Extracellular Vesicles, Influential Players of Intercellular Communication within Adult Neurogenic Niches
Abstract
1. Introduction
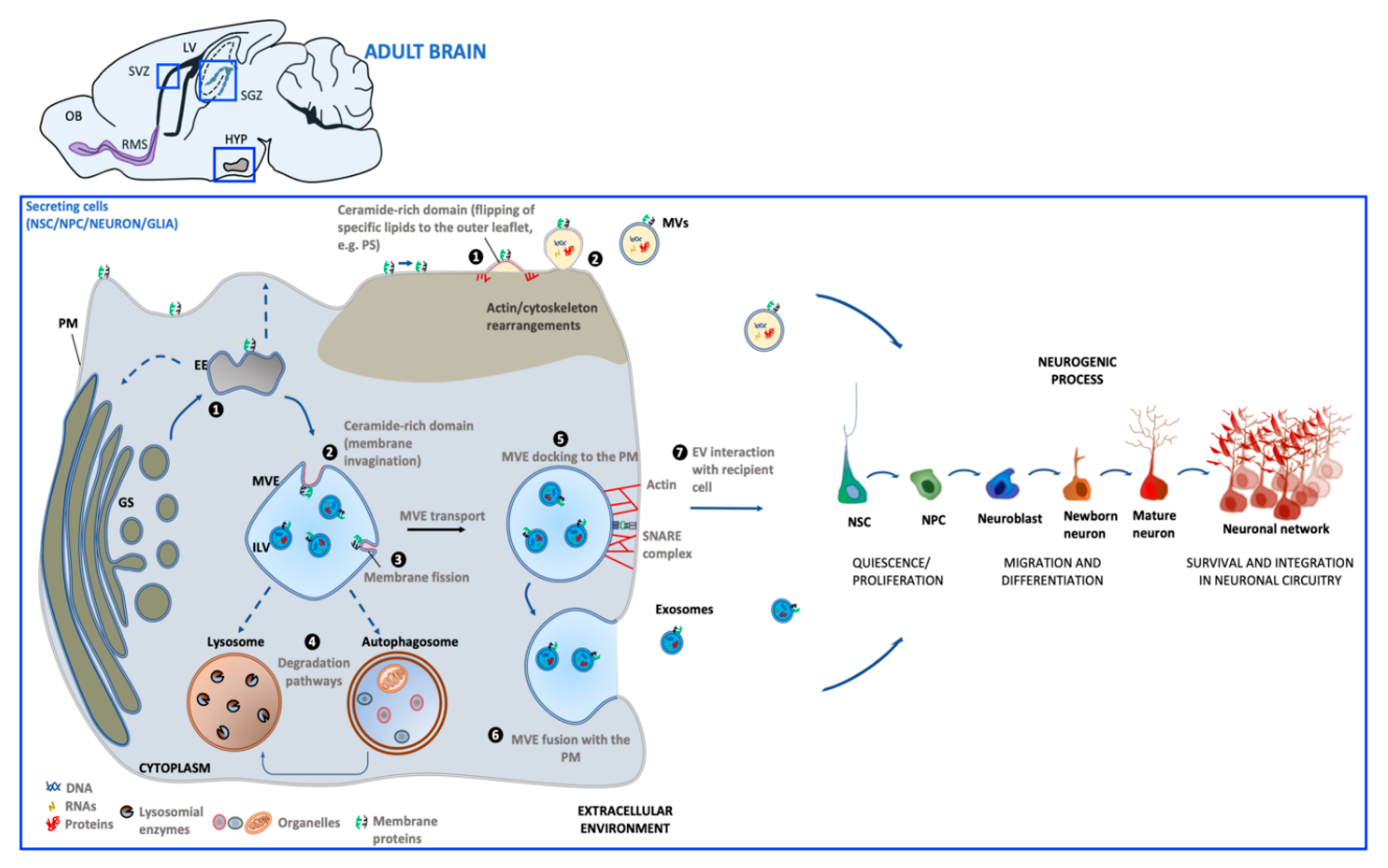
2. Extracellular Vesicles
Biogenesis and Function
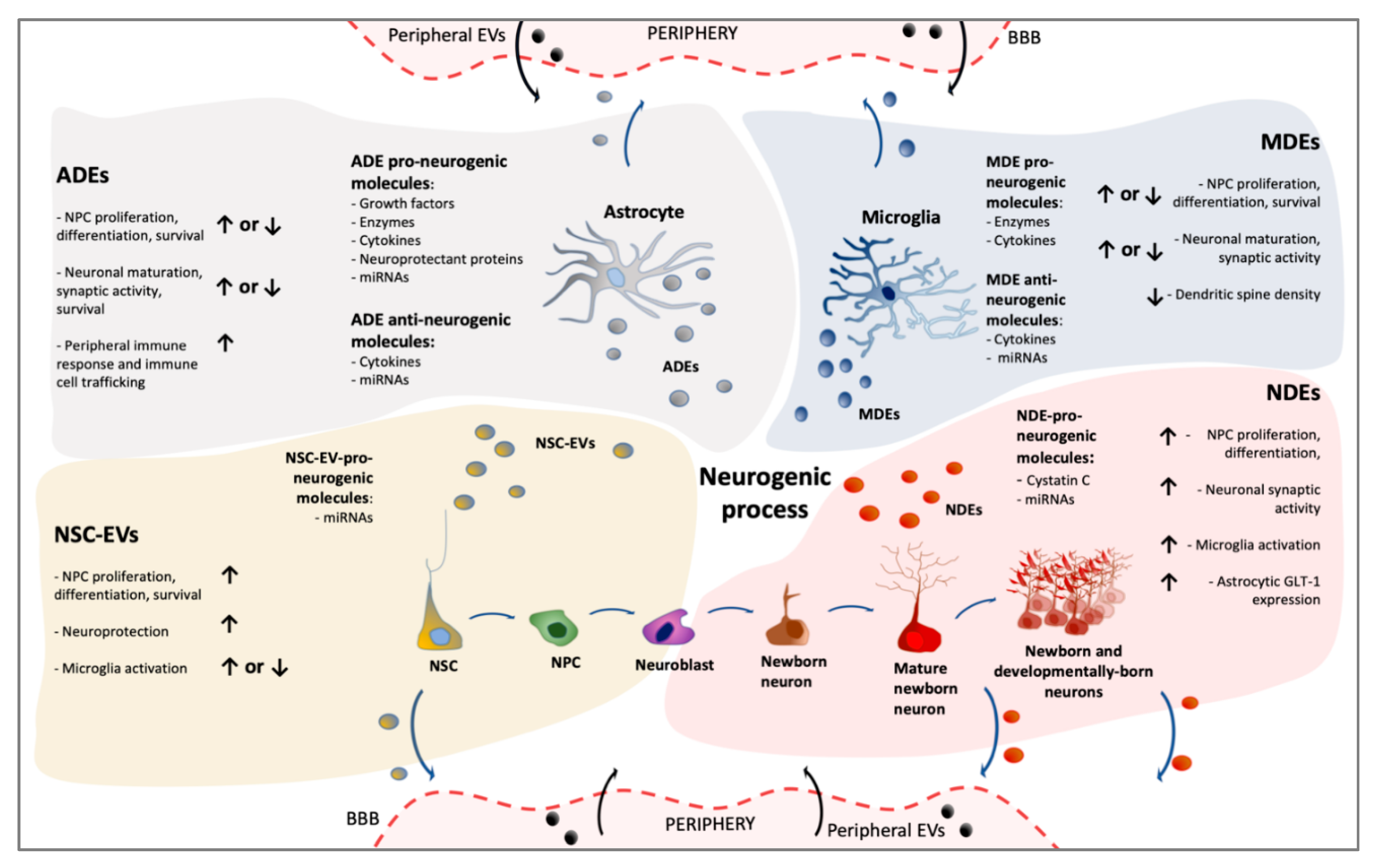
3. Extracellular Vesicles Generated in Adult Neurogenic Niches
3.1. NSC-Derived Extracellular Vesicles
3.1.1. NSC-EVs: Effects on Adult NSC and Their Neuronal Progeny
3.1.2. NSC-EVs: Effects on Glial Cells
3.1.3. NSC-EVs: Endocrine Functions
3.2. Neuron-Derived EVs in Neurogenic Niches
3.2.1. NDE: Effects on NSC and Their Progeny
3.2.2. NDE: Effects on Glial Cells
3.3. Glia-Derived Extracellular Vesicles
3.3.1. EV-Associated Growth Factors
3.3.2. EV-Associated Enzymes and Transporters
3.3.3. EV-Associated Neuroprotective Proteins
3.3.4. EV-Associated Cytokines
3.3.5. EV-Associated miRNAs
4. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eriksson, P.S.; Perfilieva, E.; Björk-Eriksson, T.; Alborn, A.M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Lepousez, G.; Valley, M.T.; Lledo, P.-M. The impact of adult neurogenesis on olfactory bulb circuits and computations. Annu. Rev. Physiol. 2013, 75, 339–363. [Google Scholar] [CrossRef] [PubMed]
- Li, W.L.; Chu, M.W.; Wu, A.; Suzuki, Y.; Imayoshi, I.; Komiyama, T. Adult-born neurons facilitate olfactory bulb pattern separation during task engagement. eLife 2018, 7, e33006. [Google Scholar] [CrossRef] [PubMed]
- Anacker, C.; Hen, R. Adult hippocampal neurogenesis and cognitive flexibility—Linking memory and mood. Nat. Rev. Neurosci. 2017, 18, 335–346. [Google Scholar] [CrossRef]
- Bortolotto, V.; Cuccurazzu, B.; Canonico, P.L.; Grilli, M. NF-κB mediated regulation of adult hippocampal neurogenesis: Relevance to mood disorders and antidepressant activity. Biomed Res. Int. 2014, 2014, 612798. [Google Scholar] [CrossRef]
- Evans, J.; Sumners, C.; Moore, J.; Huentelman, M.J.; Deng, J.; Gelband, C.H.; Shaw, G. Characterization of mitotic neurons derived from adult rat hypothalamus and brain stem. J. Neurophysiol. 2002, 87, 1076–1085. [Google Scholar] [CrossRef]
- Cheng, M.-F. Hypothalamic neurogenesis in the adult brain. Front. Neuroendocrinol. 2013, 34, 167–178. [Google Scholar] [CrossRef]
- Sousa-Ferreira, L.; de Almeida, L.P.; Cavadas, C. Role of hypothalamic neurogenesis in feeding regulation. Trends Endocrinol. Metab. 2014, 25, 80–88. [Google Scholar] [CrossRef]
- Kim, K.; Choe, H.K. Role of hypothalamus in aging and its underlying cellular mechanisms. Mech. Ageing Dev. 2019, 177, 74–79. [Google Scholar] [CrossRef]
- Lledo, P.-M.; Alonso, M.; Grubb, M.S. Adult neurogenesis and functional plasticity in neuronal circuits. Nat. Rev. Neurosci. 2006, 7, 179–193. [Google Scholar] [CrossRef]
- Toda, T.; Parylak, S.L.; Linker, S.B.; Gage, F.H. The role of adult hippocampal neurogenesis in brain health and disease. Mol. Psychiatry 2019, 24, 67–87. [Google Scholar] [CrossRef] [PubMed]
- Cassé, F.; Richetin, K.; Toni, N. Astrocytes’ Contribution to Adult Neurogenesis in Physiology and Alzheimer’s Disease. Front. Cell. Neurosci. 2018, 12, 432. [Google Scholar] [CrossRef] [PubMed]
- Cvijetic, S.; Bortolotto, V.; Manfredi, M.; Ranzato, E.; Marengo, E.; Salem, R.; Canonico, P.L.; Grilli, M. Cell autonomous and noncell-autonomous role of NF-κB p50 in astrocyte-mediated fate specification of adult neural progenitor cells. Glia 2017, 65, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, S.F.; Bortolotto, V.; Canonico, P.L.; Sortino, M.A.; Grilli, M. Astrocyte-Derived Paracrine Signals: Relevance for Neurogenic Niche Regulation and Blood-Brain Barrier Integrity. Front. Pharmacol. 2019, 10, 1346. [Google Scholar] [CrossRef]
- Lim, D.A.; Alvarez-Buylla, A. Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc. Natl. Acad. Sci. USA 1999, 96, 7526–7531. [Google Scholar] [CrossRef]
- Ashton, R.S.; Conway, A.; Pangarkar, C.; Bergen, J.; Lim, K.-I.; Shah, P.; Bissell, M.; Schaffer, D.V. Astrocytes regulate adult hippocampal neurogenesis through ephrin-B signaling. Nat. Neurosci. 2012, 15, 1399–1406. [Google Scholar] [CrossRef]
- Wilhelmsson, U.; Faiz, M.; de Pablo, Y.; Sjöqvist, M.; Andersson, D.; Widestrand, A.; Potokar, M.; Stenovec, M.; Smith, P.L.P.; Shinjyo, N.; et al. Astrocytes negatively regulate neurogenesis through the Jagged1-mediated Notch pathway. Stem Cells 2012, 30, 2320–2329. [Google Scholar] [CrossRef]
- Krzisch, M.; Temprana, S.G.; Mongiat, L.A.; Armida, J.; Schmutz, V.; Virtanen, M.A.; Kocher-Braissant, J.; Kraftsik, R.; Vutskits, L.; Conzelmann, K.-K.; et al. Pre-existing astrocytes form functional perisynaptic processes on neurons generated in the adult hippocampus. Brain Struct. Funct. 2015, 220, 2027–2042. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Matteoli, M.; Parpura, V.; Mothet, J.-P.; Zorec, R. Astrocytes as secretory cells of the central nervous system: Idiosyncrasies of vesicular secretion. EMBO J. 2016, 35, 239–257. [Google Scholar] [CrossRef]
- Diaz-Aparicio, I.; Paris, I.; Sierra-Torre, V.; Plaza-Zabala, A.; Rodríguez-Iglesias, N.; Márquez-Ropero, M.; Beccari, S.; Huguet, P.; Abiega, O.; Alberdi, E.; et al. Microglia actively remodel adult hippocampal neurogenesis through the phagocytosis secretome. J. Neurosci. 2020, 40, 1453–1482. [Google Scholar] [CrossRef]
- Sierra, A.; Encinas, J.M.; Deudero, J.J.P.; Chancey, J.H.; Enikolopov, G.; Overstreet-Wadiche, L.S.; Tsirka, S.E.; Maletic-Savatic, M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 2010, 7, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Wang, Y.; Kokovay, E.; Lin, G.; Chuang, S.-M.; Goderie, S.K.; Roysam, B.; Temple, S. Adult SVZ stem cells lie in a vascular niche: A quantitative analysis of niche cell-cell interactions. Cell Stem Cell 2008, 3, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Tavazoie, M.; Van der Veken, L.; Silva-Vargas, V.; Louissaint, M.; Colonna, L.; Zaidi, B.; Garcia-Verdugo, J.M.; Doetsch, F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell 2008, 3, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Butovsky, O.; Ziv, Y.; Schwartz, A.; Landa, G.; Talpalar, A.E.; Pluchino, S.; Martino, G.; Schwartz, M. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol. Cell. Neurosci. 2006, 31, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Ekdahl, C.T.; Kokaia, Z.; Lindvall, O. Brain inflammation and adult neurogenesis: The dual role of microglia. Neuroscience 2009, 158, 1021–1029. [Google Scholar] [CrossRef]
