An Immunological Approach to the Biocompatibility of Mesoporous SiO2-CaO Nanospheres
Abstract
:1. Introduction
2. Results and Discussion
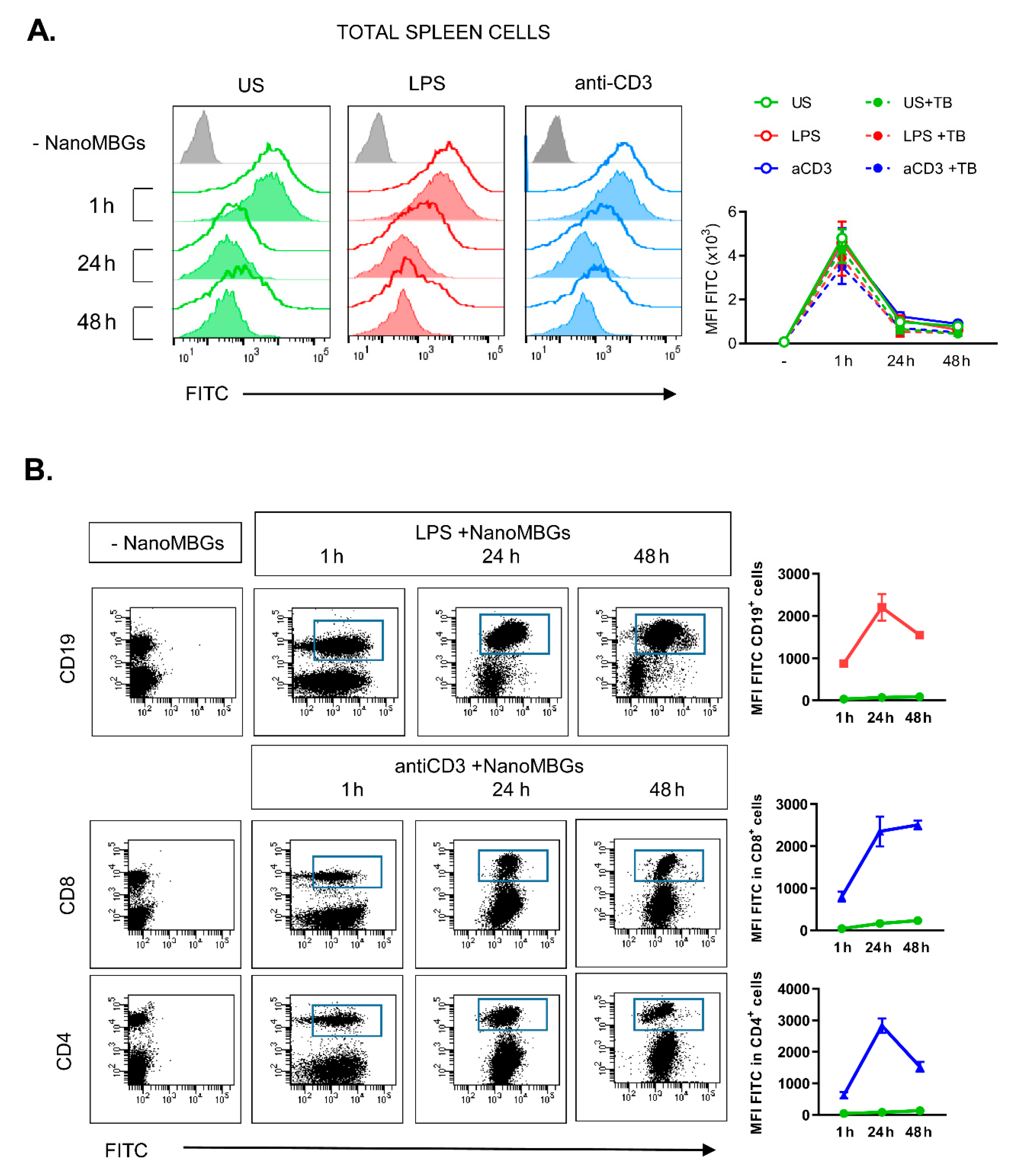
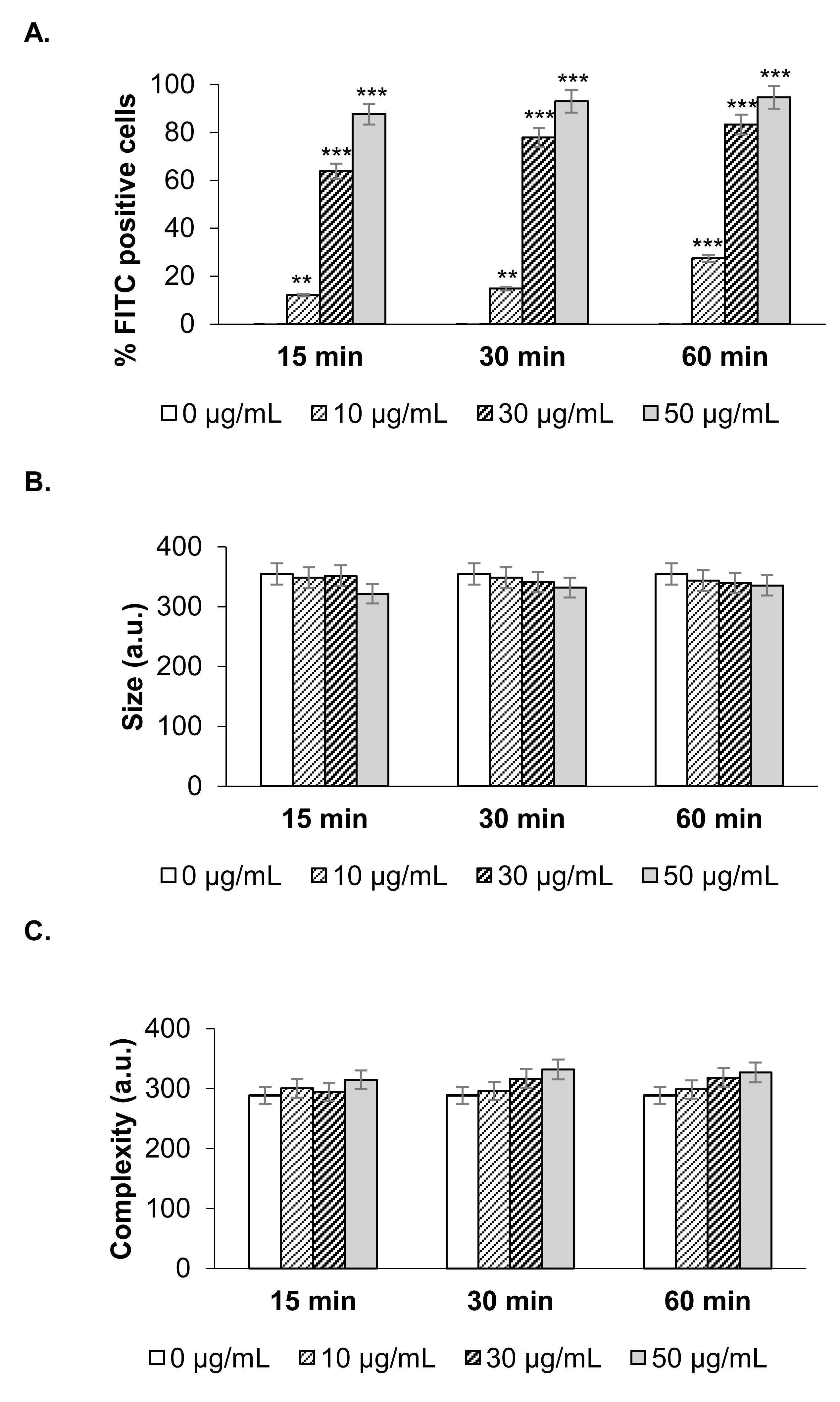
2.1. Incorporation of NanoMBGs by Murine Spleen Cells
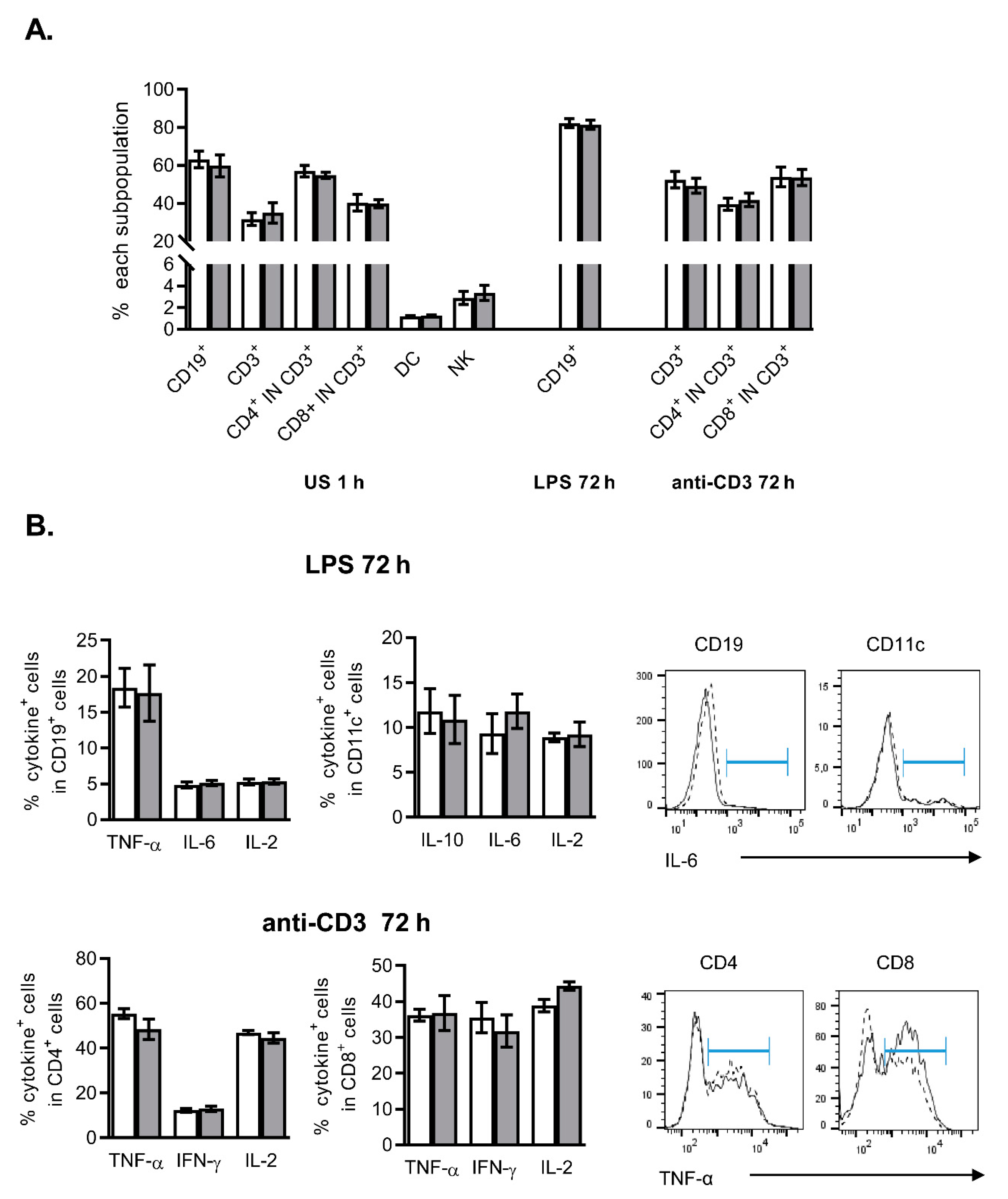
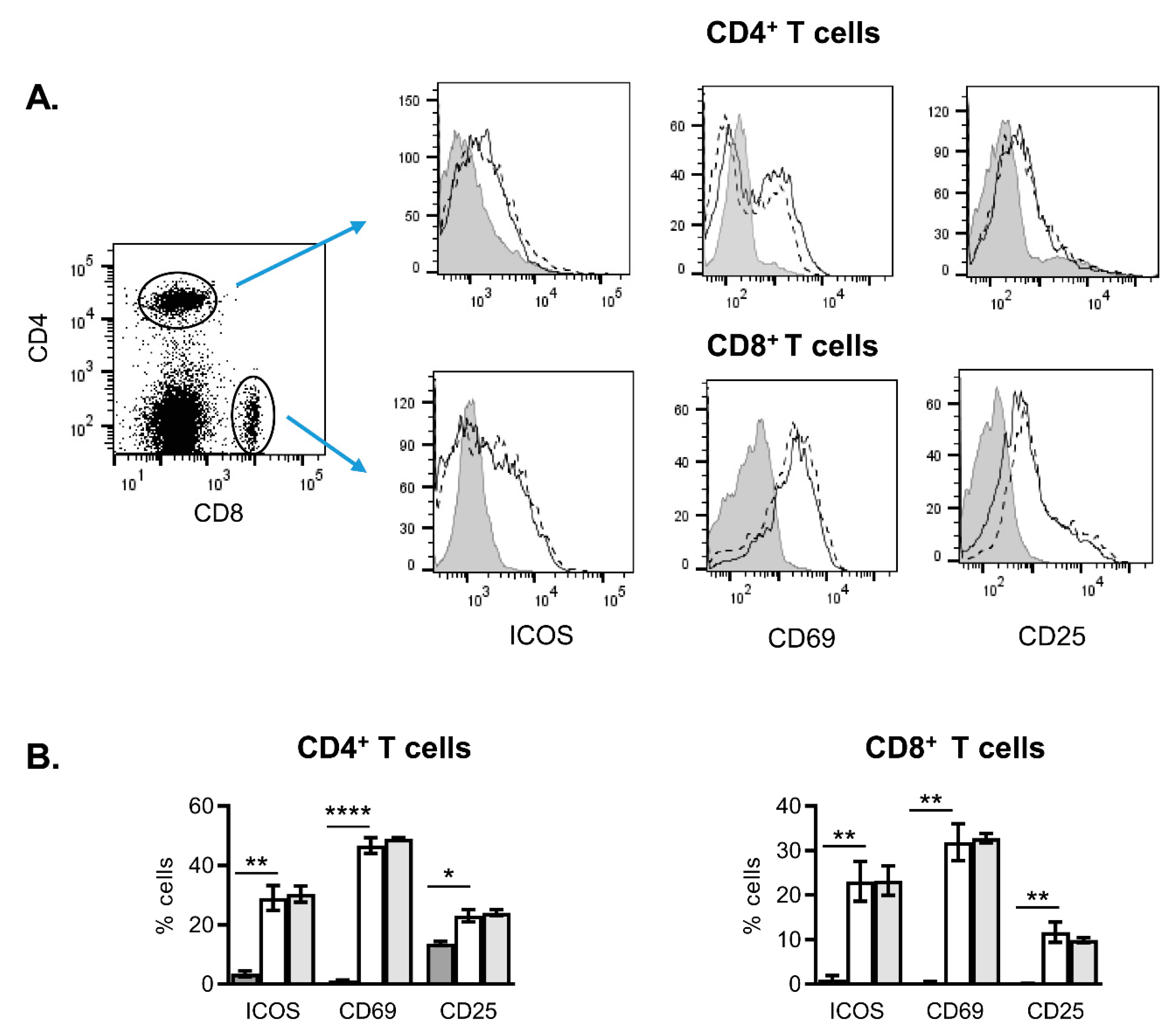
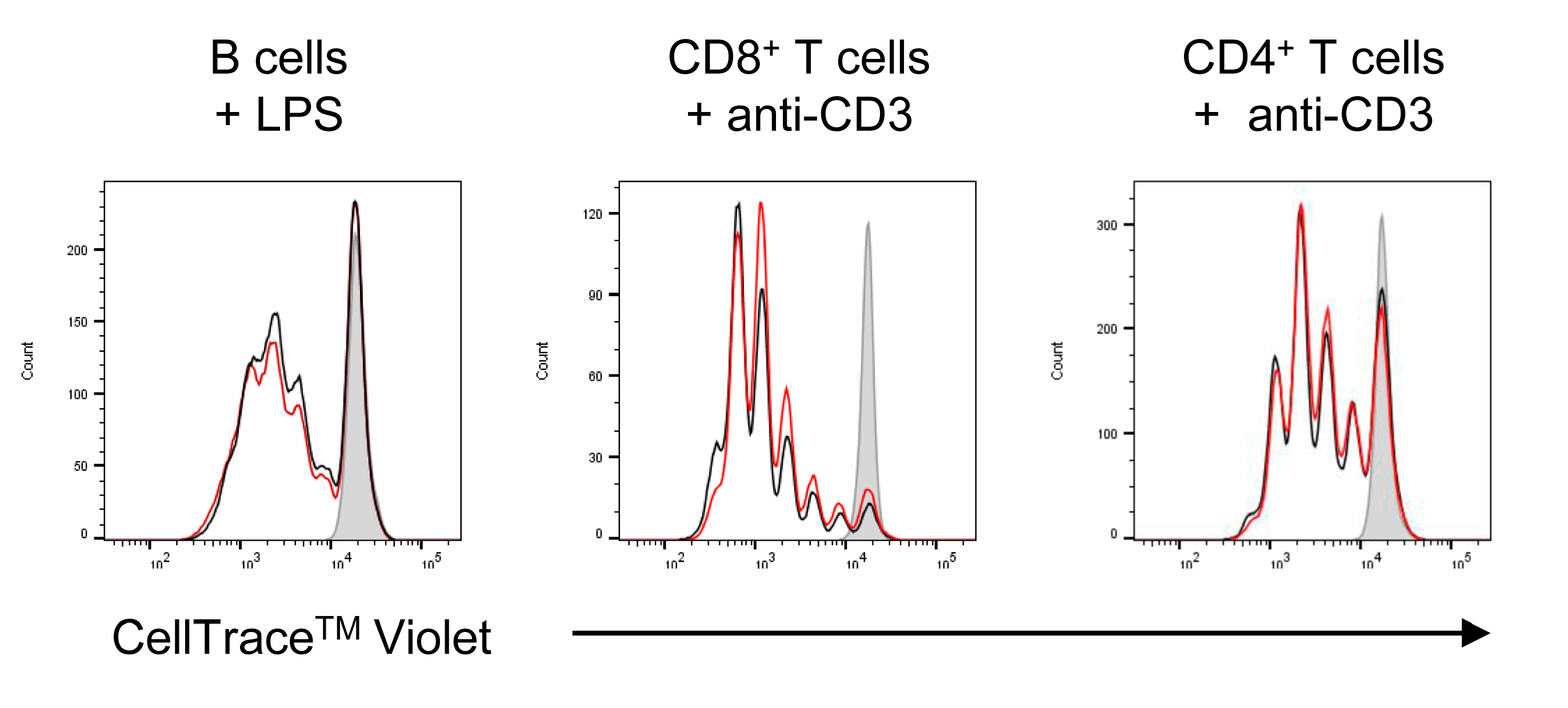
2.2. Effects of NanoMBGs on Cell Subpopulations, Cytokine Expression and Proliferation of Activated Mouse Spleen Cells
2.3. Effects of NanoMBGs on the Th2 CD4+ SR.D10 Murine Cell Line
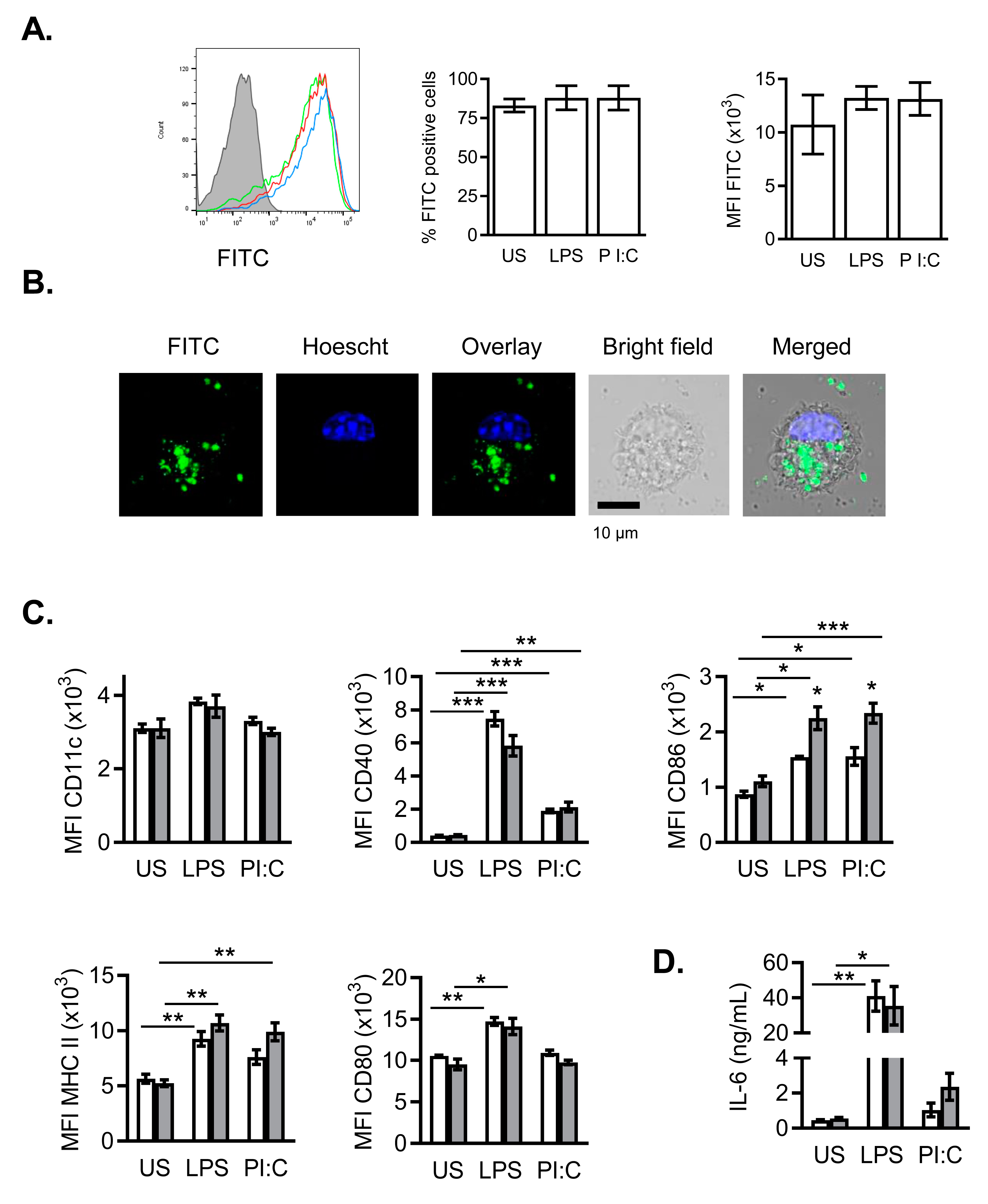
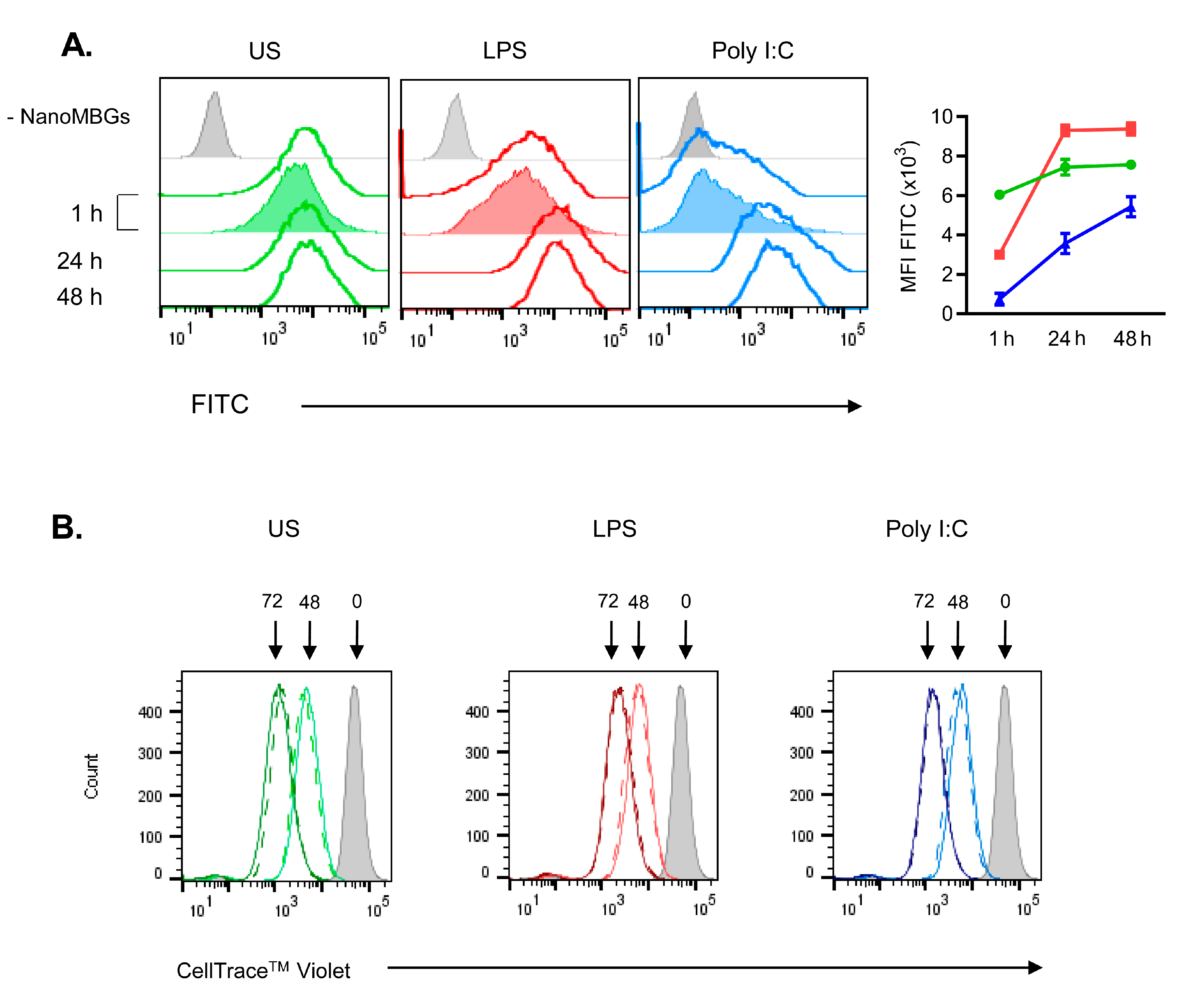
2.4. Effects of NanoMBGs in Murine Bone Marrow-Derived Dendritic Cells during the Maturation Process
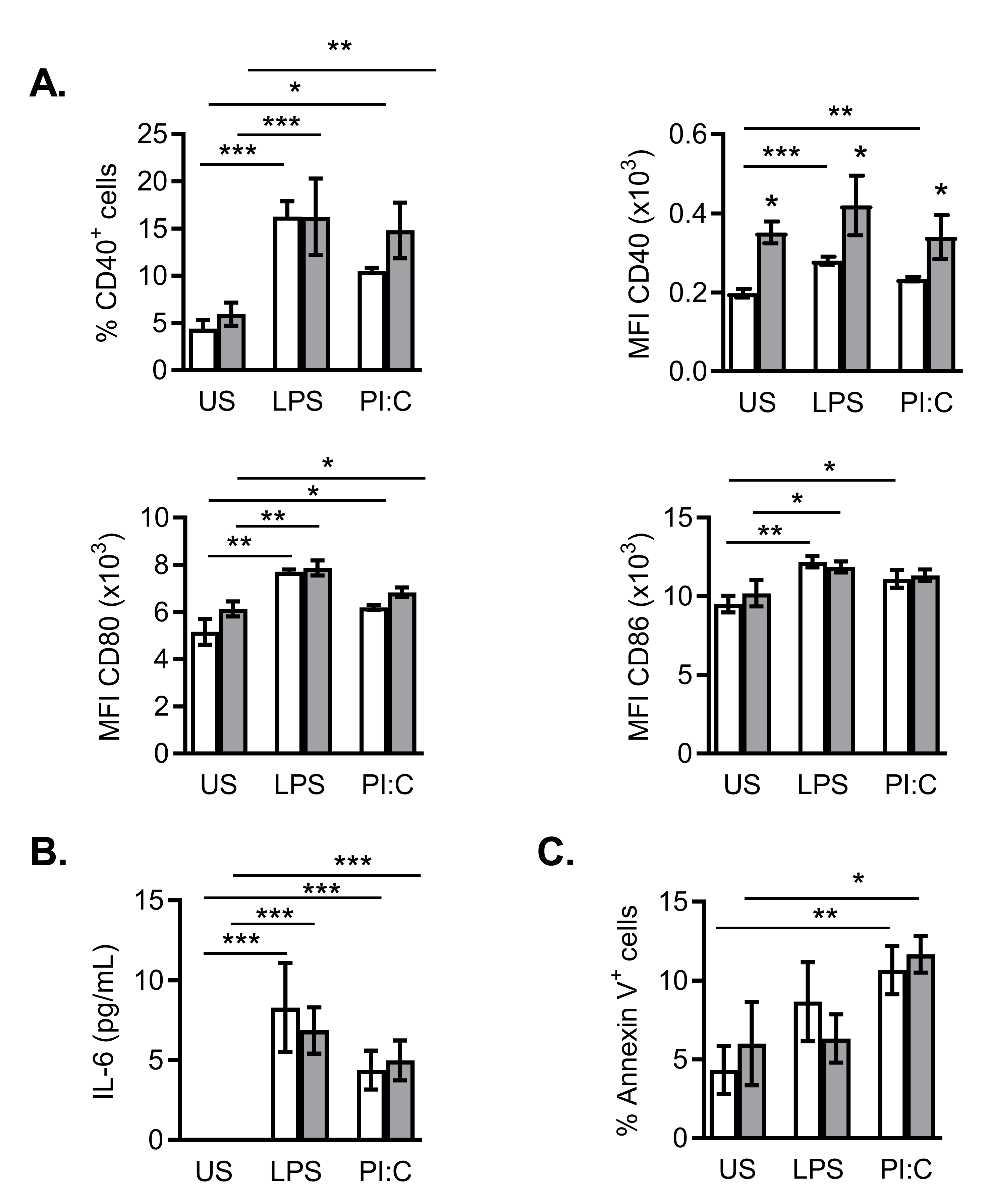
2.5. Effects of NanoMBGs on DC2.4 Murine Line
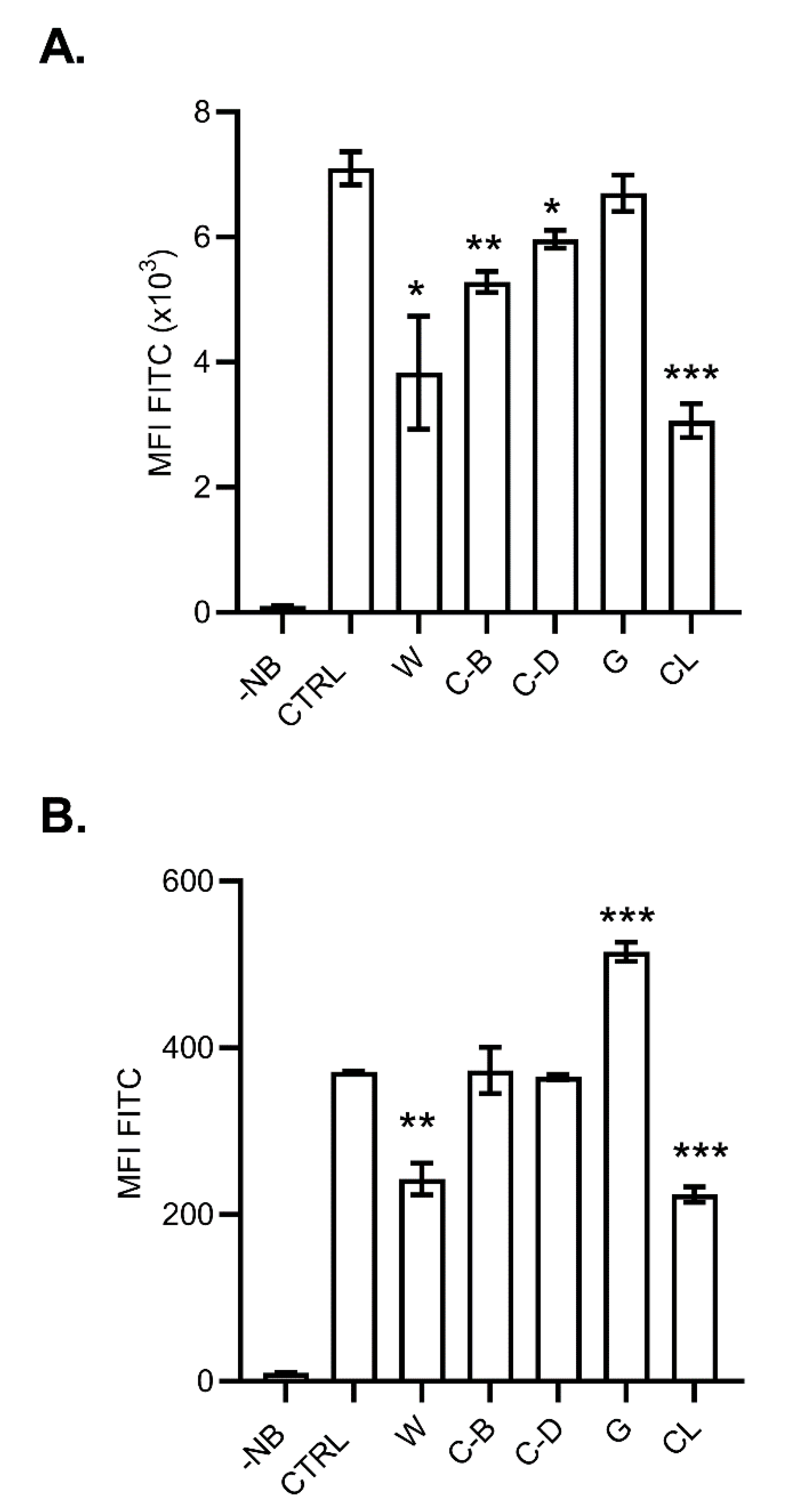
2.6. Mechanisms of NanoMBG Incorporation into the Cells
3. Materials and Methods
3.1. Synthesis and Characterization of FITC-NanoMBGs
3.2. Animals
3.3. Culture and Activation of Murine Cells
3.4. Flow Cytometry Assays
3.5. Analysis of Intracellular Cytokine Expression in Murine Spleen Cells
3.6. Analysis of Cell Proliferation by Dye Dilution
3.7. Detection of Spontaneous Apoptosis in Cell Cultures
3.8. Cytokine Quantification
3.9. Analysis of FITC-NanoMBG-Cell Interaction
3.10. Confocal Microscopy
3.11. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NanoMBGs | Nanospheres of mesoporous SiO2-CaO bioactive glass |
| DC | Dendritic cell |
| BMDC | Bone marrow derived dendritic cell |
| APC | Antigen presenting cells |
| TLR | Toll-like receptors |
