Enhanced Dopamine Transmission and Hyperactivity in the Dopamine Transporter Heterozygous Mice Lacking the D3 Dopamine Receptor
Abstract
:1. Introduction
2. Results
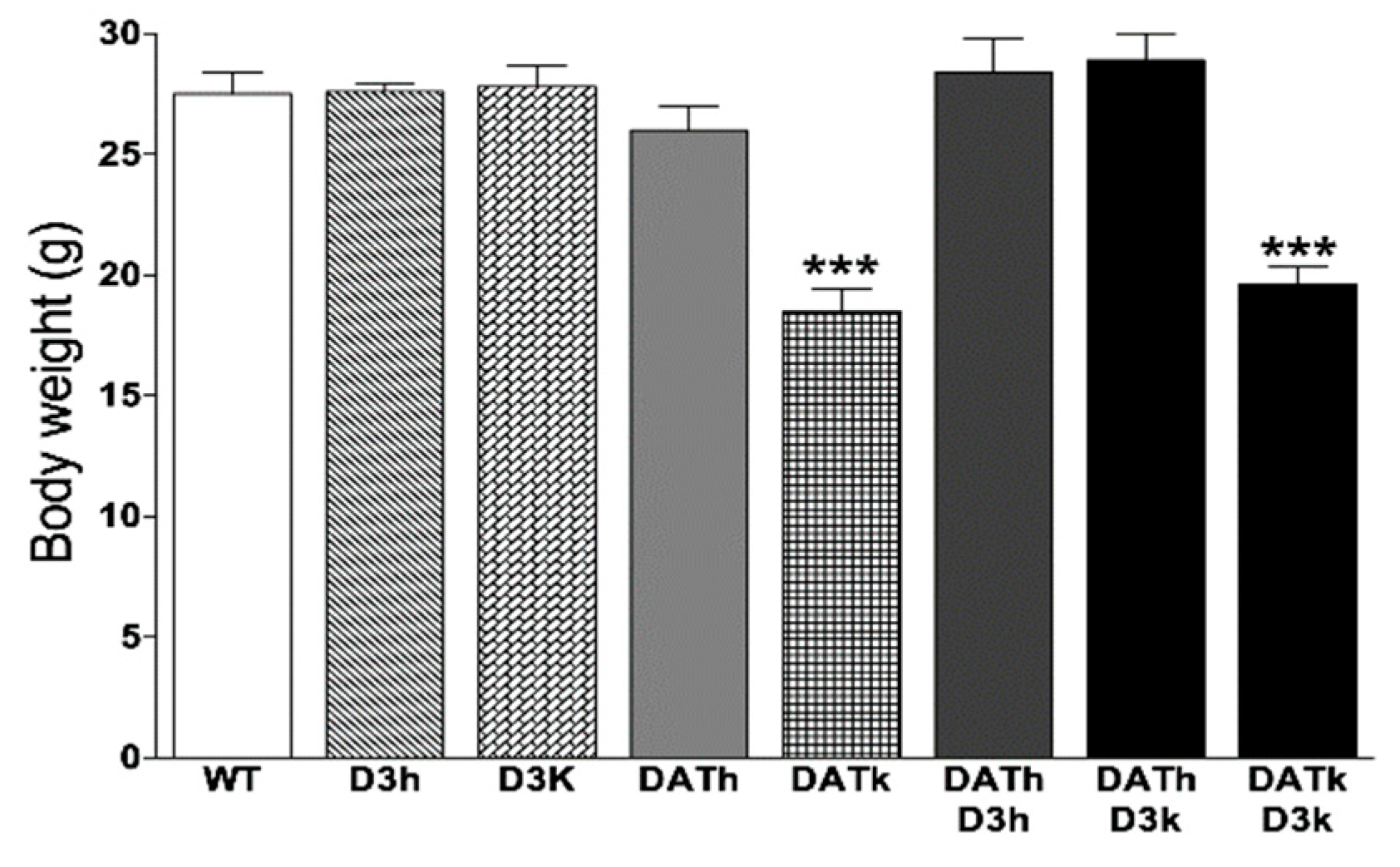
2.1. Bodyweight
2.2. Temperature
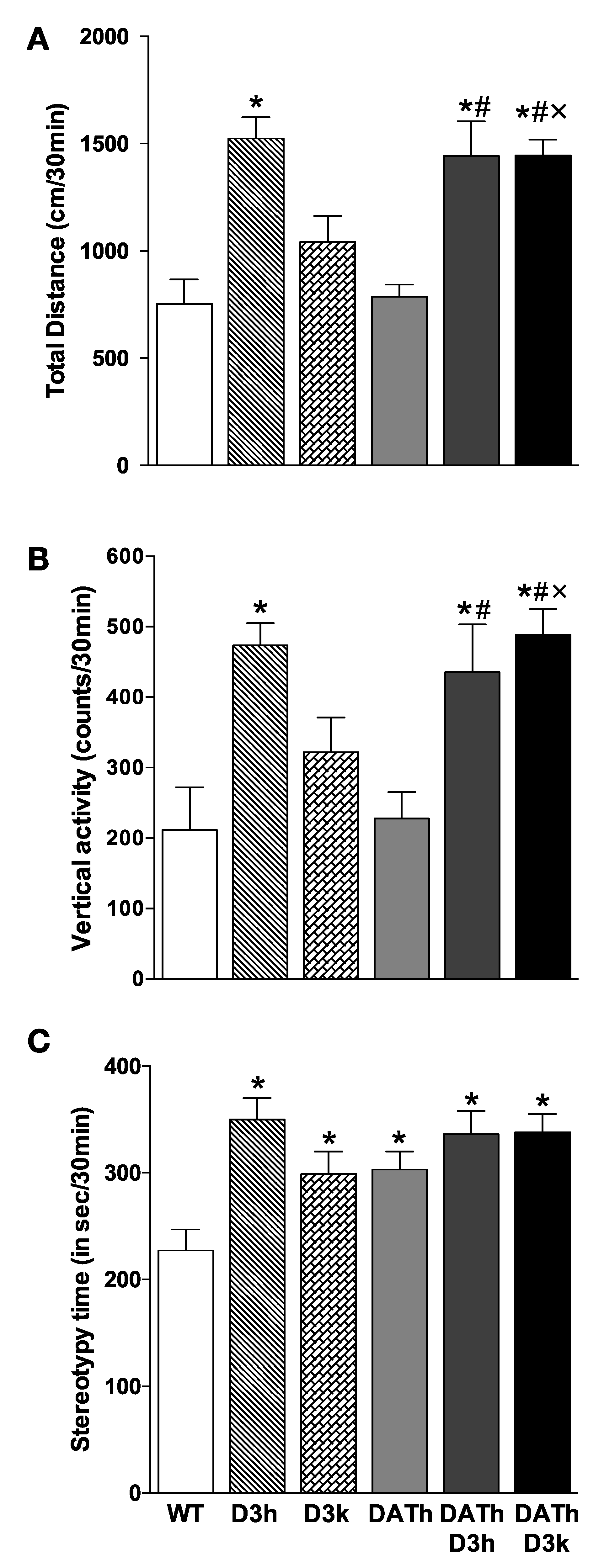
2.3. Locomotor Activity
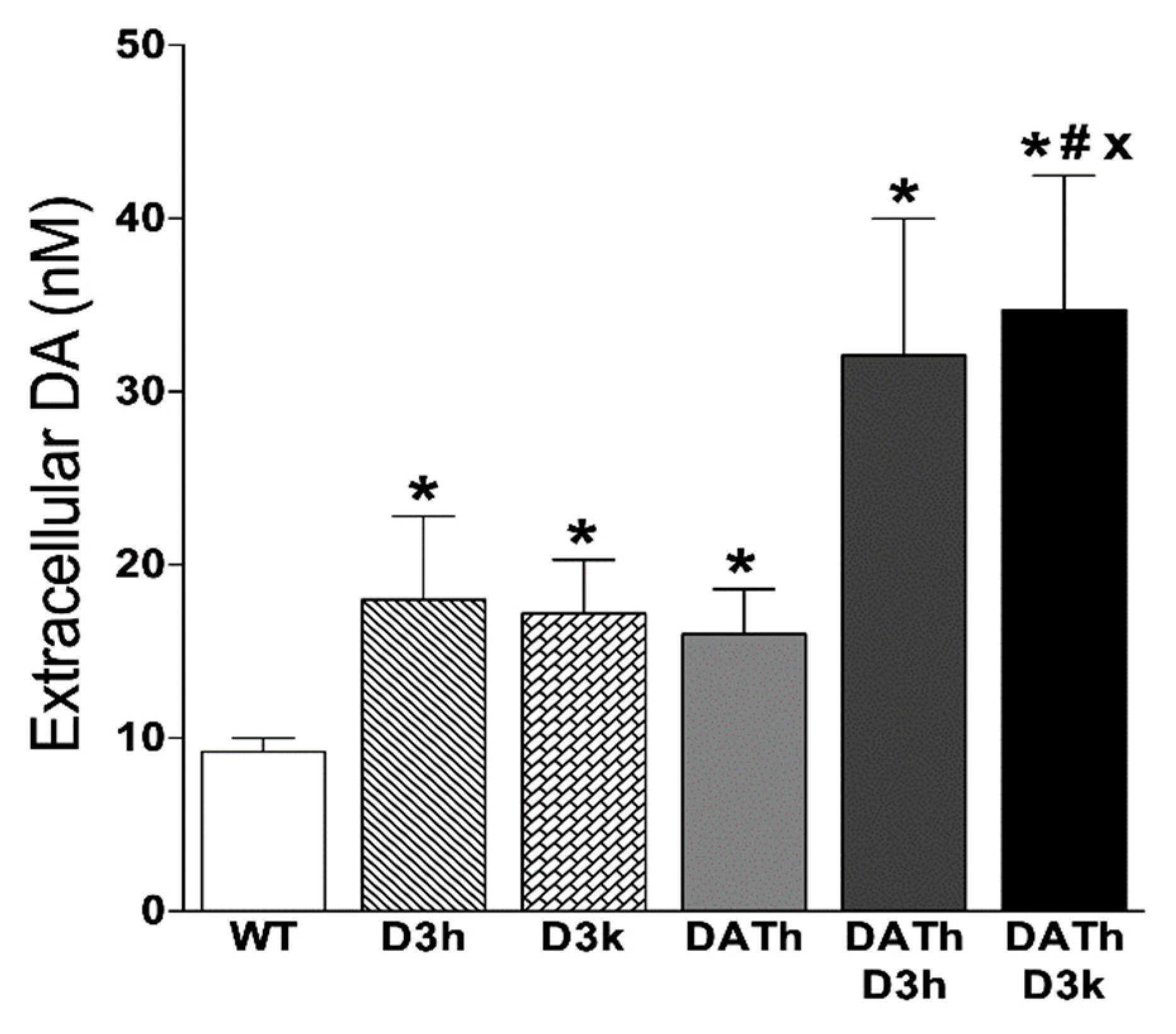
2.4. Microdialysis Measurements of Striatal Extracellular Dopamine Levels
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Locomotion
4.3. Temperature
4.4. In Vivo Microdialysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beaulieu, J.-M.; Gainetdinov, R.R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011, 63, 182–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Efimova, E.V.; Gainetdinov, R.R.; Budygin, E.A.; Sotnikova, T.D. Dopamine transporter mutant animals: A translational perspective. J. Neurogenet. 2016, 30, 5–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, S.R.; Gainetdinov, R.R.; Jaber, M.; Giros, B.; Wightman, R.M.; Caron, M.G. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc. Natl. Acad. Sci. USA 1998, 95, 4029–4034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leo, D.; Sukhanov, I.; Zoratto, F.; Illiano, P.; Caffino, L.; Sanna, F.; Messa, G.; Emanuele, M.; Esposito, A.; Dorofeikova, M.; et al. Pronounced hyperactivity, cognitive dysfunction and BDNF dysregulations in dopamine transporter knockout rats. J. Neurosci. 2018, 1959–1972. [Google Scholar] [CrossRef] [Green Version]
- Gainetdinov, R.R.; Jones, S.R.; Caron, M.G. Functional hyperdopaminergia in dopamine transporter knock-out mice. Biol. Psychiatry 1999, 46, 303–311. [Google Scholar] [CrossRef]
- Bossé, R.; Fumagalli, F.; Jaber, M.; Giros, B.; Gainetdinov, R.R.; Wetsel, W.C.; Missale, C.; Caron, M.G. Anterior pituitary hypoplasia and dwarfism in mice lacking the dopamine transporter. Neuron 1997, 19, 127–138. [Google Scholar] [CrossRef] [Green Version]