- Monje, M.L.; Toda, H.; Palmer, T.D. Inflammatory Blockade Restores Adult Hippocampal Neurogenesis. Science 2003, 302, 1760–1765. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Chiba, K. Diversity and plasticity of microglial cells in psychiatric and neurological disorders. Pharmacol. Ther. 2015, 154, 21–35. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Asrican, B.; Wooten, J.; Li, Y.-D.; Quintanilla, L.; Zhang, F.; Wander, C.; Bao, H.; Yeh, C.-Y.; Luo, Y.-J.; Olsen, R.; et al. Neuropeptides Modulate Local Astrocytes to Regulate Adult Hippocampal Neural Stem Cells. Neuron 2020, 108, 349–366.e6. [Google Scholar] [CrossRef]
- Song, J.; Zhong, C.; Bonaguidi, M.A.; Sun, G.J.; Hsu, D.; Gu, Y.; Meletis, K.; Huang, Z.J.; Ge, S.; Enikolopov, G.; et al. Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature 2012, 489, 150–154. [Google Scholar] [CrossRef]
- Pardal, R.; López Barneo, J. Mature neurons modulate neurogenesis through chemical signals acting on neural stem cells. Dev. Growth Differ. 2016, 58, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Deatherage, B.L.; Cookson, B.T. Membrane vesicle release in bacteria, eukaryotes, and archaea: A conserved yet underappreciated aspect of microbial life. Infect. Immun. 2012, 80, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Iavello, A.; Frech, V.S.L.; Gai, C.; Deregibus, M.C.; Quesenberry, P.J.; Camussi, G. Role of Alix in miRNA packaging during extracellular vesicle biogenesis. Int. J. Mol. Med. 2016, 37, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Adell, M.A.Y.; Vogel, G.F.; Pakdel, M.; Müller, M.; Lindner, H.; Hess, M.W.; Teis, D. Coordinated binding of Vps4 to ESCRT-III drives membrane neck constriction during MVB vesicle formation. J. Cell Biol. 2014, 205, 33–49. [Google Scholar] [CrossRef]
- Stuffers, S.; Sem Wegner, C.; Stenmark, H.; Brech, A. Multivesicular Endosome Biogenesis in the Absence of ESCRTs. Traffic 2009, 10, 925–937. [Google Scholar] [CrossRef]
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular Vesicles in Cancer: Exosomes, Microvesicles and the Emerging Role of Large Oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51. [Google Scholar] [CrossRef]
- Muralidharan-Chari, V.; Clancy, J.W.; Sedgwick, A.; D’Souza-Schorey, C. Microvesicles: Mediators of extracellular communication during cancer progression. J. Cell Sci. 2010, 123, 1603–1611. [Google Scholar] [CrossRef]
- Tricarico, C.; Clancy, J.; D’Souza-Schorey, C. Biology and biogenesis of shed microvesicles. Small GTPases 2017, 8, 220–232. [Google Scholar] [CrossRef]
- Escrevente, C.; Keller, S.; Altevogt, P.; Costa, J. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer 2011, 11, 108. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, J.; Miekus, K.; Kucia, M.; Zhang, J.; Reca, R.; Dvorak, P.; Ratajczak, M.Z. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: Evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006, 20, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Montecalvo, A.; Larregina, A.T.; Shufesky, W.J.; Beer Stolz, D.; Sullivan, M.L.G.; Karlsson, J.M.; Baty, C.J.; Gibson, G.A.; Erdos, G.; Wang, Z.; et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood 2012, 119, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, P.; Werner, N.; Jansen, F. Role and Function of MicroRNAs in Extracellular Vesicles in Cardiovascular Biology. Biomed Res. Int. 2015, 2015, 161393. [Google Scholar] [CrossRef] [PubMed]
- Xavier, L.; Anne-Clémence, V.; Tedgui, A.; Boulanger, C.M. Microvesicles as Cell–Cell Messengers in Cardiovascular Diseases. Circ. Res. 2014, 114, 345–353. [Google Scholar] [CrossRef]
- Nouraee, N.; Mowla, S.J. miRNA therapeutics in cardiovascular diseases: Promises and problems. Front. Genet. 2015, 6, 232. [Google Scholar] [CrossRef]
- Gatti, S.; Bruno, S.; Deregibus, M.C.; Sordi, A.; Cantaluppi, V.; Tetta, C.; Camussi, G. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol. Dial. Transplant. 2011, 26, 1474–1483. [Google Scholar] [CrossRef]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Leijendekker, R.; Harding, C.V.; Melief, C.J.M.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef]
- Del Conde, I.; Shrimpton, C.N.; Thiagarajan, P.; López, J.A. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood 2005, 106, 1604–1611. [Google Scholar] [CrossRef]
- Record, M.; Carayon, K.; Poirot, M.; Silvente-Poirot, S. Exosomes as new vesicular lipid transporters involved in cell–cell communication and various pathophysiologies. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2014, 1841, 108–120. [Google Scholar] [CrossRef]
- Chivet, M.; Hemming, F.; Pernet-Gallay, K.; Fraboulet, S.; Sadoul, R. Emerging role of neuronal exosomes in the central nervous system. Front. Physiol. 2012, 3, 145. [Google Scholar] [CrossRef] [PubMed]
- Coleman, B.M.; Hill, A.F. Extracellular vesicles—Their role in the packaging and spread of misfolded proteins associated with neurodegenerative diseases. Semin. Cell Dev. Biol. 2015, 40, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Gámez-Valero, A.; Beyer, K.; Borràs, F.E. Extracellular vesicles, new actors in the search for biomarkers of dementias. Neurobiol. Aging 2019, 74, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Fevrier, B.; Vilette, D.; Archer, F.; Loew, D.; Faigle, W.; Vidal, M.; Laude, H.; Raposo, G. Cells release prions in association with exosomes. Proc. Natl. Acad. Sci. USA 2004, 101, 9683–9688. [Google Scholar] [CrossRef]
- Li, B.; Liu, J.; Gu, G.; Han, X.; Zhang, Q.; Zhang, W. Impact of neural stem cell-derived extracellular vesicles on mitochondrial dysfunction, sirtuin 1 level, and synaptic deficits in Alzheimer’s disease. J. Neurochem. 2020, 154, e15001. [Google Scholar] [CrossRef]
- Webb, R.L.; Kaiser, E.E.; Scoville, S.L.; Thompson, T.A.; Fatima, S.; Pandya, C.; Sriram, K.; Swetenburg, R.L.; Vaibhav, K.; Arbab, A.S.; et al. Human Neural Stem Cell Extracellular Vesicles Improve Tissue and Functional Recovery in the Murine Thromboembolic Stroke Model. Transl. Stroke Res. 2018, 9, 530–539. [Google Scholar] [CrossRef]
- Mahdavipour, M.; Hassanzadeh, G.; Seifali, E.; Mortezaee, K.; Aligholi, H.; Shekari, F.; Sarkoohi, P.; Zeraatpisheh, Z.; Nazari, A.; Movassaghi, S.; et al. Effects of neural stem cell-derived extracellular vesicles on neuronal protection and functional recovery in the rat model of middle cerebral artery occlusion. Cell Biochem. Funct. 2020, 38, 373–383. [Google Scholar] [CrossRef]
