Molecular Aspects of Mycotoxins—A Serious Problem for Human Health
Abstract
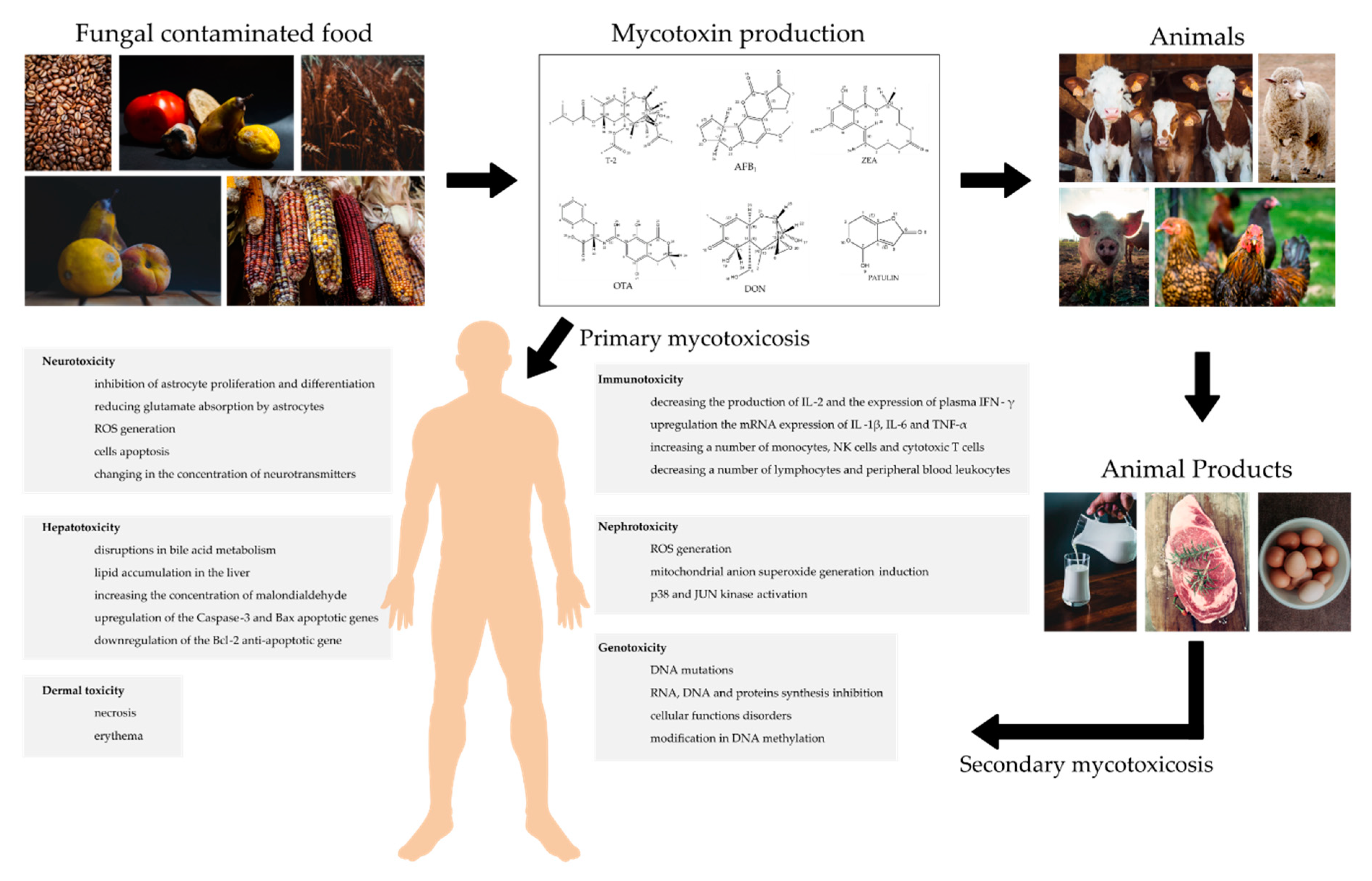
:1. Introduction
2. Aflatoxins
3. Ochratoxin A
4. T-2 Toxin
5. Deoxynivalenol
6. Patulin
7. Zearalenone
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreaviations
| α-ZOL | α-zearalenol |
| β-ZOL | β-zearalenol |
| AFB1 | Aflatoxin B1 |
| AFB2 | Aflatoxin B2 |
| AFG1 | Aflatoxin G1 |
| AFG2 | Aflatoxin G2 |
| AFM1 | Aflatoxin M1 |
| AFP1 | Aflatoxin P1 |
| AFQ1 | Aflatoxin Q1 |
| AST | Aspartate transaminase |
| ATA | Alimentary toxic aleukia |
| BBB | Blood-brain barrier |
| CYP450 | Cytochrome P450 |
| DON | Deoxynivalenol |
| EC | European Commission |
| ER | Endoplasmic reticulum |
| EU | European Union |
| FAO | Food and Agriculture Organization of the United Nations |
| FDA | Food and Drug Administration |
| FHB | Fusarium head blight |
| GLAST | Glutamate aspartate transporter |
| GLT1 | Glutamate transporter 1 |
| GSH | Glutathione |
| HBV | Hepatitis B virus |
| HCC | Hepatocellular carcinoma |
| IARC | International Agency for Research on Cancer |
| JECFA | Joint FAO/WHO Expert Committee on Food Additives |
| JNK | C-jun amino-terminal-kinase |
| KBD | Kashin–Beck disease |
| MAPK | Mitogen-activated protein kinases |
| MDA | Malondialdehyde |
| MFO | Microsomal-mixed function oxidase |
| MMP-9 | Matrix metallopeptidase 9 |
| OTA | Ochratoxin A |
| OTB | Ochratoxin B |
| OTC | Ochratoxin C |
| ROS | Reactive oxygen species |
| TDI | Tolerable daily intake |
| TJP | Tight junction proteins |
| UDPGT | Uridine diphosphate glucuronyl transferases |
| WHO | World Health Organization |
| ZEA | Zearalenone |
References
- Haque, A.; Wang, Y.; Shen, Z.; Li, X.; Saleemi, M.K.; He, C. Mycotoxin contamination and control strategy in human, domestic animal and poultry: A review. Microb. Pathog. 2020, 142, 104095. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Wang, X.; Fu, R.; Yan, H.; Han, S.; Gerelt, K.; Cui, P.; Chen, J.; Qi, K.; Zhou, Y. Determination of six groups of mycotoxins in Chinese dark tea and the associated risk assessment. Environ. Pollut. 2020, 261, 114180. [Google Scholar] [CrossRef] [PubMed]
- De Ruyck, K.; Huybrechts, I.; Yang, S.; Arcella, D.; Claeys, L.; Abbeddou, S.; De Keyzer, W.; De Vries, J.; Ocke, M.; Ruprich, J.; et al. Mycotoxin exposure assessments in a multi-center European validation study by 24-hour dietary recall and biological fluid sampling. Environ. Int. 2020, 137, 105539. [Google Scholar] [CrossRef]
- Deepa, N.; Sreenivasa, M.Y. Chapter 9-Sustainable approaches for biological control of mycotoxigenic fungi and mycotoxins in cereals. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, J.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 149–161. [Google Scholar]
- Huong, B.T.M.; Tuyen, L.D.; Do, T.T.; Madsen, H.; Brimer, L.; Dalsgaard, A. Aflatoxins and fumonisins in rice and maize staple cereals in Northern Vietnam and dietary exposure in different ethnic groups. Food Control 2016, 70, 191–200. [Google Scholar] [CrossRef]
- Sun, Z.; Xu, J.; Wang, G.; Song, A.; Li, C.; Zheng, S. Hydrothermal fabrication of rectorite based biocomposite modified by chitosan derived carbon nanoparticles as efficient mycotoxins adsorbents. Appl. Clay Sci. 2020, 184, 105373. [Google Scholar] [CrossRef]
- Schlosser, O.; Robert, S.; Noyon, N. Airborne mycotoxins in waste recycling and recovery facilities: Occupational exposure and health risk assessment. Waste Manag. 2020, 105, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Thanushree, M.; Sailendri, D.; Yoha, K.; Moses, J.; Anandharamakrishnan, C. Mycotoxin contamination in food: An exposition on spices. Trends Food Sci. Technol. 2019, 93, 69–80. [Google Scholar] [CrossRef]
- Adebiyi, J.A.; Kayitesi, E.; Njobeh, P.B. Mycotoxins reduction in dawadawa (an African fermented condiment) produced from Bambara groundnut (Vigna subterranea). Food Control 2020, 112, 107141. [Google Scholar] [CrossRef]
- Al-Jaal, B.; Salama, S.; Al-Qasmi, N.; Jaganjac, M. Mycotoxin contamination of food and feed in the Gulf Cooperation Council countries and its detection. Toxicon 2019, 171, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Patial, V.; Asrani, R.K.; Thakur, M. Chapter 9 - Food-Borne Mycotoxicoses: Pathologies and Public Health Impact. In Foodborne Diseases; Holban, A.M., Grumezescu, A.M., Eds.; Academic Press: Cambrigde, MA, USA, 2018; pp. 239–274. [Google Scholar]
- Xu, X.; Xu, X.; Han, M.; Qiu, S.; Hou, X. Development of a modified QuEChERS method based on magnetic multiwalled carbon nanotubes for the simultaneous determination of veterinary drugs, pesticides and mycotoxins in eggs by UPLC-MS/MS. Food Chem. 2019, 276, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Ma, J.; Cheng, Y.; Wang, H.; Sun, J.; Yan, Y. One-step rapid detection of fumonisin B1, dexyonivalenol and zearalenone in grains. Food Control 2020, 117, 107107. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.-H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [Green Version]
- Pitt, J.I.; Miller, J.D. A Concise History of Mycotoxin Research. J. Agric. Food Chem. 2017, 65, 7021–7033. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.J.; Sanchis, V.; Marín, S. The prehistory of mycotoxins: Related cases from ancient times to the discovery of aflatoxins. World Mycotoxin J. 2011, 4, 101–112. [Google Scholar] [CrossRef]