| CC | Click´s culture medium |
| Ab | Antibody |
| LPS | Lipopolysaccharide |
| Poly I:C | Polyinosinic-polycitidylic acid |
| FCSi | Heat-inactivated Fetal Calf serum |
| PI3K | Phosphoinositide 3-kinase |
| PMA | Phorbol 12-myristate 13-acetate |
| PS-b-PAA | Poly(styrene)-block-poly(acrylic acid) |
| THF | Tetrahydrofuran |
| CTAB | Hexadecyl trimethyl ammonium bromide |
| TEP | Triethyl phosphate |
| TEOS | Tetraethyl ortosilane |
| FITC | Fluorescein isothiocyanate |
References
- Alyassin, Y.; Sayed, E.G.; Mehta, P.; Ruparelia, K.; Arshad, M.S.; Rasekh, M.; Shepherd, J.; Kucuk, I.; Wilson, P.B.; Singh, N.; et al. Application of mesoporous silica nanoparticles as drug delivery carriers for chemotherapeutic agents. Drug Discov. Today 2020, 25, 1513–1520. [Google Scholar] [CrossRef]
- Gisbert-Garzarán, M.; Manzano, M.; Vallet-Regí, M. pH-Responsive Mesoporous Silica and Carbon Nanoparticles for Drug Delivery. Bioengineering 2017, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Manzano, M.; Vallet-Regí, M. Mesoporous silica nanoparticles in nanomedicine applications. J. Mater. Sci. Mater. Med. 2018, 29, 65. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Carmona, M.; Colilla, M.; Vallet-Regí, M. Smart Mesoporous Nanomaterials for Antitumor Therapy. Nanomaterials 2015, 5, 1906–1937. [Google Scholar] [CrossRef] [Green Version]
- Vallet-Regí, M.; González, B.; Izquierdo-Barba, I. Nanomaterials as Promising Alternative in the Infection Treatment. Int. J. Mol. Sci. 2019, 20, 3806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Ma, Y.; Zhao, H.; Yuan, Y.; Kim, B.Y.S. Nanotechnology platforms for cancer immunotherapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 12, e1590. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M. Mesoporous Silica Nanoparticles for Drug Delivery: Current Insights. Molecules 2017, 23, 47. [Google Scholar] [CrossRef] [Green Version]
