Regulation of Connexin Gap Junctions and Hemichannels by Calcium and Calcium Binding Protein Calmodulin
Abstract
:1. Lens and Connexins
2. Calcium and Calmodulin in Regulation of Lens Homeostasis and Pathology
3. Ca2+ and Calmodulin in Regulation of Cx Formed Gap Junction Channels
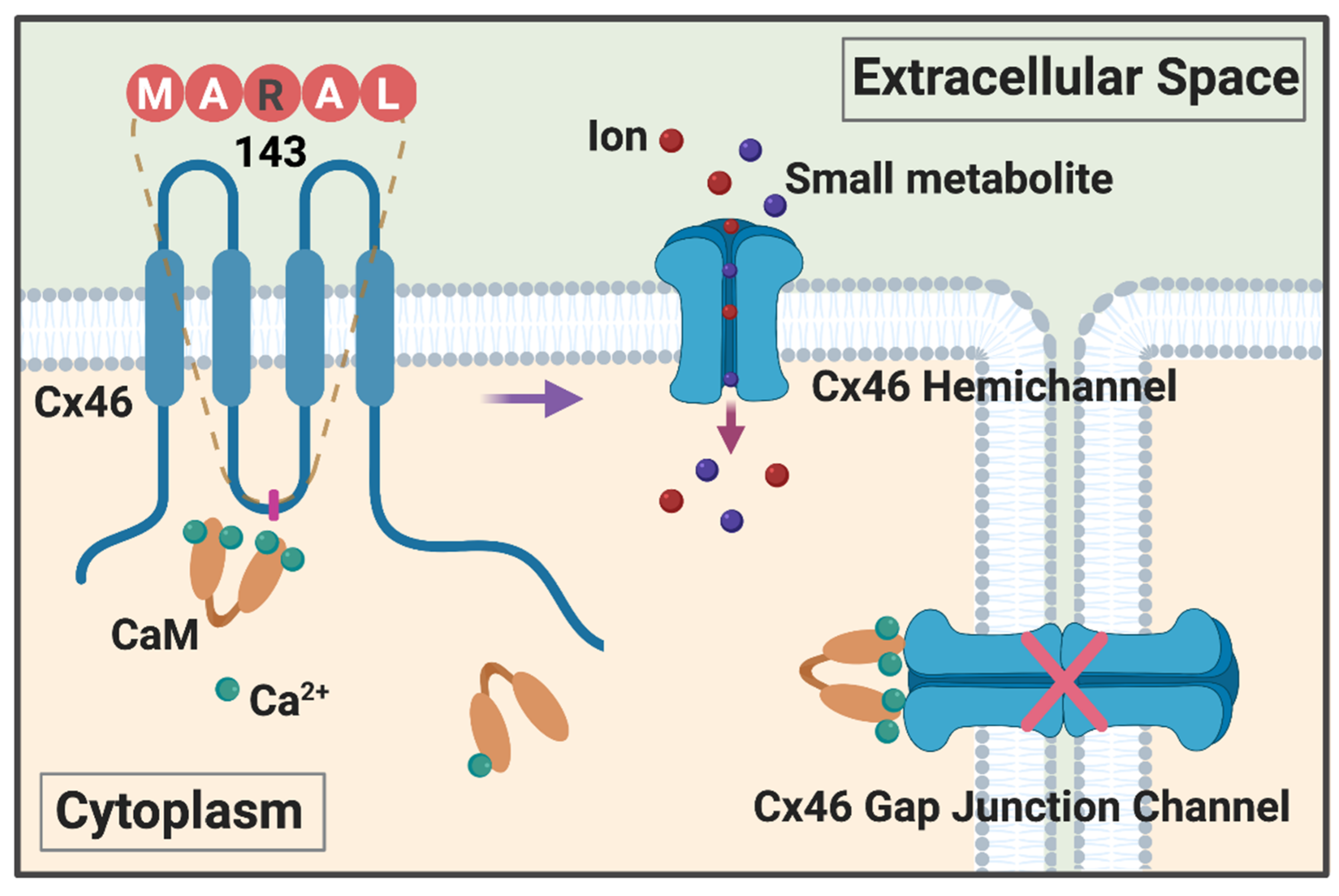
4. New Mechanism of Ca2+/CaM in Regulation of Cx46 Hemichannels
5. Impacts of Lens Ca2+ Homeostasis and Its Dysregulation on Cx46 Channels and Vice Visa
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shui, Y.B.; Fu, J.J.; Garcia, C.; Dattilo, L.K.; Rajagopal, R.; McMillan, S.; Mak, G.; Holekamp, N.M.; Lewis, A.; Beebe, D.C. Oxygen distribution in the rabbit eye and oxygen consumption by the lens. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1571–1580. [Google Scholar] [CrossRef] [Green Version]
- Bassnett, S.; Shi, Y.; Vrensen, G.F. Biological glass: Structural determinants of eye lens transparency. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 1250–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, B.E.; Klein, R.; Lee, K.E. Incidence of age-related cataract over a 10-year interval: The Beaver Dam Eye Study. Ophthalmology 2002, 109, 2052–2057. [Google Scholar] [CrossRef]
- Peracchia, C.; Girsch, S.J.; Bernardini, G.; Peracchia, L.L. Lens junctions are communicating junctions. Curr. Eye Res. 1985, 4, 1155–1169. [Google Scholar] [CrossRef]
- Mathias, R.T.; White, T.W.; Gong, X. Lens gap junctions in growth, differentiation, and homeostasis. Physiol. Rev. 2010, 90, 179–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.X. Gap junctions or hemichannel-dependent and independent roles of connexins in cataractogenesis and lens development. Curr. Mol. Med. 2010, 10, 851–863. [Google Scholar] [CrossRef]
- Gu, S.; Biswas, S.; Rodriguez, L.; Li, Z.; Li, Y.; Riquelme, M.A.; Shi, W.; Wang, K.; White, T.W.; Reilly, M.; et al. Connexin 50 and AQP0 are Essential in Maintaining Organization and Integrity of Lens Fibers. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4021–4032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, D.L.; Ebihara, L.; Takemoto, L.J.; Swenson, K.I.; Goodenough, D.A. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J. Cell Biol. 1991, 115, 1077–1089. [Google Scholar] [CrossRef] [Green Version]
- Mathias, R.T.; Kistler, J.; Donaldson, P. The lens circulation. J. Membr. Biol. 2007, 216, 1–16. [Google Scholar] [CrossRef]
- Goodenough, D.A.; Dick, J.S., 2nd; Lyons, J.E. Lens metabolic cooperation: A study of mouse lens transport and permeability visualized with freeze-substitution autoradiography and electron microscopy. J. Cell Biol. 1980, 86, 576–589. [Google Scholar] [CrossRef] [Green Version]
- Berthoud, V.M.; Gao, J.; Minogue, P.J.; Jara, O.; Mathias, R.T.; Beyer, E.C. Connexin Mutants Compromise the Lens Circulation and Cause Cataracts through Biomineralization. Int. J. Mol. Sci. 2020, 21, 5822. [Google Scholar] [CrossRef]
- Merriman-Smith, R.; Donaldson, P.; Kistler, J. Differential expression of facilitative glucose transporters GLUT1 and GLUT3 in the lens. Investig. Ophthalmol. Vis. Sci. 1999, 40, 3224–3230. [Google Scholar]
