Eicosanoids in Skin Wound Healing
Abstract
1. Introduction
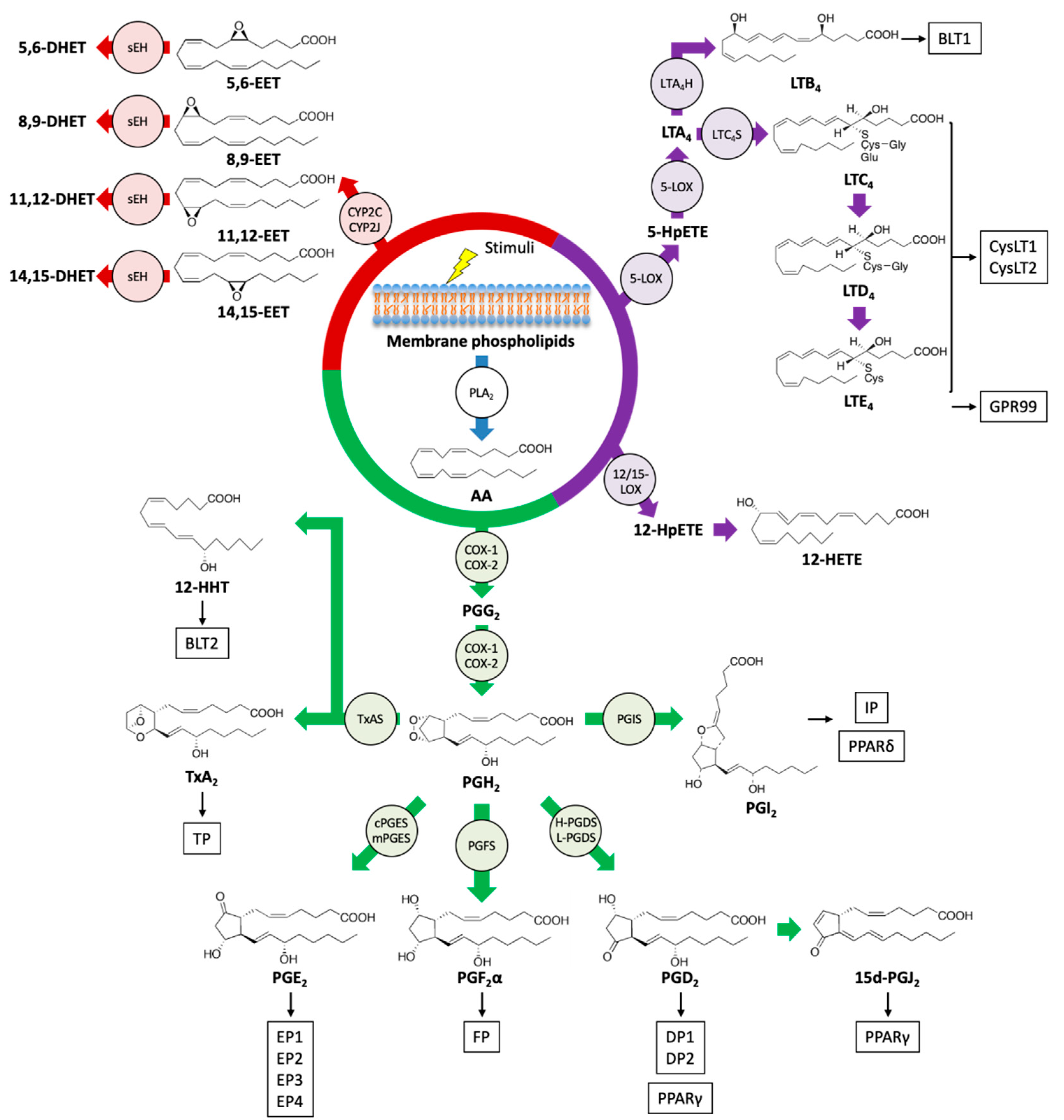
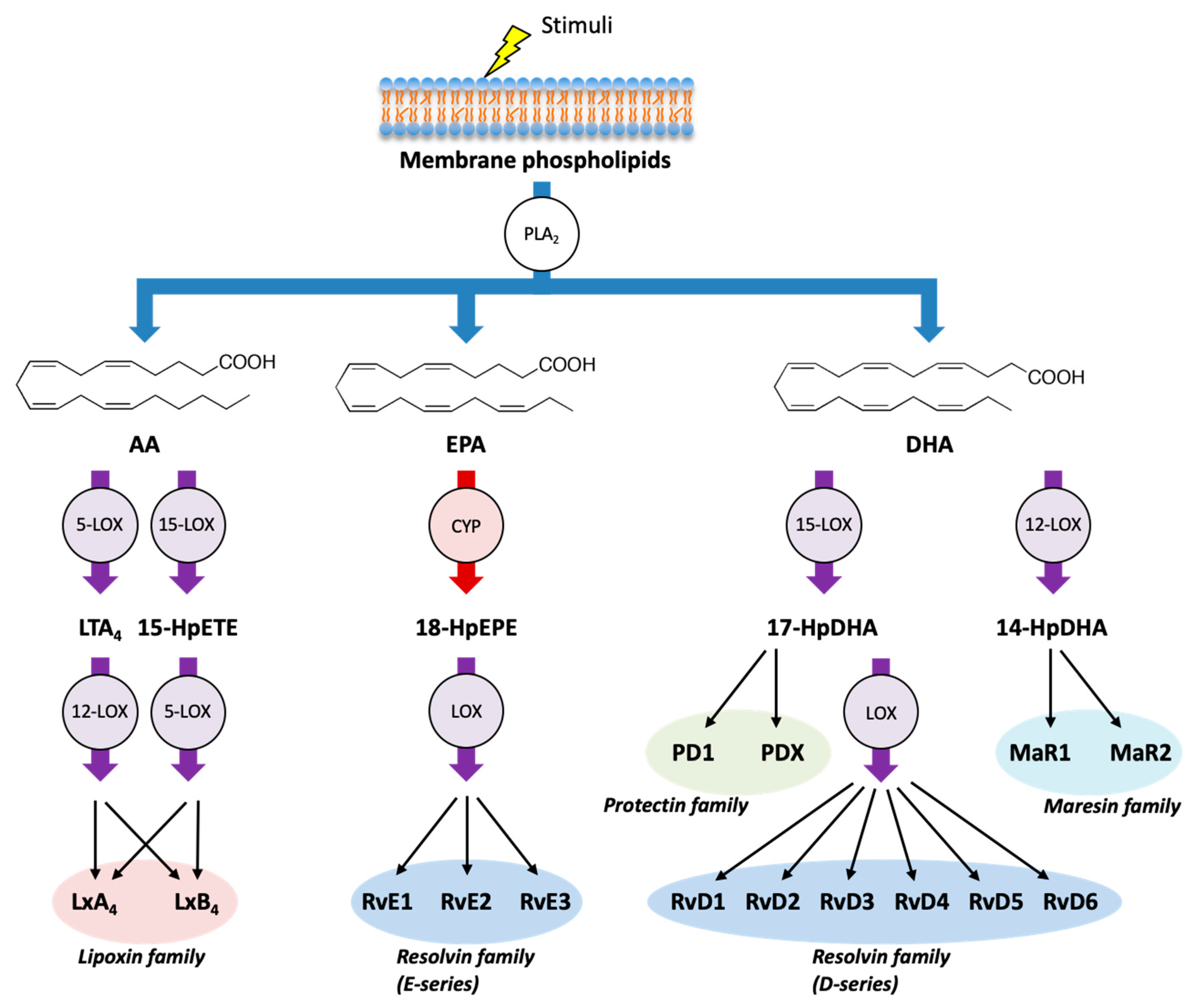
2. Biosynthetic Pathway of Eicosanoids and SPMs
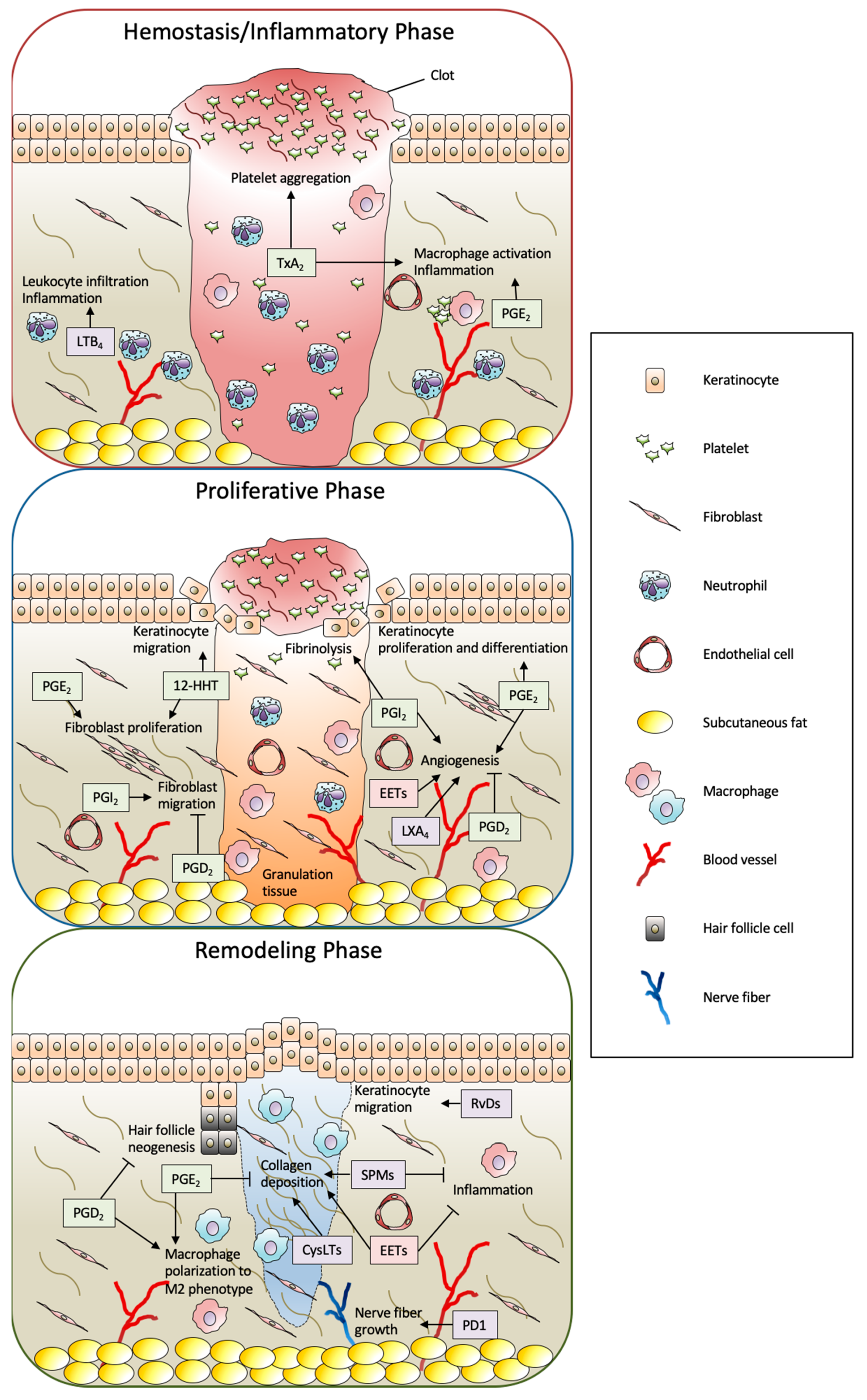
3. Functions of Eicosanoids and SPMs in Skin Wound Healing
3.1. COX Metabolites
3.1.1. TxA2
3.1.2. 12(S)-Hydroxyheptadeca-5Z,8E,10E-Trienoic Acid (12-HHT)
3.1.3. PGE2
3.1.4. PGD2
3.1.5. PGF2α
3.1.6. PGI2
3.2. LOX Metabolites
3.2.1. Leukotrienes (LTB4 and CysLTs; LTC4, LTD4, and LTE4)
3.2.2. HETEs
3.3. CYP Metabolites (EETs)
3.4. SPMs (Lipoxins, Resolvins, Protectins, and Maresins)
4. Summary and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PUFA | polyunsaturated fatty acid |
| PLA2 | phospholipase A2 |
| PG | Prostaglandin |
| TxA2 | thromboxane A2 |
| AA | arachidonic acid |
| COX | Cyclooxygenase |
| LT | Leukotriene |
| HETE | hydroxyeicosatetraenoic acid |
| LX | Lipoxin |
| LOX | lipoxygenase |
| EET | epoxyeicosatrienoic acid |
| CYP | cytochrome P450 |
| SPM | specialized pro-resolving mediator |
| EPA | eicosapentaenoic acid |
| DHA | docosahexaenoic acid |
| NSAID | nonsteroidal anti-inflammatory drug |
| HpETE | hydroperoxyeicosatetraenoic acid |
| CysLT | cysteinyl leukotriene |
| LTA4H | LTA4 hydrolase |
| LTC4S | LTC4 synthase |
| MaR1 | maresin 1 |
| TxAS | thromboxane A synthase |
| IL-6, 1β | interleukin-6, 1β |
| 12-HHT | 12(S)-hydroxyheptadeca-5Z,8E,10E-trienoic acid |
| TNFα | tumor necrosis factor α |
| MMP | matrix metalloproteinase |
| TGF-β1 | transforming growth factor-β1 |
| bFGF | basic fibroblast growth factor |
| cPGES | cytosolic prostaglandin E synthase |
| mPGES | membrane-associated prostaglandin E synthase |
| GPCR | G-protein coupled receptor |
| PGT | prostaglandin transporter |
| OSM | oncostatin M |
| CREB | cyclic AMP-responsive element binding |
| KLF4 | Krupple-like factor 4 |
| TIMP | tissue inhibitor of matrix metalloproteinase |
| H-PGDS | hematopoietic-type prostaglandin D synthase |
| L-PGDS | lipocalin-type prostaglandin D synthase |
| PPARγ, δ | peroxisome proliferator-activated receptor γ, δ |
| PGIS | prostacyclin synthase |
| uPA | urokinase-type plasminogen activator |
| VEGF | vascular endothelial growth factor |
| ROS | reactive oxygen species |
| HO-1 | heme oxygenase-1 |
| GM-CSF | granulocyte macrophage colony-stimulating factor |
| sEH | soluble epoxide hydrolase |
| DHET | dihydroxyeicosatrienoic acids |
| SDF-1α | stromal cell-derived factor 1α |
References
- Jean, K. Anatomy, histology and immunohistochemistry of normal human skin. Eur. J. Dermatol. 2002, 12, 390–401. [Google Scholar]
- Ludriksone, L.; Garcia Bartels, N.; Kanti, V.; Blume-Peytavi, U.; Kottner, J. Skin barrier function in infancy: A systematic review. Arch. Dermatol. Res 2014, 306, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Canedo-Dorantes, L.; Canedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflamm. 2019, 2019, 3706315. [Google Scholar] [CrossRef] [PubMed]
- Shaw, T.J.; Martin, P. Wound repair at a glance. J. Cell Sci. 2009, 122, 3209–3213. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Falanga, V.; Grinnell, F.; Gilchrest, B.; Maddox, Y.T.; Moshell, A. Workshop on the Pathogenesis of Chronic Wounds. J. Investig. Dermatol. 1994, 102, 125–127. [Google Scholar] [CrossRef]
- Singer, A.J.; Clark, R.A.F. Cutaneous Wound Healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef]
- Rosique, R.G.; Rosique, M.J.; Farina Junior, J.A. Curbing Inflammation in Skin Wound Healing: A Review. Int. J. Inflamm. 2015, 2015, 316235. [Google Scholar] [CrossRef]
- Moulin, V. Growth factors in skin wound healing. Eur. J. Cell Biol. 1995, 68, 1–7. [Google Scholar]
- Steed, D.L. The Role of Growth Factors in Wound Healing. Surg. Clin. 1997, 77, 575–586. [Google Scholar] [CrossRef]
- Ridiandries, A.; Tan, J.T.M.; Bursill, C.A. The Role of Chemokines in Wound Healing. Int. J. Mol. Sci. 2018, 19, 3217. [Google Scholar] [CrossRef] [PubMed]
- Esser-von Bieren, J. Eicosanoids in tissue repair. Immunol. Cell Biol. 2019, 97, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.-P.; Yin, J.; Yu, F.-S.X. Lysophosphatidic Acid Promoting Corneal Epithelial Wound Healing by Transactivation of Epidermal Growth Factor Receptor. Investig. Ophthalmol. Vis. Sci. 2007, 48, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Aoki, H.; Mukhopadhyay, P.; Tsuge, T.; Yamamoto, H.; Matsumoto, N.M.; Toyohara, E.; Okubo, Y.; Ogawa, R.; Takabe, K. Sphingosine-1-Phosphate Facilitates Skin Wound Healing by Increasing Angiogenesis and Inflammatory Cell Recruitment with Less Scar Formation. Int. J. Mol. Sci. 2019, 20, 3381. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.M.; Han, H.J. Autotaxin-LPA axis regulates hMSC migration by adherent junction disruption and cytoskeletal rearrangement via LPAR1/3-dependent PKC/GSK3beta/beta-catenin and PKC/Rho GTPase pathways. Stem Cells 2015, 33, 819–832. [Google Scholar] [CrossRef]