- Sun, X.; Jung, J.-H.; Arvola, O.; Santoso, M.R.; Giffard, R.G.; Yang, P.C.; Stary, C.M. Stem Cell-Derived Exosomes Protect Astrocyte Cultures From in vitro Ischemia and Decrease Injury as Post-stroke Intravenous Therapy. Front. Cell. Neurosci. 2019, 13, 394. [Google Scholar] [CrossRef]
- Rong, Y.; Liu, W.; Wang, J.; Fan, J.; Luo, Y.; Li, L.; Kong, F.; Chen, J.; Tang, P.; Cai, W. Neural stem cell-derived small extracellular vesicles attenuate apoptosis and neuroinflammation after traumatic spinal cord injury by activating autophagy. Cell Death Dis. 2019, 10, 340. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, K.; Pan, J.; Fan, Z.; Tian, C.; Deng, X.; Ma, K.; Xia, X.; Huang, Y.; Zheng, J.C. Induced neural progenitor cells abundantly secrete extracellular vesicles and promote the proliferation of neural progenitors via extracellular signal-regulated kinase pathways. Neurobiol. Dis. 2019, 124, 322–334. [Google Scholar] [CrossRef]
- Palmer, T.D.; Willhoite, A.R.; Gage, F.H. Vascular niche for adult hippocampal neurogenesis. J. Comp. Neurol. 2000, 425, 479–494. [Google Scholar] [CrossRef]
- Louissaint, A.J.; Rao, S.; Leventhal, C.; Goldman, S.A. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron 2002, 34, 945–960. [Google Scholar] [CrossRef]
- Zhong, D.; Cao, Y.; Li, C.-J.; Li, M.; Rong, Z.-J.; Jiang, L.; Guo, Z.; Lu, H.-B.; Hu, J.-Z. Neural stem cell-derived exosomes facilitate spinal cord functional recovery after injury by promoting angiogenesis. Exp. Biol. Med. 2020, 245, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Mesci, P.; Carromeu, C.; McClatchy, D.R.; Schiapparelli, L.; Yates, J.R.; Muotri, A.R.; Cline, H.T. Exosomes regulate neurogenesis and circuit assembly. Proc. Natl. Acad. Sci. USA 2019, 116, 16086–16094. [Google Scholar] [CrossRef] [PubMed]
- Taupin, P.; Ray, J.; Fischer, W.H.; Suhr, S.T.; Hakansson, K.; Grubb, A.; Gage, F.H. FGF-2-Responsive Neural Stem Cell Proliferation Requires CCg, a Novel Autocrine/Paracrine Cofactor. Neuron 2000, 28, 385–397. [Google Scholar] [CrossRef]
- Ghidoni, R.; Paterlini, A.; Albertini, V.; Glionna, M.; Monti, E.; Schiaffonati, L.; Benussi, L.; Levy, E.; Binetti, G. Cystatin C is released in association with exosomes: A new tool of neuronal communication which is unbalanced in Alzheimer’s disease. Neurobiol. Aging 2011, 32, 1435–1442. [Google Scholar] [CrossRef]
- Putz, U.; Howitt, J.; Lackovic, J.; Foot, N.; Kumar, S.; Silke, J.; Tan, S.-S. Nedd4 family-interacting protein 1 (Ndfip1) is required for the exosomal secretion of Nedd4 family proteins. J. Biol. Chem. 2008, 283, 32621–32627. [Google Scholar] [CrossRef]
- Korkut, C.; Li, Y.; Koles, K.; Brewer, C.; Ashley, J.; Yoshihara, M.; Budnik, V. Regulation of postsynaptic retrograde signaling by presynaptic exosome release. Neuron 2013, 77, 1039–1046. [Google Scholar] [CrossRef]
- Lee, S.H.; Shin, S.M.; Zhong, P.; Kim, H.-T.; Kim, D.-I.; Kim, J.M.; Do Heo, W.; Kim, D.-W.; Yeo, C.-Y.; Kim, C.-H.; et al. Reciprocal control of excitatory synapse numbers by Wnt and Wnt inhibitor PRR7 secreted on exosomes. Nat. Commun. 2018, 9, 3434. [Google Scholar] [CrossRef]
- Palenzuela, R.; Gutiérrez, Y.; Draffin, J.E.; Lario, A.; Benoist, M.; Esteban, J.A. MAP1B Light Chain Modulates Synaptic Transmission via AMPA Receptor Intracellular Trapping. J. Neurosci. 2017, 37, 9945–9963. [Google Scholar] [CrossRef]
- Goldie, B.J.; Dun, M.D.; Lin, M.; Smith, N.D.; Verrills, N.M.; Dayas, C.V.; Cairns, M.J. Activity-associated miRNA are packaged in Map1b-enriched exosomes released from depolarized neurons. Nucleic Acids Res. 2014, 42, 9195–9208. [Google Scholar] [CrossRef] [PubMed]
- Iraci, N.; Gaude, E.; Leonardi, T.; Costa, A.S.H.; Cossetti, C.; Peruzzotti-Jametti, L.; Bernstock, J.D.; Saini, H.K.; Gelati, M.; Vescovi, A.L.; et al. Extracellular vesicles are independent metabolic units with asparaginase activity. Nat. Chem. Biol. 2017, 13, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.M.; Duan, X.; Huang, A.S.; Liu, C.Y.; Ming, G.; Song, H.; Snyder, S.H. Aspartate racemase, generating neuronal D-aspartate, regulates adult neurogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 3175–3179. [Google Scholar] [CrossRef] [PubMed]
- Vicini, S. The role of GABA and glutamate on adult neurogenesis. J. Physiol. 2008, 586, 3737–3738. [Google Scholar] [CrossRef] [PubMed]
- Hidano, S.; Randall, L.M.; Dawson, L.; Dietrich, H.K.; Konradt, C.; Klover, P.J.; John, B.; Harris, T.H.; Fang, Q.; Turek, B.; et al. STAT1 Signaling in Astrocytes Is Essential for Control of Infection in the Central Nervous System. MBio 2016, 7, e01881-16. [Google Scholar] [CrossRef]
- Tsuda, M.; Masuda, T.; Kitano, J.; Shimoyama, H.; Tozaki-Saitoh, H.; Inoue, K. IFN-gamma receptor signaling mediates spinal microglia activation driving neuropathic pain. Proc. Natl. Acad. Sci. USA 2009, 106, 8032–8037. [Google Scholar] [CrossRef]
- Cossetti, C.; Iraci, N.; Mercer, T.R.; Leonardi, T.; Alpi, E.; Drago, D.; Alfaro-Cervello, C.; Saini, H.K.; Davis, M.P.; Schaeffer, J.; et al. Extracellular Vesicles from Neural Stem Cells Transfer IFN-γ via Ifngr1 to Activate Stat1 Signaling in Target Cells. Mol. Cell 2014, 56, 193–204. [Google Scholar] [CrossRef]
- Ma, Y.; Li, C.; Huang, Y.; Wang, Y.; Xia, X.; Zheng, J.C. Exosomes released from neural progenitor cells and induced neural progenitor cells regulate neurogenesis through miR-21a. Cell Commun. Signal. 2019, 17, 96. [Google Scholar] [CrossRef]
- Zhao, C.; Sun, G.; Li, S.; Shi, Y. A feedback regulatory loop involving microRNA-9 and nuclear receptor TLX in neural stem cell fate determination. Nat. Struct. Mol. Biol. 2009, 16, 365–371. [Google Scholar] [CrossRef]
- Zhao, C.; Sun, G.; Li, S.; Lang, M.-F.; Yang, S.; Li, W.; Shi, Y. MicroRNA regulates neural stem cell proliferation and differentiation by targeting nuclear receptor TLX signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 1876–1881. [Google Scholar] [CrossRef]
- Bielefeld, P.; Mooney, C.; Henshall, D.C.; Fitzsimons, C.P. miRNA-Mediated Regulation of Adult Hippocampal Neurogenesis; Implications for Epilepsy. Brain Plast. 2017, 3, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Seguro, B.; Diogo, M.; Fitzsimons, C. Neural Stem Cell-Derived Extracellular Vesicles as Paracrine Regulators in the Hippocampus. Extended Abstract. 2018. Available online: https://fenix.tecnico.ulisboa.pt/downloadFile/1689244997258826/Extended_Abstract.pdf (accessed on 25 November 2018).