- Vincent, S.G.; Waddell, A.E.; Caron, M.G.; Walker, J.K.L.; Fisher, J.T. A murine model of hyperdopaminergic state displays altered respiratory control. FASEB J. 2007, 21, 1463–1471. [Google Scholar] [CrossRef]
- Fauchey, V.; Jaber, M.; Caron, M.G.; Bloch, B.; Le Moine, C. Differential regulation of the dopamine D1, D2 and D3 receptor gene expression and changes in the phenotype of the striatal neurons in mice lacking the dopamine transporter. Eur. J. Neurosci. 2000, 12, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Sokoloff, P.; Giros, B.; Martres, M.-P.; Bouthenet, M.-L.; Schwartz, J.-C. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature 1990, 347, 146–151. [Google Scholar] [CrossRef]
- Accili, D.; Fishburn, C.S.; Drago, J.; Steiner, H.; Lachowicz, J.E.; Park, B.H.; Gauda, E.B.; Lee, E.J.; Cool, M.H.; Sibley, D.R.; et al. A targeted mutation of the D3 dopamine receptor gene is associated with hyperactivity in mice. Proc. Natl. Acad. Sci. USA 1996, 93, 1945–1949. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Koeltzow, T.E.; Santiago, G.T.; Moratalla, R.; Cooper, D.C.; Hu, X.-T.; White, N.M.; Graybiel, A.M.; White, F.J.; Tonegawa, S. Dopamine D3 Receptor Mutant Mice Exhibit Increased Behavioral Sensitivity to Concurrent Stimulation of D1 and D2 Receptors. Neuron 1997, 19, 837–848. [Google Scholar] [CrossRef] [Green Version]
- Koeltzow, T.E.; Xu, M.; Cooper, D.C.; Hu, X.-T.; Tonegawa, S.; Wolf, M.E.; White, F.J. Alterations in Dopamine Release But Not Dopamine Autoreceptor Function in Dopamine D3 Receptor Mutant Mice. J. Neurosci. 1998, 18, 2231–2238. [Google Scholar] [CrossRef] [Green Version]
- Joseph, J.D.; Wang, Y.-M.; Miles, P.R.; Budygin, E.A.; Picetti, R.; Gainetdinov, R.R.; Caron, M.G.; Wightman, R.M. Dopamine autoreceptor regulation of release and uptake in mouse brain slices in the absence of D(3) receptors. Neuroscience 2002, 112, 39–49. [Google Scholar] [CrossRef]