- Aoki, M.; Aoki, H.; Ramanathan, R.; Hait, N.C.; Takabe, K. Sphingosine-1-Phosphate Signaling in Immune Cells and Inflammation: Roles and Therapeutic Potential. Mediat. Inflamm. 2016, 2016, 8606878. [Google Scholar] [CrossRef]
- Dennis, E.A. Phospholipase A2 in eicosanoid generation. Am. J. Respir. Crit. Care Med. 2000, 161, S32–S35. [Google Scholar] [CrossRef]
- Shimizu, T. Lipid mediators in health and disease: Enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 123–150. [Google Scholar] [CrossRef]
- Esser-von Bieren, J. Immune-regulation and -functions of eicosanoid lipid mediators. Biol. Chem. 2017, 398, 1177–1191. [Google Scholar] [CrossRef]
- López, D.E.; Ballaz, S.J. The Role of Brain Cyclooxygenase-2 (Cox-2) Beyond Neuroinflammation: Neuronal Homeostasis in Memory and Anxiety. Mol. Neurobiol. 2020, 57, 5167–5176. [Google Scholar] [CrossRef]
- Ferreira, S.H.; Vane, J.R. Prostaglandins: Their Disappearance from and Release into the Circulation. Nature 1967, 216, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.A.; Kirkby, N.S. Eicosanoids, prostacyclin and cyclooxygenase in the cardiovascular system. Br. J. Pharmacol. 2019, 176, 1038–1050. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, B. Leukotrienes: Mediators of immediate hypersensitivity reactions and inflammation. Science 1983, 220, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Powell, W.S.; Rokach, J. Biosynthesis, biological effects, and receptors of hydroxyeicosatetraenoic acids (HETEs) and oxoeicosatetraenoic acids (oxo-ETEs) derived from arachidonic acid. Biochim. Biophys. Acta 2015, 1851, 340–355. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, J.; Chacos, N.; Werringloer, J.; Prough, R.A.; Estabrook, R.W. Liver microsomal cytochrome P-450 and the oxidative metabolism of arachidonic acid. Proc. Natl. Acad. Sci. USA 1981, 78, 5362–5366. [Google Scholar] [CrossRef]
- Jamieson, K.L.; Endo, T.; Darwesh, A.M.; Samokhvalov, V.; Seubert, J.M. Cytochrome P450-derived eicosanoids and heart function. Pharmacol. Ther. 2017, 179, 47–83. [Google Scholar] [CrossRef]
- Hellmann, J.; Sansbury, B.E.; Wong, B.; Li, X.; Singh, M.; Nuutila, K.; Chiang, N.; Eriksson, E.; Serhan, C.N.; Spite, M. Biosynthesis of D-Series Resolvins in Skin Provides Insights into their Role in Tissue Repair. J. Investig. Dermatol. 2018, 138, 2051–2060. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef]
- Dennis, E.A. Diversity of group types, regulation, and function of phospholipase A2. J. Biol. Chem. 1994, 269, 13057–13060. [Google Scholar]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef]
- Hirata, T.; Narumiya, S. Prostanoids as regulators of innate and adaptive immunity. Adv. Immunol. 2012, 116, 143–174. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.R.; Bakhle, Y.S.; Botting, R.M. Cyclooxygenases 1 AND 2. Annu. Rev. Pharmacol. Toxicol. 1998, 38, 97–120. [Google Scholar] [CrossRef] [PubMed]
- Penning, T.D.; Talley, J.J.; Bertenshaw, S.R.; Carter, J.S.; Collins, P.W.; Docter, S.; Graneto, M.J.; Lee, L.F.; Malecha, J.W.; Miyashiro, J.M.; et al. Synthesis and Biological Evaluation of the 1,5-Diarylpyrazole Class of Cyclooxygenase-2 Inhibitors: Identification of 4-[5-(4-Methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide (SC-58635, Celecoxib). J. Med. Chem. 1997, 40, 1347–1365. [Google Scholar] [CrossRef] [PubMed]
- Futagami, A.; Ishizaki, M.; Fukuda, Y.; Kawana, S.; Yamanaka, N. Wound healing involves induction of cyclooxygenase-2 expression in rat skin. Lab. Investig. 2002, 82, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Arai, I.; Futaki, N.; Hashimoto, Y.; Honma, Y.; Nakaike, S. Role of COX-1 and COX-2 on skin PGs biosynthesis by mechanical scratching in mice. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 1–8. [Google Scholar] [CrossRef]
- Buckman, S.Y.; Gresham, A.; Hale, P.; Hruza, G.; Anast, J.; Masferrer, J.; Pentland, A.P. COX-2 expression is induced by UVB exposure in human skin: Implications for the development of skin cancer. Carcinogenesis 1998, 19, 723–729. [Google Scholar] [CrossRef]
