The Transcription Factor HAND1 Is Involved in Cortical Bone Mass through the Regulation of Collagen Expression
Abstract
1. Introduction
2. Results
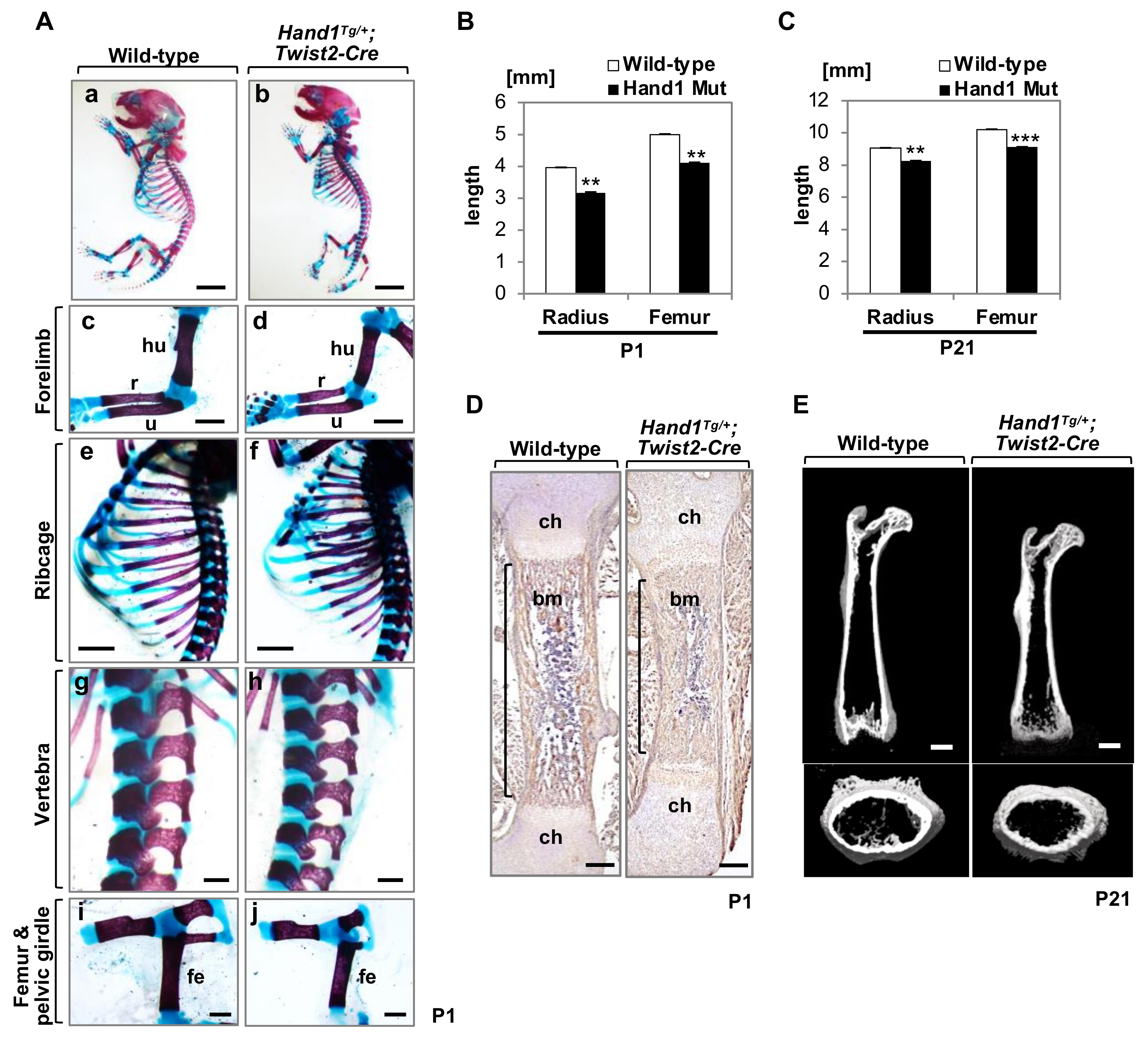
2.1. Overexpression of Hand1 Induces Developmental Defects in the Skeletal Bones
2.2. Micro-CT Analysis of Long Bones
2.3. Expression of Bone-Related Collagens in the Diaphyses
2.4. miR-196 Are Upregulated in Hand1-Overexpressing Long Bones
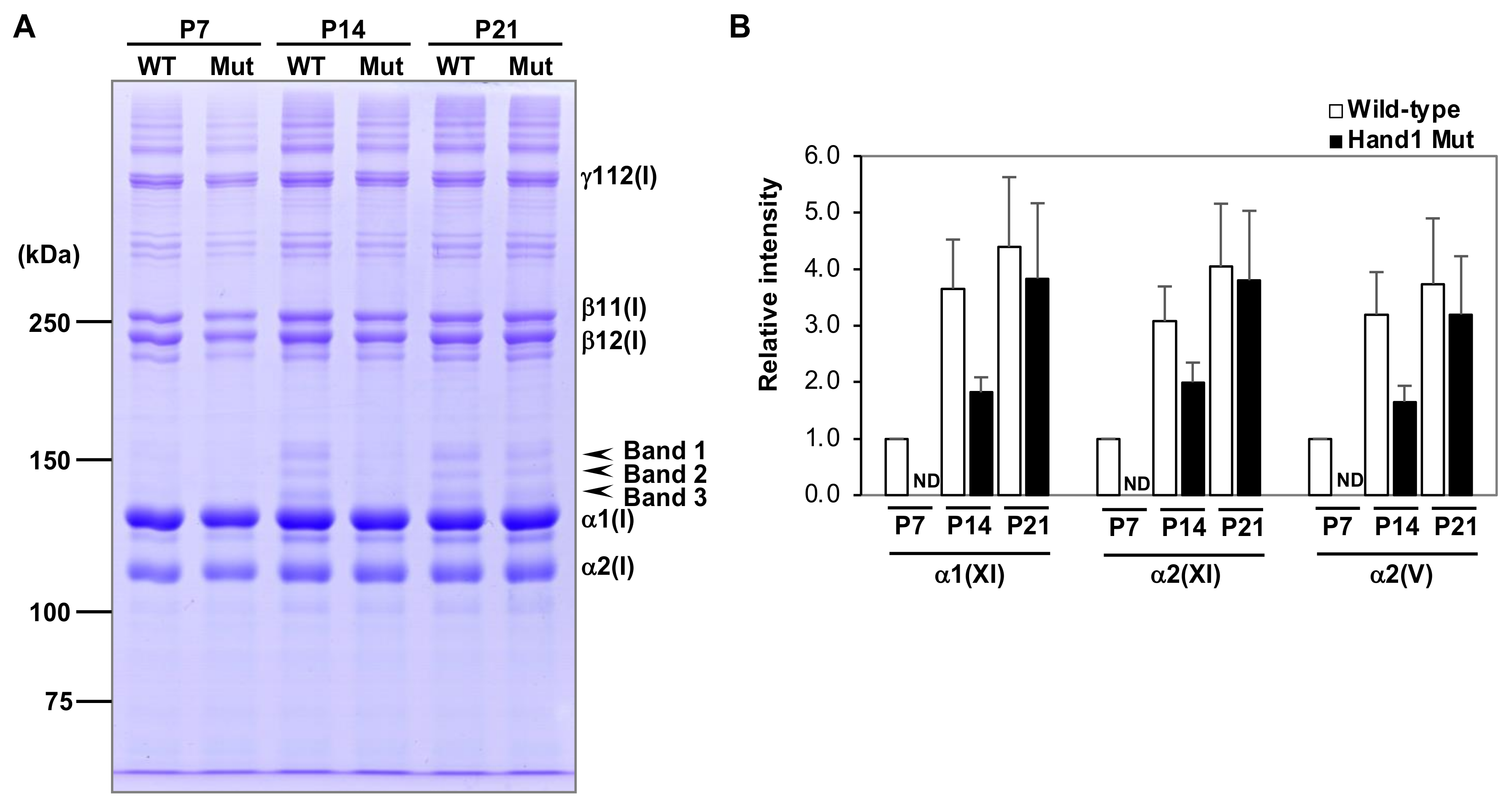
2.5. SDS-PAGE Analysis of Purified Collagen Samples
3. Discussion
3.1. Type I Collagen Expression in Hand1-Overexpressing Mice
3.2. Expression of Type V and XI Collagens in Hand1-Overexpressing Mice
4. Materials and Methods
4.1. Mice Conditionally Overexpressing Hand1
4.2. Bone Staining, Histology, and Immunohistochemistry
4.3. Micro-Computed Tomography
4.4. Real-Time Quantitative PCR
4.5. Extraction and Purification of Cortical Bone Collagens
4.6. Protein Identification Using in-Gel Digestion Followed by Mass Spectrometry
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BMC | Bone mineral content |
| BMC/TV | Volumetric bone mineral density |
| BV | Bone volume |
| ECM | Extracellular matrix |
| HAND1 | Heart and neural crest derivatives expressed protein 1 |
| MMP13 | Matrix metallopeptidase 13 |
| Micro-CT | Micro-computed tomography |
| OMIM | Online Mendelian Inheritance in Man |
| P | Postnatal day |
| S.E.M | Standard error of mean |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| TMD | Bone tissue mineral density |
| TV | Total volume of interest |
| UTR | Untranslated regions |
| bHLH | Basic helix-loop-helix |
| miRNA | MicroRNA |
| qRT-PCR | Real-time quantitative PCR |
References
- Orioli, I.M.; Castilla, E.E.; Barbosa-Neto, J.G. The birth prevalence rates for the skeletal dysplasias. J. Med. Genet. 1986, 23, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Niyibizi, C.; Eyre, D.R. Structural Characteristics of Cross-Linking Sites in type V Collagen of Bone: Chain Specificities and Heterotypic Links to Type I Collagen. Eur. J. Biochem. 1994, 224, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Krakow, D.; Rimoin, D.L. The skeletal dysplasias. Genet. Med. 2010, 12, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Viguet-Carrin, S.; Garnero, P.; Delmas, P.D. The role of collagen in bone strength. Osteoporos. Int. 2006, 17, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Weis, M.A.; Kim, L.S.; Carter, B.G.; Eyre, D.R. Differences in chain usage and cross-linking specificities of cartilage type V/XI collagen isoforms with age and tissue. J. Biol. Chem. 2009, 284, 5539–5545. [Google Scholar] [CrossRef] [PubMed]