- Le Foll, B.; Diaz, J.; Sokoloff, P. Neuroadaptations to hyperdopaminergia in dopamine D3 receptor-deficient mice. Life Sci. 2005, 76, 1281–1296. [Google Scholar] [CrossRef]
- Moraga-Amaro, R.; Gonzalez, H.; Pacheco, R.; Stehberg, J. Dopamine receptor D3 deficiency results in chronic depression and anxiety. Behav. Brain Res. 2014, 274, 186–193. [Google Scholar] [CrossRef]
- Giros, B.; Jaber, M.; Jones, S.R.; Wightman, R.M.; Caron, M.G. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 1996, 379, 606–612. [Google Scholar] [CrossRef]
- Smith, A.D.; Olson, R.J.; Justice, J.B. Quantitative microdialysis of dopamine in the striatum: Effect of circadian variation. J. Neurosci. Methods 1992, 44, 33–41. [Google Scholar] [CrossRef]
- Nagashima, T.; Oami, E.; Kutsuna, N.; Ishiura, S.; Suo, S. Dopamine regulates body size in Caenorhabditis elegans. Dev. Biol. 2016, 412, 128–138. [Google Scholar] [CrossRef]
- Kelly, M.A.; Rubinstein, M.; Phillips, T.J.; Lessov, C.N.; Burkhart-Kasch, S.; Zhang, G.; Bunzow, J.R.; Fang, Y.; Gerhardt, G.A.; Grandy, D.K.; et al. Locomotor activity in D2 dopamine receptor-deficient mice is determined by gene dosage, genetic background, and developmental adaptations. J. Neurosci. 1998, 18, 3470–3479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noain, D.; Perez-Millan, M.I.; Bello, E.P.; Luque, G.M.; Casas Cordero, R.; Gelman, D.M.; Peper, M.; Tornadu, I.G.; Low, M.J.; Becu-Villalobos, D.; et al. Central Dopamine D2 Receptors Regulate Growth-Hormone-Dependent Body Growth and Pheromone Signaling to Conspecific Males. J. Neurosci. 2013, 33, 5834–5842. [Google Scholar] [CrossRef] [PubMed]
- Chaperon, F.; Tricklebank, M.D.; Unger, L.; Neijt, H.C. Evidence for regulation of body temperature in rats by dopamine D2 receptor and possible influence of D1 but not D3 and D4 receptors. Neuropharmacology 2003, 44, 1047–1053. [Google Scholar] [CrossRef]