- Higashi, Y.; Kanekura, T.; Kanzaki, T. Enhanced expression of cyclooxygenase (COX)-2 in human skin epidermal cancer cells: Evidence for growth suppression by inhibiting COX-2 expression. Int. J. Cancer 2000, 86, 667–671. [Google Scholar] [CrossRef]
- Noverr, M.C.; Erb-Downward, J.R.; Huffnagle, G.B. Production of eicosanoids and other oxylipins by pathogenic eukaryotic microbes. Clin. Microbiol. Rev. 2003, 16, 517–533. [Google Scholar] [CrossRef]
- Lawrence, T.; Willoughby, D.A.; Gilroy, D.W. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat. Rev. Immunol. 2002, 2, 787–795. [Google Scholar] [CrossRef]
- Yokomizo, T.; Nakamura, M.; Shimizu, T. Leukotriene receptors as potential therapeutic targets. J. Clin. Investig. 2018, 128, 2691–2701. [Google Scholar] [CrossRef]
- Kuhn, H.; Banthiya, S.; van Leyen, K. Mammalian lipoxygenases and their biological relevance. Biochim. Biophys. Acta 2015, 1851, 308–330. [Google Scholar] [CrossRef] [PubMed]
- Rådmark, O.; Werz, O.; Steinhilber, D.; Samuelsson, B. 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Sheppard, K.A. Lipoxin formation during human neutrophil-platelet interactions. Evidence for the transformation of leukotriene A4 by platelet 12-lipoxygenase in vitro. J. Clin. Investig. 1990, 85, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Chiang, N.; Serhan, C.N.; Dahlen, S.E.; Drazen, J.M.; Hay, D.W.; Rovati, G.E.; Shimizu, T.; Yokomizo, T.; Brink, C. The lipoxin receptor ALX: Potent ligand-specific and stereoselective actions in vivo. Pharmacol. Rev. 2006, 58, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Spector, A.A. Arachidonic acid cytochrome P450 epoxygenase pathway. J. Lipid Res. 2009, 50, S52–S56. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Svensson, J.; Hamberg, M.; Samuelsson, B. On the formation and effects of thromboxane A2 in human platelets. Acta Physiol. Scand. 1976, 98, 285–294. [Google Scholar] [CrossRef]
- Ogletree, M.L. Overview of physiological and pathophysiological effects of thromboxane A2. Fed. Proc 1987, 46, 133–138. [Google Scholar]
- Thomas, D.W.; Mannon, R.B.; Mannon, P.J.; Latour, A.; Oliver, J.A.; Hoffman, M.; Smithies, O.; Koller, B.H.; Coffman, T.M. Coagulation defects and altered hemodynamic responses in mice lacking receptors for thromboxane A2. J. Clin. Investig. 1998, 102, 1994–2001. [Google Scholar] [CrossRef]
- Lau, C.S.; Khan, F.; McLaren, M.; Bancroft, A.; Walker, M.; Belch, J.J.F. The effects of thromboxane receptor blockade on platelet aggregation and digital skin blood flow in patients with secondary Raynaud’s syndrome. Rheumatol. Int. 1991, 11, 163–168. [Google Scholar] [CrossRef]
- Leslie, M. Beyond Clotting: The Powers of Platelets. Science 2010, 328, 562–564. [Google Scholar] [CrossRef] [PubMed]
- Mekaj, Y.H. The roles of platelets in inflammation, immunity, wound healing and malignancy. Int. J. Clin. Exp. Med. 2016, 9, 5347–5358. [Google Scholar]
- Pierre, S.; Linke, B.; Suo, J.; Tarighi, N.; Del Turco, D.; Thomas, D.; Ferreiros, N.; Stegner, D.; Frölich, S.; Sisignano, M.; et al. GPVI and Thromboxane Receptor on Platelets Promote Proinflammatory Macrophage Phenotypes during Cutaneous Inflammation. J. Investig. Dermatol. 2017, 137, 686–695. [Google Scholar] [CrossRef]
- Daniel, T.O.; Liu, H.; Morrow, J.D.; Crews, B.C.; Marnett, L.J. Thromboxane A2 is a mediator of cyclooxygenase-2-dependent endothelial migration and angiogenesis. Cancer Res. 1999, 59, 4574–4577. [Google Scholar] [PubMed]
- Nie, D.; Lamberti, M.; Zacharek, A.; Li, L.; Szekeres, K.; Tang, K.; Chen, Y.; Honn, K.V. Thromboxane A2 Regulation of Endothelial Cell Migration, Angiogenesis, and Tumor Metastasis. Biochem. Biophys. Res. Commun. 2000, 267, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Okuno, T.; Iizuka, Y.; Okazaki, H.; Yokomizo, T.; Taguchi, R.; Shimizu, T. 12(S)-Hydroxyheptadeca-5Z, 8E, 10E-trienoic acid is a natural ligand for leukotriene B4 receptor 2. J. Exp. Med. 2008, 205, 759–766. [Google Scholar] [CrossRef]
- Liu, M.; Saeki, K.; Matsunobu, T.; Okuno, T.; Koga, T.; Sugimoto, Y.; Yokoyama, C.; Nakamizo, S.; Kabashima, K.; Narumiya, S.; et al. 12-Hydroxyheptadecatrienoic acid promotes epidermal wound healing by accelerating keratinocyte migration via the BLT2 receptor. J. Exp. Med. 2014, 211, 1063–1078. [Google Scholar] [CrossRef]
