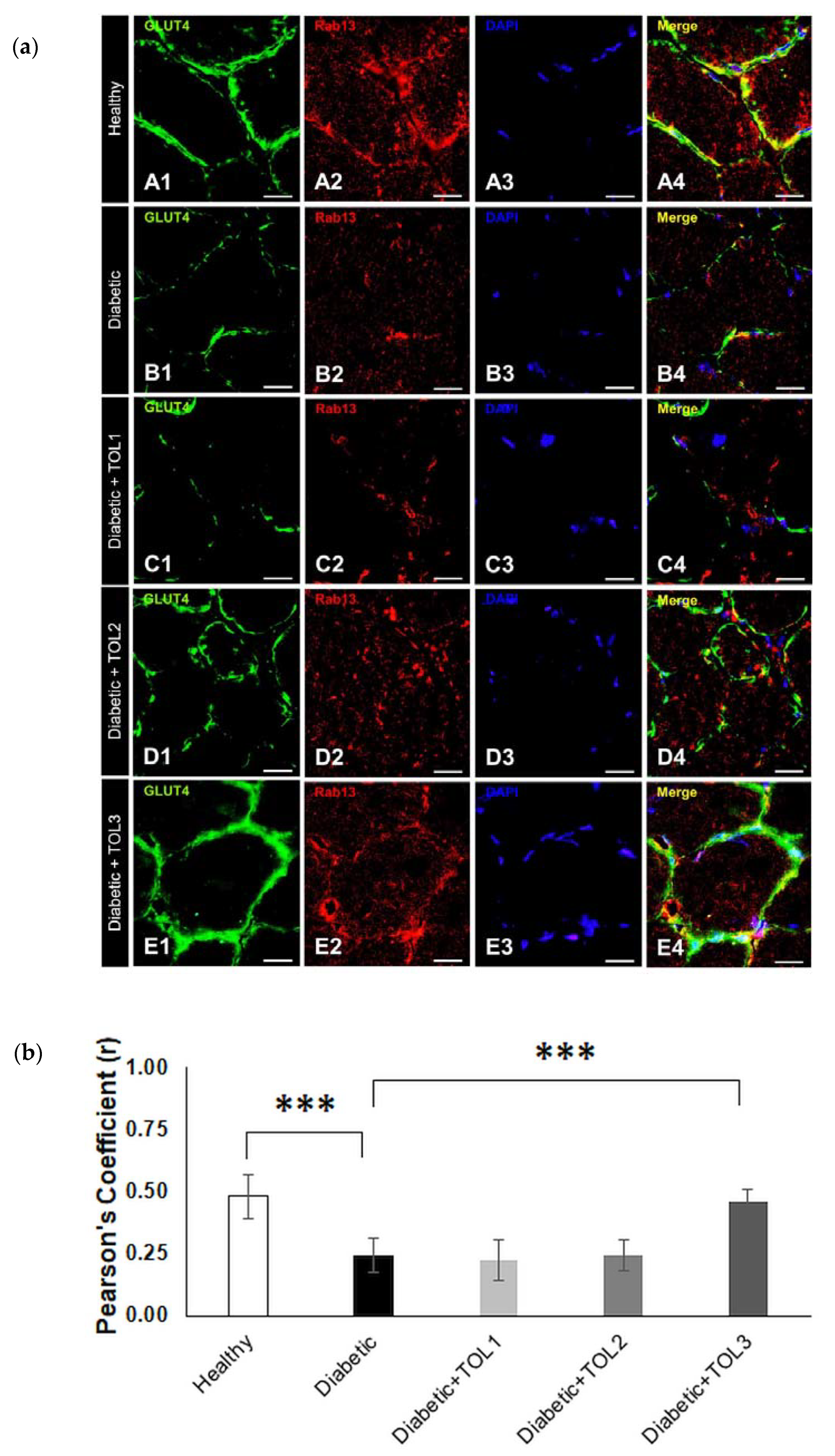
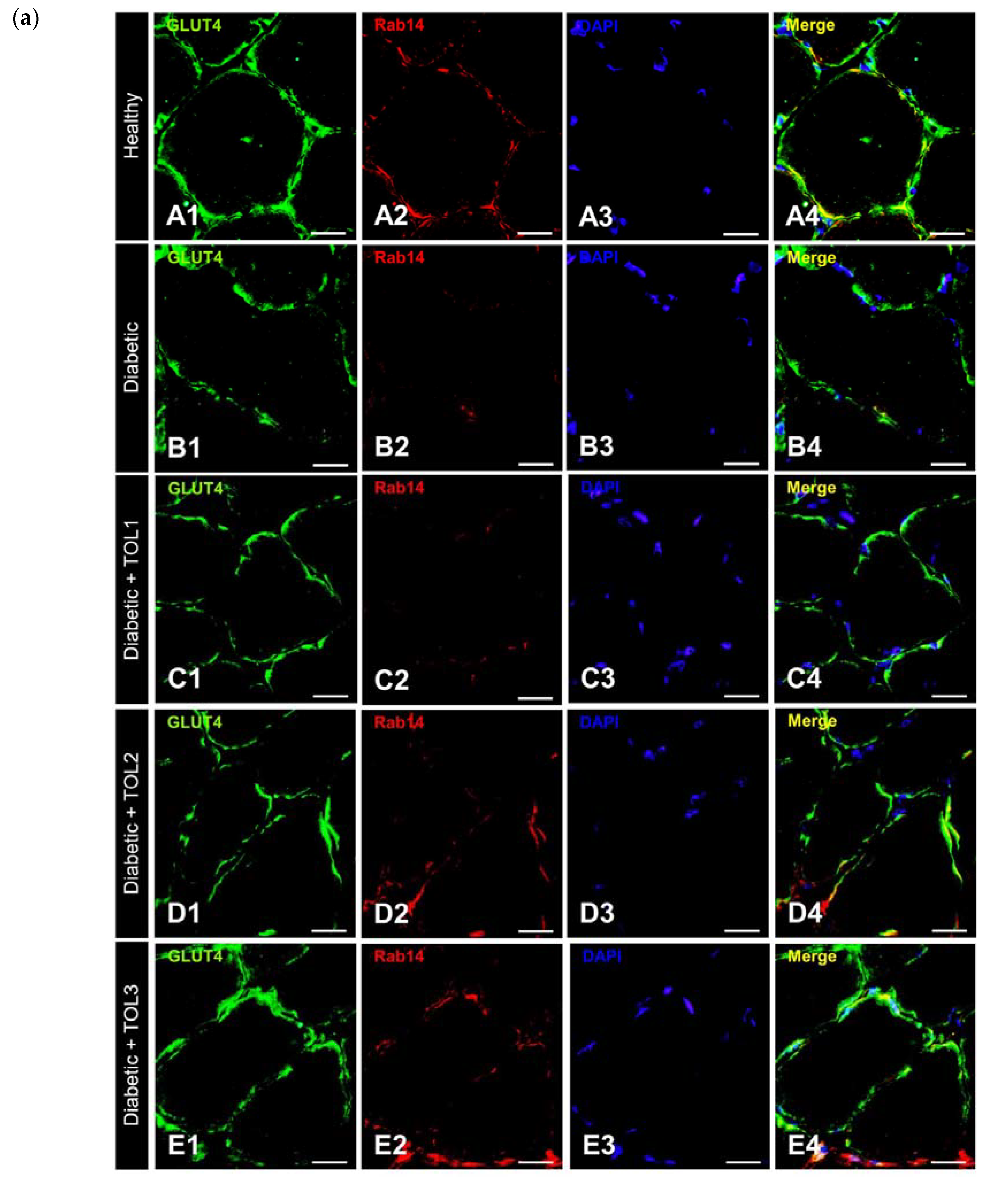
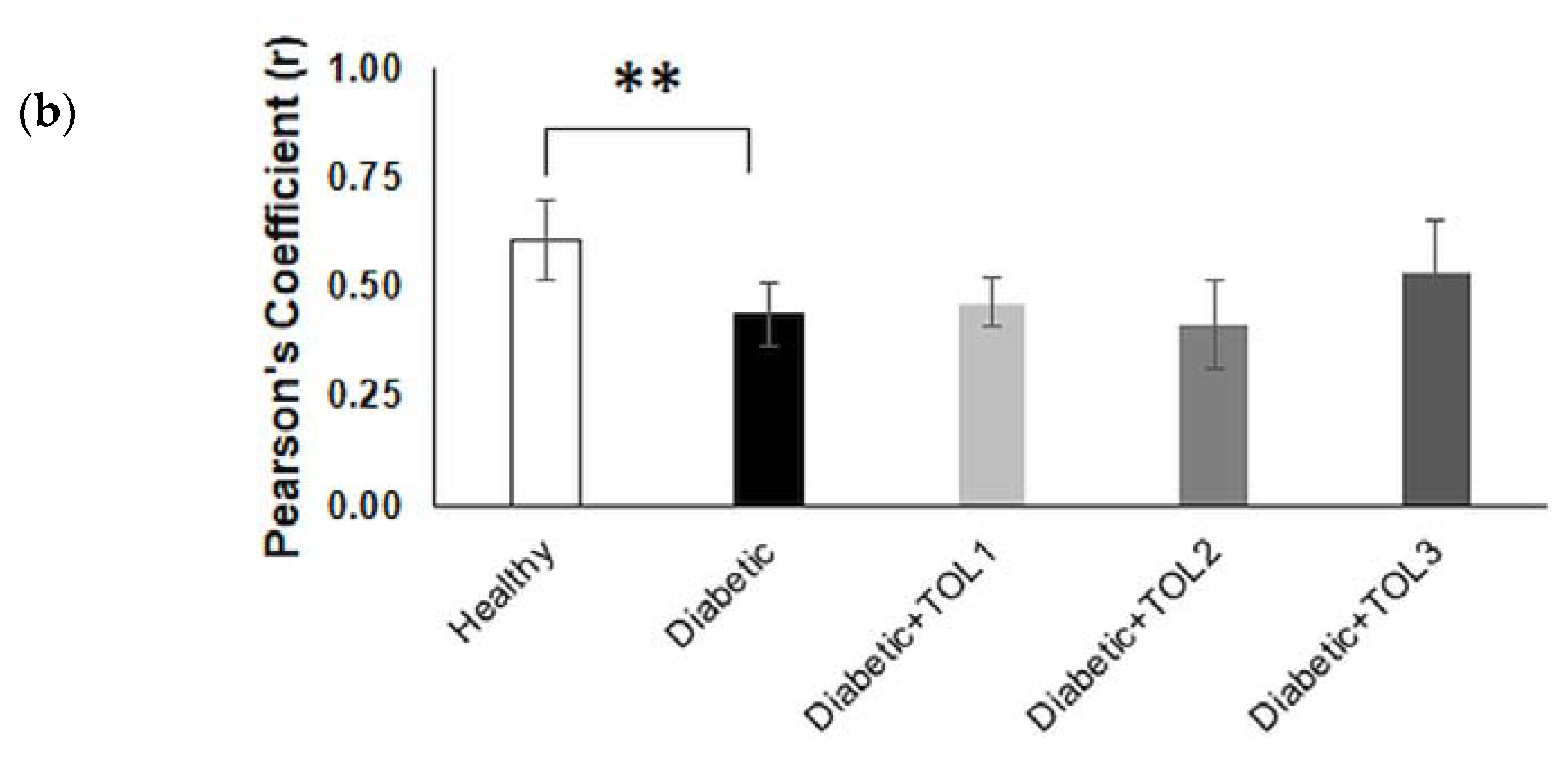
2.5. GLUT4/Rab8A, GLUT4/Rab13, and GLUT4/Rab14 Colocalizations on Muscle Fiber Membrane
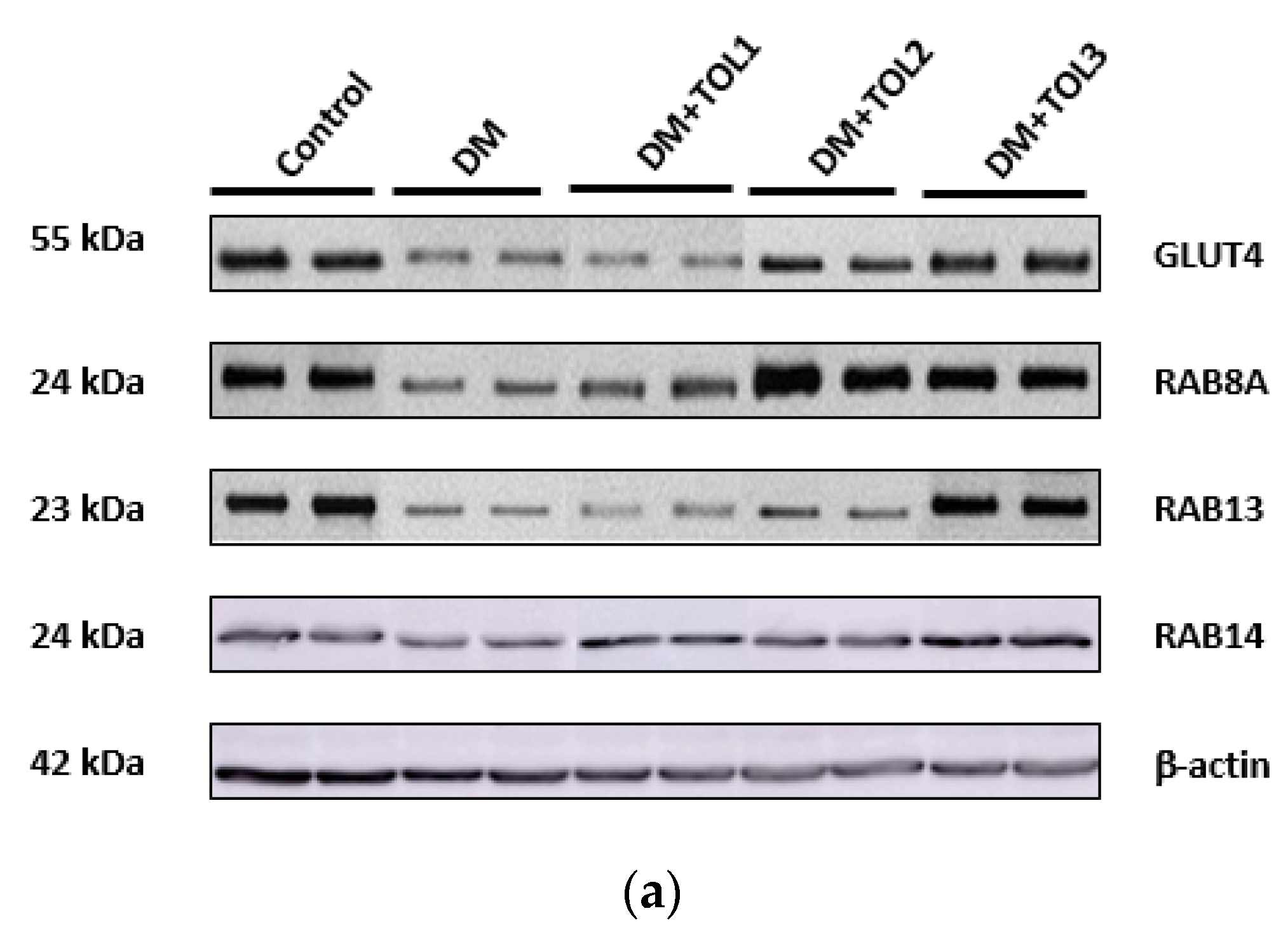
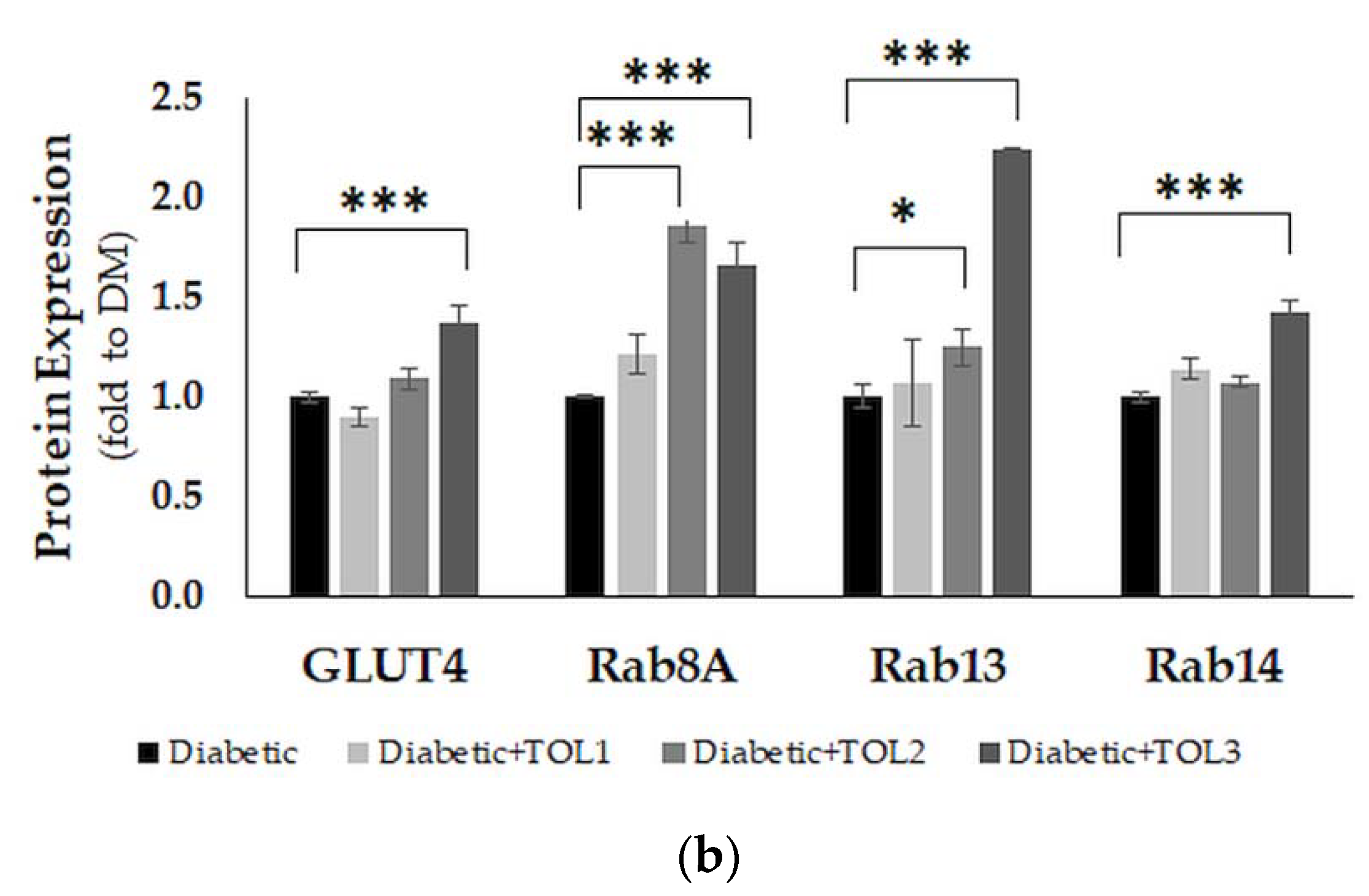
2.6. GLUT4, Rab8A, Rab13, and Rab14 Expression in Rat Soleus
3. Discussion
3.1. Olive Leaf Polyphenols Reduced Hyperglycemia and Hyperlipidemia
3.2. Histochemical Analysis of Soleus Muscle in Diabetic Rat after Olive Leaf Polyphenols (OLPs) Treatments
3.3. Rab8A, Rab13, and Rab14 in Regulation of GLUT4 Translocation in Rat Skeletal Muscle with Diabetes and Following OLP Therapy
4. Material and Methods
4.1. Materials
4.2. Phenolic Extraction and Analysis
4.2.1. Preparation of the Olea Europaea Leaf Extract (OLE)
4.2.2. UHPLC-DAD Analysis of Oleuropein in the OLE
4.3. Experimental Protocols
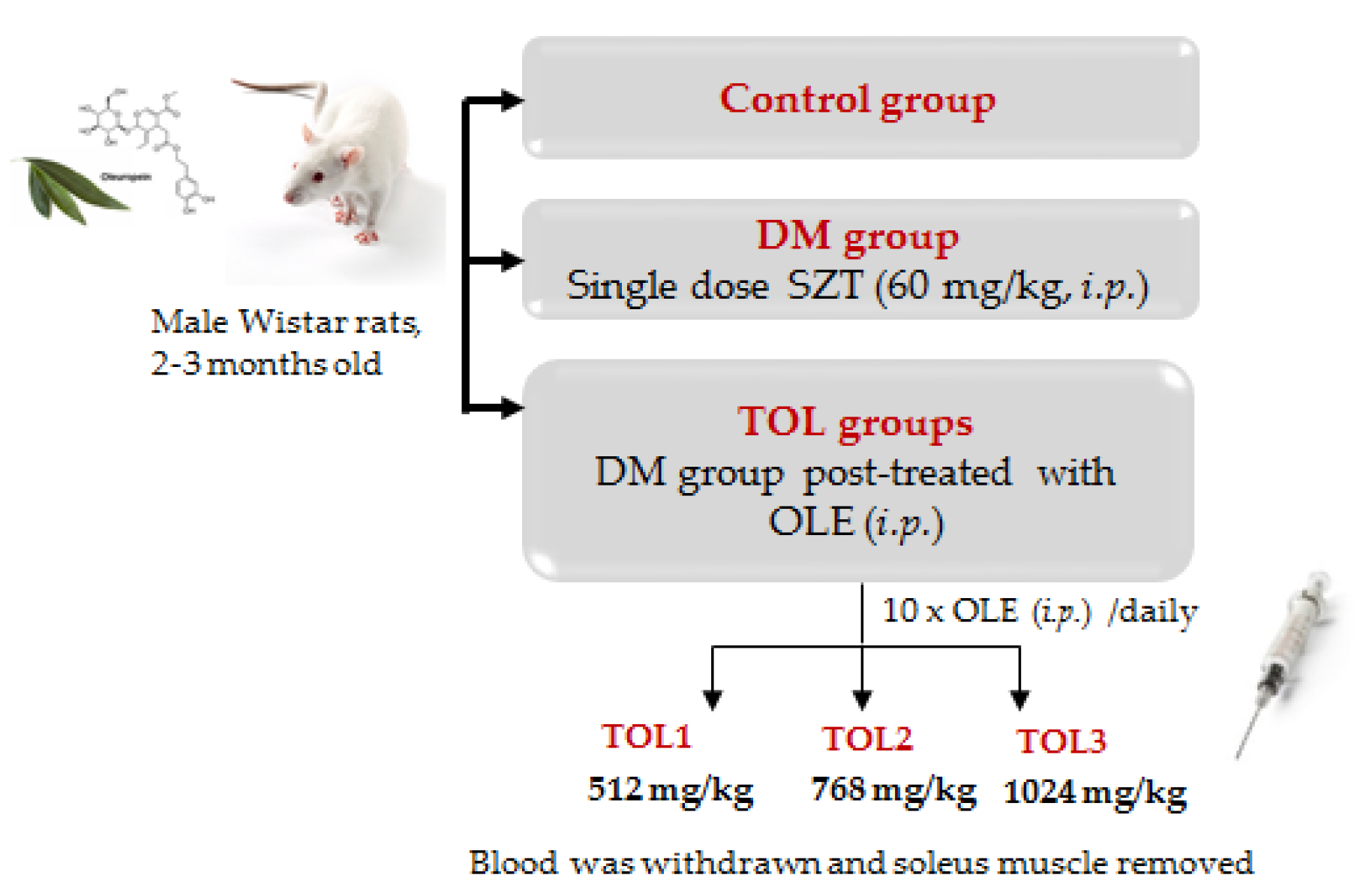
4.3.1. Animals Experimental Design and Treatments
4.3.2. Glucose Tolerance Test (GTT)
4.3.3. Blood Biochemistry
4.3.4. Tissue Muscle Homogenization
4.3.5. H&E and Immunofluorescence Staining
4.3.6. SDS-PAGE and Western Blot
4.3.7. Statistical Analysis
5. Conclusions and Future Research
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | Acetyl-CoA carboxylase |
| AMPK | 5’ adenosine monophosphate-activated protein kinase |
| AS160 (or TBC1D4) | 160 kDa substrate of the Akt Ser/Thr kinase |
| C/EBPα | CCAAT/enhancer-binding protein alpha is a transcription factor |
| CPT-1 | Carnitine O-palmitoyltransferase 1 |
| DM | Diabetes mellitus |
| ERK1/2 | Extracellular signal-regulated kinases 1 and 2 |
| FASN | Fatty acid synthase gene |
| GGT | Glucose tolerance test |
| GLUT1 | Glucose transporter 1 |
| GLUT4 | Glucose transporter 4 |
| GSV | Intracellular storage vesicles |
| GTPases | Guanosine triphosphate (GTP) ases |
| HbA1c | Glycated hemoglobin A 1c |
| HDL-C | High-density lipoprotein cholesterol |
| IL-10 | Interleukin-10 |
| IL-6 | Interleukin-6 |
| IR | Insulin receptor |
| iNOS | Nitric oxide synthase, inducible |
| IRS-1 | Insulin receptor substrate 1 |
| JAK/STAT | Janus kinases/signal transducer and activator of transcription proteins |
| LDL-C | Low-density lipoprotein cholesterol |
| MAPK3/1 | Mitogen-Activated Protein Kinase 3 and 1 |
| MCAD | Medium-chain specific acyl-CoA dehydrogenase |
| MCP-1 | Monocyte chemoattractant protein 1 |
| MyoVa | Myosin V heavy-chain gene (a class of actin-based motor proteins) |
| NEFA | Non-esterified fatty acids |
| NF-κB | Nuclear factor NF-kappa-B |
| OLE | Olive leaf extract |
| p-ACC | Phospho-Acetyl-CoA carboxylase |
| p-Akt | Phosphorylated version of AKT |
| p-AMPK | Phospho-AMPK |
| PARP | Poly (ADP-ribose) polymerase |
| PEPCK | Phosphoenolpyruvate carboxykinase |
| p-ERα | Phosphorylated estrogen receptor alpha |
| PGC-1α | Peroxisome proliferator-activated receptor gamma co-activator 1 alpha |
| p-GSK-3β | Phosphorylated glycogen synthase kinase-3 beta |
| p-IR | Phosphorylated insulin receptor |
| PI3K/AKT | Phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) signaling pathway |
| p-JNK | Phospho-JNK |
| PKC λ/ξ | Protein kinase C λ/ξ |
| PLC-PKC | Phospholipase C-protein kinase C |
| PM | Plasma membrane |
| p-mTOR | Phosphorylated mammalian target of rapamycin (mTOR) |
| p-p38 MAPK | Phospho-p38 MAPK |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| PPARα | Peroxisome proliferator-activated receptor alpha |
| p-PKC | Phosphorylated protein kinase C |
| Rab13 | Ras-related protein Rab-13 |
| Rab14 | Ras-related protein Rab-14 |
| Rab8A | Ras-related protein Rab-8A |
| Rac 1 | Ras-related C3 botulinum toxin substrate 1 |
| SREBP-1c | Sterol regulatory element-binding protein-1c |
| SZT | Streptozotocin |
| TNFα | Tumor necrosis factor alpha |
| TOL1 | Treatment with 512 mg/kg OLE |
| TOL2 | Treatment with 768 mg/kg OLE |
| TOL3 | Treatment with 1024 mg/kg OLE |
Appendix A
| Compound | Model | Protein Expression | Glucose Uptake (GU)/ Blood Glucose (BG)/ Insulin Sensitivity (IS) | Additional Findings | Treatment | Reference |
|---|---|---|---|---|---|---|
| Chlorogenic acid | L6 myotubes | GLUT4 | GU | ↑ PPARγ protein | 25 μM for 5 h: cytotoxicity > (50 μM) | [63] |
| Cinnamon and extracts | C2C12 myotubes | p-AMPK | GU | 100 or 1000 μg/mL for 3 h | [64] | |
| C2C12 myotubes | 30 μg/mL for 4 h | [65] | ||||
| Wistar rats (WR) (gastrocnemius muscle) | GLUT4 | ↑ NEFA (serum), ↓ serum creatine ↑ GLUT4 | 30 mg/kg/day for 22 days | [66] | ||
| C57BL/6J mice | BG | ↓ NEFA (serum), ↓ LDL-C, ↓ insulin | Overnight fast. 400 mg/kg/day for 21 days | [67] | ||