- Kannan, R.; Yi, J.R.; Zlokovic, B.V.; Kaplowitz, N. Molecular characterization of a reduced glutathione transporter in the lens. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1785–1792. [Google Scholar]
- Fan, X.; Monnier, V.M.; Whitson, J. Lens glutathione homeostasis: Discrepancies and gaps in knowledge standing in the way of novel therapeutic approaches. Exp. Eye Res. 2017, 156, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Sun, X.; Martinez-Wittinghan, F.J.; Gong, X.; White, T.W.; Mathias, R.T. Connections between connexins, calcium, and cataracts in the lens. J. Gen. Physiol. 2004, 124, 289–300. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Minogue, P.J.; Beyer, E.C.; Mathias, R.T.; Berthoud, V.M. Disruption of the lens circulation causes calcium accumulation and precipitates in connexin mutant mice. Am. J. Physiol. Cell Physiol. 2018, 314, C492–C503. [Google Scholar] [CrossRef] [Green Version]
- Slavi, N.; Rubinos, C.; Li, L.; Sellitto, C.; White, T.W.; Mathias, R.; Srinivas, M. Connexin 46 (cx46) gap junctions provide a pathway for the delivery of glutathione to the lens nucleus. J. Biol. Chem. 2014, 289, 32694–32702. [Google Scholar] [CrossRef] [Green Version]
- Clark, J.I.; Mengel, L.; Bagg, A.; Benedek, G.B. Cortical opacity, calcium concentration and fiber membrane structure in the calf lens. Exp. Eye Res. 1980, 31, 399–410. [Google Scholar] [CrossRef]
- Nemet, A.Y.; Hanhart, J.; Kaiserman, I.; Vinker, S. Are cataracts associated with osteoporosis? Clin. Ophthalmol. 2013, 7, 2079–2084. [Google Scholar] [CrossRef] [Green Version]
- Beyer, E.C.; Berthoud, V.M. Connexin hemichannels in the lens. Front. Physiol. 2014, 5, 20. [Google Scholar] [CrossRef] [Green Version]
- Bassnett, S.; Kuszak, J.R.; Reinisch, L.; Brown, H.G.; Beebe, D.C. Intercellular communication between epithelial and fiber cells of the eye lens. J. Cell Sci. 1994, 107, 799–811. [Google Scholar]
- Miller, T.M.; Goodenough, D.A. Evidence for two physiologically distinct gap junctions expressed by the chick lens epithelial cell. J. Cell Biol. 1986, 102, 194–199. [Google Scholar] [CrossRef] [Green Version]
- Rae, J.L.; Kuszak, J.R. The electrical coupling of epithelium and fibers in the frog lens. Exp. Eye Res. 1983, 36, 317–326. [Google Scholar] [CrossRef]
- Ebihara, L.; Tong, J.J.; Vertel, B.; White, T.W.; Chen, T.L. Properties of connexin 46 hemichannels in dissociated lens fiber cells. Investig. Ophthalmol. Vis. Sci. 2011, 52, 882–889. [Google Scholar] [CrossRef]
- Shi, W.; Riquelme, M.A.; Gu, S.; Jiang, J.X. Connexin hemichannels mediate glutathione transport and protect lens fiber cells from oxidative stress. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, J.D.; Sanderson, J. The mechanisms of calcium homeostasis and signalling in the lens. Exp. Eye Res. 2009, 88, 226–234. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Sun, X.; Yatsula, V.; Wymore, R.S.; Mathias, R.T. Isoform-specific function and distribution of Na/K pumps in the frog lens epithelium. J. Membr. Biol. 2000, 178, 89–101. [Google Scholar] [CrossRef]