- Luo, L.; Tanaka, R.; Kanazawa, S.; Lu, F.; Hayashi, A.; Yokomizo, T.; Mizuno, H. A synthetic leukotriene B4 receptor type 2 agonist accelerates the cutaneous wound healing process in diabetic rats by indirect stimulation of fibroblasts and direct stimulation of keratinocytes. J. Diabetes Its Complicat. 2017, 31, 13–20. [Google Scholar] [CrossRef][Green Version]
- Pollack, S.V. Systemic drugs and nutritional aspects of wound healing. Clin. Dermatol. 1984, 2, 68–80. [Google Scholar] [CrossRef]
- Kaushal, M.; Gopalan Kutty, N.; Mallikarjuna Rao, C. Wound healing activity of NOE-aspirin: A pre-clinical study. Nitric Oxide 2007, 16, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Tanioka, T.; Nakatani, Y.; Semmyo, N.; Murakami, M.; Kudo, I. Molecular identification of cytosolic prostaglandin E2 synthase that is functionally coupled with cyclooxygenase-1 in immediate prostaglandin E2 biosynthesis. J. Biol. Chem. 2000, 275, 32775–32782. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Naraba, H.; Tanioka, T.; Semmyo, N.; Nakatani, Y.; Kojima, F.; Ikeda, T.; Fueki, M.; Ueno, A.; Oh, S.; et al. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J. Biol. Chem. 2000, 275, 32783–32792. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, K.; Inazumi, T.; Shimamoto, A.; Sugimoto, Y. Molecular mechanisms underlying prostaglandin E2-exacerbated inflammation and immune diseases. Int. Immunol. 2019, 31, 597–606. [Google Scholar] [CrossRef]
- Sakai, Y.; Fujita, K.; Sakai, H.; Mizuno, K. Prostaglandin E2 regulates the expression of basic fibroblast growth factor messenger RNA in normal human fibroblasts. Kobe J. Med. Sci. 2001, 47, 35–45. [Google Scholar]
- Fairweather, M.; Heit, Y.I.; Buie, J.; Rosenberg, L.M.; Briggs, A.; Orgill, D.P.; Bertagnolli, M.M. Celecoxib inhibits early cutaneous wound healing. J. Surg. Res. 2015, 194, 717–724. [Google Scholar] [CrossRef]
- Kämpfer, H.; Schmidt, R.; Geisslinger, G.; Pfeilschifter, J.; Frank, S. Wound inflammation in diabetic ob/ob mice: Functional coupling of prostaglandin biosynthesis to cyclooxygenase-1 activity in diabetes-impaired wound healing. Diabetes 2005, 54, 1543–1551. [Google Scholar] [CrossRef]
- Syeda, M.M.; Jing, X.; Mirza, R.H.; Yu, H.; Sellers, R.S.; Chi, Y. Prostaglandin transporter modulates wound healing in diabetes by regulating prostaglandin-induced angiogenesis. Am. J. Pathol. 2012, 181, 334–346. [Google Scholar] [CrossRef]
- Ganesh, K.; Das, A.; Dickerson, R.; Khanna, S.; Parinandi, N.L.; Gordillo, G.M.; Sen, C.K.; Roy, S. Prostaglandin E(2) induces oncostatin M expression in human chronic wound macrophages through Axl receptor tyrosine kinase pathway. J. Immunol. 2012, 189, 2563–2573. [Google Scholar] [CrossRef]
- Lowe, N.J.; Stoughton, R.B. Effects of topical prostaglandin E analogue on normal hairless mouse epidermal DNA synthesis. J. Investig. Dermatol. 1977, 68, 134–137. [Google Scholar] [CrossRef]
- Pentland, A.P.; Needleman, P. Modulation of keratinocyte proliferation in vitro by endogenous prostaglandin synthesis. J. Clin. Investig. 1986, 77, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.B.; Pillai, S.; Goldyne, M.E. Endogenous prostaglandin E2 modulates calcium-induced differentiation in human skin keratinocytes. Prostaglandins Leukot. Essent. Fat. Acids 1993, 49, 777–781. [Google Scholar] [CrossRef]
- Konger, R.L.; Malaviya, R.; Pentland, A.P. Growth regulation of primary human keratinocytes by prostaglandin E receptor EP2 and EP3 subtypes. Biochim. Biophys. Acta 1998, 1401, 221–234. [Google Scholar] [CrossRef]
- Wang, P.H.; Huang, B.S.; Horng, H.C.; Yeh, C.C.; Chen, Y.J. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef]
- Kotwal, G.J.; Chien, S. Macrophage Differentiation in Normal and Accelerated Wound Healing. Results Probl. Cell Differ. 2017, 62, 353–364. [Google Scholar] [CrossRef]
- Luan, B.; Yoon, Y.-S.; Le Lay, J.; Kaestner, K.H.; Hedrick, S.; Montminy, M. CREB pathway links PGE2 signaling with macrophage polarization. Proc. Natl. Acad. Sci. USA 2015, 112, 15642–15647. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Zhang, X.; Zhu, D.; Qi, X.; Cao, X.; Fang, Y.; Che, Y.; Han, Z.C.; He, Z.X.; et al. Prostaglandin E2 hydrogel improves cutaneous wound healing via M2 macrophages polarization. Theranostics 2018, 8, 5348–5361. [Google Scholar] [CrossRef]
- Zhao, J.; Shu, B.; Chen, L.; Tang, J.; Zhang, L.; Xie, J.; Liu, X.; Xu, Y.; Qi, S. Prostaglandin E2 inhibits collagen synthesis in dermal fibroblasts and prevents hypertrophic scar formation in vivo. Exp. Dermatol. 2016, 25, 604–610. [Google Scholar] [CrossRef]
