A Single Cysteine Residue in the Translocation Pathway of the Mitosomal ADP/ATP Carrier from Cryptosporidium parvum Confers a Broad Nucleotide Specificity
Abstract
1. Introduction
2. Results
2.1. Mitochondrial Carriers of Cryptosporidium parvum
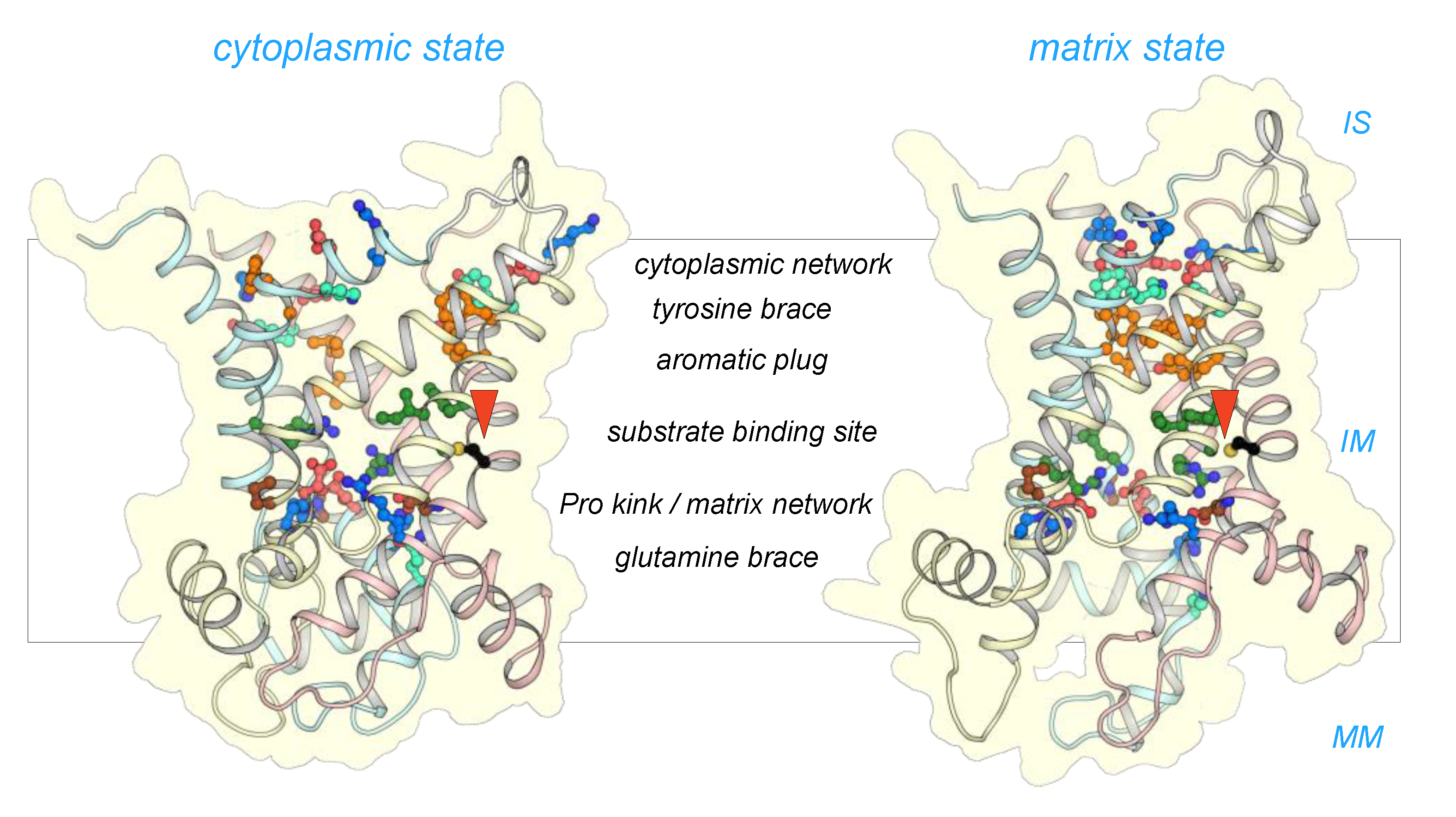
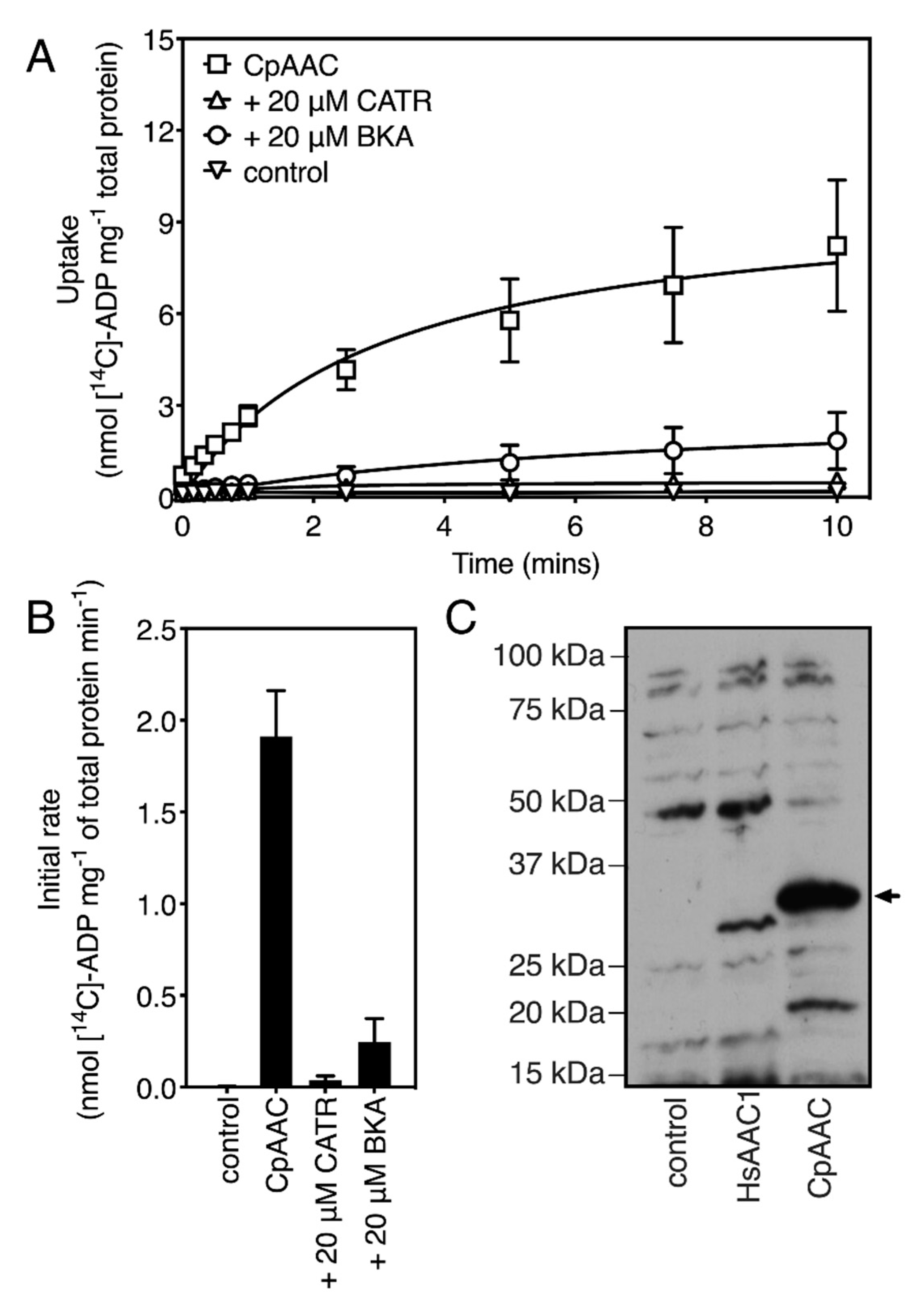
2.2. The Mitosomal ADP/ATP Carrier Retains Structural and Functional Characteristics of the Mitochondrial AAC
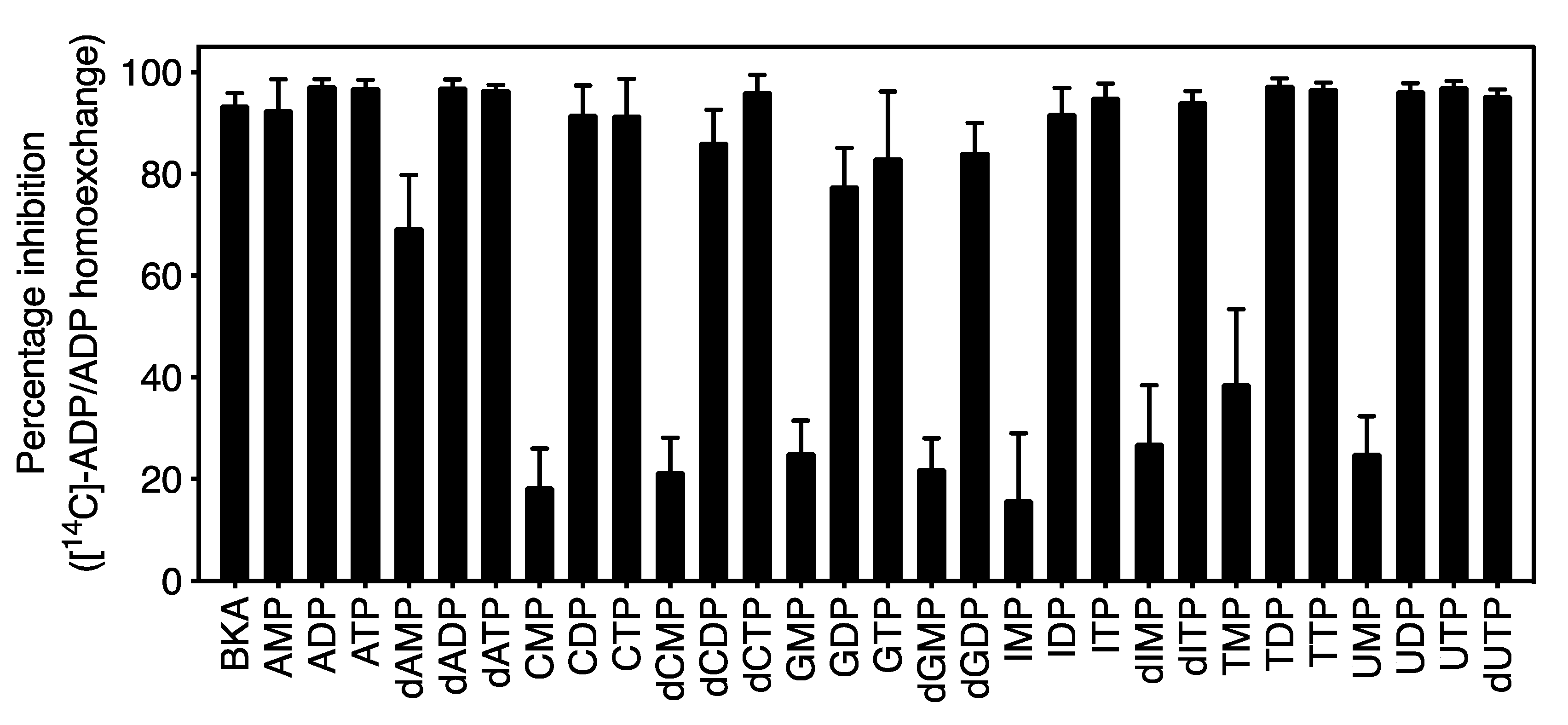
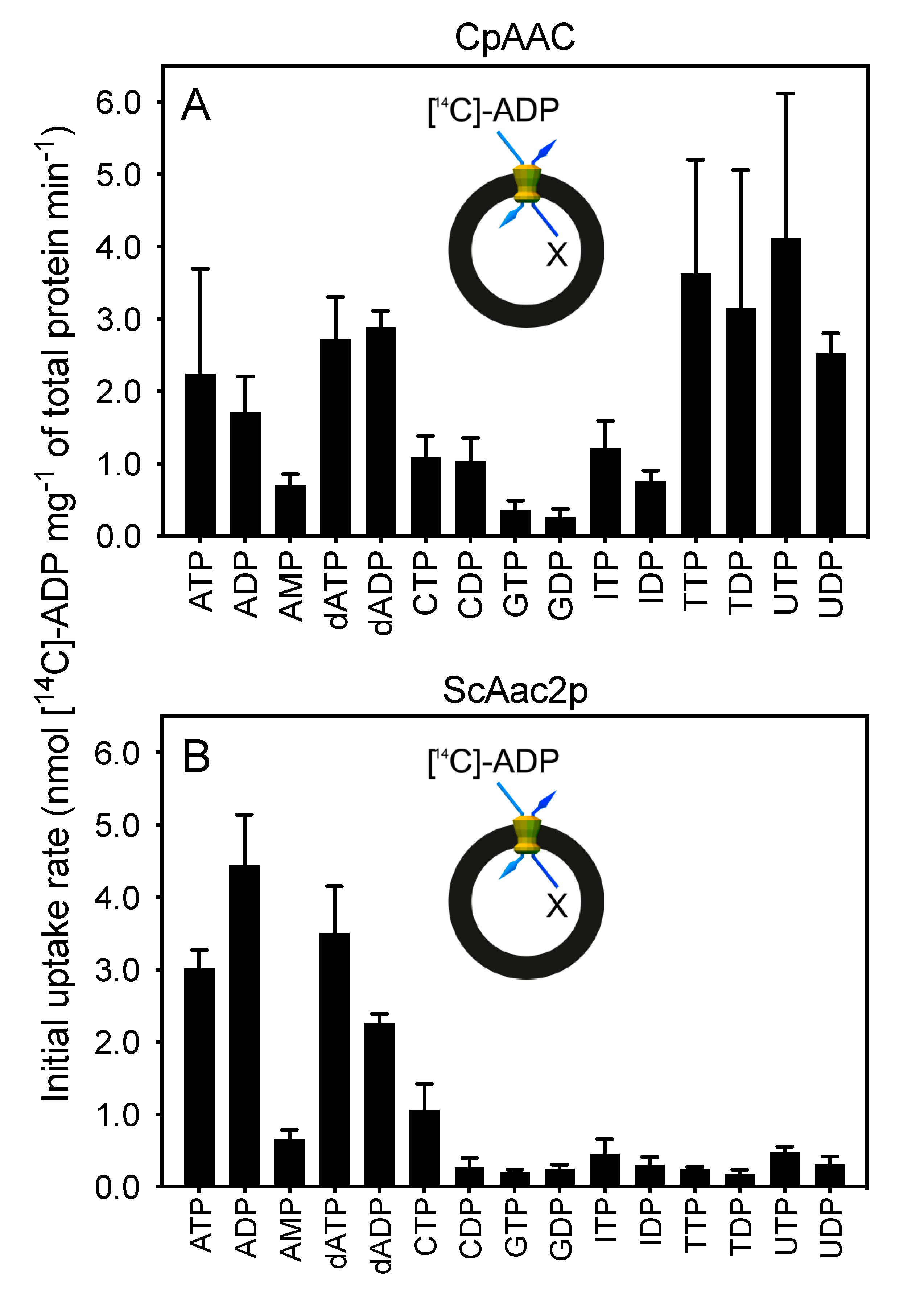
2.3. The Mitosomal ADP/ATP Carrier from Cryptosporidium parvum Has a Broad Nucleotide Specificity Profile
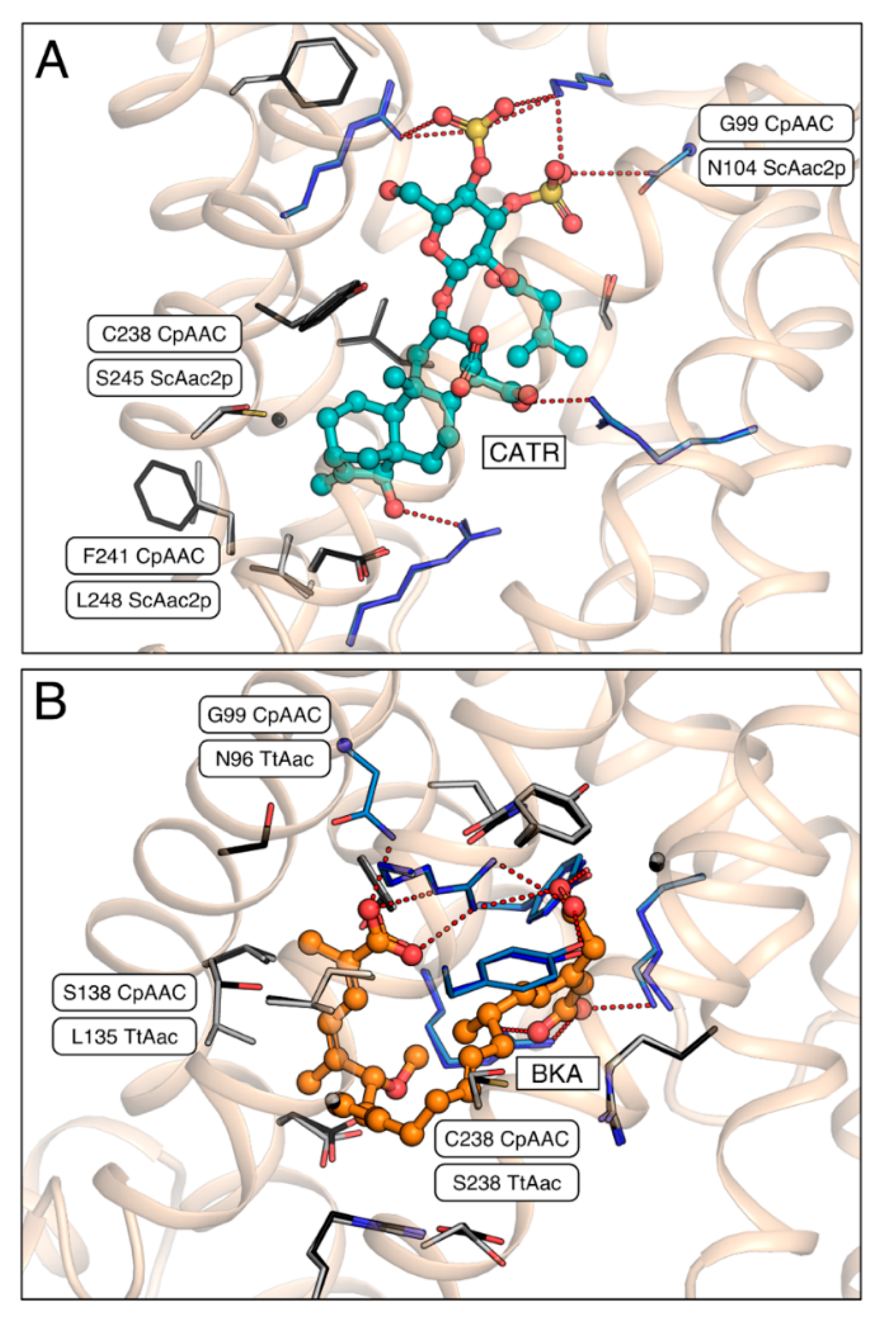
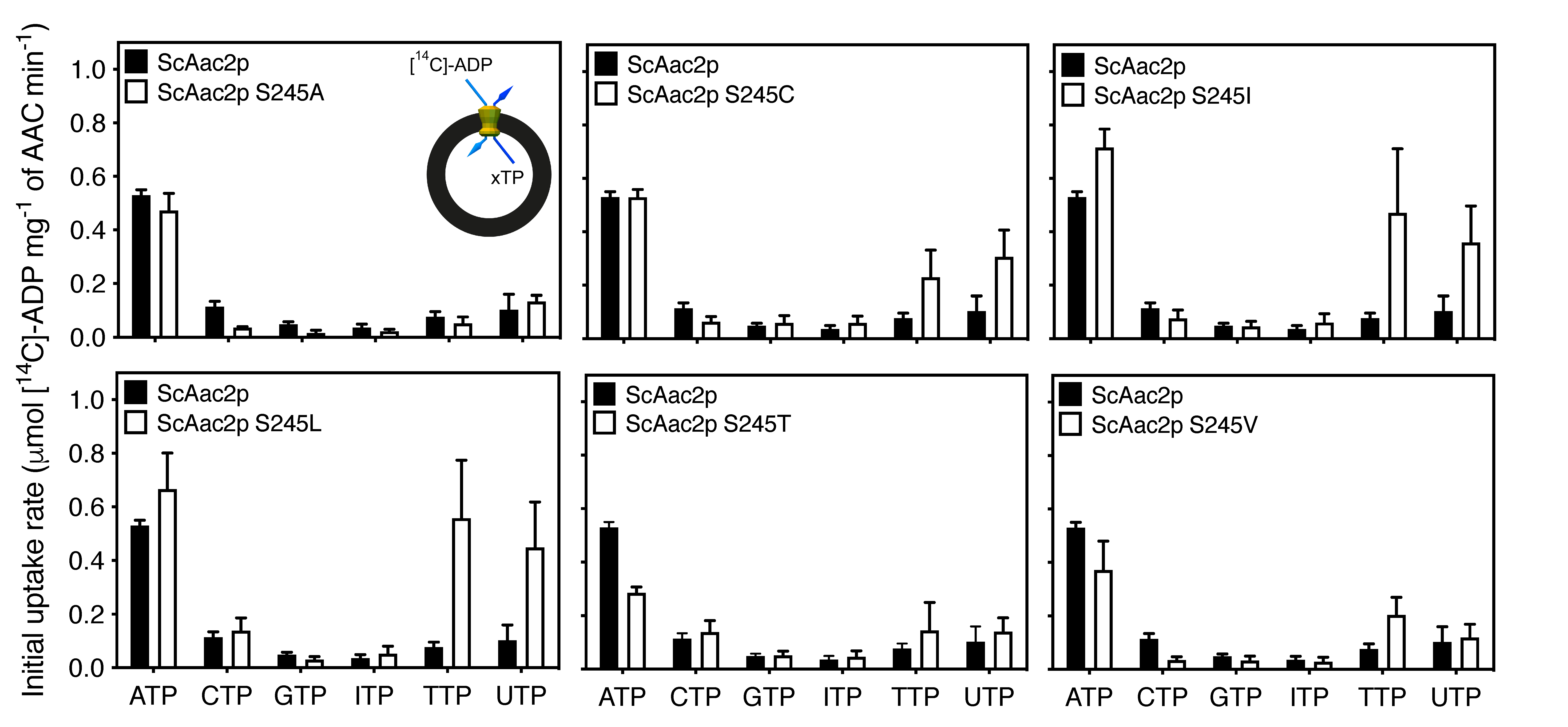
2.4. A Single Residue in the Substrate Translocation Pathway Can Broaden the Substrate Selectivity of the Yeast ADP/ATP Carrier
3. Discussion
4. Materials and Methods
4.1. In Silico Analysis
4.2. DNA Constructs and Mutagenesis
4.3. Growth of Lactococcus lactis and Membrane Isolation
4.4. SDS-PAGE and Western Blotting
4.5. Membrane Vesicle Fusions
4.6. Transport Assays
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BKA | Bongkrekic acid |
| CATR | Carboxyatractyloside |
| BtAAC1 | ADP/ATP carrier isoform 1 from Bos taurus |
| CpAAC | ADP/ATP carrier from Cryptosporidium parvum |
| HsAAC1 | ADP/ATP carrier isoform 1 from Homo sapiens |
| EhAAC | ADP/ATP carrier from Entamoeba histolytica |
| PDB | Protein Data Bank |
| ScAac2p | ADP/ATP carrier isoform 2 from Saccharomyces cerevisiae |
| TtAac | ADP/ATP carrier from Thermothelomyces thermophila |
References
- Chalmers, R.M.; Davies, A.P. Minireview: Clinical cryptosporidiosis. Exp. Parasitol. 2010, 124, 138–146. [Google Scholar] [CrossRef]
- Abubakar, I.; Aliyu, S.H.; Arumugam, C.; Usman, N.K.; Hunter, P.R. Treatment of cryptosporidiosis in immunocompromised individuals: Systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2007, 63, 387–393. [Google Scholar] [CrossRef]