- Marian, M.J.; Mukhopadhyay, P.; Borchman, D.; Paterson, C.A. Plasma membrane Ca-ATPase isoform expression in human cataractous lenses compared to age-matched clear lenses. Ophthalmic Res. 2008, 40, 86–93. [Google Scholar] [CrossRef]
- Duncan, G.; Jacob, T.J. Calcium and the physiology of cataract. Ciba Found Symp 1984, 106, 132–152. [Google Scholar] [CrossRef]
- Paterson, C.A.; Zeng, J.; Husseini, Z.; Borchman, D.; Delamere, N.A.; Garland, D.; Jimenez-Asensio, J. Calcium ATPase activity and membrane structure in clear and cataractous human lenses. Curr. Eye Res. 1997, 16, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Borchman, D.; Yappert, M.C.; Vrensen, G.F.; Rasi, V. Influence of age, diabetes, and cataract on calcium, lipid-calcium, and protein-calcium relationships in human lenses. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2059–2066. [Google Scholar] [CrossRef] [Green Version]
- Bernardini, G.; Peracchia, C. Gap junction crystallization in lens fibers after an increase in cell calcium. Investig. Ophthalmol. Vis. Sci. 1981, 21, 291–299. [Google Scholar]
- Sanderson, J.; Gandolfi, S.A.; Duncan, G. Calmodulin antagonists induce changes in lens permeability and transparency. Curr. Eye Res. 1994, 13, 219–224. [Google Scholar] [CrossRef]
- Moncrief, N.D.; Kretsinger, R.H.; Goodman, M. Evolution of EF-hand calcium-modulated proteins. I. Relationships based on amino acid sequences. J. Mol. Evol. 1990, 30, 522–562. [Google Scholar] [CrossRef]
- Beckingham, K. Use of site-directed mutations in the individual Ca2(+)-binding sites of calmodulin to examine Ca2(+)-induced conformational changes. J. Biol. Chem. 1991, 266, 6027–6030. [Google Scholar]
- Halling, D.B.; Liebeskind, B.J.; Hall, A.W.; Aldrich, R.W. Conserved properties of individual Ca2+-binding sites in calmodulin. Proc. Natl. Acad. Sci. USA 2016, 113, E1216–E1225. [Google Scholar] [CrossRef] [Green Version]
- Iimuro, A.; Takehana, M.; Iwata, S. Influence of calmodulin antagonists on Ca2+ transport in the lens. Ophthalmic Res. 1987, 19, 95–100. [Google Scholar] [CrossRef]
- Reichow, S.L.; Gonen, T. Noncanonical binding of calmodulin to aquaporin-0: Implications for channel regulation. Structure 2008, 16, 1389–1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fields, J.B.; Nemeth-Cahalan, K.L.; Freites, J.A.; Vorontsova, I.; Hall, J.E.; Tobias, D.J. Calmodulin Gates Aquaporin 0 Permeability through a Positively Charged Cytoplasmic Loop. J. Biol. Chem. 2017, 292, 185–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girsch, S.J.; Peracchia, C. Lens cell-to-cell channel protein: I. Self-assembly into liposomes and permeability regulation by calmodulin. J. Membr. Biol. 1985, 83, 217–225. [Google Scholar] [CrossRef]
- Girsch, S.J.; Peracchia, C. Lens cell-to-cell channel protein: II. Conformational change in the presence of calmodulin. J. Membr. Biol. 1985, 83, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Peracchia, C. Calcium effects on gap junction structure and cell coupling. Nature 1978, 271, 669–671. [Google Scholar] [CrossRef]