- Helliwell, R.J.; Adams, L.F.; Mitchell, M.D. Prostaglandin synthases: Recent developments and a novel hypothesis. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 101–113. [Google Scholar] [CrossRef]
- Joo, M.; Sadikot, R.T. PGD synthase and PGD2 in immune resposne. Mediat. Inflamm. 2012, 2012, 503128. [Google Scholar] [CrossRef]
- Arai, I.; Takano, N.; Hashimoto, Y.; Futaki, N.; Sugimoto, M.; Takahashi, N.; Inoue, T.; Nakaike, S. Prostanoid DP1 receptor agonist inhibits the pruritic activity in NC/Nga mice with atopic dermatitis. Eur. J. Pharmacol. 2004, 505, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Honma, Y.; Arai, I.; Hashimoto, Y.; Futaki, N.; Sugimoto, M.; Tanaka, M.; Nakaike, S. Prostaglandin D2 and prostaglandin E2 accelerate the recovery of cutaneous barrier disruption induced by mechanical scratching in mice. Eur. J. Pharmacol. 2005, 518, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Honma, Y.; Arai, I.; Sakurai, T.; Futaki, N.; Hashimoto, Y.; Sugimoto, M.; Nakanishi, Y.; Nakaike, S. Effects of indomethacin and dexamethasone on mechanical scratching-induced cutaneous barrier disruption in mice. Exp. Dermatol. 2006, 15, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, S.A.; Lenhard, J.M.; Willson, T.M.; Patel, I.; Morris, D.C.; Lehmann, J.M. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell 1995, 83, 813–819. [Google Scholar] [CrossRef]
- Forman, B.M.; Tontonoz, P.; Chen, J.; Brun, R.P.; Spiegelman, B.M.; Evans, R.M. 15-Deoxy-Δ12,14-Prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ. Cell 1995, 83, 803–812. [Google Scholar] [CrossRef]
- Bouhlel, M.A.; Derudas, B.; Rigamonti, E.; Dièvart, R.; Brozek, J.; Haulon, S.; Zawadzki, C.; Jude, B.; Torpier, G.; Marx, N.; et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007, 6, 137–143. [Google Scholar] [CrossRef]
- Chen, H.; Shi, R.; Luo, B.; Yang, X.; Qiu, L.; Xiong, J.; Jiang, M.; Liu, Y.; Zhang, Z.; Wu, Y. Macrophage peroxisome proliferator-activated receptor B deficiency delays skin wound healing through impairing apoptotic cell clearance in mice. Cell Death Dis. 2015, 6, e1597. [Google Scholar] [CrossRef]
- Mirza, R.E.; Fang, M.M.; Novak, M.L.; Urao, N.; Sui, A.; Ennis, W.J.; Koh, T.J. Macrophage PPARgamma and impaired wound healing in type 2 diabetes. J. Pathol. 2015, 236, 433–444. [Google Scholar] [CrossRef]
- Kohyama, T.; Liu, X.D.; Wen, F.Q.; Kim, H.J.; Takizawa, H.; Rennard, S.I. Prostaglandin D2 inhibits fibroblast migration. Eur. Respir. J. 2002, 19, 684–689. [Google Scholar] [CrossRef]
- Murata, T.; Lin, M.I.; Aritake, K.; Matsumoto, S.; Narumiya, S.; Ozaki, H.; Urade, Y.; Hori, M.; Sessa, W.C. Role of prostaglandin D2 receptor DP as a suppressor of tumor hyperpermeability and angiogenesis in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 20009–20014. [Google Scholar] [CrossRef]
- Nelson, A.M.; Loy, D.E.; Lawson, J.A.; Katseff, A.S.; Fitzgerald, G.A.; Garza, L.A. Prostaglandin D2 inhibits wound-induced hair follicle neogenesis through the receptor, Gpr44. J. Investig. Dermatol. 2013, 133, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Garza, L.A.; Liu, Y.; Yang, Z.; Alagesan, B.; Lawson, J.A.; Norberg, S.M.; Loy, D.E.; Zhao, T.; Blatt, H.B.; Stanton, D.C.; et al. Prostaglandin D2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia. Sci. Transl. Med. 2012, 4, 126ra134. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.G. Induction of term labor with intravenous PGF2α: A review. Prostaglandins 1973, 4, 765–774. [Google Scholar] [CrossRef]
- Lee, P.Y.; Shao, H.; Xu, L.A.; Qu, C.K. The effect of prostaglandin F2 alpha on intraocular pressure in normotensive human subjects. Investig. Ophthalmol. Vis. Sci. 1988, 29, 1474–1477. [Google Scholar]
- Hernández-Cueto, C.; Vieira, D.N.; Girela, E.; Marques, E.; Calvo, M.D.; Villalobos, M.; Oliveira de Sà, F.; Villanueva, E. Prostaglandin F2a (PGF2a): An inadequate marker of the vitality of wounds? Int. J. Legal Med. 1994, 106, 312–314. [Google Scholar] [CrossRef]
- Muller, K.; Krieg, P.; Marks, F.; Furstenberger, G. Expression of PGF2α receptor mRNA in normal, hyperplastic and neoplastic skin. Carcinogenesis 2000, 21, 1063–1066. [Google Scholar] [CrossRef][Green Version]
- Lee, H.-C.; Tominaga, M.; Yasukawa, K.; Ohba, M.; Takahashi, N.; Honda, K.; Okuno, T.; Takamori, K.; Yokomizo, T. Dietary supplementation of omega-3 fatty acid eicosapentaenoic acid does not ameliorate pruritus in murine models of atopic dermatitis and psoriasis. J. Dermatol. Sci. 2019, 95, 130–133. [Google Scholar] [CrossRef]