- Andrikopoulos, K.; Liu, X.; Keene, D.R.; Jaenisch, R.; Ramirez, F. Targeted mutation in the col5a2 gene reveals a regulatory role for type V collagen during matrix assembly. Nat. Genet. 1995, 9, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Niyibizi, C.; Eyre, D.R. Identification of the cartilage α 1(XI) chain in type V collagen from bovine bone. FEBS Lett. 1989, 242, 314–318. [Google Scholar] [CrossRef]
- Birk, D.E. Type V collagen: Heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron 2001, 32, 223–237. [Google Scholar]
- Roulet, M.; Ruggiero, F.; Karsenty, G.; LeGuellec, D. A comprehensive study of the spatial and temporal expression of the col5a1 gene in mouse embryos: A clue for understanding collagen V function in developing connective tissues. Cell Tissue Res. 2007, 327, 323–332. [Google Scholar] [CrossRef]
- Chung, U.I.; Lanske, B.; Lee, K.; Li, E.; Kronenberg, H. The parathyroid hormone/parathyroid hormone-related peptide receptor coordinates endochondral bone development by directly controlling chondrocyte differentiation. Proc. Natl. Acad. Sci. USA 1998, 95, 13030–13035. [Google Scholar] [CrossRef]
- Chung, U.I.; Schipani, E.; McMahon, A.P.; Kronenberg, H.M. Indian hedgehog couples chondrogenesis to osteogenesis in endochondral bone development. J. Clin. Investig. 2001, 107, 295–304. [Google Scholar] [CrossRef] [PubMed]
- St-Jacques, B.; Hammerschmidt, M.; McMahon, A.P. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999, 13, 2072–2086. [Google Scholar] [CrossRef] [PubMed]
- Maes, C.; Kobayashi, T.; Selig, M.K.; Torrekens, S.; Roth, S.I.; Mackem, S.; Carmeliet, G.; Kronenberg, H.M. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev. Cell 2010, 19, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- Komori, T.; Yagi, H.; Nomura, S.; Yamaguchi, A.; Sasaki, K.; Deguchi, K.; Shimizu, Y.; Bronson, R.T.; Gao, Y.H.; Inada, M.; et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 1997, 89, 755–764. [Google Scholar] [CrossRef]
- Ducy, P.; Zhang, R.; Geoffroy, V.; Ridall, A.L.; Karsenty, G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell 1997, 89, 747–754. [Google Scholar] [CrossRef]
- Kern, B.; Shen, J.; Starbuck, M.; Karsenty, G. Cbfa1 Contributes to the Osteoblast-specific Expression of type I collagen Genes. J. Biol. Chem. 2001, 276, 7101–7107. [Google Scholar] [CrossRef]
- Ortuño, M.J.; Susperregui, A.R.G.; Artigas, N.; Rosa, J.L.; Ventura, F. Osterix induces Col1a1 gene expression through binding to Sp1 sites in the bone enhancer and proximal promoter regions. Bone 2013, 52, 548–556. [Google Scholar] [CrossRef]