- Cicirello, H.G.; Kehl, K.S.; Addiss, D.G.; Chusid, M.J.; Glass, R.I.; Davis, J.P.; Havens, P.L. Cryptosporidiosis in children during a massive waterborne outbreak in Milwaukee, Wisconsin: Clinical, laboratory and epidemiologic findings. Epidemiol. Infect. 1997, 119, 53–60. [Google Scholar] [CrossRef]
- Mac Kenzie, W.R.; Hoxie, N.J.; Proctor, M.E.; Gradus, M.S.; Blair, K.A.; Peterson, D.E.; Kazmierczak, J.J.; Addiss, D.G.; Fox, K.R.; Rose, J.B.; et al. A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. N. Engl. J. Med. 1994, 331, 161–167. [Google Scholar] [CrossRef]
- Chalmers, R.M. Waterborne outbreaks of cryptosporidiosis. Annali dell’Istituto Superiore di Sanita 2012, 48, 429–446. [Google Scholar] [CrossRef]
- Current, W.L.; Garcia, L.S. Cryptosporidiosis. Clinics in Laboratory Medicine. Clin. Lab. Med. 2020, 40, 873–897. [Google Scholar] [CrossRef]
- Savioli, L.; Smith, H.; Thompson, A. Giardia and Cryptosporidium join the ‘Neglected Diseases Initiative’. Trends. Parasitol. 2006, 22, 203–208. [Google Scholar] [CrossRef]
- Makiuchi, T.; Nozaki, T. Highly divergent mitochondrion-related organelles in anaerobic parasitic protozoa. Biochimie 2014, 100, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Mogi, T.; Kita, K. Diversity in mitochondrial metabolic pathways in parasitic protists Plasmodium and Cryptosporidium. Parasitol. Int. 2010, 59, 305–312. [Google Scholar] [CrossRef]
- Abrahamsen, M.S.; Templeton, T.J.; Enomoto, S.; Abrahante, J.E.; Zhu, G.; Lancto, C.A.; Deng, M.; Liu, C.; Widmer, G.; Tzipori, S.; et al. Complete genome sequence of the apicomplexan, Cryptosporidium parvum. Science 2004, 304, 441–445. [Google Scholar] [CrossRef]