- Stjernschantz, J.W. From PGF2α-Isopropyl Ester to Latanoprost: A Review of the Development of Xalatan The Proctor Lecture. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1134–1145. [Google Scholar]
- Vane, J.; Corin, R.E. Prostacyclin: A Vascular Mediator. Eur. J. Vasc. Endovasc. Surgery 2003, 26, 571–578. [Google Scholar] [CrossRef]
- Jackson, W.F.; König, A.; Dambacher, T.; Busse, R. Prostacyclin-induced vasodilation in rabbit heart is mediated by ATP-sensitive potassium channels. Am. J. Physiol. 1993, 264, H238–H243. [Google Scholar] [CrossRef]
- Hatane, T.; Yoshida, E.; Kawano, J.; Sugiki, M.; Onitsuka, T.; Maruyama, M. Prostaglandin I2 analog enhances the expression of urokinase-type plasminogen activator and wound healing in cultured human fibroblast. Biochim. Biophys. Acta 1998, 1403, 189–198. [Google Scholar] [CrossRef]
- Pola, R.; Gaetani, E.; Flex, A.; Aprahamian, T.R.; Bosch-MarcÈ, M.; Losordo, D.; Smith, R.C.; Pola, P. Comparative analysis of the in vivo angiogenic properties of stable prostacyclin analogs: A possible role for peroxisome proliferator-activated receptors. J. Mol. Cell. Cardiol. 2004, 36, 363–370. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Lu, T.; d’Uscio, L.V.; Lam, C.F.; Lee, H.C.; Katusic, Z.S. Angiogenic function of prostacyclin biosynthesis in human endothelial progenitor cells. Circ. Res. 2008, 103, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Yokomizo, T.; Izumi, T.; Chang, K.; Takuwa, Y.; Shimizu, T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature 1997, 387, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Brandt, S.L.; Wang, S.; Dejani, N.N.; Klopfenstein, N.; Winfree, S.; Filgueiras, L.; McCarthy, B.P.; Territo, P.R.; Serezani, C.H. Excessive localized leukotriene B4 levels dictate poor skin host defense in diabetic mice. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Yokomizo, T.; Kato, K.; Terawaki, K.; Izumi, T.; Shimizu, T. A second leukotriene B(4) receptor, BLT2. A new therapeutic target in inflammation and immunological disorders. J. Exp. Med. 2000, 192, 421–432. [Google Scholar] [CrossRef]
- Kanaoka, Y.; Boyce, J.A. Cysteinyl leukotrienes and their receptors: Cellular distribution and function in immune and inflammatory responses. J. Immunol. 2004, 173, 1503–1510. [Google Scholar] [CrossRef]
- Maekawa, A.; Kanaoka, Y.; Xing, W.; Austen, K.F. Functional recognition of a distinct receptor preferential for leukotriene E4 in mice lacking the cysteinyl leukotriene 1 and 2 receptors. Proc. Natl. Acad. Sci. USA 2008, 105, 16695–16700. [Google Scholar] [CrossRef]
- Kanaoka, Y.; Maekawa, A.; Austen, K.F. Identification of GPR99 protein as a potential third cysteinyl leukotriene receptor with a preference for leukotriene E4 ligand. J. Biol. Chem. 2013, 288, 10967–10972. [Google Scholar] [CrossRef]
- Brogliato, A.R.; Moor, A.N.; Kesl, S.L.; Guilherme, R.F.; Georgii, J.L.; Peters-Golden, M.; Canetti, C.; Gould, L.J.; Benjamim, C.F. Critical role of 5-lipoxygenase and heme oxygenase-1 in wound healing. J. Invest. Dermatol. 2014, 134, 1436–1445. [Google Scholar] [CrossRef]
- Guimaraes, F.R.; Sales-Campos, H.; Nardini, V.; da Costa, T.A.; Fonseca, M.T.C.; Junior, V.R.; Sorgi, C.A.; da Silva, J.S.; Chica, J.E.L.; Faccioli, L.H.; et al. The inhibition of 5-Lipoxygenase (5-LO) products leukotriene B4 (LTB4) and cysteinyl leukotrienes (cysLTs) modulates the inflammatory response and improves cutaneous wound healing. Clin. Immunol. 2018, 190, 74–83. [Google Scholar] [CrossRef]
- Ramalho, T.; Filgueiras, L.; Silva-Jr, I.A.; Pessoa, A.F.M.; Jancar, S. Impaired wound healing in type 1 diabetes is dependent on 5-lipoxygenase products. Sci. Rep. 2018, 8, 14164. [Google Scholar] [CrossRef]
- Oyoshi, M.K.; He, R.; Kanaoka, Y.; ElKhal, A.; Kawamoto, S.; Lewis, C.N.; Austen, K.F.; Geha, R.S. Eosinophil-derived leukotriene C4 signals via type 2 cysteinyl leukotriene receptor to promote skin fibrosis in a mouse model of atopic dermatitis. Proc. Natl. Acad. Sci. USA 2012, 109, 4992–4997. [Google Scholar] [CrossRef]
- Raja, S.K.; Sivamani, K.; Garcia, M.S.; Isseroff, R.R. Wound re-epithelialization: Modulating keratinocyte migration in wound healing. Front. Biosci. 2007, 12, 2849–2868. [Google Scholar] [CrossRef]
- Chan, C.C.; Duhamel, L.; Ford-Hutchison, A. Leukotriene B4 and 12-hydroxyeicosatetraenoic acid stimulate epidermal proliferation in vivo in the guinea pig. J. Investig. Dermatol. 1985, 85, 333–334. [Google Scholar] [CrossRef]
