Suppression of Non-Random Fertilization by MHC Class I Antigens
Abstract
1. Introduction
2. Results
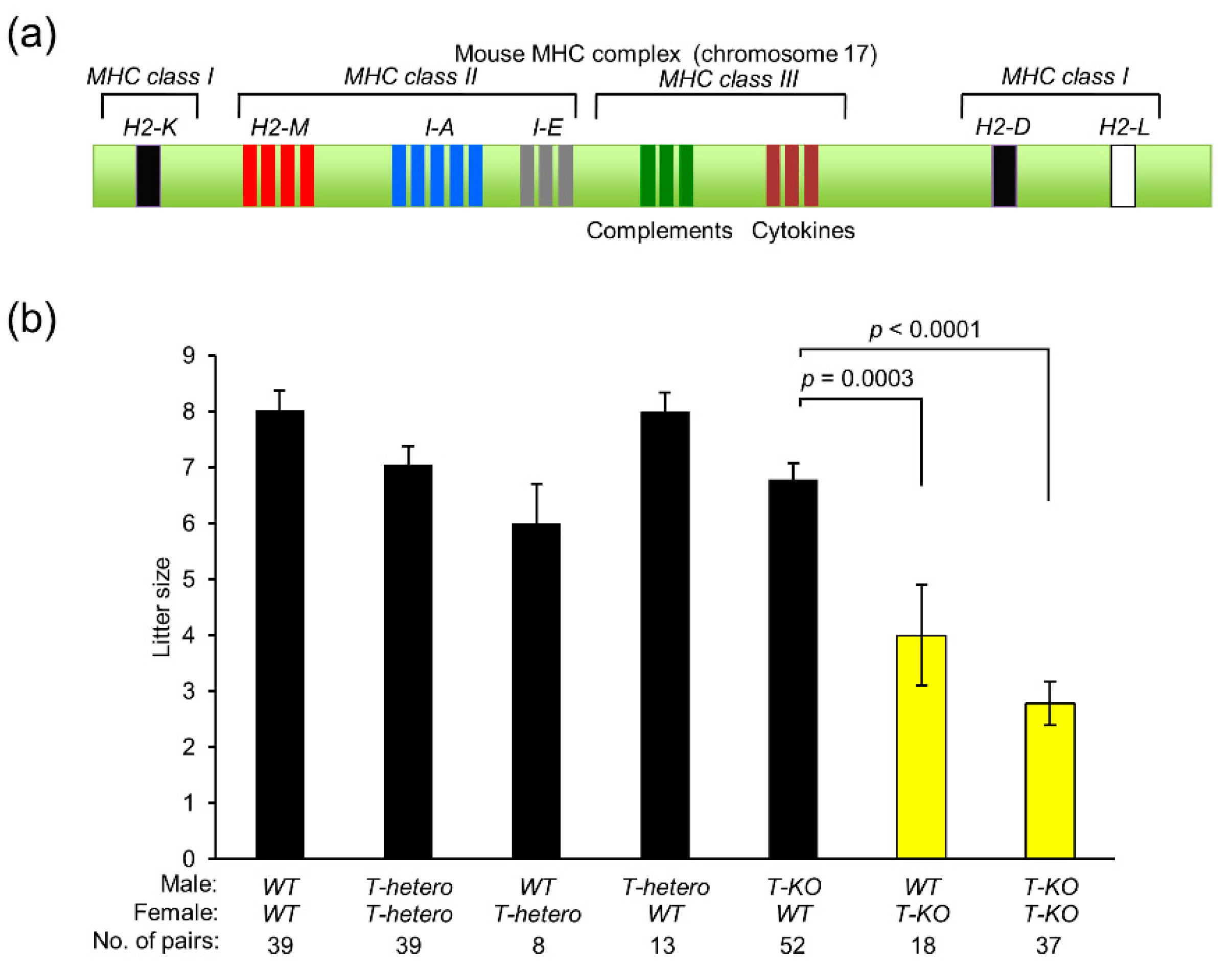
2.1. Contribution of MHC Class I Antigens to Mouse Fertility
2.2. In Vitro Fertilization
2.3. Multiple Sperm Fusion
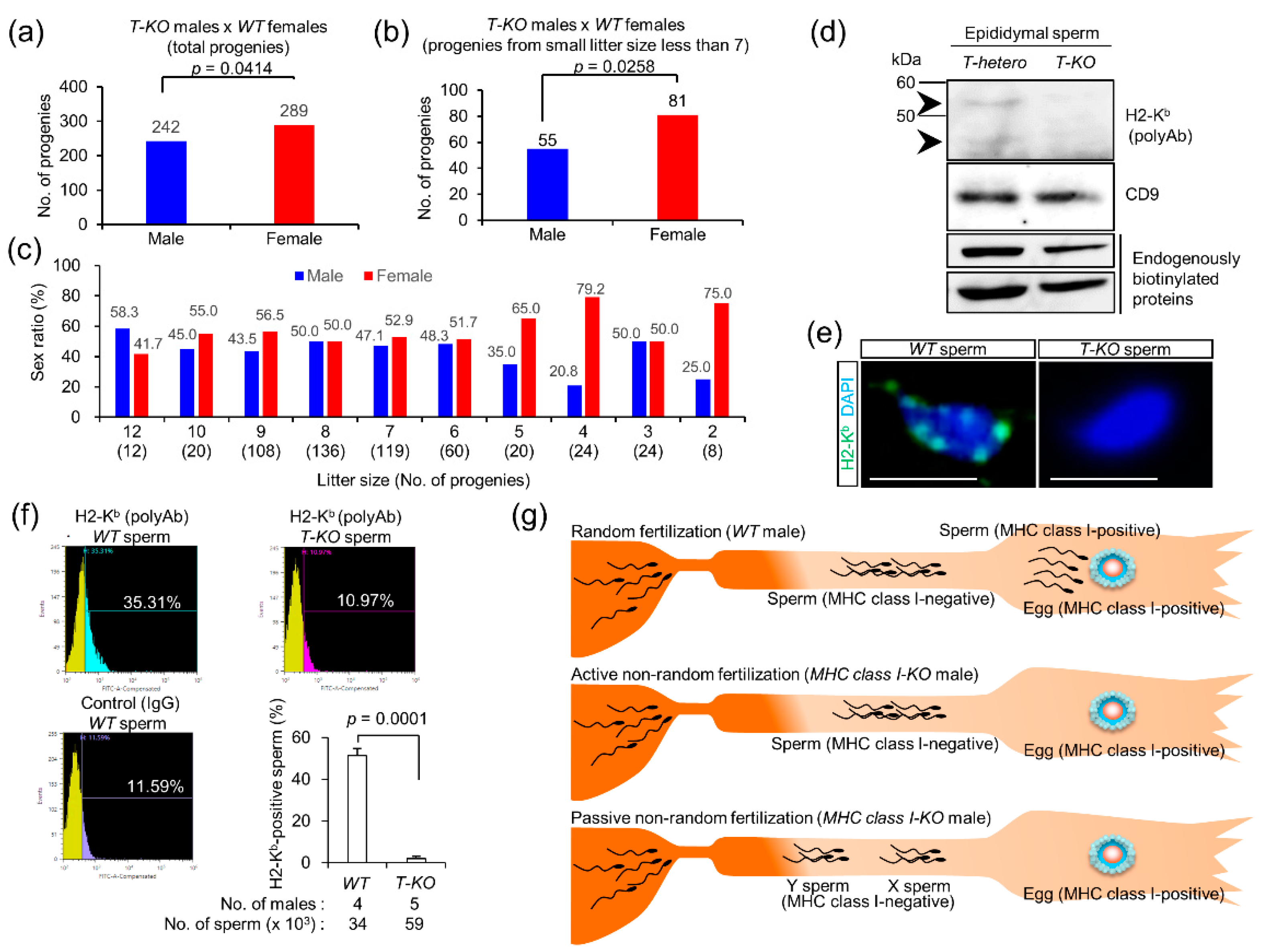
2.4. Progeny from T-KO Males and WT Females
2.5. Expression of MHC Class I Antigens on Sperm
2.6. Possible Occurrence of Non-Random Fertilization
3. Discussion
3.1. Sperm–Egg Compatibility
3.2. Sperm Heterogeneity
3.3. Differences between X and Y Sperm
3.4. Random Fertilization vs. Non-Random Fertilization
4. Materials and Methods
4.1. Antibodies
4.2. MHC-Class-I- and β2M-Deficient Mice
4.3. Immunoblotting
4.4. Flow Cytometric and Immunocytochemical Analyses
4.5. IVF
4.6. Examination of Sperm–Egg Fusion
4.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| β2M | β2-microglobulin |
| BF | bright-field microscopy |
| DAPI | 4′,6-diamidino-2-phenylindole |
| EN | egg nucleus |
| FBS | fetal bovine serum |
| H2 | histocompatibility 2 |
| hCG | human chorionic gonadotropin |
| HEPES | 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid |
| HLA | human leukocyte antigen |
| HRP | horseradish peroxidase |
| IgG | immunoglobulin G |
| IVF | in vitro fertilization |
| MHC | major histocompatibility complex |
| mAb | monoclonal antibody |
| polyAb | polyclonal antibody |
| SDS-PAGE | sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| SEM | standard error of the mean |
| SN | sperm nucleus |
| T-hetero | triple-heterozygous |
| T-KO | triple-homozygous |
| TLR | toll-like receptor |
| TYH | Toyoda, Yokoyama, Hoshi medium |
| WT | wild-type |
| BSA | bovine serum albumin |
References
- Ben-Shlomo, R. The molecular basis of allorecognition in ascidians. Bioessays 2008, 30, 1048–1051. [Google Scholar] [CrossRef]
- Harada, Y.; Sawada, H. Allorecognition mechanisms during ascidian fertilization. Int. J. Dev. Biol. 2008, 52, 637–645. [Google Scholar] [CrossRef]
- Fujii, S.; Kubo, K.; Takayama, S. Non-self- and self-recognition models in plant self-incompatibility. Nat. Plants 2016, 2, 16130. [Google Scholar] [CrossRef]