- Palmieri, F.; Scarcia, P.; Monné, M. Diseases Caused by Mutations in Mitochondrial Carrier Genes SLC25: A Review. Biomolecules 2020, 10, 655. [Google Scholar] [CrossRef]
- Kunji, E.R.S.; King, M.S.; Ruprecht, J.J.; Thangaratnarajah, C. The SLC25 Carrier Family: Important Transport Proteins in Mitochondrial Physiology and Pathology. Physiology 2020, 35, 302–327. [Google Scholar] [CrossRef]
- Ruprecht, J.J.; Kunji, E.R.S. The SLC25 Mitochondrial Carrier Family: Structure and Mechanism. Trends Biochem. Sci. 2020, 45, 244–258. [Google Scholar] [CrossRef]
- Klingenberg, M. The ADP and ATP transport in mitochondria and its carrier. Biochim. Biophys. Acta 2008, 1778, 1978–2021. [Google Scholar] [CrossRef]
- Pebay-Peyroula, E.; Dahout-Gonzalez, C.; Kahn, R.; Trezeguet, V.; Lauquin, G.J.; Brandolin, G. Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature 2003, 426, 39–44. [Google Scholar] [CrossRef]
- Ruprecht, J.J.; Hellawell, A.M.; Harding, M.; Crichton, P.G.; McCoy, A.J.; Kunji, E.R.S. Structures of yeast mitochondrial ADP/ATP carriers support a domain-based alternating-access transport mechanism. Proc. Natl. Acad. Sci. USA 2014, 111, E426–E434. [Google Scholar] [CrossRef]
- Kunji, E.R.S.; Aleksandrova, A.; King, M.S.; Majd, H.; Ashton, V.L.; Cerson, E.; Springett, R.; Kibalchenko, M.; Tavoulari, S.; Crichton, P.G.; et al. The transport mechanism of the mitochondrial ADP/ATP carrier. Biochim. Biophys. Acta 2016, 1863, 2379–2393. [Google Scholar] [CrossRef]
- Kunji, E.R.S.; Ruprecht, J.J. The mitochondrial ADP/ATP carrier exists and functions as a monomer. Biochem. Soc. Trans. 2020, 48, 1419–1432. [Google Scholar] [CrossRef]
- Kunji, E.R.S.; Crichton, P.G. Mitochondrial carriers function as monomers. Biochim. Biophys. Acta 2010, 1797, 817–831. [Google Scholar] [CrossRef]