- Yano, H.; Hamanaka, R.; Nakamura-Ota, M.; Adachi, S.; Zhang, J.J.; Matsuo, N.; Yoshioka, H. Sp7/Osterix induces the mouse pro-α2(I) collagen gene (Col1a2) expression via the proximal promoter in osteoblastic cells. Biochem. Biophys. Res. Commun. 2014, 452, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Thirunavukkarasu, K.; Zhou, L.; Pastore, L.; Baldini, A.; Hecht, J.; Geoffroy, V.; Ducy, P.; Karsenty, G. Missense mutations abolishing DNA binding of the osteoblast-specific transcription factor OSF2/CBFA1 in cleidocranial dysplasia. Nat. Genet. 1997, 16, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Mundlos, S.; Otto, F.; Mundlos, C.; Mulliken, J.B.; Aylsworth, A.S.; Albright, S.; Lindhout, D.; Cole, W.G.; Henn, W.; Knoll, J.H.; et al. Mutations involving the transcription factor CBFA1 cause cleidocranial dysplasia. Cell 1997, 89, 773–779. [Google Scholar] [CrossRef]
- Otto, F.; Thornell, A.P.; Crompton, T.; Denzel, A.; Gilmour, K.C.; Rosewell, I.R.; Stamp, G.W.; Beddington, R.S.; Mundlos, S.; Olsen, B.R.; et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 1997, 89, 765–771. [Google Scholar] [CrossRef]
- Laurie, L.E.; Kokubo, H.; Nakamura, M.; Saga, Y.; Funato, N. The transcription factor Hand1 is involved in Runx2-Ihh-regulated endochondral ossification. PLoS ONE 2016, 11, e0150263. [Google Scholar] [CrossRef] [PubMed]
- Firulli, B.A.; Milliar, H.; Toolan, K.P.; Harkin, J.; Fuchs, R.K.; Robling, A.G.; Firulli, A.B. Defective Hand1 phosphoregulation uncovers essential roles for Hand1 in limb morphogenesis. Development 2017, 2480–2489. [Google Scholar] [CrossRef]
- Funato, N.; Chapman, S.L.; McKee, M.D.; Funato, H.; Morris, J.A.; Shelton, J.M.; Richardson, J.A.; Yanagisawa, H. Hand2 controls osteoblast differentiation in the branchial arch by inhibiting DNA binding of Runx2. Development 2009, 136, 615–625. [Google Scholar] [CrossRef]
- Barbosa, A.C.; Funato, N.; Chapman, S.; McKee, M.D.; Richardson, J.A.; Olson, E.N.; Yanagisawa, H. Hand transcription factors cooperatively regulate development of the distal midline mesenchyme. Dev. Biol. 2007, 310, 154–168. [Google Scholar] [CrossRef][Green Version]
- Otsuka, T.; Fujinaka, H.; Imamura, M.; Tanaka, Y.; Hayakawa, H.; Tomizawa, S. Duplication of chromosome 4q: Renal pathology of two siblings. Am. J. Med. Genet. A 2005, 134, 330–333. [Google Scholar] [CrossRef]
- Tamura, M.; Hosoya, M.; Fujita, M.; Iida, T.; Amano, T.; Maeno, A.; Kataoka, T.; Otsuka, T.; Tanaka, S.; Tomizawa, S.; et al. Overdosage of Hand2 causes limb and heart defects in the human chromosomal disorder partial trisomy distal 4q. Hum. Mol. Genet. 2013, 22, 2471–2481. [Google Scholar] [CrossRef]