- Łukaszuk, C.; Krajewska-Kulak, E.; Guzowski, A.; Kraszyńska, B.; Grassmann, M.; Dobrowolski, R. Analysis of the incidence fungi in a crypt cemetery. J. Air Waste Manag. Assoc. 2015, 65, 1141–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Z.; Nie, D.; Ediage, E.N.; Yang, X.; Wang, J.; Chen, B.; Li, S.; On, S.L.; De Saeger, S.; Wu, A. Cumulative health risk assessment of co-occurring mycotoxins of deoxynivalenol and its acetyl derivatives in wheat and maize: Case study, Shanghai, China. Food Chem. Toxicol. 2014, 74, 334–342. [Google Scholar] [CrossRef]
- Heyndrickx, E.; Sioen, I.; Huybrechts, B.; Callebaut, A.; De Henauw, S.; De Saeger, S. Human biomonitoring of multiple mycotoxins in the Belgian population: Results of the BIOMYCO study. Environ. Int. 2015, 84, 82–89. [Google Scholar] [CrossRef]
- Janik, E.; Ceremuga, M.; Saluk-Bijak, J.; Bijak, M. Biological Toxins as the Potential Tools for Bioterrorism. Int. J. Mol. Sci. 2019, 20, 1181. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A Global Concern for Food Safety, Human Health and Their Management. Front. Microbiol. 2017, 7, 2170. [Google Scholar] [CrossRef] [Green Version]
- Jurišić, N.; E Schwartz-Zimmermann, H.; Kunz-Vekiru, E.; Moll, W.D.; Schweiger, W.; Fowler, J.; Berthiller, F. Determination of aflatoxin biomarkers in excreta and ileal content of chickens. Poult. Sci. 2019, 98, 5551–5561. [Google Scholar] [CrossRef]
- Aristil, J.; Venturini, G.; Maddalena, G.; Toffolatti, S.L.; Spada, A. Fungal contamination and aflatoxin content of maize, moringa and peanut foods from rural subsistence farms in South Haiti. J. Stored Prod. Res. 2020, 85, 101550. [Google Scholar] [CrossRef]
- Wei, T.; Ren, P.; Huang, L.; Ouyang, Z.; Wang, Z.; Kong, X.; Li, T.; Yin, Y.; Wu, Y.; He, Q. Simultaneous detection of aflatoxin B1, ochratoxin A, zearalenone and deoxynivalenol in corn and wheat using surface plasmon resonance. Food Chem. 2019, 300, 125176. [Google Scholar] [CrossRef]
- Gizachew, D.; Chang, C.-H.; Szonyi, B.; De La Torre, S.; Ting, W.-T.E. Aflatoxin B1 (AFB1) production by Aspergillus flavus and Aspergillus parasiticus on ground Nyjer seeds: The effect of water activity and temperature. Int. J. Food Microbiol. 2019, 296, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Wang, H.; Gu, X.; Zheng, X.; Wang, Y.; Diao, J.; Peng, Y.; Zhang, H. Screening and Identification of Novel Ochratoxin A-Producing Fungi from Grapes. Toxins 2016, 8, 333. [Google Scholar] [CrossRef]
- Bragulat, M.; Abarca, M.L.; Castellá, G.; Cabañes, F. Intraspecific variability of growth and ochratoxin A production by Aspergillus carbonarius from different foods and geographical areas. Int. J. Food Microbiol. 2019, 306, 108273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Wu, F. The need to revisit ochratoxin A risk in light of diabetes, obesity, and chronic kidney disease prevalence. Food Chem. Toxicol. 2017, 103, 79–85. [Google Scholar] [CrossRef]
- Nogaim, Q.A.; Bugata, L.S.P.; Prabhakar, P.V.; Reddy, U.A.; Mangala, G.P.; Indu, K.S.; Mahboob, M. Protective effect of Yemeni green coffee powder against the oxidative stress induced by Ochratoxin A. Toxicol. Rep. 2020, 7, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Singh, D.; Raisuddin, S.; Kumar, R. Amelioration of ochratoxin-A induced cytotoxicity by prophylactic treatment of N-Acetyl-l-Tryptophan in human embryonic kidney cells. Toxicology 2019, 429, 152324. [Google Scholar] [CrossRef] [PubMed]
- Kunene, K.; Weber, M.; Sabela, M.; Voiry, D.; Kanchi, S.; Bisetty, K.; Bechelany, M. Highly-efficient electrochemical label-free immunosensor for the detection of ochratoxin A in coffee samples. Sens. Actuators B Chem. 2020, 305, 127438. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, X.; Tang, Z.; Chen, Q.; Liu, X. Development of a biotin-streptavidin-amplified nanobody-based ELISA for ochratoxin A in cereal. Ecotoxicol. Environ. Saf. 2019, 171, 382–388. [Google Scholar] [CrossRef]
- Ryu, D.; Kowalski, R.J.; Ganjyal, G.; Lee, H.J. Reduction of ochratoxin A in oats and rice by twin-screw extrusion processing with baking soda. Food Control 2019, 105, 21–28. [Google Scholar] [CrossRef]
- Freire, L.; Braga, P.A.; Furtado, M.M.; Delafiori, J.; Dias-Audibert, F.L.; Pereira, G.E.; Reyes, F.G.; Catharino, R.R.; Sant’Ana, A.S. From grape to wine: Fate of ochratoxin A during red, rose, and white winemaking process and the presence of ochratoxin derivatives in the final products. Food Control 2020, 113, 107167. [Google Scholar] [CrossRef]
- Do, T.H.; Tran, S.C.; Le, C.D.; Nguyen, B.T.H.; Le, T.T.P.; Le, T.D.; Thai-Nguyen, T.H. Dietary exposure and health risk characterization of aflatoxin B1, ochratoxin A, fumonisin B1, and zearalenone in food from different provinces in Northern Vietnam. Food Control 2020, 112, 107108. [Google Scholar] [CrossRef]
- Kang, R.; Perveen, A.; Li, C. Effects of maternal T-2 toxin exposure on the hepatic glycolipid metabolism in young mice. Ecotoxicol. Environ. Saf. 2020, 196, 110530. [Google Scholar] [CrossRef]
- Sun, Y.; Li, S.; Chen, R.; Wu, P.; Liang, J. Ultrasensitive and rapid detection of T-2 toxin using a target-responsive DNA hydrogel. Sens. Actuators B Chem. 2020, 311, 127912. [Google Scholar] [CrossRef]
- Hemmati, A.A.; Kalantari, H.; Jalali, A.; Rezai, S.; Zadeh, H.H. Healing effect of quince seed mucilage on T-2 toxin-induced dermal toxicity in rabbit. Exp. Toxicol. Pathol. 2012, 64, 181–186. [Google Scholar] [CrossRef]
- Ravindran, J.; Agrawal, M.; Gupta, N.; Rao, P.L. Alteration of blood brain barrier permeability by T-2 toxin: Role of MMP-9 and inflammatory cytokines. Toxicology 2011, 280, 44–52. [Google Scholar] [CrossRef]
- Ec. Commission Recommendation 2013/165/EU of 27 March 2013 on the presence of T-2 and HT-2 toxin in cereals and cereal products. Off. J. Eur. Union 2013, 91, 12–15. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32013H0165&from=EN (accessed on 29 July 2020).
