Compression Bioreactor-Based Mechanical Loading Induces Mobilization of Human Bone Marrow-Derived Mesenchymal Stromal Cells into Collagen Scaffolds In Vitro
Abstract
:1. Introduction
1.1. Therapeutic Approaches for Chondral Defects
1.2. Matrix-Augmented Bone Marrow Stimulation
1.3. Biomechanics in the Knee Joint and the Bioreactor
1.4. Migration and Cell Morphology
1.5. Focus of the Study
2. Results
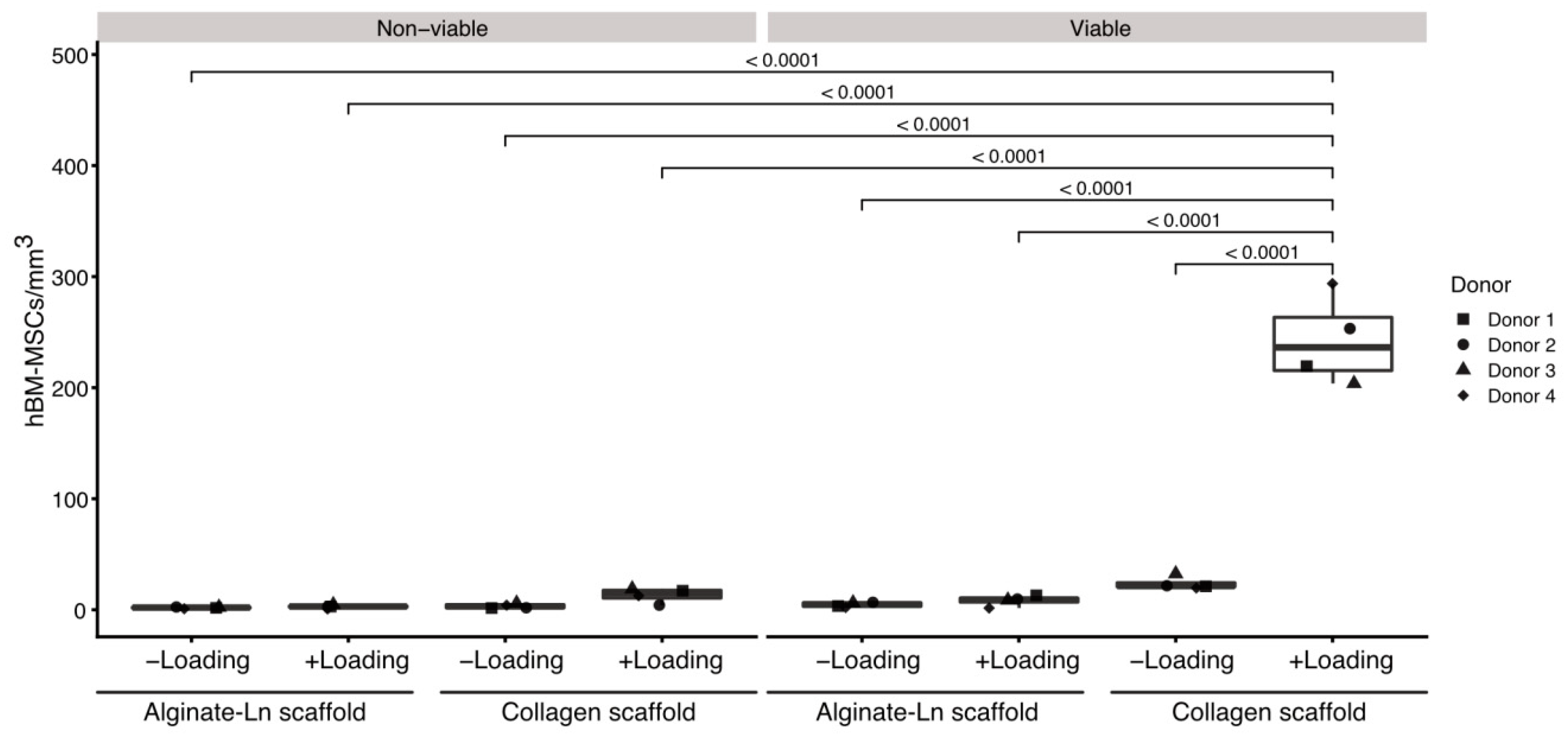
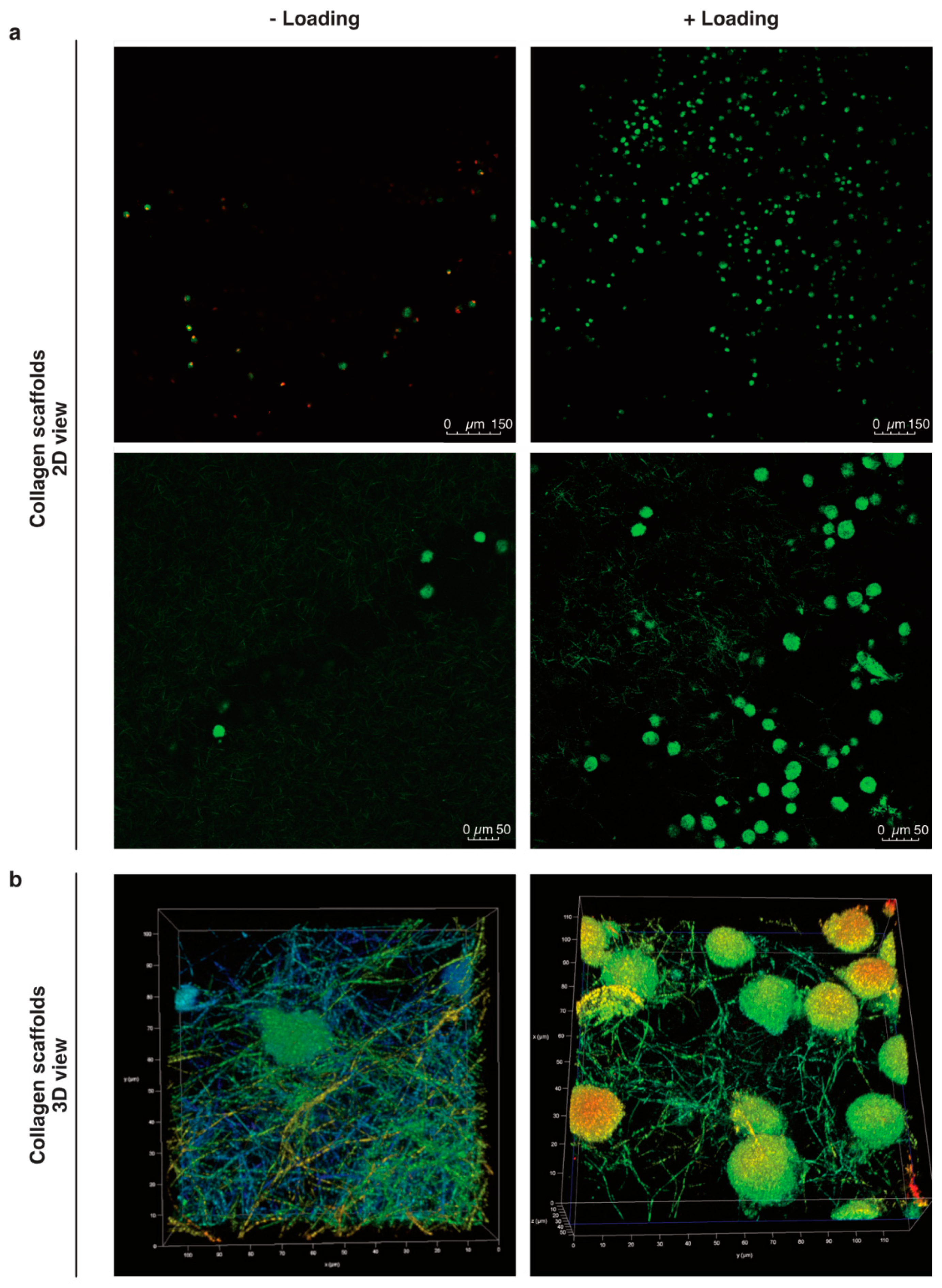
2.1. Cells Found in the Scaffolds after Mechanical Stimulation
2.2. Biomechanical Stimulation Provided by the Compression Bioreactor
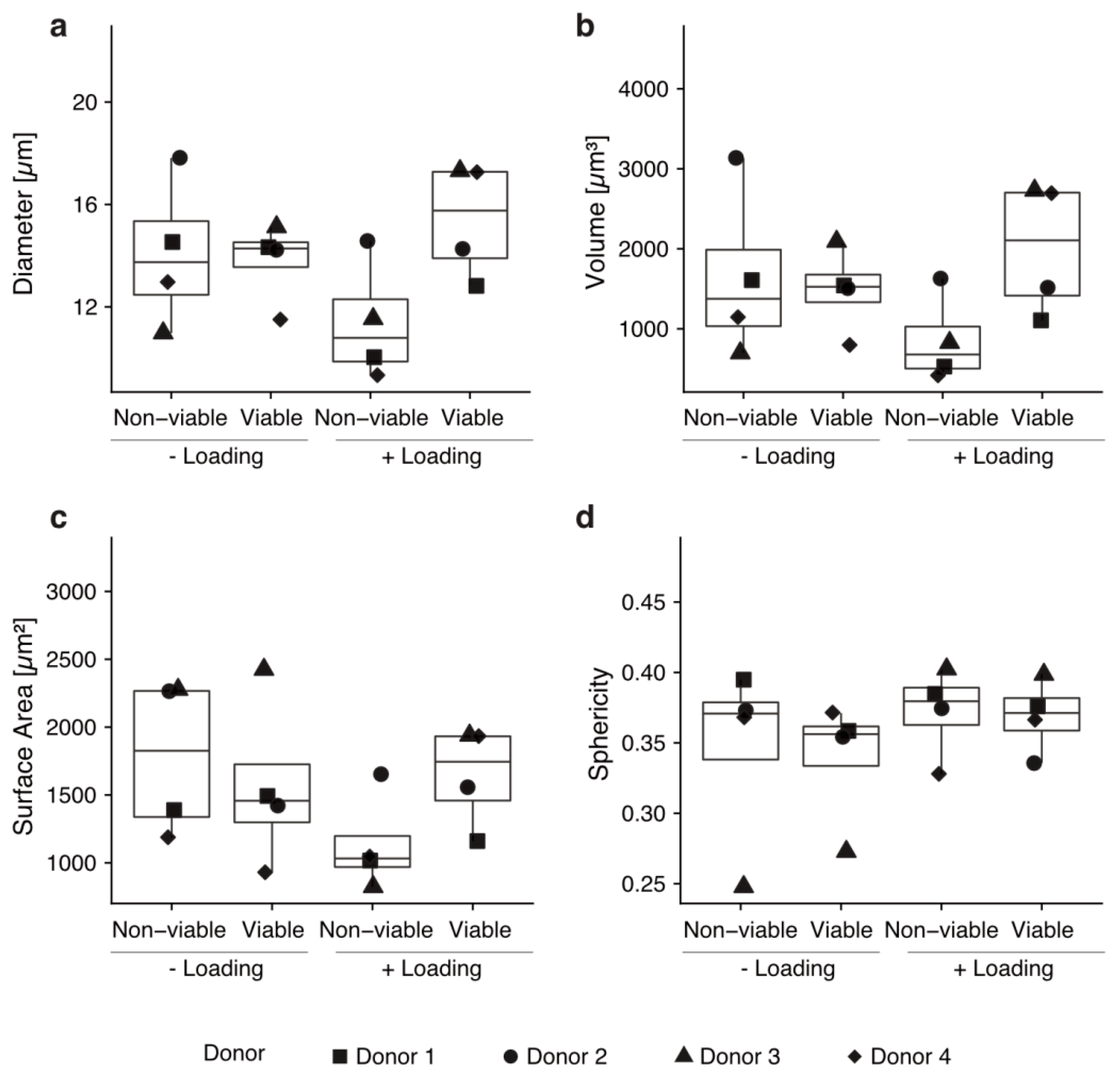
2.3. Morphometry of Mobilized Cells into Col-I Scaffolds
3. Discussion
3.1. Mechanical Stimulation and Mobilized Cells
3.2. Migration of Cells
3.3. Col-I Scaffolds
3.4. Relevance and Drawbacks of Our Study
4. Materials and Methods
4.1. Cells
4.2. Fabrication of the Scaffolds
4.3. Biomechanical Stimulation
4.4. Mechanical Data Analysis
4.5. Confocal Microscopy
4.6. Cell Quantification in 3D and Cell Morphometric Evaluation
4.7. Statistical Analyses
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Articular cartilage |
| Alginate-Ln | Alginate-Laminin |
| BM | Bone marrow |
| BDDGE | 1,4-Butanediol diglycidyl ether |
| C-AM | Calcein-AM |
| Col-I | Collagen type-I |
| Col-II | Collagen type-II |
| ECM | Extracellular matrix |
| EthD-1 | Ethidium homodimer-1 |
| GLM | General linear model |
| hBM-MSCs | Human bone marrow derived mesenchymal stromal cells |
| LAS X | Leica Application Suite X |
| Ln | Laminin |
| pBM-MSCs | Porcine bone marrow derived mesenchymal stromal cells |
| MABMS | Matrix-augmented bone marrow stimulation |
| MACI | Matrix-augmented chondrocytes implantation |
| MF | Microfracture |
| MSCs | Mesenchymal stem/stromal cells |
References
- Akkiraju, H.; Nohe, A. Role of Chondrocytes in Cartilage Formation, Progression of Osteoarthritis and Cartilage Regeneration. J. Dev. Biol. 2015, 3, 177–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grässel, S. Collagens in Hyaline Cartilage. In Cartilage Volume 1: Physiology and Development; Grässel, S., Aszódi, A., Eds.; Springer: Cham, Switzerland, 2016; Volume 1, p. 267. [Google Scholar]
- Madeira, C.; Santhagunam, A.; Salgueiro, J.B.; Cabral, J.M. Advanced cell therapies for articular cartilage regeneration. Trends Biotechnol. 2015, 33, 35–42. [Google Scholar] [CrossRef]