- Loewenstein, W.R.; Nakas, M.; Socolar, S.J. Junctional membrane uncoupling. Permeability transformations at a cell membrane junction. J. Gen. Physiol. 1967, 50, 1865–1891. [Google Scholar] [CrossRef]
- Rose, B.; Loewenstein, W.R. Permeability of a cell junction and the local cytoplasmic free ionized calcium concentration: A study with aequorin. J. Membr. Biol. 1976, 28, 87–119. [Google Scholar] [CrossRef]
- Deleze, J.; Loewenstein, W.R. Permeability of a cell junction during intracellular injection of divalent cations. J. Membr. Biol. 1976, 28, 71–86. [Google Scholar] [CrossRef]
- Dahl, G.; Isenberg, G. Decoupling of heart muscle cells: Correlation with increased cytoplasmic calcium activity and with changes of nexus ultrastructure. J. Membr. Biol. 1980, 53, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Crow, J.M.; Atkinson, M.M.; Johnson, R.G. Micromolar levels of intracellular calcium reduce gap junctional permeability in lens cultures. Investig. Ophthalmol. Vis. Sci. 1994, 35, 3332–3341. [Google Scholar]
- Peracchia, C. Chemical gating of gap junction channels; roles of calcium, pH and calmodulin. Biochim. Biophys. Acta 2004, 1662, 61–80. [Google Scholar] [CrossRef] [Green Version]
- Peracchia, C.; Wang, X.; Li, L.; Peracchia, L.L. Inhibition of calmodulin expression prevents low-pH-induced gap junction uncoupling in Xenopus oocytes. Pflugers Arch. 1996, 431, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Lazrak, A.; Peracchia, C. Gap junction gating sensitivity to physiological internal calcium regardless of pH in Novikoff hepatoma cells. Biophys. J. 1993, 65, 2002–2012. [Google Scholar] [CrossRef] [Green Version]
- Peracchia, C.; Girsch, S.J. Functional modulation of cell coupling: Evidence for a calmodulin-driven channel gate. Am. J. Physiol. 1985, 248, H765–H782. [Google Scholar] [CrossRef]
- Lurtz, M.M.; Louis, C.F. Calmodulin and protein kinase C regulate gap junctional coupling in lens epithelial cells. Am. J. Physiol. Cell Physiol. 2003, 285, C1475–C1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Q.; Kopp, R.F.; Chen, Y.; Yang, J.J.; Roe, M.W.; Veenstra, R.D. Gating of connexin 43 gap junctions by a cytoplasmic loop calmodulin binding domain. Am. J. Physiol. Cell Physiol. 2012, 302, C1548–C1556. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Yang, W.; Lurtz, M.M.; Ye, Y.; Huang, Y.; Lee, H.W.; Chen, Y.; Louis, C.F.; Yang, J.J. Identification of the calmodulin binding domain of connexin 43. J. Biol. Chem. 2007, 282, 35005–35017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, J.; Salarian, M.; Chen, Y.; Zhuo, Y.; Brown, N.E.; Hepler, J.R.; Yang, J.J. Direct visualization of interaction between calmodulin and connexin45. Biochem. J. 2017, 474, 4035–4051. [Google Scholar] [CrossRef] [PubMed]
- Peracchia, C. Calmodulin-Mediated Regulation of Gap Junction Channels. Int. J. Mol. Sci. 2020, 21, 485. [Google Scholar] [CrossRef] [Green Version]
- Saez, J.C.; Berthoud, V.M.; Branes, M.C.; Martinez, A.D.; Beyer, E.C. Plasma membrane channels formed by connexins: Their regulation and functions. Physiol. Rev. 2003, 83, 1359–1400. [Google Scholar]