- Harada, Y.; Takagaki, Y.; Sunagawa, M.; Saito, T.; Yamada, L.; Taniguchi, H.; Shoguchi, E.; Sawada, H. Mechanism of self-sterility in a hermaphroditic chordate. Science 2008, 320, 548–550. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carlisle, J.A.; Swanson, W.J. Molecular mechanisms and evolution of fertilization proteins. J. Exp. Zool. B Mol. Dev. Evol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Marino, R.; Pinto, M.R.; Cotelli, F.; Lamia, C.L.; De Santis, R. The hsp70 protein is involved in the acquisition of gamete self-sterility in the ascidian Ciona intestinalis. Development 1998, 125, 899–907. [Google Scholar]
- Benacerraf, B. Role of MHC gene products in immune regulation. Science 1981, 212, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Ljunggren, H.G.; Karre, K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol. Today 1990, 11, 237–244. [Google Scholar] [CrossRef]
- Davis, M.M.; Bjorkman, P.J. T-cell antigen receptor genes and T-cell recognition. Nature 1988, 334, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Spies, T.; Bresnahan, M.; Bahram, S.; Arnold, D.; Blanck, G.; Mellins, E.; Pious, D.; DeMars, R. A gene in the human major histocompatibility complex class II region controlling the class I antigen presentation pathway. Nature 1990, 348, 744–747. [Google Scholar] [CrossRef]
- Gomes, A.Q.; Correia, D.V.; Silva-Santos, B. Non-classical major histocompatibility complex proteins as determinants of tumour immunosurveillance. EMBO Rep. 2007, 8, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Shiina, T.; Blancher, A.; Inoko, H.; Kulski, J.K. Comparative genomics of the human, macaque and mouse major histocompatibility complex. Immunology 2017, 150, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Vugmeyster, Y.; Glas, R.; Perarnau, B.; Lemonnier, F.A.; Eisen, H.; Ploegh, H. Major histocompatibility complex (MHC) class I KbDb -/- deficient mice possess functional CD8+ T cells and natural killer cells. Proc. Natl. Acad. Sci. USA 1998, 95, 12492–12497. [Google Scholar] [CrossRef] [PubMed]
- Springer, S. Transport and quality control of MHC class I molecules in the early secretory pathway. Curr. Opin. Immunol. 2015, 34, 83–90. [Google Scholar] [CrossRef]
- Shrestha, D.; Szollosi, J.; Jenei, A. Bare lymphocyte syndrome: An opportunity to discover our immune system. Immunol. Lett. 2012, 141, 147–157. [Google Scholar] [CrossRef]
- Hanna, S.; Etzioni, A. MHC class I and II deficiencies. J. Allergy Clin. Immunol. 2014, 134, 269–275. [Google Scholar] [CrossRef]