- Morton, M.C.; Neckles, V.N.; Seluzicki, C.M.; Holmberg, J.C.; Feliciano, D.M. Neonatal Subventricular Zone Neural Stem Cells Release Extracellular Vesicles that Act as a Microglial Morphogen. Cell Rep. 2018, 23, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, S.M.; Krüger, C.; Park, B.; Derkow, K.; Rosenberger, K.; Baumgart, J.; Trimbuch, T.; Eom, G.; Hinz, M.; Kaul, D.; et al. An unconventional role for miRNA: Let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat. Neurosci. 2012, 15, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Ma, R.; Yang, L.; Hu, G.; Chen, X.; Duan, M.; Kook, Y.; Niu, F.; Liao, K.; Fu, M.; et al. MiR-9 promotes microglial activation by targeting MCPIP1. Nat. Commun. 2014, 5, 4386. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bhatia, H.S.; de Oliveira, A.C.P.; Fiebich, B.L. microRNA-26a modulates inflammatory response induced by toll-like receptor 4 stimulation in microglia. J. Neurochem. 2015, 135, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Y.-J.; Wu, X.-Y.; Hong, Z.; Wei, W.-S. MicroRNA-181c negatively regulates the inflammatory response in oxygen-glucose-deprived microglia by targeting Toll-like receptor 4. J. Neurochem. 2015, 132, 713–723. [Google Scholar] [CrossRef]
- Mollinari, C.; Racaniello, M.; Berry, A.; Pieri, M.; de Stefano, M.C.; Cardinale, A.; Zona, C.; Cirulli, F.; Garaci, E.; Merlo, D. miR-34a regulates cell proliferation, morphology and function of newborn neurons resulting in improved behavioural outcomes. Cell Death Dis. 2015, 6, e1622. [Google Scholar] [CrossRef]
- Sarkar, S.; Jun, S.; Rellick, S.; Quintana, D.D.; Cavendish, J.Z.; Simpkins, J.W. Expression of microRNA-34a in Alzheimer’s disease brain targets genes linked to synaptic plasticity, energy metabolism, and resting state network activity. Brain Res. 2016, 1646, 139–151. [Google Scholar] [CrossRef]
- Åkerblom, M.; Sachdeva, R.; Barde, I.; Verp, S.; Gentner, B.; Trono, D.; Jakobsson, J. MicroRNA-124 Is a Subventricular Zone Neuronal Fate Determinant. J. Neurosci. 2012, 32, 8879–8889. [Google Scholar] [CrossRef]
- Cheng, L.-C.; Pastrana, E.; Tavazoie, M.; Doetsch, F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat. Neurosci. 2009, 12, 399–408. [Google Scholar] [CrossRef]
- Veremeyko, T.; Kuznetsova, I.S.; Dukhinova, M.; Yung, A.W.Y.; Kopeikina, E.; Barteneva, N.S.; Ponomarev, E.D. Neuronal extracellular microRNAs miR-124 and miR-9 mediate cell–cell communication between neurons and microglia. J. Neurosci. Res. 2019, 97, 162–184. [Google Scholar] [CrossRef] [PubMed]
- Men, Y.; Yelick, J.; Jin, S.; Tian, Y.; Chiang, M.S.R.; Higashimori, H.; Brown, E.; Jarvis, R.; Yang, Y. Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS. Nat. Commun. 2019, 10, 4136. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wei, Q.; Huang, Y.; Xia, W.; Zhou, Y.; Wang, S. The effects of astrocytes on differentiation of neural stem cells are influenced by knock-down of the glutamate transporter, GLT-1. Neurochem. Int. 2013, 63, 498–506. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Danbolt, N.C. Glutamate uptake. Prog. Neurobiol. 2001, 65, 1–105. [Google Scholar] [CrossRef]
- Yin, Z.; Han, Z.; Hu, T.; Zhang, S.; Ge, X.; Huang, S.; Wang, L.; Yu, J.; Li, W.; Wang, Y.; et al. Neuron-derived exosomes with high miR-21-5p expression promoted polarization of M1 microglia in culture. Brain. Behav. Immun. 2020, 83, 270–282. [Google Scholar] [CrossRef]
- Stappert, L.; Klaus, F.; Brüstle, O. MicroRNAs Engage in Complex Circuits Regulating Adult Neurogenesis. Front. Neurosci. 2018, 12, 707. [Google Scholar] [CrossRef]
- Stevanato, L.; Thanabalasundaram, L.; Vysokov, N.; Sinden, J.D. Investigation of Content, Stoichiometry and Transfer of miRNA from Human Neural Stem Cell Line Derived Exosomes. PLoS ONE 2016, 11, e0146353. [Google Scholar] [CrossRef]
- Zhang, Y.; Kim, M.S.; Jia, B.; Yan, J.; Zuniga-Hertz, J.P.; Han, C.; Cai, D. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature 2017, 548, 52–57. [Google Scholar] [CrossRef]
- Cimadamore, F.; Amador-Arjona, A.; Chen, C.; Huang, C.-T.; Terskikh, A. V SOX2–LIN28/let-7 pathway regulates proliferation and neurogenesis in neural precursors. Proc. Natl. Acad. Sci. USA 2013, 110, E3017–E3026. [Google Scholar] [CrossRef]
- Moon, C.; Yoo, J.-Y.; Matarazzo, V.; Sung, Y.K.; Kim, E.J.; Ronnett, G. V Leukemia inhibitory factor inhibits neuronal terminal differentiation through STAT3 activation. Proc. Natl. Acad. Sci. USA 2002, 99, 9015–9020. [Google Scholar] [CrossRef]
- Shi, Y.; Chichung Lie, D.; Taupin, P.; Nakashima, K.; Ray, J.; Yu, R.T.; Gage, F.H.; Evans, R.M. Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature 2004, 427, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Sanuki, R.; Onishi, A.; Koike, C.; Muramatsu, R.; Watanabe, S.; Muranishi, Y.; Irie, S.; Uneo, S.; Koyasu, T.; Matsui, R.; et al. miR-124a is required for hippocampal axogenesis and retinal cone survival through Lhx2 suppression. Nat. Neurosci. 2011, 14, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, J.; Purkayastha, S.; Tang, Y.; Zhang, H.; Yin, Y.; Li, B.; Liu, G.; Cai, D. Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature 2013, 497, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Molofsky, A.V.; Slutsky, S.G.; Joseph, N.M.; He, S.; Pardal, R.; Krishnamurthy, J.; Sharpless, N.E.; Morrison, S.J. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature 2006, 443, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Encinas, J.M.; Michurina, T.V.; Peunova, N.; Park, J.-H.; Tordo, J.; Peterson, D.A.; Fishell, G.; Koulakov, A.; Enikolopov, G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 2011, 8, 566–579. [Google Scholar] [CrossRef]
- Villeda, S.A.; Luo, J.; Mosher, K.I.; Zou, B.; Britschgi, M.; Bieri, G.; Stan, T.M.; Fainberg, N.; Ding, Z.; Eggel, A.; et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 2011, 477, 90–94. [Google Scholar] [CrossRef]
- Chivet, M.; Javalet, C.; Laulagnier, K.; Blot, B.; Hemming, F.J.; Sadoul, R. Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J. Extracell. Vesicles 2014, 3, 24722. [Google Scholar] [CrossRef]
- Fauré, J.; Lachenal, G.; Court, M.; Hirrlinger, J.; Chatellard-Causse, C.; Blot, B.; Grange, J.; Schoehn, G.; Goldberg, Y.; Boyer, V.; et al. Exosomes are released by cultured cortical neurones. Mol. Cell. Neurosci. 2006, 31, 642–648. [Google Scholar] [CrossRef]
- Lachenal, G.; Pernet-Gallay, K.; Chivet, M.; Hemming, F.J.; Belly, A.; Bodon, G.; Blot, B.; Haase, G.; Goldberg, Y.; Sadoul, R. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol. Cell. Neurosci. 2011, 46, 409–418. [Google Scholar] [CrossRef]
- Wang, Y.; Balaji, V.; Kaniyappan, S.; Krüger, L.; Irsen, S.; Tepper, K.; Chandupatla, R.; Maetzler, W.; Schneider, A.; Mandelkow, E.; et al. The release and trans-synaptic transmission of Tau via exosomes. Mol. Neurodegener. 2017, 12, 5. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, Y.; Du, X.-F.; Li, J.; Zi, H.-X.; Bu, J.-W.; Yan, Y.; Han, H.; Du, J.-L. Neurons secrete miR-132-containing exosomes to regulate brain vascular integrity. Cell Res. 2017, 27, 882–897. [Google Scholar] [CrossRef] [PubMed]
- Arantes, R.M.E.; Andrews, N.W. A role for synaptotagmin VII-regulated exocytosis of lysosomes in neurite outgrowth from primary sympathetic neurons. J. Neurosci. 2006, 26, 4630–4637. [Google Scholar] [CrossRef] [PubMed]
- Dellarole, A.; Grilli, M. Adult dorsal root ganglia sensory neurons express the early neuronal fate marker doublecortin. J. Comp. Neurol. 2008, 511, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Stolt, C.C.; Lommes, P.; Sock, E.; Chaboissier, M.-C.; Schedl, A.; Wegner, M. The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev. 2003, 17, 1677–1689. [Google Scholar] [CrossRef] [PubMed]
- Visvanathan, J.; Lee, S.; Lee, B.; Lee, J.W.; Lee, S.-K. The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes Dev. 2007, 21, 744–749. [Google Scholar] [CrossRef]
- Bahrini, I.; Song, J.; Diez, D.; Hanayama, R. Neuronal exosomes facilitate synaptic pruning by up-regulating complement factors in microglia. Sci. Rep. 2015, 5, 7989. [Google Scholar] [CrossRef]