- Casarrubios, L.; Gómez-Cerezo, N.; Feito, M.J.; Vallet-Regí, M.; Arcos, D.; Portolés, M. Incorporation and effects of mesoporous SiO2-CaO nanospheres loaded with ipriflavone on osteoblast/osteoclast cocultures. Eur. J. Pharm. Biopharm. 2018, 133, 258–268. [Google Scholar] [CrossRef] [Green Version]
- Gera, S.S.A.S.D.S.; Sampathi, S.; Dodoala, S. Role of Nanoparticles in Drug Delivery and Regenerative Therapy for Bone Diseases. Curr. Drug Deliv. 2017, 14, 904–916. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Babensee, J.E. Interaction of dendritic cells with biomaterials. Semin. Immunol. 2008, 20, 101–108. [Google Scholar] [CrossRef]
- Sefton, M.V.; Babensee, J.E.; Woodhouse, K.A. Innate and adaptive immune responses in tissue engineering. Semin. Immunol. 2008, 20, 83–85. [Google Scholar] [CrossRef]
- Przekora, A. The summary of the most important cell-biomaterial interactions that need to be considered during in vitro biocompatibility testing of bone scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2019, 97, 1036–1051. [Google Scholar] [CrossRef]
- Remes, A.; Williams, D. Immune response in biocompatibility. Biomaterials 1992, 13, 731–743. [Google Scholar] [CrossRef]
- Boraschi, D.; Italiani, P.; Palomba, R.; Decuzzi, P.; Duschl, A.; Fadeel, B.; Moghimi, S.M. Nanoparticles and innate immunity: New perspectives on host defence. Semin. Immunol. 2017, 34, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.P.; Bottini, M.; Fadeel, B. Graphene and the Immune System: A Romance of Many Dimensions. Front. Immunol. 2017, 8, 673. [Google Scholar] [CrossRef] [Green Version]