- Makris, E.A.; Gomoll, A.H.; Malizos, K.N.; Hu, J.C.; Athanasiou, K.A. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 2015, 11, 21–34. [Google Scholar] [CrossRef]
- Caldwell, K.L.; Wang, J. Cell-based articular cartilage repair: The link between development and regeneration. Osteoarthr. Cartil. 2015, 23, 351–362. [Google Scholar] [CrossRef] [Green Version]
- Jacobi, M.; Villa, V.; Magnussen, R.A.; Neyret, P. MACI—A new era? Sports Med. Arthrosc. Rehabil. Ther. Technol. 2011, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- Iseki, T.; Rothrauff, B.B.; Kihara, S.; Sasaki, H.; Yoshiya, S.; Fu, F.H.; Tuan, R.S.; Gottardi, R. Dynamic Compressive Loading Improves Cartilage Repair in an In Vitro Model of Microfracture: Comparison of 2 Mechanical Loading Regimens on Simulated Microfracture Based on Fibrin Gel Scaffolds Encapsulating Connective Tissue Progenitor Cells. Am. J. Sports Med. 2019, 47, 2188–2199. [Google Scholar] [CrossRef] [Green Version]
- Sommerfeldt, M.F.; Magnussen, R.A.; Hewett, T.E.; Kaeding, C.C.; Flanigan, D.C. Microfracture of Articular Cartilage. JBJS Rev. 2016, 4. [Google Scholar] [CrossRef]
- Richter, W. Mesenchymal stem cells and cartilage in situ regeneration. J. Intern. Med. 2009, 266, 390–405. [Google Scholar] [CrossRef]
- Choi, J.R.; Yong, K.W.; Choi, J.Y. Effects of mechanical loading on human mesenchymal stem cells for cartilage tissue engineering. J. Cell. Physiol. 2018, 233, 1913–1928. [Google Scholar] [CrossRef]
- Niemeyer, P.; Becher, C.; Brucker, P.; Buhs, M.; Fickert, S.; Gelse, K.; Günther, D.; Kaelin, R.; Kreuz, P.; Lützner, J.; et al. Significance of Matrix-augmented Bone Marrow Stimulation for Treatment of Cartilage Defects of the Knee: A Consensus Statement of the DGOU Working Group on Tissue Regeneration. Z. Orthop. Unfall. 2018, 156, 513–532. [Google Scholar] [CrossRef]
- Erggelet, C.; Endres, M.; Neumann, K.; Morawietz, L.; Ringe, J.; Haberstroh, K.; Sittinger, M.; Kaps, C. Formation of cartilage repair tissue in articular cartilage defects pretreated with microfracture and covered with cell-free polymer-based implants. J. Orthop. Res. 2009, 27, 1353–1360. [Google Scholar] [CrossRef]
- Kramer, J.; Bohrnsen, F.; Lindner, U.; Behrens, P.; Schlenke, P.; Rohwedel, J. In vivo matrix-guided human mesenchymal stem cells. Cell. Mol. Life Sci. 2006, 63, 616–626. [Google Scholar] [CrossRef]