- Baladi, M.G.; Newman, A.H.; Nielsen, S.M.; Hanson, G.R.; Fleckenstein, A.E. Dopamine D(3) receptors contribute to methamphetamine-induced alterations in dopaminergic neuronal function: Role of hyperthermia. Eur. J. Pharmacol. 2014, 732, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Cox, B.; Lee, T.F. Further evidence for a physiological role for hypothalamic dopamine in thermoregulation in the rat. J. Physiol. 1980, 300, 7–17. [Google Scholar] [CrossRef]
- Crandall, C.G.; Vongpatanasin, W.; Victor, R.G. Mechanism of Cocaine-Induced Hyperthermia in Humans. Ann. Intern. Med. 2002, 136, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, F.; Gainetdinov, R.R.; Valenzano, K.J.; Caron, M.G. Role of dopamine transporter in methamphetamine-induced neurotoxicity: Evidence from mice lacking the transporter. J. Neurosci. 1998, 18, 4861–4869. [Google Scholar] [CrossRef]
- Sonsalla, P.K.; Gibb, J.W.; Hanson, G.R. Roles of D1 and D2 dopamine receptor subtypes in mediating the methamphetamine-induced changes in monoamine systems. J. Pharmacol. Exp. Ther. 1986, 238, 932–937. [Google Scholar]
- Bowyer, J.F.; Davies, D.L.; Schmued, L.; Broening, H.W.; Newport, G.D.; Slikker, W.; Holson, R.R. Further studies of the role of hyperthermia in methamphetamine neurotoxicity. J. Pharmacol. Exp. Ther. 1994, 268, 1571–1580. [Google Scholar]
- Salmi, P. Independent roles of dopamine D1 and D2/3 receptors in rat thermoregulation. Brain Res. 1998, 781, 188–193. [Google Scholar] [CrossRef]
- Albers, D.S.; Sonsalla, P.K. Methamphetamine-induced hyperthermia and dopaminergic neurotoxicity in mice: Pharmacological profile of protective and nonprotective agents. J. Pharmacol. Exp. Ther. 1995, 275, 1104–1114. [Google Scholar]
- Boulay, D.; Depoortere, R.; Rostene, W.; Perrault, G.; Sanger, D. Dopamine D3 receptor agonists produce similar decreases in body temperature and locomotor activity in D3 knock-out and wild-type mice. Neuropharmacology 1999, 38, 555–565. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, C.; Zhu, L.; Li, J.; Guan, F.; Chen, T. Depletion of D3 dopamine receptor affects methamphetamine-induced expression patterns of Pde4b and Atf3. Neurosci. Lett. 2018, 665, 54–60. [Google Scholar] [CrossRef]