| db/db mice | BG | ↓ NEFA (serum) ↓ fasting blood glucose level | 6 h fast. 400 mg/kg/day for 14 days | |||
| C57BL/6J mice | BG | ↑ IRS-1, IR protein | Overnight fast. 150 mg/kg/day for 14 days | [68] | ||
| C57BLKS/J db/db mice | BG | ↑ HDL-C levels (serum) ↓ p-Akt, Upregulated mRNA GLUT4 ↑ p-Akt | 20 mg/kg/day (p.o.) for 4 weeks | [69] | ||
| Curcumin | C2C12 myotubes | p-Akt, p-AMPK | ↑ p-ACC protein ↑ GU ↑ GLUT4 translocation ↑ p-AMPK ↑ p-ACC ↑ p-Akt (insulin-induced) | 40 μM for 24 h: cytotoxicity > 40 μM 40 µM for 1 h | [70] | |
| WR (soleus muscle) | p-AMPK | BG | 1 μM for 30 min or 60 mg/kg | [71] | ||
| C2C12 cells | ↑ GU ↓ p-IRS-1 ↓ p-ACC ↑ p-Akt ↑ p-ERK1/2 ↑ p-p38 MAPK | 20 µM for 2 h | [72] | |||
| C2 murine myoblasts | ↑ Apoptosis ↓ Cell viability ↑ PARP fragmentation ↑ p-JNK | 50 µM for 24 h | [73] | |||
| L6myc skeletal muscle cells | ↑ GLUT4 translocation ↑ p-Akt ↑ p-GSK-3β ↓ TNF-α, IL-6 and MCP-1 levels ↑ IL-10 levels | 25 µM for 16 h | [74] | |||
| ECGC | L6 myotubes | p-Akt, p-AMPK | GU | 40 μM for 3 h | [75] | |
| SD rats (soleus muscle) | 12 h fast. 75 mg/kg for 1 h, or 100 nM for 15 min | [29] | ||||
| C57BL/6 mice (soleus muscle) | 12 h fast. 75 mg/kg for 1 h, or 100 nM for 15 min | |||||
| L6 myotubes | GU | 100 nM for 15 min | ||||
| C2C12 myotubes | p-Akt, p-AMPK | ↑ p-p38 MAPK, ↑ p-ACC | 20 μM up to 72 h: treatment not cytotoxic up to 48 h | [72] | ||
| L6 myotube | GLUT4 | ↑ PI3K, ↓ PKC λ/ξ ↑ GLUT4 RNA, ↑ Rac1 | 1 nM | [76] | ||
| ICR mice | GLUT4, PI3K, p-AMPK | ↑ GLUT4, glycogen accumulation in skeletal muscle | Oral administration postprandial hyperglycemia | |||
| C2C12 myotubes (ßGlud1−/−) | p-AMPK | ↑ p-AMPK, ↑ GU, ↑ IS | 20 µM EGCG for 10 min | [77] | ||
| Ellagic acid | 3T3-L1 adipocytes and C2C12 myotubes | GLUT4, p-AMPK | GU | ↑ GLUT4 ↑ p-AMPK ↑ p-ERK1/2 ↑ p-PKC ζ/λ | 50 μg/mL for 1 h 1, 10, 100, 500 nM | [33] |
| Ferulic acid | L6 myotubes | GLUT4 | GU | ↑ PI3K protein | 5 μM for 5 h: cytotoxicity > (50 μM) | [63] |
| Gingerol | L6 myotubes | GU | 40 μg/mL for 48 h: treatment not cytotoxic | [78] | ||
| Naringenin | L6 myotubes | p-AMPK | GU | 150 μM for 2 h | [79] | |
| Quercetin | ob/ob mice (gastrocnemius muscle) | GLUT4 | BG, IS | ↑ GLUT4 RNA, ↓ TNF-α, ↓ iNOS RNA, ↓ NF κB activation | 30 mg/kg alternating days for 10 weeks | [80] |
| L6 myotubes | p-Akt | GU | 200 μM for 48 h | |||
| Kunming mice (gastrocnemius muscle) | GLUT4, p-AMPK | BG | ↑ p-ACC, ↓ blood triacylglycerol, ↓ total cholesterol, | 12-h fast. 5, 10, 20 mg/kg/day for 13 weeks | [81] | |
| C2C12 myotubes | GLUT4, p-AMPK | GU | ↑ PPARα, ACC, MCAD, CPT-1, GLUT4, PGC-1α RNA, p-ACC protein | 10 μM for 24 h: treatment not cytotoxic | ||
| C2C12 myotubes | GU | ↑ p-ACC protein | 100 μM for 18 h | [81] | ||
| C2C12 myotubes | p-AMPK | GU | enhanced glucose uptake by 38–59% stimulated uptake by 37%, stimulated the AMPK pathway (25–100 mM) inhibited ATP synthase (in mitochondria) by 34 and 79% | quercetin-3-O-glycosides (50 mM; 18 h treatment) in the absence of insulin quercetin aglycone and quercetin glycosides quercetin aglycone by 25 and 100 mM | [82] | |
| L6 myotubes | CaMKKβ/AMPK, IRS1/PI3K/Akt JAK/STAT | GU | ↑ GU (0.1 nM and 1 nM quercetin or 1 nM isorhamnetin) ↑ JAK/STAT (1 nM and 10 nM isorhamnetin) ↑ p-AMPK (quercetin) ↑ JAK2/STAT (isorhamnetin) ↑ IRS-1 (at 10 nM) | [34] | ||
| ICR mice | GLUT4 | GU | quercetin aglycone form were 4.95 and 6.80 nM (plasma concentration) | 10, 100 and 1000 mg/kg body weight | ||
| Resveratrol | L6 myotubes | p-AMPK | GU | 100 μM for 4 h | [83] | |
| db/db mice | BG | ↑ Glucose tolerance | 5 mg/mL/100g body weight for 3 weeks † | |||
| SD rats (soleus muscle) | GU, BR | Overnight fast. 10 mg/kg/day for 16 weeks | [84] | |||
| C2C12 myotubes | GU | 10 μM for 24 h | ||||
| L6 myotubes | p-AMPK | GU | 100 μM for 2 h: cell morphology unaltered up to 125 μM | [85] | ||
| SD rats (soleus muscle) | GU | ↑ p-ERα, ↑ p-IR, ↓ serum cholesterol, ↓ triglycerides, ↓ uric acid | 1 mg/kg/day for 15 days or 15 weeks | [86] | ||
| C2C12 myotubes | p-Akt | GU | ↑ p-ERα, ↑ p-p38 MAPK, ↑ p-ERK, ↑ p-IR | 0.1 μM for 14 h: treatment not cytotoxic | ||
| WR (soleus muscle) | GLUT4, p-Akt | BG | ↑ PEPCK | Overnight fast. 0.05–10 mg/kg/day for 7 days | [87] | |
| C2C12 myotubes | GU | 30 μM for 30min | ||||
| C2C12 myocytes | p-AMPK | ↑ PGC-1α RNA | 50 μM for 24 h: cytotoxicity > (50 μM) | [88] | ||
| SIRT1 knockout mice | BG, IS | ↓Mitochondrial content and respiration | 100 mg/kg day for 9 weeks | [89] | ||
| Green tea | Wistar rats (WR) | GLUT4 | ↓ Triacylglycerols (plasma) ↓ NEFA, ↓ HbA1c, ↑ GLUT4 | 50 mg/kg body weight for 12 days | [90] | |