- Del Corsso, C.; Iglesias, R.; Zoidl, G.; Dermietzel, R.; Spray, D.C. Calmodulin dependent protein kinase increases conductance at gap junctions formed by the neuronal gap junction protein connexin36. Brain Res. 2012, 1487, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.Y.; Laing, J.G.; Kanter, E.M.; Berthoud, V.M.; Bao, M.; Rohrs, H.W.; Townsend, R.R.; Yamada, K.A. Identification of CaMKII phosphorylation sites in Connexin43 by high-resolution mass spectrometry. J. Proteome Res. 2011, 10, 1098–1109. [Google Scholar] [CrossRef] [Green Version]
- Alev, C.; Urschel, S.; Sonntag, S.; Zoidl, G.; Fort, A.G.; Höher, T.; Matsubara, M.; Willecke, K.; Spray, D.C.; Dermietzel, R. The neuronal connexin36 interacts with and is phosphorylated by CaMKII in a way similar to CaMKII interaction with glutamate receptors. Proc. Natl. Acad. Sci. USA 2008, 105, 20964–20969. [Google Scholar] [CrossRef] [Green Version]
- Frederikse, P.; Nandanoor, A.; Kasinathan, C. PTBP-dependent PSD-95 and CamKIIα alternative splicing in the lens. Mol. Vis. 2014, 20, 1660–1667. [Google Scholar] [PubMed]
- Peracchia, C. Calmodulin-Cork Model of Gap Junction Channel Gating-One Molecule, Two Mechanisms. Int. J. Mol. Sci. 2020, 21, 4938. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Riquelme, M.A.; Wang, B.; Bugay, V.; Brenner, R.; Gu, S.; Jiang, J.X. Cataract-associated connexin 46 mutation alters its interaction with calmodulin and function of hemichannels. J. Biol. Chem. 2018, 293, 2573–2585. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Yang, W.; Lurtz, M.M.; Chen, Y.; Jiang, J.; Huang, Y.; Louis, C.F.; Yang, J.J. Calmodulin mediates the Ca2+-dependent regulation of Cx44 gap junctions. Biophys. J. 2009, 96, 2832–2848. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Qu, X.; Su, S.; Guan, L.; Liu, P. A novel mutation in GJA3 associated with congenital Coppock-like cataract in a large Chinese family. Mol. Vis. 2012, 18, 2114–2118. [Google Scholar]
- Ren, Q.; Riquelme, M.A.; Xu, J.; Yan, X.; Nicholson, B.J.; Gu, S.; Jiang, J.X. Cataract-causing mutation of human connexin 46 impairs gap junction, but increases hemichannel function and cell death. PLoS ONE 2013, 8, e74732. [Google Scholar] [CrossRef] [Green Version]
- Trexler, E.B.; Bennett, M.V.; Bargiello, T.A.; Verselis, V.K. Voltage gating and permeation in a gap junction hemichannel. Proc. Natl. Acad. Sci. USA 1996, 93, 5836–5841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngezahayo, A.; Zeilinger, C.; Todt, I.; Marten, I.; Kolb, H.A. Inactivation of expressed and conducting rCx46 hemichannels by phosphorylation. Pflugers Archiv Eur. J. Physiol. 1998, 436, 627–629. [Google Scholar] [CrossRef]
- Jedamzik, B.; Marten, I.; Ngezahayo, A.; Ernst, A.; Kolb, H.A. Regulation of lens rCx46-formed hemichannels by activation of protein kinase C, external Ca(2+) and protons. J. Membr. Biol. 2000, 173, 39–46. [Google Scholar] [CrossRef]