- Sereshki, N.; Andalib, A.; Ghahiri, A.; Mehrabian, F.; Sherkat, R.; Rezaei, A.; Wilkinson, D. The expression of human leukocyte antigen by human ejaculated spermatozoa. Mol. Genet. Genom. Med. 2019, 7, e1005. [Google Scholar] [CrossRef] [PubMed]
- Guillaudeux, T.; Gomez, E.; Onno, M.; Drenou, B.; Segretain, D.; Alberti, S.; Lejeune, H.; Fauchet, R.; Jegou, B.; Le Bouteiller, P. Expression of HLA class I genes in meiotic and post-meiotic human spermatogenic cells. Biol. Reprod 1996, 55, 99–110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bianchi, E.; Wright, G.J. Sperm meets egg: The genetics of mammalian fertilization. Annu. Rev. Genet. 2016, 50, 93–111. [Google Scholar] [CrossRef] [PubMed]
- Tokuhiro, K.; Dean, J. Glycan-independent gamete recognition triggers egg zinc sparks and ZP2 cleavage to prevent polyspermy. Dev. Cell 2018, 46, 627–640. [Google Scholar] [CrossRef]
- Rahman, M.S.; Pang, M.G. New biological insights on X and Y chromosome-bearing spermatozoa. Front. Cell Dev. Biol. 2019, 7, 388. [Google Scholar] [CrossRef] [PubMed]
- Miyado, K.; Yamada, G.; Yamada, S.; Hasuwa, H.; Nakamura, Y.; Ryu, F.; Suzuki, K.; Kosai, K.; Inoue, K.; Ogura, A.; et al. Requirement of CD9 on the egg plasma membrane for fertilization. Science 2000, 287, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Miyado, K.; Yoshida, K.; Yamagata, K.; Sakakibara, K.; Okabe, M.; Wang, X.; Miyamoto, K.; Akutsu, H.; Kondo, T.; Takahashi, Y.; et al. The fusing ability of sperm is bestowed by CD9-containing vesicles released from eggs in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 12921–12926. [Google Scholar] [CrossRef] [PubMed]
- Miyado, M.; Kang, W.; Kawano, N.; Miyado, K. Microexosomes versus exosomes: Shared components but distinct structures. Regen Ther. 2019, 11, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Ito, C.; Yamatoya, K.; Yoshida, K.; Maekawa, M.; Miyado, K.; Toshimori, K. Tetraspanin family protein CD9 in the mouse sperm: Unique localization, appearance, behavior and fate during fertilization. Cell Tissue Res. 2010, 340, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Brewis, I.A.; Gadella, B.M. Sperm surface proteomics: From protein lists to biological function. Mol. Hum. Reprod. 2010, 16, 68–79. [Google Scholar] [CrossRef]