| KK-Ay mice | GLUT4 | IS | ↓ Triacylglycerols (plasma) | 4 weeks | ||
| Procyanidins (dimer to tetramer) from black soybean seeds | ICR mice (soleus muscle) | GLUT4 | GU | ↑ GLUT4 ↑ p-IRS-1 ↑ p-AMPK ↑ plasma insulin level ↑ plasma adiponectin ↓ plasma glucose | EC and C3G in water at 10 μg/kg body weight | [91] |
| Procyanidins from cocoa liquor (CLPr) | L6 myoblasts | GLUT4 | GU | ↑ GLUT4 (7 days) GLUT1 unchanged | 250 mg/mL CLPr in DMSO | [92] |
| ICR mice (soleus muscle) | GLUT4 | GU | ↑ GLUT4 (7 days) GLUT1 unchanged | 1, 5, 10 μg/mL single oral administration | ||
| High-molecular-weight cocoa procyanidins | human primary skeletal muscle cells | GLUT4, PI3K/AKT, p-AMPK | GU | ↑ glycogen synthesis (cocoa extract), ↑ glycogen synthase (GS) (monomers 30% at 10 μM, oligomers 62% at 10 μM, polymers 16% at 10 μM and 32% at 25 μM) ↑ GU (all doses) ↓ PI3K/AKT (CE, 25 μM; oligomer, 25 μM; polymer 10 and 25 μM) | 10 and 25 μM | [93] |
| Sinapic acid | SZT-diabetic rats (soleus muscle) | GLUT4 | GU | ↑ reduced glucose infusion rate (GIR) | 5 mg/kg, 10 mg/kg, and 25 mg/kg | [94] |
| L6 cells | GU | ↓ PLC-PKC signals ↑ glucose uptake | ||||
| Gallic acid | 3T3-L1 cells | GLUT4 | GU | ↑ GLUT4 ↑ PKCζ/λ | 1 µM, 10 µM, 20 µM | [95] |
| HFD SZT-diabetic rats (adipose tissue) | GLUT4 | GU | ↑ PPARγ ↑ GLUT4 ↑ PI3K/p-Akt ↑ GU | 20 mg/kg | [96] | |
| Black tea polyphenols (theaflavin) | L6 myotubes | GLUT4, p-AMPK | GU | ↑ p-IRS1 ↑ p-AMPK/AMPK ↑ p-GSK-3β ↑ PI3K | 0.1, 1.0, 10 BTP µg/mL | [32] |
| Rosemary extract | L6 myotubes | p-AMPK, GLUT4 | GU | ↑ GU (5 µg/mL) ↑ p-AMPK ↑ p-ACC protein ↑ p-PKC | 5 µM RA for 4 h (maximum) | [97] |
| Carnosol | L6 myotubes GLUT4myc | p-AMPK, GLUT4, p-Akt | GU | ↑ p-Akt ↑ p-mTOR ↑ p-ACC protein ↑ GLUT4 | 25 µM carnosol (4 h) | [31] |
References
- Aronoff, S.L.; Berkowitz, K.; Shreiner, B. Glucose Metabolism and Regulation: Beyond Insulin and Glucagon. Diabetes Spectr. 2004, 17, 183–190. [Google Scholar] [CrossRef]
- Rowland, A.F.; Fazakerley, D.J.; James, D.E. Mapping Insulin/GLUT4 Circuitry. Traffic 2011, 12, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Jaldin-Fincati, J.R.; Pavarotti, M.; Frendo-Cumbo, S.; Bilan, P.J.; Klip, A. Update on GLUT4 Vesicle Traffic: A Cornerstone of Insulin Action. Trends Endocrinol. Metab. 2017, 28, 597–611. [Google Scholar] [CrossRef] [PubMed]
- Klip, A.; Sun, Y.; Chiu, T.T.; Foley, K.P. Signal transduction meets vesicle traffic: The software and hardware of GLUT4 translocation. AJP Cell Physiol. 2014, 306, C879–C886. [Google Scholar] [CrossRef] [PubMed]
- Schultze, S.M.; Hemmings, B.A.; Niessen, M.; Tschopp, O. PI3K/AKT, MAPK and AMPK signalling: Protein kinases in glucose homeostasis. Expert Rev. Mol. Med. 2012, 14. [Google Scholar] [CrossRef]
- Alsadat, S.; Khorami, H. PI3K / AKT pathway in modulating glucose homeostasis and its alteration in Diabetes. Ann. Med. Biomed. Sci. 2015, 1, 46–55. [Google Scholar]
- Banworth, M.J.; Li, G. Consequences of Rab GTPase dysfunction in genetic or acquired human diseases. Small GTPases 2018, 9, 158–181. [Google Scholar] [CrossRef]
- Kramer, H.F.; Witczak, C.A.; Taylor, E.B.; Fujii, N.; Hirshman, M.F.; Goodyear, L.J. AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J. Biol. Chem. 2006, 281, 31478–31485. [Google Scholar] [CrossRef]
- Middelbeek, R.J.W.; Chambers, M.A.; Tantiwong, P.; Treebak, J.T.; An, D.; Hirshman, M.F.; Musi, N.; Goodyear, L.J. Insulin stimulation regulates AS160 and TBC1D1 phosphorylation sites in human skeletal muscle. Nutr. Diabetes 2013, 3, 6–9. [Google Scholar] [CrossRef]
- Sun, Y.; Bilan, P.J.; Liu, Z.; Klip, A. Rab8A and Rab13 are activated by insulin and regulate GLUT4 translocation in muscle cells. Proc. Natl. Acad. Sci. USA 2010, 107, 19909–19914. [Google Scholar] [CrossRef]
- Ishikura, S.; Bilan, P.J.; Klip, A. Rabs 8A and 14 are targets of the insulin-regulated Rab-GAP AS160 regulating GLUT4 traffic in muscle cells. Biochem. Biophys. Res. Commun. 2007, 353, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Brewer, P.D.; Habtemichael, E.N.; Romenskaia, I.; Coster, A.C.; Mastick, C.C. Rab14 limits the sorting of Glut4 from endosomes into insulin-sensitive regulated secretory compartments in adipocytes. Biochem. J. 2016, 473, 1315–1327. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lippincott-Schwartz, J. Insulin triggers surface-directed trafficking of sequestered GLUT4 storage vesicles marked by Rab10. Small GTPases 2013, 4, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Sadacca, L.A.; Bruno, J.; Wen, J.; Xiong, W.; McGraw, T.E. Specialized sorting of GLUT4 and its recruitment to the cell surface are independently regulated by distinct Rabs. Mol. Biol. Cell 2013, 24, 2544–2557. [Google Scholar] [CrossRef] [PubMed]