- Kushiro, M. Effects of Milling and Cooking Processes on the Deoxynivalenol Content in Wheat. Int. J. Mol. Sci. 2008, 9, 2127–2145. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Tan, Y.; Liu, N.; Liao, Y.; Sun, C.; Wang, S.; Wu, A. Functional Agents to Biologically Control Deoxynivalenol Contamination in Cereal Grains. Front. Microbiol. 2016, 7, 395. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, S.; Hou, W.; Xiao, P.; Chen, N.; Qiu, P.; Peng, Z.; Liao, Y.; Wang, L.; Li, D.; et al. High contamination levels of deoxynivalenol-induced erythrocyte damage in different models. Trends Food Sci. Technol. 2019, 86, 41–50. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; DiNovi, M.; Grasl-Kraupp, B.; et al. Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2017, 15, e04718. [Google Scholar] [CrossRef]
- Wang, J.-J.; Zhang, R.-Q.; Zhai, Q.-Y.; Liu, J.-C.; Li, N.; Liu, W.-X.; Li, L.; Shen, W. Metagenomic analysis of gut microbiota alteration in a mouse model exposed to mycotoxin deoxynivalenol. Toxicol. Appl. Pharmacol. 2019, 372, 47–56. [Google Scholar] [CrossRef]
- Yu, M.; Chen, L.; Peng, Z.; Nussler, A.K.; Wu, Q.; Liu, L.; Yang, W. Mechanism of deoxynivalenol effects on the reproductive system and fetus malformation: Current status and future challenges. Toxicol. In Vitro 2017, 41, 150–158. [Google Scholar] [CrossRef]
- Barad, S.; Sionov, E.; Prusky, D. Role of patulin in post-harvest diseases. Fungal Biol. Rev. 2016, 30, 24–32. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Malik, S.; Asi, M.R.; Selamat, J.; Malik, N. Natural occurrence of patulin in different fruits, juices and smoothies and evaluation of dietary intake in Punjab, Pakistan. Food Control 2018, 84, 370–374. [Google Scholar] [CrossRef]
- Altunay, N.; Elik, A.; Gürkan, R. A novel, green and safe ultrasound-assisted emulsification liquid phase microextraction based on alcohol-based deep eutectic solvent for determination of patulin in fruit juices by spectrophotometry. J. Food Compos. Anal. 2019, 82, 103256. [Google Scholar] [CrossRef]
- Song, E.; Xia, X.; Su, C.; Dong, W.; Xian, Y.; Wang, W.; Song, Y. Hepatotoxicity and genotoxicity of patulin in mice, and its modulation by green tea polyphenols administration. Food Chem. Toxicol. 2014, 71, 122–127. [Google Scholar] [CrossRef]
- Saleh, I.; Goktepe, I. Health risk assessment of Patulin intake through apples and apple-based foods sold in Qatar. Heliyon 2019, 5, e02754. [Google Scholar] [CrossRef]
- Rogowska, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Złoch, M.; Walczak, J.; Buszewski, B. A study of zearalenone biosorption and metabolisation by prokaryotic and eukaryotic cells. Toxicon 2019, 169, 81–90. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, D.; Peng, J.; Cui, Y.; Shi, Y.; He, H. Magnetic hyperbranched molecularly imprinted polymers for selective enrichment and determination of zearalenone in wheat proceeded by HPLC-DAD analysis. Talanta 2020, 209, 120555. [Google Scholar] [CrossRef]
- Abassi, H.; Ayed-Boussema, I.; Shirley, S.; Abid, S.; Bacha, H.; Micheau, O. The mycotoxin zearalenone enhances cell proliferation, colony formation and promotes cell migration in the human colon carcinoma cell line HCT116. Toxicol. Lett. 2016, 254, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Frizzell, C.; Uhlig, S.; Miles, C.O.; Verhaegen, S.; Elliott, C.T.; Eriksen, G.S.; Sørlie, M.; Ropstad, E.; Connolly, L. Biotransformation of zearalenone and zearalenols to their major glucuronide metabolites reduces estrogenic activity. Toxicol. In Vitro 2015, 29, 575–581. [Google Scholar] [CrossRef]
- Iram, W.; Anjum, T.; Iqbal, M.; Ghaffar, A.; Abbas, M.; Khan, A.M. Structural Analysis and Biological Toxicity of Aflatoxins B1 and B2 Degradation Products Following Detoxification by Ocimum basilicum and Cassia fistula Aqueous Extracts. Front. Microbiol. 2016, 7, 1105. [Google Scholar] [CrossRef]
- Liu, X.; Guan, X.; Xing, F.; Lv, C.; Dai, X.; Liu, Y. Effect of water activity and temperature on the growth of Aspergillus flavus, the expression of aflatoxin biosynthetic genes and aflatoxin production in shelled peanuts. Food Control 2017, 82, 325–332. [Google Scholar] [CrossRef]
- Iram, W.; Anjum, T.; Iqbal, M.; Ghaffar, A.; Abbas, M. Mass spectrometric identification and toxicity assessment of degraded products of aflatoxin B1 and B2 by Corymbia citriodora aqueous extracts. Sci. Rep. 2015, 5, 14672. [Google Scholar] [CrossRef] [Green Version]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin B1 and M1: Biological Properties and Their Involvement in Cancer Development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef]