- Bamber, L.; Slotboom, D.-J.; Kunji, E.R.S. Yeast mitochondrial ADP/ATP carriers are monomeric in detergents as demonstrated by differential affinity purification. J. Mol. Biol. 2007, 371, 388–395. [Google Scholar] [CrossRef]
- Bamber, L.; Harding, M.; Monné, M.; Slotboom, D.-J.; Kunji, E.R.S. The yeast mitochondrial ADP/ATP carrier functions as a monomer in mitochondrial membranes. Proc. Natl. Acad. Sci. USA 2007, 104, 10830–10834. [Google Scholar] [CrossRef] [PubMed]
- Bamber, L.; Harding, M.; Butler, P.J.G.; Kunji, E.R.S. Yeast mitochondrial ADP/ATP carriers are monomeric in detergents. Proc. Natl. Acad. Sci. USA 2006, 103, 16224–16229. [Google Scholar] [CrossRef] [PubMed]
- Saraste, M.; Walker, J.E. Internal sequence repeats and the path of polypeptide in mitochondrial ADP/ATP translocase. FEBS Lett. 1982, 144, 250–254. [Google Scholar] [CrossRef]
- Kunji, E.R.S.; Harding, M. Projection structure of the atractyloside-inhibited mitochondrial ADP/ATP carrier of Saccharomyces cerevisiae. J. Biol. Chem. 2003, 278, 36985–36988. [Google Scholar] [CrossRef]
- Nelson, D.R.; Felix, C.M.; Swanson, J.M. Highly conserved charge-pair networks in the mitochondrial carrier family. J. Mol. Biol. 1998, 277, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.J.; Overy, C.; Kunji, E.R.S. The mechanism of transport by mitochondrial carriers based on analysis of symmetry. Proc. Natl. Acad. Sci. USA 2008, 105, 17766–17771. [Google Scholar] [CrossRef] [PubMed]
- King, M.S.; Kerr, M.; Crichton, P.G.; Springett, R.; Kunji, E.R.S. Formation of a cytoplasmic salt bridge network in the matrix state is a fundamental step in the transport mechanism of the mitochondrial ADP/ATP carrier. Biochim. Biophys. Acta 2016, 1857, 14–22. [Google Scholar] [CrossRef]
- Ruprecht, J.J.; King, M.S.; Zogg, T.; Aleksandrova, A.A.; Pardon, E.; Crichton, P.G.; Steyaert, J.; Kunji, E.R.S. The molecular mechanism of transport by the mitochondrial ADP/ATP carrier. Cell 2019, 176, 435–447. [Google Scholar] [CrossRef]