- Sano, H.; Eguez, L.; Teruel, M.N.; Fukuda, M.; Chuang, T.D.; Chavez, J.A.; Lienhard, G.E.; McGraw, T.E. Rab10, a target of the AS160 Rab GAP, is required for insulin-stimulated translocation of GLUT4 to the adipocyte plasma membrane. Cell. Metab. 2007, 5, 293–303. [Google Scholar] [CrossRef]
- Bruno, J.; Brumfield, A.; Chaudhary, N.; Iaea, D.; McGraw, T.E. SEC16A is a RAB10 effector required for insulin-stimulated GLUT4 trafficking in adipocytes. J. Cell Biol. 2016, 214, 61–76. [Google Scholar] [CrossRef]
- Ishikura, S.; Klip, A. Muscle cells engage Rab8A and myosin Vb in insulin-dependent GLUT4 translocation. Am. J. Physiol. Cell Physiol. 2008, 295, 1016–1025. [Google Scholar] [CrossRef]
- Chen, Q.; Rong, P.; Xu, D.; Zhu, S.; Chen, L.; Xie, B.; Du, Q.; Quan, C.; Sheng, Y.; Zhao, T.J.; et al. Rab8a deficiency in skeletal muscle causes hyperlipidemia and hepatosteatosis by impairing muscle lipid uptake and storage. Diabetes 2017, 66, 2387–2399. [Google Scholar] [CrossRef]
- Wu, L.; Xu, D.; Zhou, L.; Xie, B.; Yu, L.; Yang, H.; Huang, L.; Ye, J.; Deng, H.; Yuan, Y.A.; et al. Rab8a-AS160-MSS4 regulatory circuit controls lipid droplet fusion and growth. Dev. Cell 2014, 30, 378–393. [Google Scholar] [CrossRef]
- Wang, H.Y.; Ducommun, S.; Quan, C.; Xie, B.; Li, M.; Wasserman, D.H.; Sakamoto, K.; Mackintosh, C.; Chen, S. AS160 deficiency causes whole-body insulin resistance via composite effects in multiple tissues. Biochem. J. 2013, 449, 479–489. [Google Scholar] [CrossRef]
- Prabhakar, K.P.; Doble, M. Mechanism of Action of Natural Products Used in the Treatment of Diabetes Mellitus. Chin. J. Integr. Tradit. West. Med. 2011, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Ploug, T.; Van Deurs, B.; Ai, H.; Cushman, S.W.; Ralston, E. Analysis of GLUT4 distribution in whole skeletal muscle fibers: Identification of distinct storage compartments that are recruited by insulin and muscle contractions. J. Cell Biol. 1998, 142, 1429–1446. [Google Scholar] [CrossRef] [PubMed]
- Gannon, N.P.; Conn, C.A.; Vaughan, R.A. Dietary stimulators of GLUT4 expression and translocation in skeletal muscle: A mini-review. Mol. Nutr. Food Res. 2015, 59, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Samad, M.B.; Mohsin, M.N.A.B.; Razu, B.A.; Hossain, M.T.; Mahzabeen, S.; Unnoor, N.; Muna, I.A.; Akhter, F.; Kabir, A.U.; Hannan, J.M.A. [6]-Gingerol, from Zingiber officinale, potentiates GLP-1 mediated glucose-stimulated insulin secretion pathway in pancreatic β-cells and increases RAB8/RAB10-regulated membrane presentation of GLUT4 transporters in skeletal muscle to improve hyperglycemia in Leprdb/db type 2 diabetic mice. BMC Complement. Altern. Med. 2017, 17, 1–13. [Google Scholar] [CrossRef]
- Wang, B.; Yang, Q.; Sun, Y.Y.; Xing, Y.F.; Wang, Y.B.; Lu, X.T.; Bai, W.W.; Liu, X.Q.; Zhao, Y.X. Resveratrol-enhanced autophagic flux ameliorates myocardial oxidative stress injury in diabetic mice. J. Cell. Mol. Med. 2014, 18, 1599–1611. [Google Scholar] [CrossRef]
- Elhassan, S.A.M.; Candasamy, M.; Chan, E.W.L.; Bhattamisra, S.K. Autophagy and GLUT4: The missing pieces. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 12, 1109–1116. [Google Scholar] [CrossRef]
- Hadrich, F.; Garcia, M.; Maalej, A.; Moldes, M.; Isoda, H.; Feve, B.; Sayadi, S. Oleuropein activated AMPK and induced insulin sensitivity in C2C12 muscle cells. Life Sci. 2016, 151, 167–173. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Tsukahara, C.; Ikeda, N.; Sone, Y.; Ishikawa, T.; Ichi, I.; Koike, T.; Aoki, Y. Oleuropein improves insulin resistance in skeletal muscle by promoting the translocation of GLUT4. J. Clin. Biochem. Nutr. 2017, 61, 196–202. [Google Scholar] [CrossRef]
- Ueda, M.; Nishiumi, S.; Nagayasu, H.; Fukuda, I.; Yoshida, K.; Ashida, H. Epigallocatechin gallate promotes GLUT4 translocation in skeletal muscle. Biochem. Biophys. Res. Commun. 2008, 377, 286–290. [Google Scholar] [CrossRef]
- Doan, K.V.; Ko, C.M.; Kinyua, A.W.; Yang, D.J.; Choi, Y.H.; Oh, I.Y.; Nguyen, N.M.; Ko, A.; Choi, J.W.; Jeong, Y.; et al. Gallic acid regulates body weight and glucose homeostasis through AMPK activation. Endocrinology 2015, 156, 157–168. [Google Scholar] [CrossRef]
- Vlavcheski, F.; Baron, D.; Vlachogiannis, I.A.; Macpherson, R.E.K.; Tsiani, E. Carnosol Increases Skeletal Muscle Cell Glucose Uptake via AMPK-Dependent GLUT4 Glucose Transporter Translocation. Int. J. Mol. Sci. 2018, 19, 1321. [Google Scholar] [CrossRef] [PubMed]
