Synergistic Effect of Beauveria bassiana and Trichoderma asperellum to Induce Maize (Zea mays L.) Defense against the Asian Corn Borer, Ostrinia furnacalis (Lepidoptera, Crambidae) and Larval Immune Response
Abstract
1. Introduction
2. Results
2.1. In Vitro Pathogenicity Bioassay
2.2. Scanning Electron Microscopy
2.3. Defense Response of Maize Plant
2.3.1. Confirmation of Endophytic Colonization of Plants
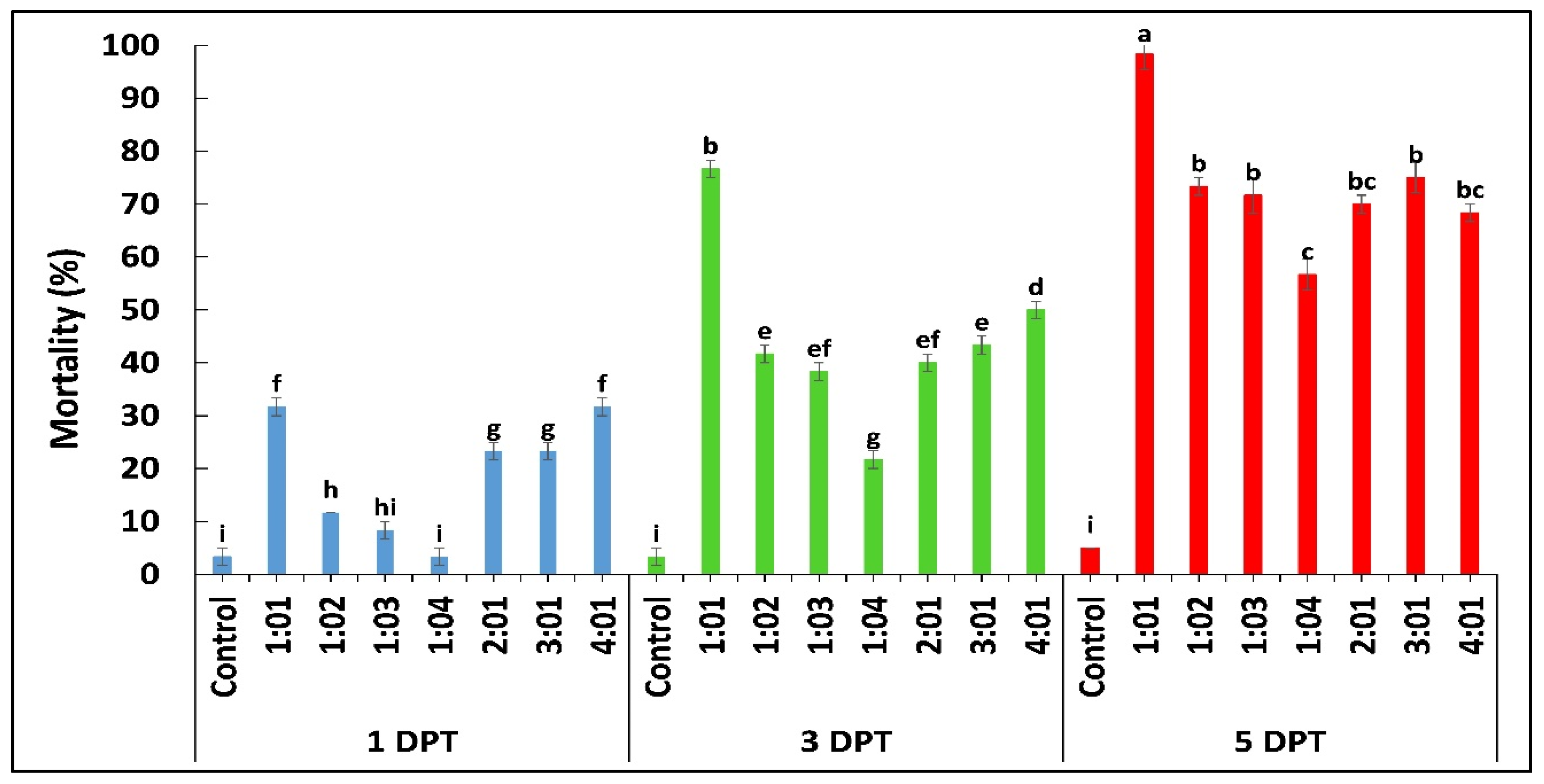
2.3.2. Larval Mortality and Maize Plant Damage Rating
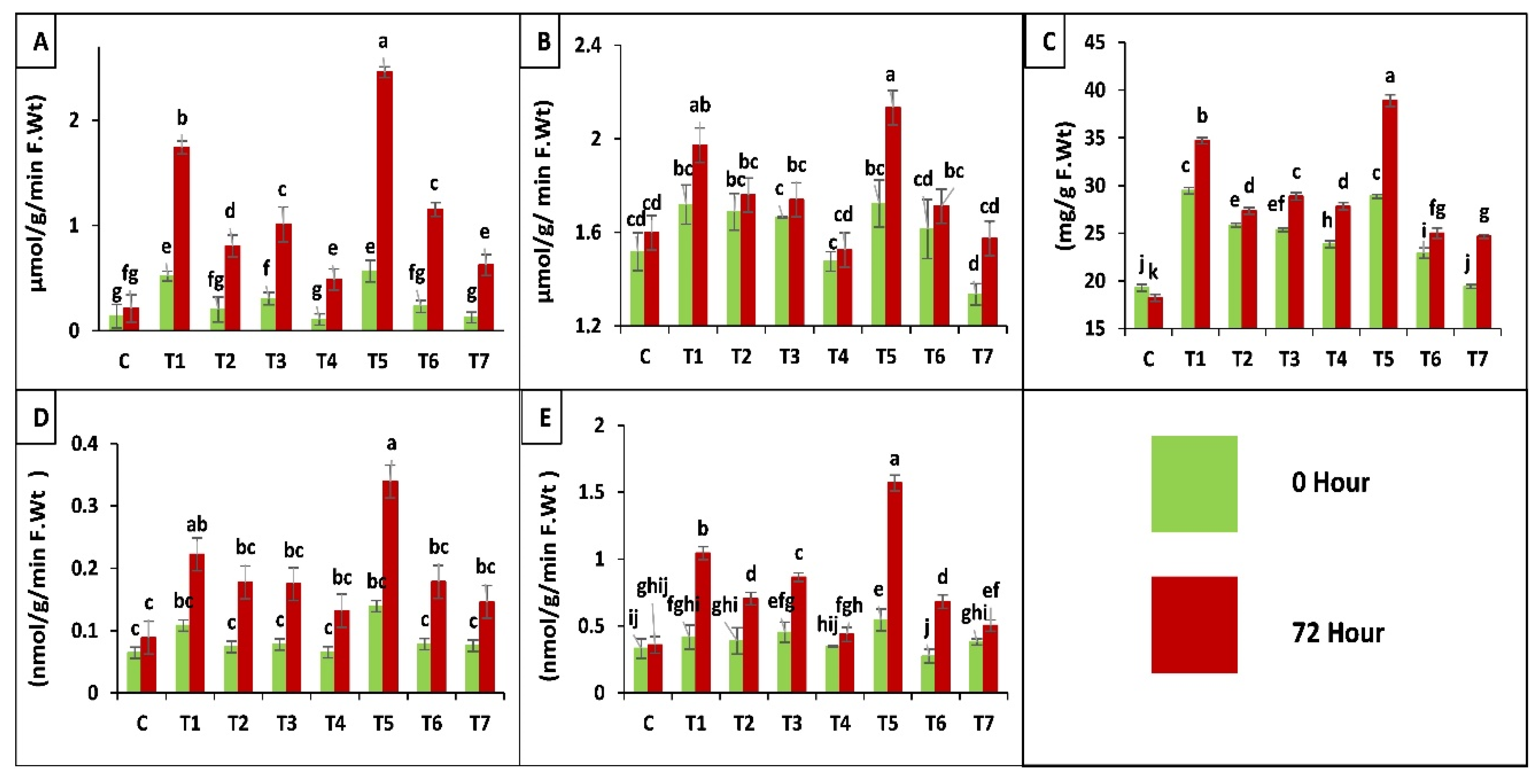
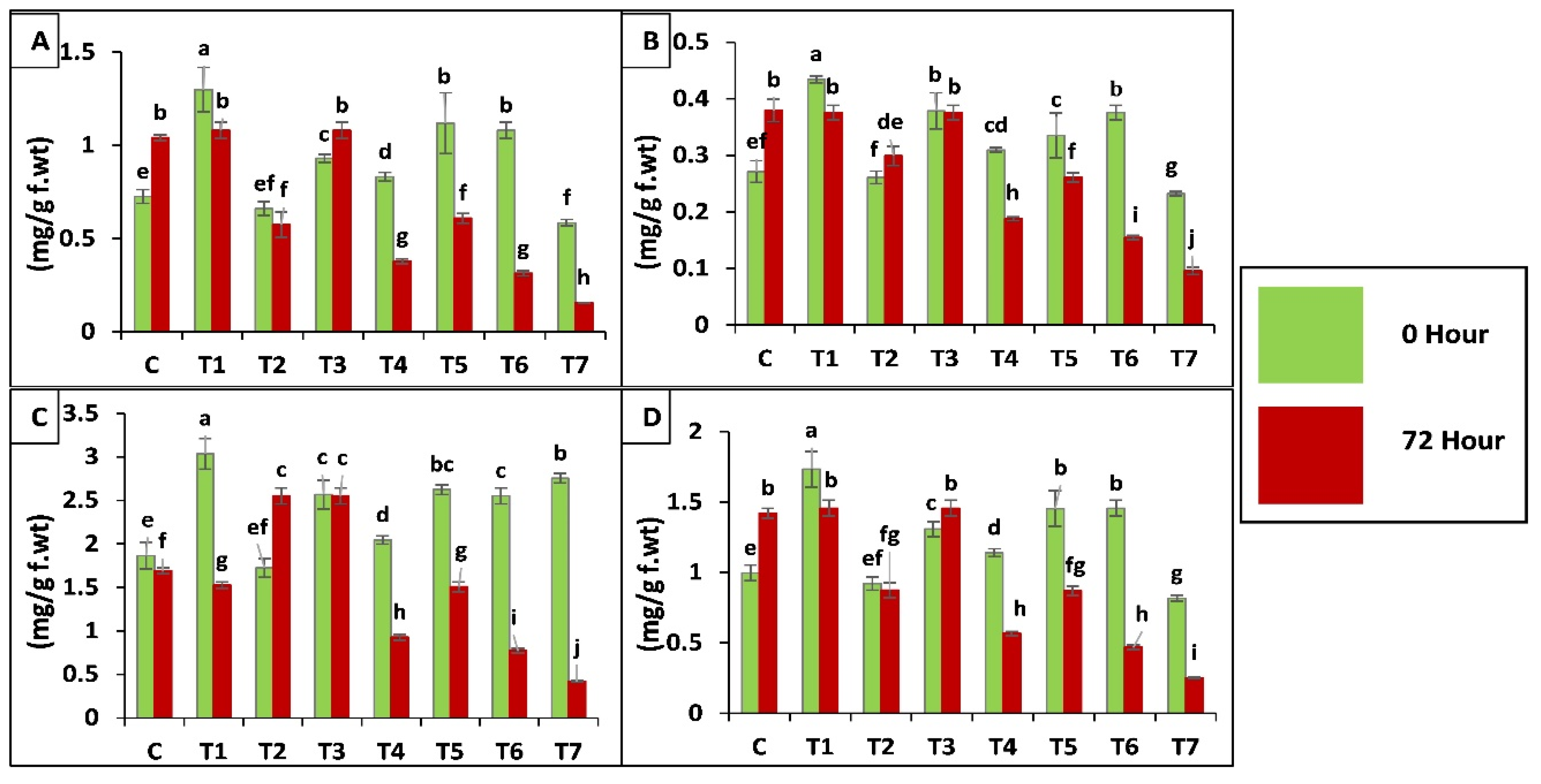
2.3.3. Biochemical Analysis of Maize Plants
Antioxidant Enzyme Assay
2.3.4. Chlorophyll a, b and Carotenoids Contents in Maize Plants
Proline Content of Leaves
Protease (EC 3.4.21.112) Activity
Polyphenol Oxidase (PPO, EC 1.10.3.1) Activity
2.4. Transcriptome Analysis and Identification of Deferentially Expressed Genes in ACB
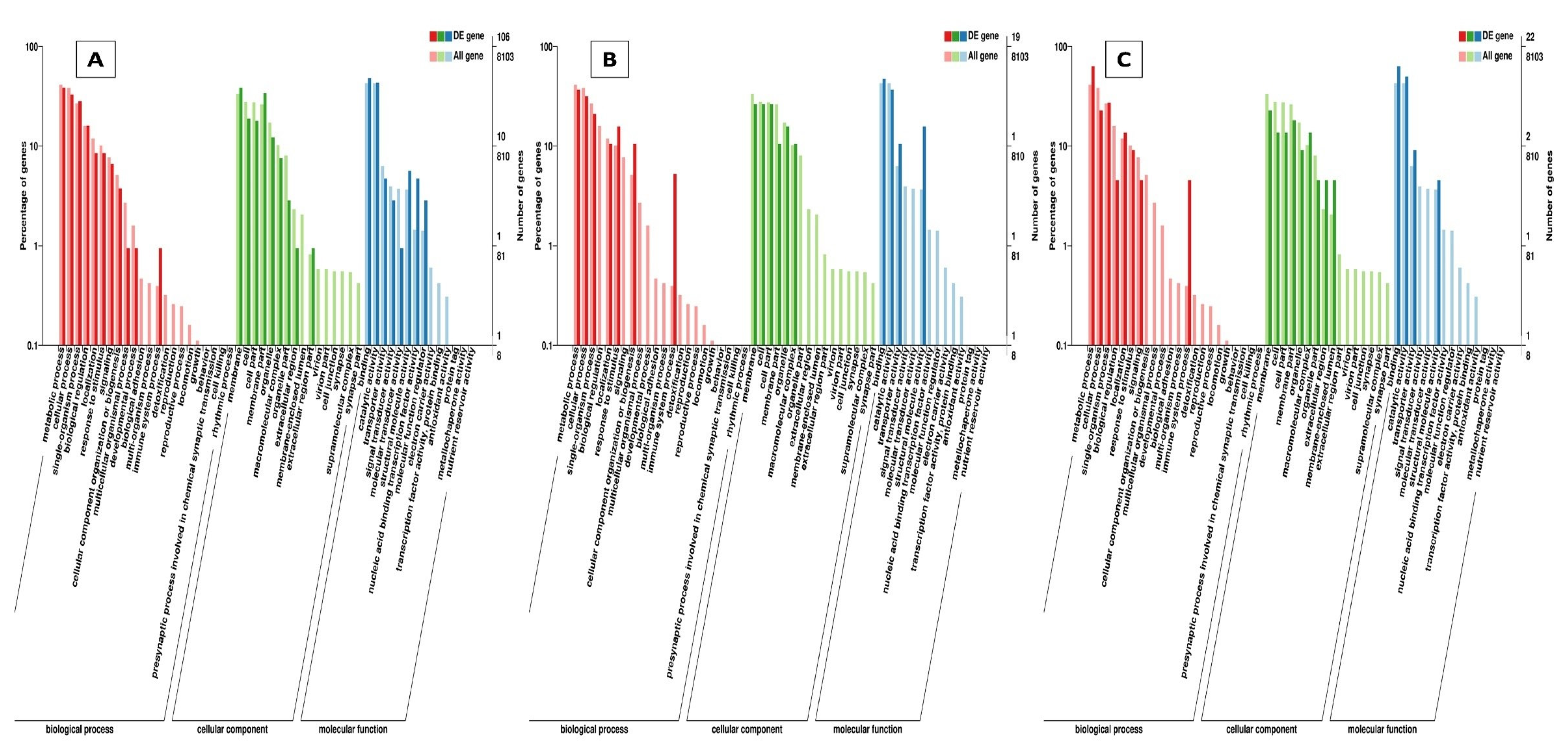
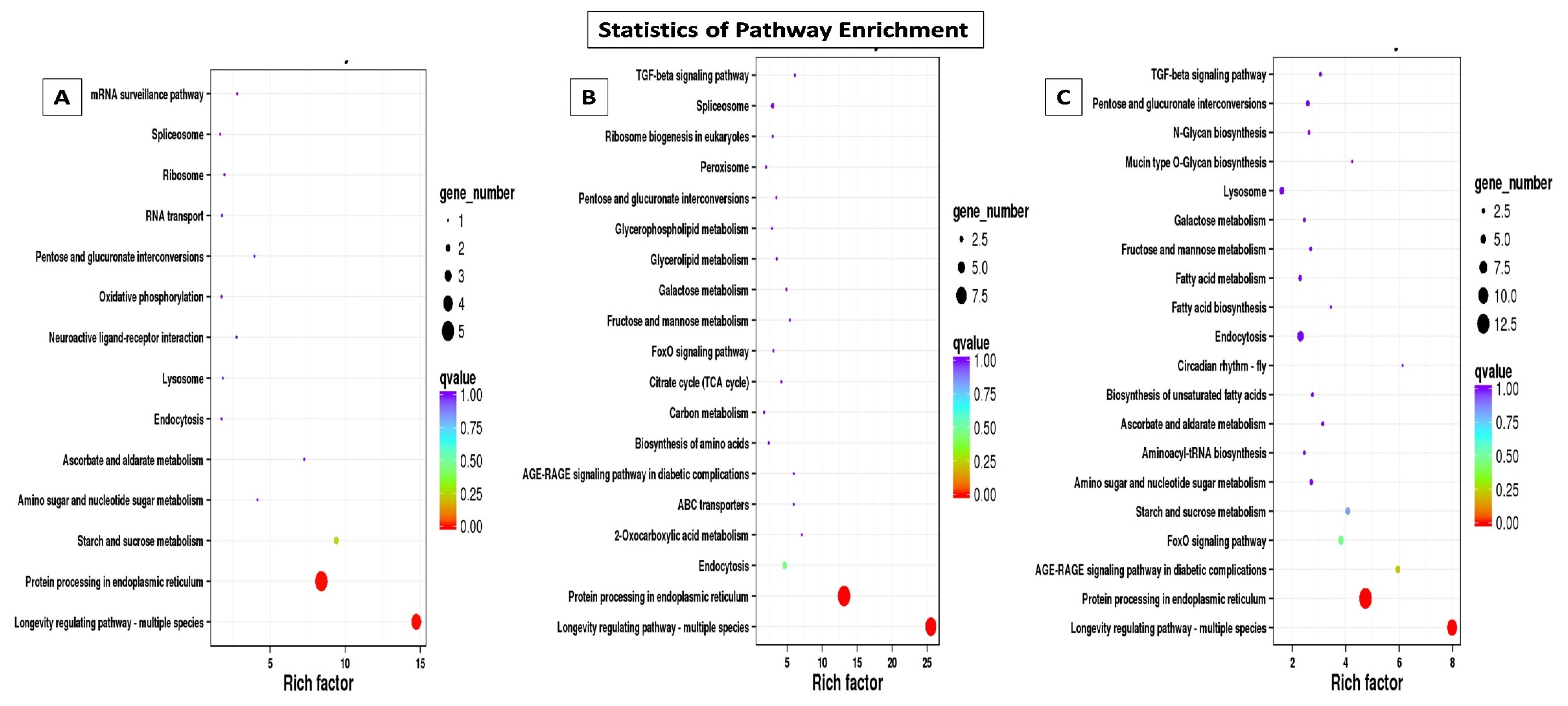
2.4.1. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Classification and Enrichment Analysis
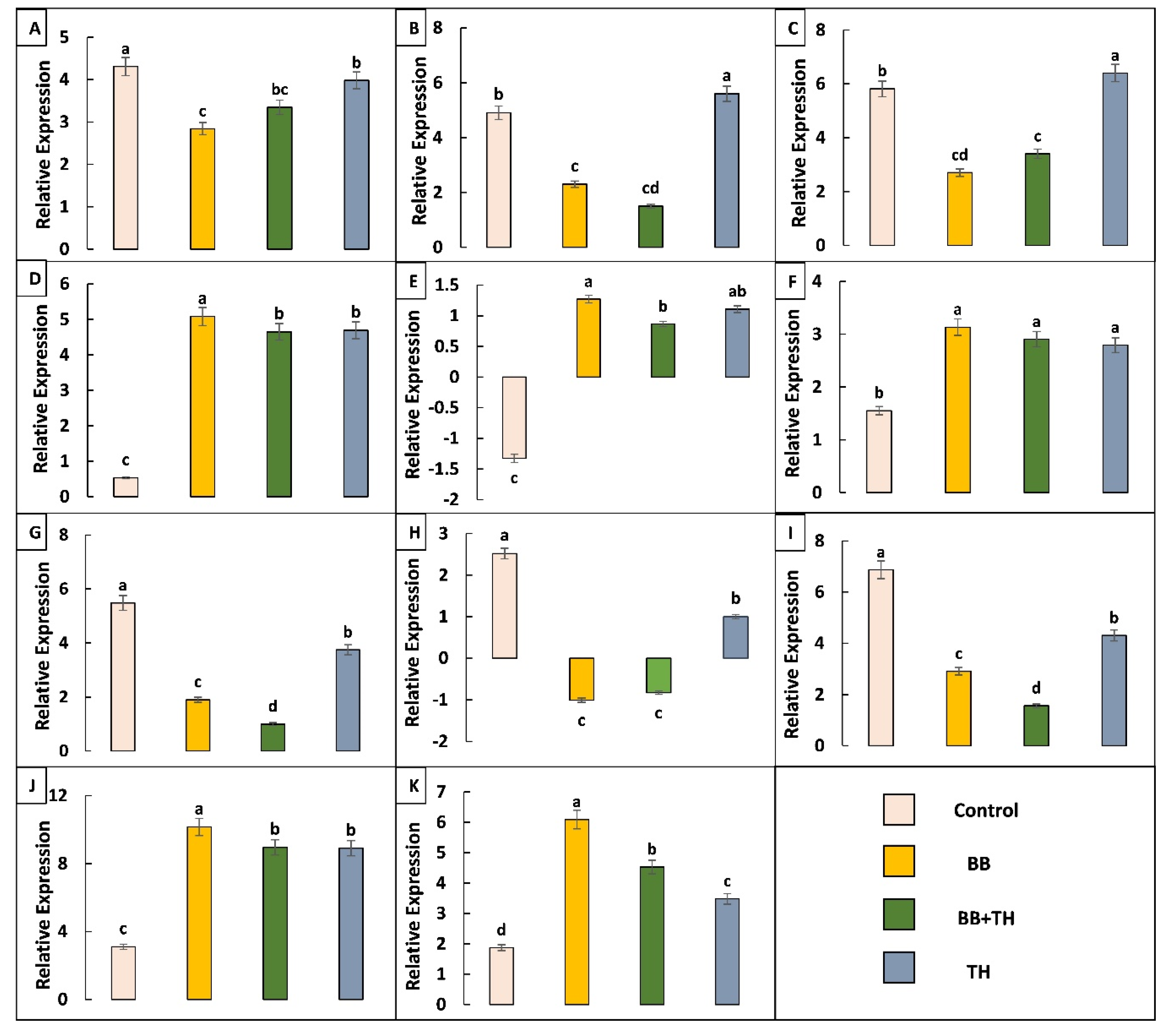
2.4.2. Expression Pattern of Immune Related Genes and qRT-PCR Validation
3. Discussion
4. Material and Methods
4.1. Source of Insect and Fungal Isolates
4.2. Conidial Suspension Preparation
4.3. In Vitro Pathogenicity Bioassay
4.4. Scanning Electron Microscopy (SEM)
4.5. Defense Response of Maize Plant
4.5.1. Application of Fungal Suspensions and Sowing
4.5.2. Confirmation of Endophytic Colonization of Plants
4.5.3. Insect Infestation and Sampling
4.5.4. Larval Mortality and Maize Plant Damage Rating
4.5.5. Physiological and Biochemical Analysis of Plants
Antioxidant Enzyme Assay
Proline Content of Leaves
Protease (EC 3.4.21.112) Activity
Polyphenol Oxidase (PPO, EC 1.10.3.1) Activity
4.5.6. Chlorophyll Content of Plants
4.6. Transcriptome Analysis
4.6.1. Sample Collection and Preparation
4.6.2. Library Preparation and Illumina Sequencing
4.6.3. Assembly and Functional Annotation
4.6.4. Differential Expression Analysis
4.6.5. Go and KEGG Pathway Enrichment Analysis
4.6.6. Validation of Defense Related DEG’s by RT-qPCR
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bohra, B.; Rathore, R.S.; Jain, M.L. Management of Fusarium stalk rot of maize caused by Fusarium moniliforme Sheldon. J. Mycol. Plant Pathol. 2001, 31, 245–247. [Google Scholar]
- Nafus, D.; Schreiner, I. Review of the biology and control of the Asian corn borer, Ostrinia furnacalis (Lep: Pyralidae). Int. J. Pest Manag. 1991, 37, 41–56. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, X.; He, K.; Zhou, D. Review of history, present situation and prospect of the Asian maize borer research in China. Yournal Shenyang Agric. Univ. 2000, 31, 402–412. [Google Scholar]
- Saranraj, P.; Jayaparakash, A. Agrobenificial entomopathogenic fungi–Beauveria bassiana: A review. Indo–Asian J. Multidiscip. Res. (IAJMR) 2017, 3, 1051–1087. [Google Scholar]
- Bano, A.; Muqarab, R. Plant defence induced by PGPR against Spodoptera litura in tomato (Solanum lycopersicum L.). Plant Biol. 2017, 19, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, V.; Viswanathan, R.; Raguchander, T.; Prakasam, V.; Samiyappan, R. Induction of systemic resistance by plant growth promoting rhizobacteria in crop plants against pests and diseases. Crop. Prot. 2001, 20, 1–11. [Google Scholar] [CrossRef]
- Majeed, M.Z.; Fiaz, M.; Ma, C.-S.; Afzal, M. Entomopathogenicity of Three Muscardine Fungi, Beauveria bassiana, Isaria fumosorosea and Metarhizium anisopliae, against the Asian Citrus Psyllid, Diaphorina citri Kuwayama (Hemiptera: Psyllidae). Egypt. J. Biol. Pest Control 2017, 27, 21–215. [Google Scholar]
- Mora, M.A.E.; Castilho, A.M.C.; Fraga, M.E. Classification and infection mechanism of entomopathogenic fungi. Arq. Do Inst. Biológico 2017, 84. [Google Scholar] [CrossRef]