- Ekdahl, C.T. Microglial activation—tuning and pruning adult neurogenesis. Front. Pharmacol. 2012, 3, 41. [Google Scholar] [CrossRef]
- Rahpeymai, Y.; Hietala, M.A.; Wilhelmsson, U.; Fotheringham, A.; Davies, I.; Nilsson, A.-K.; Zwirner, J.; Wetsel, R.A.; Gerard, C.; Pekny, M.; et al. Complement: A novel factor in basal and ischemia-induced neurogenesis. EMBO J. 2006, 25, 1364–1374. [Google Scholar] [CrossRef]
- Upadhya, R.; Zingg, W.; Shetty, S.; Shetty, A.K. Astrocyte-derived extracellular vesicles: Neuroreparative properties and role in the pathogenesis of neurodegenerative disorders. J. Control. Release 2020, 323, 225–239. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Bergamini, G.; Rajendran, L. Cell-to-cell Communication by Extracellular Vesicles: Focus on Microglia. Neuroscience 2019, 405, 148–157. [Google Scholar] [CrossRef]
- Ohkubo, Y.; Uchida, A.O.; Shin, D.; Partanen, J.; Vaccarino, F.M. Fibroblast growth factor receptor 1 is required for the proliferation of hippocampal progenitor cells and for hippocampal growth in mouse. J. Neurosci. 2004, 24, 6057–6069. [Google Scholar] [CrossRef] [PubMed]
- Proia, P.; Schiera, G.; Mineo, M.; Ingrassia Maria Rita, A.; Santoro, G.; Savettieri, G.; Di Liegro, I. Astrocytes shed extracellular vesicles that contain fibroblast growth factor-2 and vascular endothelial growth factor. Int. J. Mol. Med. 2008, 21, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Espósito, M.S.; Piatti, V.C.; Laplagne, D.A.; Morgenstern, N.A.; Ferrari, C.C.; Pitossi, F.J.; Schinder, A.F. Neuronal Differentiation in the Adult Hippocampus Recapitulates Embryonic Development. J. Neurosci. 2005, 25, 10074–10086. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Goh, E.L.K.; Sailor, K.A.; Kitabatake, Y.; Ming, G.; Song, H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 2006, 439, 589–593. [Google Scholar] [CrossRef]
- Bordey, A. Enigmatic GABAergic networks in adult neurogenic zones. Brain Res. Rev. 2007, 53, 124–134. [Google Scholar] [CrossRef]
- Gosselin, R.-D.; Meylan, P.; Decosterd, I. Extracellular microvesicles from astrocytes contain functional glutamate transporters: Regulation by protein kinase C and cell activation. Front. Cell. Neurosci. 2013, 7, 251. [Google Scholar] [CrossRef]
- Gampe, K.; Stefani, J.; Hammer, K.; Brendel, P.; Pötzsch, A.; Enikolopov, G.; Enjyoji, K.; Acker-Palmer, A.; Robson, S.C.; Zimmermann, H. NTPDase2 and purinergic signaling control progenitor cell proliferation in neurogenic niches of the adult mouse brain. Stem Cells 2015, 33, 253–264. [Google Scholar] [CrossRef]
- Chauhan, G.; Ray, K.; Sahu, S.; Roy, K.; Jain, V.; Wadhwa, M.; Panjwani, U.; Kishore, K.; Singh, S.B. Adenosine A1 receptor antagonist mitigates deleterious effects of sleep deprivation on adult neurogenesis and spatial reference memory in rats. Neuroscience 2016, 337, 107–116. [Google Scholar] [CrossRef]
- Benito-Muñoz, M.; Matute, C.; Cavaliere, F. Adenosine A1 receptor inhibits postnatal neurogenesis and sustains astrogliogenesis from the subventricular zone. Glia 2016, 64, 1465–1478. [Google Scholar] [CrossRef]
- Ceruti, S.; Colombo, L.; Magni, G.; Viganò, F.; Boccazzi, M.; Deli, M.A.; Sperlágh, B.; Abbracchio, M.P.; Kittel, Á. Oxygen–glucose deprivation increases the enzymatic activity and the microvesicle-mediated release of ectonucleotidases in the cells composing the blood–brain barrier. Neurochem. Int. 2011, 59, 259–271. [Google Scholar] [CrossRef]
- Eisch, A.J.; Barrot, M.; Schad, C.A.; Self, D.W.; Nestler, E.J. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc. Natl. Acad. Sci. USA 2000, 97, 7579–7584. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Kim, J.-E.; Lee, R.; Malberg, J.E.; Chen, J.; Steffen, C.; Zhang, Y.-J.; Nestler, E.J.; Duman, R.S. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J. Neurosci. 2002, 22, 3673–3682. [Google Scholar] [CrossRef] [PubMed]
- Arguello, A.A.; Harburg, G.C.; Schonborn, J.R.; Mandyam, C.D.; Yamaguchi, M.; Eisch, A.J. Time course of morphine’s effects on adult hippocampal subgranular zone reveals preferential inhibition of cells in S phase of the cell cycle and a subpopulation of immature neurons. Neuroscience 2008, 157, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zheng, H.; Loh, H.H.; Law, P.-Y. Morphine Promotes Astrocyte-Preferential Differentiation of Mouse Hippocampal Progenitor Cells via PKCε-Dependent ERK Activation and TRBP Phosphorylation. Stem Cells 2015, 33, 2762–2772. [Google Scholar] [CrossRef]
- Potolicchio, I.; Carven, G.J.; Xu, X.; Stipp, C.; Riese, R.J.; Stern, L.J.; Santambrogio, L. Proteomic Analysis of Microglia-Derived Exosomes: Metabolic Role of the Aminopeptidase CD13 in Neuropeptide Catabolism. J. Immunol. 2005, 175, 2237–2243. [Google Scholar] [CrossRef]
- Lev-Vachnish, Y.; Cadury, S.; Rotter-Maskowitz, A.; Feldman, N.; Roichman, A.; Illouz, T.; Varvak, A.; Nicola, R.; Madar, R.; Okun, E. L-Lactate Promotes Adult Hippocampal Neurogenesis. Front. Neurosci. 2019, 13, 403. [Google Scholar] [CrossRef]
- Barbieri, R.; Contestabile, A.; Ciardo, M.G.; Forte, N.; Marte, A.; Baldelli, P.; Benfenati, F.; Onofri, F. Synapsin I and Synapsin II regulate neurogenesis in the dentate gyrus of adult mice. Oncotarget 2018, 9, 18760–18774. [Google Scholar] [CrossRef]
- Jovanovic, J.N.; Benfenati, F.; Siow, Y.L.; Sihra, T.S.; Sanghera, J.S.; Pelech, S.L.; Greengard, P.; Czernik, A.J. Neurotrophins stimulate phosphorylation of synapsin I by MAP kinase and regulate synapsin I-actin interactions. Proc. Natl. Acad. Sci. USA 1996, 93, 3679–3683. [Google Scholar] [CrossRef]
- Hilfiker, S.; Pieribone, V.A.; Czernik, A.J.; Kao, H.T.; Augustine, G.J.; Greengard, P. Synapsins as regulators of neurotransmitter release. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1999, 354, 269–279. [Google Scholar] [CrossRef]
- Wang, S.; Cesca, F.; Loers, G.; Schweizer, M.; Buck, F.; Benfenati, F.; Schachner, M.; Kleene, R. Synapsin I Is an Oligomannose-Carrying Glycoprotein, Acts As an Oligomannose-Binding Lectin, and Promotes Neurite Outgrowth and Neuronal Survival When Released via Glia-Derived Exosomes. J. Neurosci. 2011, 31, 7275–7290. [Google Scholar] [CrossRef]
- Evgen’ev, M.B.; Krasnov, G.S.; Nesterova, I.V.; Garbuz, D.G.; Karpov, V.L.; Morozov, A.V.; Snezhkina, A.V.; Samokhin, A.N.; Sergeev, A.; Kulikov, A.M.; et al. Molecular Mechanisms Underlying Neuroprotective Effect of Intranasal Administration of Human Hsp70 in Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 59, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Kim, W.; Jung, H.Y.; Kang, M.S.; Kim, J.W.; Hahn, K.R.; Yoo, D.Y.; Yoon, Y.S.; Hwang, I.K.; Kim, D.W. Heat shock protein 70 increases cell proliferation, neuroblast differentiation, and the phosphorylation of CREB in the hippocampus. Lab. Anim. Res. 2019, 35, 21. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.R.; Robinson, M.B.; Gifondorwa, D.J.; Tytell, M.; Milligan, C.E. Regulation of heat shock protein 70 release in astrocytes: Role of signaling kinases. Dev. Neurobiol. 2007, 67, 1815–1829. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Cheng, C.; Liu, Y.; Liu, N.; Lo, E.H.; Wang, X. Neuroglobin promotes neurogenesis through Wnt signaling pathway. Cell Death Dis. 2018, 9, 945. [Google Scholar] [CrossRef] [PubMed]
- Venturini, A.; Passalacqua, M.; Pelassa, S.; Pastorino, F.; Tedesco, M.; Cortese, K.; Gagliani, M.C.; Leo, G.; Maura, G.; Guidolin, D.; et al. Exosomes From Astrocyte Processes: Signaling to Neurons. Front. Pharmacol. 2019, 10, 1452. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.D.; Hein, A.M.; Moravan, M.J.; Shaftel, S.S.; Olschowka, J.A.; O’Banion, M.K. Adult murine hippocampal neurogenesis is inhibited by sustained IL-1β and not rescued by voluntary running. Brain Behav. Immun. 2012, 26, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.D.; Montgomery, S.L.; Rivera-Escalera, F.; Olschowka, J.A.; O’Banion, M.K. Sustained IL-1β expression impairs adult hippocampal neurogenesis independent of IL-1 signaling in nestin+ neural precursor cells. Brain Behav. Immun. 2013, 32, 9–18. [Google Scholar] [CrossRef]