- Irawan, V.; Sung, T.C.; Higuchi, A.; Ikoma, T. Collagen Scaffolds in Cartilage Tissue Engineering and Relevant Approaches for Future Development. Tissue Eng. Regen. Med. 2018, 15, 673–697. [Google Scholar] [CrossRef]
- Park, M.S.; Kim, Y.H.; Jung, Y.; Kim, S.H.; Park, J.C.; Yoon, D.S.; Kim, S.-H.; Lee, J.W. In Situ Recruitment of Human Bone Marrow-Derived Mesenchymal Stem Cells Using Chemokines for Articular Cartilage Regeneration. Cell Transplant. 2015, 24, 1067–1083. [Google Scholar] [CrossRef] [PubMed]
- Rodin, S.; Antonsson, L.; Hovatta, O.; Tryggvason, K. Monolayer culturing and cloning of human pluripotent stem cells on laminin-521-based matrices under xeno-free and chemically defined conditions. Nat. Protoc. 2014, 9, 2354–2368. [Google Scholar] [CrossRef]
- Rodin, S.; Antonsson, L.; Niaudet, C.; Simonson, O.E.; Salmela, E.; Hansson, E.M.; Domogatskaya, A.; Xiao, Z.; Damdimopoulou, P.; Sheikhi, M.; et al. Clonal culturing of human embryonic stem cells on laminin-521/E-cadherin matrix in defined and xeno-free environment. Nat. Commun. 2014, 5, 3195. [Google Scholar] [CrossRef]
- Gamez, C.; Schneider-Wald, B.; Schuette, A.; Mack, M.; Hauk, L.; Khan, A.U.M.; Gretz, N.; Stoffel, M.; Bieback, K.; Schwarz, M.J. Bioreactor for mobilization of mesenchymal stem/stromal cells into scaffolds under mechanical stimulation: Preliminary results. PLoS ONE 2020, 15, e0227553. [Google Scholar] [CrossRef]
- Fahy, N.; Alini, M.; Stoddart, M.J. Mechanical stimulation of mesenchymal stem cells: Implications for cartilage tissue engineering. J. Orthop. Res. 2018, 36, 52–63. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Adams, J.; Leddy, H.A.; McNulty, A.L.; O’Conor, C.J.; Guilak, F. The mechanobiology of articular cartilage: Bearing the burden of osteoarthritis. Curr. Rheumatol. Rep. 2014, 16, 451. [Google Scholar] [CrossRef] [Green Version]
- Anderson, D.E.; Johnstone, B. Dynamic Mechanical Compression of Chondrocytes for Tissue Engineering: A Critical Review. Front. Bioeng. Biotechnol. 2017, 5, 76. [Google Scholar] [CrossRef] [Green Version]
- Coleman, J.L.; Widmyer, M.R.; Leddy, H.A.; Utturkar, G.M.; Spritzer, C.E.; Moorman, C.T.; Guilak, F.; DeFrate, L.E. Diurnal variations in articular cartilage thickness and strain in the human knee. J. Biomech. 2013, 46, 541–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzgerald, J.B.; Jin, M.; Grodzinsky, A.J. Shear and compression differentially regulate clusters of functionally related temporal transcription patterns in cartilage tissue. J. Biol. Chem. 2006, 281, 24095–24103. [Google Scholar] [CrossRef] [Green Version]