- Walter, W.J.; Zeilinger, C.; Bintig, W.; Kolb, H.A.; Ngezahayo, A. Phosphorylation in the C-terminus of the rat connexin46 (rCx46) and regulation of the conducting activity of the formed connexons. J. Bioenerg. Biomembr. 2008, 40, 397–405. [Google Scholar] [CrossRef]
- Retamal, M.A.; Yin, S.; Altenberg, G.A.; Reuss, L. Modulation of Cx46 hemichannels by nitric oxide. Am. J. Physiol. Cell Physiol. 2009, 296, C1356–C1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Retamal, M.A.; Evangelista-Martínez, F.; León-Paravic, C.G.; Altenberg, G.A.; Reuss, L. Biphasic effect of linoleic acid on connexin 46 hemichannels. Pflugers Archiv Eur. J. Physiol. 2011, 461, 635–643. [Google Scholar] [CrossRef] [Green Version]
- Retamal, M.A.; Fiori, M.C.; Fernandez-Olivares, A.; Linsambarth, S.; Peña, F.; Quintana, D.; Stehberg, J.; Altenberg, G.A. 4-Hydroxynonenal induces Cx46 hemichannel inhibition through its carbonylation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158705. [Google Scholar] [CrossRef]
- Pogoda, K.; Kameritsch, P.; Retamal, M.A.; Vega, J.L. Regulation of gap junction channels and hemichannels by phosphorylation and redox changes: A revision. BMC Cell Biol. 2016, 17 (Suppl. 1), 11. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; De Bock, M.; Decrock, E.; Bol, M.; Gadicherla, A.; Bultynck, G.; Leybaert, L. Connexin targeting peptides as inhibitors of voltage- and intracellular Ca2+-triggered Cx43 hemichannel opening. Neuropharmacology 2013, 75, 506–516. [Google Scholar]
- Zou, J.; Salarian, M.; Chen, Y.; Veenstra, R.; Louis, C.F.; Yang, J.J. Gap junction regulation by calmodulin. FEBS Lett. 2014, 588, 1430–1438. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zou, T.; Liu, Y.; Qi, Y. The gating effect of calmodulin and calcium on the connexin50 hemichannel. Biol. Chem. 2006, 387, 595–601. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, Y. Role of intramolecular interaction in connexin50: Mediating the Ca2+-dependent binding of calmodulin to gap junction. Arch. Biochem. Biophys. 2005, 440, 111–117. [Google Scholar] [CrossRef]
- Rundus, V.R.; Marshall, G.B.; Parker, S.B.; Bales, E.S.; Hertzberg, E.L.; Minkoff, R. Association of cell and substrate adhesion molecules with connexin43 during intramembranous bone formation. Histochem. J. 1998, 30, 879–896. [Google Scholar] [CrossRef]
- Flores, C.E.; Cachope, R.; Nannapaneni, S.; Ene, S.; Nairn, A.C.; Pereda, A.E. Variability of distribution of Ca(2+)/calmodulin-dependent kinase II at mixed synapses on the mauthner cell: Colocalization and association with connexin 35. J. Neurosci. 2010, 30, 9488–9499. [Google Scholar] [CrossRef]
- Sotkis, A.; Wang, X.G.; Yasumura, T.; Peracchia, L.L.; Persechini, A.; Rash, J.E.; Peracchia, C. Calmodulin colocalizes with connexins and plays a direct role in gap junction channel gating. Cell Commun. Adhes. 2001, 8, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Billaud, M.; Lohman, A.W.; Johnstone, S.R.; Biwer, L.A.; Mutchler, S.; Isakson, B.E. Regulation of cellular communication by signaling microdomains in the blood vessel wall. Pharmacol. Rev. 2014, 66, 513–569. [Google Scholar] [CrossRef] [Green Version]