- Ruzicka, T. The role of the epidermal 12-hydroxyeicosatetraenoic acid receptor in the skin. Eicosanoids 1992, 5, S63–S65. [Google Scholar]
- Yokomizo, T.; Kato, K.; Hagiya, H.; Izumi, T.; Shimizu, T. Hydroxyeicosanoids bind to and activate the low affinity leukotriene B4 receptor, BLT2. J. Biol. Chem. 2001, 276, 12454–12459. [Google Scholar] [CrossRef]
- Panigrahy, D.; Kalish, B.T.; Huang, S.; Bielenberg, D.R.; Le, H.D.; Yang, J.; Edin, M.L.; Lee, C.R.; Benny, O.; Mudge, D.K.; et al. Epoxyeicosanoids promote organ and tissue regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 13528–13533. [Google Scholar] [CrossRef]
- Sander, A.L.; Sommer, K.; Neumayer, T.; Fleming, I.; Marzi, I.; Barker, J.H.; Frank, J.; Jakob, H. Soluble epoxide hydrolase disruption as therapeutic target for wound healing. J. Surg. Res. 2013, 182, 362–367. [Google Scholar] [CrossRef]
- Sommer, K.; Jakob, H.; Badjlan, F.; Henrich, D.; Frank, J.; Marzi, I.; Sander, A.L. 11,12 and 14,15 epoxyeicosatrienoic acid rescue deteriorated wound healing in ischemia. PLoS ONE 2019, 14, e0209158. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, J.; Chai, J.; Zhang, Y.; Yu, C.; Pan, Z.; Gao, P.; Zong, C.; Guan, Q.; Fu, Y.; et al. Cytochrome P450 (CYP) epoxygenases as potential targets in the management of impaired diabetic wound healing. Lab. Investig. 2017, 97, 782–791. [Google Scholar] [CrossRef]
- Supp, D.M.; Hahn, J.M.; McFarland, K.L.; Combs, K.A.; Lee, K.S.; Inceoglu, B.; Wan, D.; Boyce, S.T.; Hammock, B.D. Soluble Epoxide Hydrolase Inhibition and Epoxyeicosatrienoic Acid Treatment Improve Vascularization of Engineered Skin Substitutes. Plast Reconstr. Surg. Glob. Open 2016, 4, e1151. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef]
- Hellmann, J.; Tang, Y.; Spite, M. Proresolving lipid mediators and diabetic wound healing. Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 104–108. [Google Scholar] [CrossRef]
- Reis, M.B.; Pereira, P.A.T.; Caetano, G.F.; Leite, M.N.; Galvao, A.F.; Paula-Silva, F.W.G.; Frade, M.A.C.; Faccioli, L.H. Lipoxin A4 encapsulated in PLGA microparticles accelerates wound healing of skin ulcers. PLoS ONE 2017, 12, e0182381. [Google Scholar] [CrossRef] [PubMed]
- Herrera, B.S.; Kantarci, A.; Zarrough, A.; Hasturk, H.; Leung, K.P.; Van Dyke, T.E. LXA4 actions direct fibroblast function and wound closure. Biochem. Biophys. Res. Commun. 2015, 464, 1072–1077. [Google Scholar] [CrossRef]
- Arita, M.; Bianchini, F.; Aliberti, J.; Sher, A.; Chiang, N.; Hong, S.; Yang, R.; Petasis, N.A.; Serhan, C.N. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J. Exp. Med. 2005, 201, 713–722. [Google Scholar] [CrossRef]
- Arita, M.; Ohira, T.; Sun, Y.P.; Elangovan, S.; Chiang, N.; Serhan, C.N. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J. Immunol. 2007, 178, 3912–3917. [Google Scholar] [CrossRef]
- Menon, R.; Krzyszczyk, P.; Berthiaume, F. Pro-Resolution Potency of Resolvins D1, D2 and E1 on Neutrophil Migration and in Dermal Wound Healing. Nano Life 2017, 07, 1750002. [Google Scholar] [CrossRef]
- Hong, S.; Tian, H.; Lu, Y.; Laborde, J.M.; Muhale, F.A.; Wang, Q.; Alapure, B.V.; Serhan, C.N.; Bazan, N.G. Neuroprotectin/protectin D1: Endogenous biosynthesis and actions on diabetic macrophages in promoting wound healing and innervation impaired by diabetes. Am. J. Physiol Cell Physiol 2014, 307, C1058–C1067. [Google Scholar] [CrossRef]
- Cezar, T.L.C.; Martinez, R.M.; Rocha, C.D.; Melo, C.P.B.; Vale, D.L.; Borghi, S.M.; Fattori, V.; Vignoli, J.A.; Camilios-Neto, D.; Baracat, M.M.; et al. Treatment with maresin 1, a docosahexaenoic acid-derived pro-resolution lipid, protects skin from inflammation and oxidative stress caused by UVB irradiation. Sci. Rep. 2019, 9, 3062. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.W.; Yu, S.H.; Fretwurst, T.; Larsson, L.; Sugai, J.V.; Oh, J.; Lehner, K.; Jin, Q.; Giannobile, W.V. Maresin 1 Promotes Wound Healing and Socket Bone Regeneration for Alveolar Ridge Preservation. J. Dent. Res. 2020, 99, 930–937. [Google Scholar] [CrossRef]
- Ito, K.; Ito, S.; Sekine, M.; Abe, M. Reconstruction of the soft tissue of a deep diabetic foot wound with artificial dermis and recombinant basic fibroblast growth factor. Plast. Reconstr. Surg. 2005, 115, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Tai, H.H.; Ensor, C.M.; Tong, M.; Zhou, H.; Yan, F. Prostaglandin catabolizing enzymes. Prostaglandins Other Lipid Mediat. 2002, 68–69, 483–493. [Google Scholar] [CrossRef]