- Grodzinsky, A.J.; Levenston, M.E.; Jin, M.; Frank, E.H. Cartilage tissue remodeling in response to mechanical forces. Annu. Rev. Biomed. 2000, 2, 691–713. [Google Scholar] [CrossRef]
- Sauerland, K.; Raiss, R.X.; Steinmeyer, J. Proteoglycan metabolism and viability of articular cartilage explants as modulated by the frequency of intermittent loading. Osteoarthr. Cartil. 2003, 11, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Bonzani, I.C.; Campbell, J.; Knight, M.M.; Williams, A.; Lee, D.A.; Bader, D.; Stevens, M.M. Dynamic compressive strain influences chondrogenic gene expression in human periosteal cells: A case study. J. Mech. Behav. Biomed. Mater. 2012, 11, 72–81. [Google Scholar] [CrossRef]
- Sauerland, K.; Steinmeyer, J. Intermittent mechanical loading of articular cartilage explants modulates chondroitin sulfate fine structure. Osteoarthr. Cartil. 2007, 15, 1403–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinmeyer, J.; Knue, S.; Raiss, R.H.; Pelzer, I. Effects of intermittently applied cyclic loading on proteoglycan metabolism and swelling behaviour of articular cartilage explants. Ostheoarthr. Cartil. 1999, 7, 155–164. [Google Scholar] [CrossRef] [Green Version]
- Nachtsheim, J.; Dursun, G.; Markert, B.; Stoffel, M. Chondrocyte colonisation of a tissue-engineered cartilage substitute under a mechanical stimulus. Med. Eng. Phys. 2019, 74, 58–64. [Google Scholar] [CrossRef]
- Mousavi, S.J.; Doweidar, M.H. Three-dimensional numerical model of cell morphology during migration in multi-signaling substrates. PLoS ONE 2015, 10, e0122094. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.H. Cell Traction Forces (CTFs) and CTF Microscopy Applications in Musculoskeletal Research. Oper. Tech. Orthop. 2010, 20, 106–109. [Google Scholar] [CrossRef] [Green Version]
- Bodor, D.L.; Ponisch, W.; Endres, R.G.; Paluch, E.K. Of Cell Shapes and Motion: The Physical Basis of Animal Cell Migration. Dev. Cell 2020, 52, 550–562. [Google Scholar] [CrossRef]
- Mousavi, S.J.; Doweidar, M.H. A novel mechanotactic 3D modeling of cell morphology. Phys. Biol. 2014, 11, 046005. [Google Scholar] [CrossRef]