- Coene, E.D.; Shaw, M.K.; Vaux, D.J. Anti-biotin antibodies offer superior organelle-specific labelling of mitochondria over avidin or streptavidin. Methods Mol. Biol 2008, 418, 157–170. [Google Scholar]
- Nadeau, J.H. Do gametes woo? Evidence for their nonrandom union at fertilization. Genetics 2017, 207, 369–387. [Google Scholar]
- Stein, K.K.; Primakoff, P.; Myles, D. Sperm-egg fusion: Events at the plasma membrane. J. Cell Sci. 2004, 117 Pt 26, 6269–6274. [Google Scholar] [CrossRef]
- Bureau, J.F.; Montagutelli, X.; Lefebvre, S.; Guenet, J.L.; Pla, M.; Brahic, M. The interaction of two groups of murine genes determines the persistence of Theiler’s virus in the central nervous system. J. Virol. 1992, 66, 4698–4704. [Google Scholar] [CrossRef]
- Lipton, H.L.; Melvold, R.; Miller, S.D.; Dal Canto, M.C. Mutation of a major histocompatibility class I locus, H-2D, leads to an increased virus burden and disease susceptibility in Theiler’s virus-induced demyelinating disease. J. Neurovirol. 1995, 1, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Martin-Villa, J.M.; Luque, I.; Martinez-Quiles, N.; Corell, A.; Regueiro, J.R.; Timon, M.; Arnaiz-Villena, A. Diploid expression of human leukocyte antigen class I and class II molecules on spermatozoa and their cyclic inverse correlation with inhibin concentration. Biol. Reprod. 1996, 55, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Clark, G.F. The mammalian zona pellucida: A matrix that mediates both gamete binding and immune recognition? Syst. Biol. Reprod. Med. 2010, 56, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Curry, A.J.; Pettigrew, G.J.; Negus, M.C.; Easterfield, A.J.; Young, J.L.; Bolton, E.M.; Bradley, J.A. Dendritic cells internalise and re-present conformationally intact soluble MHC class I alloantigen for generation of alloantibody. Eur. J. Immunol. 2007, 37, 696–705. [Google Scholar] [CrossRef]
- Griswold, M.D. Spermatogenesis: The Commitment to Meiosis. Physiol Rev. 2016, 96, 1–17. [Google Scholar] [CrossRef]
- Satouh, Y.; Ikawa, M. New insights into the molecular events of mammalian fertilization. Trends Biochem. Sci. 2018, 43, 818–828. [Google Scholar] [CrossRef]