- Koo, J.W.; Duman, R.S. IL-1β is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc. Natl. Acad. Sci. USA 2008, 105, 751–756. [Google Scholar] [CrossRef]
- Wang, X.; Fu, S.; Wang, Y.; Yu, P.; Hu, J.; Gu, W.; Xu, X.-M.; Lu, P. Interleukin-1β mediates proliferation and differentiation of multipotent neural precursor cells through the activation of SAPK/JNK pathway. Mol. Cell. Neurosci. 2007, 36, 343–354. [Google Scholar] [CrossRef]
- Bianco, F.; Pravettoni, E.; Colombo, A.; Schenk, U.; Möller, T.; Matteoli, M.; Verderio, C. Astrocyte-Derived ATP Induces Vesicle Shedding and IL-1β Release from Microglia. J. Immunol. 2005, 174, 7268–7277. [Google Scholar] [CrossRef]
- Bianco, F.; Perrotta, C.; Novellino, L.; Francolini, M.; Riganti, L.; Menna, E.; Saglietti, L.; Schuchman, E.H.; Furlan, R.; Clementi, E.; et al. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J. 2009, 28, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- McPherson, C.A.; Aoyama, M.; Harry, G.J. Interleukin (IL)-1 and IL-6 regulation of neural progenitor cell proliferation with hippocampal injury: Differential regulatory pathways in the subgranular zone (SGZ) of the adolescent and mature mouse brain. Brain. Behav. Immun. 2011, 25, 850–862. [Google Scholar] [CrossRef] [PubMed]
- Vallières, L.; Campbell, I.L.; Gage, F.H.; Sawchenko, P.E. Reduced Hippocampal Neurogenesis in Adult Transgenic Mice with Chronic Astrocytic Production of Interleukin-6. J. Neurosci. 2002, 22, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Storer, M.A.; Gallagher, D.; Fatt, M.P.; Simonetta, J.V.; Kaplan, D.R.; Miller, F.D. Interleukin-6 Regulates Adult Neural Stem Cell Numbers during Normal and Abnormal Post-natal Development. Stem Cell Rep. 2018, 10, 1464–1480. [Google Scholar] [CrossRef]
- Bowen, K.K.; Dempsey, R.J.; Vemuganti, R. Adult interleukin-6 knockout mice show compromised neurogenesis. Neuroreport 2011, 22, 126–130. [Google Scholar] [CrossRef]
- Chen, Y.; Xia, K.; Chen, L.; Fan, D. Increased Interleukin-6 Levels in the Astrocyte-Derived Exosomes of Sporadic Amyotrophic Lateral Sclerosis Patients. Front. Neurosci. 2019, 13, 574. [Google Scholar] [CrossRef]
- Yang, Y.; Boza-Serrano, A.; Dunning, C.J.R.; Clausen, B.H.; Lambertsen, K.L.; Deierborg, T. Inflammation leads to distinct populations of extracellular vesicles from microglia. J. Neuroinflamm. 2018, 15, 168. [Google Scholar] [CrossRef]
- Chen, Z.; Palmer, T.D. Differential roles of TNFR1 and TNFR2 signaling in adult hippocampal neurogenesis. Brain. Behav. Immun. 2013, 30, 45–53. [Google Scholar] [CrossRef]
- Iosif, R.E.; Ekdahl, C.T.; Ahlenius, H.; Pronk, C.J.H.; Bonde, S.; Kokaia, Z.; Jacobsen, S.-E.W.; Lindvall, O. Tumor Necrosis Factor Receptor 1 Is a Negative Regulator of Progenitor Proliferation in Adult Hippocampal Neurogenesis. J. Neurosci. 2006, 26, 9703–9712. [Google Scholar] [CrossRef]
- Yang, S.-L.; Yang, M.; Herrlinger, S.; Liang, C.; Lai, F.; Chen, J.-F. MiR-302/367 regulate neural progenitor proliferation, differentiation timing, and survival in neurulation. Dev. Biol. 2015, 408, 140–150. [Google Scholar] [CrossRef]
- Jovičić, A.; Gitler, A.D. Distinct repertoires of microRNAs present in mouse astrocytes compared to astrocyte-secreted exosomes. PLoS ONE 2017, 12, e0171418. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.D.; Dastgheyb, R.M.; Yoo, S.-W.; Trout, A.; Talbot, C.C.J.; Hao, H.; Witwer, K.W.; Haughey, N.J. TNFα and IL-1β modify the miRNA cargo of astrocyte shed extracellular vesicles to regulate neurotrophic signaling in neurons. Cell Death Dis. 2018, 9, 363. [Google Scholar] [CrossRef] [PubMed]
- De Chevigny, A.; Coré, N.; Follert, P.; Gaudin, M.; Barbry, P.; Béclin, C.; Cremer, H. miR-7a regulation of Pax6 controls spatial origin of forebrain dopaminergic neurons. Nat. Neurosci. 2012, 15, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Morgado, A.L.; Rodrigues, C.M.P.; Solá, S. MicroRNA-145 Regulates Neural Stem Cell Differentiation Through the Sox2–Lin28/let-7 Signaling Pathway. Stem Cells 2016, 34, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Jovičić, A.; Roshan, R.; Moisoi, N.; Pradervand, S.; Moser, R.; Pillai, B.; Luthi-Carter, R. Comprehensive Expression Analyses of Neural Cell-Type-Specific miRNAs Identify New Determinants of the Specification and Maintenance of Neuronal Phenotypes. J. Neurosci. 2013, 33, 5127–5137. [Google Scholar] [CrossRef]
- Prada, I.; Gabrielli, M.; Turola, E.; Iorio, A.; D’Arrigo, G.; Parolisi, R.; De Luca, M.; Pacifici, M.; Bastoni, M.; Lombardi, M.; et al. Glia-to-neuron transfer of miRNAs via extracellular vesicles: A new mechanism underlying inflammation-induced synaptic alterations. Acta Neuropathol. 2018, 135, 529–550. [Google Scholar] [CrossRef]
- Yang, L.; Niu, F.; Yao, H.; Liao, K.; Chen, X.; Kook, Y.; Ma, R.; Hu, G.; Buch, S. Exosomal miR-9 Released from HIV Tat Stimulated Astrocytes Mediates Microglial Migration. J. Neuroimmune Pharmacol. 2018, 13, 330–344. [Google Scholar] [CrossRef]
- Xue, Q.; Yu, C.; Wang, Y.; Liu, L.; Zhang, K.; Fang, C.; Liu, F.; Bian, G.; Song, B.; Yang, A.; et al. miR-9 and miR-124 synergistically affect regulation of dendritic branching via the AKT/GSK3β pathway by targeting Rap2a. Sci. Rep. 2016, 6, 26781. [Google Scholar] [CrossRef]
- Li, D.; Huang, S.; Yin, Z.; Zhu, J.; Ge, X.; Han, Z.; Tan, J.; Zhang, S.; Zhao, J.; Chen, F.; et al. Increases in miR-124-3p in Microglial Exosomes Confer Neuroprotective Effects by Targeting FIP200-Mediated Neuronal Autophagy Following Traumatic Brain Injury. Neurochem. Res. 2019, 44, 1903–1923. [Google Scholar] [CrossRef]
- Liu, C.; Teng, Z.-Q.; Santistevan, N.J.; Szulwach, K.E.; Guo, W.; Jin, P.; Zhao, X. Epigenetic regulation of miR-184 by MBD1 governs neural stem cell proliferation and differentiation. Cell Stem Cell 2010, 6, 433–444. [Google Scholar] [CrossRef]
- Mao, S.; Sun, Q.; Xiao, H.; Zhang, C.; Li, L. Secreted miR-34a in astrocytic shedding vesicles enhanced the vulnerability of dopaminergic neurons to neurotoxins by targeting Bcl-2. Protein Cell 2015, 6, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Brett, J.O.; Renault, V.M.; Rafalski, V.A.; Webb, A.E.; Brunet, A. The microRNA cluster miR-106b~25 regulates adult neural stem/progenitor cell proliferation and neuronal differentiation. Aging 2011, 3, 108–124. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Zhu, Y.; Sun, Y.; Mao, X.O.; Xie, L.; Greenberg, D.A. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 11946–11950. [Google Scholar] [CrossRef] [PubMed]
- Rai, K.S.; Hattiangady, B.; Shetty, A.K. Enhanced production and dendritic growth of new dentate granule cells in the middle-aged hippocampus following intracerebroventricular FGF-2 infusions. Eur. J. Neurosci. 2007, 26, 1765–1779. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.H.; Tripps, W.K.C.; Feldmann, R.E.; Kuschinsky, W. Expression of vascular endothelial growth factor and its receptors in rat neural stem cells. Neurosci. Lett. 2003, 344, 165–168. [Google Scholar] [CrossRef]
- Schänzer, A.; Wachs, F.-P.; Wilhelm, D.; Acker, T.; Cooper-Kuhn, C.; Beck, H.; Winkler, J.; Aigner, L.; Plate, K.H.; Kuhn, H.G. Direct Stimulation of Adult Neural Stem Cells In Vitro and Neurogenesis In Vivo by Vascular Endothelial Growth Factor. Brain Pathol. 2004, 14, 237–248. [Google Scholar] [CrossRef]
- Suyama, S.; Sunabori, T.; Kanki, H.; Sawamoto, K.; Gachet, C.; Koizumi, S.; Okano, H. Purinergic Signaling Promotes Proliferation of Adult Mouse Subventricular Zone Cells. J. Neurosci. 2012, 32, 9238–9247. [Google Scholar] [CrossRef]
- Leeson, H.C.; Kasherman, M.A.; Chan-Ling, T.; Lovelace, M.D.; Brownlie, J.C.; Toppinen, K.M.; Gu, B.J.; Weible, M.W., II. P2X7 Receptors Regulate Phagocytosis and Proliferation in Adult Hippocampal and SVZ Neural Progenitor Cells: Implications for Inflammation in Neurogenesis. Stem Cells 2018, 36, 1764–1777. [Google Scholar] [CrossRef]