- Schabo, D.C.; Martins, L.M.; Maciel, J.F.; Iamanaka, B.T.; Taniwaki, M.H.; Schaffner, D.W.; Magnani, M. Production of aflatoxin B1 and B2 by Aspergillus flavus in inoculated wheat using typical craft beer malting conditions. Food Microbiol. 2020, 89, 103456. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, M.; Wang, L.; Liu, M.; Jiang, K.; Xia, S.; Qi, C.; Wang, B. Analysis of the expression of metabolism-related genes and histopathology of the hepatopancreas of white shrimp Litopenaeus vannamei fed with aflatoxin B1. Aquaculture 2018, 485, 191–196. [Google Scholar] [CrossRef]
- Lv, C.; Jin, J.; Wang, P.; Dai, X.; Liu, Y.; Zheng, M.; Xing, F. Interaction of water activity and temperature on the growth, gene expression and aflatoxin production by Aspergillus flavus on paddy and polished rice. Food Chem. 2019, 293, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.; Magnoli, A.; Pereyra, M.G.; Cavaglieri, L. Probiotic bacteria and yeasts adsorb aflatoxin M1 in milk and degrade it to less toxic AFM1-metabolites. Toxicon 2019, 172, 1–7. [Google Scholar] [CrossRef]
- Arenas-Huertero, F.; Zaragoza-Ojeda, M.; Sánchez-Alarcón, J.; Milić, M.; Klarić, M. Šegvić; Montiel-González, J.M.; Valencia-Quintana, R. Involvement of Ahr Pathway in Toxicity of Aflatoxins and Other Mycotoxins. Front. Microbiol. 2019, 10, 2347. [Google Scholar] [CrossRef] [Green Version]
- Rotimi, O.A.; Rotimi, S.O.; Goodrich, J.M.; Adelani, I.B.; Agbonihale, E.; Talabi, G. Time-Course Effects of Acute Aflatoxin B1 Exposure on Hepatic Mitochondrial Lipids and Oxidative Stress in Rats. Front. Pharmacol. 2019, 10, 467. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Chang, P.-K.; Kong, Q.; Shan, S.; Wei, Q. Comparison of aflatoxin production of Aspergillus flavus at different temperatures and media: Proteome analysis based on TMT. Int. J. Food Microbiol. 2019, 310, 108313. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Zheng, N.; Guo, L.; Song, X.; Zhao, S.; Wang, J. Biological System Responses of Dairy Cows to Aflatoxin B1 Exposure Revealed with Metabolomic Changes in Multiple Biofluids. Toxins 2019, 11, 77. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, G.; Evangelista, S.R.; Passamani, F.R.F.; Santiago, W.D.; Cardoso, M.D.G.; Batista, L.R. Influence of temperature and water activity on Ochratoxin A production by Aspergillus strain in coffee south of Minas Gerais/Brazil. LWT 2019, 102, 1–7. [Google Scholar] [CrossRef]
- Bui-Klimke, T.R.; Wu, F. Ochratoxin A and Human Health Risk: A Review of the Evidence. Crit. Rev. Food Sci. Nutr. 2015, 55, 1860–1869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alborch, L.; Bragulat, M.R.; Abarca, M.L.; Cabañes, F.J. Effect of water activity, temperature and incubation time on growth and ochratoxin A production by Aspergillus niger and Aspergillus carbonarius on maize kernels. Int. J. Food Microbiol. 2011, 147, 53–57. [Google Scholar] [CrossRef]
- Giancarlo, B.; Elisabetta, B.; Edmondo, C.; Valeriana, C.; Giuseppina, T. Determination of ochratoxin A in eggs and target tissues of experimentally drugged hens using HPLC–FLD. Food Chem. 2011, 126, 1278–1282. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Nisar, S.; Asi, M.R.; Jinap, S. Natural incidence of aflatoxins, ochratoxin A and zearalenone in chicken meat and eggs. Food Control 2014, 43, 98–103. [Google Scholar] [CrossRef]
- Huong, B.T.M.; Tuyen, L.D.; Tuan, D.H.; Brimer, L.; Dalsgaard, A. Dietary exposure to aflatoxin B 1, ochratoxin A and fuminisins of adults in Lao Cai province, Viet Nam: A total dietary study approach. Food Chem. Toxicol. 2016, 98, 127–133. [Google Scholar] [CrossRef]
- El Khoury, A.; Atoui, A. Ochratoxin A: General Overview and Actual Molecular Status. Toxins 2010, 2, 461–493. [Google Scholar] [CrossRef] [Green Version]
- Khaneghah, A.M.; Fakhri, Y.; Abdi, L.; Coppa, C.C.; Franco, L.T.; Oliveira, C.A.F. The concentration and prevalence of ochratoxin A in coffee and coffee-based products: A global systematic review, meta-analysis and meta-regression. Fungal Biol. 2019, 123, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Freire, L.; Furtado, M.M.; Guerreiro, T.M.; Da Graça, J.S.; Da Silva, B.S.; Oliveira, D.N.; Catharino, R.R.; Sant’Ana, A.S. The presence of ochratoxin A does not influence Saccharomyces cerevisiae growth kinetics but leads to the formation of modified ochratoxins. Food Chem. Toxicol. 2019, 133, 110756. [Google Scholar] [CrossRef]
- Reddy, L.; Bhoola, K. Ochratoxins—Food Contaminants: Impact on Human Health. Toxins 2010, 2, 771–779. [Google Scholar] [CrossRef] [Green Version]