- Sarma, B.K.; Yadav, S.K.; Singh, S.; Singh, H.B. Microbial consortium-mediated plant defense against phytopathogens: Readdressing for enhancing efficacy. Soil Biol. Biochem. 2015, 87, 25–33. [Google Scholar] [CrossRef]
- Zimmermann, G. Review on safety of the entomopathogenic fungi Beauveria bassiana and Beauveria Brongniartii. Biocontrol. Sci. Technol. 2007, 17, 553–596. [Google Scholar] [CrossRef]
- Ownley, B.H.; Gwinn, K.D.; Vega, F.E. Endophytic fungal entomopathogens with activity against plant pathogens: Ecology and evolution. BioControl 2010, 55, 113–128. [Google Scholar] [CrossRef]
- Lopez, D.C.; Sword, G.A. The endophytic fungal entomopathogens Beauveria bassiana and Purpureocillium lilacinum enhance the growth of cultivated cotton (Gossypium hirsutum) and negatively affect survival of the cotton bollworm (Helicoverpa zea). Biol. Control 2015, 89, 53–60. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Horwitz, B.A.; Herrera-Estrella, A.; Schmoll, M.; Kenerley, C.M. Trichoderma research in the genome era. Annu. Rev. Phytopathol. 2013, 51, 105–129. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Jurado, I.; Fernández-Bravo, M.; Campos, C.; Quesada-Moraga, E. Diversity of entomopathogenic Hypocreales in soil and phylloplanes of five Mediterranean cropping systems. J. Invertebr. Pathol. 2015, 130, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Howell, C. Mechanisms employed by Trichoderma species in the biological control of plant diseases: The history and evolution of current concepts. Plant Dis. 2003, 87, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.R. Beauveria Bassiana, a Cotton Endophyte with Biocontrol Activity against Seedling Disease. Ph.D. Thesis, University of Tennessee, Knoxville, TN, USA, 2007. [Google Scholar]
- Quesada-Moraga, E.; López-Díaz, C.; Landa, B.B. The hidden habit of the entomopathogenic fungus Beauveria bassiana: First demonstration of vertical plant transmission. PLoS ONE 2014, 9, e89278. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, A.R.; Raya-Díaz, S.; Zamarreño, Á.M.; García-Mina, J.M.; del Campillo, M.C.; Quesada-Moraga, E. An endophytic Beauveria bassiana strain increases spike production in bread and durum wheat plants and effectively controls cotton leafworm (Spodoptera littoralis) larvae. Biol. Control 2018, 116, 90–102. [Google Scholar] [CrossRef]
- Sharma, V.; Salwan, R.; Sharma, P.; Gulati, A. Integrated translatome and proteome: Approach for accurate portraying of widespread multifunctional aspects of Trichoderma. Front. Microbiol. 2017, 8, 1602. [Google Scholar] [CrossRef]
- Simon, J.C.; Biere, A.; Sugio, A. The promises and challenges of research on plant–insect–microbe interactions. Insect Sci. 2017, 24, 904–909. [Google Scholar] [CrossRef]
- Lacey, L.; Grzywacz, D.; Shapiro-Ilan, D.; Frutos, R.; Brownbridge, M.; Goettel, M. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef]
- Gonzalez, F.; Tkaczuk, C.; Dinu, M.M.; Fiedler, Ż.; Vidal, S.; Zchori-Fein, E.; Messelink, G.J. New opportunities for the integration of microorganisms into biological pest control systems in greenhouse crops. J. Pest Sci. 2016, 89, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.A.; Reichhart, J.-M. Drosophila innate immunity: An evolutionary perspective. Nat. Immunol. 2002, 3, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shen, D.; Zhou, F.; Wang, G.; An, C. Identification of immunity-related genes in Ostrinia furnacalis against entomopathogenic fungi by RNA-seq analysis. PLoS ONE 2014, 9, e86436. [Google Scholar]
- El Chamy, L.; Leclerc, V.; Caldelari, I.; Reichhart, J.-M. Sensing of ‘danger signals’ and pathogen-associated molecular patterns defines binary signaling pathways ‘upstream’ of Toll. Nat. Immunol. 2008, 9, 1165–1170. [Google Scholar] [CrossRef]
- Söderhäll, K.; Cerenius, L. Role of the prophenoloxidase-activating system in invertebrate immunity. Curr. Opin. Immunol. 1998, 10, 23–28. [Google Scholar] [CrossRef]