- Jia, J.; Zhang, Y.; Xin, Y.; Jiang, C.; Yan, B.; Zhai, S. Interactions Between Nanoparticles and Dendritic Cells: From the Perspective of Cancer Immunotherapy. Front. Oncol. 2018, 8, 404. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H.-J.; Hang, T.; Yu, Y.; Liu, G.; He, G.; Xiao, S.; Yang, B.-R.; Yang, C.; Liu, F.; et al. Physical activation of innate immunity by spiky particles. Nat. Nanotechnol. 2018, 13, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nat. Cell Biol. 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Kapsenberg, M.L. Dendritic-cell control of pathogen-driven T-cell polarization. Nat. Rev. Immunol. 2003, 3, 984–993. [Google Scholar] [CrossRef]
- Na, H.; Cho, M.; Chung, Y. Regulation of Th2 Cell Immunity by Dendritic Cells. Immune Netw. 2016, 16, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Curato, C.; Bernshtein, B.; Zupancič, E.; Dufner, A.; Jaitin, D.; Giladi, A.; David, E.; Chappell-Maor, L.; Leshkowitz, D.; Knobeloch, K.-P.; et al. DC Respond to Cognate T Cell Interaction in the Antigen-Challenged Lymph Node. Front. Immunol. 2019, 10, 863. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Gerber, M.H.; Babensee, J.E. Phenotype and polarization of autologous T cells by biomaterial-treated dendritic cells. J. Biomed. Mater. Res. Part A 2014, 103, 170–184. [Google Scholar] [CrossRef] [Green Version]