- Cao, X.; Li, L.-P.; Qin, X.-H.; Li, S.-J.; Zhang, M.; Wang, Q.; Hu, H.-H.; Fang, Y.-Y.; Gao, Y.-B.; Li, X.-W.; et al. Astrocytic Adenosine 5′-Triphosphate Release Regulates the Proliferation of Neural Stem Cells in the Adult Hippocampus. Stem Cells 2013, 31, 1633–1643. [Google Scholar] [CrossRef]
- Cheng, Q.; Song, S.-H.; Augustine, G.J. Molecular Mechanisms of Short-Term Plasticity: Role of Synapsin Phosphorylation in Augmentation and Potentiation of Spontaneous Glutamate Release. Front. Synaptic Neurosci. 2018, 10, 33. [Google Scholar] [CrossRef]
- Shevtsov, M.A.; Nikolaev, B.P.; Yakovleva, L.Y.; Dobrodumov, A.V.; Dayneko, A.S.; Shmonin, A.A.; Vlasov, T.D.; Melnikova, E.V.; Vilisov, A.D.; Guzhova, I.V.; et al. Neurotherapeutic activity of the recombinant heat shock protein Hsp70 in a model of focal cerebral ischemia in rats. Drug Des. Dev. Ther. 2014, 8, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Pratt, W.B.; Gestwicki, J.E.; Osawa, Y.; Lieberman, A.P. Targeting Hsp90/Hsp70-based protein quality control for treatment of adult onset neurodegenerative diseases. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 353–371. [Google Scholar] [CrossRef] [PubMed]
- Repalli, J.; Meruelo, D. Screening strategies to identify HSP70 modulators to treat Alzheimer’s disease. Drug Des. Dev. Ther. 2015, 9, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jin, K.; Peel, A.; Mao, X.O.; Xie, L.; Greenberg, D.A. Neuroglobin protects the brain from experimental stroke in vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 3497–3500. [Google Scholar] [CrossRef] [PubMed]
- Guidolin, D.; Agnati, L.F.; Tortorella, C.; Marcoli, M.; Maura, G.; Albertin, G.; Fuxe, K. Neuroglobin as a regulator of mitochondrial-dependent apoptosis: A bioinformatics analysis. Int. J. Mol. Med. 2014, 33, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, Z.P.; Luyckx, E.; Dewilde, S. Neuroglobin Expression in the Brain: A Story of Tissue Homeostasis Preservation. Mol. Neurobiol. 2019, 56, 2101–2122. [Google Scholar] [CrossRef] [PubMed]
- Fuster-Matanzo, A.; Llorens-Martín, M.; Hernández, F.; Avila, J. Role of Neuroinflammation in Adult Neurogenesis and Alzheimer Disease: Therapeutic Approaches. Mediat. Inflamm. 2013, 2013, 260925. [Google Scholar] [CrossRef]
- Pereira, L.; Font-Nieves, M.; Van den Haute, C.; Baekelandt, V.; Planas, A.M.; Pozas, E. IL-10 regulates adult neurogenesis by modulating ERK and STAT3 activity. Front. Cell. Neurosci. 2015, 9, 57. [Google Scholar] [CrossRef]
- Zhang, J.; Rong, P.; Zhang, L.; He, H.; Zhou, T.; Fan, Y.; Mo, L.; Zhao, Q.; Han, Y.; Li, S.; et al. IL4-driven microglia modulate stress resilience through BDNF-dependent neurogenesis. bioRxiv 2020. [Google Scholar] [CrossRef]
- Fitzgerald, W.; Freeman, M.L.; Lederman, M.M.; Vasilieva, E.; Romero, R.; Margolis, L. A System of Cytokines Encapsulated in ExtraCellular Vesicles. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Casella, G.; Colombo, F.; Finardi, A.; Descamps, H.; Ill-Raga, G.; Spinelli, A.; Podini, P.; Bastoni, M.; Martino, G.; Muzio, L.; et al. Extracellular Vesicles Containing IL-4 Modulate Neuroinflammation in a Mouse Model of Multiple Sclerosis. Mol. Ther. 2018, 26, 2107–2118. [Google Scholar] [CrossRef] [PubMed]
- Drago, F.; Lombardi, M.; Prada, I.; Gabrielli, M.; Joshi, P.; Cojoc, D.; Franck, J.; Fournier, I.; Vizioli, J.; Verderio, C. ATP Modifies the Proteome of Extracellular Vesicles Released by Microglia and Influences Their Action on Astrocytes. Front. Pharmacol. 2017, 8, 910. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, F.; Turola, E.; Riganti, L.; Caleo, M.; Gabrielli, M.; Perrotta, C.; Novellino, L.; Clementi, E.; Giussani, P.; Viani, P.; et al. Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J. 2012, 31, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Borgmann, K.; Edara, V.V.; Stacy, S.; Ghorpade, A.; Ikezu, T. Activated human astrocyte-derived extracellular vesicles modulate neuronal uptake, differentiation and firing. J. Extracell. Vesicles 2020, 9, 1706801. [Google Scholar] [CrossRef] [PubMed]
- Lepousez, G.; Nissant, A.; Lledo, P.-M. Adult Neurogenesis and the Future of the Rejuvenating Brain Circuits. Neuron 2015, 86, 387–401. [Google Scholar] [CrossRef]
- Datta Chaudhuri, A.; Dasgheyb, R.M.; DeVine, L.R.; Bi, H.; Cole, R.N.; Haughey, N.J. Stimulus-dependent modifications in astrocyte-derived extracellular vesicle cargo regulate neuronal excitability. Glia 2020, 68, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Dickens, A.M.; Tovar-y-Romo, L.B.; Yoo, S.-W.; Trout, A.L.; Bae, M.; Kanmogne, M.; Megra, B.; Williams, D.W.; Witwer, K.W.; Gacias, M.; et al. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci. Signal. 2017, 10, eaai7696. [Google Scholar] [CrossRef]
- Ziv, Y.; Ron, N.; Butovsky, O.; Landa, G.; Sudai, E.; Greenberg, N.; Cohen, H.; Kipnis, J.; Schwartz, M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat. Neurosci. 2006, 9, 268–275. [Google Scholar] [CrossRef]
- Wolf, S.A.; Steiner, B.; Akpinarli, A.; Kammertoens, T.; Nassenstein, C.; Braun, A.; Blankenstein, T.; Kempermann, G. CD4-positive T lymphocytes provide a neuroimmunological link in the control of adult hippocampal neurogenesis. J. Immunol. 2009, 182, 3979–3984. [Google Scholar] [CrossRef]
- Vinuesa, A.; Bentivegna, M.; Calfa, G.; Filipello, F.; Pomilio, C.; Bonaventura, M.M.; Lux-Lantos, V.; Matzkin, M.E.; Gregosa, A.; Presa, J.; et al. Early Exposure to a High-Fat Diet Impacts on Hippocampal Plasticity: Implication of Microglia-Derived Exosome-like Extracellular Vesicles. Mol. Neurobiol. 2019, 56, 5075–5094. [Google Scholar] [CrossRef]
- Mansouri, S.; Ortsäter, H.; Pintor Gallego, O.; Darsalia, V.; Sjöholm, Å.; Patrone, C. Pituitary adenylate cyclase-activating polypeptide counteracts the impaired adult neural stem cell viability induced by palmitate. J. Neurosci. Res. 2012, 90, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, D.; Kuo, W.P.; Frühbeis, C.; Sun, J.-J.; Zehendner, C.M.; Luhmann, H.J.; Pinto, S.; Toedling, J.; Trotter, J.; Krämer-Albers, E.-M. Multifaceted effects of oligodendroglial exosomes on neurons: Impact on neuronal firing rate, signal transduction and gene regulation. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130510. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.-Y.; Zhang, P.-R.; Zheng, M.-H.; Cao, X.-L.; Cao, Y.; Zhang, Y.-Z.; Zhang, Y.-F.; Wu, H.-N.; Lu, Z.-H.; Liang, L.; et al. Neurons can upregulate Cav-1 to increase intake of endothelial cells-derived extracellular vesicles that attenuate apoptosis via miR-1290. Cell Death Dis. 2019, 10, 869. [Google Scholar] [CrossRef] [PubMed]
- Winner, B.; Winkler, J. Adult neurogenesis in neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2015, 7, a021287. [Google Scholar] [CrossRef]
- Villata, S.; Canta, M.; Cauda, V. EVs and Bioengineering: From Cellular Products to Engineered Nanomachines. Int. J. Mol. Sci. 2020, 21, 6068. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. Discovery of Double-Stranded Genomic DNA in Circulating Exosomes. In Cold Spring Harbor Symposia on Quantitative Biology; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2016; Volume 81, pp. 275–280. [Google Scholar] [CrossRef]
- Chiasserini, D.; van Weering, J.R.T.; Piersma, S.R.; Pham, T.V.; Malekzadeh, A.; Teunissen, C.E.; de Wit, H.; Jiménez, C.R. Proteomic analysis of cerebrospinal fluid extracellular vesicles: A comprehensive dataset. J. Proteom. 2014, 106, 191–204. [Google Scholar] [CrossRef]
- Vacchi, E.; Burrello, J.; Di Silvestre, D.; Burrello, A.; Bolis, S.; Mauri, P.; Vassalli, G.; Cereda, C.W.; Farina, C.; Barile, L.; et al. Immune profiling of plasma-derived extracellular vesicles identifies Parkinson disease. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e866. [Google Scholar] [CrossRef]
- Ohmichi, T.; Mitsuhashi, M.; Tatebe, H.; Kasai, T.; Ali El-Agnaf, O.M.; Tokuda, T. Quantification of brain-derived extracellular vesicles in plasma as a biomarker to diagnose Parkinson’s and related diseases. Parkinsonism Relat. Disord. 2019, 61, 82–87. [Google Scholar] [CrossRef]