- Hultmark, D. Drosophila immunity: Paths and patterns. Curr. Opin. Immunol. 2003, 15, 12–19. [Google Scholar] [CrossRef]
- Osta, M.A.; Christophides, G.K.; Vlachou, D.; Kafatos, F.C. Innate immunity in the malaria vector Anopheles gambiae: Comparative and functional genomics. J. Exp. Biol. 2004, 207, 2551–2563. [Google Scholar] [CrossRef]
- Butt, T.M. Use of entomogenous fungi for the control of insect pests. In Agricultural Applications; Springer: Berlin/Heidelberg, Germany, 2002; pp. 111–134. [Google Scholar]
- Vey, A.; Matha, V.; Dumas, C. Effects of the peptide mycotoxin destruxin E on insect haemocytes and on dynamics and efficiency of the multicellular immune reaction. J. Invertebr. Pathol. 2002, 80, 177–187. [Google Scholar] [CrossRef]
- Vinale, F.; Manganiello, G.; Nigro, M.; Mazzei, P.; Piccolo, A.; Pascale, A.; Ruocco, M.; Marra, R.; Lombardi, N.; Lanzuise, S. A novel fungal metabolite with beneficial properties for agricultural applications. Molecules 2014, 19, 9760–9772. [Google Scholar] [CrossRef]
- Rasmann, S.; Bennett, A.; Biere, A.; Karley, A.; Guerrieri, E. Root symbionts: Powerful drivers of plant above-and belowground indirect defenses. Insect Sci. 2017, 24, 947–960. [Google Scholar] [CrossRef]
- Nazir, T.; Basit, A.; Hanan, A.; Majeed, M.; Qiu, D. In Vitro Pathogenicity of Some Entomopathogenic Fungal Strains against Green Peach Aphid Myzus persicae (Homoptera: Aphididae). Agronomy 2019, 9, 7. [Google Scholar] [CrossRef]
- Karuppiah, V.; Vallikkannu, M.; Li, T.; Chen, J. Simultaneous and sequential based co-fermentations of Trichoderma asperellum GDFS1009 and Bacillus amyloliquefaciens 1841: A strategy to enhance the gene expression and metabolites to improve the bio-control and plant growth promoting activity. Microb. Cell Factories 2019, 18, 185. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ying, S.-H.; Shan, L.-T.; Feng, M.-G. A new non-hydrophobic cell wall protein (CWP10) of Metarhizium anisopliae enhances conidial hydrophobicity when expressed in Beauveria Bassiana. Appl. Microbiol. Biotechnol. 2010, 85, 975–984. [Google Scholar] [CrossRef]
- Ansari, M.; Vestergaard, S.; Tirry, L.; Moens, M. Selection of a highly virulent fungal isolate, Metarhizium anisopliae CLO 53, for controlling Hoplia philanthus. J. Invertebr. Pathol. 2004, 85, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Feng, M.-G. Advances in fundamental and applied studies in China of fungal biocontrol agents for use against arthropod pests. Biol. Control 2014, 68, 129–135. [Google Scholar] [CrossRef]
- Gabarty, A.; Salem, H.; Fouda, M.; Abas, A.; Ibrahim, A. Pathogencity induced by the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in Agrotis ipsilon (Hufn.). J. Radiat. Res. Appl. Sci. 2014, 7, 95–100. [Google Scholar] [CrossRef]
- Asensio, L.; Lopez-Llorca, L.; López-Jiménez, J. Use of light, scanning electron microscopy and bioassays to evaluate parasitism by entomopathogenic fungi of the red scale insect of palms (Phoenicococcus marlatti Ckll., 1899). Micron 2005, 36, 169–175. [Google Scholar] [CrossRef]
- Lefebvre, C.L. Penetration and development of the fungus, Beauveria bassiana, in the tissues of the corn borer. Ann. Bot. 1934, 48, 441–452. [Google Scholar] [CrossRef]
- Lewis, L.C.; Berry, E.C.; Obrycki, J.J.; Bing, L.A. Aptness of insecticides (Bacillus thuringiensis and carbofuran) with endophytic Beauveria bassiana, in suppressing larval populations of the European corn borer. Agric. Ecosyst. Environ. 1996, 57, 27–34. [Google Scholar] [CrossRef]
- Elad, Y. Biological control of foliar pathogens by means of Trichoderma harzianum and potential modes of action. Crop. Prot. 2000, 19, 709–714. [Google Scholar] [CrossRef]