- Elefteriou, F.; Yang, X. Genetic mouse models for bone studies-Strengths and limitations. Bone 2011, 49, 1242–1254. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Xu, J.; Liu, Z.; Sosic, D.; Shao, J.; Olson, E.N.; Towler, D.A.; Ornitz, D.M. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development 2003, 130, 3063–3074. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.A.; Kim, Y.; Czubryt, M.P.; Phan, D.; McAnally, J.; Qi, X.; Shelton, J.M.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. MEF2C transcription factor controls chondrocyte hypertrophy and bone development. Dev. Cell 2007, 12, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, M.J.G.; Balbín, M.; López, J.M.; Alvarez, J.; Komori, T.; López-Otín, C. Collagenase 3 Is a Target of Cbfa1, a Transcription Factor of the runt Gene Family Involved in Bone Formation. Mol. Cell. Biol. 1999, 19, 4431–4442. [Google Scholar] [CrossRef]
- Porte, D.; Tuckermann, J.; Becker, M.; Baumann, B.; Teurich, S.; Higgins, T.; Owen, M.J.; Schorpp-Kistner, M.; Angel, P. Both AP-1 and Cbfa1-like factors are required for the induction of interstitial collagenase by parathyroid hormone. Oncogene 1999, 18, 667–678. [Google Scholar] [CrossRef]
- Mattot, V.; Raes, M.B.; Henriet, P.; Eeckhout, Y.; Stehelin, D.; Vandenbunder, B.; Desbiens, X. Expression of interstitial collagenase is restricted to skeletal tissue during mouse embryogenesis. J. Cell Sci. 1995, 108, 529–535. [Google Scholar] [PubMed]
- Fuller, K.; Chambers, T.J. Localisation of mRNA for collagenase in osteocytic, bone surface and chondrocytic cells but not osteoclasts. J. Cell Sci. 1995, 108, 2221–2230. [Google Scholar]
- Ståhle-Bäckdahl, M.; Sandstedt, B.; Bruce, K.; Lindahl, A.; Jiménez, M.G.; Vega, J.A.; López-Otín, C. Collagenase-3 (MMP-13) is expressed during human fetal ossification and re-expressed in postnatal bone remodeling and in rheumatoid arthritis. Lab. Investig. 1997, 76, 717–728. [Google Scholar]
- Park, A.C.; Phillips, C.L.; Pfeiffer, F.M.; Roenneburg, D.A.; Kernien, J.F.; Adams, S.M.; Davidson, J.M.; Birk, D.E.; Greenspan, D.S. Homozygosity and Heterozygosity for Null Col5a2 Alleles Produce Embryonic Lethality and a Novel Classic Ehlers-Danlos Syndrome-Related Phenotype. Am. J. Pathol. 2015, 185, 2000–2011. [Google Scholar] [CrossRef]
- Forlino, A.; Porter, F.D.; Eric, J.L.; Westphal, H.; Marini, J.C. Use of the Cre/lox recombination system to develop a non-lethal knock-in murine model for osteogenesis imperfecta with an α1(I) G349C substitution. Variability in phenotype in BrtlIV mice. J. Biol. Chem. 1999, 274, 37923–37931. [Google Scholar] [CrossRef]