- Nagano, T.; Hayashibara, K.; Ueda-Wakagi, M.; Yamashita, Y.; Ashida, H. Black Tea Polyphenols Promotes GLUT4 Translocation through Both PI3K-and AMPK-dependent Pathways in Skeletal Muscle Cells. Food Sci. Technol. Res. 2015, 21, 489–494. [Google Scholar] [CrossRef]
- Poulose, N.; Prasad, C.V.; Haridas, P.N.; Anilkumar, G. Ellagic Acid Stimulates Glucose Transport in Adipocytes and Muscles through AMPK Mediated Pathway. J. Diabetes Metab. 2011, 2. [Google Scholar] [CrossRef]
- Jiang, H.; Yamashita, Y.; Nakamura, A.; Croft, K.; Ashida, H. Quercetin and its metabolite isorhamnetin promote glucose uptake through different signalling pathways in myotubes. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Keogh, J.; Clifton, P. Polyphenols and Glycemic Control. Nutrients 2016, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhu, F. Antidiabetic dietary materials and animal models. Food Res. Int. 2016, 85, 315–331. [Google Scholar] [CrossRef]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef]
- Velayutham, P.; Babu, A.; Liu, D.; Gilbert, E.R. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J. Nutr. Biochem. 2013, 24, 1777–1789. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Dietary polyphenols as potential nutraceuticals in management of diabetes: A review. J. Diabetes Metab. Disord. 2013, 12, 43. [Google Scholar] [CrossRef]
- Cumaoǧlu, A.; Rackova, L.; Stefek, M.; Kartal, M.; Maechler, P.; Karasu, Ç. Effects of olive leaf polyphenols against H2O2 toxicity in insulin secreting β-cells. Acta Biochim. Pol. 2011, 58, 45–50. [Google Scholar]
- López de las Hazas, M.-C.; Piñol, C.; Macià, A. Differential absorption and metabolism of hydroxytyrosol and its precursors oleuropein and secoiridoids. J. Funct. Foods 2016, 22, 52–63. [Google Scholar] [CrossRef]
- Poudyal, H.; Campbell, F.; Brown, L. Olive Leaf Extract Attenuates Cardiac, Hepatic, and Metabolic Changes in High Carbohydrate–, High Fat– Fed Rats. J. Nutr. 2010, 140, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Xu, J.; Zou, X.; Li, Y.; Chen, C.; Zheng, A.; Li, H.; Li, H.; Szeto, I.M.Y.; Shi, Y.; et al. Hydroxytyrosol prevents diet-induced metabolic syndrome and attenuates mitochondrial abnormalities in obese mice. Free Radic. Biol. Med. 2014, 67, 396–407. [Google Scholar] [CrossRef]
- Murotomi, K.; Umeno, A.; Yasunaga, M.; Shichiri, M.; Ishida, N.; Koike, T.; Matsuo, T.; Abe, H.; Yoshida, Y.; Nakajima, Y. Oleuropein-Rich Diet Attenuates Hyperglycemia and Impaired Glucose Tolerance in Type 2 Diabetes Model Mouse. J. Agric. Food Chem. 2015, 63, 6715–6722. [Google Scholar] [CrossRef]
- Shen, Y.; Song, S.J.; Keum, N.; Park, T. Olive leaf extract attenuates obesity in high-fat diet-fed mice by modulating the expression of molecules involved in adipogenesis and thermogenesis. Evid.-Based Complement. Altern. Med. 2014, 2014, 12. [Google Scholar] [CrossRef] [PubMed]
- Drira, R.; Chen, S.; Sakamoto, K. Oleuropein and hydroxytyrosol inhibit adipocyte differentiation in 3 T3-L1 cells. Life Sci. 2011, 89, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.K. Skeletal muscle as a therapeutic target for delaying type 1 diabetic complications. World J. Diabetes 2015, 6, 1323. [Google Scholar] [CrossRef]
- Perry, B.D.; Caldow, M.K.; Brennan-Speranza, T.C.; Sbaraglia, M.; Jerums, G.; Garnham, A.; Wong, C.; Levinger, P.; ul Haq, A.M.; Hare, D.L.; et al. Muscle atrophy in patients with Type 2 Diabetes Mellitus: Roles of inflammatory pathways, physical activity and exercise. Exerc. Immunol. Rev. 2016, 22, 94–109. [Google Scholar]
- Calábria, L.K.; Vieira, A.; José, R.; Deconte, S.R.; Nascimento, R.; de Carvalho, W.J.; de Oliveira, V.N.; Alberto, C.; Filho, A.; Rezende, L.; et al. Myosins Are Differentially Expressed under Oxidative Stress in Chronic Streptozotocin-Induced Diabetic Rat Brains. Neuroscience 2013, 10. [Google Scholar] [CrossRef]
- Aragno, M.; Mastrocola, R.; Catalano, M.G.; Brignardello, E.; Danni, O.; Boccuzzi, G. Oxidative Stress Impairs Skeletal Muscle Repair in Diabetic Rats. Diabetes 2004, 1082–1088. [Google Scholar] [CrossRef]
- Shrilatha, B. Occurrence of oxidative impairments, response of antioxidant defences and associated biochemical perturbations in male reproductive milieu in the Streptozotocin-diabetic rat. Int. J. Androl. 2007, 30, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ran, L.; Lang, H.; Zhou, M.; Yu, L.; Yi, L.; Zhu, J.; Liu, L.; Mi, M. Myricetin improves endurance capacity by inducing muscle fiber type conversion via miR-499. Nutr. Metab. 2019, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Cheng, X.; Cui, Y.; Xia, Q.; Yan, X.; Zhang, M.; Lan, G.; Liu, J.; Shan, T.; Huang, Y. Resveratrol regulates skeletal muscle fibers switching through the AdipoR1-AMPK-PGC-1α pathway. Food Funct. 2019, 10, 3334–3343. [Google Scholar] [CrossRef] [PubMed]