- Cicuéndez, M.; Portolés, P.; Montes-Casado, M.; Izquierdo-Barba, I.; Vallet-Regí, M.; Portolés, M.T. Effects of 3D nanocomposite bioceramic scaffolds on the immune response. J. Mater. Chem. B 2014, 2, 3469–3479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franz, S.; Rammelt, S.; Scharnweber, D.; Simon, J.C. Immune responses to implants–A review of the implications for the design of immunomodulatory biomaterials. Biomaterials 2011, 32, 6692–6709. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.-J.; Tong, Y.-L.; Sheng, Z.-Y.; Yao, Y. Role of dendritic cells in the host response to biomaterials and their signaling pathways. Acta Biomater. 2019, 94, 132–144. [Google Scholar] [CrossRef]
- Shen, Z.; Reznikoff, G.; Dranoff, G.; Rock, K.L. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J. Immunol. 1997, 158, 2723–2730. [Google Scholar]
- Liu, Y.; Shreder, K.R.; Gai, W.; Corral, S.; Ferris, D.K.; Rosenblum, J.S. Wortmannin, a Widely Used Phosphoinositide 3-Kinase Inhibitor, also Potently Inhibits Mammalian Polo-like Kinase. Chem. Biol. 2005, 12, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Soldati, T.; Schliwa, M. Powering membrane traffic in endocytosis and recycling. Nat. Rev. Mol. Cell Biol. 2006, 7, 897–908. [Google Scholar] [CrossRef]
- Chen, F.; Zhu, L.; Zhang, Y.; Kumar, D.; Cao, G.; Hu, X.; Liang, Z.; Kuang, S.; Xue, R.; Gong, C. Clathrin-mediated endocytosis is a candidate entry sorting mechanism for Bombyx mori cypovirus. Sci. Rep. 2018, 8, 7268. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Wu, Y. Genistein and HIV Infection. In HIV/AIDS. Oxidative Stress and Dietary Antioxidants; Preedy, V.R., Watson, R.R., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 125–132. [Google Scholar]