- Daley, E.; Streeten, E.A.; Sorkin, J.D.; Kuznetsova, N.; Shapses, S.A.; Carleton, S.M.; Shuldiner, A.R.; Marini, J.C.; Phillips, C.L.; Goldstein, S.A.; et al. Variable bone fragility associated with an Amish COL1A2 variant and a knock-in mouse model. J. Bone Miner. Res. 2010, 25, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Honda, N.; Jinnin, M.; Kajihara, I.; Makino, T.; Makino, K.; Masuguchi, S.; Fukushima, S.; Okamoto, Y.; Hasegawa, M.; Fujimoto, M.; et al. TGF-β–Mediated Downregulation of MicroRNA-196a Contributes to the Constitutive Upregulated Type I Collagen Expression in Scleroderma Dermal Fibroblasts. J. Immunol. 2012, 188, 3323–3331. [Google Scholar] [CrossRef] [PubMed]
- Takemura, Y.; Mizuno, K.; Imamura, Y.; Hayashi, T. Preferential Liberation of Type V Collagen from Bovine Corneal Stroma by Limited Treatment with Protease. Connect. Tissue 2003, 35, 133–139. [Google Scholar]
- Ducy, P.; Starbuck, M.; Priemel, M.; Shen, J.; Pinero, G.; Geoffroy, V.; Amling, M.; Karsenty, G. A Cbfa1-dependent genetic pathway controls bone formation beyond embryonic development. Genes Dev. 1999, 13, 1025–1036. [Google Scholar] [CrossRef]
- Nishio, Y.; Dong, Y.; Paris, M.; O’Keefe, R.J.; Schwarz, E.M.; Drissi, H. Runx2-mediated regulation of the zinc finger Osterix/Sp7 gene. Gene 2006, 372, 62–70. [Google Scholar] [CrossRef]
- Goto, T.; Matsui, Y.; Fernandes, R.J.; Hanson, D.A.; Kubo, T.; Yukata, K.; Michigami, T.; Komori, T.; Fujita, T.; Yang, L.; et al. Sp1 family of transcription factors regulates the human alpha2 (XI) collagen gene (COL11A2) in Saos-2 osteoblastic cells. J. Bone Miner. Res. 2006, 21, 661–673. [Google Scholar] [CrossRef]
- Funato, N. New Insights Into Cranial Synchondrosis Development: A Mini Review. Front. Cell Dev. Biol. 2020, 8, 1–9. [Google Scholar] [CrossRef]
- Tabeta, K.; Du, X.; Arimatsu, K.; Yokoji, M.; Takahashi, N.; Amizuka, N.; Hasegawa, T.; Crozat, K.; Maekawa, T.; Miyauchi, S.; et al. An ENU-induced splice site mutation of mouse Col1a1 causing recessive osteogenesis imperfecta and revealing a novel splicing rescue. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Linsenmayer, T.F.; Gibney, E.; Igoe, F.; Gordon, M.K.; Fitch, J.M.; Fessler, L.I.; Birk, D.E. Type V collagen: Molecular structure and fibrillar organization of the chicken α1(V) NH2-terminal domain, a putative regulator of corneal fibrillogenesis. J. Cell Biol. 1993, 121, 1181–1189. [Google Scholar] [CrossRef]