- Gainetdinov, R.R.; Sotnikova, T.D.; Grekhova, T.V.; Rayevsky, K.S. In vivo evidence for preferential role of dopamine D3 receptor in the presynaptic regulation of dopamine release but not synthesis. Eur. J. Pharmacol. 1996, 308, 261–269. [Google Scholar] [CrossRef]
- Gobert, A.; Lejeune, F.; Rivet, J.M.; Cistarelli, L.; Millan, M.J. Dopamine D3 (auto) receptors inhibit dopamine release in the frontal cortex of freely moving rats in vivo. J. Neurochem. 1996, 66, 2209–2212. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.; Pilon, C.; Le Foll, B.; Gros, C.; Triller, A.; Schwartz, J.C.; Sokoloff, P. Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. J. Neurosci. 2000, 20, 8677–8684. [Google Scholar] [CrossRef] [Green Version]
- Maina, F.K.; Mathews, T.A. A functional fast scan cyclic voltammetry assay to characterize dopamine D2 and D3 autoreceptors in the mouse striatum. ACS Chem. Neurosci. 2010, 1, 450–462. [Google Scholar] [CrossRef]
- McGinnis, M.M.; Siciliano, C.A.; Jones, S.R. Dopamine D3 autoreceptor inhibition enhances cocaine potency at the dopamine transporter. J. Neurochem. 2016, 138, 821–829. [Google Scholar] [CrossRef]
- Jones, S.R.; Gainetdinov, R.R.; Wightman, R.M.; Caron, M.G. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J. Neurosci. 1998, 18, 1979–1986. [Google Scholar] [CrossRef]
- Jones, S.R.; Gainetdinov, R.R.; Hu, X.T.; Cooper, D.C.; Wightman, R.M.; White, F.J.; Caron, M.G. Loss of autoreceptor functions in mice lacking the dopamine transporter. Nat. Neurosci. 1999, 2, 649–655. [Google Scholar] [CrossRef]
- Sotnikova, T.D.; Caron, M.G.; Gainetdinov, R.R. DDD mice, a novel acute mouse model of Parkinson’s disease. Neurology 2006, 67, S12–S17. [Google Scholar] [CrossRef]