- De Marcos Lousa, C.; Trézéguet, V.; Dianoux, A.-C.; Brandolin, G.; Lauquin, G.J.-M. The human mitochondrial ADP/ATP carriers: Kinetic properties and biogenesis of wild-type and mutant proteins in the yeast S. cerevisiae. Biochemistry 2002, 41, 14412–14420. [Google Scholar] [CrossRef]
- Dolce, V.; Scarcia, P.; Iacopetta, D.; Palmieri, F. A fourth ADP/ATP carrier isoform in man: Identification, bacterial expression, functional characterization and tissue distribution. FEBS Lett. 2004, 579, 633–637. [Google Scholar] [CrossRef]
- Pfaff, E.; Klingenberg, M. Adenine nucleotide translocation of mitochondria. 1. Specificity and control. Eur. J. Biochem. 1968, 6, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Mifsud, J.; Ravaud, S.; Krammer, E.-M.; Chipot, C.; Kunji, E.R.S.; Pebay-Peyroula, E.; Dehez, F. The substrate specificity of the human ADP/ATP carrier AAC1. Mol. Membr. Biol. 2013, 30, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Kunji, E.R.S.; Robinson, A.J. The conserved substrate binding site of mitochondrial carriers. Biochim. Biophys. Acta 1757, 1237–1248. [Google Scholar] [CrossRef]
- Robinson, A.J.; Kunji, E.R.S. Mitochondrial carriers in the cytoplasmic state have a common substrate binding site. Proc. Natl. Acad. Sci. USA 2006, 103, 2617–2622. [Google Scholar] [CrossRef]
- Dehez, F.; Pebay-Peyroula, E.; Chipot, C. Binding of ADP in the mitochondrial ADP/ATP carrier is driven by an electrostatic funnel. J. Am. Chem. Soc. 2008, 130, 12725–12733. [Google Scholar] [CrossRef]
- Wang, Y.; Tajkhorshid, E. Electrostatic funneling of substrate in mitochondrial inner membrane carriers. Proc. Natl. Acad. Sci. USA 2008, 105, 9598–9603. [Google Scholar] [CrossRef]