- Seegmiller, R.; Fraser, F.C.; Sheldon, H. A New Chondrodystrophic Mutant in Mice. J. Cell Biol. 1971, 48, 580–593. [Google Scholar] [CrossRef]
- Bonaventure, J.; Zylberberg, L.; Cohen-Solal, L.; Allain, J.C.; Lasselin, C.; Maroteaux, P. A new lethal brittle bone syndrome with increased amount of type V collagen in a patient. Am. J. Med. Genet. 1989, 33, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Hida, M.; Sasaki, T.; Yano, H.; Kawano, K.; Yoshioka, H.; Matsuo, N. Sp1 upregulates the proximal promoter activity of the mouse collagen α1(XI) gene (Col11a1) in chondrocytes. In Vitro Cell. Dev. Biol. Anim. 2016, 52, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Funato, N.; Kokubo, H.; Nakamura, M.; Yanagisawa, H.; Saga, Y. Specification of jaw identity by the Hand2 transcription factor. Sci. Rep. 2016, 6, 28405. [Google Scholar] [CrossRef] [PubMed]
- Funato, N.; Nakamura, M.; Richardson, J.A.; Srivastava, D.; Yanagisawa, H. Tbx1 regulates oral epithelial adhesion and palatal development. Hum. Mol. Genet. 2012, 21, 2524–2537. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, A.; Tomas, H.; Havliš, J.; Olsen, J.V.; Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2007, 1, 2856–2860. [Google Scholar] [CrossRef] [PubMed]
- Taga, Y.; Kusubata, M.; Ogawa-Goto, K.; Hattori, S. Development of a Novel Method for Analyzing Collagen O-glycosylations by Hydrazide Chemistry. Mol. Cell. Proteom. 2012, 11. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Funato, N.; Taga, Y.; Laurie, L.E.; Tometsuka, C.; Kusubata, M.; Ogawa-Goto, K. The Transcription Factor HAND1 Is Involved in Cortical Bone Mass through the Regulation of Collagen Expression. Int. J. Mol. Sci. 2020, 21, 8638. https://doi.org/10.3390/ijms21228638
Funato N, Taga Y, Laurie LE, Tometsuka C, Kusubata M, Ogawa-Goto K. The Transcription Factor HAND1 Is Involved in Cortical Bone Mass through the Regulation of Collagen Expression. International Journal of Molecular Sciences. 2020; 21(22):8638. https://doi.org/10.3390/ijms21228638
Chicago/Turabian StyleFunato, Noriko, Yuki Taga, Lindsay E. Laurie, Chisa Tometsuka, Masashi Kusubata, and Kiyoko Ogawa-Goto. 2020. "The Transcription Factor HAND1 Is Involved in Cortical Bone Mass through the Regulation of Collagen Expression" International Journal of Molecular Sciences 21, no. 22: 8638. https://doi.org/10.3390/ijms21228638
APA StyleFunato, N., Taga, Y., Laurie, L. E., Tometsuka, C., Kusubata, M., & Ogawa-Goto, K. (2020). The Transcription Factor HAND1 Is Involved in Cortical Bone Mass through the Regulation of Collagen Expression. International Journal of Molecular Sciences, 21(22), 8638. https://doi.org/10.3390/ijms21228638