- Kőszegi, T.; Poór, M. Ochratoxin A: Molecular Interactions, Mechanisms of Toxicity and Prevention at the Molecular Level. Toxins 2016, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Giromini, C.; Rebucci, R.; Fusi, E.; Rossi, L.; Saccone, F.; Baldi, A. Cytotoxicity, apoptosis, DNA damage and methylation in mammary and kidney epithelial cell lines exposed to ochratoxin A. Cell Biol. Toxicol. 2016, 32, 249–258. [Google Scholar] [CrossRef]
- Solcan, C.; Timofte, D.; Floristean, V.; Carter, S.D.; Solcan, G. Ultrastructural lesions and immunohistochemical analysis of Bcl-2 protein expression in the kidney of chickens with experimental ochratoxicosis. Acta Vet. Hung. 2013, 61, 344–353. [Google Scholar] [CrossRef]
- Hope, J.H.; Hope, B.E. A Review of the Diagnosis and Treatment of Ochratoxin A Inhalational Exposure Associated with Human Illness and Kidney Disease including Focal Segmental Glomerulosclerosis. J. Environ. Public Health 2011, 2012, 1–10. [Google Scholar] [CrossRef]
- Rutigliano, L.; Valentini, L.; Martino, N.A.; Pizzi, F.; Zanghì, A.; Dell’Aquila, M.E.; Minervini, F. Ochratoxin A at low concentrations inhibits in vitro growth of canine umbilical cord matrix mesenchymal stem cells through oxidative chromatin and DNA damage. Reprod. Toxicol. 2015, 57, 121–129. [Google Scholar] [CrossRef]
- Sorrenti, V.; Di Giacomo, C.; Acquaviva, R.; Barbagallo, I.; Bognanno, M.; Galvano, F. Toxicity of Ochratoxin A and Its Modulation by Antioxidants: A Review. Toxins 2013, 5, 1742–1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, H.S.; Lee, H.J.; Pyo, M.C.; Ryu, D.; Lee, K.-W. Ochratoxin A-Induced Hepatotoxicity through Phase I and Phase II Reactions Regulated by AhR in Liver Cells. Toxins 2019, 11, 377. [Google Scholar] [CrossRef] [Green Version]
- Solcan, C.; Pavel, G.; Floristean, V.; Chiriac, I.S.B.; Şlencu, B.G.; Solcan, G. Effect of ochratoxin A on the intestinal mucosa and mucosa-associated lymphoid tissues in broiler chickens. Acta Vet. Hung. 2015, 63, 30–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Lim, W.; You, S.; Song, G. Ochratoxin A exerts neurotoxicity in human astrocytes through mitochondria-dependent apoptosis and intracellular calcium overload. Toxicol. Lett. 2019, 313, 42–49. [Google Scholar] [CrossRef]
- Razafimanjato, H.; Garmy, N.; Guo, X.-J.; Varini, K.; Di Scala, C.; Di Pasquale, E.; Taïeb, N.; Maresca, M. The food-associated fungal neurotoxin ochratoxin A inhibits the absorption of glutamate by astrocytes through a decrease in cell surface expression of the excitatory amino-acid transporters GLAST and GLT-1. Neurotoxicology 2010, 31, 475–484. [Google Scholar] [CrossRef]
- Bhat, P.V.; Pandareesh; Khanum, F.; Tamatam, A. Cytotoxic Effects of Ochratoxin A in Neuro-2a Cells: Role of Oxidative Stress Evidenced by N-acetylcysteine. Front. Microbiol. 2016, 7, 1142. [Google Scholar] [CrossRef] [Green Version]
- Karami-Osboo, R. Nanofluid extraction of Ochratoxin A in food. J. Food Compos. Anal. 2020, 87, 103425. [Google Scholar] [CrossRef]
- Ling, A.; Sun, L.; Guo, W.; Sun, S.; Yang, J.; Zhao, Z. Individual and combined cytotoxic effects of T-2 toxin and its four metabolites on porcine Leydig cells. Food Chem. Toxicol. 2020, 139, 111277. [Google Scholar] [CrossRef]
- McCormick, S.P.; Price, N.P.J.; Kurtzman, C.P. Glucosylation and Other Biotransformations of T-2 Toxin by Yeasts of the Trichomonascus Clade. Appl. Environ. Microbiol. 2012, 78, 8694–8702. [Google Scholar] [CrossRef] [Green Version]
- Guo, P.; Liu, A.; Huang, D.; Wu, Q.; Fatima, Z.; Tao, Y.; Cheng, G.; Wang, X.; Yuan, Z. Brain damage and neurological symptoms induced by T-2 toxin in rat brain. Toxicol. Lett. 2018, 286, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Dong, Y.; Guo, J.; Jiang, X.; Liu, J.; Xu, S.; Wang, H. Monoclonal antibody production and the development of an indirect competitive enzyme-linked immunosorbent assay for screening T-2 toxin in milk. Toxicon 2018, 156, 1–6. [Google Scholar] [CrossRef]
- Li, D.; Han, J.; Guo, X.; Qu, C.; Yu, F.; Wu, X. The effects of T-2 toxin on the prevalence and development of Kashin–Beck disease in China: A meta-analysis and systematic review. Toxicol. Res. 2016, 5, 731–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pleadin, J.; Vasilj, V.; Kudumija, N.; Petrović, D.; Vilušić, M.; Škrivanko, M. Survey of T-2/HT-2 toxins in unprocessed cereals, food and feed coming from Croatia and Bosnia & Herzegovina. Food Chem. 2017, 224, 153–159. [Google Scholar] [CrossRef]
- Adhikari, M.; Negi, B.; Kaushik, N.; Adhikari, A.; Al-Khedhairy, A.A.; Kaushik, N.K.; Choi, E.H. T-2 mycotoxin: Toxicological effects and decontamination strategies. Oncotarget 2017, 8, 33933–33952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Königs, M.; Mulac, D.; Schwerdt, G.; Gekle, M.; Humpf, H.U. Metabolism and cytotoxic effects of T-2 toxin and its metabolites on human cells in primary culture. Toxicology 2009, 258, 106–115. [Google Scholar] [CrossRef]