- Li, Y.Y.; Tang, J.; Fu, K.H.; Gao, S.G.; Wu, Q.; Chen, J. Construction of transgenic Trichoderma koningi with chit42 of Metarhizium anisopliae and analysis of its activity against the Asian corn borer. J. Environ. Sci. Health Part B 2012, 47, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Wagner, B.L.; Lewis, L.C. Colonization of corn, Zea mays, by the entomopathogenic fungus Beauveria bassiana. Appl. Environ. Microbiol. 2000, 66, 3468–3473. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.; Saini, R.; Pagadala, V.; Kumar, N.; Sharma, D.; Saini, A. Analysis of plant growth promoting potential of endophytes isolated from Echinacea purpurea and Lonicera japonica. J. Soil Sci. Plant Nutr. 2016, 16, 558–577. [Google Scholar] [CrossRef]
- Muvea, A.M.; Meyhöfer, R.; Subramanian, S.; Poehling, H.-M.; Ekesi, S.; Maniania, N.K. Colonization of onions by endophytic fungi and their impacts on the biology of Thrips tabaci. PLoS ONE 2014, 9, e108242. [Google Scholar] [CrossRef]
- Bing, L.A.; Lewis, L.C. Temporal relationships between Zea mays, ostrinia nubilalis (Lep.: Pyralidae) and endophytic Beauveria bassiana. Entomophaga 1992, 37, 525–536. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; Landa, B.; Muñoz-Ledesma, J.; Jiménez-Diáz, R.; Santiago-Alvarez, C. Endophytic colonisation of opium poppy, Papaver somniferum, by an entomopathogenic Beauveria bassiana strain. Mycopathologia 2006, 161, 323–329. [Google Scholar] [CrossRef]
- Umadevi, P.; Anandaraj, M.; Benjamin, S. Endophytic interactions of Trichoderma harzianum in a tropical perennial rhizo-ecosystem. Res. J. Biotechnol. Vol. 2017, 12, 3. [Google Scholar]
- Hardy, T.N.; Clay, K.; Hammond Jr, A.M. Leaf age and related factors affecting endophyte-mediated resistance to fall armyworm (Lepidoptera: Noctuidae) in tall fescue. Environ. Entomol. 1986, 15, 1083–1089. [Google Scholar] [CrossRef]
- Leckie, B.M. Effects of Beauveria bassiana Mycelia and Metabolites Incorporated into Synthetic Diet and Fed to Larval Helicoverpa Zea; and Detection of Endophytic Beauveria Bassiana in Tomato Plants Using PCR and ITS Primers. Master’s Thesis, University of Tennessee, Knoxville, TN, USA, 2002. [Google Scholar]
- Shakeri, J.; Foster, H.A. Proteolytic activity and antibiotic production by Trichoderma harzianum in relation to pathogenicity to insects. Enzym. Microb. Technol. 2007, 40, 961–968. [Google Scholar] [CrossRef]
- Netzker, T.; Fischer, J.; Weber, J.; Mattern, D.J.; König, C.C.; Valiante, V.; Schroeckh, V.; Brakhage, A.A. Microbial communication leading to the activation of silent fungal secondary metabolite gene clusters. Front. Microbiol. 2015, 6, 299. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M. Nitrogen containing compounds and adaptation of plants to salinity stress. Biol. Plant. 2000, 43, 491–500. [Google Scholar] [CrossRef]
- El-Khallal, S.M. Induction and modulation of resistance in tomato plants against Fusarium wilt disease by bioagent fungi (arbuscular mycorrhiza) and/or hormonal elicitors (jasmonic acid & salicylic acid): 1-Changes in growth, some metabolic activities and endogenous hormones related to defence mechanism. Aust. J. Basic Appl. Sci. 2007, 1, 691–705. [Google Scholar]
- Sharma, P.; Dubey, R.S. Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regul. 2005, 46, 209–221. [Google Scholar] [CrossRef]
- Joe, M.M.; Muthukumaran, N. Role of Certain Elicitors on the Chemical Induction of Resistance in Tomato against the Leaf Caterpillar Spodoptera litura Fab. Not. Bot. Horti Agrobot. Cluj-Napoca 2008, 36, 71–75. [Google Scholar]
- Chandrasekar, V.; Sairam, R.K.; Srivastava, G. Physiological and biochemical responses of hexaploid and tetraploid wheat to drought stress. J. Agron. Crop. Sci. 2000, 185, 219–227. [Google Scholar] [CrossRef]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq Boyce, A. Role of plant growth promoting rhizobacteria in agricultural sustainability—A review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef]