- Simpliciano, C.; Clark, L.; Asi, B.; Chu, N.; Mercado, M.; Diaz, S.; Goedert, M.; Mobed-Miremadi, M. Cross-Linked Alginate Film Pore Size Determination Using Atomic Force Microscopy and Validation Using Diffusivity Determinations. J. Surf. Eng. Mater. Adv. Technol. 2013, 3, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Adams, G.; Buttery, L.; Falcone, F.H.; Stolnik, S. Alginate encapsulation technology supports embryonic stem cells differentiation into insulin-producing cells. J. Biotechnol. 2009, 144, 304–312. [Google Scholar] [CrossRef]
- Noort, W.A.; Oerlemans, M.I.F.J.; Rozemuller, H.; Feyen, D.; Jaksani, S.; Stecher, D.; Naaijkens, B.; Martens, A.C.; Bühring, H.J.; Doevendans, P.A.; et al. Human versus porcine mesenchymal stromal cells: Phenotype, differentiation potential, immunomodulation and cardiac improvement after transplantation. J. Cell. Mol. Med. 2012, 16, 1827–1839. [Google Scholar] [CrossRef] [Green Version]
- Gamez, C.; Khan, A.M.; Torelli, A.; Schneider-Wald, B.; Gretz, N.; Wolf, I.; Bieback, K.; Schwarz, M.L. Image processing workflow to visualize and quantify MSCs in 3D. In Proceedings of the German Congress of Orthopedic and Trauma Surgery (DKOU 2018), Berlin, Germany, 19–23 October 2018. [Google Scholar]
- Wang, Z.C.; Sun, H.J.; Li, K.H.; Fu, C.; Liu, M.Z. Icariin promotes directed chondrogenic differentiation of bone marrow mesenchymal stem cells but not hypertrophy in vitro. Exp. Ther. Med. 2014, 8, 1528–1534. [Google Scholar] [CrossRef]
- Schiavi, J.; Reppel, L.; Charif, N.; De Isla, N.; Mainard, D.; Benkirane-Jessel, N.; Stoltz, J.; Rahouadj, R.; Huselstein, C. Mechanical stimulations on human bone marrow mesenchymal stem cells enhance cells differentiation in a three-dimensional layered scaffold. J. Tissue Eng. Regen. Med. 2018, 12, 360–369. [Google Scholar] [CrossRef]
- Wang, K.C.; Frank, R.M.; Cotter, E.J.; Christian, D.R.; Cole, B.J. Arthroscopic Management of Isolated Tibial Plateau Defect With Microfracture and Micronized Allogeneic Cartilage-Platelet-Rich Plasma Adjunct. Arthrosc. Tech. 2017, 6, e1613–e1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matziolis, G.; Tuischer, J.; Kasper, G.; Thompson, M.; Bartmeyer, B.; Krocker, D.; Perka, C.; Duda, G. Simulation of Cell Differentiation in Fracture Healing: Mechanically Loaded Composite Scaffolds in a Novel Bioreactor System. Tissue Eng. 2006, 12, 201–208. [Google Scholar] [CrossRef]
- Ode, A.; Kopf, J.; Kurtz, A.; Schmidt-Bleek, K.; Schrade, P.; Kolar, P.; Buttgerei, F.; Lehmann, K.; Hutmacher, D.W.; Duda, G.; et al. CD73 and CD29 concurrently mediate the mechanically induced decrease of migratory capacity of mesenchymal stromal cells. Eur. Cells Mater. 2011, 22, 26–42. [Google Scholar] [CrossRef]