- Gaster, M.; Staehr, P.; Beck-Nielsen, H.; Schrøder, H.D.; Handberg, A. GLUT4 Is Reduced in Slow Muscle Fibers of Type 2 Diabetic Patients. Diabetes 2001, 1324–1329. [Google Scholar] [CrossRef]
- Sun, Y.; Chiu, T.T.; Foley, K.P.; Bilan, P.J.; Klip, A. Myosin Va mediates Rab8A-regulated GLUT4 vesicle exocytosis in insulin-stimulated muscle cells. Mol. Biol. Cell 2014, 25, 1159–1170. [Google Scholar] [CrossRef]
- Wasserman, D.H.; Kang, L.; Ayala, J.E.; Fueger, P.T.; Lee-young, R.S. The physiological regulation of glucose flux into muscle in vivo. J. Exp. Biol. 2011, 214, 254–262. [Google Scholar] [CrossRef]
- Lauritzen, H.; Galbo, H.; Brandauer, J.; Laurie, G.; Thorkil, P. Large GLUT4 Vesicles Are Stationary While Locally and. Diabetes 2008, 57, 315–324. [Google Scholar] [CrossRef]
- Lizunov, V.A.; Stenkula, K.G.; Lisinski, I.; Gavrilova, O.; Yver, D.R.; Chadt, A.; Al-Hasani, H.; Zimmerberg, J.; Cushman, S.W. Insulin stimulates fusion, but not tethering, of GLUT4 vesicles in skeletal muscle of HA-GLUT4-GFP transgenic mice. AJP Endocrinol. Metab. 2012, 302, E950–E960. [Google Scholar] [CrossRef]
- Sreekumar, R.; Halvatsiotis, P.; Schimke, J.C.; Sreekumaran Nair, K. Gene expression profile in skeletal muscle of type 2 diabetes and the effect of insulin treatment. Diabetes 2002, 51, 1913–1920. [Google Scholar] [CrossRef]
- Li, H.; Ou, L.; Fan, J.; Xiao, M.; Kuang, C.; Liu, X.; Sun, Y.; Xu, Y. Rab8A regulates insulin-stimulated GLUT4 translocation in C2C12 myoblasts. FEBS Lett. 2017, 591. [Google Scholar] [CrossRef]
- Giacometti, J.; Žauhar, G.; Žuvić, M. Optimization of Ultrasonic-Assisted Extraction of Major Phenolic Compounds from Olive Leaves (Olea europaea L.) Using Response Surface Methodology. Foods 2018, 7, 149. [Google Scholar] [CrossRef]
- Giacometti, J.; Grubić-Kezele, T. Olive Leaf Polyphenols Attenuate the Clinical Course of Experimental Autoimmune Encephalomyelitis and Provide Neuroprotection by Reducing Oxidative Stress, Regulating Microglia and SIRT1, and Preserving Myelin Integrity. Oxid. Med. Cell. Longev. 2020, 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, P.K.; Doble, M. Synergistic effect of phytochemicals in combination with hypoglycemic drugs on glucose uptake in myotubes. Phytomedicine 2009, 16, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Honma, N.; Kobayashi, K.; Jia, L.N.; Hosono, T.; Shindo, K.; Ariga, T.; Seki, T. Cinnamon extract enhances glucose uptake in 3T3-L1 adipocytes and C2C12 myocytes by inducing LKB1-AMP-activated protein kinase signaling. PLoS ONE 2014, 9, e87894. [Google Scholar] [CrossRef] [PubMed]
- Absalan, A.; Mohiti-Ardakani, J.; Hadinedoushan, H.; Khalili, M.A. Hydro-alcoholic cinnamon extract, enhances glucose transporter isotype-4 translocation from intracellular compartments into the cytoplasmic membrane of C2C12 myotubes. Indian J. Clin. Biochem. 2012, 27, 351–356. [Google Scholar] [CrossRef][Green Version]
- Shen, Y.; Fukushima, M.; Ito, Y.; Muraki, E.; Hosono, T.; Seki, T.; Ariga, T. Verification of the antidiabetic effects of cinnamon (Cinnamomum zeylanicum) using insulin-uncontrolled type 1 diabetic rats and cultured adipocytes. Biosci. Biotechnol. Biochem. 2010, 74, 2418–2425. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Zhang, Y.; Gong, Z.; Huang, C.; Zang, Y.Q. Improved insulin resistance and lipid metabolism by cinnamon extract through activation of peroxisome proliferator-activated receptors. PPAR Res. 2008, 2008, 581348. [Google Scholar] [CrossRef]
- Kim, W.; Khil, L.Y.; Clark, R.; Bok, S.H.; Kim, E.E.; Lee, S.; Jun, H.S.; Yoon, J.W. Naphthalenemethyl ester derivative of dihydroxyhydrocinnamic acid, a component of cinnamon, increases glucose disposal by enhancing translocation of glucose transporter 4. Diabetologia 2006, 49, 2437–2448. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, T.; Wang, L.; Guo, X.; Wu, L.; Qin, L.; Sun, W. Antihyperglycemic and antihyperlipidemic action of cinnamaldehyde in C57BLKS/J db/db mice. J. Tradit. Chin. Med. 2012, 32, 446–452. [Google Scholar] [CrossRef]
- Kang, C.; Kim, E. Synergistic effect of curcumin and insulin on muscle cell glucose metabolism. Food Chem. Toxicol. 2010, 48, 2366–2373. [Google Scholar] [CrossRef]