- Rahdari, P.; Tavakoli, S.; Hosseini, S.M. Studying of salinity stress effect on germination, proline, sugar, protein, lipid and chlorophyll content in purslane (Portulaca oleracea L.) leaves. J. Stress Physiol. Biochem. 2012, 8, 182–193. [Google Scholar]
- Jung, S.; Kim, J.S.; Cho, K.Y.; Tae, G.S.; Kang, B.G. Antioxidant responses of cucumber (Cucumis sativus) to photoinhibition and oxidative stress induced by norflurazon under high and low PPFDs. Plant Sci. 2000, 153, 145–154. [Google Scholar] [CrossRef]
- Delon, I.; Payre, F. Evolution of larval morphology in flies: Get in shape with shavenbaby. Trends Genet. 2004, 20, 305–313. [Google Scholar] [CrossRef]
- Moussian, B. Recent advances in understanding mechanisms of insect cuticle differentiation. Insect Biochem. Mol. Biol. 2010, 40, 363–375. [Google Scholar] [CrossRef]
- Zhang, T.; Coates, B.S.; Wang, Y.; Wang, Y.; Bai, S.; Wang, Z.; He, K. Down-regulation of aminopeptidase N and ABC transporter subfamily G transcripts in Cry1Ab and Cry1Ac resistant Asian corn borer, Ostrinia furnacalis (Lepidoptera: Crambidae). Int. J. Biol. Sci. 2017, 13, 835. [Google Scholar] [CrossRef] [PubMed]
- Teets, N.M.; Denlinger, D.L. Surviving in a frozen desert: Environmental stress physiology of terrestrial Antarctic arthropods. J. Exp. Biol. 2014, 217, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Taoka, M.; Shinkawa, T.; Yamauchi, Y.; Isobe, T.; Sato, D. Identification of a cuticle protein with unique repeated motifs in the silkworm, Bombyx Mori. Insect Biochem. Mol. Biol. 2013, 43, 344–351. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Botelho, J.M.; McNall, R.J.; Belozerov, V.; Dunn, W.A.; Mize, T.; Orlando, R.; Willis, J.H. Proteomic analysis of cast cuticles from Anopheles gambiae by tandem mass spectrometry. Insect Biochem. Mol. Biol. 2007, 37, 135–146. [Google Scholar] [CrossRef]
- Ali, S.; Huang, Z.; Ren, S. Production of cuticle degrading enzymes by Isaria fumosorosea and their evaluation as a biocontrol agent against diamondback moth. J. Pest Sci. 2010, 83, 361–370. [Google Scholar] [CrossRef]
- Pal, S.; Leger, R.J.S.; Wu, L.P. Fungal peptide Destruxin A plays a specific role in suppressing the innate immune response in Drosophila melanogaster. J. Biol. Chem. 2007, 282, 8969–8977. [Google Scholar] [CrossRef]
- Xiong, G.-H.; Xing, L.-S.; Lin, Z.; Saha, T.T.; Wang, C.; Jiang, H.; Zou, Z. High throughput profiling of the cotton bollworm Helicoverpa armigera immunotranscriptome during the fungal and bacterial infections. BMC Genom. 2015, 16, 1–21. [Google Scholar] [CrossRef]
- Yoshida, H.; Kinoshita, K.; Ashida, M. Purification of a peptidoglycan recognition protein from hemolymph of the silkworm, Bombyx mori. J. Biol. Chem. 1996, 271, 13854–13860. [Google Scholar] [CrossRef]
- Tabashnik, B.E. ABCs of insect resistance to Bt. PLoS Genet. 2015, 11, e1005646. [Google Scholar] [CrossRef]
- Dermauw, W.; Van Leeuwen, T. The ABC gene family in arthropods: Comparative genomics and role in insecticide transport and resistance. Insect Biochem. Mol. Biol. 2014, 45, 89–110. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, M.Z.; Zhang, T.; Wang, Z.; He, K. Transcriptome and proteome alternation with resistance to Bacillus thuringiensis Cry1Ah toxin in Ostrinia Furnacalis. Front. Physiol. 2019, 10, 27. [Google Scholar] [CrossRef]
- Li, X.; Schuler, M.A.; Berenbaum, M.R. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 2007, 52, 231–253. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Rui, C.; Yang, D.; Wang, Z.; Yuan, H. De novo transcriptome and expression profile analyses of the Asian corn borer (Ostrinia furnacalis) reveals relevant flubendiamide response genes. BMC Genom. 2017, 18, 20. [Google Scholar] [CrossRef]
- Xu, L.; Ferry, N.; Wang, Z.; Zhang, J.; Edwards, M.G.; Gatehouse, A.M.; He, K. A proteomic approach to study the mechanism of tolerance to Bt toxins in Ostrinia furnacalis larvae selected for resistance to Cry1Ab. Transgenic Res. 2013, 22, 1155–1166. [Google Scholar] [CrossRef]