- Dutta, D.; Donaldson, J.G. Search for inhibitors of endocytosis. Cell. Logist. 2012, 2, 203–208. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, S.; McElhanon, K.E.; Gushchina, L.V.; Weisleder, N. Role of phosphatidylinositol-4,5-bisphosphate 3-kinase signaling in vesicular trafficking. Life Sci. 2016, 167, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Guermonprez, P.; Valladeau, J.; Zitvogel, L.; Théry, C.; Amigorena, S. Antigen Presentation and T Cell Stimulation by Dendritic Cells. Annu. Rev. Immunol. 2002, 20, 621–667. [Google Scholar] [CrossRef]
- Linares, J.; Matesanz, M.C.; Vila, M.; Feito, M.J.; Gonçalves, G.; Vallet-Regí, M.; Marques, P.A.A.P.; Portolés, M.T. Endocytic Mechanisms of Graphene Oxide Nanosheets in Osteoblasts, Hepatocytes and Macrophages. ACS Appl. Mater. Interfaces 2014, 6, 13697–13706. [Google Scholar] [CrossRef]
- Peck, A.B.; Bach, F.H. A miniaturized mouse mixed leukocyte culture in serum-free and mouse serum supplemented media. J. Immunol. Methods 1973, 3, 147–163. [Google Scholar] [CrossRef]
- Inaba, K.; Inaba, M.; Romani, N.; Aya, H.; Deguchi, M.; Ikehara, S.; Muramatsu, S.; Steinman, R.M. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1992, 176, 1693–1702. [Google Scholar] [CrossRef]
- Zhu, J.; Martinez, J.; Huang, X.; Yang, Y. Innate immunity against vaccinia virus is mediated by TLR2 and requires TLR-independent production of IFN-beta. Blood 2007, 109, 619–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojeda, G.; Ronda, M.; Ballester, S.; Díez-Orejas, R.; Feito, M.J.; Garcia-Albert, L.; Rojo, J.M.; Portolés, P.; Díez-Orejas, R. A Hyperreactive Variant of a CD4+ T Cell Line Is Activated by Syngeneic Antigen Presenting Cells in the Absence of Antigen. Cell. Immunol. 1995, 164, 265–278. [Google Scholar] [CrossRef]