- Nathanail, A.V.; Varga, E.; Meng-Reiterer, J.; Bueschl, C.; Michlmayr, H.; Malachova, A.; Fruhmann, P.; Jestoi, M.; Peltonen, K.; Adam, G.; et al. Metabolism of the Fusarium Mycotoxins T-2 Toxin and HT-2 Toxin in Wheat. J. Agric. Food Chem. 2015, 63, 7862–7872. [Google Scholar] [CrossRef]
- Chaudhary, M.; Rao, P.L. Brain oxidative stress after dermal and subcutaneous exposure of T-2 toxin in mice. Food Chem. Toxicol. 2010, 48, 3436–3442. [Google Scholar] [CrossRef]
- Behrens, M.; Hüwel, S.; Galla, H.-J.; Humpf, H.-U. Blood-Brain Barrier Effects of the Fusarium Mycotoxins Deoxynivalenol, 3 Acetyldeoxynivalenol, and Moniliformin and Their Transfer to the Brain. PLoS ONE 2015, 10, e0143640. [Google Scholar] [CrossRef]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and its toxicity. Interdiscip. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Lim, W.; Park, S.; Kim, J.; You, S.; Song, G. Deoxynivalenol induces apoptosis and disrupts cellular homeostasis through MAPK signaling pathways in bovine mammary epithelial cells. Environ. Pollut. 2019, 252, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Bertero, A.; Moretti, A.; Spicer, L.J.; Caloni, F. Fusarium Molds and Mycotoxins: Potential Species-Specific Effects. Toxins 2018, 10, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinton, P.; Tsybulskyy, D.; Lucioli, J.; Laffitte, J.; Callu, P.; Lyazhri, F.; Grosjean, F.; Bracarense, A.P.; Kolf-Clauw, M.; Oswald, I.P. Toxicity of Deoxynivalenol and Its Acetylated Derivatives on the Intestine: Differential Effects on Morphology, Barrier Function, Tight Junction Proteins, and Mitogen-Activated Protein Kinases. Toxicol. Sci. 2012, 130, 180–190. [Google Scholar] [CrossRef]
- Yang, W.; Yu, M.; Fu, J.; Bao, W.; Wang, D.; Hao, L.; Yao, P.; Nüssler, A.K.; Yan, H.; Liu, L. Deoxynivalenol induced oxidative stress and genotoxicity in human peripheral blood lymphocytes. Food Chem. Toxicol. 2014, 64, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Döll, S.; Dänicke, S.; Valenta, H. Residues of deoxynivalenol (DON) in pig tissue after feeding mash or pellet diets containing low concentrations. Mol. Nutr. Food Res. 2008, 52, 727–734. [Google Scholar] [CrossRef]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens—the IARC Monographs classification. Mycotoxin Res. 2016, 33, 65–73. [Google Scholar] [CrossRef]
- Cunha, S.; Faria, M.; Pereira, V.L.; Oliveira, T.; Lima, A.; Pinto, E. Patulin assessment and fungi identification in organic and conventional fruits and derived products. Food Control 2014, 44, 185–190. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, H.; Yang, Q.; Ren, R. Efficacy of Pichia caribbica in controlling blue mold rot and patulin degradation in apples. Int. J. Food Microbiol. 2013, 162, 167–173. [Google Scholar] [CrossRef]
- Tang, H.; Li, X.; Zhang, F.; Meng, X.; Liu, B. Biodegradation of the mycotoxin patulin in apple juice by Orotate phosphoribosyltransferase from Rhodotorula mucilaginosa. Food Control 2019, 100, 158–164. [Google Scholar] [CrossRef]
- Zhu, R.; Feussner, K.; Wu, T.; Yan, F.; Karlovsky, P.; Zheng, X. Detoxification of mycotoxin patulin by the yeast Rhodosporidium paludigenum. Food Chem. 2015, 179, 1–5. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Li, X.; Zhang, Q.H. Determination of trace patulin in apple-based food matrices. Food Chem. 2017, 233, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Saleh, I.; Goktepe, I. The characteristics, occurrence, and toxicological effects of patulin. Food Chem. Toxicol. 2019, 129, 301–311. [Google Scholar] [CrossRef]
- Ahmadi, A.; Danesh, N.M.; Ramezani, M.; Alibolandi, M.; Lavaee, P.; Emrani, A.S.; Abnous, K.; Taghdisi, S.M. A rapid and simple ratiometric fluorescent sensor for patulin detection based on a stabilized DNA duplex probe containing less amount of aptamer-involved base pairs. Talanta 2019, 204, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Pi, F.; Zhang, H.; Sun, J.; Zhang, Y.; Sun, X. A novel molecularly imprinted electrochemical sensor modified with carbon dots, chitosan, gold nanoparticles for the determination of patulin. Biosens. Bioelectron. 2017, 98, 299–304. [Google Scholar] [CrossRef]
- Papp, G.; Horváth, E.; Mike, N.; Gazdag, Z.; Belágyi, J.; Gyöngyi, Z.; Bánfalvi, G.; Hornok, L.; Pesti, M. Regulation of patulin-induced oxidative stress processes in the fission yeast Schizosaccharomyces pombe. Food Chem. Toxicol. 2012, 50, 3792–3798. [Google Scholar] [CrossRef]