- Xu, X.; Yu, L.; Wu, Y. Disruption of a cadherin gene associated with resistance to Cry1Ac δ-endotoxin of Bacillus thuringiensis in Helicoverpa armigera. Appl. Environ. Microbiol. 2005, 71, 948–954. [Google Scholar] [CrossRef]
- Vilcinskas, A.; Matha, V.; Götz, P. Effects of the entomopathogenic fungus Metarhizium anisopliae and its secondary metabolites on morphology and cytoskeleton of plasmatocytes isolated from the greater wax moth, Galleria Mellonella. J. Insect Physiol. 1997, 43, 1149–1159. [Google Scholar] [CrossRef]
- Soberon, M.; Gill, S.; Bravo, A. Signaling versus punching hole: How do Bacillus thuringiensis toxins kill insect midgut cells? Cell. Mol. Life Sci. 2009, 66, 1337–1349. [Google Scholar] [CrossRef]
- Athman, S.Y. Host-Endophyte-Pest Interactions of Endophytic Fusarium Oxysporum Antagonistic to Radopholus Similis in Banana (Musa Spp.); University of Pretoria: Pretoria, South Africa, 2006. [Google Scholar]
- Backman, P.A.; Sikora, R.A. Endophytes: An emerging tool for biological control. Biol. Control 2008, 46, 1–3. [Google Scholar] [CrossRef]
- Tibbets, T.; Faeth, S.H. Neotyphodium endophytes in grasses: Deterrents or promoters of herbivory by leaf-cutting ants? Oecologia 1999, 118, 297–305. [Google Scholar] [CrossRef]
- Marcelino, J.; Giordano, R.; Gouli, S.; Gouli, V.; Parker, B.L.; Skinner, M.; TeBeest, D.; Cesnik, R. Colletotrichum acutatum var. fioriniae (teleomorph: Glomerella acutata var. fioriniae var. nov.) infection of a scale insect. Mycologia 2008, 100, 353–374. [Google Scholar] [CrossRef]
- Ambethgar, V. Potential of entomopathogenic fungi in insecticide resistance management (IRM): A review. J. Biopestic. 2009, 2, 177–193. [Google Scholar]
- Araujo, E.S.; Benatto, A.; Rizzato, F.B.; Poltronieri, A.S.; Poitevin, C.G.; Zawadneak, M.A.; Pimentel, I.C. Combining biocontrol agents with different mechanisms of action to control Duponchelia fovealis, an invasive pest in South America. Crop. Prot. 2020, 134, 105184. [Google Scholar] [CrossRef]
- Dhawan, M.; Joshi, N. Enzymatic comparison and mortality of Beauveria bassiana against cabbage caterpillar Pieris brassicae LINN. Braz. J. Microbiol. 2017, 48, 522–529. [Google Scholar] [CrossRef]
- Yeo, H.; Pell, J.K.; Alderson, P.G.; Clark, S.J.; Pye, B.J. Laboratory evaluation of temperature effects on the germination and growth of entomopathogenic fungi and on their pathogenicity to two aphid species. Pest Manag. Sci. Former. Pestic. Sci. 2003, 59, 156–165. [Google Scholar] [CrossRef]
- Wiegand, H.; Finney, D.J. Probit analysis. 3. Aufl. Cambridge University Press, Cambridge 1971. XV, 333 S., 41 Rechenbeispiele, 20 Diagr., 8 Tab., 231 Lit., L 5.80. Biom. Z. 1972, 14, 72. [Google Scholar] [CrossRef]
- Li, Y.; Fu, K.; Gao, S.; Wu, Q.; Fan, L.; Li, Y.; Chen, J. Impact on bacterial community in midguts of the asian corn borer larvae by transgenic Trichoderma Strain overexpressing a heterologous chit42 gene with chitin-binding domain. PLoS ONE 2013, 8, e55555. [Google Scholar] [CrossRef]
- Motholo, L.F. Endophytic Establishment of Beauveria Bassiana in Wheat (Triticum aestivum) and Its Impact on Diuraphis Noxia; North-West University, Potchefstroom Campus: Potchefstroom, South Africa, 2019. [Google Scholar]
- Cherry, A.J.; Banito, A.; Djegui, D.; Lomer, C. Suppression of the stem-borer Sesamia calamistis (Lepidoptera; Noctuidae) in maize following seed dressing, topical application and stem injection with African isolates of Beauveria Bassiana. Int. J. Pest Manag. 2004, 50, 67–73. [Google Scholar] [CrossRef]
- Xie, H.; Liu, K.; Sun, D.; Wang, Z.; Lu, X.; He, K. A field experiment with elevated atmospheric CO 2-mediated changes to C 4 crop-herbivore interactions. Sci. Rep. 2015, 5, 13923. [Google Scholar] [CrossRef][Green Version]