- Wiedemann, M.K.; Kohn, F.P.M.; Wolfgang, H.R.; Hanke, R.L. Effects of Altered Gravity on the Actin and Microtubule Cytoskeleton, Cell Migration and Neurite Outgrowth. In Self-Organization and Pattern-Formation in Neuronal Systems Under Conditions of Variable Gravity; Springer: Berlin/Heidelberg, Germany, 2011; pp. 167–186. [Google Scholar]
- Ahn, C.B.; Lee, J.-H.; Han, D.G.; Kang, H.-W.; Lee, S.-H.; Son, K.H.; Lee, J.W. Simulated microgravity with floating environment promotes migration of non-small cell lung cancers. Sci. Rep. 2019, 9, 14553. [Google Scholar] [CrossRef]
- Yamada, K.M.; Sixt, M. Mechanisms of 3D cell migration. Nat. Rev. Mol. Cell Biol. 2019, 20, 738–752. [Google Scholar] [CrossRef]
- Charras, G.; Sahai, E. Physical influences of the extracellular environment on cell migration. Nat. Rev. Mol Cell Biol. 2014, 15, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Duan, L.; Zhu, W.; Xiong, J.; Wang, D. Extracellular matrix production in vitro in cartilage tissue engineering. J. Transl. Med. 2014, 12. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef] [Green Version]
- Krishnakumar, G.S.; Gostynska, N.; Dapporto, M.; Campodoni, E.; Montesi, M.; Panseri, S.; Tampieri, A.; Kon, E.; Marcacci, M.; Sprio, S.; et al. Evaluation of different crosslinking agents on hybrid biomimetic collagen-hydroxyapatite composites for regenerative medicine. Int. J. Biol. Macromol. 2018, 106, 739–748. [Google Scholar] [CrossRef]
- De Boulle, K.; Glogau, R.; Kono, T.; Nathan, M.; Tezel, A.; Roca-Martinez, J.-X.; Paliwal, S.; Stroumpoulis, D. A review of the metabolism of 1,4-butanediol diglycidyl ether-crosslinked hyaluronic acid dermal fillers. Dermatol. Surg. 2013, 39, 1758–1766. [Google Scholar] [CrossRef] [Green Version]
- Breil-Wirth, A.; Engelhardt, L.V.; Lobner, S.; Jerosch, J. Retrospektive Untersuchung einer zellfreien Matrix zur Knorpeltherapie. OUP 2016, 9, 515–520. [Google Scholar]
- Roessler, P.P.; Pfister, B.; Gesslein, M.; Figiel, J.; Heyse, T.J.; Colcuc, C.; Lorbach, O.; Efe, T.; Schüttler, K.F. Short-term follow up after implantation of a cell-free collagen type I matrix for the treatment of large cartilage defects of the knee. Int. Orthop. 2015, 39, 2473–2479. [Google Scholar] [CrossRef]