- Kaye, J.; Porcelli, S.; Tite, J.; Jones, B.; Janeway, C.A., Jr. Both a monoclonal antibody and antisera specific for determinants unique to individual cloned helper T cell lines can substitute for antigen and antigen-presenting cells in the activation of T cells. J. Exp. Med. 1983, 158, 836–856. [Google Scholar] [CrossRef] [Green Version]
- Alcaide, M.; Portolés, P.; López-Noriega, A.; Arcos, D.; Vallet-Regi, M.; Portolés, M. Interaction of an ordered mesoporous bioactive glass with osteoblasts, fibroblasts and lymphocytes, demonstrating its biocompatibility as a potential bone graft material. Acta Biomater. 2010, 6, 892–899. [Google Scholar] [CrossRef]
- Portolés, P.; Rojo, J.; Golby, A.; Bonneville, M.; Gromkowski, S.; Greenbaum, L.; Janeway, C.A., Jr.; Murphy, D.B.; Bottomly, K. Monoclonal antibodies to murine CD3e define distinct epitopes, one of which may interact with CD4 during T cell activation. J. Immunol. 1989, 142, 4169–4175. [Google Scholar] [PubMed]
- Montes-Casado, M.; Ojeda, G.; Aragoneses-Fenoll, L.; López, D.; De Andrés, B.; Gaspar, M.L.; Dianzani, U.; Rojo, J.M.; Portolés, P. ICOS deficiency hampers the homeostasis, development and function of NK cells. PLoS ONE 2019, 14, e0219449. [Google Scholar] [CrossRef] [Green Version]
| Name | Effect | References |
|---|---|---|
| Wortmannin | Pan-inhibitor of phosphoinositide 3-kinases (PI3-K). It displays a similar potency in vitro for class I, II and III PI3K | [28] |
| Cytochalasin B and D | Inhibits actin polymerization and network formation by actin filaments. Affect most endocytic pathways. | [29] |