- Jayashree, G.V.; Krupashree, K.; Rachitha, P.; Khanum, F. Patulin Induced Oxidative Stress Mediated Apoptotic Damage in Mice, and its Modulation by Green Tea Leaves. J. Clin. Exp. Hepatol. 2017, 7, 127–134. [Google Scholar] [CrossRef]
- Boussabbeh, M.; Ben Salem, I.; Prola, A.; Guilbert, A.; Bacha, H.; Abid-Essefi, S.; Lemaire, C. Patulin Induces Apoptosis through ROS-Mediated Endoplasmic Reticulum Stress Pathway. Toxicol. Sci. 2015, 144, 328–337. [Google Scholar] [CrossRef] [Green Version]
- De Melo, F.T.; De Oliveira, I.M.; Greggio, S.; Dacosta, J.C.; Guecheva, T.N.; Saffi, J.; Henriques, J.A.P.; Rosa, R.M. DNA damage in organs of mice treated acutely with patulin, a known mycotoxin. Food Chem. Toxicol. 2012, 50, 3548–3555. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.; Bahuguna, A.; Kim, M. The effects of mycotoxin patulin on cells and cellular components. Trends Food Sci. Technol. 2019, 83, 99–113. [Google Scholar] [CrossRef]
- Vidal, A.; Ouhibi, S.; Ghali, R.; Hedhili, A.; De Saeger, S.; De Boevre, M. The mycotoxin patulin: An updated short review on occurrence, toxicity and analytical challenges. Food Chem. Toxicol. 2019, 129, 249–256. [Google Scholar] [CrossRef]
- Faisal, Z.; Garai, E.; Csepregi, R.; Bakos, K.; Fliszár-Nyúl, E.; Szente, L.; Balázs, A.; Cserháti, M.; Kőszegi, T.; Urbányi, B.; et al. Protective effects of beta-cyclodextrins vs. zearalenone-induced toxicity in HeLa cells and Tg(vtg1:mCherry) zebrafish embryos. Chemosphere 2020, 240, 124948. [Google Scholar] [CrossRef]
- Złoch, M.; Rogowska, A.; Pomastowski, P.; Railean-Plugaru, V.; Walczak-Skierska, J.; Rudnicka, J.; Buszewski, B. Use of Lactobacillus paracasei strain for zearalenone binding and metabolization. Toxicon 2020, 181, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.-G.; Wang, Y.-D.; Huang, W.-F.; Liu, J.; Yang, X. Molecular reaction mechanism for elimination of zearalenone during simulated alkali neutralization process of corn oil. Food Chem. 2020, 307, 125546. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, L.; Chen, J.; Wang, W.; Zhang, R.; Li, Y.; Zhang, Q.; Wang, W. Degradation mechanism for Zearalenone ring-cleavage by Zearalenone hydrolase RmZHD: A QM/MM study. Sci. Total. Environ. 2020, 709, 135897. [Google Scholar] [CrossRef]
- Malekinejad, H.; Maas-Bakker, R.; Fink-Gremmels, J. Species differences in the hepatic biotransformation of zearalenone. Vet. J. 2006, 172, 96–102. [Google Scholar] [CrossRef]
- Tan, S.-J.; Ge, W.; Wang, J.-J.; Liu, W.-X.; Zhao, Y.; Shen, W.; Li, L. Zearalenone-induced aberration in the composition of the gut microbiome and function impacts the ovary reserve. Chemosphere 2020, 244, 125493. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, W.; Hu, J.; Fan, B.; Shi, J.; Xu, J. Preparative isolation and purification of zearalenone from rice culture by combined use of macroporous resin column and high-speed counter-current chromatography. J. Chromatogr. B 2019, 43–50. [Google Scholar] [CrossRef]
- Dellafiora, L.; Oswald, I.P.; Dorne, J.-L.; Galaverna, G.; Battilani, P.; Dall’Asta, C. An in silico structural approach to characterize human and rainbow trout estrogenicity of mycotoxins: Proof of concept study using zearalenone and alternariol. Food Chem. 2020, 312, 126088. [Google Scholar] [CrossRef] [PubMed]
- Poór, M.; Kunsági-Máté, S.; Sali, N.; Kőszegi, T.; Szente, L.; Peles-Lemli, B. Interactions of zearalenone with native and chemically modified cyclodextrins and their potential utilization. J. Photochem. Photobiol. B 2015, 151, 63–68. [Google Scholar] [CrossRef]
- Zhang, G.-L.; Feng, Y.-L.; Song, J.-L.; Zhou, X.-S. Zearalenone: A Mycotoxin With Different Toxic Effect in Domestic and Laboratory Animals’ Granulosa Cells. Front. Genet. 2018, 9, 667. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yu, H.; Shan, A.; Jin, Y.; Fang, H.; Zhao, Y.; Shen, J.; Zhou, C.; Zhou, Y.; Fu, Y.; et al. Toxic effects of Zearalenone on intestinal microflora and intestinal mucosal immunity in mice. Food Agric. Immunol. 2018, 29, 1002–1011. [Google Scholar] [CrossRef]
- Karaman, E.F.; Zeybel, M.; Ozden, S. Evaluation of the epigenetic alterations and gene expression levels of HepG2 cells exposed to zearalenone and α-zearalenol. Toxicol. Lett. 2020, 326, 52–60. [Google Scholar] [CrossRef]
- Pinton, P.; Oswald, I.P. Effect of Deoxynivalenol and Other Type B Trichothecenes on the Intestine: A Review. Toxins 2014, 6, 1615–1643. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha, M.E.B.; Freire, F.D.C.O.; Maia, F.E.F.; Guedes, M.I.F.; Rondina, D. Mycotoxins and their effects on human and animal health. Food Control 2014, 36, 159–165. [Google Scholar] [CrossRef]