- Ruprecht, J.J.; Kunji, E.R.S. Structural changes in the transport cycle of the mitochondrial ADP/ATP carrier. Curr. Opin. Struct. Biol. 2019, 57, 135–144. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; Beer, T.A.P.D.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Kunji, E.R.S.; Chan, K.W.; Slotboom, D.J.; Floyd, S.; O’Connor, R.; Monné, M. Eukaryotic membrane protein overproduction in Lactococcus lactis. Curr. Opin. Biotechnol. 2005, 16, 546–551. [Google Scholar] [CrossRef]
- Kunji, E.R.S.; Slotboom, D.-J.; Poolman, B. Lactococcus lactis as host for overproduction of functional membrane proteins. Biochim. Biophys. Acta 2003, 1610, 97–108. [Google Scholar] [CrossRef]
- Monné, M.; Chan, K.W.; Slotboom, D.-J.; Kunji, E.R.S. Functional expression of eukaryotic membrane proteins in Lactococcus lactis. Protein Sci. 2005, 14, 3048–3056. [Google Scholar] [CrossRef]
- Booty, L.M.; King, M.S.; Thangaratnarajah, C.; Majd, H.; James, A.M.; Kunji, E.R.S.; Murphy, M.P. The mitochondrial dicarboxylate and 2-oxoglutarate carriers do not transport glutathione. FEBS Lett. 2015, 589, 621–628. [Google Scholar] [CrossRef]
- Chan, K.W.; Slotboom, D.J.; Cox, S.; Embley, T.M.; Fabre, O.; van der Giezen, M.; Harding, M.; Horner, D.S.; Kunji, E.R.S.; Leon-Avila, G.; et al. A novel ADP/ATP transporter in the mitosome of the microaerophilic human parasite Entamoeba histolytica. Curr. Biol. 2005, 15, 737–742. [Google Scholar] [CrossRef]
- Majd, H.; King, M.S.; Smith, A.C.; Kunji, E.R.S. Pathogenic mutations of the human mitochondrial citrate carrier SLC25A1 lead to impaired citrate export required for lipid, dolichol, ubiquinone and sterol synthesis. Biochim. Biophys. Acta. 2018, 1859, 1–7. [Google Scholar] [CrossRef]
- King, M.S.; Boes, C.; Kunji, E.R.S. Membrane Protein Expression in Lactococcus lactis. Methods Enzymol. 2015, 556, 77–97. [Google Scholar] [CrossRef]