| Genistein | Tyrosine kinases inhibitor. Inhibits caveolae-mediated endocytosis; also antioxidant, antiangiogenic, and immunosuppressive activities. | [30,31] |
| Chlorpromazine | Cationic amphipathic drug that blocks clathrin-mediated endocytosis | [32] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montes-Casado, M.; Sanvicente, A.; Casarrubios, L.; Feito, M.J.; Rojo, J.M.; Vallet-Regí, M.; Arcos, D.; Portolés, P.; Portolés, M.T. An Immunological Approach to the Biocompatibility of Mesoporous SiO2-CaO Nanospheres. Int. J. Mol. Sci. 2020, 21, 8291. https://doi.org/10.3390/ijms21218291
Montes-Casado M, Sanvicente A, Casarrubios L, Feito MJ, Rojo JM, Vallet-Regí M, Arcos D, Portolés P, Portolés MT. An Immunological Approach to the Biocompatibility of Mesoporous SiO2-CaO Nanospheres. International Journal of Molecular Sciences. 2020; 21(21):8291. https://doi.org/10.3390/ijms21218291
Chicago/Turabian StyleMontes-Casado, María, Adrian Sanvicente, Laura Casarrubios, María José Feito, José M. Rojo, María Vallet-Regí, Daniel Arcos, Pilar Portolés, and María Teresa Portolés. 2020. "An Immunological Approach to the Biocompatibility of Mesoporous SiO2-CaO Nanospheres" International Journal of Molecular Sciences 21, no. 21: 8291. https://doi.org/10.3390/ijms21218291
APA StyleMontes-Casado, M., Sanvicente, A., Casarrubios, L., Feito, M. J., Rojo, J. M., Vallet-Regí, M., Arcos, D., Portolés, P., & Portolés, M. T. (2020). An Immunological Approach to the Biocompatibility of Mesoporous SiO2-CaO Nanospheres. International Journal of Molecular Sciences, 21(21), 8291. https://doi.org/10.3390/ijms21218291