- Edwards, S.G.; Kharbikar, L.L.; Dickin, E.T.; Macdonald, S.; Scudamore, K.A. Impact of pre-harvest rainfall on the distribution of fusarium mycotoxins in wheat mill fractions. Food Control 2018, 89, 150–156. [Google Scholar] [CrossRef]
- Magan, N.; Aldred, D. Post-harvest control strategies: Minimizing mycotoxins in the food chain. Int. J. Food Microbiol. 2007, 119, 131–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, L.; Gan, F.; Zhou, X.; Zhou, Y.; Qian, G.; Liu, Z.; Huang, K. Immunotoxicity of ochratoxin A and aflatoxin B1 in combination is associated with the nuclear factor kappa B signaling pathway in 3D4/21 cells. Chemosphere 2018, 199, 718–727. [Google Scholar] [CrossRef]
- Mitchell, N.J.; Bowers, E.; Hurburgh, C.; Wu, F. Potential economic losses to the US corn industry from aflatoxin contamination. Food Addit. Contam. Part A 2016, 33, 540–550. [Google Scholar] [CrossRef]
- Janssen, E.M.; Mourits, M.; Van Der Fels-Klerx, H.J.; Lansink, A.O. Pre-harvest measures against Fusarium spp. infection and related mycotoxins implemented by Dutch wheat farmers. Crop. Prot. 2019, 122, 9–18. [Google Scholar] [CrossRef]
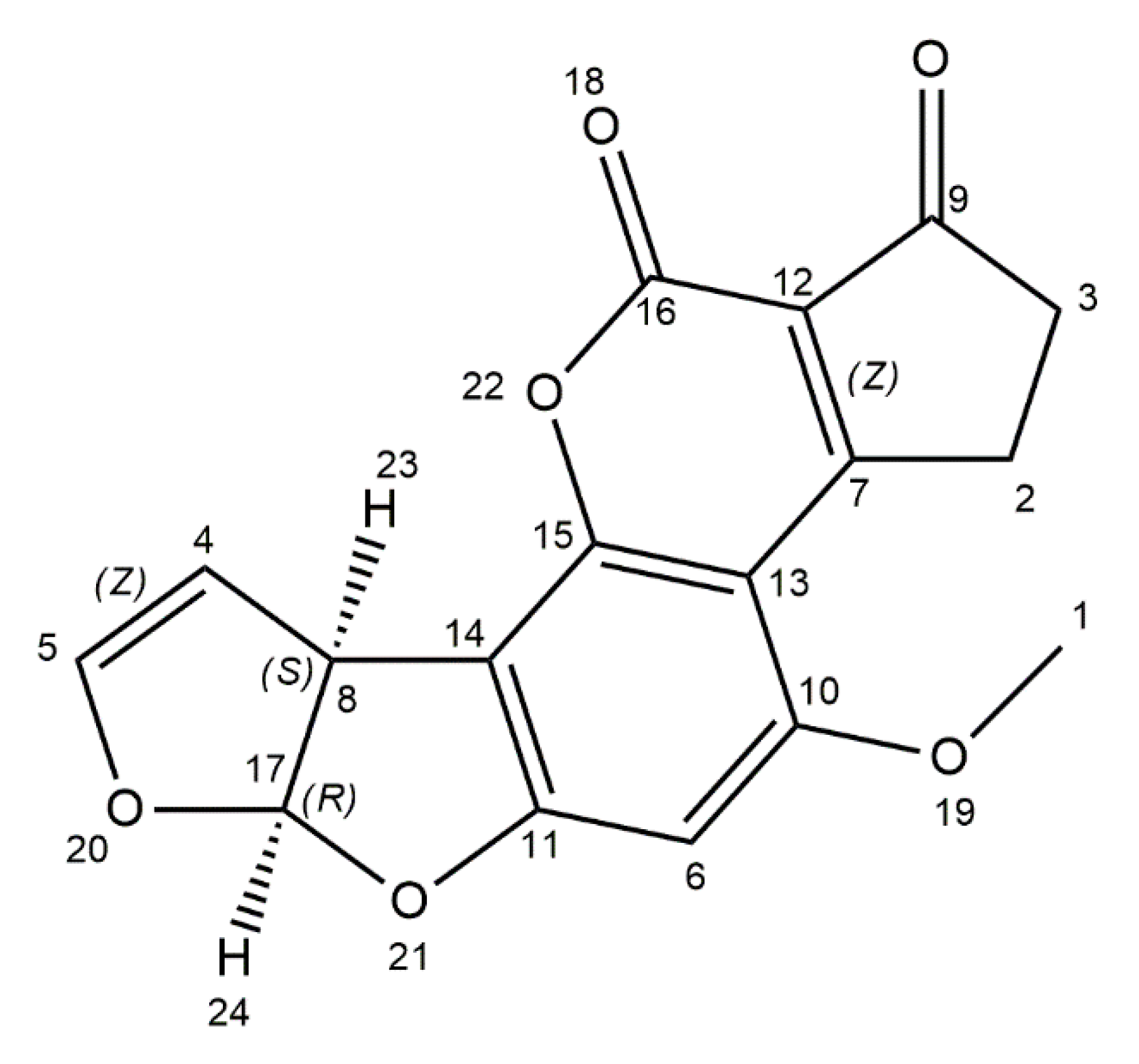
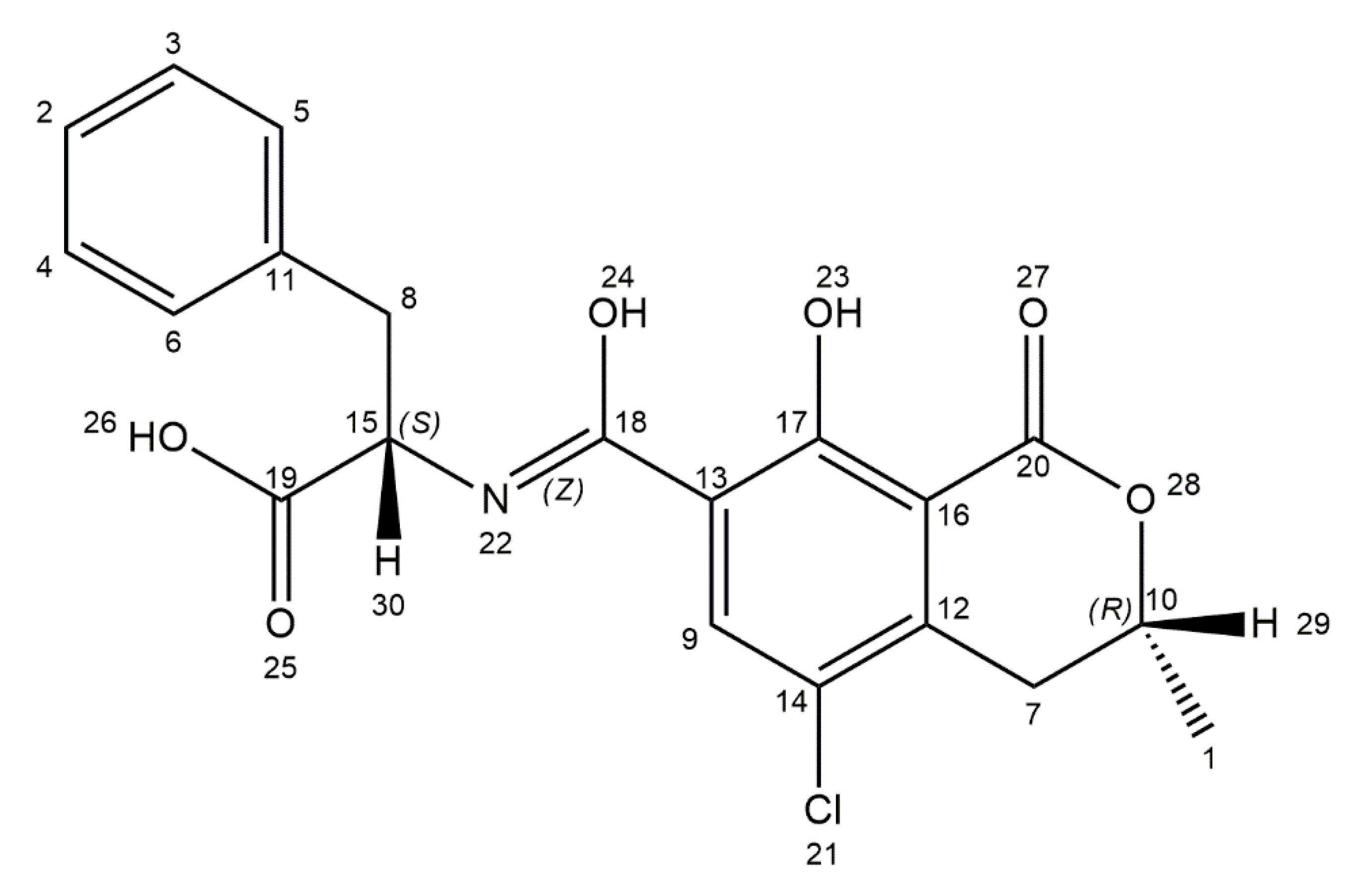

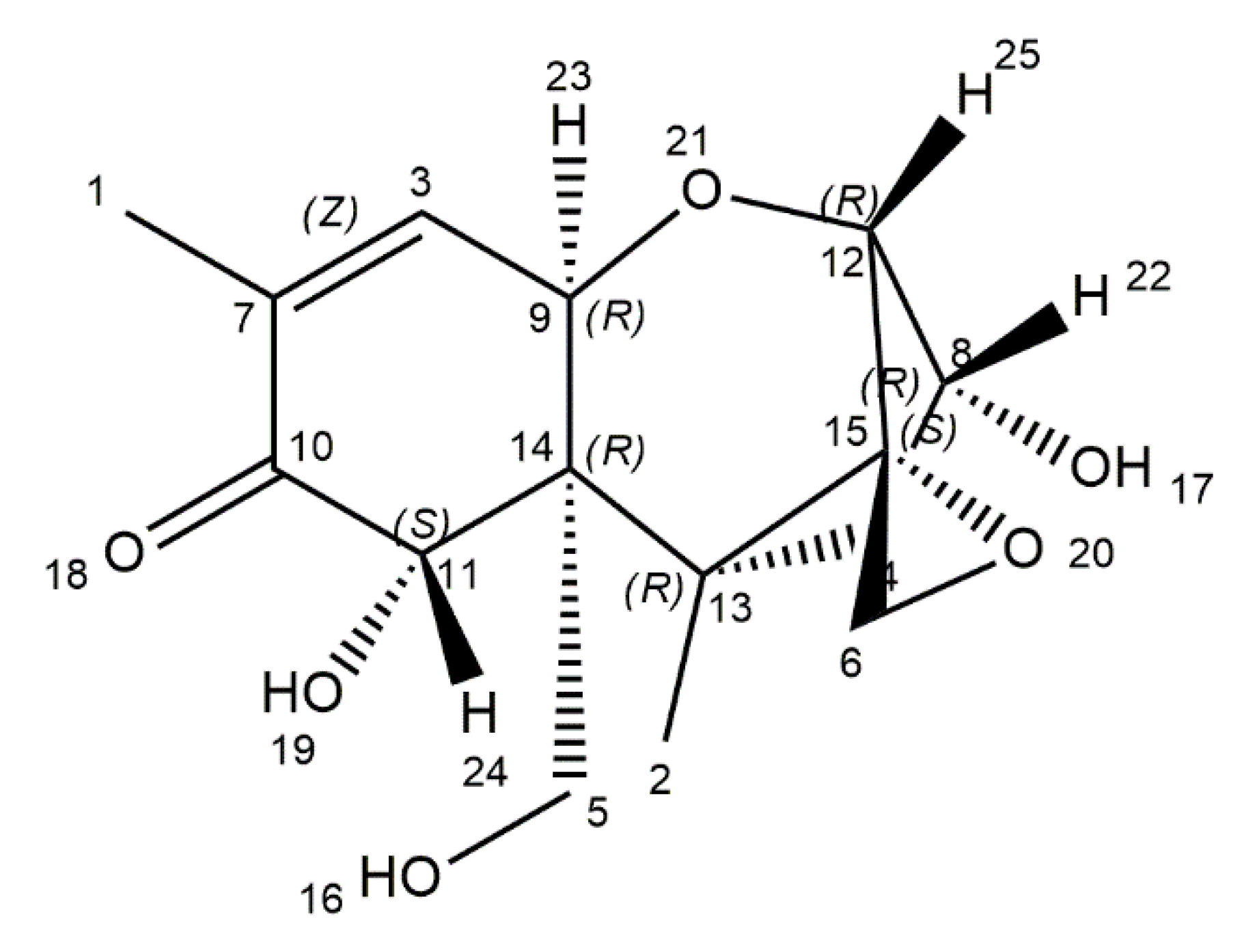


| Mycotoxin | Mould Species | Food Commodity | Pathological Effects | Regulation Levels in Food | References |
|---|---|---|---|---|---|
| Aflatoxins | Aspergillus flavus, A. parasiticus, A. nomius | Wheat, walnut, maize, cotton, peanuts, maize, eggs, milk, meat | Hepatotoxicity, teratogenicity, immunotoxicity, carcinogenicity | European Union (EU): 2 μg/kg (cereals, all cereal derived products) Food and Drug Administration (FDA): 20 μg/kg (dairy animal feed) China: 5 μg/kg (barley, wheat) 20 μg/kg (corn and corn products) | [21,22,23,24,25] |
| Ochratoxin A | Aspergillus ochraceus, A. carbonarius, Penicillium verrucosum, P. nordicum | Coffee beans, oats, wheat, maize, wine, dried fruits, spices, eggs, meat | Nephrotoxicity, hepatotoxicity, genotoxicity, teratogenicity, immunotoxicity neurotoxicity | EU: 3 μg/kg (cereal products), 5 μg/kg (unprocessed cereal), 10 μg/kg (dried fruits), 15 μg/kg (spices) European Commission (EC): 5 ng/kg (coffee beans), 10 ng/kg (instant coffee), 0.5 μg/kg (cereal-based food) 2 μg/kg (wines) Joint FAO/WHO Expert Committee on Food Additives (JECFA): 0.1 μg/kg b. w. per week | [26,27,28,29,30,31,32,33,34,35] |
| T-2 toxin | Fusarium sporotrichoides, F. poae, F. acuminatum, F. equiseti | Barley, oats, wheat | Dermal toxicity, immunotoxicity, hepatotoxicity, neurotoxicity | EU: 100 ng/kg body weight per day | [36,37,38,39,40] |
| Deoxynivalenol | Fusarium graminearum, F. culmorum | Wheat, maize | Immunotoxicity, reproductive system toxicity, genotoxicity, gastrointestinal toxicity, neurotoxicity | JECFA: 1 μg/kg b. w. per day | [41,42,43,44,45,46] |
| Patulin | Penicillium expansum, P. carneum, P. coprobium, Aspergillus clavatus, A. giganteus, Byssochlamys nivea, Paecilomyces saturatus | Apples, grapes, plums, peaches, pears, tomatoes | Hepatotoxicity, nephrotoxicity, immunotoxicity, genotoxicity, teratogenicity, neurotoxicity | World Health Organization (WHO): 50 μg/kg (apples), 50 μg/L (apple juice), and 10 μg/L (young children and infants apple-based food) | [47,48,49,50,51] |
| Zearalenone | Fusarium graminearum, F. cerealis, F. CulmorumF. equiseti | Maize, barley, oats, sorghum and wheat | Reproductive system disorders, hepatotoxicity, immunotoxicity, genotoxicity, | JECFA: 0.5 μg/kg body weight | [52,53,54,55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janik, E.; Niemcewicz, M.; Ceremuga, M.; Stela, M.; Saluk-Bijak, J.; Siadkowski, A.; Bijak, M. Molecular Aspects of Mycotoxins—A Serious Problem for Human Health. Int. J. Mol. Sci. 2020, 21, 8187. https://doi.org/10.3390/ijms21218187
Janik E, Niemcewicz M, Ceremuga M, Stela M, Saluk-Bijak J, Siadkowski A, Bijak M. Molecular Aspects of Mycotoxins—A Serious Problem for Human Health. International Journal of Molecular Sciences. 2020; 21(21):8187. https://doi.org/10.3390/ijms21218187
Chicago/Turabian StyleJanik, Edyta, Marcin Niemcewicz, Michal Ceremuga, Maksymilian Stela, Joanna Saluk-Bijak, Adrian Siadkowski, and Michal Bijak. 2020. "Molecular Aspects of Mycotoxins—A Serious Problem for Human Health" International Journal of Molecular Sciences 21, no. 21: 8187. https://doi.org/10.3390/ijms21218187
APA StyleJanik, E., Niemcewicz, M., Ceremuga, M., Stela, M., Saluk-Bijak, J., Siadkowski, A., & Bijak, M. (2020). Molecular Aspects of Mycotoxins—A Serious Problem for Human Health. International Journal of Molecular Sciences, 21(21), 8187. https://doi.org/10.3390/ijms21218187