- Ramon, M.; Jimenez-Rabadan, P.; Garcia-Alvarez, O.; Maroto-Morales, A.; Soler, A.J.; Fernandez-Santos, M.R.; Perez-Guzman, M.D.; Garde, J.J. Understanding sperm heterogeneity: Biological and practical implications. Reprod. Domest. Anim. 2014, 49 (Suppl. 4), 30–36. [Google Scholar] [CrossRef]
- Teitz, L.S.; Pyntikova, T.; Skaletsky, H.; Page, D.C. Selection has countered high mutability to preserve the ancestral copy number of y chromosome amplicons in diverse human lineages. Am. J. Hum. Genet. 2018, 103, 261–275. [Google Scholar] [CrossRef]
- Hotaling, J.M. Genetics of male infertility. Urol. Clin. North. Am. 2014, 41, 1–17. [Google Scholar] [CrossRef]
- Umehara, T.; Tsujita, N.; Shimada, M. Activation of Toll-like receptor 7/8 encoded by the X chromosome alters sperm motility and provides a novel simple technology for sexing sperm. PLoS Biol. 2019, 17, e3000398. [Google Scholar] [CrossRef]
- Yamatoya, K.; Ito, C.; Araki, M.; Furuse, R.; Toshimori, K. One-step collagenase method for zona pellucida removal in unfertilized eggs: Easy and gentle method for large-scale preparation. Reprod. Med. Biol. 2011, 10, 97–103. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamiya, J.; Kang, W.; Yoshida, K.; Takagi, R.; Kanai, S.; Hanai, M.; Nakamura, A.; Yamada, M.; Miyamoto, Y.; Miyado, M.; et al. Suppression of Non-Random Fertilization by MHC Class I Antigens. Int. J. Mol. Sci. 2020, 21, 8731. https://doi.org/10.3390/ijms21228731
Kamiya J, Kang W, Yoshida K, Takagi R, Kanai S, Hanai M, Nakamura A, Yamada M, Miyamoto Y, Miyado M, et al. Suppression of Non-Random Fertilization by MHC Class I Antigens. International Journal of Molecular Sciences. 2020; 21(22):8731. https://doi.org/10.3390/ijms21228731
Chicago/Turabian StyleKamiya, Junki, Woojin Kang, Keiichi Yoshida, Ryota Takagi, Seiya Kanai, Maito Hanai, Akihiro Nakamura, Mitsutoshi Yamada, Yoshitaka Miyamoto, Mami Miyado, and et al. 2020. "Suppression of Non-Random Fertilization by MHC Class I Antigens" International Journal of Molecular Sciences 21, no. 22: 8731. https://doi.org/10.3390/ijms21228731
APA StyleKamiya, J., Kang, W., Yoshida, K., Takagi, R., Kanai, S., Hanai, M., Nakamura, A., Yamada, M., Miyamoto, Y., Miyado, M., Kuroki, Y., Hayashi, Y., Umezawa, A., Kawano, N., & Miyado, K. (2020). Suppression of Non-Random Fertilization by MHC Class I Antigens. International Journal of Molecular Sciences, 21(22), 8731. https://doi.org/10.3390/ijms21228731