- Schneider, U. Controlled, randomized multicenter study to compare compatibility and safety of ChondroFiller liquid (cell free 2-component collagen gel) with microfracturing of patients with focal cartilage defects of the knee joint. Video J. Orthop. Surg. 2016, 1, 1–8. [Google Scholar] [CrossRef]
- Jansen, K.A.; Licup, A.J.; Sharma, A.; Rens, R.; MacKintosh, F.C.; Koenderink, G.H. The Role of Network Architecture in Collagen Mechanics. Biophys. J. 2018, 114, 2665–2678. [Google Scholar] [CrossRef]
- Zeeman, R.; Dijkstra, P.J.; Van Wachem, P.B.; Van Luyn, M.J.A.; Hendriks, M.; Cahalan, P.T.; Feijen, J. The kinetics of 1,4-butanediol diglycidyl ether crosslinking of dermal sheep collagen. J. Biomed. Mater. Res. 2000. [Google Scholar] [CrossRef]
- Hadjipanayi, E.; Mudera, V.; Brown, R.A. Guiding cell migration in 3D: A collagen matrix with graded directional stiffness. Cell Motil. Cytoskelet. 2009, 66, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Im, G.I. Endogenous Cartilage Repair by Recruitment of Stem Cells. Tissue Eng. Part B Rev. 2016, 22, 160–171. [Google Scholar] [CrossRef]
- Kern, S.; Eichler, H.; Stoeve, J.; Kluter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Shankar, K.G.; Gostynska, N.; Montesi, M.; Panseri, S.; Sprio, S.; Kon, E.; Marcacci, M.; Tampieri, A.; Sandri, M. Investigation of different cross-linking approaches on 3D gelatin scaffolds for tissue engineering application: A comparative analysis. Int. J. Biol. Macromol. 2017, 95, 1199–1209. [Google Scholar] [CrossRef]
- Decherchi, P.; Cochard, P.; Gauthier, P. Dual staining assessment of Schwann cell viability within wholeperipheral nerves using calcein-AM and ethidium homodimer. J. Neurosci. Methods 1997, 71, 205–213. [Google Scholar] [CrossRef]
- Leica Mycrosistems. Leica LAS X.; Leica Mycrosistems: Mannheim, Germany, 2017. [Google Scholar]
- Dorland, Y.L.; Cornelissen, A.S.; Kuijk, C.; Tol, S.; Hoogenboezem, M.; Van Buul, J.D.; Nolte, M.A.; Voermans, C.; Huveneers, S. Nuclear shape, protrusive behaviour and in vivo retention of human bone marrow mesenchymal stromal cells is controlled by Lamin-A/C expression. Sci. Rep. 2019, 9, 14401. [Google Scholar] [CrossRef]
- Yin, L.; Wu, Y.; Yang, Z.; Tee, C.A.; Denslin, V.; Lai, Z.; Lim, C.T.; Lee, E.H.; Ouyang, W. Microfluidic label-free selection of mesenchymal stem cell subpopulation during culture expansion extends the chondrogenic potential in vitro. Lab Chip 2018, 18, 878–889. [Google Scholar] [CrossRef] [Green Version]