- Cheng, T.C.; Lin, C.S.; Hsu, C.C.; Chen, L.J.; Cheng, K.C.; Cheng, J.T. Activation of muscarinic M-1 cholinoceptors by curcumin to increase glucose uptake into skeletal muscle isolated from Wistar rats. Neurosci. Lett. 2009, 465, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.-T.; Chang, T.-W.; Lee, M.-S.; Lin, J.-K. Suppression of free fatty acid-induced insulin resistance by phytopolyphenols in C2C12 mouse skeletal muscle cells. J. Agric. Food Chem. 2012, 60, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Peleli, M.; Aggeli, I.-K.; Matralis, A.N.; Kourounakis, A.P.; Beis, I.; Gaitanaki, C. Evaluation of two novel antioxidants with differential effects on curcumin-induced apoptosis in C2 skeletal myoblasts; involvement of JNKs. Bioorg. Med. Chem. 2015, 23, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Tamrakar, A.K.; Mahajan, S.; Prasad, G.B.K.S. Chitosan encapsulated nanocurcumin induces GLUT-4 translocation and exhibits enhanced anti-hyperglycemic function. Life Sci. 2018, 213, 226–235. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Li, Q.; Liang, J.; Dai, X.Q.; Ding, Y.; Wang, J.B.; Li, Y. Epigallocatechin-3-O-gallate (EGCG) protects the insulin sensitivity in rat L6 muscle cells exposed to dexamethasone condition. Phytomedicine 2010, 17, 14–18. [Google Scholar] [CrossRef]
- Ueda-Wakagi, M.; Hayashibara, K.; Nagano, T.; Ikeda, M.; Yuan, S.; Ueda, S.; Shirai, Y.; Yoshida, K.I.; Ashida, H. Epigallocatechin gallate induces GLUT4 translocation in skeletal muscle through both PI3K- and AMPK-dependent pathways. Food Funct. 2018, 15, 4223–4233. [Google Scholar] [CrossRef]
- Pournourmohammadi, S.; Grimaldi, M.; Stridh, M.H.; Lavallard, V.; Waagepetersen, H.S.; Wollheim, C.B.; Maechler, P. Epigallocatechin-3-gallate (EGCG) activates AMPK through the inhibition of glutamate dehydrogenase in muscle and pancreatic ß-cells: A potential beneficial effect in the pre-diabetic state? Int. J. Biochem. Cell Biol. 2017, 88, 220–225. [Google Scholar] [CrossRef]
- Li, Y.; Tran, V.H.; Duke, C.C.; Roufogalis, B.D. Gingerols of Zingiber officinale enhance glucose uptake by increasing cell surface GLUT4 in cultured L6 myotubes. Planta Med. 2012, 78, 1549–1555. [Google Scholar] [CrossRef]
- Zygmunt, K.; Faubert, B.; MacNeil, J.; Tsiani, E. Naringenin, a citrus flavonoid, increases muscle cell glucose uptake via AMPK. Biochem. Biophys. Res. Commun. 2010, 398, 178–183. [Google Scholar] [CrossRef]
- Anhê, G.F.; Okamoto, M.M.; Kinote, A.; Sollon, C.; Lellis-Santos, C.; Anhê, F.F.; Lima, G.A.; Hirabara, S.M.; Velloso, L.A.; Bordin, S.; et al. Quercetin decreases inflammatory response and increases insulin action in skeletal muscle of ob/ob mice and in L6 myotubes. Eur. J. Pharmacol. 2012, 689, 285–293. [Google Scholar] [CrossRef]
- Shen, J.Z.; Ma, L.N.; Han, Y.; Liu, J.X.; Yang, W.Q.; Chen, L.; Liu, Y.; Hu, Y.; Jin, M.W. Pentamethylquercetin generates beneficial effects in monosodium glutamate-induced obese mice and C2C12 myotubes by activating AMP-activated protein kinase. Diabetologia 2012, 55, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Eid, H.M.; Martineau, L.C.; Saleem, A.; Muhammad, A.; Vallerand, D.; Benhaddou-Andaloussi, A.; Nistor, L.; Afshar, A.; Arnason, J.T.; Haddad, P.S. Stimulation of AMP-activated protein kinase and enhancement of basal glucose uptake in muscle cells by quercetin and quercetin glycosides, active principles of the antidiabetic medicinal plant vaccinium vitis-idaea. Mol. Nutr. Food Res. 2010, 54, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Minakawa, M.; Miura, Y.; Yagasaki, K. Piceatannol, a resveratrol derivative, promotes glucose uptake through glucose transporter 4 translocation to plasma membrane in L6 myocytes and suppresses blood glucose levels in type 2 diabetic model db/db mice. Biochem. Biophys. Res. Commun. 2012, 422, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Zhou, L.J.; Mu, P.W.; Liu, S.P.; Chen, S.J.; Fu, X.D.; Wang, T.H. Caveolin-3 is involved in the protection of resveratrol against high-fat-diet- induced insulin resistance by promoting GLUT4 translocation to the plasma membrane in skeletal muscle of ovariectomized rats. J. Nutr. Biochem. 2012, 23, 1716–1724. [Google Scholar] [CrossRef]
- Breen, D.M.; Sanli, T.; Giacca, A.; Tsiani, E. Stimulation of muscle cell glucose uptake by resveratrol through sirtuins and AMPK. Biochem. Biophys. Res. Commun. 2008, 374, 117–122. [Google Scholar] [CrossRef]
- Deng, J.Y.; Hsieh, P.S.; Huang, J.P.; Lu, L.S.; Hung, L.M. Activation of estrogen receptor is crucial for resveratrol-stimulating muscular glucose uptake via both insulin-dependent and -independent pathways. Diabetes 2008, 57, 1814–1823. [Google Scholar] [CrossRef]