- Clish, C.B.; Levy, B.D.; Chiang, N.; Tai, H.H.; Serhan, C.N. Oxidoreductases in lipoxin A4 metabolic inactivation: A novel role for 15-onoprostaglandin 13-reductase/leukotriene B4 12-hydroxydehydrogenase in inflammation. J. Biol. Chem. 2000, 275, 25372–25380. [Google Scholar] [CrossRef] [PubMed]
- Nicolete, R.; Lima, K.d.M.; Júnior, J.M.R.; Baruffi, M.D.; de Medeiros, A.I.; Bentley, M.V.L.B.; Silva, C.L.; Faccioli, L.H. In vitro and in vivo activities of leukotriene B4-loaded biodegradable microspheres. Prostaglandins Other Lipid Mediat. 2007, 83, 121–129. [Google Scholar] [CrossRef]
- Park, S.K.; Herrnreiter, A.; Pfister, S.L.; Gauthier, K.M.; Falck, B.A.; Falck, J.R.; Campbell, W.B. GPR40 is a low-affinity epoxyeicosatrienoic acid receptor in vascular cells. J. Biol. Chem. 2018, 293, 10675–10691. [Google Scholar] [CrossRef]
- Hansen, T.V.; Vik, A.; Serhan, C.N. The Protectin Family of Specialized Pro-resolving Mediators: Potent Immunoresolvents Enabling Innovative Approaches to Target Obesity and Diabetes. Front. Pharmacol. 2018, 9, 1582. [Google Scholar] [CrossRef]
- Bang, S.; Xie, Y.K.; Zhang, Z.J.; Wang, Z.; Xu, Z.Z.; Ji, R.R. GPR37 regulates macrophage phagocytosis and resolution of inflammatory pain. J. Clin. Investig. 2018, 128, 3568–3582. [Google Scholar] [CrossRef]
- Chiang, N.; Libreros, S.; Norris, P.C.; de la Rosa, X.; Serhan, C.N. Maresin 1 activates LGR6 receptor promoting phagocyte immunoresolvent functions. J. Clin. Investig. 2019, 129, 5294–5311. [Google Scholar] [CrossRef]
| Eicosanoid and SPM | Source | Biosynthetic Pathway | Function Related to Skin Wound Healing | Reference |
|---|---|---|---|---|
| TxA2 | AA | COX-1/2 TxAS | Platelet aggregation (Hemostasis) ↑ Inflammation ↑ Endothelial migration and angiogenesis ↑ (in vitro) | [50,51] [54] [55,56] |
| 12-HHT | AA | COX-1/2 TxAS | Keratinocyte migration ↑ Fibroblast proliferation ↑ | [58] [59] |
| PGE2 | AA | COX-1/2 cPGES or mPGES | Angiogenesis ↑ Fibroblast proliferation ↑ Inflammation ↓ Keratinocyte proliferation and differentiation ↑ Macrophage polarization to M2 phenotype ↑ Collagen synthesis/Fibrosis ↓ | [65,66] [65,66] [69] [70,71,72,73,74] [76,77] [78] |
| PGD2 | AA | COX-1/2 H-PGDS or L-PGDS | Cutaneous barrier function ↑ Macrophage polarization to M2 phenotype ↑ Fibroblast migration ↓ (in vitro) Angiogenesis ↓ Hair follicle neogenesis ↓ | [82,83] [84,85,86,87,88] [89] [90] [91,92] |
| PGF2α | AA | COX-1/2 PGFS | Unknown | - |
| PGI2 | AA | COX-1/2 PGIS | Fibrinolysis ↑ Fibroblast migration ↑ (in vitro) Angiogenesis ↑ | [101] [101] [102,103] |
| LTB4 | AA | 5-LOX LTA4H | ROS production ↑ Inflammation ↑ Macrophage polarization to M2 phenotype ↓ | [110] [111] [112] |
| CysLTs (LTC4, LTD4, and LTE4) | AA | 5-LOX LTC4S | ROS production ↑ Inflammation ↑ Macrophage polarization to M2 phenotype ↓ Collagen deposition ↑ | [110] [111] [112] [113] |
| 12-HETE | AA | 12/15-LOX | Keratinocyte proliferation and migration ↑ (in vitro) | [115,116] |
| EETs (5,6-, 8,9-, 11,12-, and 14,15-EET) | AA | CYP2C or CYP2J | Angiogenesis ↑ Inflammation ↓ Collagen deposition ↑ | [118,121,122] [119,120,121] [121] |
| LXA4 | AA | 5-LOX 12- or 15-LOX | Inflammation ↓ Angiogenesis ↑ Collagen deposition ↑ Fibroblast proliferation and migration ↓ (in vitro) | [125] [125] [125] [126] |
| RvE1 | EPA | CYP LOX | Inflammation ↓ Collagen deposition ↑ | [127,128] [129] |
| RvD1, RvD2, and RvD4 | DHA | LOX | Collagen deposition ↑ Keratinocyte migration ↑ Fibroblast proliferation and migration ↓ (in vitro) | [129] [27] [126] |
| PD1 | DHA | LOX | Inflammation ↓ Nerve fiber growth ↑ Re-epithelialization ↑ Collagen deposition ↑ | [130] [130] [130] [130] |
| MaR1 | DHA | LOX | Inflammation ↓ Re-epithelialization ↑ Macrophage polarization to M2 phenotype ↑ | [131,132] [132] [132] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasukawa, K.; Okuno, T.; Yokomizo, T. Eicosanoids in Skin Wound Healing. Int. J. Mol. Sci. 2020, 21, 8435. https://doi.org/10.3390/ijms21228435
Yasukawa K, Okuno T, Yokomizo T. Eicosanoids in Skin Wound Healing. International Journal of Molecular Sciences. 2020; 21(22):8435. https://doi.org/10.3390/ijms21228435
Chicago/Turabian StyleYasukawa, Ken, Toshiaki Okuno, and Takehiko Yokomizo. 2020. "Eicosanoids in Skin Wound Healing" International Journal of Molecular Sciences 21, no. 22: 8435. https://doi.org/10.3390/ijms21228435
APA StyleYasukawa, K., Okuno, T., & Yokomizo, T. (2020). Eicosanoids in Skin Wound Healing. International Journal of Molecular Sciences, 21(22), 8435. https://doi.org/10.3390/ijms21228435