- Reddy, K.; Subhani, S.; Khan, P.; Kumar, K. Effect of light and benzyladenine on dark-treated growing rice (Oryza sativa) leaves II. Changes in peroxidase activity. Plant Cell Physiol. 1985, 26, 987–994. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- McDonald, C.; Chen, L.L. The Lowry modification of the Folin reagent for determination of proteinase activity. Anal. Biochem. 1965, 10, 175–177. [Google Scholar] [CrossRef]
- Kar, M.; Mishra, D. Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol. 1976, 57, 315–319. [Google Scholar] [CrossRef]
- Saeidi, M.; Zabihi-e-Mahmoodabad, R. Evaluation of drought stress on relative water content and chlorophyll content of sesame (Sesamum indicum L.) genotypes at early flowering stage. Res. J. Environ. Sci. 2009, 3, 345–350. [Google Scholar]
- Götz, S.; García-Gómez, J.M.; Terol, J.; Williams, T.D.; Nagaraj, S.H.; Nueda, M.J.; Robles, M.; Talón, M.; Dopazo, J.; Conesa, A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008, 36, 3420–3435. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2007, 36, D480–D484. [Google Scholar] [CrossRef]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef]
- Alexa, A.; Rahnenfuhrer, J. TopGO: Enrichment analysis for gene ontology. R. Package Version 2010, 2, 2010. [Google Scholar]
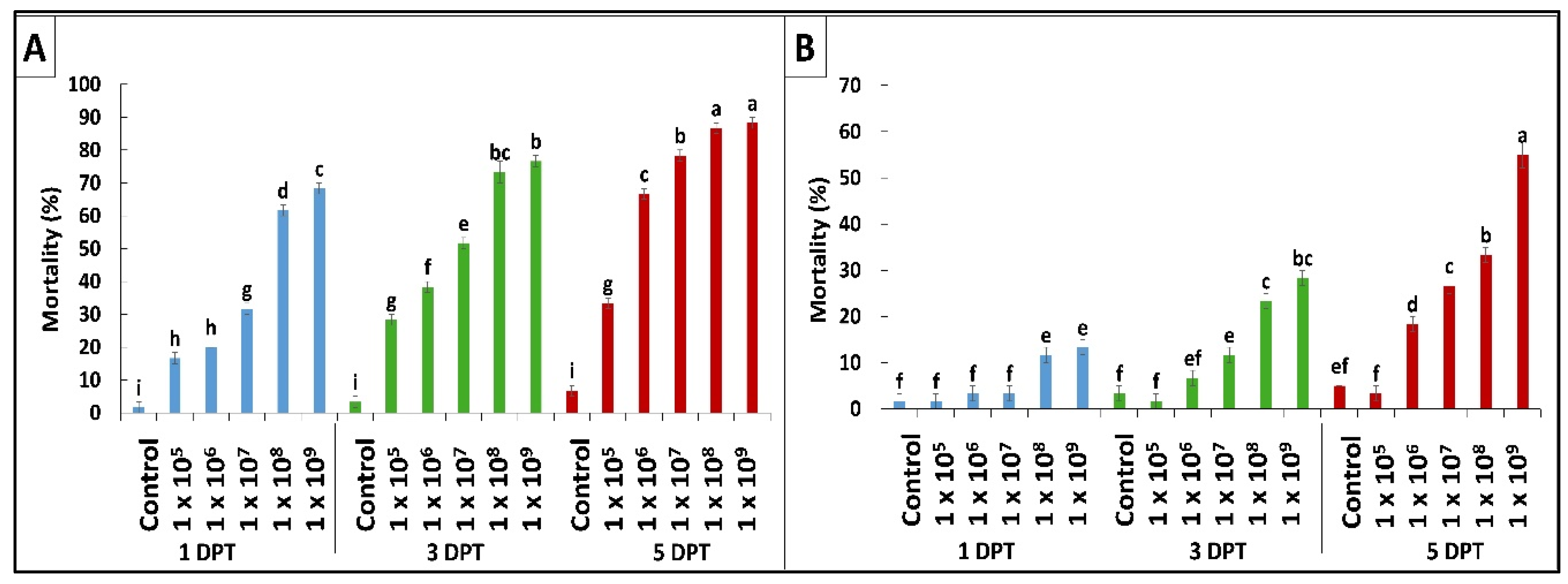

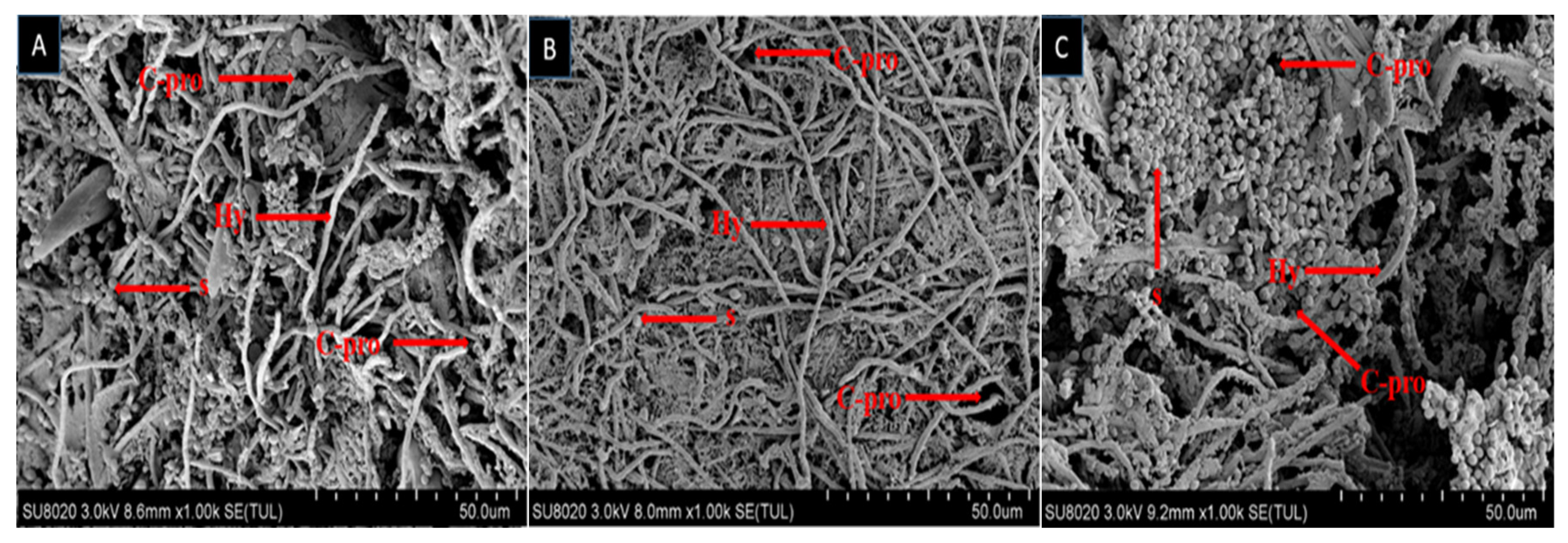
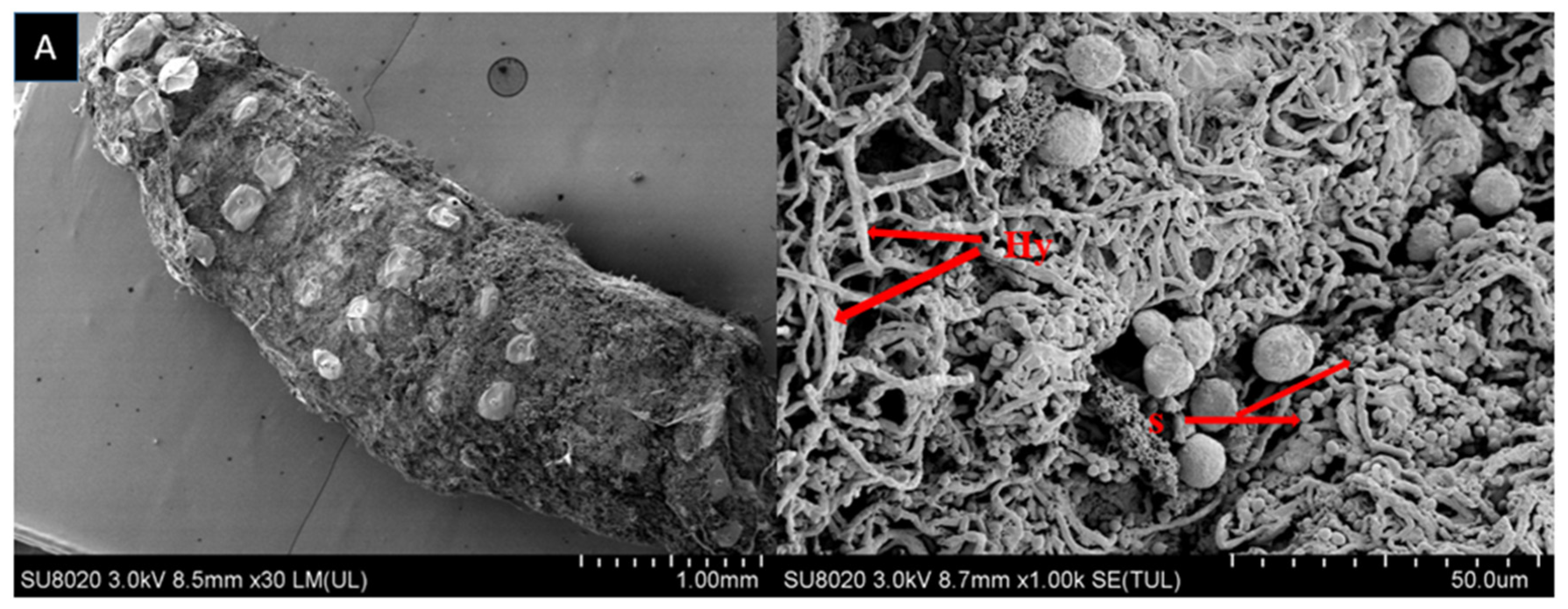


| Fungal Strain | LC50 (%) | 95% FL | Slope ± SE | X2 (DF = 13) | p-Value |
|---|---|---|---|---|---|
| Beauveria bassiana OFDH1-5 | 5.9 × 105 | 143015.917–1582815.025 | 0.436 ± 0.065 | 7.89 | ≤0.01 |
| Trichoderma asperellum GDFS1009 | 8.4 × 108 | 273575649.988–5512644984.830 | 0.454 ± 0.079 | 6.221 | ≤0.01 |
| Treatments | Mortality (%) | Tunnel Number | Tunnel Length (cm) |
|---|---|---|---|
| C | 0 ± 0 e | 0 ± 0 e | 0 ± 0 d |
| T1 | 85 ± 1.6 a | 1.2 ± 0.79 c,d | 0.765 ± 0.75 c,d |
| T2 | 71.6 ± 4.3 b,c | 1.7 ± 0.82 b,c | 1.14 ± 0.55 b,c,d |
| T3 | 53.3 ± 2.2 c | 2.3 ± 0.48 b | 2.08 ± 0.81 b,c |
| T4 | 46.67 ± 2.1 d | 2.3 ± 0.5 b | 2.476 ± 1.06 b |
| T5 | 91.7 ± 3.7 a | 0.6 ± 0.84 d,e | 0.49 ± 0.72 d |
| T6 | 81.6 ± 0.74 a,b | 1.1 ± 0.57 c,d | 1.208 ± 0.68 b,c,d |
| T7 | 8.7 ± 2.7 e | 4.7 ± 1.16 a | 4.64 ± 1.96 a |
| DEG Set | Total | Upregulated | Downregulated |
|---|---|---|---|
| CONTROL vs. BB | 218 | 168 | 50 |
| CONTROL vs. BB-TH | 38 | 13 | 25 |
| CONTROL vs. TH | 45 | 17 | 28 |
| Gene ID | Function | Expression (Log2) | ||
|---|---|---|---|---|
| CONTROL vs. BB | CONTROL vs. BB+TH | CONTROL vs. TH | ||
| gene-LOC114351388 | Signal transduction mechanisms | 2.891216 | --- | -- |
| gene-LOC114359283 | serine/threonine-protein kinase | 1.801724 | -- | -- |
| gene-LOC114365584 | Signal transduction mechanisms | 1.71629 | -- | -- |
| gene-LOC114363167 | Signal transduction mechanisms | 1.111284 | -- | -- |
| gene-LOC114352123 | peptidoglycan recognition protein | 1.783481 | -- | -- |
| gene-LOC114359877 | chitinase-3 | 3.204765 | -- | -- |
| gene-LOC114354452 | Signal transduction mechanisms | −1.1389 | -- | -- |
| gene-LOC114351571 | Signal transduction mechanisms | 1.841207 | -- | -- |
| gene-LOC114354308 | Defense mechanisms (ABC transporter) | 1.421725 | -- | -- |
| gene-LOC114366083 | Signal transduction mechanisms | 2.038954 | -- | -- |
| gene-LOC114350700 | Signal transduction mechanisms | 2.649408 | -- | -- |
| gene-LOC114356220 | heat shock protein 83 | −1.72546 | −1.8703 | -- |
| gene-LOC114353130 | Defense mechanisms (ABC transporter) | 2.170025 | -- | -- |
| gene-LOC114358387 | heat shock protein 20.4 | −2.57941 | −2.9913 | -- |
| gene-LOC114353313 | UDP-glucosidase | 1.953112 | -- | 1.78757 |
| gene-LOC114362284 | Signal transduction mechanisms | 1.597204 | -- | -- |
| gene-LOC114359576 | serine/threonine-protein kinase | 1.717456 | -- | -- |
| gene-LOC114362676 | serine/threonine-protein kinase | 1.821126 | -- | -- |
| gene-LOC114365247 | Signal transduction mechanisms | 1.355035 | -- | -- |
| gene-LOC114364856 | Signal transduction mechanisms | 1.350309 | -- | -- |