- Chi, T.C.; Chen, W.P.; Chi, T.L.; Kuo, T.F.; Lee, S.S.; Cheng, J.T.; Su, M.J. Phosphatidylinositol-3-kinase is involved in the antihyperglycemic effect induced by resveratrol in streptozotocin-induced diabetic rats. Life Sci. 2007, 80, 1713–1720. [Google Scholar] [CrossRef]
- Price, N.L.; Gomes, A.P.; Ling, A.J.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef]
- Menzies, K.J.; Singh, K.; Saleem, A.; Hood, D.A. Sirtuin 1-mediated effects of exercise and resveratrol on mitochondrial biogenesis. J. Biol. Chem. 2013, 288, 6968–6979. [Google Scholar] [CrossRef]
- Ueda-Wakagi, M.; Nagayasu, H.; Yamashita, Y.; Ashida, H. Green Tea Ameliorates Hyperglycemia by Promoting the Translocation of Glucose Transporter 4 in the Skeletal Muscle of Diabetic Rats. Int. J. Mol. Sci. 2019, 20, 2436. [Google Scholar] [CrossRef]
- Yamashita, Y.; Wang, L.; Nanba, F.; Ito, C.; Toda, T.; Ashida, H. Procyanidin promotes translocation of glucose transporter 4 in muscle of mice through activation of insulin and AMPK signaling pathways. PLoS ONE 2016, 11, e0161704. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Okabe, M.; Natsume, M.; Ashida, H. Cacao liquor procyanidin extract improves glucose tolerance by enhancing GLUT4 translocation and glucose uptake in skeletal muscle. J. Nutr. Sci. 2012, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bowser, S.M.; Moore, W.T.; McMillan, R.P.; Dorenkott, M.R.; Goodrich, K.M.; Ye, L.; O’Keefe, S.F.; Hulver, M.W.; Neilson, A.P. High-molecular-weight cocoa procyanidins possess enhanced insulin-enhancing and insulin mimetic activities in human primary skeletal muscle cells compared to smaller procyanidins. J. Nutr. Biochem. 2017, 39, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Cherng, Y.; Tsai, C.; Chung, H.; Lai, Y.; Kuo, S.; Cheng, J. Antihyperglycemic Action of Sinapic Acid in Diabetic Rats. J. Agric. Food Chem. 2013, 61, 12053–12059. [Google Scholar] [CrossRef]
- Prasad, V.C.N.; Anjana, T.; Banerji, A.; Gopalakrishnapillai, A. Gallic acid induces GLUT4 translocation and glucose uptake activity in 3T3-L1 cells. FEBS Lett. 2010, 584, 531–536. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Jothi, G.; Antony, P.J.; Balakrishna, K.; Paulraj, M.G.; Ignacimuthu, S.; Stalin, A.; Al-Dhabi, N.A. Gallic acid attenuates high-fat diet fed-streptozotocin-induced insulin resistance via partial agonism of PPARγ in experimental type 2 diabetic rats and enhances glucose uptake through translocation and activation of GLUT4 in PI3K/p-Akt signaling pathway. Eur. J. Pharmacol. 2014, 745, 201–216. [Google Scholar] [CrossRef]
- Vlavcheski, F.; Naimi, M.; Murphy, B.; Hudlicky, T.; Tsiani, E. Rosmarinic Acid, a Rosemary Extract Polyphenol, Increases Skeletal Muscle Cell Glucose Uptake and Activates AMPK. Molecules 2017, 22, 1669. [Google Scholar] [CrossRef]

| Group | |||||
|---|---|---|---|---|---|
| Control | DM | TOL1 | TOL2 | TOL3 | |
| Weight, g | 298.8 ± 21.38 | 241.6 ± 37.45 # | 220.0 ± 20.00 | 240.67 ± 31.88 | 235.3 ± 4.51 |
| Glucose, mg/dL | 116 ± 22.3 | 309 ± 40.7 # | 261 ± 8.3 * | 228.2 ± 36.9 * | 257 ± 27.9 * |
| Cholesterol, mg/dL | 165 ± 6.2 | 166 ± 4.3 | 163 ± 5.1 | 160 ± 1.1 | 164 ± 6.9 |
| Triglycerides, mg/dL | 104 ± 2.1 | 193 ± 114.1 # | 113 ± 7.6 * | 119.5 ± 4.7 | 100 ± 15.5 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giacometti, J.; Muhvić, D.; Grubić-Kezele, T.; Nikolić, M.; Šoić-Vranić, T.; Bajek, S. Olive Leaf Polyphenols (OLPs) Stimulate GLUT4 Expression and Translocation in the Skeletal Muscle of Diabetic Rats. Int. J. Mol. Sci. 2020, 21, 8981. https://doi.org/10.3390/ijms21238981
Giacometti J, Muhvić D, Grubić-Kezele T, Nikolić M, Šoić-Vranić T, Bajek S. Olive Leaf Polyphenols (OLPs) Stimulate GLUT4 Expression and Translocation in the Skeletal Muscle of Diabetic Rats. International Journal of Molecular Sciences. 2020; 21(23):8981. https://doi.org/10.3390/ijms21238981
Chicago/Turabian StyleGiacometti, Jasminka, Damir Muhvić, Tanja Grubić-Kezele, Marina Nikolić, Tamara Šoić-Vranić, and Snježana Bajek. 2020. "Olive Leaf Polyphenols (OLPs) Stimulate GLUT4 Expression and Translocation in the Skeletal Muscle of Diabetic Rats" International Journal of Molecular Sciences 21, no. 23: 8981. https://doi.org/10.3390/ijms21238981
APA StyleGiacometti, J., Muhvić, D., Grubić-Kezele, T., Nikolić, M., Šoić-Vranić, T., & Bajek, S. (2020). Olive Leaf Polyphenols (OLPs) Stimulate GLUT4 Expression and Translocation in the Skeletal Muscle of Diabetic Rats. International Journal of Molecular Sciences, 21(23), 8981. https://doi.org/10.3390/ijms21238981