- Šidák, Z. Rectangular Confidence Regions for the Means of Multivariate Normal Distributions. J. Am. Stat. Assoc. 1967, 62, 626–633. [Google Scholar] [CrossRef]
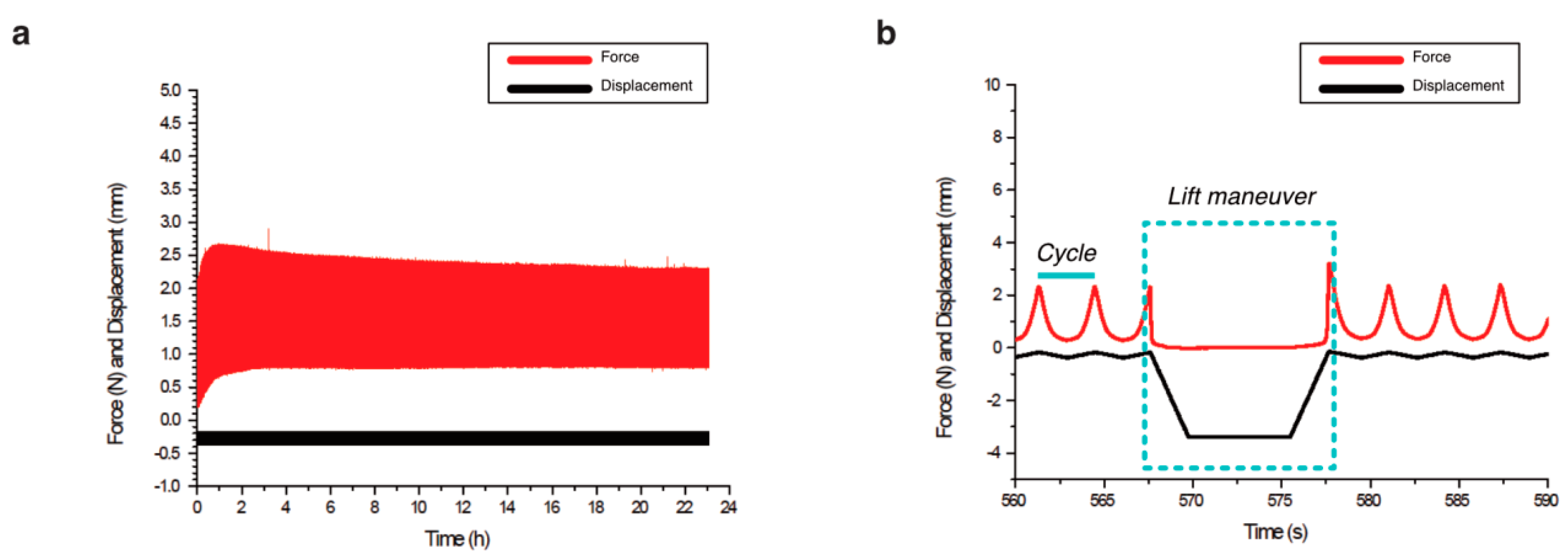
| Scaffold | Data | n | Mean | Std Dev | Median | Max | Min |
|---|---|---|---|---|---|---|---|
| Alginate-Ln | Force (N) | 12 | 1.16 | 0.42 | 1.15 | 2.23 | 0.69 |
| Displacement (µm) | 12 | 277.90 | 53.01 | 287.93 | 359.01 | 174.05 | |
| Number of periods | 12 | 142.58 | 16.42 | 151.50 | 156.00 | 108.00 | |
| Time (h) | 12 | 24.13 | 0.08 | 24.16 | 24.21 | 23.95 | |
| Col-I | Force (N) | 11 * | 1.08 | 0.13 | 1.09 | 1.25 | 0.88 |
| Displacement (µm) | 11 * | 202.20 | 11.10 | 199.68 | 225.73 | 193.18 | |
| Number of periods | 11 * | 133.27 | 10.73 | 141.00 | 143.00 | 118.00 | |
| Time (h) | 11 * | 23.64 | 0.86 | 23.40 | 24.95 | 22.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamez, C.; Schneider-Wald, B.; Bieback, K.; Schuette, A.; Büttner, S.; Hafner, M.; Gretz, N.; Schwarz, M.L. Compression Bioreactor-Based Mechanical Loading Induces Mobilization of Human Bone Marrow-Derived Mesenchymal Stromal Cells into Collagen Scaffolds In Vitro. Int. J. Mol. Sci. 2020, 21, 8249. https://doi.org/10.3390/ijms21218249
Gamez C, Schneider-Wald B, Bieback K, Schuette A, Büttner S, Hafner M, Gretz N, Schwarz ML. Compression Bioreactor-Based Mechanical Loading Induces Mobilization of Human Bone Marrow-Derived Mesenchymal Stromal Cells into Collagen Scaffolds In Vitro. International Journal of Molecular Sciences. 2020; 21(21):8249. https://doi.org/10.3390/ijms21218249
Chicago/Turabian StyleGamez, Carolina, Barbara Schneider-Wald, Karen Bieback, Andy Schuette, Sylvia Büttner, Mathias Hafner, Norbert Gretz, and Markus L. Schwarz. 2020. "Compression Bioreactor-Based Mechanical Loading Induces Mobilization of Human Bone Marrow-Derived Mesenchymal Stromal Cells into Collagen Scaffolds In Vitro" International Journal of Molecular Sciences 21, no. 21: 8249. https://doi.org/10.3390/ijms21218249
APA StyleGamez, C., Schneider-Wald, B., Bieback, K., Schuette, A., Büttner, S., Hafner, M., Gretz, N., & Schwarz, M. L. (2020). Compression Bioreactor-Based Mechanical Loading Induces Mobilization of Human Bone Marrow-Derived Mesenchymal Stromal Cells into Collagen Scaffolds In Vitro. International Journal of Molecular Sciences, 21(21), 8249. https://doi.org/10.3390/ijms21218249