- Tong, X.; Lopez, W.; Ramachandran, J.; Ayad, W.A.; Liu, Y.; Lopez-Rodriguez, A.; Harris, A.L.; Contreras, J.E. Glutathione release through connexin hemichannels: Implications for chemical modification of pores permeable to large molecules. J. Gen. Physiol. 2015, 146, 245–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebihara, L.; Korzyukov, Y.; Kothari, S.; Tong, J.-J. Cx46 hemichannels contribute to the sodium leak conductance in lens fiber cells. Am. J. Physiol. Cell Physiol. 2014, 306, C506–C513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Zhou, Y.; Lin, X.; Wong, H.C.; Xu, Q.; Jiang, J.; Wang, S.; Lurtz, M.M.; Louis, C.F.; Veenstra, R.D.; et al. Molecular interaction and functional regulation of connexin50 gap junctions by calmodulin. Biochem. J. 2011, 435, 711–722. [Google Scholar] [CrossRef]
- Berthoud, V.M.; Ngezahayo, A. Focus on lens connexins. BMC Cell Biol. 2017, 18, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Retamal, M.A.; Froger, N.; Palacios-Prado, N.; Ezan, P.; Sáez, P.J.; Sáez, J.C.; Giaume, C. Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J. Neurosci. 2007, 27, 13781–13792. [Google Scholar] [CrossRef]
- Kar, R.; Riquelme, M.A.; Werner, S.; Jiang, J.X. Connexin 43 channels protect osteocytes against oxidative stress-induced cell death. J. Bone Miner. Res. 2013, 28, 1611–1621. [Google Scholar] [CrossRef] [Green Version]
- Rovegno, M.; Sáez, J.C. Role of astrocyte connexin hemichannels in cortical spreading depression. Biochim. Biophys. Acta Biomembr. 2018, 1860, 216–223. [Google Scholar] [CrossRef]
- Lundgaard, I.; Osório, M.J.; Kress, B.T.; Sanggaard, S.; Nedergaard, M. White matter astrocytes in health and disease. Neuroscience 2014, 276, 161–173. [Google Scholar] [CrossRef] [Green Version]
- Davidson, J.O.; Green, C.R.; Bennet, L.; Nicholson, L.F.; Danesh-Meyer, H.; O’Carroll, S.J.; Gunn, A.J. A key role for connexin hemichannels in spreading ischemic brain injury. Curr. Drug Targets 2013, 14, 36–46. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Riquelme, M.A.; Gu, S.; Jiang, J.X. Regulation of Connexin Gap Junctions and Hemichannels by Calcium and Calcium Binding Protein Calmodulin. Int. J. Mol. Sci. 2020, 21, 8194. https://doi.org/10.3390/ijms21218194
Hu Z, Riquelme MA, Gu S, Jiang JX. Regulation of Connexin Gap Junctions and Hemichannels by Calcium and Calcium Binding Protein Calmodulin. International Journal of Molecular Sciences. 2020; 21(21):8194. https://doi.org/10.3390/ijms21218194
Chicago/Turabian StyleHu, Zhengping, Manuel A. Riquelme, Sumin Gu, and Jean X. Jiang. 2020. "Regulation of Connexin Gap Junctions and Hemichannels by Calcium and Calcium Binding Protein Calmodulin" International Journal of Molecular Sciences 21, no. 21: 8194. https://doi.org/10.3390/ijms21218194
APA StyleHu, Z., Riquelme, M. A., Gu, S., & Jiang, J. X. (2020). Regulation of Connexin Gap Junctions and Hemichannels by Calcium and Calcium Binding Protein Calmodulin. International Journal of Molecular Sciences, 21(21), 8194. https://doi.org/10.3390/ijms21218194