- Kunji, E.R.S. The role and structure of mitochondrial carriers. FEBS Lett. 2004, 564, 239–244. [Google Scholar] [CrossRef]
- Gardner, M.J.; Hall, N.; Fung, E.; White, O.; Berriman, M.; Hyman, R.W.; Carlton, J.M.; Pain, A.; Nelson, K.E.; Bowman, S.; et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 2002, 419, 498–511. [Google Scholar] [CrossRef]
- Springett, R.; King, M.S.; Crichton, P.G.; Kunji, E.R.S. Modelling the free energy profile of the mitochondrial ADP/ATP carrier. Biochim. Biophys. Acta 2017, 1858, 906–914. [Google Scholar] [CrossRef]
- Majd, H.; King, M.S.; Palmer, S.M.; Smith, A.C.; Elbourne, L.D.; Paulsen, I.T.; Sharples, D.; Henderson, P.J.; Kunji, E.R.S. Screening of candidate substrates and coupling ions of transporters by thermostability shift assays. eLife 2018, 7. [Google Scholar] [CrossRef]
- Lucas, X.; Bauza, A.; Frontera, A.; Quinonero, D. A thorough anion-pi interaction study in biomolecules: On the importance of cooperativity effects. Chem. Sci. 2016, 7, 1038–1050. [Google Scholar] [CrossRef]
- Seeber, F.; Limenitakis, J.; Soldati-Favre, D. Apicomplexan mitochondrial metabolism: A story of gains, losses and retentions. Trends Parasitol. 2008, 24, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Tovar, J.; Leon-Avila, G.; Sanchez, L.B.; Sutak, R.; Tachezy, J.; van der Giezen, M.; Hernandez, M.; Muller, M.; Lucocq, J.M. Mitochondrial remnant organelles of Giardia function in iron-sulphur protein maturation. Nature 2003, 426, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.A.P.; Hirt, R.P.; Lucocq, J.M.; Embley, T.M. A mitochondrial remnant in the microsporidian Trachipleistophora hominis. Nat. Cell Biol. 2002, 418, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Striepen, B.; Pruijssers, A.J.; Huang, J.; Li, C.; Gubbels, M.J.; Umejiego, N.N.; Hedstrom, L.; Kissinger, J.C. Gene transfer in the evolution of parasite nucleotide biosynthesis. Proc. Natl. Acad. Sci. USA 2004, 101, 3154–3159. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; Mcgettigan, P.; McWilliam, H.; Valentin, F.A.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Huang, Y.; Niu, B.; Gao, Y.; Fu, L.; Li, W. CD-HIT Suite: A web server for clustering and comparing biological sequences. Bioinformatics 2010, 26, 680–682. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
King, M.S.; Tavoulari, S.; Mavridou, V.; King, A.C.; Mifsud, J.; Kunji, E.R.S. A Single Cysteine Residue in the Translocation Pathway of the Mitosomal ADP/ATP Carrier from Cryptosporidium parvum Confers a Broad Nucleotide Specificity. Int. J. Mol. Sci. 2020, 21, 8971. https://doi.org/10.3390/ijms21238971
King MS, Tavoulari S, Mavridou V, King AC, Mifsud J, Kunji ERS. A Single Cysteine Residue in the Translocation Pathway of the Mitosomal ADP/ATP Carrier from Cryptosporidium parvum Confers a Broad Nucleotide Specificity. International Journal of Molecular Sciences. 2020; 21(23):8971. https://doi.org/10.3390/ijms21238971
Chicago/Turabian StyleKing, Martin S., Sotiria Tavoulari, Vasiliki Mavridou, Alannah C. King, John Mifsud, and Edmund R. S. Kunji. 2020. "A Single Cysteine Residue in the Translocation Pathway of the Mitosomal ADP/ATP Carrier from Cryptosporidium parvum Confers a Broad Nucleotide Specificity" International Journal of Molecular Sciences 21, no. 23: 8971. https://doi.org/10.3390/ijms21238971
APA StyleKing, M. S., Tavoulari, S., Mavridou, V., King, A. C., Mifsud, J., & Kunji, E. R. S. (2020). A Single Cysteine Residue in the Translocation Pathway of the Mitosomal ADP/ATP Carrier from Cryptosporidium parvum Confers a Broad Nucleotide Specificity. International Journal of Molecular Sciences, 21(23), 8971. https://doi.org/10.3390/ijms21238971