- Boulay, D.; Depoortere, R.; Perrault, G.; Borrelli, E.; Sanger, D.J. Dopamine D2 receptor knock-out mice are insensitive to the hypolocomotor and hypothermic effects of dopamine D2/D3 receptor agonists. Neuropharmacology 1999, 38, 1389–1396. [Google Scholar] [CrossRef]
- Kelley, A.E. Measurement of rodent stereotyped behavior. Curr. Protoc. Neurosci. 2001, 4, 8.8.1–8.8.13. [Google Scholar] [CrossRef]
- Berridge, K.C.; Aldridge, J.W.; Houchard, K.R.; Zhuang, X. Sequential super-stereotypy of an instinctive fixed action pattern in hyper-dopaminergic mutant mice: A model of obsessive compulsive disorder and Tourette’s. BMC Biol. 2005, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Devine, D.P. Animal Models of Self-Injurious Behavior: An Update. Methods Mol. Biol. 2019, 2011, 41–60. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sotnikova, T.D.; Efimova, E.V.; Gainetdinov, R.R. Enhanced Dopamine Transmission and Hyperactivity in the Dopamine Transporter Heterozygous Mice Lacking the D3 Dopamine Receptor. Int. J. Mol. Sci. 2020, 21, 8216. https://doi.org/10.3390/ijms21218216
Sotnikova TD, Efimova EV, Gainetdinov RR. Enhanced Dopamine Transmission and Hyperactivity in the Dopamine Transporter Heterozygous Mice Lacking the D3 Dopamine Receptor. International Journal of Molecular Sciences. 2020; 21(21):8216. https://doi.org/10.3390/ijms21218216
Chicago/Turabian StyleSotnikova, Tatyana D., Evgeniya V. Efimova, and Raul R. Gainetdinov. 2020. "Enhanced Dopamine Transmission and Hyperactivity in the Dopamine Transporter Heterozygous Mice Lacking the D3 Dopamine Receptor" International Journal of Molecular Sciences 21, no. 21: 8216. https://doi.org/10.3390/ijms21218216
APA StyleSotnikova, T. D., Efimova, E. V., & Gainetdinov, R. R. (2020). Enhanced Dopamine Transmission and Hyperactivity in the Dopamine Transporter Heterozygous Mice Lacking the D3 Dopamine Receptor. International Journal of Molecular Sciences, 21(21), 8216. https://doi.org/10.3390/ijms21218216