- Svenningsen, P.; Sabaratnam, R.; Jensen, B.L. Urinary extracellular vesicles: Origin, role as intercellular messengers and biomarkers; efficient sorting and potential treatment options. Acta Physiol. 2020, 228, e13346. [Google Scholar] [CrossRef]
- Street, J.M.; Barran, P.E.; Mackay, C.L.; Weidt, S.; Balmforth, C.; Walsh, T.S.; Chalmers, R.T.A.; Webb, D.J.; Dear, J.W. Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J. Transl. Med. 2012, 10, 5. [Google Scholar] [CrossRef]
| Class of Molecule | Molecules | Cellular Process/Molecular Target | EV Type |
|---|---|---|---|
| Growth factors | Growth factor receptor cysteine-rich domain, EGF-like domain, EGF-like calcium-binding domain | ↑ NSC proliferation by activating the down-stream extracellular signal-regulated kinase (ERK) pathways [60] | NSC-EVs [60] |
| VEGF | ↑ NSC proliferation in SGZ [61]; ↑ survival and integration of newborn neurons in the forebrain [62] | NSC-EVs [63] | |
| Proteins | Flotillin, GAP43, Cadherin 2 L1CAM | Regulate NSC proliferation and neuronal differentiation [64] | NDEs [64] |
| Cystatin C | ↑ NSC proliferation by cooperating with FGF-2 [65] | NDEs [66] | |
| Ndfip1 | ↑ Removal of protein during stress [67] | NDEs [67] | |
| Synaptotagmin 4 | ↑ Retrograde signaling in pre-synaptic cells by releasing Syt4-bound exosomes [68] | NDEs [68] | |
| PRR7 | ↑ Removal of excitatory synapses by acting as a Wnt inhibitor [69] | NDEs [69] | |
| MAP1b | ↑ synaptic transmission and plasticity [70] | NDEs [71] | |
| Enzymes | Asrgl1 | ↑ levels of aspartate/glutamate [72] which regulate adult neurogenesis [73,74] | NSC-EVs [72] |
| Cytokines | INFγ | Regulate function of microglia and astrocytes by activating Stat1 in target cells [75,76] | NSC-EVs [77] |
| miRNAs | miR-21a | ↑ NSC proliferation by targeting Sox2 and Stat3 [78] | NSC-EVs [78] |
| miR-9 | ↓ NSC proliferation and ↑ neural differentiation by targeting the stem cell regulator TLX [79] | NSC-EVs [78] | |
| miR-let-7b | ↓ NSC proliferation and ↑ neural differentiation by targeting the stem cell regulator TLX and the cell cycle regulator cyclin D1 [80] | NSC-EVs [78] | |
| miR-124 miR-137 | Regulate NSC activation/proliferation, fate specification and differentiation by cooperatively targeting the pro-apoptotic protein BCL2L13 [81] | NSC-EVs [82] | |
| miR-let-7 | Regulate microglia activation which negatively affect NSC proliferation in SVZ [83] | NSC-EVs [83] | |
| miR-9, miR-let-7, miR-26a, and miR-181c | Regulate microglia morphology and physiology [84,85,86,87] | NSC-EVs [83] | |
| miR-34a | Regulate NSC proliferation and morphology and function of newborn neurons by interacting with DCX [88] Target genes linked to the regulation of neuronal excitability, mitochondria oxidative phosphorylation, glycolysis, and resting state functional connectivity [89] | NDEs [89] | |
| miR-124 | ↑ NSC neuronal differentiation in SVZ [90] ↑ NSC neuronal differentiation in SVZ by targeting SOX9 [91] | NDEs [92] | |
| miR-124-3p | ↑ GLT-1 expression in astrocytes [93] which ↑ NSC differentiation in vitro [94] and regulate synaptic transmission [95] | NDEs [93] | |
| miR-21-5p | ↑ M1 polarization in microglia [96] | NDEs [96] |
| Class of Molecule | Molecules | Cellular Process/Molecular Target | Glial EV Type |
|---|---|---|---|
| Growth Factor | FGF-2 | ↑ NSC proliferation and differentiation in SGZ and SVZ [122] | ADEs [123] |
| VEGF | ↑ NSC proliferation in SGZ [61]; ↑ survival and integration of newborn neurons in the forebrain [62] | ADEs [123] | |
| Enzymes | EAAT-1 | ↑ NSC differentiation, maturation and integration of newly formed neurons in synaptic network in SGZ and SVZ through regulation of extracellular glutamate [124] and GABA [125,126] levels | ADEs [127] |
| NTPDases | ↓ NSC proliferation in SGZ and SVZ by regulating nucleotide ATP and adenosine levels [128] ↓ NSC proliferation in hippocampus [129] and in vitro neuronal differentiation of SVZ NSCs [130] through adenosine production | ADEs [131] | |
| CD13 | ↑ NSC proliferation, differentiation and survival through regulation of cAMP levels [132,133,134,135] | MDEs [136] | |
| MCT-1 | ↑ NSC survival of newly generated neurons [137] | MDEs [136] | |
| Neuroprotectant proteins | Synapsins | ↑ NSC proliferation and survival in adult DG [138] ↑ synapse development [139], neurotransmitter release [140], neurite outgrowth after oxygen-glucose deprivation (OGD)/oxidative stress [141] | ADEs [141] |
| HSP70 | ↑ expression of genes involved in neuronal differentiation, synaptic activity, regulation of neuronal synaptic plasticity in Alzheimer’s disease [142] ↑ NSC proliferation, differentiation in DG via enhanced CREB phosphorylation and improve novel object recognition in mice [143] | ADEs [144] | |
| Neuroglobin | ↑ NSC proliferation and differentiation in SVZ via Wnt signaling in murine stroke model [145] | ADEs [146] | |
| Cytokines | IL-1β | ↓ neurogenesis in DG by reducing the number of DCX+ cells [147] ↓ neurogenesis in DG by reducing the number of Nestin+ cells [148] ↓ hippocampal NSC proliferation in vitro via the nuclear factor-κB signaling pathway [149] ↑ NSC proliferation and differentiation through the activation of SAPK/JNK pathway [150] | MDEs [151], ADEs [152] |
| IL-6 | ↓ DG NSC proliferation in vitro [153] ↓ NSC proliferation, differentiation and survival in DG [154] ↑ NSC self-renewal and maintenance in SVZ [155] ↑ NSC proliferation and neuronal maturation in SVZ and SGZ [156] | ADEs [157], MDEs [158] | |
| TNFα | ↑ NSC proliferation and survival through TNFR2 in vitro and in vivo [159] ↓ NSC proliferation and ↑ cell death through TNFR1 in vitro and in vivo [159,160] | MDEs [158] | |
| miRNAs | miR-302 | ↑ NSC proliferation, differentiation, survival through Cyclin D1/D2 and Fgf15 [161] | ADEs [162] |
| miR-let-7d, miR-let-7a | ↓ NSC proliferation and ↑ neural differentiation by targeting TLX receptor gene [163] ↑ NSC dopaminergic differentiation in olfactory bulb by PAX6 targeting (miR-let-7a, [164]) | ADEs [163] | |
| miR-145 | ↑ NSC differentiation through Sox2-Lin28/let-7 signaling pathway [165] | ADEs [163] | |
| miR-146a-5p | ↓ NSC neural specification and synaptogenesis by targeting neuroligin 1 (Nlg1) and synaptotagmin 1 (Syt1) [166] | MDEs [167] | |
| miR-9 | ↓ NSC proliferation, ↑ NSC neural differentiation by targeting TLX receptor [79] | ADEs [168] | |
| miR-9, miR-124 | ↑NSC neural differentiation and dendritic branching of differentiated neurons by targeting the small GTP-binding protein Rap2a [169] | ADEs [168], MDEs [170] | |
| miR-184 | ↑ NSC proliferation, ↓ differentiation in SGZ by targeting Numblike [171] | ADEs [162] | |
| miR-34a | ↑ NSC proliferation, ↓ dendrite branching and neuronal maturation by targeting DCX [88] | ADEs [172], MDEs [167] | |
| miR-106b, miR-93, miR-25 | ↑ NSC proliferation and differentiation toward neuronal lineage in vitro through insulin/IGF-FoxO pathway [173] | ADEs [162] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Losurdo, M.; Grilli, M. Extracellular Vesicles, Influential Players of Intercellular Communication within Adult Neurogenic Niches. Int. J. Mol. Sci. 2020, 21, 8819. https://doi.org/10.3390/ijms21228819
Losurdo M, Grilli M. Extracellular Vesicles, Influential Players of Intercellular Communication within Adult Neurogenic Niches. International Journal of Molecular Sciences. 2020; 21(22):8819. https://doi.org/10.3390/ijms21228819
Chicago/Turabian StyleLosurdo, Morris, and Mariagrazia Grilli. 2020. "Extracellular Vesicles, Influential Players of Intercellular Communication within Adult Neurogenic Niches" International Journal of Molecular Sciences 21, no. 22: 8819. https://doi.org/10.3390/ijms21228819
APA StyleLosurdo, M., & Grilli, M. (2020). Extracellular Vesicles, Influential Players of Intercellular Communication within Adult Neurogenic Niches. International Journal of Molecular Sciences, 21(22), 8819. https://doi.org/10.3390/ijms21228819