| gene-LOC114357915 | Signal transduction mechanisms | 1.705776 | -- | -- |
| gene-LOC114357147 | Signal transduction mechanisms | 1.634834 | -- | -- |
| gene-LOC114362187 | Signal transduction mechanisms | 1.942793 | -- | -- |
| gene-LOC114351255 | serine/threonine-protein phosphatase | 1.745418 | -- | -- |
| gene-LOC114355543 | Signal transduction mechanisms | 1.109096 | -- | -- |
| gene-LOC114366552 | Insect cuticle protein | −1.75469 | -- | -- |
| gene-LOC114354721 | heat shock protein 24.3 | −2.38213 | −3.15665 | -- |
| gene-LOC114351436 | serine proteinase inhibitor | 2.446235 | -- | -- |
| gene-LOC114352122 | peptidoglycan-recognition protein-S | 2.992085 | -- | -- |
| gene-LOC114354585 | peptidoglycan-recognition protein-S | 1.810414 | -- | -- |
| gene-LOC114356221 | Heat shock protein 70 | −2.19843 | −2.84068 | −1.96491 |
| gene-LOC114354346 | Signal transduction mechanisms | 1.478152 | -- | -- |
| gene-LOC114351799 | Signal transduction mechanisms | 1.927021 | -- | -- |
| gene-LOC114351742 | Signal transduction mechanisms | 1.262353 | -- | -- |
| gene-LOC114366353 | Insect cuticle protein | −2.04885 | −1.34725 | 2.27946 |
| gene-LOC114362562 | Cadherin | 1.536785 | 0.9054481 | 0.7932067 |
| gene-LOC114366367 | Insect cuticle protein | −1.66763 | −1.26854 | 1.5968267 |
| gene-LOC114354308 | ACB transporter | 1.421725 | 1.1354 | 1.11238 |
| gene-LOC114350074 | UDP-glucuronosyltransferase | −1.41575 | −0.9498 | −1.30987 |
| gene-LOC114359337 | Signal transduction mechanisms | -- | 2.726825 | -- |
| gene-LOC114356222 | Protein lethal essential for life (HSP-20) | -- | −2.80833 | −2.34904 |
| gene-LOC114363773 | Heat shock protein 68 | −4.13625 | −3.49075 | −3.89546 |
| gene-LOC114353087 | Cytochrome P450 | −0.99134 | −1.6274 | −0.23413 |
| gene-LOC114354720 | Protein lethal (2) essential for life | -- | −2.25955 | −1.99824 |
| gene-LOC114352113 | Peptidoglycan-recognition protein-B | −2.05588 | −2.1622 | |
| gene-LOC114353764 | Protein lethal essential for life (HSP- 20) | -- | −3.00427 | −1.69466 |
| gene-LOC114358386 | Protein lethal essential for life (HSP−20) | -- | −2.45793 | -- |
| gene-LOC114357364 | Defense mechanisms (ABC transporter) | -- | 3.555454 | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batool, R.; Umer, M.J.; Wang, Y.; He, K.; Zhang, T.; Bai, S.; Zhi, Y.; Chen, J.; Wang, Z. Synergistic Effect of Beauveria bassiana and Trichoderma asperellum to Induce Maize (Zea mays L.) Defense against the Asian Corn Borer, Ostrinia furnacalis (Lepidoptera, Crambidae) and Larval Immune Response. Int. J. Mol. Sci. 2020, 21, 8215. https://doi.org/10.3390/ijms21218215
Batool R, Umer MJ, Wang Y, He K, Zhang T, Bai S, Zhi Y, Chen J, Wang Z. Synergistic Effect of Beauveria bassiana and Trichoderma asperellum to Induce Maize (Zea mays L.) Defense against the Asian Corn Borer, Ostrinia furnacalis (Lepidoptera, Crambidae) and Larval Immune Response. International Journal of Molecular Sciences. 2020; 21(21):8215. https://doi.org/10.3390/ijms21218215
Chicago/Turabian StyleBatool, Raufa, Muhammad Jawad Umer, Yangzhou Wang, Kanglai He, Tiantao Zhang, Shuxiong Bai, Yang Zhi, Jie Chen, and Zhenying Wang. 2020. "Synergistic Effect of Beauveria bassiana and Trichoderma asperellum to Induce Maize (Zea mays L.) Defense against the Asian Corn Borer, Ostrinia furnacalis (Lepidoptera, Crambidae) and Larval Immune Response" International Journal of Molecular Sciences 21, no. 21: 8215. https://doi.org/10.3390/ijms21218215
APA StyleBatool, R., Umer, M. J., Wang, Y., He, K., Zhang, T., Bai, S., Zhi, Y., Chen, J., & Wang, Z. (2020). Synergistic Effect of Beauveria bassiana and Trichoderma asperellum to Induce Maize (Zea mays L.) Defense against the Asian Corn Borer, Ostrinia furnacalis (Lepidoptera, Crambidae) and Larval Immune Response. International Journal of Molecular Sciences, 21(21), 8215. https://doi.org/10.3390/ijms21218215







